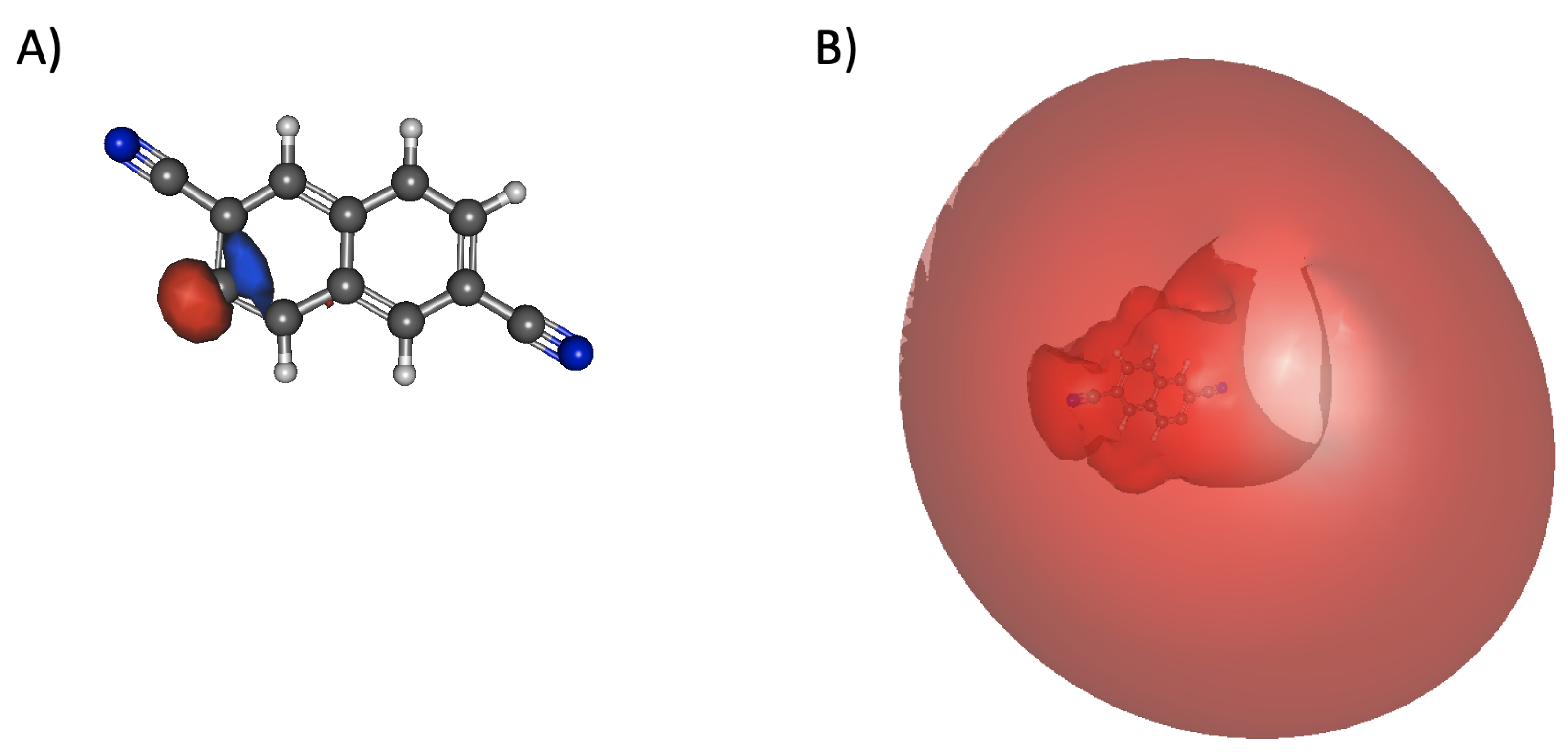
Valence-, Dipole- and Quadropole-Bound Electronically Excited States of Closed-Shell Anions Formed by Deprotonation of Cyano- and Ethynyl-Disubstituted Polycyclic Aromatic Hydrocarbons
Abstract
:1. Introduction
2. Computational Details
3. Results
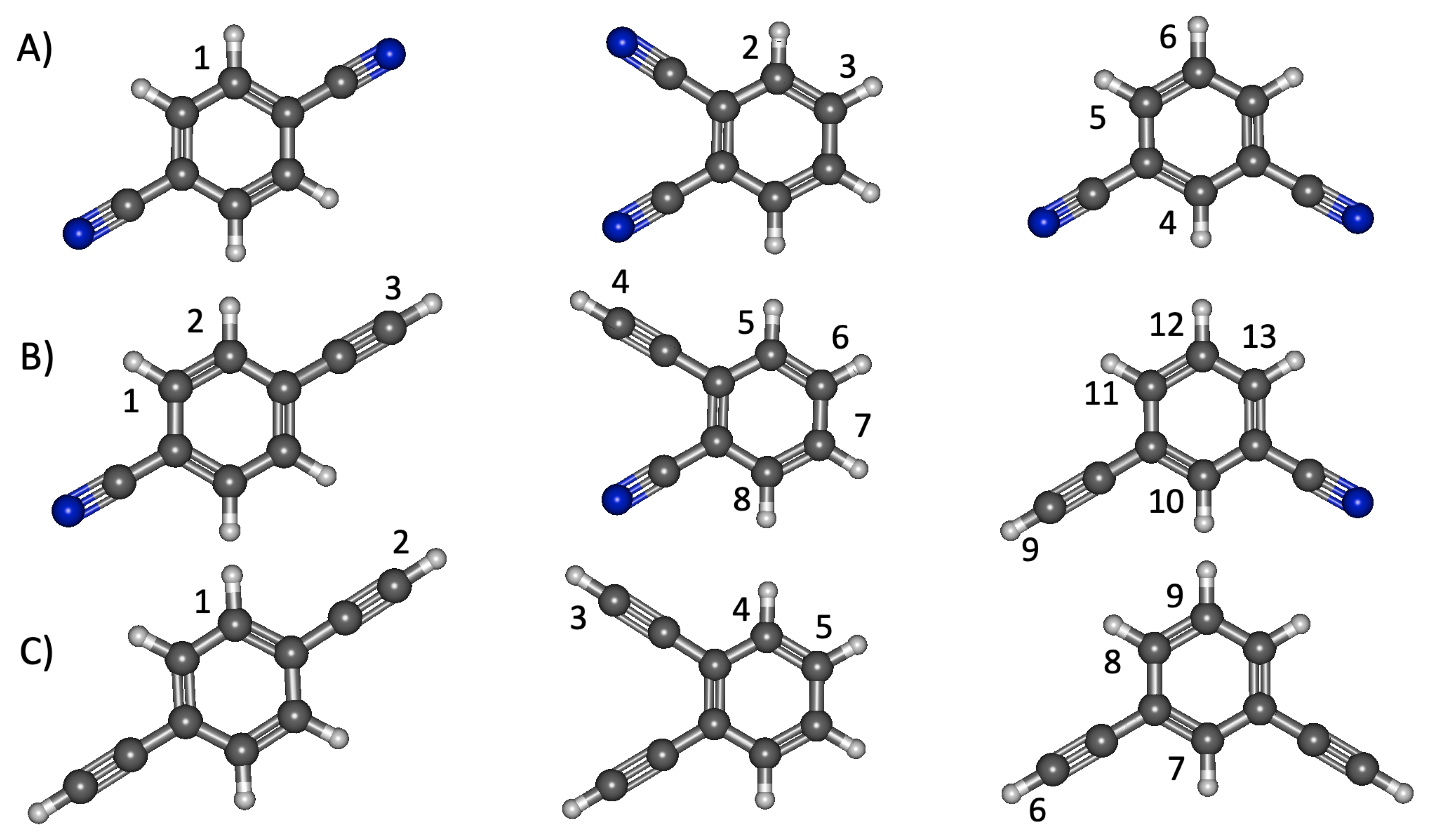
3.1. Benzene Derivatives
3.1.1. Relative Energies and Electrostatic Properties
3.1.2. Vertical Excitation Energies
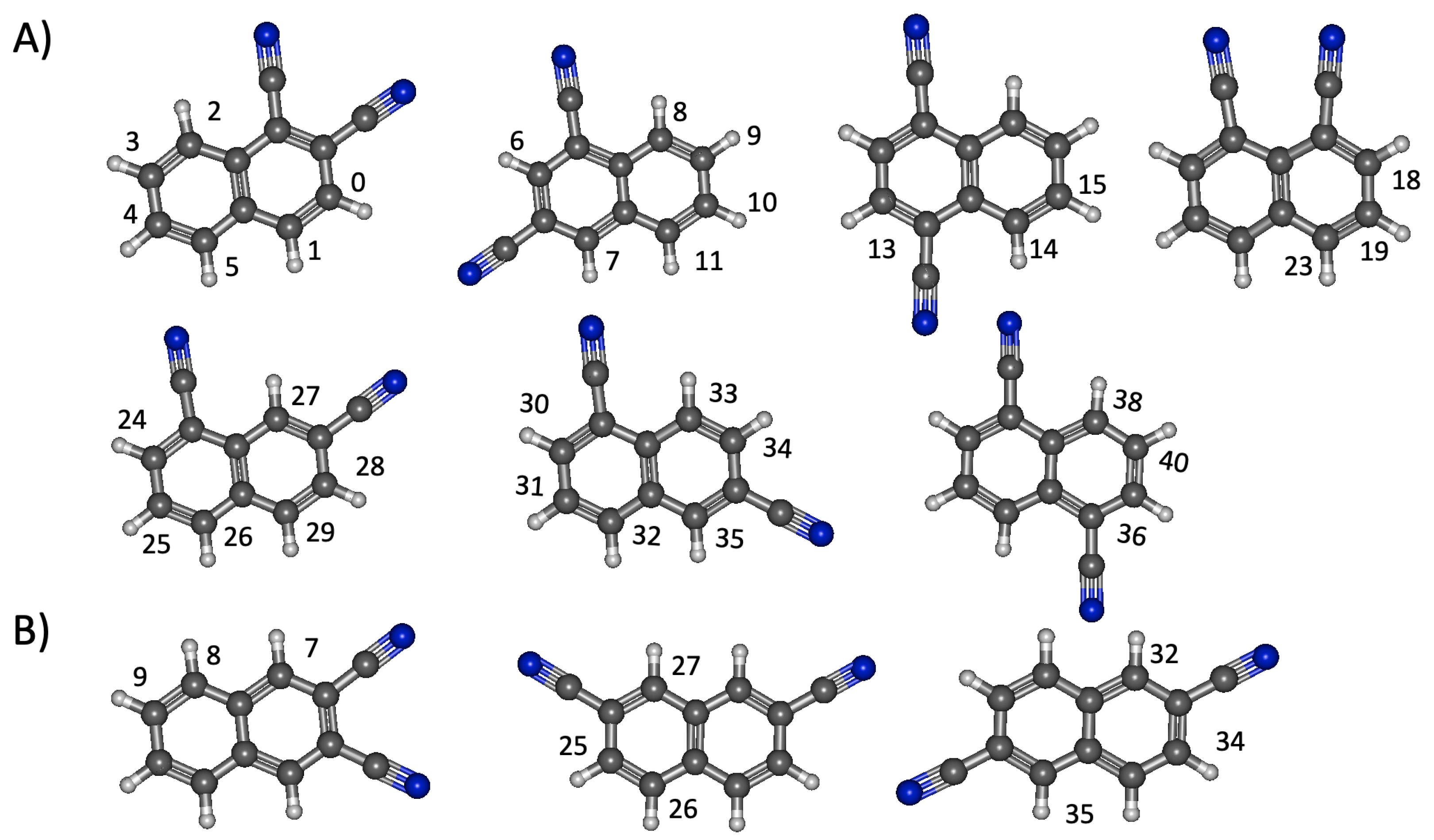
3.2. Dicyanonaphthalene
3.2.1. Relative Energies and Electrostatic Properties
3.2.2. Vertical Excitation Energies
3.3. Cyanoethynylnaphthalene and Diethynylnaphthalene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ISM | Interstellar Medium |
| eBE | Electron Binding Energy |
| VBS | Valence-Bound State |
| DBS | Dipole-Bound State |
| QBS | Quadrupole-Bound State |
| DIBs | Diffuse Interstellar Bands |
References
- Compton, R.N.; Carman, H.S., Jr.; Desfrançois, C.; Hendricks, J.; Lyapustina, S.A.; Bowen, K.H. On the Binding of Electrons to Nitromethane: Dipole and Valence Bound Anions. J. Chem. Phys. 1996, 105, 3472–3478. [Google Scholar] [CrossRef] [Green Version]
- Jordan, K.D.; Wang, F. Theory of Dipole-Bound Anions. Ann. Rev. Phys. Chem. 2003, 54, 367–396. [Google Scholar] [CrossRef]
- Simons, J. Molecular Anions. J. Phys. Chem. A 2008, 112, 6401–6511. [Google Scholar] [CrossRef]
- Ard, S.; Garrett, W.R.; Compton, R.N.; Adamowicz, L.; Stepanian, S.G. Rotational States of Dipole-Bound Anions of Hydrogen Cyanide. Chem. Phys. Lett. 2009, 473, 223–226. [Google Scholar] [CrossRef]
- Simons, J. Theoretical Study of Negative Molecular Anions. Annu. Rev. Phys. Chem. 2011, 62, 107–128. [Google Scholar] [CrossRef] [Green Version]
- Fortenberry, R.C. Interstellar Anions: The Role of Quantum Chemistry. J. Phys. Chem. A 2015, 119, 9941–9953. [Google Scholar] [CrossRef]
- Brinkman, E.A.; Gunther, E.; Schafer, O.; Brauman, J.I. Bound Excited Electronic States of Anions. J. Chem. Phys. 1993, 100, 1840–1848. [Google Scholar] [CrossRef]
- Lakin, N.M.; Guthe, F.; Tulej, M.; Pachkov, M.; Maier, J.P. Spectroscopy of Excited States of Carbon Anions above the Photodetachment Threshold. Faraday Discuss. 2000, 115, 383–393. [Google Scholar] [CrossRef]
- Gutowski, M.; Skurski, P.; Li, X.; Wang, L.S. (MgO)2n-(n = 1-5) Clusters: Multipole-Bound Anions and Photodetachment Spectroscopy. Phys. Rev. Lett. 2000, 85, 3145–3148. [Google Scholar] [CrossRef]
- Chomicz, L.; Rak, J.; Paneth, P.; Sevilla, M.; Ko, Y.J.; Wang, H.; Bowen, K.H. Valence Anions of N-Acetylproline in the Gas Phase: Computational and Anion Photoelectron Spectroscopic Studies. J. Chem. Phys. 2011, 135, 114301. [Google Scholar] [CrossRef] [Green Version]
- Fortenberry, R.C.; Crawford, T.D. Electronically Excited States in Interstellar Chemistry. Annu. Rep. Comput. Chem. 2011, 7, 195–214. [Google Scholar]
- McCarthy, M.C.; Gottlieb, C.A.; Gupta, H.; Thaddeus, P. Laboratory and Astronomical Identification of the Negative Molecular Ion C6H−. Astrophys. J. 2006, 652, L141–L144. [Google Scholar] [CrossRef]
- Cernicharo, J.; Guèlin, M.; Agùndez, M.; Kawaguchi, K.; McCarthy, M.; Thaddeus, P. Astronomical Detection of C4H−, the Second Interstellar Anion. Astron. Astrophys. 2007, 467, L37–L40. [Google Scholar] [CrossRef]
- Brünken, S.; Gupta, H.; Gottlieb, C.A.; McCarthy, M.C.; Thaddeus, P. Detection of the Carbon Chain Negative Ion C8H− in TMC-1. Astrophys. J. 2007, 664, L43–L46. [Google Scholar] [CrossRef] [Green Version]
- Remijan, A.J.; Hollis, J.M.; Lovas, F.J.; Cordiner, M.A.; Millar, T.J.; Markwick-Kemper, A.J.; Jewell, P.R. Detection of C8H− and comparison with C8H toward IRC+10216. Astrophys. J. 2007, 664, L47–L50. [Google Scholar] [CrossRef] [Green Version]
- Thaddeus, P.; Gottlieb, C.A.; Gupta, H.; Brünken, S.; McCarthy, M.C.; Agùndez, M.; Guèlin, M.; Cernicharo, J. Laboratory and Astronomical Detection of the Negative Molecular Ion C3N−. Astrophys. J. 2008, 677, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Cernicharo, J.; Guèlin, M.; Agundez, M.; McCarthy, M.C.; Thaddeus, P. Detection of C5N− and Vibrationally Excited C6H in IRC+10216. Astrophys. J. 2008, 688, L83–L86. [Google Scholar] [CrossRef]
- Agùndez, M.; Cernicharo, J.; Guèlin, M.; Kahane, C.; Roueff, E.; Klos, J.; Aoiz, F.J.; Lique, F.; Marcelino, N.; Goicoechea, J.R.; et al. Astronomical Identification of CN−, the Smallest Observed Molecular Anion. Astron. Astrophys. 2010, 517, L2. [Google Scholar] [CrossRef] [Green Version]
- Cordiner, M.A.; Charnley, S.B.; Buckle, J.V.; Walsh, C.; Millar, T.J. Discovery of Interstellar Anions in Cepheus and Auriga. Astrophys. J. Lett. 2011, 730, L18. [Google Scholar] [CrossRef] [Green Version]
- Cordiner, M.A.; Buckle, J.V.; Wirström, E.S.; Olofsson, A.O.H.; Charnley, S.B. On the Ubiquity of Molecular Anions in the Dense Interstellar Medium. Astrophys. J. 2013, 770, 48. [Google Scholar] [CrossRef] [Green Version]
- McGuire, B.A. 2018 Census of Interstellar, Circumstellar, Extragalactic, Protoplanetary Disk, and Exoplanetary Molecules. Astrophys. J. Suppl. Ser. 2018, 239, 17. [Google Scholar] [CrossRef]
- Fermi, E.; Teller, E. The Capture of Negative Mesotrons in Matter. Phys. Rev. 1947, 72, 399–408. [Google Scholar] [CrossRef]
- Crawford, O.H.; Dalgarno, A. Bound States of an Electron in a Dipole Field. Chem. Phys. Lett. 1967, 1, 23. [Google Scholar] [CrossRef]
- Jordan, K.D.; Luken, W. Theoretical Study of the Binding of an Electron to a Molecular Dipole: LiCl−. J. Chem. Phys. 1976, 64, 2760. [Google Scholar] [CrossRef]
- Crawford, O.H.; Garrett, W.R. Electron Affinities of Polar Molecules. J. Chem. Phys. 1977, 66, 4968. [Google Scholar] [CrossRef]
- Turner, J.E. Minimum Dipole Moment Required to Bind an Electron: Molecular Theorists Rediscover Phenomenon Mentioned in Fermi–Teller Paper Twenty Years Earlier. Am. J. Phys. 1977, 45, 758. [Google Scholar] [CrossRef]
- Gutowski, M.; Skurksi, P.; Boldyrev, A.I.; Simons, J.; Jordan, K.D. The Contribution of Electron Correlation to the Stability of Dipole-Bound Anionic States. Phys. Rev. 1996, 54, 1906. [Google Scholar] [CrossRef] [Green Version]
- Hoshina, K.; Kohguchi, H.; Ohshima, Y.; Endo, Y. Laser-Induced Fluorescence Spectroscopy of the C4H and C4D Radicals in a Supersonic Jet. J. Chem. Phys. 1998, 108, 3465–3477. [Google Scholar] [CrossRef]
- Fortenberry, R.C.; King, R.A.; Stanton, J.F.; Crawford, T.D. A Benchmark Study of the Vertical Electronic Spectra of the Linear Chain Radicals C2H and C4H. J. Chem. Phys. 2010, 132, 144303. [Google Scholar] [CrossRef] [Green Version]
- Agúndez, M.; Cernicharo, J.; Guélin, M.; Gerin, M.; McCarthy, M.C.; Thaddeus, P. Search for Anions in Molecular Sources: C4H− Detection in L1527. Astron. Astrophys. 2008, 478, L19–L22. [Google Scholar] [CrossRef] [Green Version]
- Gupta, H.; Gottlieb, C.A.; McCarthy, M.C.; Thaddeus, P. A Survery of C4H, C6H and C6H− with the Green Bank Telescope. Astrophys. J. 2009, 691, 1494–1500. [Google Scholar] [CrossRef] [Green Version]
- Tucker, K.D.; Kutner, M.L.; Thaddeus, P. The Ethynyl Radical C2H—A New Interstellar Molecule. Astrophys. J. 1974, 193, L115–L119. [Google Scholar] [CrossRef]
- Heikkilä, A.; Johansson, L.E.B.; Olofsson, H. Molecular Abundance Variations in the Magellanic Clouds. Astron. Astrophys. 1999, 344, 817–847. [Google Scholar]
- Peeters, E.; Hony, S.; Van Kerckhoven, C.; Tielens, A.G.G.M.; Allamandola, L.J.; Hudgins, D.M.; Bauschlicher, C.W. The Rich 6 to 9 μm Spectrum of Interstellar PAHs. Astron. Astrophys. 2002, 390, 1089–1113. [Google Scholar] [CrossRef] [Green Version]
- Tielens, A.G.G.M. Interstellar Polycyclic Aromatic Hydrocarbon Molecules. Annu. Rev. Astron. Astrophys. 2008, 46, 289–337. [Google Scholar] [CrossRef] [Green Version]
- Allamandola, L.J. PAHs and Astrobiology. In PAHs and the Universe: A Symposium to Celebrate the 25th Anniversary of the PAH Hypothesis; Joblin, C., Tielens, A.G.G.M., Eds.; EAS Publication Series; EDP Sciences: Cambridge, UK, 2011. [Google Scholar]
- Andrews, H.; Boersma, C.; Werner, M.W.; Livingston, J.; Allamandola, L.J.; Tielens, A.G.G.M. PAH Emission at the Bright Locations of PDRs: The grandPAH Hypothesis. Astrophys. J. 2015, 807, 99. [Google Scholar] [CrossRef] [Green Version]
- McGuire, B.A.; Burkhardt, A.M.; Kalenskii, S.; Shingledecker, C.N.; Remijan, A.J.; Herbst, E.; McCarthy, M.C. Detection of the Aromatic Molecule Benzonitrile (c-C6H5CN) in the Interstellar Medium. Science 2018, 359, 202–205. [Google Scholar] [CrossRef] [Green Version]
- McGuire, B.A.; Loomis, R.A.; Burkhardt, A.M.; Lee, K.L.K.; Shingledecker, C.N.; Charnley, S.B.; Cooke, I.R.; Cordiner, M.A.; Herbst, E.; Kalenskii, S.; et al. Detection of Two Interstellar Polycyclic Aromatic Hydrocarbons via Spectral Matched Filtering. Science 2021, 371, 1265. [Google Scholar] [CrossRef]
- Cernicharo, J.; Agúndez, M.; Cabezas, C.; Tercero, B.; Marcelino, N.; Pardo, J.R.; de Vicente, P. Pure Hydrocarbon Cycles in TMC-1: Discovery of Ethynyl Cyclopropenylidene, Cyclopentadiene, and Indene. Astron. Astrophys. 2021, 649, L15. [Google Scholar] [CrossRef]
- Cernicharo, J.; Agúndez, M.; Kaiser, R.I.; Cabezas, C.; Tercero, B.; Marcelino, N.; Pardo, J.R.; de Vicente, P. Discovery of Two Isomers of Ethynyl Cyclopentadiene in TMC-1: Abundances of CCH and CN Derivatives of Hydrocarbon Cycles. Astron. Astrophys. 2021, 655, L1. [Google Scholar] [CrossRef]
- Douglas, A.E. Origin of Diffuse Interstellar Lines. Nature 1977, 269, 130–132. [Google Scholar] [CrossRef]
- Smith, W.H. Astrophysics and Space Science Library. In The Diffuse Interstellar Bands; Kluwer: Dordrecht, The Netherlands, 1995; pp. 3–12. [Google Scholar]
- Sarre, P.J. The Diffuse Interstellar Bands: A Major Problem in Astronomical Spectroscopy. J. Mol. Spectrosc. 2006, 238, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Heger, M.L. The spectra of certain class B stars in the regions 5630 Å-6680 Åand 3280 Å-3380 Å. Lick Obs. Bull. 1922, 10, 146. [Google Scholar]
- Merrill, P.W. Unidentified Interstellar Lines. Publ. Astron. Soc. Pacific 1934, 46, 206–207. [Google Scholar] [CrossRef] [Green Version]
- Merrill, P.W. Stationary Lines in the Spectrum of the Binary Star Boss 6142. Astrophys. J. 1936, 83, 126–128. [Google Scholar] [CrossRef]
- Hobbs, L.M.; York, D.G.; Snow, T.P.; Oka, T.; Thorburn, J.A.; Bishof, M.; Friedman, S.D.; McCall, B.J.; Rachford, B.; Sonnentrucker, P.; et al. A Catalog of Diffuse Interstellar Bands in the Spectrum of HD 204827. Astrophys. J. 2008, 620, 1256–1270. [Google Scholar] [CrossRef]
- Campbell, E.K.; Holz, M.; Gerlich, D.; Maier, J.P. Laboratory confirmation of C60+ as the carrier of two diffuse interstellar bands. Nature 2015, 523, 322–324. [Google Scholar] [CrossRef]
- Cordinder, M.A.; Linnartz, H.; Cox, N.L.J.; Cami, J.; Najarro, F.; Proffitt, C.R.; Lallement, R.; Ehrenfreund, P.; Foing, B.H.; Gull, T.R.; et al. Confirming Interstellar Using the Hubble Space Telescope. Astrophys. J. Lett. 2019, 875, L28. [Google Scholar] [CrossRef] [Green Version]
- Lykhin, A.O.; Ahmadvand, S.; Varganov, S.A. Electronic Transitions Responsible for C60+ Diffuse Interstellar Bands. J. Phys. Chem. Lett. 2019, 10, 115–120. [Google Scholar] [CrossRef]
- McCall, B.J.; Drosback, M.M.; Thorburn, J.A.; York, D.G.; Friedman, S.D.; Hobbs, L.M.; Rachford, B.L.; Snow, T.P.; Sonnetrucker, P.; Welty, D.E. Studies on the Diffuse Interstellar Bands. IV. The Nearly Perfect Correlation between λλ 6196.0 and 6613.6. Astrophys. J. 2010, 708, 1628–1638. [Google Scholar] [CrossRef] [Green Version]
- Sarre, P.J. The Diffuse Interstellar Bands: A Dipole-Bound Hypothesis. Mon. Not. R. Astron. Soc. 2000, 313, L14–L16. [Google Scholar] [CrossRef] [Green Version]
- Cordiner, M.A.; Sarre, P.J. The CH2CN− Molecule: Carrier of the λ8037 Diffuse Interstellar Band. Astron. Astrophys. 2007, 472, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Fortenberry, R.C. Theoretical Electronic and Rovibrational Studies for Anions of Interest to the DIBs. In IAU Symposium 297: The Diffuse Interstellar Bands; Cami, J., Cox, N.L.J., Eds.; Campbridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Fortenberry, R.C.; Crawford, T.D. Theoretical Prediction of New Dipole-Bound States for Anions of Interstellar Interest. J. Chem. Phys. 2011, 134, 154304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortenberry, R.C.; Crawford, T.D. Singlet Excited States of Silicon-Containing Anions Relevant to Interstellar Chemistry. J. Phys. Chem. A 2011, 115, 8119–8124. [Google Scholar] [CrossRef]
- Fortenberry, R.C. Singlet Excited States of Anions with Higher Main Group Elements. Mol. Phys. 2013, 111, 3265–3275. [Google Scholar] [CrossRef] [Green Version]
- Salama, F.; Galazutdinov, G.A.; Krełowski, J.; Allamandola, L.J.; Musaev, F.A. Polycyclic Aromatic Hydrocarbons and the Diffuse Interstellar Bands: A Survey. Astrophys. J. 1999, 526, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Theis, M.L.; Candian, A.; Tielens, A.G.G.M.; Lee, T.J.; Fortenberry, R.C. Electronically Excited States of PANH Anions. Phys. Chem. Chem. Phys. 2015, 17, 14761–14772. [Google Scholar] [CrossRef]
- Theis, M.L.; Candian, A.; Tielens, A.G.G.M.; Lee, T.J.; Fortenberry, R.C. Electronically Excited States of Anistropically Extended PAH Anions. J. Phys. Chem. A 2015, 119, 13048–13054. [Google Scholar] [CrossRef]
- Fortenberry, R.C.; Moore, M.M.; Lee, T.J. Excited State Trends in Bidirectionally Expanded Closed-Shell PAH and PANH Anions. J. Phys. Chem. A 2016, 120, 7327–7334. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.Z.; Liu, Y.; Wang, L.S. Observation of Excited Quadrupole-Bound States in Cold Anions. Phys. Rev. Lett. 2017, 119, 023002. [Google Scholar] [CrossRef] [Green Version]
- Santaloci, T.; Fortenberry, R. Electronically Excited States of Closed-Shell, Cyano-Functionalized Polycyclic Aromatic Hydrocarbon Anions. Chemistry 2021, 3, 296–313. [Google Scholar] [CrossRef]
- Santaloci, T.J.; Strauss, M.E.; Fortenberry, R.C. Electronically Excited States of Potential Interstellar, Anionic Building Blocks for Astrobiological Nucleic Acids. Fron. Astron. Space Sci. 2021, 8, 212. [Google Scholar] [CrossRef]
- Santaloci, T.J.; Fortenberry, R.C. On the Possibility of Electronically Excited States in Stable Amine Anions: Dicyanoamine, Cyanoethynylamine, and Diethynylamine. Molec. Astrophys. 2020, 19, 100070. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical tool box. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- The Open Babel Package. Available online: https://openbabel.org/ (accessed on 15 May 2020).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Werner, H.J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M.; Celani, P.; Györffy, W.; Kats, D.; Korona, T.; Lindh, R.; et al. MOLPRO, Version 2019.2, a Package of ab Initio Programs. 2019. Available online: http://www.molpro.net (accessed on 10 June 2020).
- Krylov, A.I. Equation-of-Motion Coupled Cluster Methods for Open-Shell and Electronically Excited Species: The Hitchiker’s Guide to Fock Space. Ann. Rev. Phys. Chem. 2007, 59, 433–463. [Google Scholar] [CrossRef] [Green Version]
- Shavitt, I.; Bartlett, R.J. Many-Body Methods in Chemistry and Physics: MBPT and Coupled-Cluster Theory; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Bartlett, R.J.; Musial, M. Coupled-cluster theory in quantum chemistry. Rev. Mod. Phys. 2007, 79, 291–352. [Google Scholar] [CrossRef] [Green Version]
- Bassett, M.K.; Fortenberry, R.C. Symmetry Breaking and Spectral Considerations of the Surprisingly Floppy c-C3H Radical and the Related Dipole-Bound Excited State of c-C3H−. J. Chem. Phys. 2017, 146, 224303. [Google Scholar] [CrossRef]
- Morgan, W.J.; Fortenberry, R.C. Additional Diffuse Functions in Basis Sets for Dipole-Bound Excited States of Anions. Theor. Chem. Acc. 2015, 134, 47. [Google Scholar] [CrossRef]
- Lykke, K.R.; Neumark, D.M.; Andersen, T.; Trapa, V.J.; Lineberger, W.C. Autodetachment Spectroscopy and Dynamics of CH2CN− and CH2CN. J. Chem. Phys. 1987, 87, 6842–6853. [Google Scholar] [CrossRef]
- Allouche, A.R. Gabedit—A Graphical User Interface for Computational Chemistry Softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef]

| Dicyano | Anion Rel. E. (eV) | Radical Rel. E. (eV) | Dipole (D) | Quad (D-Å) |
|---|---|---|---|---|
| p-CNCN-1 | 0.073 | 0.029 | 0.87 | 26.69 |
| o-CNCN-2 | 0.225 | 0.122 | 7.36 | 3.02 |
| o-CNCN-3 | 0.277 | 0.075 | 6.35 | 4.32 |
| m-CNCN-4 | 0.000 | 0.095 | 5.07 | 18.44 |
| m-CNCN-5 | 0.077 | 0.042 | 4.15 | 18.05 |
| m-CNCN-6 | 0.167 | 0.000 | 3.31 | 16.45 |
| Cyanoethynyl | ||||
| p-CNC2H-1 | 0.973 | 0.022 | 5.00 | 9.24 |
| p-CNC2H-2 | 1.048 | 0.018 | 3.94 | 9.24 |
| p-CNC2H-3 | 0.000 | 0.931 | 2.34 | 32.45 |
| o-CNC2H-4 | 0.210 | 0.697 | 8.68 | 5.14 |
| o-CNC2H-5 | 1.163 | 0.075 | 4.12 | 10.80 |
| o-CNC2H-6 | 1.136 | 0.028 | 3.56 | 12.51 |
| o-CNC2H-7 | 1.135 | 0.049 | 4.14 | 8.64 |
| o-CNC2H-8 | 1.085 | 0.078 | 5.06 | 9.71 |
| m-CNC2H-9 | 0.143 | 0.631 | 5.56 | 21.61 |
| m-CNC2H-10 | 0.982 | 0.091 | 5.00 | 9.88 |
| m-CNC2H-11 | 1.055 | 0.037 | 3.47 | 11.17 |
| m-CNC2H-12 | 1.068 | 0.000 | 4.05 | 9.92 |
| m-CNC2H-13 | 0.976 | 0.060 | 5.01 | 9.81 |
| Diethynyl | ||||
| p-C2HC2H-1 | 1.106 | 0.016 | 0.96 | 7.93 |
| p-C2HC2H-2 | 0.000 | 0.474 | 7.42 | 15.45 |
| o-C2HC2H-3 | 0.187 | 0.576 | 6.63 | 13.93 |
| o-C2HC2H-4 | 1.213 | 0.072 | 1.19 | 8.81 |
| o-C2HC2H-5 | 1.183 | 0.044 | 0.49 | 7.81 |
| m-C2HC2H-6 | 0.112 | 0.565 | 6.61 | 14.37 |
| m-C2HC2H-7 | 1.120 | 0.087 | 1.48 | 8.01 |
| m-C2HC2H-8 | 1.112 | 0.054 | 0.91 | 7.57 |
| m-C2HC2H-9 | 1.127 | 0.000 | 0.42 | 7.71 |
| Excited States | eBE (eV) | ||||
|---|---|---|---|---|---|
| Dicyano | 1 B/1 A | 2 B/2 A | 2 A/2 A | 3 A/3 A | |
| p-CNCN-1 | 2.327 | 3.012 | 3.003 | 3.005 | 3.005 |
| o-CNCN-2 | 2.384 | 2.942 | 2.829 | 2.933 | 2.934 |
| o-CNCN-3 | 2.442 | 2.863 | 2.779 | 2.855 | 2.855 |
| m-CNCN-4 | 3.178 | 3.204 | 3.107 | 3.171 | 3.171 |
| m-CNCN-5 | 2.815 | 3.076 | 3.010 | 3.069 | 3.068 |
| m-CNCN-6 | 2.851 | 2.877 | 2.817 | 2.844 | 2.836 |
| Cyanoethynyl | |||||
| p-CNC2H-1 | 2.393 | 2.596 | 2.585 | 2.589 | 2.587 |
| p-CNC2H-2 | 2.266 | 2.504 | 2.494 | 2.497 | 2.494 |
| p-CNC2H-3 | 3.801 | 3.827 | 3.817 | 3.699 | 3.825 |
| o-CNC2H-4 | 3.599 | 3.732 | 3.741 | 3.747 | 3.735 |
| o-CNC2H-5 | 2.361 | 2.436 | 2.405 | 2.429 | 2.427 |
| o-CNC2H-6 | 2.450 | 2.480 | 2.430 | 2.443 | 2.440 |
| o-CNC2H-7 | 2.450 | 2.479 | 2.426 | 2.443 | 2.441 |
| o-CNC2H-8 | 2.468 | 2.528 | 2.494 | 2.521 | 2.519 |
| m-CNC2H-9 | 3.666 | 3.759 | 3.732 | 3.766 | 3.759 |
| m-CNC2H-10 | 2.669 | 2.675 | 2.654 | 2.669 | 2.667 |
| m-CNC2H-11 | 2.576 | 2.605 | 2.561 | 2.569 | 2.563 |
| m-CNC2H-12 | 2.326 | 2.433 | 2.420 | 2.426 | 2.424 |
| m-CNC2H-13 | 2.669 | 2.697 | 2.649 | 2.662 | 2.661 |
| Diethynyl | |||||
| p-C2HC2H-1 | 2.105 | 2.137 | 2.097 | 2.102 | 2.097 |
| p-C2HC2H-2 | 3.443 | 3.492 | 3.438 | 3.451 | 3.400 |
| o-C2HC2H-3 | 3.271 | 3.320 | 3.327 | 3.353 | 3.319 |
| o-C2HC2H-4 | 2.046 | 2.078 | 2.038 | 2.041 | 2.038 |
| o-C2HC2H-5 | 2.061 | 2.094 | 2.054 | 2.057 | 2.053 |
| m-C2HC2H-6 | 3.372 | 3.405 | 3.412 | 3.437 | 3.404 |
| m-C2HC2H-7 | 2.188 | 2.220 | 2.180 | 2.183 | 2.180 |
| m-C2HC2H-8 | 2.179 | 2.212 | 2.172 | 2.176 | 2.172 |
| m-C2HC2H-9 | 2.034 | 2.067 | 2.027 | 2.031 | 2.027 |
| 1-Dicyano | Anion Rel. E. (eV) | Radical Rel. E. (eV) | Dipole (D) | Quad(D-Å) |
|---|---|---|---|---|
| 0 | 0.296 | 0.198 | 8.22 | 7.40 |
| 1 | 0.267 | 0.135 | 7.24 | 8.04 |
| 2 | 0.591 | 0.154 | 8.62 | 7.68 |
| 3 | 0.660 | 0.123 | 7.73 | 7.96 |
| 4 | 0.602 | 0.119 | 6.96 | 11.79 |
| 5 | 0.486 | 0.131 | 7.20 | 10.63 |
| 6 | 0.046 | 0.146 | 6.40 | 10.83 |
| 7 | 0.000 | 0.085 | 5.62 | 9.39 |
| 8 | 0.461 | 0.061 | 6.21 | 9.80 |
| 9 | 0.493 | 0.024 | 5.12 | 12.19 |
| 10 | 0.492 | 0.031 | 4.66 | 8.63 |
| 11 | 0.329 | 0.040 | 5.37 | 9.37 |
| 13 | 0.107 | 0.095 | 2.17 | 18.61 |
| 14 | 0.482 | 0.053 | 1.77 | 21.83 |
| 15 | 0.588 | 0.023 | 0.73 | 19.82 |
| 18 | 0.607 | 0.319 | 8.95 | 8.32 |
| 19 | 0.630 | 0.303 | 8.10 | 12.83 |
| 23 | 0.509 | 0.280 | 7.69 | 15.57 |
| 24 | 0.202 | 0.057 | 7.65 | 6.05 |
| 25 | 0.281 | 0.027 | 6.69 | 5.95 |
| 26 | 0.192 | 0.016 | 6.79 | 5.08 |
| 27 | 0.219 | 0.096 | 8.21 | 4.90 |
| 28 | 0.310 | 0.075 | 7.85 | 5.44 |
| 29 | 0.246 | 0.026 | 6.88 | 5.08 |
| 30 | 0.155 | 0.038 | 4.12 | 26.22 |
| 31 | 0.275 | 0.015 | 3.37 | 23.38 |
| 32 | 0.148 | 0.000 | 4.02 | 24.18 |
| 33 | 0.335 | 0.032 | 4.88 | 24.19 |
| 34 | 0.312 | 0.059 | 5.28 | 23.71 |
| 35 | 0.083 | 0.054 | 4.33 | 25.94 |
| 36 | 0.233 | 0.040 | 0.80 | 24.18 |
| 38 | 0.301 | 0.023 | 0.86 | 29.02 |
| 40 | 0.364 | 0.013 | 0.98 | 26.58 |
| 2-Dicyano | ||||
| 7 | 0.107 | 0.160 | 8.84 | 7.34 |
| 8 | 0.370 | 0.116 | 8.61 | 7.90 |
| 9 | 0.468 | 0.103 | 7.68 | 11.31 |
| 25 | 0.156 | 0.044 | 4.15 | 29.30 |
| 26 | 0.155 | 0.000 | 3.41 | 27.18 |
| 27 | 0.000 | 0.048 | 5.08 | 27.82 |
| 32 | 0.137 | 0.000 | 0.93 | 25.31 |
| 34 | 0.176 | 0.046 | 0.94 | 26.68 |
| 35 | 0.016 | 0.043 | 0.76 | 27.97 |
| Excited States | eBE | ||||
|---|---|---|---|---|---|
| 1-Dicyano | 1 A | 2 A | 2 A | 3 A | |
| 0 | 3.011 | 3.013 | 2.967 | 3.010 | 3.011 |
| 1 | 3.014 | 3.016 | 2.996 | 3.013 | 3.013 |
| 2 | 2.677 | 2.680 | 2.648 | 2.676 | 2.677 |
| 3 | 2.329 | 2.595 | 2.511 | 2.592 | 2.594 |
| 4 | 2.669 | 2.671 | 2.638 | 2.667 | 2.668 |
| 5 | 2.755 | 2.757 | 2.727 | 2.754 | 2.754 |
| 6 | 3.258 | 3.260 | 3.233 | 3.257 | 3.257 |
| 7 | 3.284 | 3.287 | 3.272 | 3.284 | 3.284 |
| 8 | 2.694 | 2.696 | 2.664 | 2.693 | 2.693 |
| 9 | 2.707 | 2.709 | 2.642 | 2.705 | 2.705 |
| 10 | 2.666 | 2.668 | 2.619 | 2.665 | 2.665 |
| 11 | 2.857 | 2.859 | 2.800 | 2.856 | 2.856 |
| 13 | 3.130 | 3.132 | 3.129 | 3.129 | 3.130 |
| 14 | 2.686 | 2.688 | 2.673 | 2.686 | 2.680 |
| 15 | 2.578 | 2.580 | 2.574 | 2.578 | 2.580 |
| 18 | 2.867 | 2.869 | 2.824 | 2.866 | 2.866 |
| 19 | 2.765 | 2.767 | 2.670 | 2.762 | 2.764 |
| 23 | 2.866 | 2.957 | 2.877 | 2.954 | 2.957 |
| 24 | 2.585 | 3.012 | 2.923 | 3.010 | 3.011 |
| 25 | 2.322 | 2.874 | 2.809 | 2.872 | 2.873 |
| 26 | 2.987 | 2.989 | 2.920 | 2.985 | 2.986 |
| 27 | 3.011 | 3.013 | 2.982 | 3.010 | 3.010 |
| 28 | 2.894 | 2.896 | 2.866 | 2.893 | 2.893 |
| 29 | 2.874 | 2.876 | 2.853 | 2.873 | 2.873 |
| 30 | 3.065 | 3.067 | 3.010 | 3.064 | 3.064 |
| 31 | 2.846 | 2.848 | 2.809 | 2.845 | 2.845 |
| 32 | 3.045 | 3.048 | 2.976 | 3.045 | 3.044 |
| 33 | 2.772 | 2.774 | 2.743 | 2.771 | 2.770 |
| 34 | 2.888 | 2.890 | 2.875 | 2.887 | 2.887 |
| 35 | 3.126 | 3.128 | 3.101 | 3.101 | 3.124 |
| 36 | 2.966 | 2.968 | 2.965 | 2.966 | 2.968 |
| 38 | 2.871 | 2.873 | 2.870 | 2.871 | 2.873 |
| 40 | 2.758 | 2.760 | 2.758 | 2.759 | 2.760 |
| 2-Dicyano | |||||
| 7 | 3.138 | 3.140 | 3.109 | 3.109 | 3.137 |
| 8 | 2.837 | 2.839 | 2.780 | 2.835 | 2.837 |
| 9 | 2.742 | 2.744 | 2.643 | 2.737 | 2.741 |
| 25 | 3.011 | 3.013 | 2.984 | 3.010 | 3.010 |
| 26 | 2.896 | 2.898 | 2.881 | 2.895 | 2.895 |
| 27 | 3.176 | 3.178 | 3.152 | 3.152 | 3.175 |
| 32 | 2.937 | 2.939 | 2.935 | 2.937 | 2.938 |
| 34 | 2.968 | 2.970 | 2.951 | 2.968 | 2.968 |
| 35 | 3.134 | 3.136 | 3.132 | 3.132 | 3.135 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strauss, M.E.; Santaloci, T.J.; Fortenberry, R.C. Valence-, Dipole- and Quadropole-Bound Electronically Excited States of Closed-Shell Anions Formed by Deprotonation of Cyano- and Ethynyl-Disubstituted Polycyclic Aromatic Hydrocarbons. Chemistry 2022, 4, 42-56. https://doi.org/10.3390/chemistry4010004
Strauss ME, Santaloci TJ, Fortenberry RC. Valence-, Dipole- and Quadropole-Bound Electronically Excited States of Closed-Shell Anions Formed by Deprotonation of Cyano- and Ethynyl-Disubstituted Polycyclic Aromatic Hydrocarbons. Chemistry. 2022; 4(1):42-56. https://doi.org/10.3390/chemistry4010004
Chicago/Turabian StyleStrauss, Marie E., Taylor J. Santaloci, and Ryan C. Fortenberry. 2022. "Valence-, Dipole- and Quadropole-Bound Electronically Excited States of Closed-Shell Anions Formed by Deprotonation of Cyano- and Ethynyl-Disubstituted Polycyclic Aromatic Hydrocarbons" Chemistry 4, no. 1: 42-56. https://doi.org/10.3390/chemistry4010004
APA StyleStrauss, M. E., Santaloci, T. J., & Fortenberry, R. C. (2022). Valence-, Dipole- and Quadropole-Bound Electronically Excited States of Closed-Shell Anions Formed by Deprotonation of Cyano- and Ethynyl-Disubstituted Polycyclic Aromatic Hydrocarbons. Chemistry, 4(1), 42-56. https://doi.org/10.3390/chemistry4010004