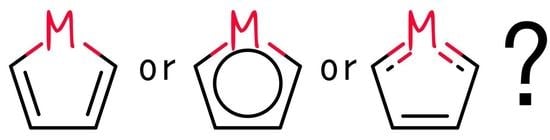
Are Metallacyclopentadienes Always Non-Aromatic?
Abstract
1. Introduction
2. Computational Methods
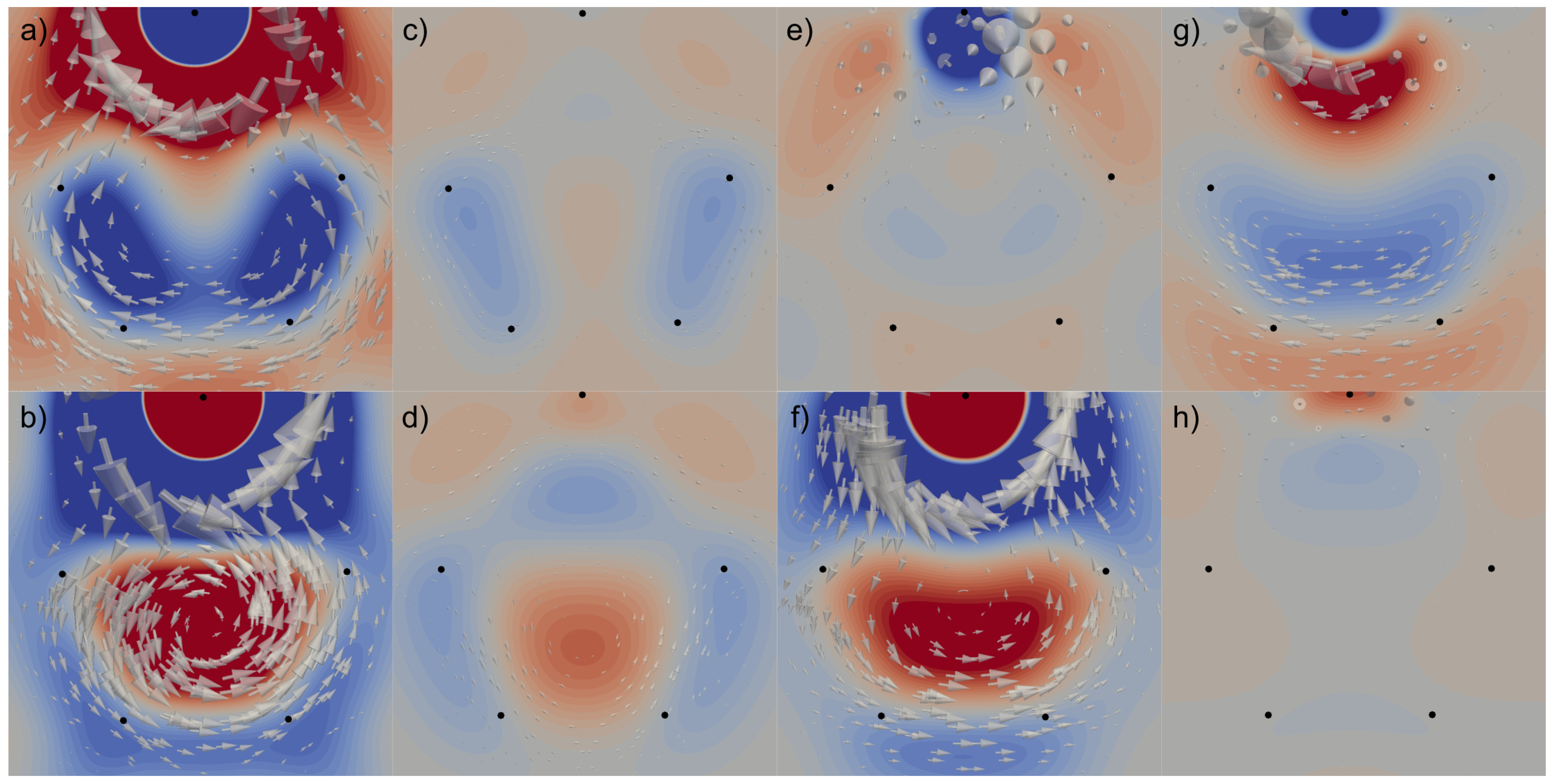
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MCP | Metallacyclopentadiene |
| MB | Metallabenzene |
| CSD | Cambridge Structural Database |
| ASE | Aromatic stabilization energy |
| NICS | Nuclear independent chemical shifts |
| ISE | Isomerization stabilization energy |
| DI | Delocalization index |
| MO | Molecular orbital |
| CTOCD-DZ | Continuous transformations of the origin of the current density-diamagnetic zero |
| GIAO | Gauge-including atomic orbital |
References
- Thorn, D.L.; Hoffmann, R. Delocalization in Metallocycles. Nouv. J. Chem. 1979, 3, 39–45. [Google Scholar]
- Mague, J.T. Crystal and molecular structure of chlorobis(triphenylstibine)tetrakis(trifluoromethyl)rhodiacyclopentadaiene-dichloromethane solvate, RhCl(Sb(C6H5)3)2C4(CF3)4.CH2Cl2. Inorg. Chem. 1970, 9, 1610–1618. [Google Scholar] [CrossRef]
- Mogue, J.T. Crystal and molecular structure of chloroaquobis(trimethylarsine)tetrakis)trifluoromethyl)rhodiacyclopentadiene. Inorg. Chem. 1973, 12, 2649–2654. [Google Scholar] [CrossRef]
- Atwood, J.L.; Hunter, W.E.; Alt, H.; Rausch, M.D. The molecular structure of 1,1-bis(η5-cyclopentadienyl)-2,3,4,5-tetraphenyltitanole and its hafnium analogue. J. Am. Chem. Soc. 1976, 98, 2454–2459. [Google Scholar] [CrossRef]
- Gastinger, R.G.; Rausch, M.D.; Sullivan, D.A.; Palenik, G.J. The synthesis and molecular structure of 1-(η5-cyclopentadienyl)-1-triphenylphosphine-2,3,4,5-tetrakis(pentafluorophenyl)rhodole. J. Organomet. Chem. 1976, 117, 355–364. [Google Scholar] [CrossRef]
- Gastinger, R.G.; Rausch, M.D.; Sullivan, D.A.; Palenik, G.J. Synthesis and molecular structure of 1-(π-cyclopentadienyl)-1-triphenylphosphine-2,3,4,5-tetrakis(pentafluorophenyl)cobaltole. J. Am. Chem. Soc. 1976, 98, 719–723. [Google Scholar] [CrossRef]
- Suzuki, H.; Itoh, K.; Ishii, Y.; Simon, K.; Ibers, J.A. Preparation structure, and role of tetrakis(methoxycarbonyl)palladiacyclopentadiene cyclic diolefin complexes in selective palladium-catalyzed cyclocotrimerization of acetylenes with olefins. J. Am. Chem. Soc. 1976, 98, 8494–8500. [Google Scholar] [CrossRef]
- Pierpont, C.G.; Downs, H.H.; Itoh, K.; Nishiyama, J.; Ishii, Y. Synthesis and structure of the triphenylphosphonium cyclopentadienylide adduct of oligomeric tetrakis(methoxycarbonyl)palladiacyclopentadiene. J. Organomet. Chem. 1977, 124, 93–101. [Google Scholar] [CrossRef]
- Brown, L.D.; Itoh, K.; Suzuki, H.; Hirai, K.; Ibers, J.A. Effects of donor molecules on the palladium-catalyzed cyclocotrimerization of acetylenes with olefins. Preparation of dimeric tetrakis(methoxycarbonyl)palladiacyclopentadiene(base) complexes and structure with base = 2,6-lutidine. J. Am. Chem. Soc. 1978, 100, 8232–8238. [Google Scholar] [CrossRef]
- Elliott, G.P.; Roper, W.R.; Waters, J.M. Metallacyclohexatrienes or ‘metallabenzenes.’ Synthesis of osmabenzene derivatives and X-ray crystal structure of [Os(CSCHCHCHCH)(CO)(PPh3)2]. J. Chem. Soc. Chem. Commun. 1982, 811–813. [Google Scholar] [CrossRef]
- Fernández, I.; Frenking, G. Aromaticity in Metallabenzenes. Chem. Eur. J. 2007, 13, 5873–5884. [Google Scholar] [CrossRef]
- Dalebrook, A.F.; Wright, L.J. Chapter Three-Metallabenzenes and Metallabenzenoids. In Advances in Organometallic Chemistry; Hill, A.F., Fink, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 60, pp. 93–177. [Google Scholar] [CrossRef]
- Landorf, C.W.; Haley, M.M. Recent Advances in Metallabenzene Chemistry. Angew. Chem. Int. Ed. 2006, 45, 3914–3936. [Google Scholar] [CrossRef]
- Fernández, I.; Frenking, G.; Merino, G. Aromaticity of metallabenzenes and related compounds. Chem. Soc. Rev. 2015, 44, 6452–6463. [Google Scholar] [CrossRef]
- Fernández, I.; Frenking, G. Theoretical Studies of Metallabenzenes: From Bonding Situation to Reactivity. In Metallabenzenes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 267–304. [Google Scholar] [CrossRef]
- Periyasamy, G.; Burton, N.A.; Hillier, I.H.; Thomas, J.M.H. Electron Delocalization in the Metallabenzenes: A Computational Analysis of Ring Currents. J. Phys. Chem. A 2008, 112, 5960–5972. [Google Scholar] [CrossRef]
- Mauksch, M.; Tsogoeva, S.B. Demonstration of “Möbius” Aromaticity in Planar Metallacycles. Chem. Eur. J. 2010, 16, 7843–7851. [Google Scholar] [CrossRef] [PubMed]
- Havenith, R.W.A.; Proft, F.D.; Jenneskens, L.W.; Fowler, P.W. Relativistic ring currents in metallabenzenes: An analysis in terms of contributions of localised orbitals. Phys. Chem. Chem. Phys. 2012, 14, 9897–9905. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feixas, F.; Matito, E.; Poater, J.; Solà, M. Metalloaromaticity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 105–122. [Google Scholar] [CrossRef]
- Dávila, K.L.; Contreras, R.R.; Fontal, B.; Torres, F.J.; Rincón, L. An alternative description of aromaticity in metallabenzenes. J. Mex. Chem. Soc. 2017, 61. [Google Scholar] [CrossRef]
- Frogley, B.J.; Wright, L.J. Recent Advances in Metallaaromatic Chemistry. Chem. Eur. J. 2018, 24, 2025–2038. [Google Scholar] [CrossRef]
- Chen, D.; Hua, Y.; Xia, H. Metallaaromatic Chemistry: History and Development. Chem. Rev. 2020, 120, 12994–13086. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, G.; Lin, Z. Understanding Nonplanarity in Metallabenzene Complexes. Organometallics 2007, 26, 1986–1995. [Google Scholar] [CrossRef]
- Chen, Z.N.; Fu, G.; Zhang, I.Y.; Xu, X. Understanding the Nonplanarity in Aromatic Metallabenzenes: A sigma-Control Mechanism. Inorg. Chem. 2018, 57, 9205–9214. [Google Scholar] [CrossRef]
- Proft, F.D.; Geerlings, P. Relative hardness as a measure of aromaticity. Phys. Chem. Chem. Phys. 2004, 6, 242–248. [Google Scholar] [CrossRef]
- Lin, R.; Lee, K.H.; Poon, K.C.; Sung, H.H.Y.; Williams, I.D.; Lin, Z.; Jia, G. Synthesis of Rhenabenzenes from the Reactions of Rhenacyclobutadienes with Ethoxyethyne. Chem. Eur. J. 2014, 20, 14885–14899. [Google Scholar] [CrossRef]
- Iron, M.A.; Lucassen, A.C.B.; Cohen, H.; van der Boom, M.E.; Martin, J.M.L. A Computational Foray into the Formation and Reactivity of Metallabenzenes. J. Am. Chem. Soc. 2004, 126, 11699–11710. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, R.; Pasdar, H. Computational study of substituent effect in para substituted platinabenzene complexes. Russ. J. Phys. Chem. A 2013, 87, 973–978. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, R.; Li, J.; Zhu, J.; Xia, H. Interconversion between Ruthenacyclohexadiene and Ruthenabenzene: A Combined Experimental and Theoretical Study. Organometallics 2014, 33, 5606–5609. [Google Scholar] [CrossRef]
- Ghiasi, R.; Amini, E. Substituent and solvent effects on geometric and electronic structure of C5H5Ir(PH3)3 iridabenzene: A theoretical insight. J. Struct. Chem. 2015, 56, 1483–1494. [Google Scholar] [CrossRef]
- Ghiasi, R.; Boshak, A. Substituent Effect in para Substituted Osmabenzene Complexes. J. Mex. Chem. Soc. 2017, 57. [Google Scholar] [CrossRef]
- Han, F.; Wang, T.; Li, J.; Zhang, H.; Xia, H. m-Metallaphenol: Synthesis and Reactivity Studies. Chem. Eur. J. 2014, 20, 4363–4372. [Google Scholar] [CrossRef]
- Ma, W.; Yu, C.; Chen, T.; Xu, L.; Zhang, W.X.; Xi, Z. Metallacyclopentadienes: Synthesis, structure and reactivity. Chem. Soc. Rev. 2017, 46, 1160–1192. [Google Scholar] [CrossRef]
- Jemmis, E.D.; Phukan, A.K.; Jiao, H.; Rosenthal, U. Structure and Neutral Homoaromaticity of Metallacyclopentene, -pentadiene, -pentyne, and -pentatriene: A Density Functional Study. Organometallics 2003, 22, 4958–4965. [Google Scholar] [CrossRef]
- Islas, R.; Poater, J.; Solà, M. Analysis of the Aromaticity of Five-Membered Heterometallacycles Containing Os, Ru, Rh, and Ir. Organometallics 2014, 33, 1762–1773. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Yang, S.Y.; Li, X.Y. An investigation of the aromaticity of transition metal heterocyclic complexes by conventional criteria and indices of aromaticity. J. Organomet. Chem. 2004, 689, 1050–1056. [Google Scholar] [CrossRef]
- Jeffreys, J.A.D.; Willis, C.M. Crystal and molecular structure of tricarbonyl-π-[1,1,1-tricarbonyl-2,3-dimethoxy-5-(diphenylmethyl) ferracyclopentadiene]iron(Fe–Fe), a product from the reaction between diphenyldiazomethane and tricarbonyl-π-[1,1,1-tricarbonyl-2,5-dimethoxyferracyclopentadiene]iron(Fe–Fe). J. Chem. Soc. Dalton Trans. 1972, 2169–2173. [Google Scholar] [CrossRef]
- King, M.; Holt, E.M.; Radnia, P.; McKennis, J.S. Metallametallocenes: Ferracobaltocene and ferrarhodocene. New aromatic species. Organometallics 1982, 1, 1718–1720. [Google Scholar] [CrossRef]
- Hong, F.E.; Lue, I.R.; Lo, S.C.; Lin, C.C. Reactions of molybdenum-cobalt complex with phenylacetylene: X-ray crystal structure of [MoCo(CO)4{CPhCHCHCPh}(η5-C5H5)]. J. Organomet. Chem. 1995, 495, 97–101. [Google Scholar] [CrossRef]
- Baxter, R.J.; Knox, G.R.; Pauson, P.L.; Spicer, M.D. Synthesis of Dicarbonyl(η4-tricarbonylcobaltacyclopentadiene)cobalt Complexes from Co2(CO)8. A General Route to Intermediates in Cobalt Carbonyl Mediated Alkyne Trimerization. Organometallics 1999, 18, 197–205. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Eckerle, P.; Miyatake, T.; Kainosho, M.; Ono, A.; Ikariya, T.; Noyori, R. Well-Controlled Polymerization of Phenylacetylenes with Organorhodium(I) Complexes: Mechanism and Structure of the Polyenes. J. Am. Chem. Soc. 1999, 121, 12035–12044. [Google Scholar] [CrossRef]
- Dennett, J.N.L.; Knox, S.A.R.; Anderson, K.M.; Charmant, J.P.H.; Orpen, A.G. The synthesis of [FeRu(CO)2(μ-CO)2(η-C5H5)(η-C5Me5)] and convenient entries to its organometallic chemistry. Dalton Trans. 2005, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Nugent, W.A.; Thorn, D.L.; Harlow, R.L. Cyclization of diacetylenes to E,E exocyclic dienes. Complementary procedures based on titanium and zirconium reagents. J. Am. Chem. Soc. 1987, 109, 2788–2796. [Google Scholar] [CrossRef]
- Hill, J.E.; Fanwick, P.E.; Rothwell, I.P. Formation, fragmentation, and isomerization of titanacycle rings supported by aryloxide ligation. Organometallics 1990, 9, 2211–2213. [Google Scholar] [CrossRef]
- Kaleta, K.; Strehler, F.; Hildebrandt, A.; Beweries, T.; Arndt, P.; Rüffer, T.; Spannenberg, A.; Lang, H.; Rosenthal, U. Synthesis and Characterization of Multiferrocenyl-Substituted Group 4 Metallocene Complexes. Chem. Eur. J. 2012, 18, 12672–12680. [Google Scholar] [CrossRef] [PubMed]
- Àrias, O.; Petrov, A.R.; Bannenberg, T.; Altenburger, K.; Arndt, P.; Jones, P.G.; Rosenthal, U.; Tamm, M. Titanocene and Zirconocene Complexes with Diaminoacetylenes: Formation of Unusual Metallacycles and Fulvene Complexes. Organometallics 2014, 33, 1774–1786. [Google Scholar] [CrossRef]
- Kiel, G.R.; Ziegler, M.S.; Tilley, T.D. Zirconacyclopentadiene-Annulated Polycyclic Aromatic Hydrocarbons. Angew. Chem. Int. Ed. 2017, 56, 4839–4844. [Google Scholar] [CrossRef] [PubMed]
- Urrego-Riveros, S.; Medina, I.M.R.Y.; Duvinage, D.; Lork, E.; Sönnichsen, F.D.; Staubitz, A. Negishi’s Reagent Versus Rosenthal’s Reagent in the Formation of Zirconacyclopentadienes. Chem. Eur. J. 2019, 25, 13318–13328. [Google Scholar] [CrossRef]
- Zeng, Y.; Feng, H.; King, R.B.; Schaefer, H.F. Metallocene versus Metallabenzene Isomers of Nickel, Palladium, and Platinum. Organometallics 2014, 33, 7193–7198. [Google Scholar] [CrossRef]
- Ebrahimi, A.A.; Ghiasi, R.; Foroutan-Nejad, C. Topological characteristics of the Ring Critical Points and the aromaticity of groups IIIA to VIA hetero-benzenes. J. Mol. Struct. THEOCHEM 2010, 941, 47–52. [Google Scholar] [CrossRef]
- Foroutan-Nejad, C. Is NICS a reliable aromaticity index for transition metal clusters? Theor. Chem. Accounts 2015, 134, 8. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Accounts Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Noro, T.; Sekiya, M.; Koga, T. Segmented contracted basis sets for atoms H through Xe: Sapporo-(DK)-nZP sets (n = D, T, Q). Theor. Chem. Accounts 2012, 131, 1124. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Keith, T.A. AIMAll, TK Gristmill Software. Overland, Park KS, USA, 2019. [Google Scholar]
- Barquera-Lozada, J.E. The vorticity of the current density tensor and 3D-aromaticity. Int. J. Quantum Chem. 2019, 119, e25848. [Google Scholar] [CrossRef]
- Barquera-Lozada, J.E. Vorticity: Simplifying the analysis of the current density. J. Comput. Chem. 2019, 40, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Rico-Sotomayor, E.M.; Barquera-Lozada, J.E. Triangulenes and their ions: Reaching the limits of Clar’s rule. Phys. Chem. Chem. Phys. 2020, 22, 24704–24711. [Google Scholar] [CrossRef]
- Keith, T.A.; Bader, R.F.W. Calculation of magnetic response properties using a continuous set of gauge transformations. Chem. Phys. Lett. 1993, 210, 223–231. [Google Scholar] [CrossRef]
- Lazzeretti, P.; Malagoli, M.; Zanasi, R. Computational approach to molecular magnetic properties by continuous transformation of the origin of the current density. Chem. Phys. Lett. 1994, 220, 299–304. [Google Scholar] [CrossRef]
- Steiner, E.; Fowler, P.W. Patterns of Ring Currents in Conjugated Molecules: A Few-Electron Model Based on Orbital Contributions. J. Phys. Chem. A 2001, 105, 9553–9562. [Google Scholar] [CrossRef]
- Monaco, G.; Summa, F.F.; Zanasi, R. Program Package for the Calculation of Origin-Independent Electron Current Density and Derived Magnetic Properties in Molecular Systems. J. Chem. Inf. Model. 2021, 61, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.E.; Atwood, J.L.; Fachinetti, G.; Floriani, C. The crystal structure of 1,1-bis(η5-cyclopentadienyl)-2,3,4,5-tetraphenylzirconole. J. Organomet. Chem. 1981, 204, 67–74. [Google Scholar] [CrossRef]
- Sala-Pala, J.; Amaudrut, J.; Guerchais, J.E.; Mercier, R.; Douglade, J.; Theobald, J.G. Chimie organométallique du niobium VIII. Etude détaillée de la réaction d’acétyléniques perfluorés avec (η5C5H5)2NbH3: Mise en evídence de la coupure de liaison C-F, de la formation de liaison Nb-F et de la reduction du niobium. Structure cristalline d’un dérivé niobiacyclopentadiène. J. Organomet. Chem. 1981, 204, 347–359. [Google Scholar] [CrossRef]
- Albers, M.O.; Waal, D.J.A.D.; Liles, D.C.; Robinson, D.J.; Singleton, E.; Wiege, M.B. The novel cyclodimerization of phenylacetylene at a ruthenium(II) centre. The synthesis and X-ray structural characterization of the first metallacyclopentatriene, [(η-C5H5)Ru(C4Ph2H2)Br], and its facile conversion into metallacyclopentadienes. J. Chem. Soc. Chem. Commun. 1986, 1680–1682. [Google Scholar] [CrossRef]
- Sánchez, G.; Vives, J.; Serrano, J.L.; Pérez, J.; López, G. New palladacyclopentadiene complexes containing an N,P-donor setting. Crystal structure of [Pd{C4(COOMe)4}(o-Ph2PC6H4CHNiPr)]. Inorganica Chim. Acta 2002, 328, 74–80. [Google Scholar] [CrossRef]
- Canovese, L.; Visentin, F.; Levi, C.; Santo, C.; Bertolasi, V. Facile synthesis and reactivity study of mixed phosphane–isocyanide Pd(II) and Pd(0) complexes. Inorganica Chim. Acta 2011, 378, 239–249. [Google Scholar] [CrossRef]
- Yi, C.S.; Torres-Lubian, J.R.; Liu, N.; Rheingold, A.L.; Guzei, I.A. Selective Linear Coupling Reaction of Acetylene and Acrylonitrile Catalyzed by the Well-Defined Metallacyclopentadiene Complex C5Me5(PPh3)(Cl)RuCHCHCHCH. Organometallics 1998, 17, 1257–1259. [Google Scholar] [CrossRef]
- Bruce, M.I.; Hall, B.C.; Skelton, B.W.; Tiekink, E.R.T.; White, A.H.; Zaitseva, N.N. Some Pentamethylcyclopentadienyl-Ruthenium Derivatives of Methyl Propiolate. Aust. J. Chem. 2000, 53, 99–107. [Google Scholar] [CrossRef]
- Yamazaki, H.; Wakatsuki, Y. Cobalt metallocycles: XIII. Preparation and x-ray crystallography of cobaltacyclopentadiene and dinuclear cobalt complexes. J. Organomet. Chem. 1984, 272, 251–263. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Yeamine, M.R.; Amin, J.; Motevalli, M.; Richards, C.J. Synthesis and 1H NMR spectroscopic properties of substituted (η4-tetraarylcyclobutadiene)(η5-cyclopentadienyl)cobalt metallocenes. J. Organomet. Chem. 2008, 693, 3668–3676. [Google Scholar] [CrossRef]
- Ernst, C.; Walter, O.; Dinjus, E.; Arzberger, S.; Görls, H. Structural characterization of Cp*Ru-intermediates of phenylacetylene cyclotrimerization. J. FüR Prakt. Chem. 1999, 341, 801–804. [Google Scholar] [CrossRef]
- Ernst, C.; Walter, O.; Dinjus, E. Cp*Ru-allylcarbene complexes by nucleophilic attack of cyclic Cp*Ru-dicarbenes. J. Organomet. Chem. 2001, 627, 249–254. [Google Scholar] [CrossRef]
- Yamada, Y.; Mizutani, J.; Kurihara, M.; Nishihara, H. Synthesis of a new bis(ferrocenyl)ruthenacyclopentatriene compound with a significant inter-metal electronic communication. J. Organomet. Chem. 2001, 637–639, 80–83. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Arakawa, T.; Ogawa, R.; Itoh, K. Ruthenium(II)-Catalyzed Selective Intramolecular [2 + 2 + 2] Alkyne Cyclotrimerizations. J. Am. Chem. Soc. 2003, 125, 12143–12160. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Curchod, B.F.E.; Campomanes, P.; Solari, E.; Scopelliti, R.; Rothlisberger, U.; Severin, K. Reactions of Alkynes with [RuCl(cyclopentadienyl)] Complexes: The Important First Steps. Chem. Eur. J. 2010, 16, 8400–8409. [Google Scholar] [CrossRef]
- Schleyer, P.V.R.; Pühlhofer, F. Recommendations for the Evaluation of Aromatic Stabilization Energies. Org. Lett. 2002, 4, 2873–2876. [Google Scholar] [CrossRef]
- O’Connor, J.M.; Merwin, R.; Rheingold, A.L.; Adams, M.L. Synthesis and Structural Characterization of a Diiridium .mu.-Acyl Complex. Organometallics 1995, 14, 2102–2105. [Google Scholar] [CrossRef]
- O’Connor, J.M.; Hiibner, K.; Rheingold, A.L.; Liable-Sands, L.M. New transition metal binding modes for creatinine: Molecular structures of [(C4R4)Ir(C4H7N3O)(PPh3)2Cl] and [(C4R4)Ir(C4H7N3O)(PPh3)2]BF4, (R = CO2CH3). Polyhedron 1997, 16, 2029–2035. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Han, F.; Long, L.; Lin, Z.; Xia, H. cine-Substitution Reactions of Metallabenzenes: An Experimental and Computational Study. Chem. Eur. J. 2013, 19, 10982–10991. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Stephens, M.E. Spatial localization of the electronic pair and number distributions in molecules. J. Am. Chem. Soc. 1975, 97, 7391–7399. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M.; Duran, M.; Fradera, X. The calculation of electron localization and delocalization indices at the Hartree–Fock, density functional and post-Hartree–Fock levels of theory. Theor. Chem. Accounts 2002, 107, 362–371. [Google Scholar] [CrossRef]
- Firme, C.L.; Antunes, O.A.C.; Esteves, P.M. Relation between bond order and delocalization index of QTAIM. Chem. Phys. Lett. 2009, 468, 129–133. [Google Scholar] [CrossRef]
- Outeiral, C.; Vincent, M.A.; Pendás, A.M.; Popelier, P.L.A. Revitalizing the concept of bond order through delocalization measures in real space. Chem. Sci. 2018, 9, 5517–5529. [Google Scholar] [CrossRef] [PubMed]
- Poater, J.; Fradera, X.; Duran, M.; Solà, M. The Delocalization Index as an Electronic Aromaticity Criterion: Application to a Series of Planar Polycyclic Aromatic Hydrocarbons. Chem. Eur. J. 2003, 9, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Jusélius, J.; Sundholm, D.; Gauss, J. Calculation of current densities using gauge-including atomic orbitals. J. Chem. Phys. 2004, 121, 3952–3963. [Google Scholar] [CrossRef] [PubMed]
- Gershoni-Poranne, R.; Stanger, A. Magnetic criteria of aromaticity. Chem. Soc. Rev. 2015, 44, 6597–6615. [Google Scholar] [CrossRef] [PubMed]
- Sundholm, D.; Fliegl, H.; Berger, R.J.F. Calculations of magnetically induced current densities: Theory and applications. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 6, 639–678. [Google Scholar] [CrossRef]
- Fliegl, H.; Sundholm, D.; Taubert, S.; Jusélius, J.; Klopper, W. Magnetically Induced Current Densities in Aromatic, Antiaromatic, Homoaromatic, and Nonaromatic Hydrocarbons. J. Phys. Chem. A 2009, 113, 8668–8676. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.; Jusélius, J.; Saue, T. 4-Component relativistic calculation of the magnetically induced current density in the group 15 heteroaromatic compounds. Chem. Phys. 2009, 356, 187–194. [Google Scholar] [CrossRef]
- Monaco, G.; Zanasi, R.; Pelloni, S.; Lazzeretti, P. Relative Weights of σ and π Ring Currents in a Few Simple Monocycles. J. Chem. Theory Comput. 2010, 6, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Cyrański, M.; Havenith, R.; Dobrowolski, M.; Gray, B.; Krygowski, T.; Fowler, P.; Jenneskens, L. The Phenalenyl Motif: A Magnetic Chameleon. Chem. Eur. J. 2007, 13, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Havenith, R.W.A.; Fowler, P.W. The origin of the ring current in the all-metal aromatic, Al42-. Phys. Chem. Chem. Phys. 2006, 8, 3383–3386. [Google Scholar] [CrossRef] [PubMed]
- Steiner, E.; Fowler, P.W. On the orbital analysis of magnetic properties. Phys. Chem. Chem. Phys. 2004, 6, 261–272. [Google Scholar] [CrossRef]
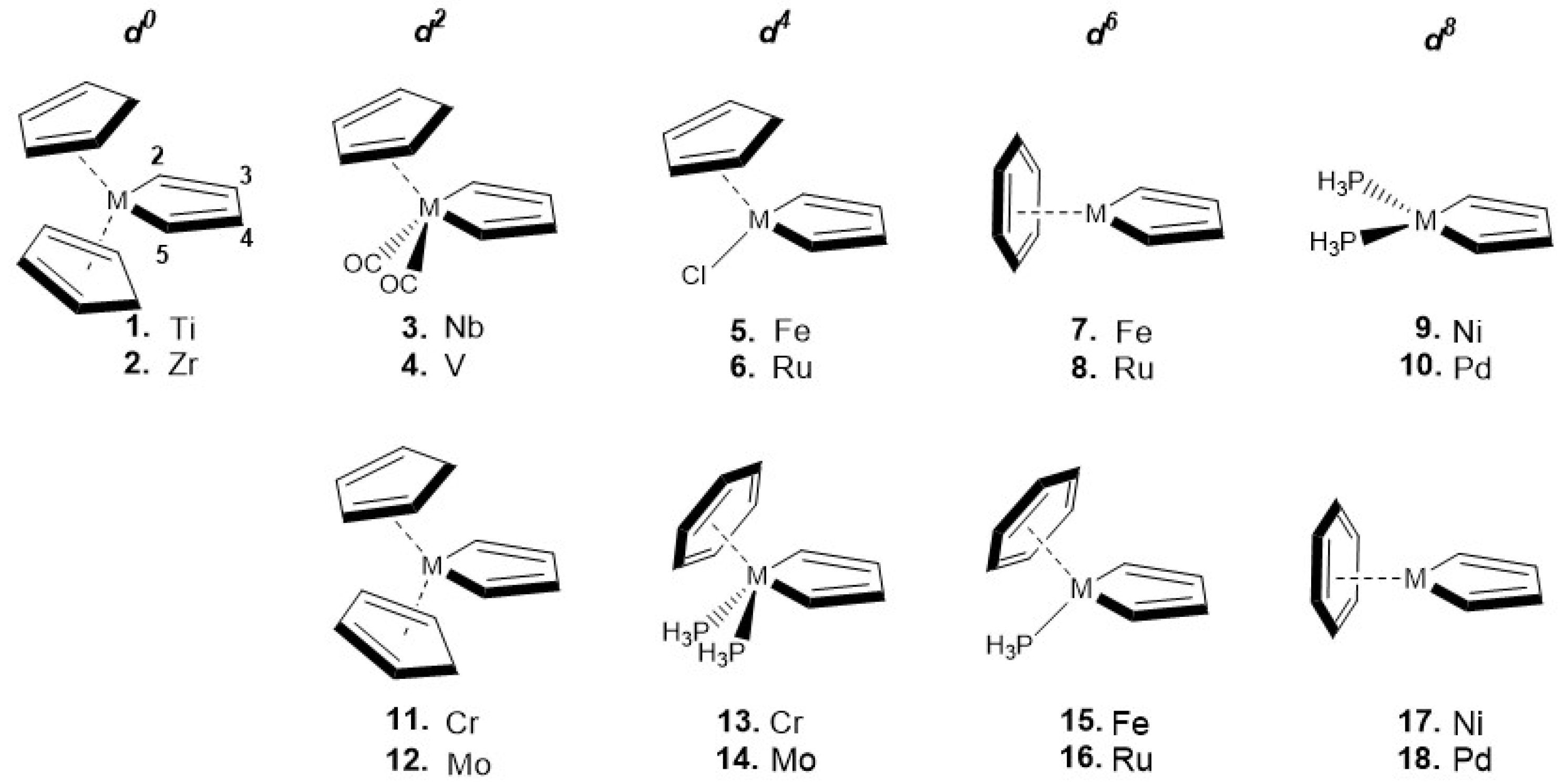

| Molecule | (Å) | (Å) | (Å) | (°) |
|---|---|---|---|---|
| 1 | 2.12 | 1.34 | 1.47 | 0.1 |
| 2 | 2.24 | 1.35 | 1.48 | 0.1 |
| 3 | 2.25 | 1.33 | 1.47 | 1.0 |
| 4 | 2.13 | 1.33 | 1.47 | 0.2 |
| 5 | 1.92 | 1.34 | 1.45 | 3.4 |
| 6 | 1.94 | 1.36 | 1.42 | 5.0 |
| 7 | 2.00 | 1.34 | 1.49 | 5.7 |
| 8 | 1.84 | 1.48 | 1.34 | 0.0 |
| 9 | 1.97 | 1.34 | 1.47 | 0.0 |
| 10 | 2.05 | 1.34 | 1.46 | 0.0 |
| 11 | 2.06 | 1.33 | 1.46 | 2.2 |
| 12 | 2.16 | 1.34 | 1.46 | 0.0 |
| 13 | 2.14 | 1.34 | 1.45 | 1.4 |
| 14 | 2.16 | 1.35 | 1.44 | 2.3 |
| 15 | 2.01 | 1.35 | 1.46 | 3.3 |
| 16 | 2.03 | 1.35 | 1.45 | 2.5 |
| 17 | 1.92 | 1.34 | 1.47 | 0.0 |
| 18 | 1.98 | 1.33 | 1.46 | 0.0 |
| Molecule | ISE (kcal/mol) | (a.u.) | (a.u.) | (a.u.) | (a.u.) | (nA/T) |
|---|---|---|---|---|---|---|
| 1 | 0.94 | 0.643 | 1.739 | 1.091 | 0.647 | −0.14 |
| 2 | −0.24 | 0.615 | 1.769 | 1.082 | 0.687 | 0.28 |
| 3 | −3.70 | 0.654 | 1.758 | 1.060 | 0.698 | −20.58 |
| 4 | −5.15 | 0.513 | 1.753 | 1.062 | 0.691 | −7.86 |
| 5 | −1.20 | 0.894 | 1.683 | 1.118 | 0.564 | 13.93 |
| 6 | 0.57 | 1.147 | 1.539 | 1.214 | 0.324 | −13.58 |
| 7 | −7.82 | 0.793 | 1.801 | 1.047 | 0.753 | 0.70 |
| 8 | −26.60 | 1.690 | 1.075 | 1.670 | −0.594 | −55.99 |
| 9 | −5.71 | 0.774 | 1.784 | 1.066 | 0.718 | −2.91 |
| 10 | −5.94 | 0.865 | 1.764 | 1.076 | 0.688 | −5.43 |
| 11 | 4.94 | 0.739 | 1.690 | 1.106 | 0.585 | 7.43 |
| 12 | 3.40 | 0.775 | 1.702 | 1.104 | 0.598 | 3.86 |
| 13 | 2.04 | 0.610 | 1.684 | 1.120 | 0.564 | 3.94 |
| 14 | 4.77 | 0.732 | 1.631 | 1.153 | 0.478 | 5.41 |
| 15 | 0.91 | 0.768 | 1.700 | 1.106 | 0.594 | 7.02 |
| 16 | 3.38 | 0.945 | 1.665 | 1.117 | 0.548 | 5.12 |
| 17 | −5.71 | 0.884 | 1.783 | 1.069 | 0.715 | −2.50 |
| 18 | −4.99 | 0.993 | 1.748 | 1.082 | 0.666 | −4.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casiano-González, R.; Barquera-Lozada, J.E. Are Metallacyclopentadienes Always Non-Aromatic? Chemistry 2021, 3, 1302-1313. https://doi.org/10.3390/chemistry3040094
Casiano-González R, Barquera-Lozada JE. Are Metallacyclopentadienes Always Non-Aromatic? Chemistry. 2021; 3(4):1302-1313. https://doi.org/10.3390/chemistry3040094
Chicago/Turabian StyleCasiano-González, Ricardo, and José Enrique Barquera-Lozada. 2021. "Are Metallacyclopentadienes Always Non-Aromatic?" Chemistry 3, no. 4: 1302-1313. https://doi.org/10.3390/chemistry3040094
APA StyleCasiano-González, R., & Barquera-Lozada, J. E. (2021). Are Metallacyclopentadienes Always Non-Aromatic? Chemistry, 3(4), 1302-1313. https://doi.org/10.3390/chemistry3040094