Investigation into the Re-Arrangement of Copper Foams Pre- and Post-CO2 Electrocatalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Copper Foam Preparation
2.3. Material Characterization
2.4. CO2 Electrocatalysis
2.5. Electrocatalysis Product Analysis
3. Results
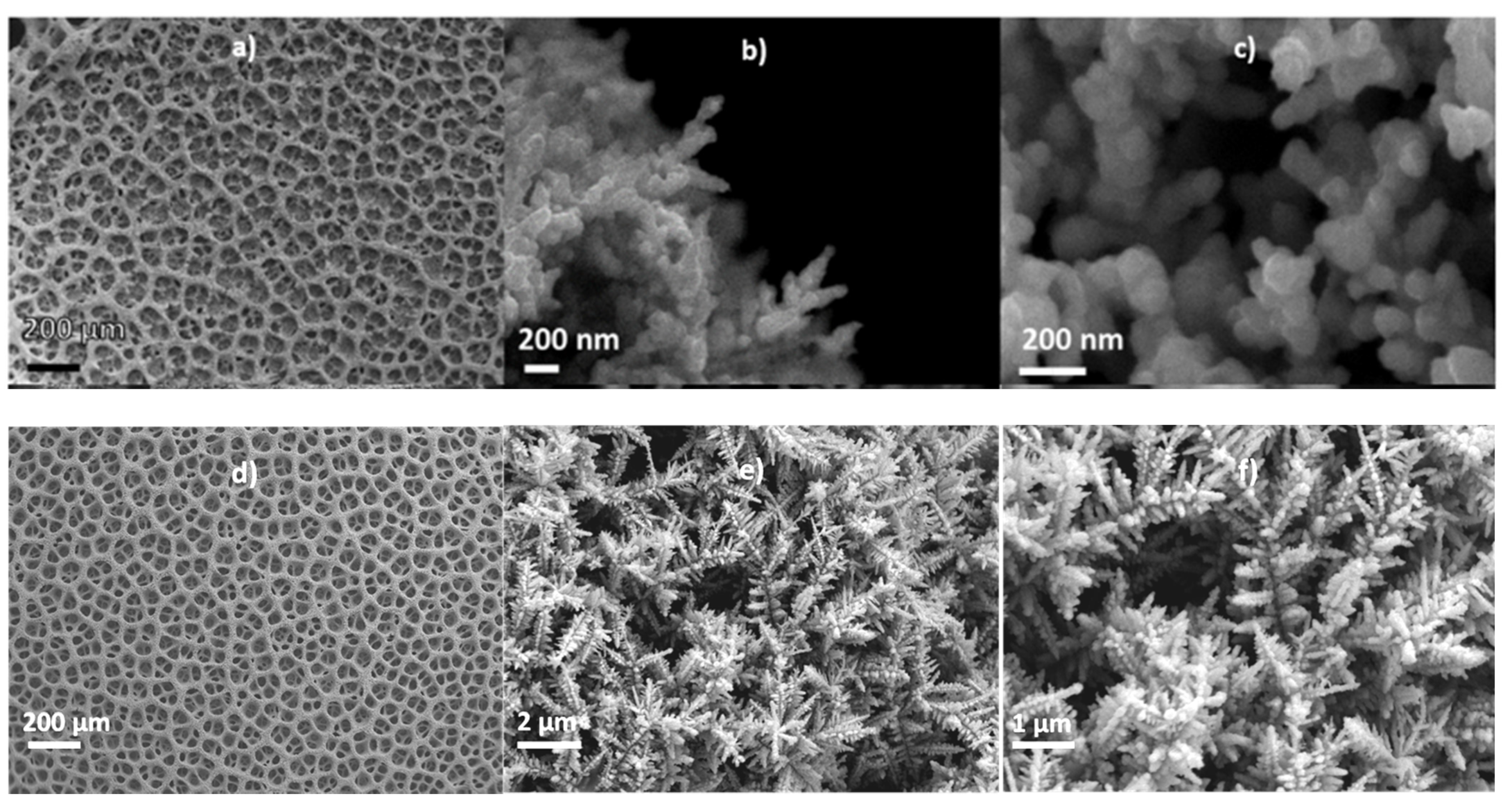
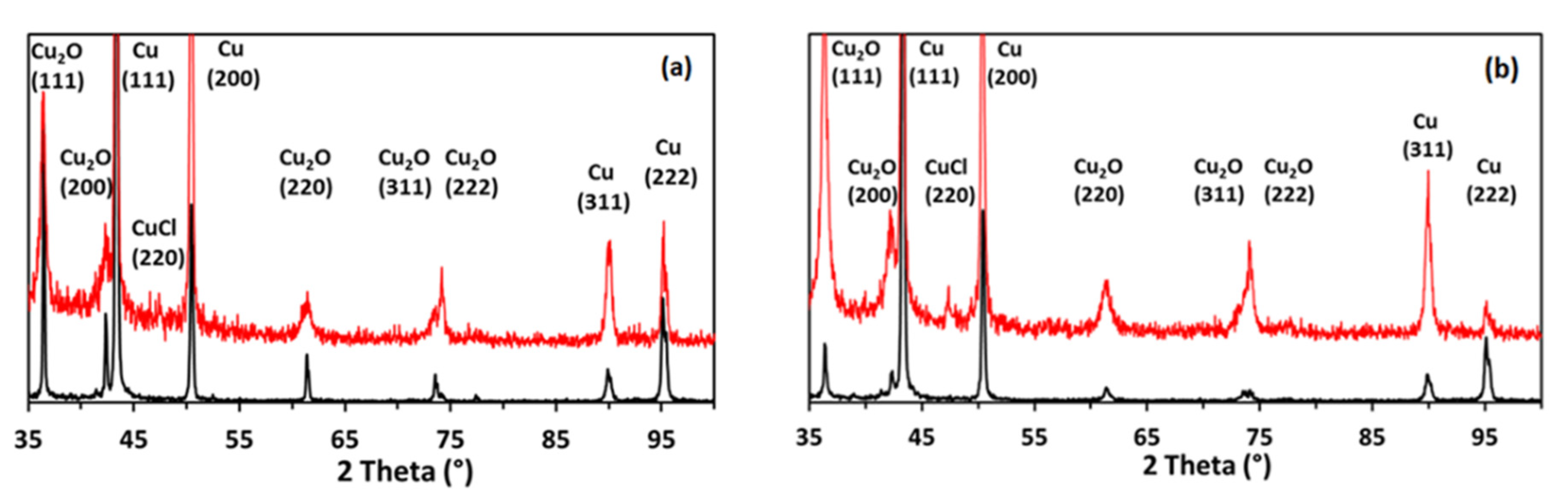
3.1. Effect of HCl on the Copper Foams
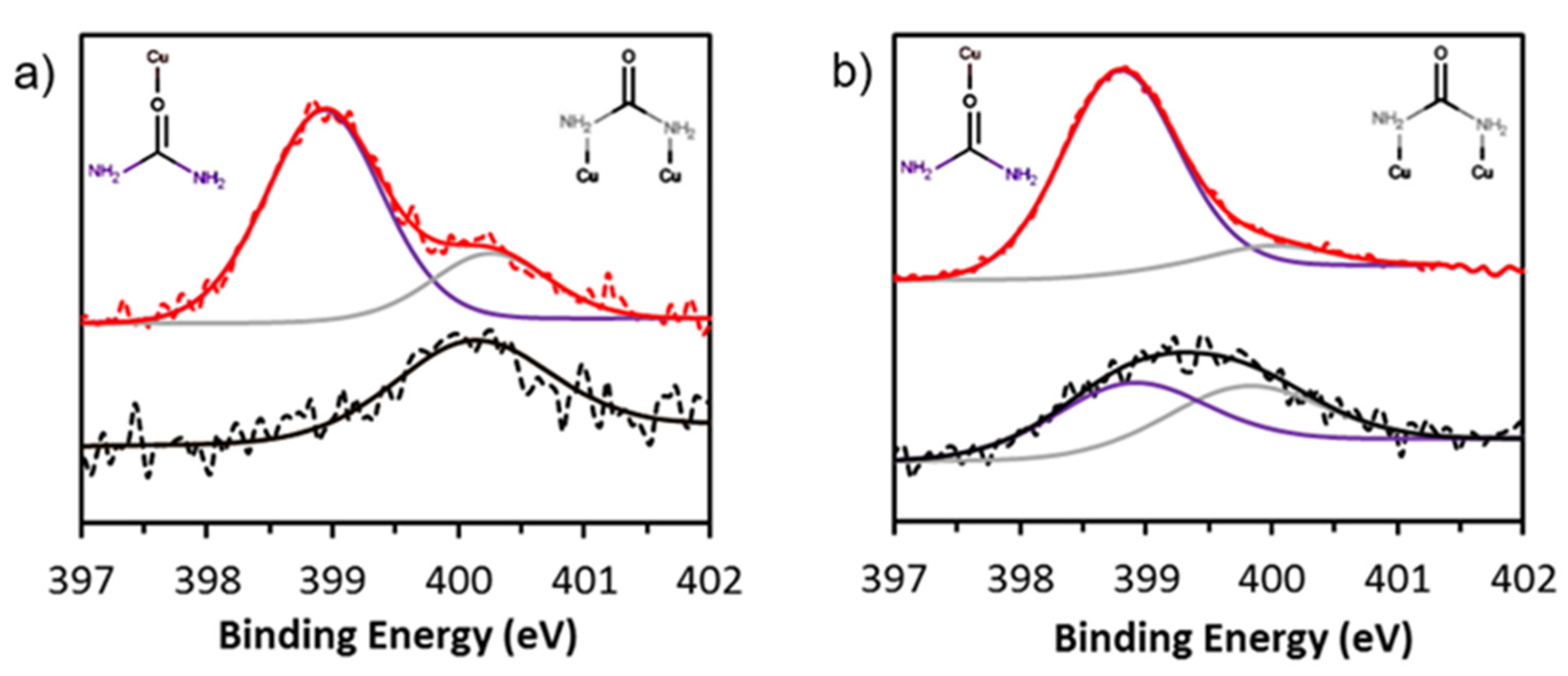
3.2. Effect of Urea on the Copper Foams
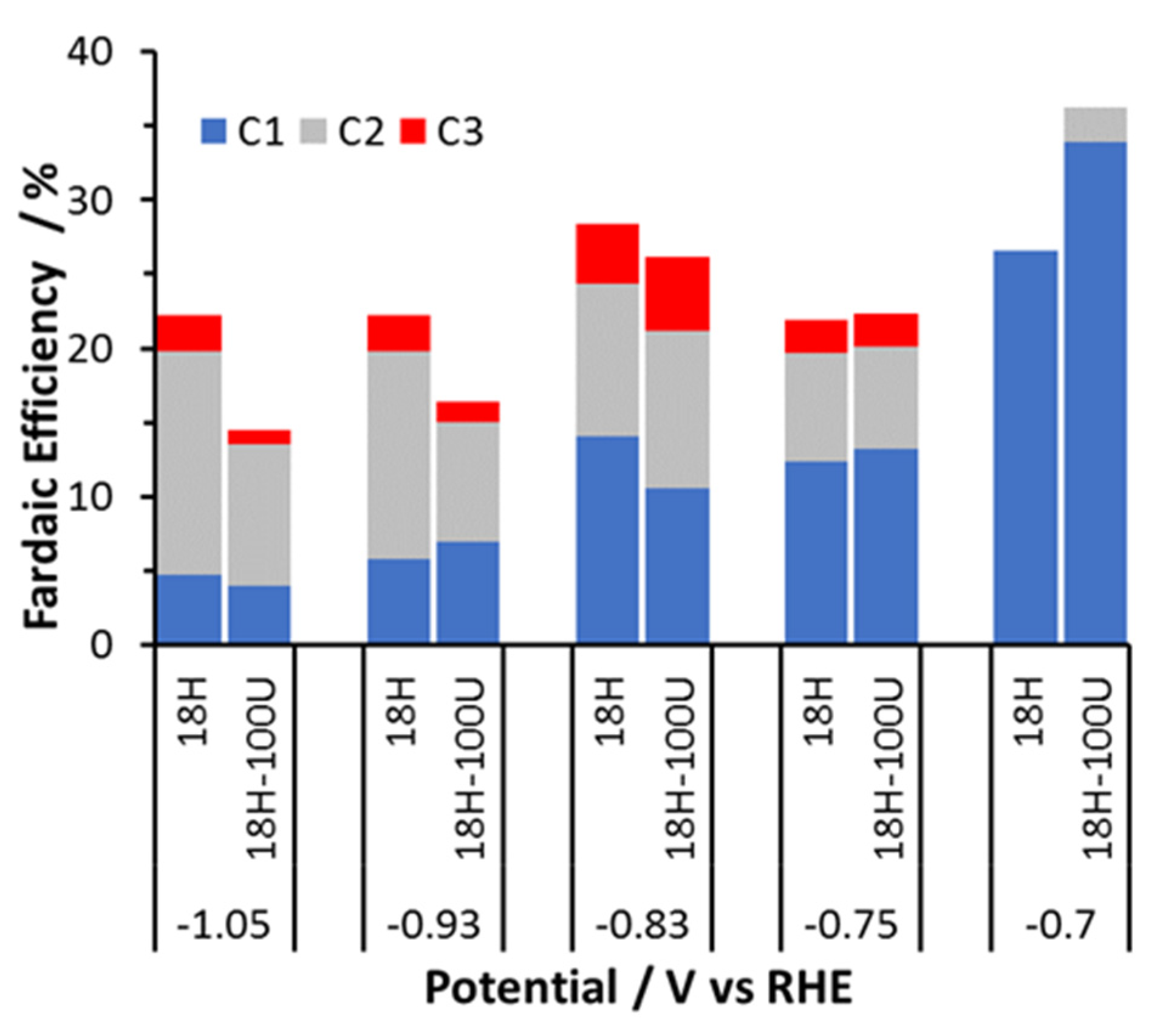
3.3. CO2 Electrocatalytic Activity of Foams
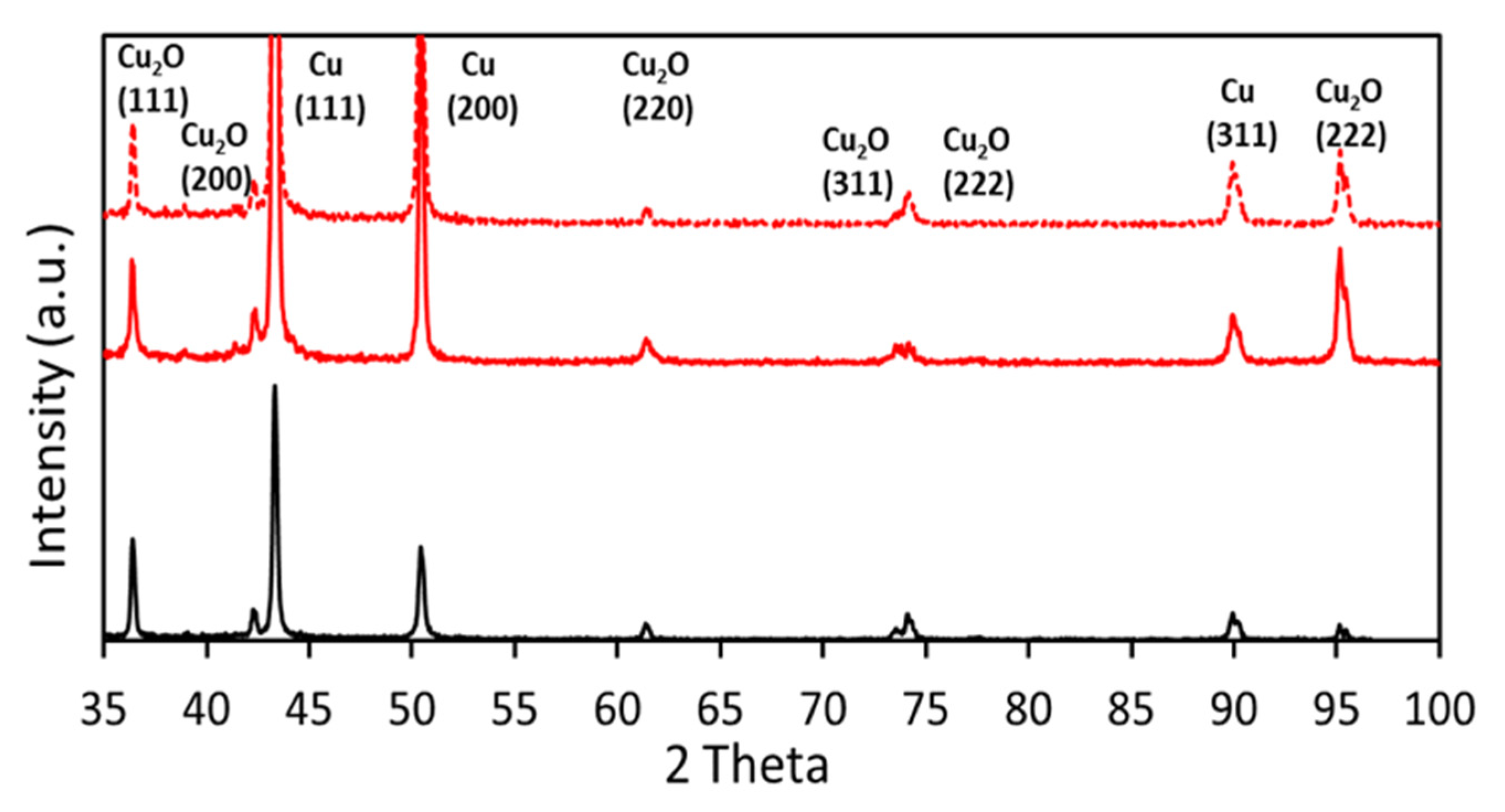
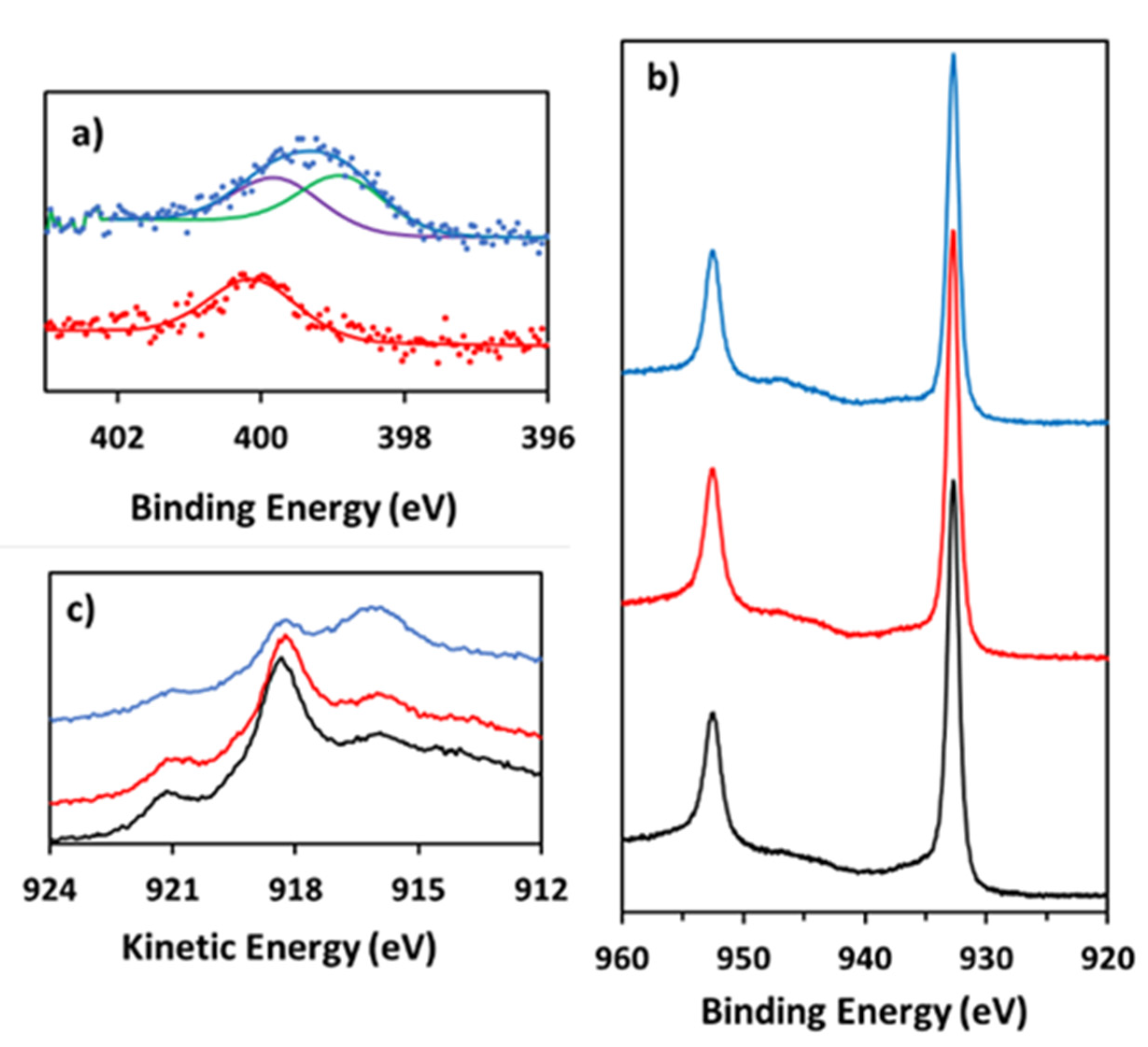
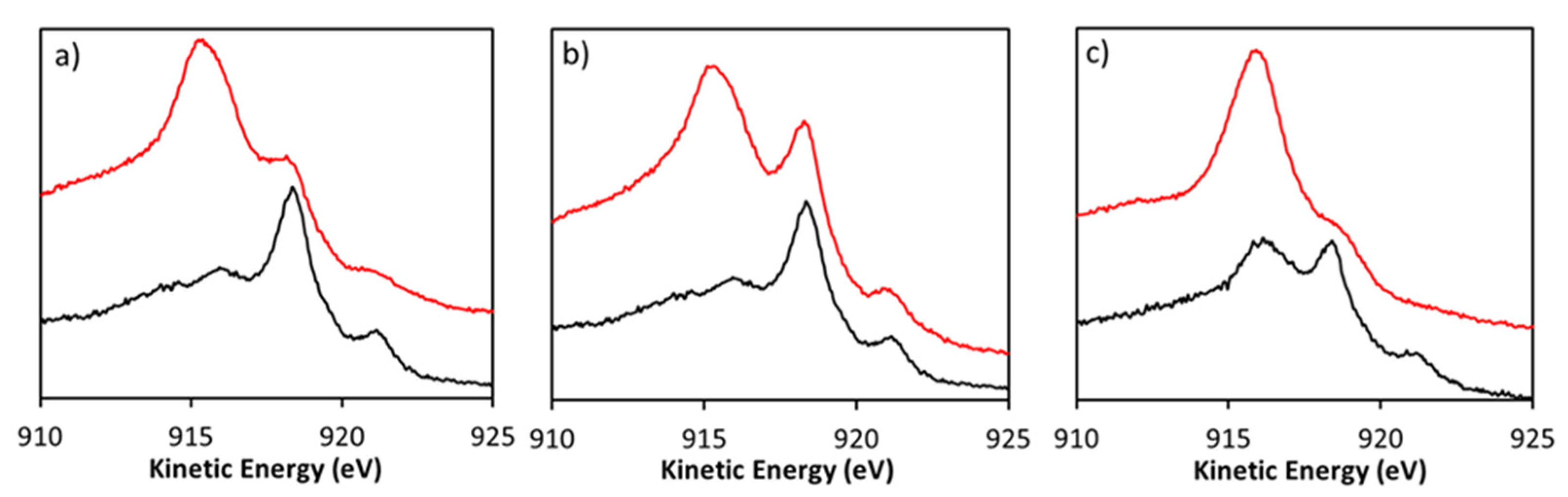
3.4. Post-Catalysis Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Graf, F. STOREandGO, Innovative large-scale energy STOragE technologies AND Power-to-Gas concepts after Optimisation, H2020. Impact 2018, 2018, 81–83. [Google Scholar] [CrossRef]
- Reisch, M. Solvay adds DuPont battery materials. Chem. Eng. News 2017, 95, 14–15. [Google Scholar] [CrossRef]
- Keith, D.W.; Holmes, G.; Angelo, D.S.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef] [Green Version]
- Holmes, G.; Nold, K.; Walsh, T.; Heidel, K.; Henderson, M.A.; Ritchie, J.; Klavins, P.; Singh, A.; Keith, D.W. Outdoor Prototype Results for Direct Atmospheric Capture of Carbon Dioxide. Energy Procedia 2013, 37, 6079–6095. [Google Scholar] [CrossRef] [Green Version]
- Zapantis, A.; Townsend, A.; Rassool, D. Policy Priorities to Incentivise Large Scale Deployment of CCS; Thought Leadership Report; Global CCS Institute: Docklands, Australia, 2019. [Google Scholar]
- Irlam, L. Global Costs of Carbon Capture and Storage; Global CCS Institute: Docklands, Australia, 2017. [Google Scholar]
- Voiry, D.; Shin, H.S.; Loh, K.P.; Chhowalla, M. Low-dimensional catalysts for hydrogen evolution and CO2 reduction. Nat. Rev. Chem. 2018, 2, 0105. [Google Scholar] [CrossRef]
- Rao, H.; Schmidt, L.C.; Bonin, J.; Robert, M. Visible-light-driven methane formation from CO2 with a molecular iron catalyst. Nat. Cell Biol. 2017, 548, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Jiao, F. Electrochemical CO2 reduction: Electrocatalyst, reaction mechanism, and process engineering. Nano Energy 2016, 29, 439–456. [Google Scholar] [CrossRef] [Green Version]
- Schlögl, R.; Abanades, C.; Aresta, M.; Azapagic, A.; Blekkan, E.A.; Cantat, T.; Centi, G.; Duic, N.; El Khamlichi, A.; Hutchings, G.; et al. Novel Carbon Capture and Utilisation Technologies: Research and Climate Aspects; SAPEA: Berlin, Germany, 2018. [Google Scholar]
- Nørskov, J.K.; Weckhuysen, B.; Centi, G.; ChorkendorffI, S.R.; Marin, G.; Grimaud, A.; Rossmeisl, J.; Strasser, P.; Koper, M.; Roldan, B.; et al. Research needs towards sustainable production of fuels and chemicals. Energy X 2019. Available online: https://www.energy-x.eu/wp-content/uploads/2019/10/Energy-X-Research-needs-report.pdf (accessed on 28 June 2021).
- Yoshio, H.; Katsuhei, K.; Shin, S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. J. Chem. Lett. 1985, 14, 1695–1698. [Google Scholar]
- Hori, Y. Modern Aspects of Electrochemistry; Springer: New York, NY, USA, 2008; Volume 2. [Google Scholar]
- Dutta, A.; Morstein, C.E.; Rahaman, M.; López, A.C.; Broekmann, P. Beyond Copper in CO2 Electrolysis: Effective Hydrocarbon Production on Silver-Nanofoam Catalysts. ACS Catal. 2018, 8, 8357–8368. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic Conversion of Carbon Dioxide to Methane and Methanol on Transition Metal Surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; pp. 89–189. [Google Scholar]
- Hori, Y.; Murata, A.; Takahashi, R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 2309–2326. [Google Scholar] [CrossRef]
- DeWulf, D.W.; Jin, T.; Bard, A.J. Electrochemical and Surface Studies of Carbon Dioxide Reduction to Methane and Ethylene at Copper Electrodes in Aqueous Solutions. J. Electrochem. Soc. 1989, 136, 1686–1691. [Google Scholar] [CrossRef]
- Montoya, J.H.; Peterson, A.A.; Nørskov, J.K. Insights into C-C Coupling in CO2 Electroreduction on Copper Electrodes. ChemCatChem 2013, 5, 737–742. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [Green Version]
- Lv, W.; Zhou, J.; Bei, J.; Zhang, R.; Wang, L.; Xu, Q.; Wang, W. Electrodeposition of nano-sized bismuth on copper foil as electrocatalyst for reduction of CO2 to formate. Appl. Surf. Sci. 2017, 393, 191–196. [Google Scholar] [CrossRef]
- Lv, W.; Zhou, J.; Kong, F.; Fang, H.; Wang, W. Porous tin-based film deposited on copper foil for electrochemical reduction of carbon dioxide to formate. Int. J. Hydrogen Energy 2016, 41, 1585–1591. [Google Scholar] [CrossRef]
- Lum, Y.; Yue, B.; Lobaccaro, P.; Bell, A.T.; Ager, J.W. Optimizing C-C Coupling on Oxide-Derived Copper Catalysts for Electrochemical CO2 Reduction. J. Phys. Chem. C 2017, 121, 14191–14203. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.; Xiao, H.; Goddard, W.A. Nature of the Active Sites for CO Reduction on Copper Nanoparticles; Suggestions for Optimizing Performance. J. Am. Chem. Soc. 2017, 139, 11642–11645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kas, R.; Kortlever, R.; Yılmaz, H.; Koper, M.T.M.; Mul, G. Manipulating the Hydrocarbon Selectivity of Copper Nanoparticles in CO2 Electroreduction by Process Conditions. ChemElectroChem 2015, 2, 354–358. [Google Scholar] [CrossRef]
- Dongare, S.; Singh, N.; Bhunia, H. Electrocatalytic reduction of CO2 to useful chemicals on copper nanoparticles. Appl. Surf. Sci. 2021, 537, 148020. [Google Scholar] [CrossRef]
- Huang, J.; Yang, T.; Zhao, K.; Chen, S.; Huang, Q.; Han, Y. Copper-comprising nanocrystals as well-defined electrocatalysts to advance electrochemical CO2 reduction. J. Energy Chem. 2021, 62, 71–102. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Jia, A.-P.; Lu, J.-Q.; Huang, W. Morphology-dependent CO Reduction Kinetic and Surface Copper Species Evolution of Cu2O Nanocrystals. J. Phys. Chem. C 2020, 124, 21568–21576. [Google Scholar] [CrossRef]
- Gorginpour, F.; Zali-Boeini, H. Synergistic effect of copper nanocrystals-nanoparticles incorporated in a porous organic polymer for the Ullmann C-O coupling reaction. Mol. Catal. 2021, 504, 111460. [Google Scholar] [CrossRef]
- Kung, C.-W.; Audu, C.O.; Peters, A.W.; Noh, H.; Farha, O.K.; Hupp, J.T. Copper Nanoparticles Installed in Metal-Organic Framework Thin Films are Electrocatalytically Competent for CO2 Reduction. ACS Energy Lett. 2017, 2, 2394–2401. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, H.J.; Lim, H.; Kwon, Y.; Jeong, H.M. Metal-organic framework-mediated strategy for enhanced methane production on copper nanoparticles in electrochemical CO2 reduction. Electrochim. Acta 2019, 306, 28–34. [Google Scholar] [CrossRef]
- Rayer, A.V.; Reid, E.; Kataria, A.; Luz, I.; Thompson, S.J.; Lail, M.; Zhou, J.; Soukri, M. Electrochemical carbon dioxide reduction to isopropanol using novel carbonized copper metal organic framework derived electrodes. J. CO2 Util. 2020, 39, 101159. [Google Scholar] [CrossRef]
- Dutta, A.; Rahaman, M.; Hecker, B.; Drnec, J.; Kiran, K.; Montiel, I.Z.; Weber, D.J.; Zanetti, A.; López, A.C.; Martens, I.; et al. CO2 electrolysis—Complementary operando XRD, XAS and Raman spectroscopy study on the stability of CuxO foam catalysts. J. Catal. 2020, 389, 592–603. [Google Scholar] [CrossRef]
- Klingan, K.; Kottakkat, T.; Jovanov, Z.P.; Jiang, S.; Pasquini, C.; Scholten, F.; Kubella, P.; Bergmann, A.; Cuenya, B.R.; Roth, C.; et al. Reactivity Determinants in Electrodeposited Cu Foams for Electrochemical CO2 Reduction. ChemSusChem 2018, 11, 3449–3459. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Yang, X.; Cao, Z.; Dong, X.; Kozlov, S.M.; Falivene, L.; Huang, J.-K.; Zhou, X.; Hedhili, M.N.; Lai, Z.; et al. Efficient electrochemical transformation of CO2 to C2/C3 chemicals on benzimidazole-functionalized copper surfaces. Chem. Commun. 2018, 54, 11324–11327. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Jouny, M.; Luc, W.W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Hernandez-Aldave, S.; Andreoli, E. Fundamentals of Gas Diffusion Electrodes and Electrolysers for Carbon Dioxide Utilisation: Challenges and Opportunities. Catalysts 2020, 10, 713. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Branković, G.; Pavlović, M.G.; Popov, K.I. The effect of hydrogen co-deposition on the morphology of copper electrodeposits. II. Correlation between the properties of electrolytic solutions and the quantity of evolved hydrogen. J. Electroanal. Chem. 2008, 621, 13–21. [Google Scholar] [CrossRef]
- Shin, H.-C.; Dong, J.; Liu, M. Nanoporous Structures Prepared by an Electrochemical Deposition Process. Adv. Mater. 2003, 15, 1610–1614. [Google Scholar] [CrossRef]
- Shin, H.-C.; Liu, M. Copper Foam Structures with Highly Porous Nanostructured Walls. Chem. Mater. 2004, 16, 5460–5464. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, R.-H.; Kwon, H.-S. Preparation of copper foam with 3-dimensionally interconnected spherical pore network by electrodeposition. Electrochem. Commun. 2008, 10, 1148–1151. [Google Scholar] [CrossRef]
- Garza, A.J.; Bell, A.T.; Head-Gordon, M. Mechanism of CO2 Reduction at Copper Surfaces: Pathways to C2 Products. ACS Catal. 2018, 8, 1490–1499. [Google Scholar] [CrossRef] [Green Version]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Han, Z.; Kortlever, R.; Chen, H.Y.; Peters, J.C.; Agapie, T. CO2 Reduction Selective for C ≥ 2 Products on Polycrystalline Copper with N-Substituted Pyridinium Additives. ACS Central Sci. 2017, 3, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Kley, C.S.; Li, Y.; Yang, P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products. Proc. Natl. Acad. Sci. USA 2017, 114, 10560–10565. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Wong, N.T.; Handoko, A.D.; Huang, Y.; Yeo, B.S. Mechanistic Insights into the Enhanced Activity and Stability of Agglomerated Cu Nanocrystals for the Electrochemical Reduction of Carbon Dioxide to n-Propanol. J. Phys. Chem. Lett. 2016, 7, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Grosse, P.; Gao, D.; Scholten, F.; Sinev, I.; Mistry, H.; Cuenya, B.R. Dynamic Changes in the Structure, Chemical State and Catalytic Selectivity of Cu Nanocubes during CO2 Electroreduction: Size and Support Effects. Angew. Chem. Int. Ed. 2018, 57, 6192–6197. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.; Klyukin, K.; Wakeham, R.J.; Rudd, J.A.; Lewis, A.R.; Alexander, S.; Carla, F.; Alexandrov, V.; Andreoli, E. Poly-Amide Modified Copper Foam Electrodes for Enhanced Electrochemical Reduction of Carbon Dioxide. ACS Catal. 2018, 8, 4132–4142. [Google Scholar] [CrossRef]
- Dutta, A.; Rahaman, M.; Mohos, M.; Zanetti, A.; Broekmann, P. Electrochemical CO2 Conversion Using Skeleton (Sponge) Type of Cu Catalysts. ACS Catal. 2017, 7, 5431–5437. [Google Scholar] [CrossRef]
- Xie, M.S.; Xia, B.Y.; Li, Y.; Yan, Y.; Yang, Y.; Sun, Q.; Chan, S.H.; Fisher, A.; Wang, X. Amino acid modified copper electrodes for the enhanced selective electroreduction of carbon dioxide towards hydrocarbons. Energy Environ. Sci. 2016, 9, 1687–1695. [Google Scholar] [CrossRef]
- Hao, G.L.; Xu, Q.P.; Han, F.S.; Li, W.D.; Bai, S.M. Processing and damping behaviour of porous copper. Powder Met. 2009, 52, 145–150. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Abbott, A.; Yang, C. Electrochemical fabrication of nanoporous copper films in choline chloride-urea deep eutectic solvent. Phys. Chem. Chem. Phys. 2015, 17, 14702–14709. [Google Scholar] [CrossRef] [PubMed]
- Wuttig, A.; Surendranath, Y. Impurity Ion Complexation Enhances Carbon Dioxide Reduction Catalysis. ACS Catal. 2015, 5, 4479–4484. [Google Scholar] [CrossRef] [Green Version]
- Coxeter, H.S.M. Regular Polytopes, 3rd ed.; Dover Publications: Methuen, MA, USA, 1973. [Google Scholar]
- Dutta, A.; Rahaman, M.; Luedi, N.C.; Mohos, M.; Broekmann, P. Morphology Matters: Tuning the Product Distribution of CO2 Electroreduction on Oxide-Derived Cu Foam Catalysts. ACS Catal. 2016, 6, 3804–3814. [Google Scholar] [CrossRef]
- Wen, N.; Brooker, M.H. Urea protonation: Raman and theoretical study. J. Phys. Chem. 1993, 97, 8608–8616. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Li, C.W.; Kanan, M.W. CO2 Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu2O Films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Cave, E.R.; Nitopi, S.; Feaster, J.; Wang, L.; Kuhl, K.P.; Jackson, A.; Johnson, N.C.; Abram, D.N.; Hatsukade, T.; et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat. Catal. 2018, 1, 764–771. [Google Scholar] [CrossRef]
- Rahaman, M.; Dutta, A.; Zanetti, A.; Broekmann, P. Electrochemical Reduction of CO2 into Multicarbon Alcohols on Activated Cu Mesh Catalysts: An Identical Location (IL) Study. ACS Catal. 2017, 7, 7946–7956. [Google Scholar] [CrossRef]
- Rahaman, M.; Kiran, K.; Montiel, I.Z.; Grozovski, V.; Dutta, A.; Broekmann, P. Selective n-propanol formation from CO2 over degradation-resistant activated PdCu alloy foam electrocatalysts. Green Chem. 2020, 22, 6497–6509. [Google Scholar] [CrossRef]
- Wang, H.; Matios, E.; Wang, C.; Luo, J.; Lu, X.; Hu, X.; Li, W. Rapid and Scalable Synthesis of Cuprous Halide-Derived Copper Nano-Architectures for Selective Electrochemical Reduction of Carbon Dioxide. Nano Lett. 2019, 19, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Guo, L. Nanocrystal Facets: Facet-Controlled Synthetic Strategy of Cu2O-Based Crystals for Catalysis and Sensing. Adv. Sci. 2015, 2. [Google Scholar] [CrossRef]
- Seah, M.P. AES: Energy calibration of electron spectrometers. IV. A re-evaluation of the reference energies. J. Electron. Spectrosc. Relat. Phenom. 1998, 97, 235–241. [Google Scholar] [CrossRef]
- Gaarenstroom, S.W.; Winograd, N. Initial and final state effects in the ESCA spectra of cadmium and silver oxides. J. Chem. Phys. 1977, 67, 3500–3506. [Google Scholar] [CrossRef]
- Battistoni, C.; Mattogno, G.; Paparazzo, E.; Naldini, L. An XPS and Auger study of some polynuclear copper compounds. Inorg. Chim. Acta 1985, 102, 1–3. [Google Scholar] [CrossRef]
- Lee, C.; Stahlberg, E.A.; Fitzgerald, G. Chemical Structure of Urea in Water. J. Phys. Chem. 1995, 99, 17737–17741. [Google Scholar] [CrossRef]
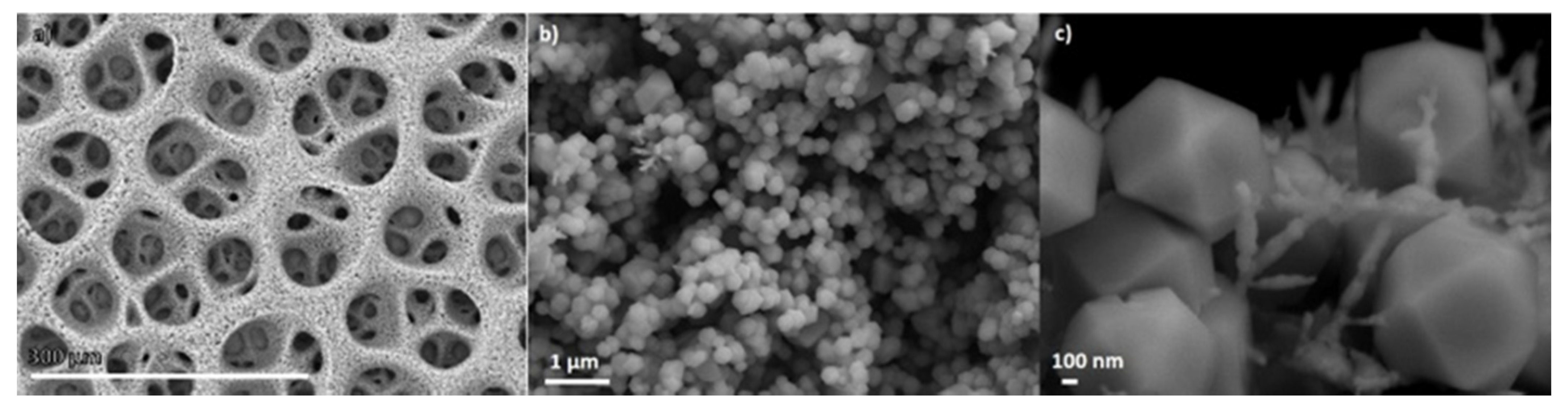
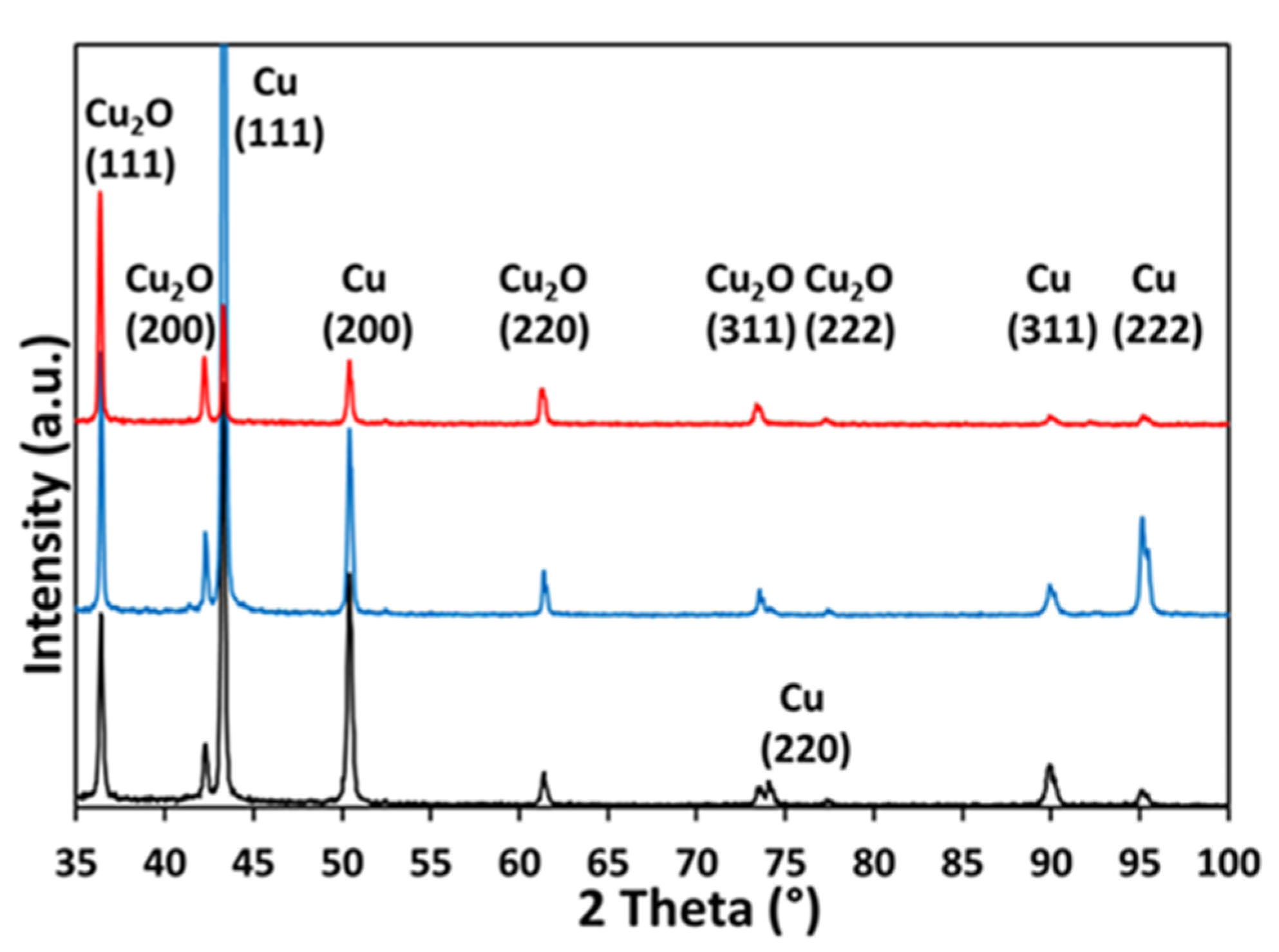
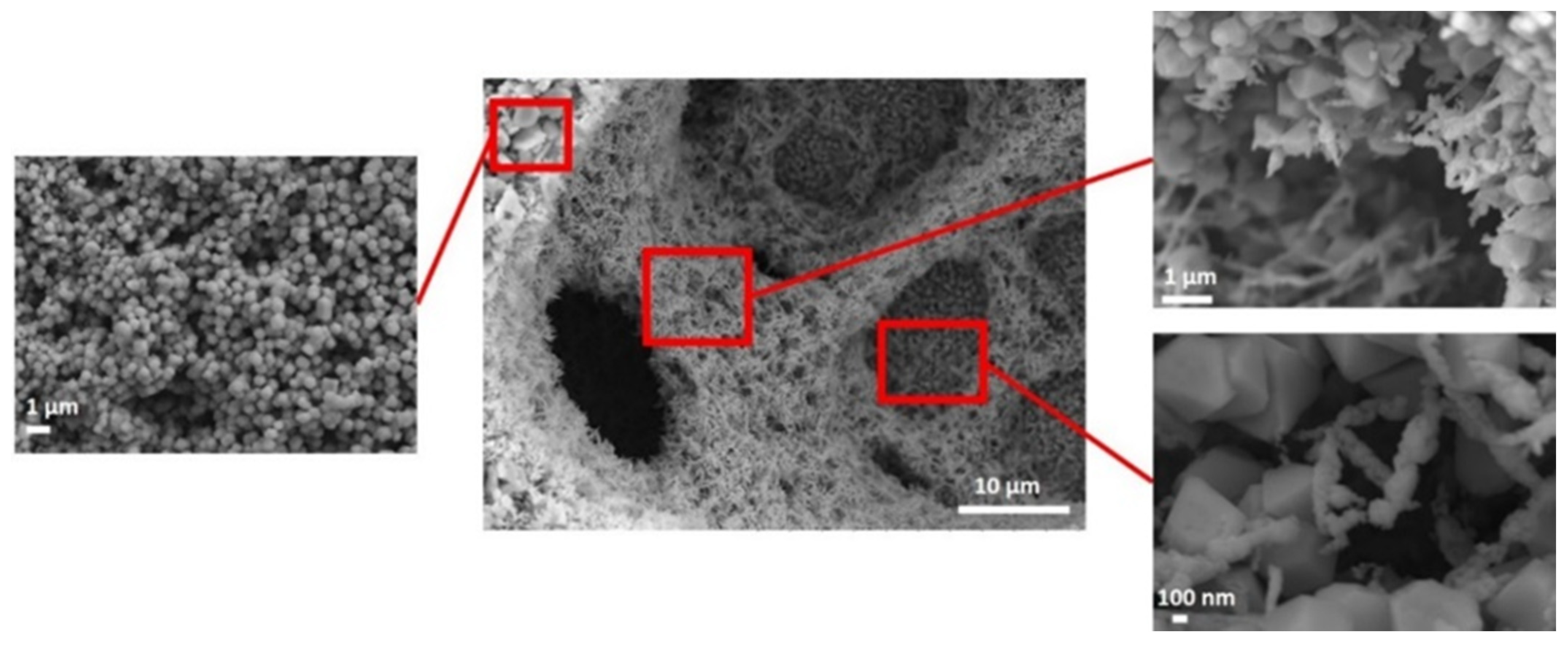
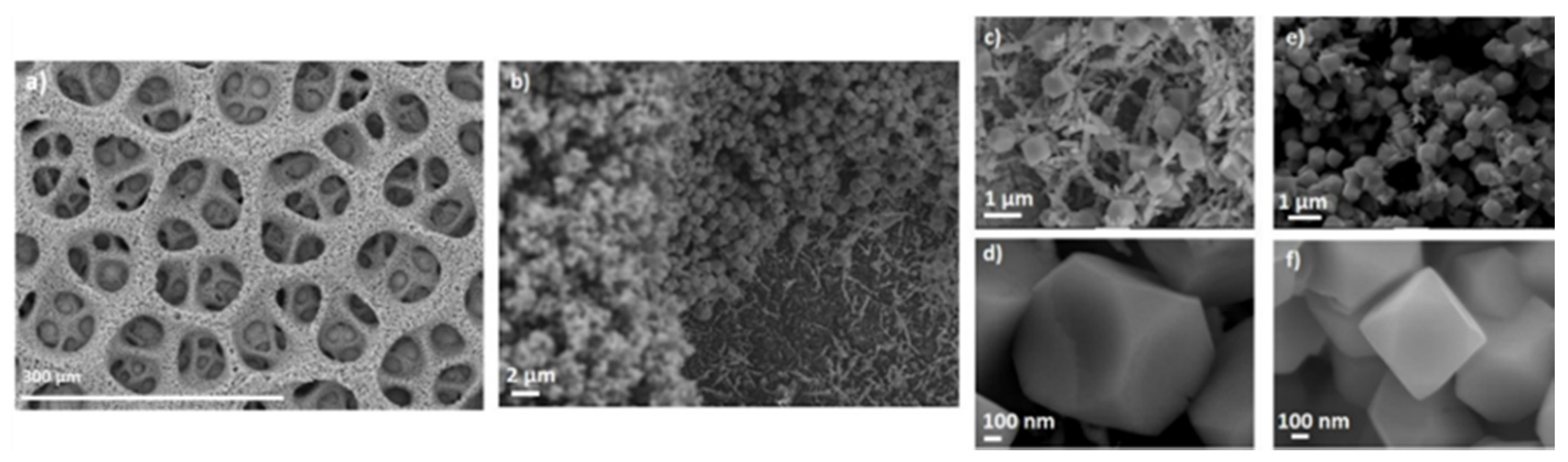

| CF-18-100U | CF-18H-100U | CF-18H-DCU | |
|---|---|---|---|
| Capacitance (µF) | 447 | 474 | 472 |
| Electrochemically active surface area (cm2) | 16.0 | 16.9 | 16.9 |
| Surface roughness factor | 225 | 238 | 237 |
| jgeometric (mA/cm2) | 31.0 | 26.8 | 38.0 |
| jECSA (mA/cm2) | 0.14 | 0.11 | 0.17 |
| Work | Potential versus RHE (V) | FE n-PrOH (%) | Total Geometric Current Density (mA/cm2) | n-Propanol Partial Geometric Current Density (mA/cm2) | Ref. |
|---|---|---|---|---|---|
| Rahaman | −0.9 | 13.1 | −19.5 | −2.56 a | [61] |
| Rahaman | −0.85 | 8.2 | −30.5 | −2.50 | [62] |
| CF-18H-100U | −0.83 | 4.93 | −37.6 | −1.85 | This work |
| Dutta | −0.87 | 7.1 | −24.2 | −1.72 | [33] |
| Ren | −0.85 | 10.6 | −14.2 | −1.50 | [47] |
| Wang | −0.80 | 7.5 | −20.0 | −1.5 | [63] |
| Wang | −0.64 | 10.0 | −12.0 | −1.2 | [63] |
| Rahaman | −0.65 | 13.7 | −8.4 | −1.15 | [62] |
| CF-18H | −0.83 | 3.34 | −29.4 | −0.98 | This work |
| Kim | −0.81 | 5.9 | −12.7 | −0.75 | [46] |
| Han | −1.10 | 11.8 | −1.0 | −0.12 | [45] |
| Grosse | −1.05 | 1.0 | −10.0 | −0.10 | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudd, J.A.; Hernandez-Aldave, S.; Kazimierska, E.; Hamdy, L.B.; Bain, O.J.E.; Barron, A.R.; Andreoli, E. Investigation into the Re-Arrangement of Copper Foams Pre- and Post-CO2 Electrocatalysis. Chemistry 2021, 3, 687-703. https://doi.org/10.3390/chemistry3030048
Rudd JA, Hernandez-Aldave S, Kazimierska E, Hamdy LB, Bain OJE, Barron AR, Andreoli E. Investigation into the Re-Arrangement of Copper Foams Pre- and Post-CO2 Electrocatalysis. Chemistry. 2021; 3(3):687-703. https://doi.org/10.3390/chemistry3030048
Chicago/Turabian StyleRudd, Jennifer A., Sandra Hernandez-Aldave, Ewa Kazimierska, Louise B. Hamdy, Odin J. E. Bain, Andrew R. Barron, and Enrico Andreoli. 2021. "Investigation into the Re-Arrangement of Copper Foams Pre- and Post-CO2 Electrocatalysis" Chemistry 3, no. 3: 687-703. https://doi.org/10.3390/chemistry3030048
APA StyleRudd, J. A., Hernandez-Aldave, S., Kazimierska, E., Hamdy, L. B., Bain, O. J. E., Barron, A. R., & Andreoli, E. (2021). Investigation into the Re-Arrangement of Copper Foams Pre- and Post-CO2 Electrocatalysis. Chemistry, 3(3), 687-703. https://doi.org/10.3390/chemistry3030048









