Unveiling the Intramolecular Ionic Diels–Alder Reactions within Molecular Electron Density Theory
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussions
3.1. Study of the Structure and Reactivity at the GS of the Reagents
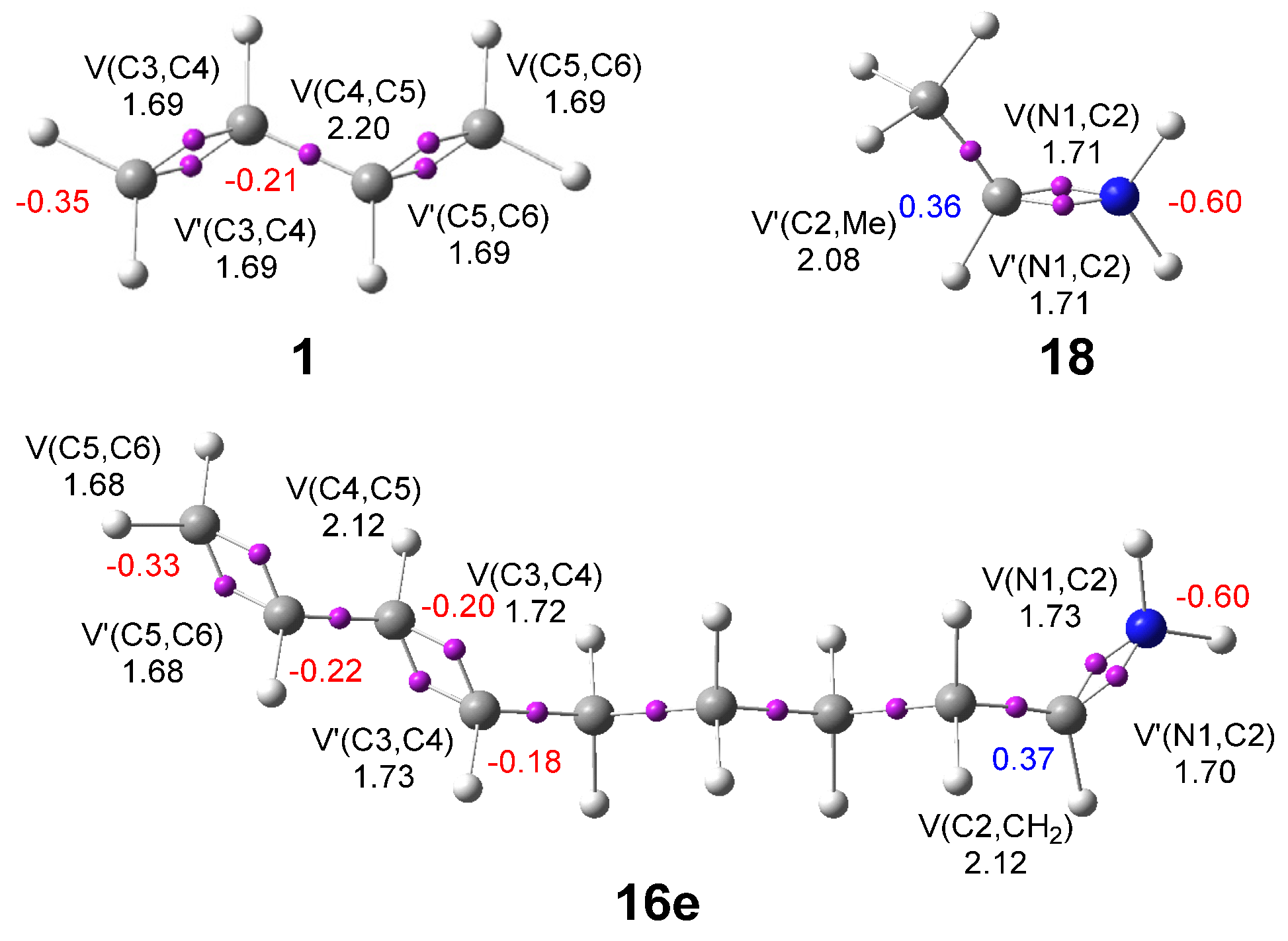
3.1.1. Analysis of the GS Electronic Structures of the Reagents
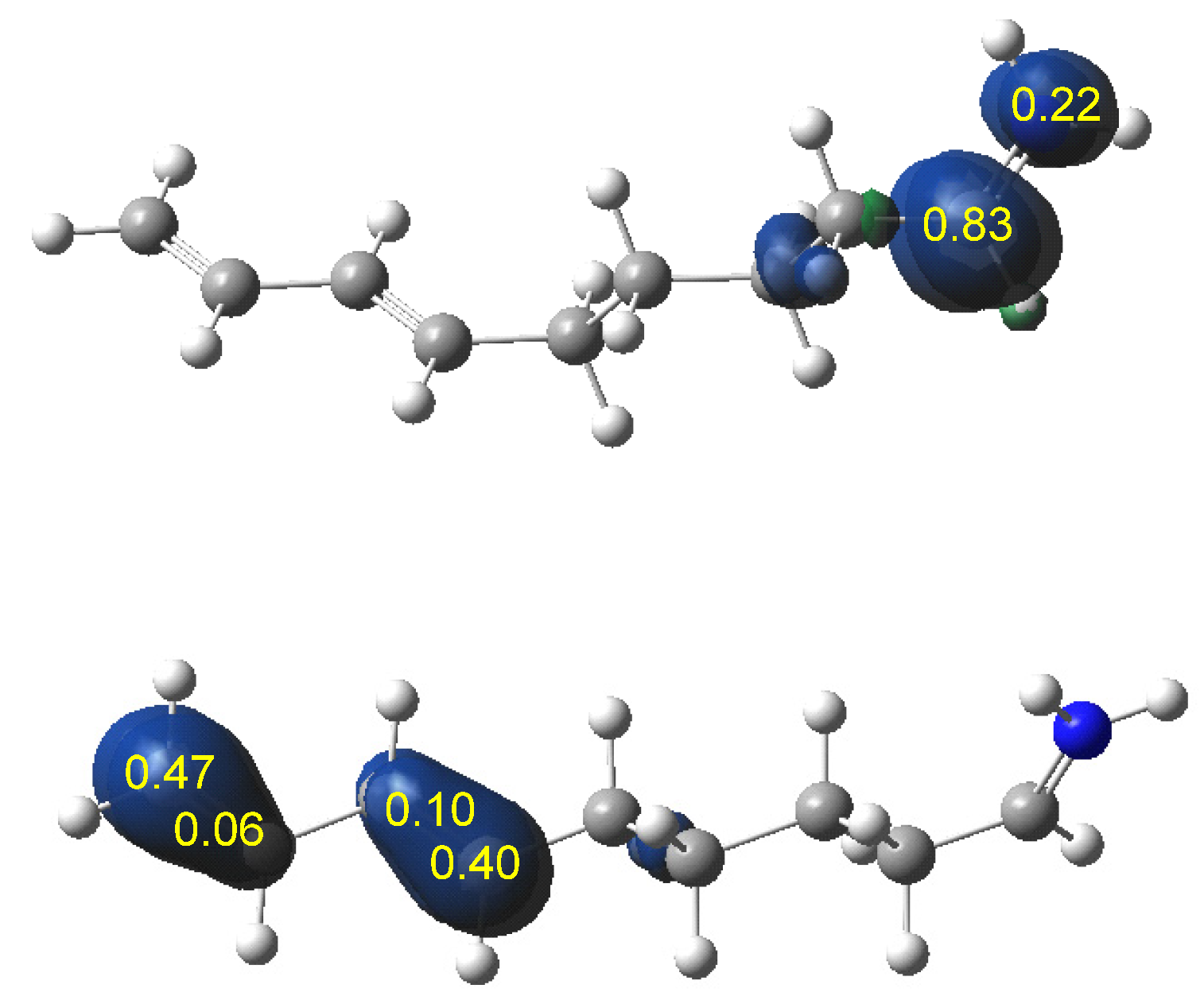
3.1.2. Analysis of the CDFT Reactivity Indices at the GS of the Reagents
3.2. Study of the I-DA Reaction between Butadiene 1 and Ethaniminium 18, and the IIDA Reactions of Dieniminiums 12 and 16
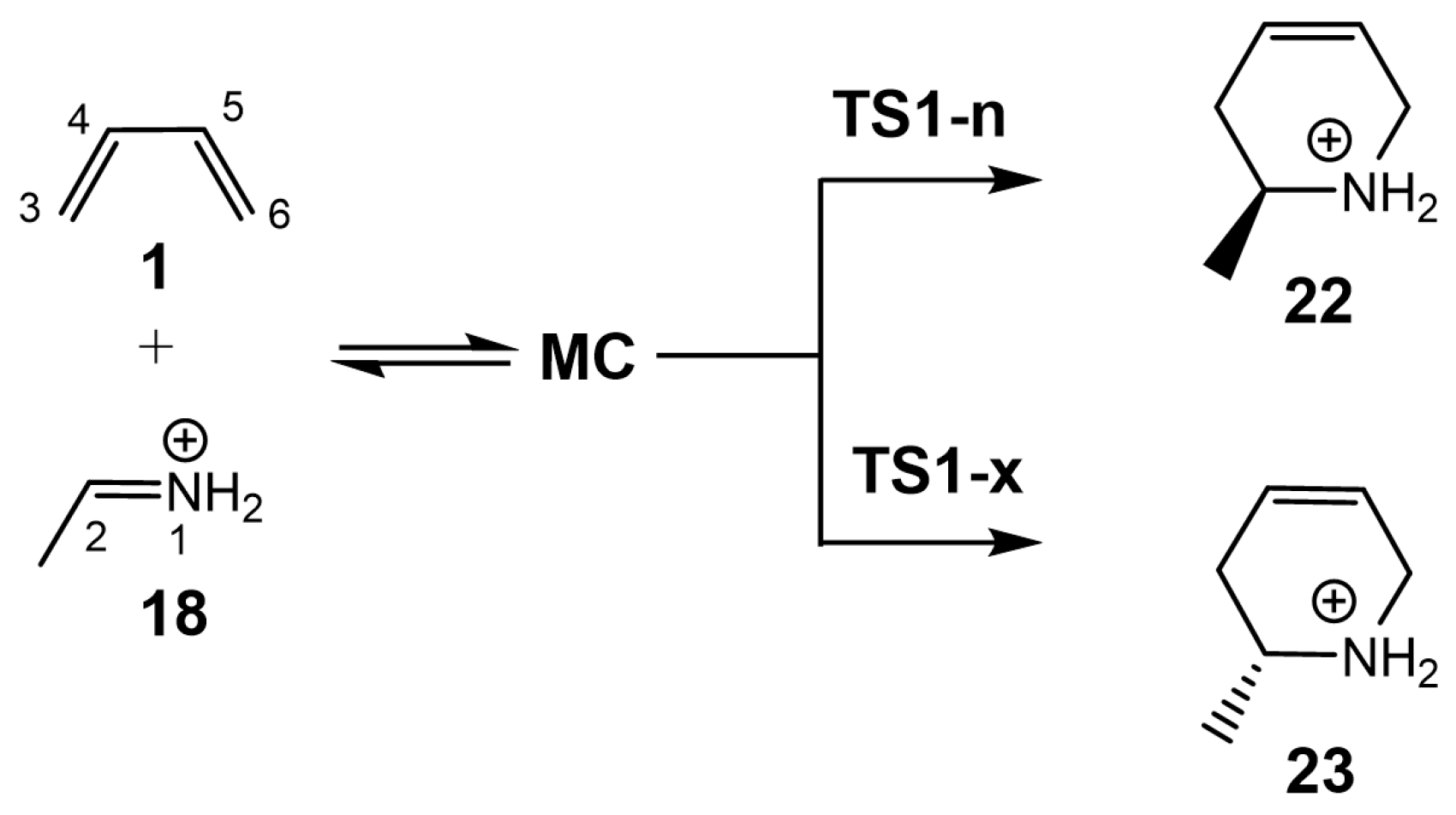
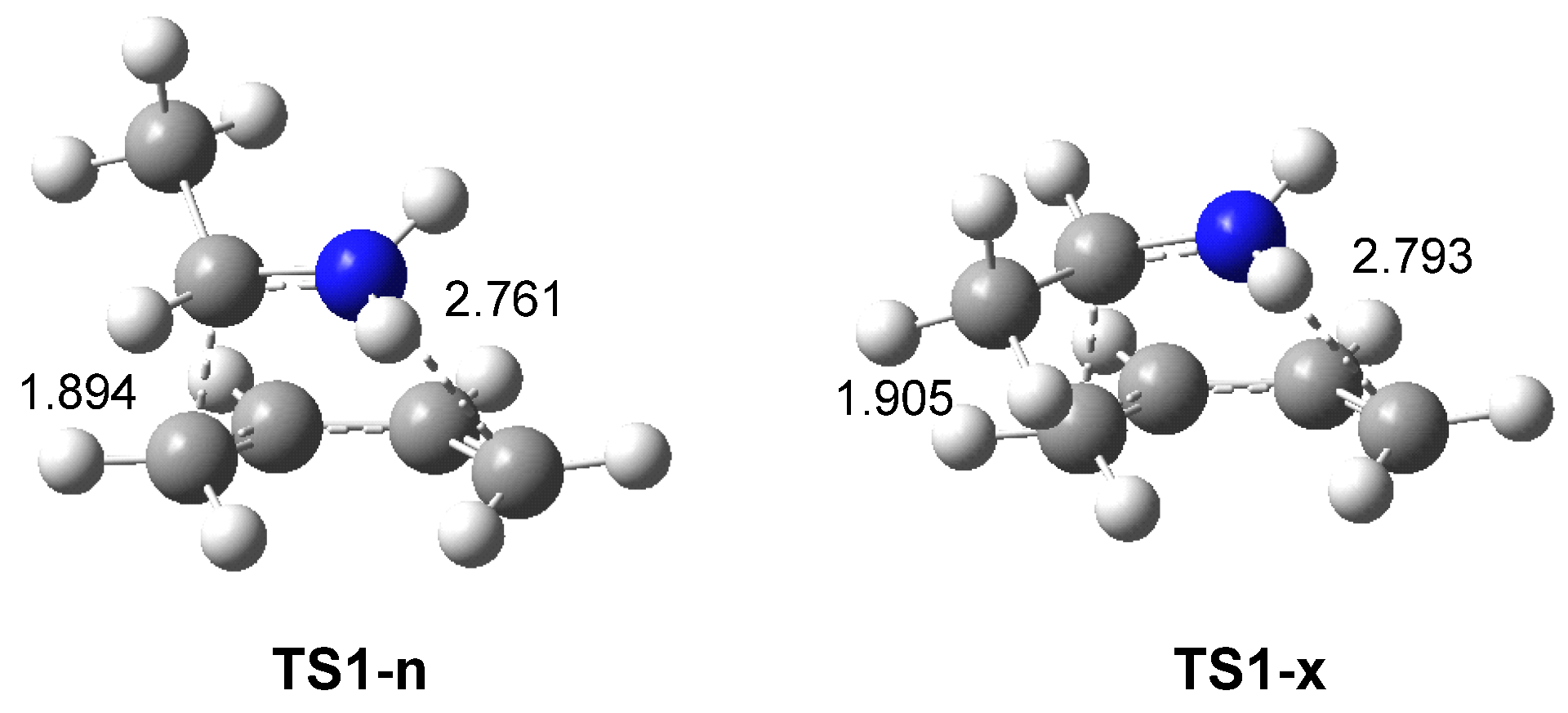
3.2.1. Study of the I-DA Reaction between Butadiene 1 and Ethaniminium 18
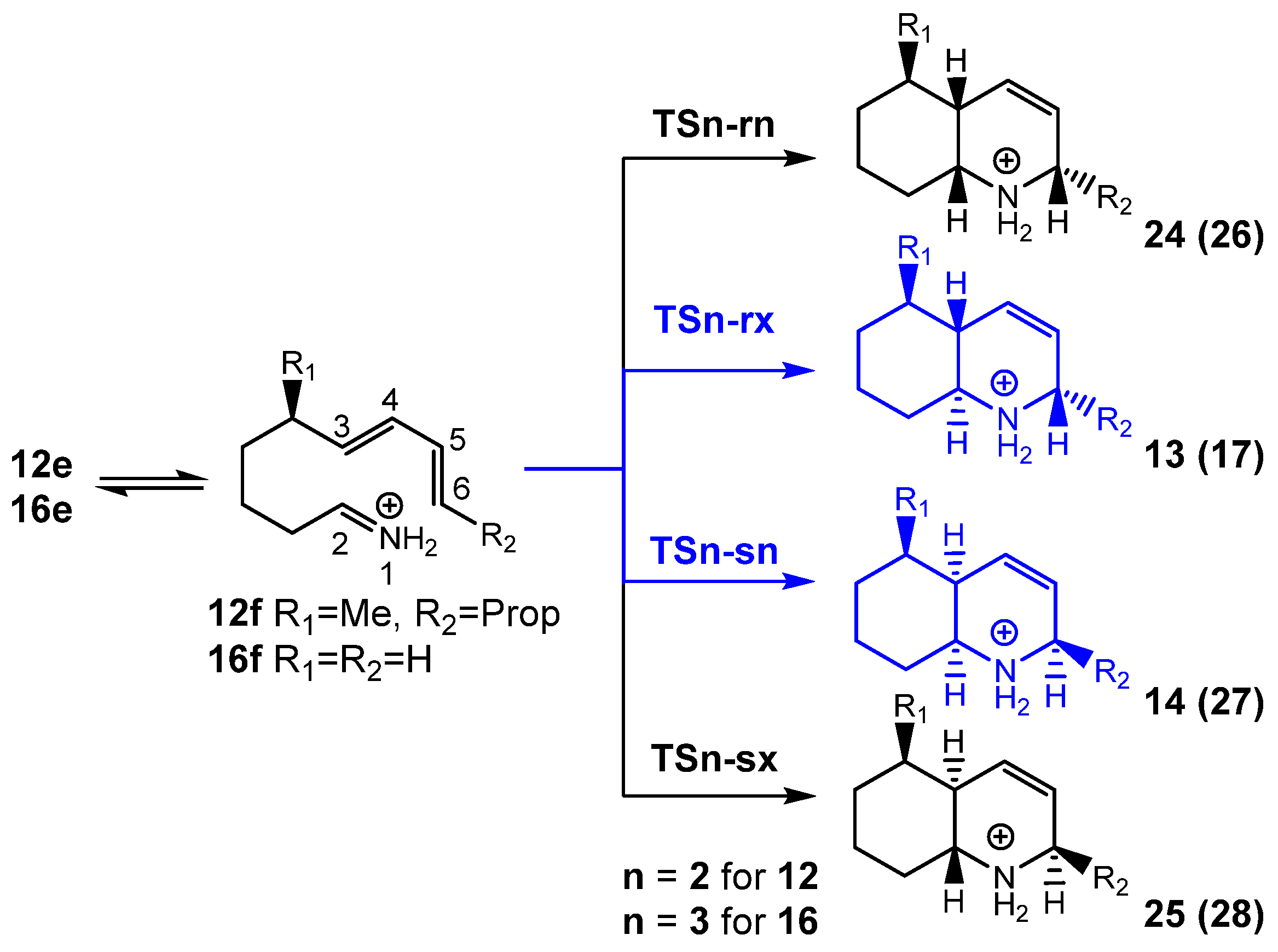
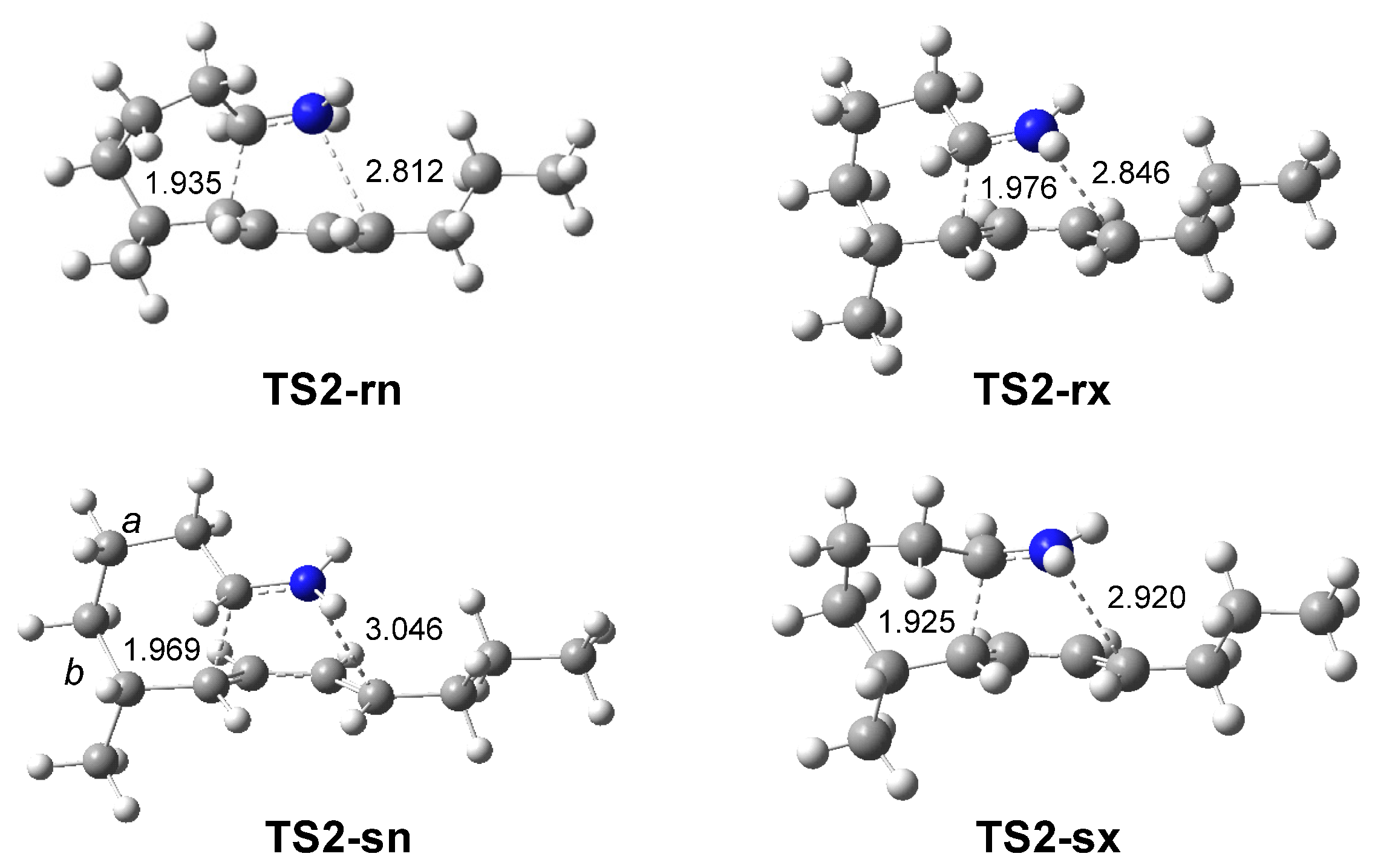
3.2.2. Study of the IIDA Reactions of Dieniminiums 12 and 16
3.3. Topological Analysis of the Bonding Changes along the IIDA Reactions
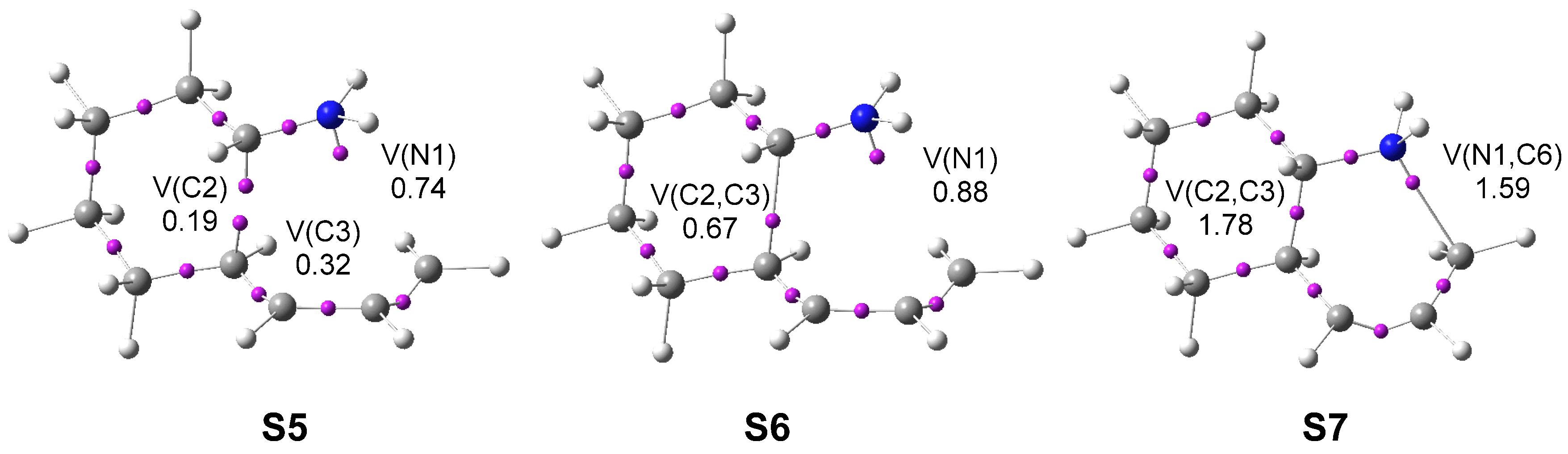
3.3.1. ELF Analysis of the N1−C6 and C2−C3 Single Bond Formation along the IIDA Reaction of Dieniminium 16
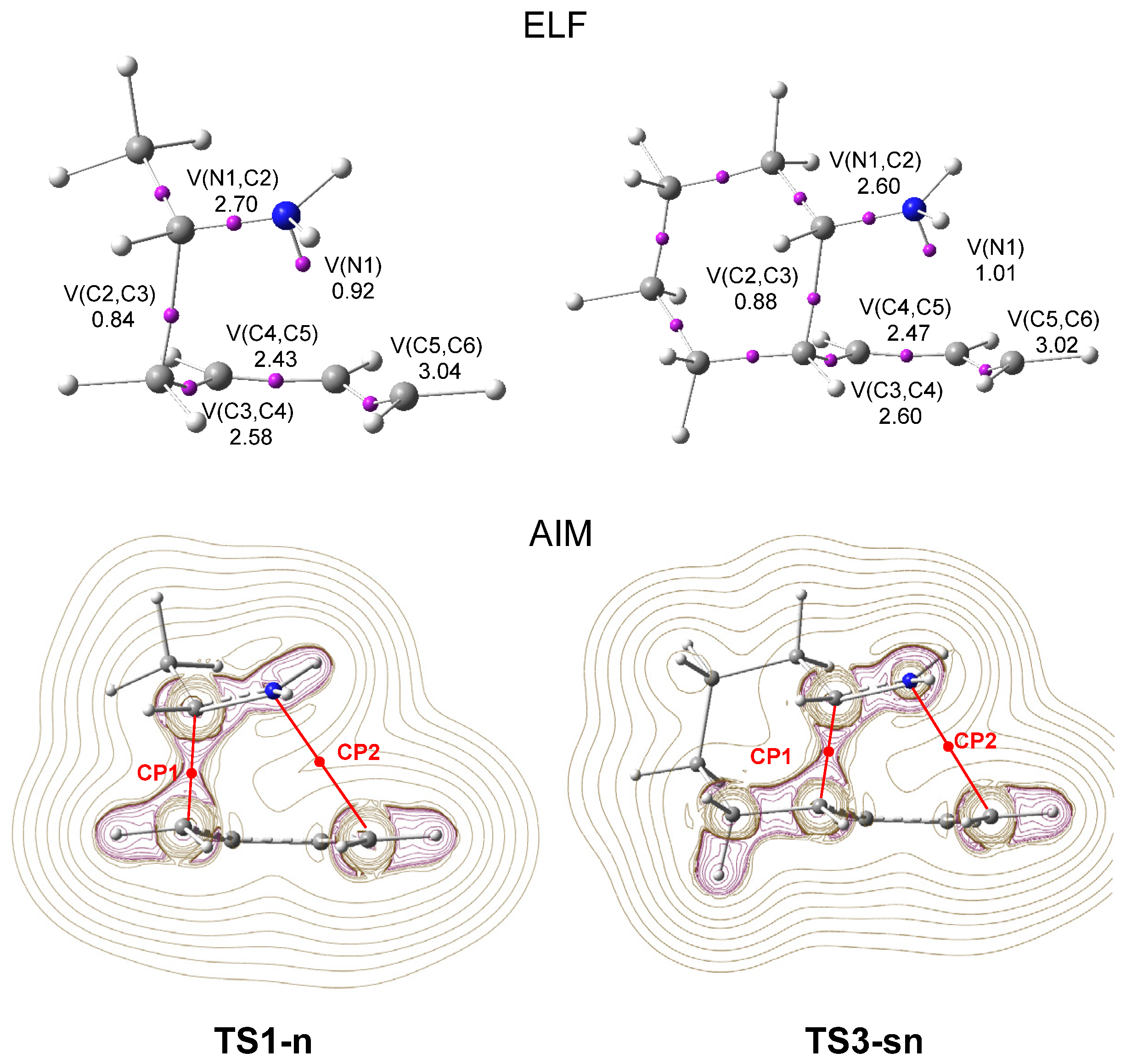
3.3.2. ELF and AIM Comparative Topological Analysis of the Most Favorable TSs Involved in the Inter and Intra I-DA Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Carruthers, W. Some Modern Methods of Organic Synthesis, 2nd ed.; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Carruthers, W. Cycloaddition Reactions in Organic Synthesis; Pergamon: Oxford, UK, 1990. [Google Scholar]
- Woodward, R.B.; Hoffmann, R. The Conservation of Orbital Symmetry. Angew. Chem. Int. Ed. Engl. 1969, 8, 781–853. [Google Scholar] [CrossRef]
- Houk, K.N.; Gonzalez, J.; Li, Y. Pericyclic Reaction Transition States: Passions and Punctilios, 1935–1995. Acc. Chem. Res. 1995, 28, 81–90. [Google Scholar] [CrossRef]
- Rowley, D.; Steiner, H. Kinetics of diene reactions at high temperatures. Discuss. Faraday Soc. 1951, 10, 198–213. [Google Scholar] [CrossRef]
- Goldstein, E.; Beno, B.; Houk, K.N. Density Functional Theory Prediction of the Relative Energies and Isotope Effects for the Concerted and Stepwise Mechanisms of the Diels−Alder Reaction of Butadiene and Ethylene. J. Am. Chem. Soc. 1996, 118, 6036–6043. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef] [Green Version]
- Domingo, L.R.; Arnó, M.; Andrés, J. Influence of Reactant Polarity on the Course of the Inverse-Electron-Demand Diels–Alder Reaction. A DFT Study of Regio- and Stereoselectivity, Presence of Lewis Acid Catalyst, and Inclusion of Solvent Effects in the Reaction between Nitroethene and Substituted Ethenes. J. Org. Chem. 1999, 64, 5867–5875. [Google Scholar]
- Domingo, L.R.; Aurell, M.J.; Perez, P.; Contreras, R. Origin of the synchronicity on the transition structures of polar Diels–Alder reactions. Are these reactions [4+2] processes? J. Org. Chem. 2003, 68, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Sáez, J.A. Understanding the mechanism of polar Diels–Alder reactions. Org. Biomol. Chem. 2009, 7, 3576–3583. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular electron density theory: A modern view of reactivity in organic chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Aurell, M.J. Unveiling the Ionic Diels–Alder Reactions within the Molecular Electron Density Theory. Molecules 2021, 26, 3638. [Google Scholar] [CrossRef] [PubMed]
- Sustmann, R.; Trill, H. Substituent Effects in 1,3-Dipolar Cycloadditions of Phenyl Azide. Angew. Chem. Int. Ed. Engl. 1972, 11, 838–840. [Google Scholar] [CrossRef]
- Fukui, K. Molecular Orbitals in Chemistry, Physics, and Biology; Löwdin, P.-O., Pullman, B., Eds.; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Houk, K.N.; Sims, J.; Watts, C.R.; Luskus, L.J. Origin of reactivity, regioselectivity, and periselectivity in 1,3-dipolar cycloadditions. J. Am. Chem. Soc. 1973, 95, 7301–7315. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Silvi, B.; Pérez, P. The Mysticism of Pericyclic Reactions. A Contemporary Rationalisation of Organic Reactivity Based on the Electron Density Analysis. Eur. J. Org. Chem. 2018, 2018, 1107–1120. [Google Scholar] [CrossRef]
- Kiselev, V.D.; Konovalov, A.I. Factors that determine the reactivity of reactants in normal and catalysed Diels–Alder reactions. Russ. Chem. Rev. 1989, 58, 230–249. [Google Scholar] [CrossRef]
- Anh, N.T.; Maurel, F. Use and misuse of frontier orbital theory. New J. Chem. 1997, 21, 861–871. [Google Scholar]
- Spino, C.; Rezaei, H.; Dory, Y.L. Characteristics of the Two Frontier Orbital Interactions in the Diels−Alder Cycloaddition. J. Org. Chem. 2004, 69, 757–764. [Google Scholar] [CrossRef]
- Domingo, L.R. Why do electron-deficient dienes react rapidly in Diels–Alder reactions with electron-deficient ethylenes? A density functional theory analysis. Eur. J. Org. Chem. 2004, 2004, 4788–4793. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Molecular Electron Density Theory Study of the Reactivity of Tetrazines in Aza-Diels–Alder Reactions. RSC Adv. 2020, 10, 15394–15405. [Google Scholar] [CrossRef] [Green Version]
- Domingo, L.R.; Kula, K.; Ríos-Gutiérrez, M. Unveiling the Reactivity of Cyclic Azomethine Ylides in [3 + 2] Cycloaddition Reactions within the Molecular Electron Density Theory. Eur. J. Org. Chem. 2020, 2020, 5938–5948. [Google Scholar] [CrossRef]
- Soto-Delgado, J.; Aizman, A.; Contreras, R.; Domingo, L.R. On the Catalytic Effect of Water in the Intramolecular Diels–Alder Reactions of Quinone System. A Theoretical Study. Molecules 2012, 17, 13687–13703. [Google Scholar] [CrossRef] [PubMed]
- Soto-Delgado, J.; Aizman, A.; Domingo, L.R.; Contreras, R. Invariance of electrophilicity of independent fragments. Application to intramolecular Diels–Alder reactions. Chem. Phys. Lett. 2010, 499, 272–277. [Google Scholar] [CrossRef]
- Soto-Delgado, J.; Domingo, L.R.; Contreras, R. Quantitative characterization of group electrophilicity and nucleophilicity for intramolecular Diels–Alder reactions. Org. Biomol. Chem. 2010, 8, 3678–3683. [Google Scholar] [CrossRef]
- Liang, Y.; Jiang, X.; Yu, Z.-X. Mechanisms of Cascade Reactions in the Syntheses of Camptothecin-Family Alkaloids: Intramolecular [4+ + 2]. Reactions of N-Arylimidates and Alkynes. Org. Lett. 2009, 22, 5303–5305. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Zheng, L.; Zhuo, l.-G.; Dang, Q.; Yu, Z.-X.; Bai, X. On-Demand Selection of the Reaction Path from Imino Diels–Alder to Ene-Type Cyclization: Synthesis of Epiminopyrimido[4,5-b]azepines. Eur. J. Org. Chem. 2014, 660, 660–669. [Google Scholar] [CrossRef]
- Grieco, P.A.; Parker, D.T. Octahydroquinoline Synthesis via Immonium Ion Based Diels–Alder Chemistry: Synthesis of (-)-8a-Epipumiliotoxin C. J. Org. Chem. 1988, 53, 3658–3662. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. The role of exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Phys. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Hehre, M.J.; Radom, L.; Schleyer, P.V.R.; Pople, J. Ab initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Schlegel, H.B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982, 3, 214–218. [Google Scholar] [CrossRef]
- Schlegel, H.B. Modern Electronic Structure Theory; Yarkony, D.R., Ed.; World Scientific Publishing: Singapore, 1994. [Google Scholar]
- Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher-order implicit algorithms. J. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, J.; Persico, M. Molecular interactions in solution: And overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Simkin, B.Y.; Sheikhet, I.I. Quantum Chemical and Statistical Theory of Solutions–Computational Approach; Ellis Horwood: London, UK, 1995. [Google Scholar]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular-systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Computational tools for the electron localization function topological analysis. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Tang, Y.H.; Tal, Y.; Biegler-König, F.W. Properties of atoms and bonds in hydrocarbon molecules. J. Am. Chem. Soc. 1982, 104, 946–952. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- GaussView, Version 6.0; Dennington, R.; Keith, T.A.; Millam, J.M. (Eds.) Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Ahrens, J.; Geveci, B.; Law, C. ParaView: An End-User Tool for Large Data Visualization. In Visualization Handbook; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Ayachit, U. The ParaView Guide: A Parallel Visualization Application; Kitware: New York, NY, USA, 2015; ISBN 978-1930934306. [Google Scholar]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Pérez, P.; Domingo, L.R.; Aizman, A.; Contreras, R. The Electrophilicity Index in Organic Chemistry. In Theoretical Aspects of Chemical Reactivity; Toro-Labbe, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 9, pp. 139–201. [Google Scholar]
- Domingo, L.R.; Pérez, P. The Nucleophilicity N Index in Organic Chemistry. Org. Biomol. Chem. 2011, 9, 7168–7175. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.v.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P. The Lithium Cation Catalysed Benzene Diels–Alder reaction. Insights on the Molecular Mechanism within the Molecular Electron Density Theory. J. Org. Chem. 2020, 85, 13121–13132. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Aurell, M.J.; Domingo, L.R.; Perez, P.; Contreras, R. A theoretical study on the regioselectivity of 1,3-dipolar cycloadditions using DFT-based reactivity indexes. Tetrahedron 2004, 60, 11503–11509. [Google Scholar] [CrossRef]
- Domingo, L.R.; Perez, P.; Sáez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 31486–31494. [Google Scholar] [CrossRef]
- Domingo, L.R.; Rios-Gutierrez, M.; Ndassa, I.A.I.M.; Nouhou, C.N.; Mbadcam, J.K. Molecular Electron Density Theory Study of Fused Regioselectivity in the Intramolecular [3+2] Cycloaddition Reaction of Nitrones. Chem. Select. 2018, 3, 5412–5420. [Google Scholar] [CrossRef]
- Krokidis, X.; Noury, S.; Silvi, B. Characterization of Elementary Chemical Processes by Catastrophe Theory. J. Phys. Chem. A 1997, 101, 7277–7282. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A.; Zaragozá, R.J.; Arnó, M. Understanding the Participation of Quadricyclane as Nucleophile in Polar [2σ + 2σ + 2π] Cycloadditions toward Electrophilic π Molecules. J. Org. Chem. 2008, 73, 8791–8799. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H⋯F–YX–H⋯F–Y system. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Sanderson, R.T. Partial charges on atoms in organic compounds. Science 1955, 121, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, R.T. Chemical Bonds and Bond Energy, 2nd ed.; Academic Press: New York, NY, USA, 1976. [Google Scholar]
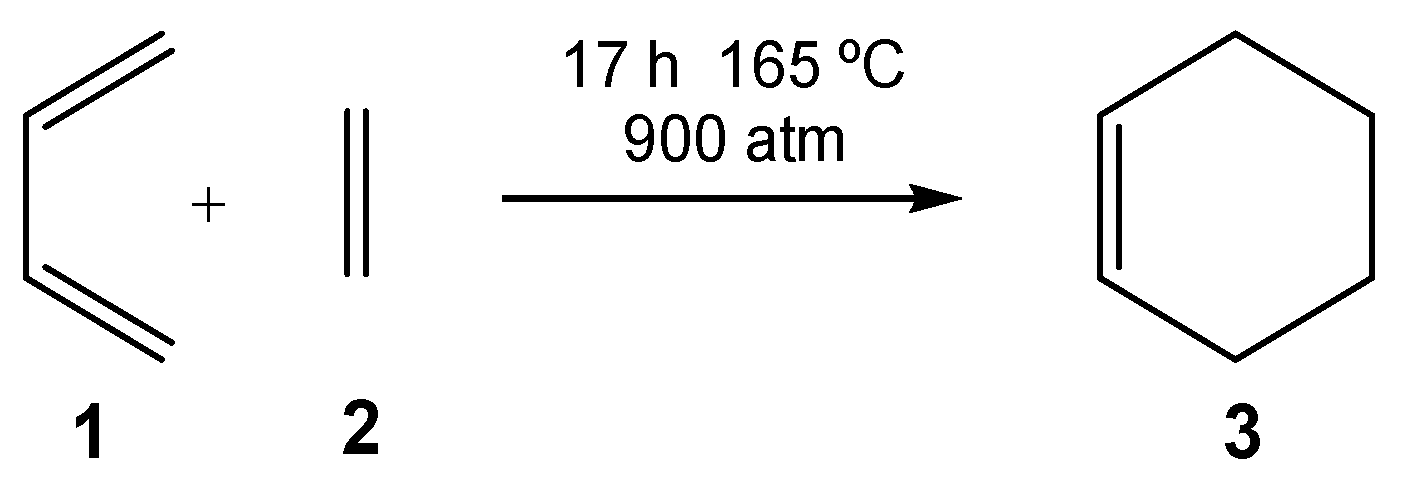
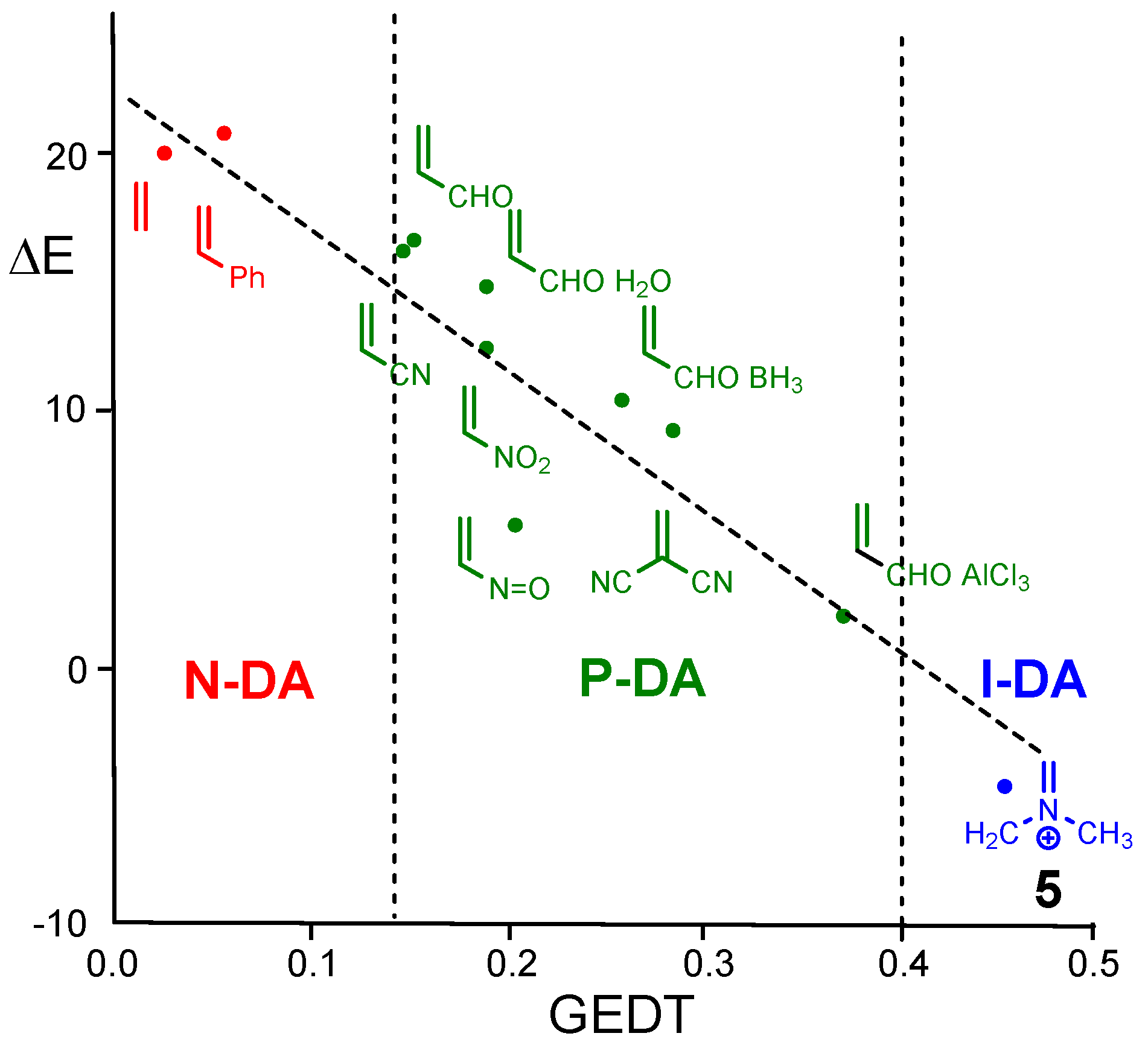

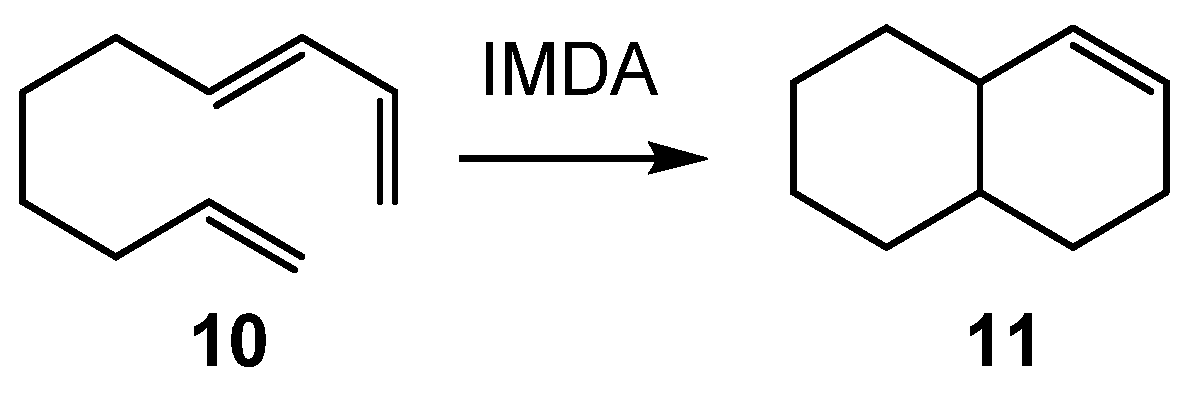

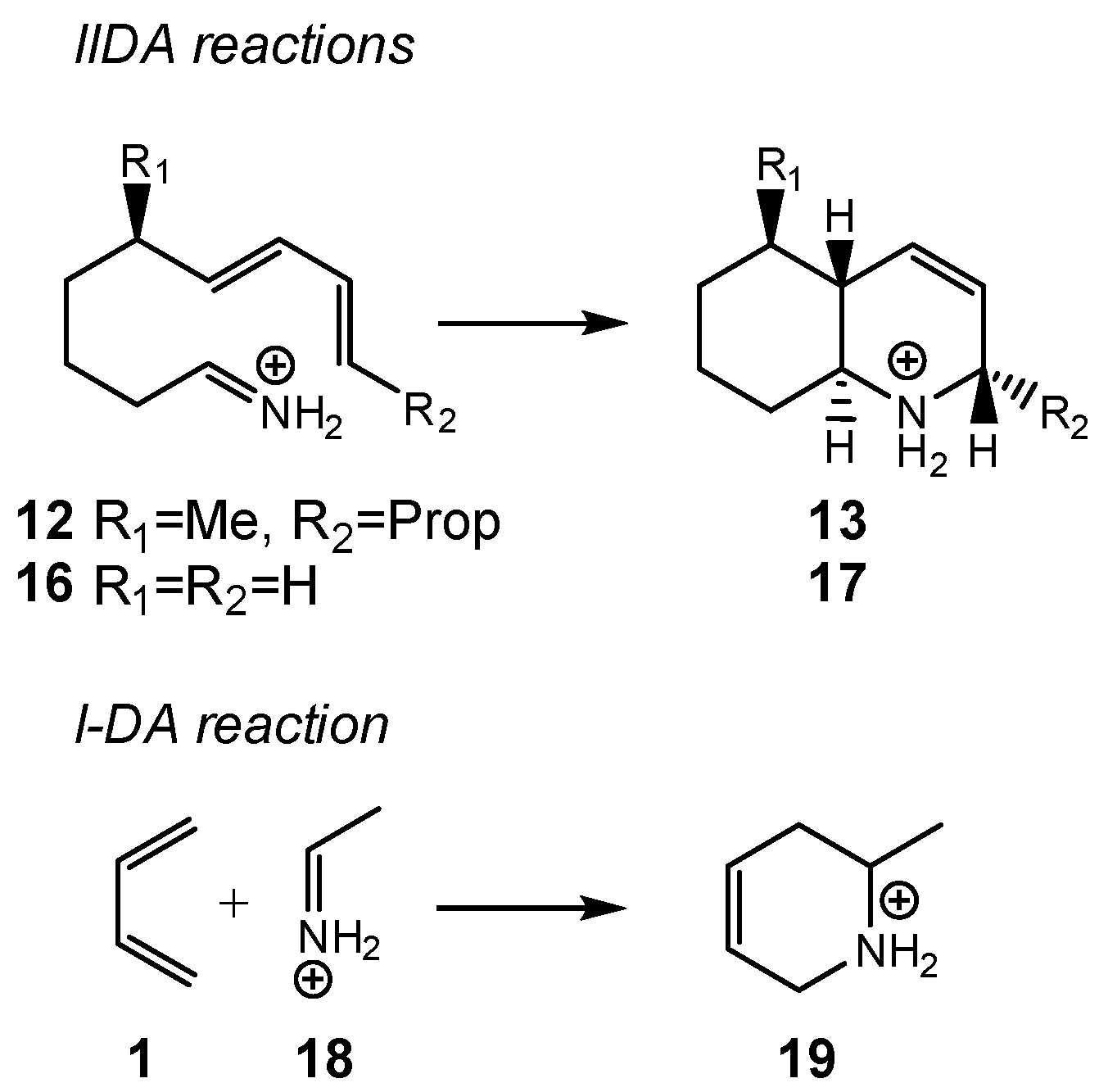
| ΔH | ΔS | ΔG | |
|---|---|---|---|
| TS-inter | 25.7 | −44.8 | 45.4 |
| 3 | −28.5 | −52.4 | −5.5 |
| TS-intra | 29.4 | −19.4 | 38.0 |
| 11 | −28.8 | −24.2 | −18.2 |
| μ | η | ω | N | |
|---|---|---|---|---|
| 12e | −7.39 | 1.02 | 26.72 | 1.22 |
| 16e | −7.65 | 1.16 | 25.19 | 0.89 |
| 16f | −8.23 | 3.76 | 9.01 | −0.99 |
| ethaniminium 18 | −11.82 | 8.27 | 8.46 | −6.83 |
| 12f | −7.85 | 3.68 | 8.36 | −0.57 |
| butadiene 1 | −3.42 | 5.62 | 1.04 | 2.89 |
| pentadiene 20 | −3.17 | 5.51 | 0.91 | 3.20 |
| dienimine 21 | −3.31 | 5.47 | 1.00 | 3.08 |
| ΔE | ΔH | ΔS | ΔG | |
|---|---|---|---|---|
| MC | −4.5 | −3.1 | −26.0 | 6.0 |
| TS1-n | 8.1 | 9.3 | −46.1 | 25.3 |
| TS1-x | 9.0 | 10.1 | −44.9 | 25.8 |
| 22 | −41.3 | −36.3 | −51.6 | −18.3 |
| 23 | −35.9 | −31.0 | −49.9 | −13.6 |
| ΔE | ΔE | ||
|---|---|---|---|
| 12e | 0.3 | 16e | 0.0 |
| 12f | 0.0 | 16f | 1.0 |
| TS2-rn | 12.9 | TS3-rn | 17.7 |
| TS2-rx | 8.8 | TS3-rx | 13.6 |
| TS2-sn | 8.9 | TS3-sn | 13.6 |
| TS2-sx | 13.9 | TS3-sx | 18.1 |
| 24 | −25.0 | 26 | −27.1 |
| 13 | −31.1 | 17 | −30.6 |
| 14 | −30.3 | 27 | −30.1 |
| 25 | −25.2 | 28 | −24.5 |
| ΔH | ΔS | ΔG | |
|---|---|---|---|
| 12e | 0.0 | 0.0 | 0.0 |
| 12f | −1.1 | −11.2 | 2.8 |
| TS2-rn | 12.0 | −16.2 | 17.6 |
| TS2-rx | 7.6 | −14.3 | 12.6 |
| TS2-sn | 7.9 | −16.6 | 13.7 |
| TS2-sx | 13.0 | −14.5 | 18.1 |
| 24 | −22.2 | −23.8 | −14.0 |
| 13 | −28.5 | −23.1 | −20.5 |
| 14 | −27.8 | −20.9 | −20.6 |
| 25 | −22.3 | −22.8 | −14.4 |
| TS1-n | TS3-sn | |||
|---|---|---|---|---|
| CP1 | CP2 | CP1 | CP2 | |
| Density ρ(r) | 0.0989 | 0.0142 | 0.1001 | 0.0176 |
| Laplacian ∇2(r) | −0.0247 | 0.0364 | −0.0274 | 0.0444 |
| G(r) | 0.0331 | 0.0079 | 0.0331 | 0.0099 |
| V(r) | −0.0723 | −0.0067 | −0.0732 | −0.0087 |
| |V(r)|/G(r) | 2.1843 | 0.8481 | 2.2115 | 0.8788 |
| H(r) | 0.0392 | 0.0012 | 0.0400 | 0.0012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingo, L.R.; Ríos-Gutiérrez, M.; Aurell, M.J. Unveiling the Intramolecular Ionic Diels–Alder Reactions within Molecular Electron Density Theory. Chemistry 2021, 3, 834-853. https://doi.org/10.3390/chemistry3030061
Domingo LR, Ríos-Gutiérrez M, Aurell MJ. Unveiling the Intramolecular Ionic Diels–Alder Reactions within Molecular Electron Density Theory. Chemistry. 2021; 3(3):834-853. https://doi.org/10.3390/chemistry3030061
Chicago/Turabian StyleDomingo, Luis R., Mar Ríos-Gutiérrez, and María José Aurell. 2021. "Unveiling the Intramolecular Ionic Diels–Alder Reactions within Molecular Electron Density Theory" Chemistry 3, no. 3: 834-853. https://doi.org/10.3390/chemistry3030061
APA StyleDomingo, L. R., Ríos-Gutiérrez, M., & Aurell, M. J. (2021). Unveiling the Intramolecular Ionic Diels–Alder Reactions within Molecular Electron Density Theory. Chemistry, 3(3), 834-853. https://doi.org/10.3390/chemistry3030061








