On the Aromaticity and 13C-NMR Pattern of Pentagonal-Pyramidal Hexamethylbenzene Dication [C6(CH3)6]2+: A {C5(CH3)5}−–{CCH3}3+ Aggregate
Abstract
:1. Introduction
2. Computational Details
3. Results and Conclusion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Minkin, V.I.; Minyaev, R.M.; Hoffmann, R. Non-classical structures of organic compounds: Unusual stereochemistry and hypercoordination. Russ. Chem. Rev. 2002, 71, 869–892. [Google Scholar] [CrossRef] [Green Version]
- Hogeveen, H.; Kwant, P.W. Chemistry and spectroscopy in strongly acidic solutions. XL. (CCH3)62+, an unusual dication. J. Am. Chem. Soc. 1974, 96, 2208–2214. [Google Scholar] [CrossRef]
- Hogeveen, H.; Kwant, P.W. Pyramidal mono- and dications. Bridge between organic and organometallic chemistry. Accounts Chem. Res. 1975, 8, 413–420. [Google Scholar] [CrossRef]
- Jemmis, E.D.; Jayasree, E.G.; Parameswaran, P. Hypercarbons in polyhedral structures. Chem. Soc. Rev. 2006, 35, 157–168. [Google Scholar] [CrossRef]
- Marx, D. CH5+: The cheshire cat smiles. Science 1999, 284, 59–61. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.C.; Crittenden, D.L.; Jordan, M.J.T. CH5+: Chemistry’s chameleon unmasked. J. Am. Chem. Soc. 2005, 127, 4954–4958. [Google Scholar] [CrossRef]
- Vassilev-Galindo, V.; Pan, S.; Donald, K.J.; Merino, G. Planar pentacoordinate carbons. Nat. Rev. Chem. 2018, 2, 0114. [Google Scholar] [CrossRef]
- Boldyrev, A.I.; Simons, J. Tetracoordinated planar carbon in pentaatomic molecules. J. Am. Chem. Soc. 1998, 120, 7967–7972. [Google Scholar] [CrossRef]
- Heine, T.; Merino, G. What is the maximum coordination number in a planar structure? Angew. Chem. Int. Ed. 2012, 51, 4275–4276. [Google Scholar] [CrossRef] [PubMed]
- Yañez, O.; Báez-Grez, R.; Garza, J.; Pan, S.; Barroso, J.; Vásquez-Espinal, A.; Merino, G.; Tiznado, W. Embedding a planar hypercoordinate carbon atom into a [4n+2] π-System. ChemPhysChem 2019, 21, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Trindle, C.; Altun, Z.; Bleda, E.A. Bonding analysis of compounds with unusual coordination of carbon: Proposed symmetric systems with six-coordinate carbon. Molecule 2020, 25, 3937. [Google Scholar] [CrossRef] [PubMed]
- Scholz, F.; Himmel, D.; Heinemann, F.W.; Schleyer, P.V.R.; Meyer, K.; Krossing, I. Crystal structure determination of the nonclassical 2-norbornyl cation. Science 2013, 341, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Malischewski, M.; Seppelt, K. Crystal structure determination of the pentagonal-pyramidal hexamethylbenzene dication C6 (CH3)6 2+. Angew. Chem. Int. Ed. 2017, 56, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, H.; Kwant, P.W. Direct observation of a remarkably stable dication of unusual structure: (CCH3)62⊕. Tetrahedron Lett. 1973, 14, 1665–1670. [Google Scholar] [CrossRef]
- Hogeveen, H.; Kwant, P.W.; Postma, J.; van Duynen, P.T. Electronic spectra of pyramidal dications, (CCH362+ and (CH)62+. Tetrahedron Lett. 1974, 15, 4351–4354. [Google Scholar] [CrossRef]
- Hogeveen, H.; Van Kruchten, E.M.G.A. Isotopic perturbation of the carbon-13 nuclear magnetic resonance spectrum of a pyramidal dication. J. Org. Chem. 1981, 46, 1350–1353. [Google Scholar] [CrossRef]
- Gershoni-Poranne, R.; Stanger, A. The NICS-XY-Scan: Identification of local and global ring currents in multi-ring systems. Chem.-A Eur. J. 2014, 20, 5673–5688. [Google Scholar] [CrossRef]
- Steiner, E.; Fowler, P.W. Ring currents in aromatic hydrocarbons. Int. J. Quantum Chem. 1996, 60, 609–616. [Google Scholar] [CrossRef]
- Islas, R.; Heine, T.; Merino, G. The induced magnetic field. Acc. Chem. Res. 2012, 45, 215–228. [Google Scholar] [CrossRef]
- Merino, G.; Heine, T.; Seifert, G. The induced magnetic field in cyclic molecules. Chem. -A Eur. J. 2004, 10, 4367–4371. [Google Scholar] [CrossRef]
- Pople, J.A.; Untch, K.G. Induced paramagnetic ring currents. J. Am. Chem. Soc. 1966, 88, 4811–4815. [Google Scholar] [CrossRef]
- Gershoni-Poranne, R.; Stanger, A. Magnetic criteria of aromaticity. Chem. Soc. Rev. 2015, 44, 6597–6615. [Google Scholar] [CrossRef] [PubMed]
- Benassi, R.; Lazzeretti, P.; Taddei, F. Magnetic criteria for aromaticity. J. Phys. Chem. 1975, 79, 848–851. [Google Scholar] [CrossRef]
- Bird, C.W. The relationship of classical and magnetic criteria of aromaticity. Tetrahedron 1996, 52, 9945–9952. [Google Scholar] [CrossRef]
- Steiner, E.; Fowler, P.W. On the orbital analysis of magnetic properties. Phys. Chem. Chem. Phys. 2004, 6, 261–272. [Google Scholar] [CrossRef]
- Von Schleyer, P.R.; Jiao, H. What is aromaticity? Pure Appl. Chem. 1996, 68, 209–218. [Google Scholar] [CrossRef]
- Kulichenko, M.; Fedik, N.; Boldyrev, A.; Muñoz-Castro, A. Expansion of magnetic aromaticity criteria to multilayer structures: Magnetic response and spherical aromaticity of matryoshka-like cluster [Sn@Cu12@Sn20]12−. Chem.-A Eur. J. 2020, 26, 2263–2268. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, M.; Kanatomi, Y.; Minoura, M.; Hatanaka, M.; Morokuma, K.; Ishimura, K.; Saito, M. Double aromaticity arising from σ- and π-rings. Commun. Chem. 2018, 1, 60. [Google Scholar] [CrossRef] [Green Version]
- Heine, T.; Islas, R.; Merino, G. σ and π contributions to the induced magnetic field: Indicators for the mobility of electrons in molecules. J. Comput. Chem. 2007, 28, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Lazzeretti, P. Assessment of aromaticity via molecular response properties. Phys. Chem. Chem. Phys. 2004, 6, 217–223. [Google Scholar] [CrossRef]
- Kaupp, M.; Bühl, M.; Malkin, V.G. Calculation of NMR and EPR Parameters: Theory and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Papadopoulos, A.G.; Charistos, N.D.; Muñoz-Castro, A. Magnetic response of aromatic rings under rotation: Aromatic shielding cone of benzene upon different orientations of the magnetic field. ChemPhysChem 2017, 18, 1499–1502. [Google Scholar] [CrossRef]
- Ehnbom, A.; Hall, M.B.; Gladysz, J.A. Origin of shielding and deshielding effects in NMR spectra of organic conjugated polyynes. Org. Lett. 2019, 21, 753–757. [Google Scholar] [CrossRef]
- Vernet, R.D.; Boekelheide, V. Nuclear magnetic resonance spectroscopy. Ring-current effects on carbon-13 chemical shifts. Proc. Natl. Acad. Sci. USA 1974, 71, 2961–2964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.E.; Bovey, F.A. Calculation of nuclear magnetic resonance spectra of aromatic hydrocarbons. J. Chem. Phys. 1958, 29, 1012–1014. [Google Scholar] [CrossRef]
- Yannoni, C.S.; Johnson, R.D.; Meijer, G.; Bethune, D.S.; Salem, J.R. Carbon-13 NMR study of the C60 cluster in the solid state: Molecular motion and carbon chemical shift anisotropy. J. Phys. Chem. 1991, 95, 9–10. [Google Scholar] [CrossRef]
- Orendt, A.M.; Facelli, J.C.; Bai, S.; Rai, A.; Gossett, M.; Scott, L.T.; Boerio-Goates, J.; Pugmire, R.J.; Grant, D.M. Carbon-13 shift tensors in polycyclic aromatic compounds. 8.1a low-temperature NMR study of coronene and corannulene. J. Phys. Chem. A 2000, 104, 149–155. [Google Scholar] [CrossRef]
- Muñoz-Castro, A. Axis-dependent magnetic behavior of C60and C6010+. consequences of spherical aromatic character. Chem. Commun. 2015, 51, 10287–10290. [Google Scholar] [CrossRef] [PubMed]
- SCM. ADF Code; Vrije Universiteit: Amsterdam, The Netherlands, 2019; Available online: http://www.scm.com (accessed on 5 October 2021).
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Schreckenbach, G.; Ziegler, T. Calculation of NMR shielding tensors using gauge-including atomic orbitals and modern density functional theory. J. Phys. Chem. 1995, 99, 606–611. [Google Scholar] [CrossRef]
- Wolff, S.K.; Ziegler, T.; Van Lenthe, E.; Baerends, E.J. Density functional calculations of nuclear magnetic shieldings using the zeroth-order regular approximation (ZORA) for relativistic effects: ZORA nuclear magnetic resonance. J. Chem. Phys. 1999, 110, 7689–7698. [Google Scholar] [CrossRef] [Green Version]
- Handy, N.C.; Cohen, A. Left-right correlation energy. Mol. Phys. 2001, 99, 403–412. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Ziarelli, F.; Caldarelli, S. Solid-state NMR as an analytical tool: Quantitative aspects. Solid State Nucl. Magn. Reson. 2006, 29, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Heine, T.; Corminboeuf, C.; Seifert, G. The magnetic shielding function of molecules and pi-electron delocalization. Chem. Rev. 2005, 105, 3889–3910. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, P.V.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J. Nucleus-independent chemical shifts: A simple and efficient aromaticity probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Cotton, F. Chemical Applications of Group Theory; Wiley-Interscience: Hoboken, NJ, USA, 1990. [Google Scholar]
- Klein, J.E.M.N.; Havenith, R.W.A.; Knizia, G. The pentagonal-pyramidal hexamethylbenzene dication: Many shades of coordination chemistry at Carbon. Chem. -A Eur. J. 2018, 24, 12340–12345. [Google Scholar] [CrossRef] [Green Version]
- Kaupp, M. Interpretation of NMR chemical shifts. In Calculation of NMR and EPR Parameters; Wiley-VCH Verlag GmbH {&} Co. KGaA: Weinheim, Germany, 2004; pp. 293–306. [Google Scholar] [CrossRef]
- Chan, J.C.C. Solid state NMR. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2012; Volume 306, ISBN 978-3-642-24802-3. [Google Scholar]
- Haeberlen, U. High resolution Nmr in solids: Selective averaging. In Advances Hin Magnetich Resolution Nmr in Solids Selective Averaginceg; Academic Press: New York, NY, USA, 1976. [Google Scholar]
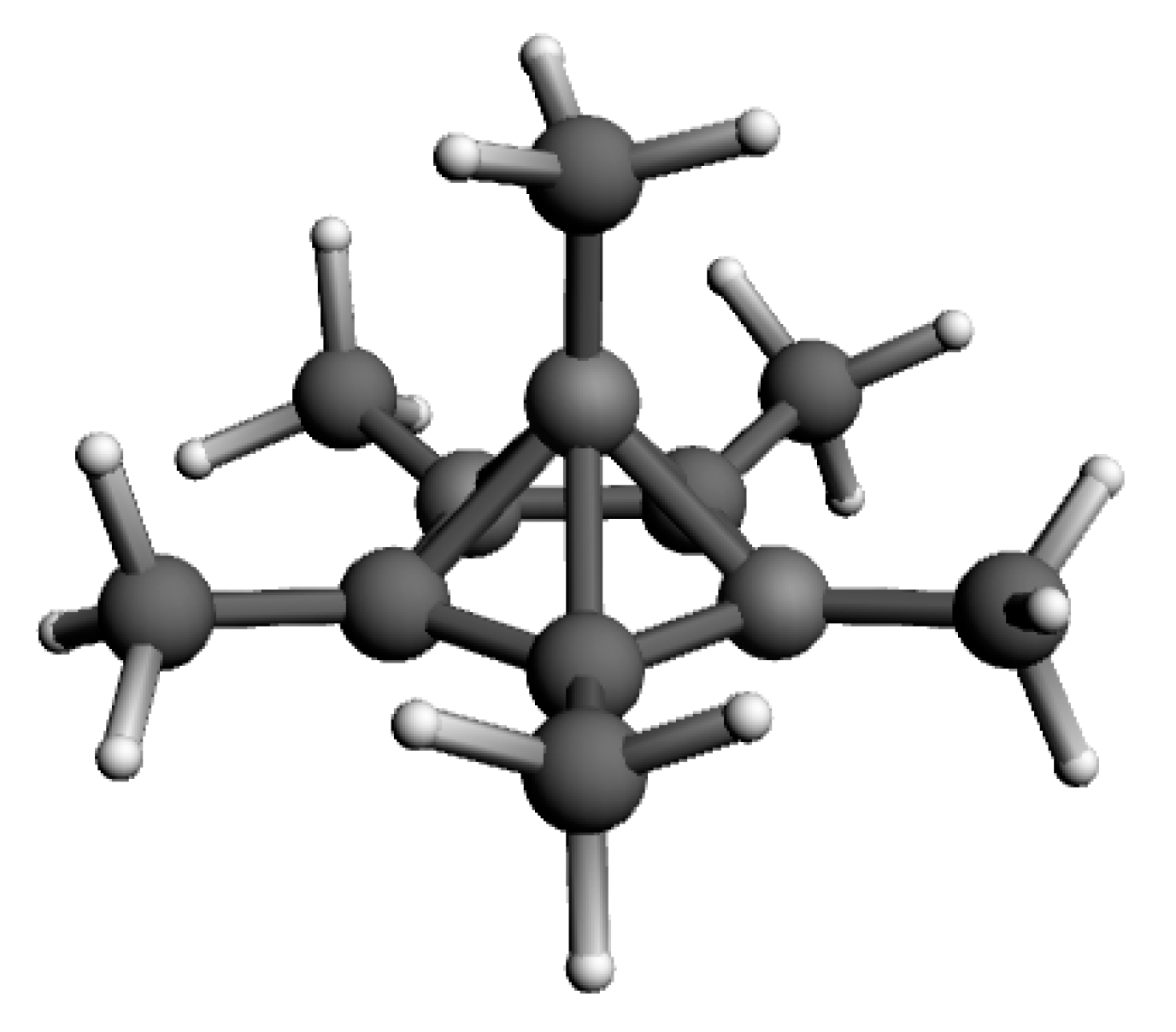


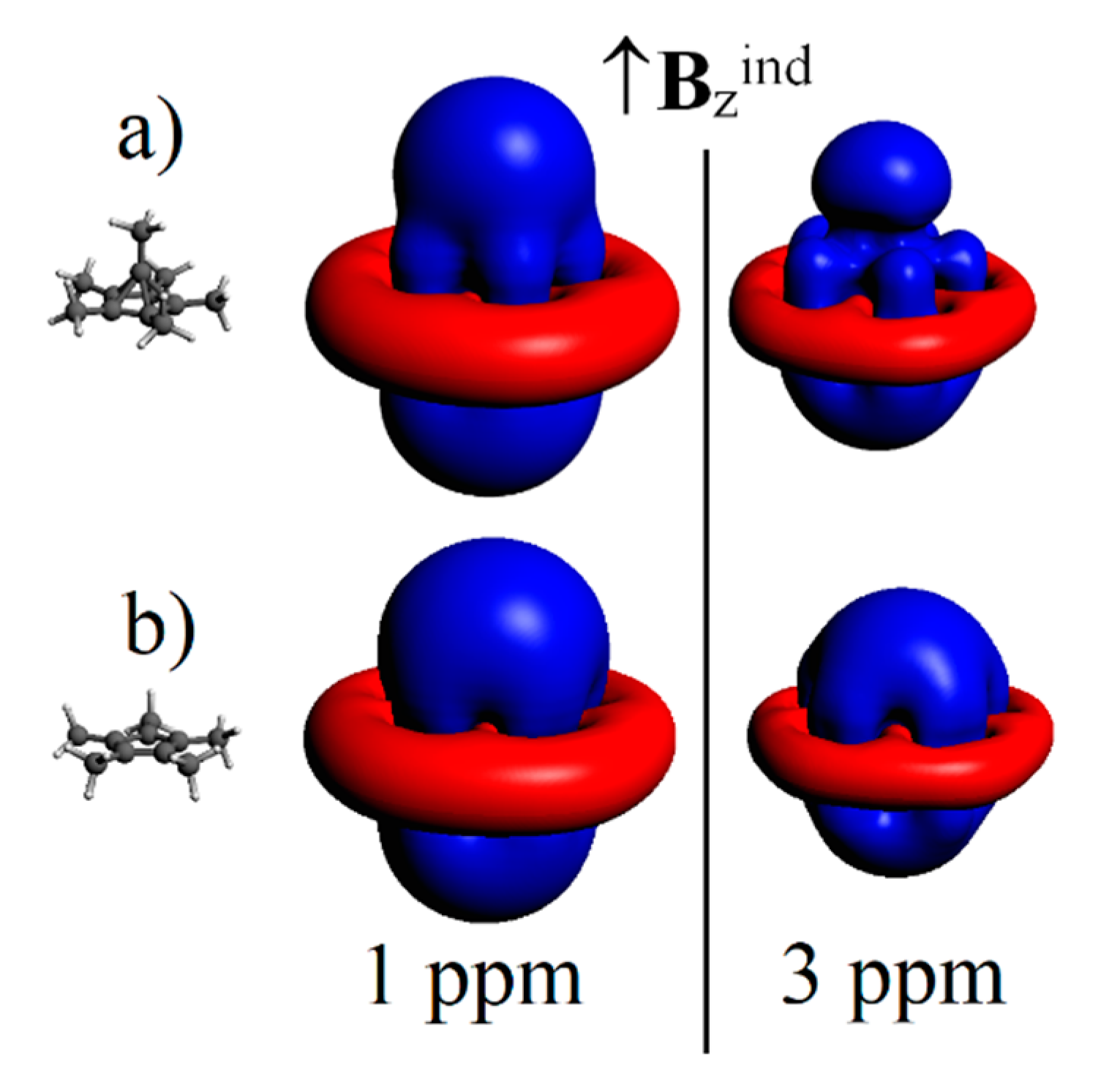
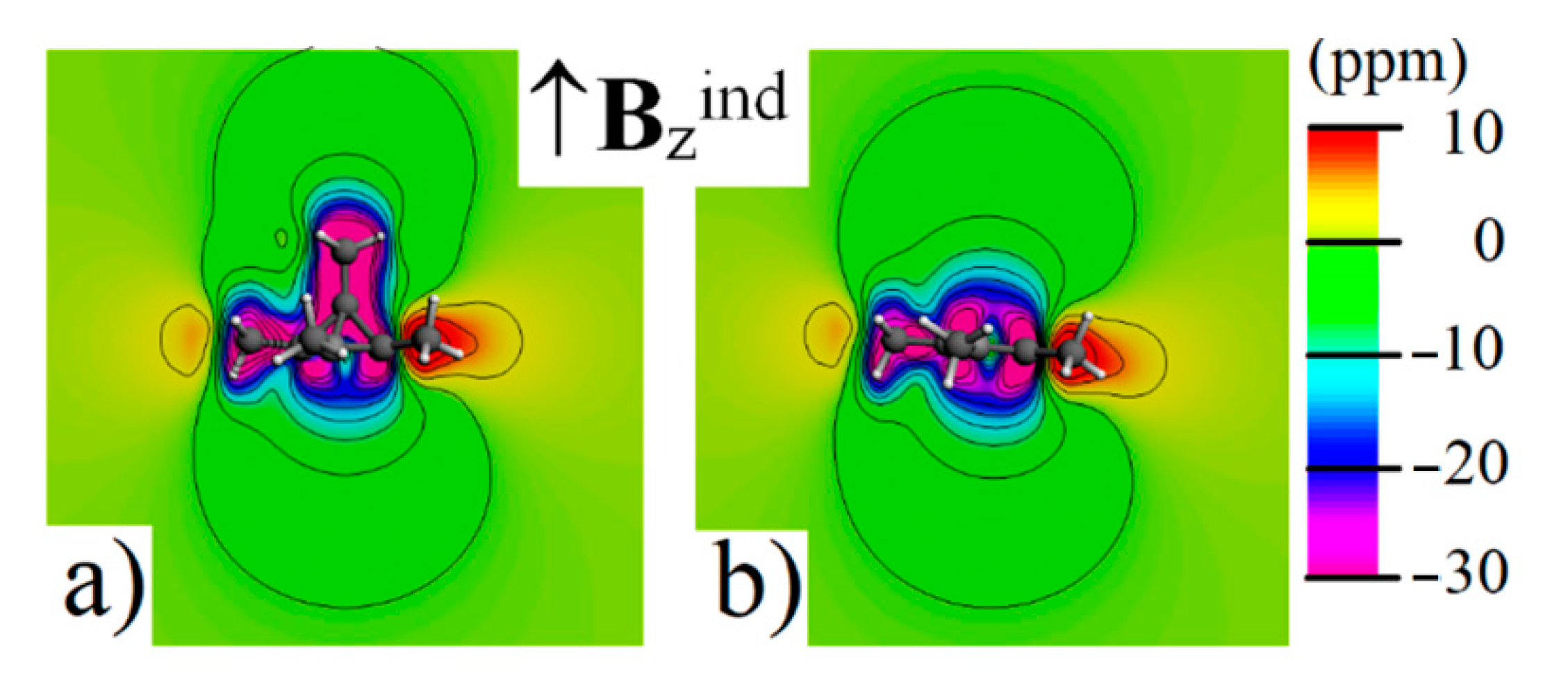
| σ11 | σ22 | σ33 | σiso | δ Shift | Exp. δ Shift a | |
|---|---|---|---|---|---|---|
| C5(CH3)5− | ||||||
| C5 b | 21.8 | 92.5 | 151.9 | 88.7 | 99.3 | |
| CMe1 b | 162.7 | 176.0 | 192.9 | 177.2 | 10.8 | |
| C6(CH3)62+ | ||||||
| C5 b | −14.7 | 45.2 | 154.9 | 61.8 | 126.2 | 125.3 |
| C b | 130.1 | 136.2 | 230.5 | 165.6 | 22.4 | 21.0 |
| CMe1 b | 163.5 | 179.2 | 194.1 | 178.9 | 9.1 | 8.2 |
| CMe2 b | 184.1 | 184.5 | 211.4 | 193.3 | −5.3 | −4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacLeod-Carey, D.; Muñoz-Castro, A. On the Aromaticity and 13C-NMR Pattern of Pentagonal-Pyramidal Hexamethylbenzene Dication [C6(CH3)6]2+: A {C5(CH3)5}−–{CCH3}3+ Aggregate. Chemistry 2021, 3, 1363-1370. https://doi.org/10.3390/chemistry3040097
MacLeod-Carey D, Muñoz-Castro A. On the Aromaticity and 13C-NMR Pattern of Pentagonal-Pyramidal Hexamethylbenzene Dication [C6(CH3)6]2+: A {C5(CH3)5}−–{CCH3}3+ Aggregate. Chemistry. 2021; 3(4):1363-1370. https://doi.org/10.3390/chemistry3040097
Chicago/Turabian StyleMacLeod-Carey, Desmond, and Alvaro Muñoz-Castro. 2021. "On the Aromaticity and 13C-NMR Pattern of Pentagonal-Pyramidal Hexamethylbenzene Dication [C6(CH3)6]2+: A {C5(CH3)5}−–{CCH3}3+ Aggregate" Chemistry 3, no. 4: 1363-1370. https://doi.org/10.3390/chemistry3040097
APA StyleMacLeod-Carey, D., & Muñoz-Castro, A. (2021). On the Aromaticity and 13C-NMR Pattern of Pentagonal-Pyramidal Hexamethylbenzene Dication [C6(CH3)6]2+: A {C5(CH3)5}−–{CCH3}3+ Aggregate. Chemistry, 3(4), 1363-1370. https://doi.org/10.3390/chemistry3040097







