Investigation of SiO2 Nanoparticle Retention in Flow Channels, Its Remediation Using Surfactants and Relevance of Artificial Intelligence in the Future
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of the Two-Step silica Nanofluid
2.3. Nanoparticle Retention Experiments
3. Results and Discussion
3.1. Synthesis of Two-Step silica Nanofluids
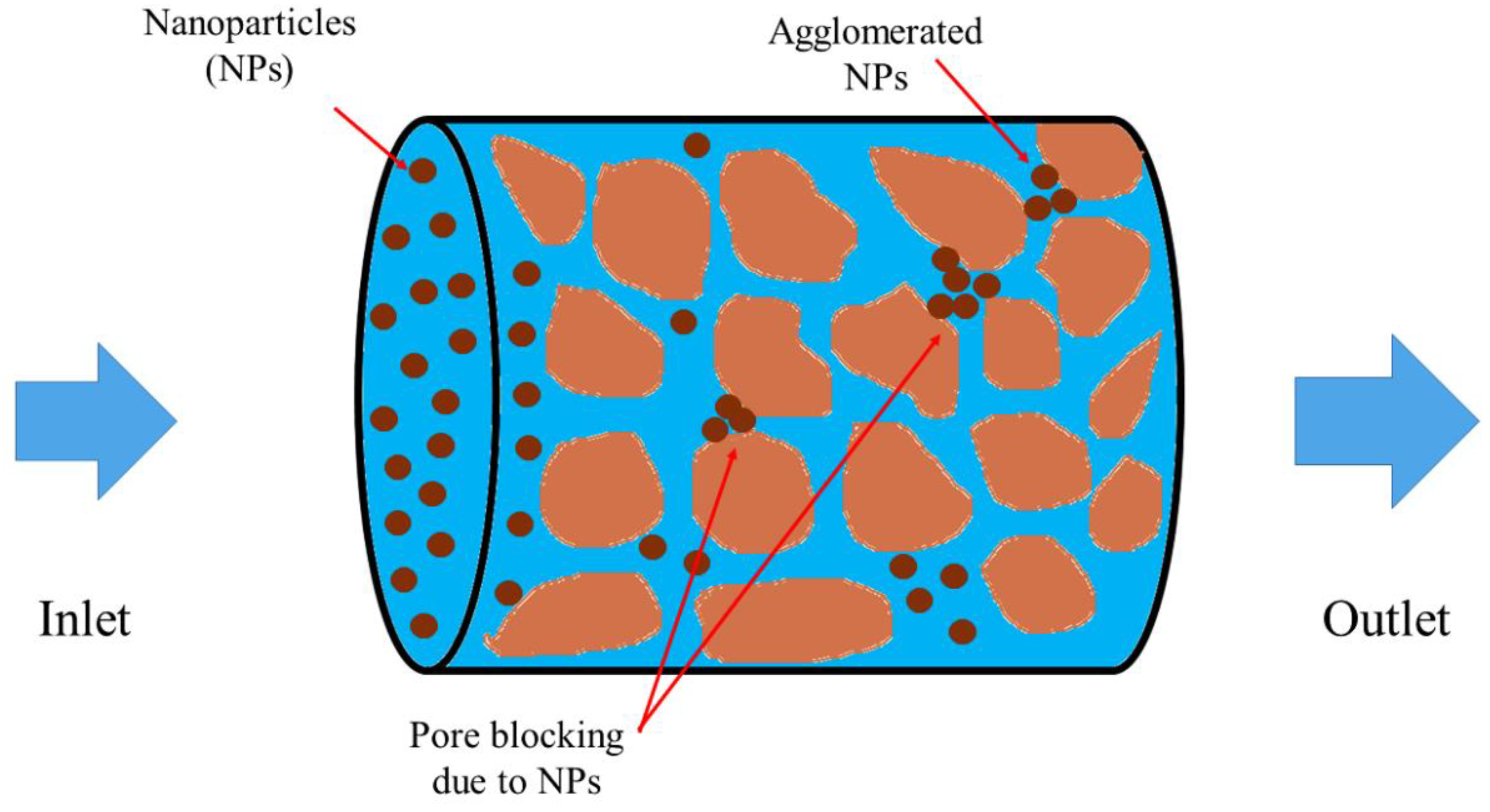
3.2. Silica NP Retention in Porous Media
3.3. Silica NP Retention in Saline Media and Its Mitigation Using Surfactants
3.4. Future Research Direction: Rise of AI
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rai, S.; Tiwari, S. Nano Silica in Cement Hydration. Mater. Today Proc. 2018, 5, 9196–9202. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Yusuff, A.S. An overview of chemical enhanced oil recovery: Recent advances and prospects. Int. Nano Lett. 2019, 9, 171–202. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, A.; Raval, A.; Chandra, S.; Shah, M.; Sircar, A. A comprehensive review of the application of nano-silica in oil well cementing. Petroleum 2020, 6, 123–129. [Google Scholar] [CrossRef]
- Fakoya, M.F.; Shah, S.N. Emergence of nanotechnology in the oil and gas industry: Emphasis on the application of silica nanoparticles. Petroleum 2017, 3, 391–405. [Google Scholar] [CrossRef]
- Chaturvedi, K.R.; Narukulla, R.; Sharma, T. CO2 capturing evaluation of single-step silica nanofluid through rheological investigation for nanofluid use in carbon utilization applications. J. Mol. Liq. 2020, 304, 112765. [Google Scholar] [CrossRef]
- Chaturvedi, K.R.; Sharma, T. Carbonated polymeric nanofluids for enhanced oil recovery from sandstone reservoir. J. Pet. Sci. Eng. 2020, 194, 107499. [Google Scholar] [CrossRef]
- Raghav Chaturvedi, K.; Kumar, R.; Trivedi, J.; Sheng, J.J.; Sharma, T. Stable Silica Nanofluids of an Oilfield Polymer for Enhanced CO2 Absorption for Oilfield Applications. Energy and Fuels 2018, 32, 12730–12741. [Google Scholar] [CrossRef]
- Shreyash, N.; Sonker, M.; Bajpai, S.; Tiwary, S.K.; Khan, M.A.; Raj, S.; Sharma, T.; Biswas, S. The Review of Carbon Capture-Storage Technologies and Developing Fuel Cells for Enhancing Utilization. Energies 2021, 14, 4978. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Zhang, X.-W.; Mo, Z.-W.; Xu, Y.-Z.; Tian, X.-Y.; Li, Y.; Chen, X.-M.; Zhang, J.-P. Adsorptive separation of carbon dioxide: From conventional porous materials to metal–organic frameworks. EnergyChem 2019, 1, 100016. [Google Scholar] [CrossRef]
- Ogolo, N.A.; Olafuyi, O.A.; Onyekonwu, M.O. Enhanced oil recovery using nanoparticles. In Proceedings of the Society of Petroleum Engineers—SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 8–11 April 2012; pp. 276–284. [Google Scholar]
- Bila, A.; Stensen, J.Å.; Torsæter, O. Experimental investigation of polymer-coated silica nanoparticles for enhanced oil recovery. Nanomaterials 2019, 9, 822. [Google Scholar] [CrossRef] [Green Version]
- Park, K.J.; Jung, D. Boiling heat transfer enhancement with carbon nanotubes for refrigerants used in building air-conditioning. Energy Build. 2007, 39, 1061–1064. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Roy, G.; Gauthier, C.; Galanis, N. Heat transfer enhancement using Al2O3-water nanofluid for an electronic liquid cooling system. Appl. Therm. Eng. 2007, 27, 1501–1506. [Google Scholar] [CrossRef]
- Trisaksri, V.; Wongwises, S. Nucleate pool boiling heat transfer of TiO2-R141b nanofluids. Int. J. Heat Mass Transf. 2009, 52, 1582–1588. [Google Scholar] [CrossRef]
- Bhattad, A.; Sarkar, J.; Ghosh, P. Improving the performance of refrigeration systems by using nanofluids: A comprehensive review. Renew. Sustain. Energy Rev. 2018, 82, 3656–3669. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, S.; Luo, Y.; Pang, H. Synthesis of confining cobalt nanoparticles within SiOx/nitrogen-doped carbon framework derived from sustainable bamboo leaves as oxygen electrocatalysts for rechargeable Zn-air batteries. Chem. Eng. J. 2020, 401, 126005. [Google Scholar] [CrossRef]
- Yuan, M.; Guo, X.; Li, N.; Li, Q.; Wang, S.; Liu, C.-S.; Pang, H. Highly dispersed and stabilized nickel nanoparticle/silicon oxide/nitrogen-doped carbon composites for high-performance glucose electrocatalysis. Sens. Actuators B Chem. 2019, 297, 126809. [Google Scholar] [CrossRef]
- Azmi, W.H.; Sharif, M.Z.; Yusof, T.M.; Mamat, R.; Redhwan, A.A.M. Potential of nanorefrigerant and nanolubricant on energy saving in refrigeration system–A review. Renew. Sustain. Energy Rev. 2017, 69, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, A.C. Performance evaluation of a refrigeration system using nanolubricant. Appl. Nanosci. 2020, 10, 1667–1678. [Google Scholar] [CrossRef]
- Kumar, R. Effect of Nanoparticles on Performance Characteristics of Refrigeration Cycle. In Low-Temperature Technologies; IntechOpen: London, UK, 2019; ISBN 978-1-83880-667-5. [Google Scholar]
- Chaturvedi, K.R.; Fogat, M.; Sharma, T. Low Temperature rheological characterization of single-step silica nanofluids: An additive in refrigeration and gas hydrate drilling applications. J. Pet. Sci. Eng. 2021, 204, 108742. [Google Scholar] [CrossRef]
- Peng, H.; Ding, G.; Jiang, W.; Hu, H.; Gao, Y. Heat transfer characteristics of refrigerant-based nanofluid flow boiling inside a horizontal smooth tube. Int. J. Refrig. 2009, 32, 1259–1270. [Google Scholar] [CrossRef]
- Kim, J.; Kang, Y.T.; Choi, C.K. Soret and Dufour effects on convective instabilities in binary nanofluids for absorption application. Int. J. Refrig. 2007, 30, 323–328. [Google Scholar] [CrossRef]
- Keykhosravi, A.; Simjoo, M. Insights into stability of silica nanofluids in brine solution coupled with rock wettability alteration: An enhanced oil recovery study in oil-wet carbonates. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 124008. [Google Scholar] [CrossRef]
- Kumar, R.S.; Chaturvedi, K.R.; Iglauer, S.; Trivedi, J.; Sharma, T. Impact of anionic surfactant on stability, viscoelastic moduli, and oil recovery of silica nanofluid in saline environment. J. Pet. Sci. Eng. 2020, 195, 107634. [Google Scholar] [CrossRef]
- Chaturvedi, K.R.; Trivedi, J.; Sharma, T. Single-step silica nanofluid for improved carbon dioxide flow and reduced formation damage in porous media for carbon utilization. Energy 2020, 197, 117276. [Google Scholar] [CrossRef]
- Rao, K.S.; El-Hami, K.; Kodaki, T.; Matsushige, K.; Makino, K. A novel method for synthesis of silica nanoparticles. J. Colloid Interface Sci. 2005, 289, 125–131. [Google Scholar] [CrossRef]
- Natarajan, E.; Sathish, R. Role of nanofluids in solar water heater. Int. J. Adv. Manuf. Technol. 2009. [Google Scholar] [CrossRef]
- Kumar, R.S.; Sharma, T. Stability and rheological properties of nanofluids stabilized by SiO2 nanoparticles and SiO2-TiO2 nanocomposites for oilfield applications. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 171–183. [Google Scholar] [CrossRef]
- Kumar, R.S.; Sharma, T. Stable SiO 2 –TiO 2 composite-based nanofluid of improved rheological behaviour for high-temperature oilfield applications. Geosystem Eng. 2020, 1–11. [Google Scholar] [CrossRef]
- Chaturvedi, K.R.; Singh, A.K.; Sharma, T. Impact of shale on properties and oil recovery potential of sandstone formation for low-salinity waterflooding applications. Asia-Pac. J. Chem. Eng. 2019, 14. [Google Scholar] [CrossRef]
- Goswami, R.; Chaturvedi, K.R.; Kumar, R.S.; Chon, B.H.; Sharma, T. Effect of ionic strength on crude emulsification and EOR potential of micellar flood for oil recovery applications in high saline environment. J. Pet. Sci. Eng. 2018, 170, 49–61. [Google Scholar] [CrossRef]
- Sharma, T.; Sangwai, J.S. Silica nanofluids in polyacrylamide with and without surfactant: Viscosity, surface tension, and interfacial tension with liquid paraffin. J. Pet. Sci. Eng. 2017, 152, 575–585. [Google Scholar] [CrossRef]
- Gosens, I.; Post, J.A.; de la Fonteyne, L.J.J.; Jansen, E.H.J.M.; Geus, J.W.; Cassee, F.R.; de Jong, W.H. Impact of agglomeration state of nano- and submicron sized gold particles on pulmonary inflammation. Part. Fibre Toxicol. 2010, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Sharma, T.; Iglauer, S.; Sangwai, J.S. Silica Nanofluids in an Oilfield Polymer Polyacrylamide: Interfacial Properties, Wettability Alteration, and Applications for Chemical Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2016, 55, 12387–12397. [Google Scholar] [CrossRef]
- Dong, X.; Xu, J.; Cao, C.; Sun, D.; Jiang, X. Aqueous foam stabilized by hydrophobically modified silica particles and liquid paraffin droplets. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 181–188. [Google Scholar] [CrossRef]
- Dehaghani, A.H.S.; Daneshfar, R. How much would silica nanoparticles enhance the performance of low-salinity water flooding? Pet. Sci. 2019, 16, 591–605. [Google Scholar] [CrossRef] [Green Version]
- Franco-Aguirre, M.; Zabala, R.D.; Lopera, S.H.; Franco, C.A.; Cortés, F.B. Interaction of anionic surfactant-nanoparticles for gas-Wettability alteration of sandstone in tight gas-condensate reservoirs. J. Nat. Gas. Sci. Eng. 2018, 51, 53–64. [Google Scholar] [CrossRef]
- Rognmo, A.U.; Horjen, H.; Fernø, M.A. Nanotechnology for improved CO2 utilization in CCS: Laboratory study of CO2-foam flow and silica nanoparticle retention in porous media. Int. J. Greenh. Gas. Control. 2017, 64, 113–118. [Google Scholar] [CrossRef]
- Babakhani, P. The impact of nanoparticle aggregation on their size exclusion during transport in porous media: One- and three-dimensional modelling investigations. Sci. Rep. 2019, 9, 14071. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.R.; Sharma, T. Rheological analysis and EOR potential of surfactant treated single-step silica nanofluid at high temperature and salinity. J. Pet. Sci. Eng. 2021, 196, 107704. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Arif, M.; Wang, S.; Barifcani, A.; Iglauer, S. Stabilising nanofluids in saline environments. J. Colloid Interface Sci. 2017, 508, 222–229. [Google Scholar] [CrossRef] [Green Version]





| Silica NP Concentration (wt%) | SDS Concentration (wt%) | Average Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| 0.1 | 0 | 264 ± 32 | −32.2 |
| 0.5 | 386 ± 47 | −31.1 | |
| 1 | 562 ± 56 | −29.4 | |
| 0.1 | 0.1 | 278 ± 36 | −33.1 |
| 0.2 | 256 ± 22 | −33.4 | |
| 0.3 | 234 ± 18 | −33.2 | |
| 0.4 | 245 ± 48 | −31.1 | |
| 0.5 | 312 ± 52 | −30.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajpai, S.; Shreyash, N.; Sonker, M.; Tiwary, S.K.; Biswas, S. Investigation of SiO2 Nanoparticle Retention in Flow Channels, Its Remediation Using Surfactants and Relevance of Artificial Intelligence in the Future. Chemistry 2021, 3, 1371-1380. https://doi.org/10.3390/chemistry3040098
Bajpai S, Shreyash N, Sonker M, Tiwary SK, Biswas S. Investigation of SiO2 Nanoparticle Retention in Flow Channels, Its Remediation Using Surfactants and Relevance of Artificial Intelligence in the Future. Chemistry. 2021; 3(4):1371-1380. https://doi.org/10.3390/chemistry3040098
Chicago/Turabian StyleBajpai, Sushant, Nehil Shreyash, Muskan Sonker, Saurabh Kr Tiwary, and Susham Biswas. 2021. "Investigation of SiO2 Nanoparticle Retention in Flow Channels, Its Remediation Using Surfactants and Relevance of Artificial Intelligence in the Future" Chemistry 3, no. 4: 1371-1380. https://doi.org/10.3390/chemistry3040098
APA StyleBajpai, S., Shreyash, N., Sonker, M., Tiwary, S. K., & Biswas, S. (2021). Investigation of SiO2 Nanoparticle Retention in Flow Channels, Its Remediation Using Surfactants and Relevance of Artificial Intelligence in the Future. Chemistry, 3(4), 1371-1380. https://doi.org/10.3390/chemistry3040098






