Abstract
The [3+2] cycloaddition (32CA) reactions of strongly nucleophilic norbornadiene (NBD), with simplest diazoalkane (DAA) and three DAAs of increased electrophilicity, have been studied within the Molecular Electron Density Theory (MEDT) at the MPWB1K/6-311G (d,p) computational level. These pmr-type 32CA reactions follow an asynchronous one-step mechanism with activation enthalpies ranging from 17.7 to 27.9 kcal·mol−1 in acetonitrile. The high exergonic character of these reactions makes them irreversible. The presence of electron-withdrawing (EW) substituents in the DAA increases the activation enthalpies, in complete agreement with the experimental slowing-down of the reactions, but contrary to the Conceptual DFT prediction. Despite the nucleophilic and electrophilic character of the reagents, the global electron density transfer at the TSs indicates rather non-polar 32CA reactions. The present MEDT study establishes the depopulation of the N–N–C core in this series of DAAs with the increase of the EW character of the substituents present at the carbon center is responsible for the experimentally found deceleration.
1. Introduction
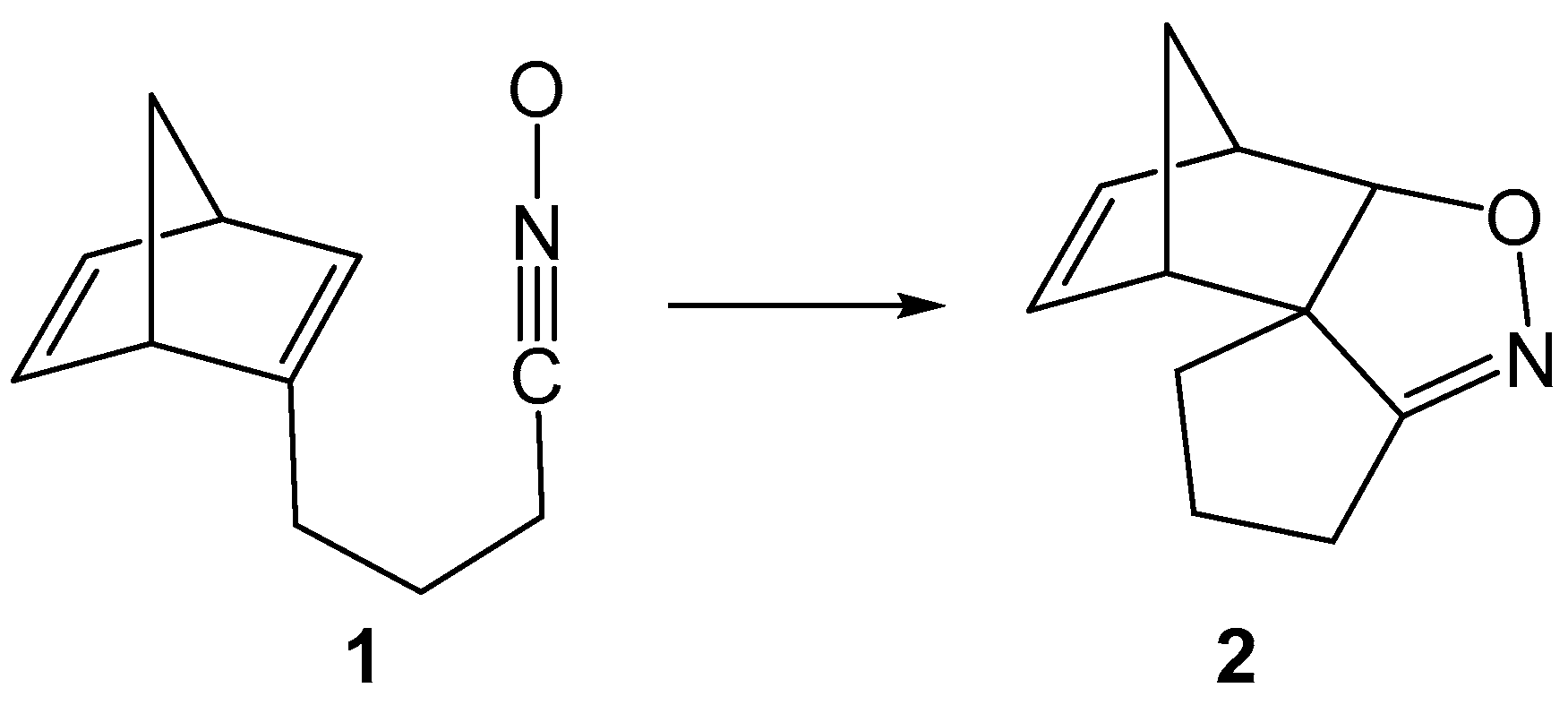
Norbornadiene (NBD) (bicyclo [2.2.1] hepta-2,5-diene), norbornene (bicyclo [2.2.1] hept-2-ene) and related compounds serve as key intermediates in natural product synthesis [1,2,3] and polymer chemistry [1,4] owing to their angularly strained unusual geometry and high reactivity. Recently, NBD has also found applications in molecular photoswitches to absorb solar radiation [5], and by 2018, more than 30 thousand publications and patents had reported their production and applications in various fields [1]. The behavior of NBD derivatives in the Wagner–Meerwein rearrangement [6,7], photochemical di-π methane rearrangement [8], Diels Alder reactions [9], catalytic reactions with alkynes [10] and several other instances [1] have established their uniqueness as organic reagents and fostered their wide synthetic applications in [3+2] cycloaddition (32CA) reactions to form isoxazolidines, triazoles, carbocycles and other heterocycles of biological and environmental relevance [11,12,13,14,15]. In 2001, Tam and coworkers [16,17] reported the highly regio- and stereoselective intramolecular 32CA reactions of NBD-tethered nitrones and nitrile oxides (Scheme 1).
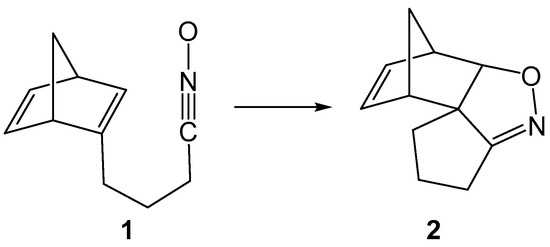
Scheme 1.
Intramolecular 32CA reaction of norbornadiene (NBD)-tethered nitrile oxide 1.
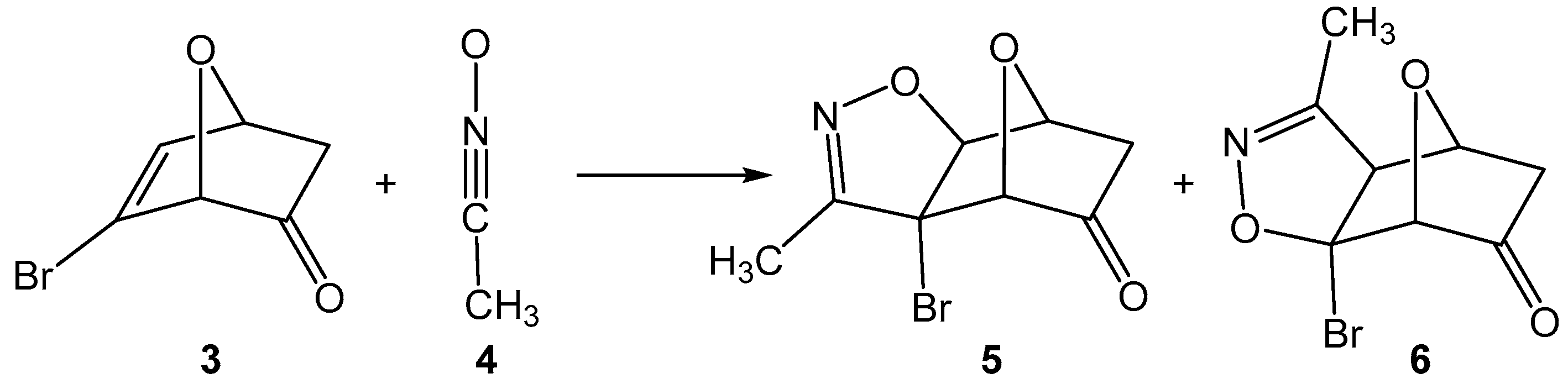
The 32CA reactions of 7-oxanorbornadiene with nitrile oxides were theoretically studied by Tajabadi et al. [18] in 2015, concluding their anti and exo-stereoselective one-step mechanism. In 2017, Domingo et al. [19] reported the complete syn facial stereoselectivity in the 32CA reactions of acetonitrile oxide and 7-oxanorborn-5-en-2-ones as an outcome of the unfavorable steric interactions along the anti approach (see Scheme 2).
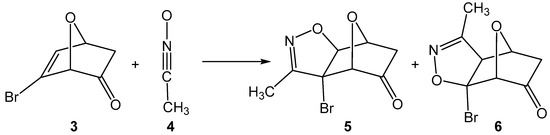
Scheme 2.
32CA reaction of 7-oxanorborn-5-en-2-one 3 with acetonitrile oxide 4.
Very recently, two different studies [20,21] have addressed the regio-, stereo-, and enantioselectivities of the 32CA reactions of 7-isopropylidenenorbornadiene and oxonorbornadiene with nitrones, azides, and nitrileimines.
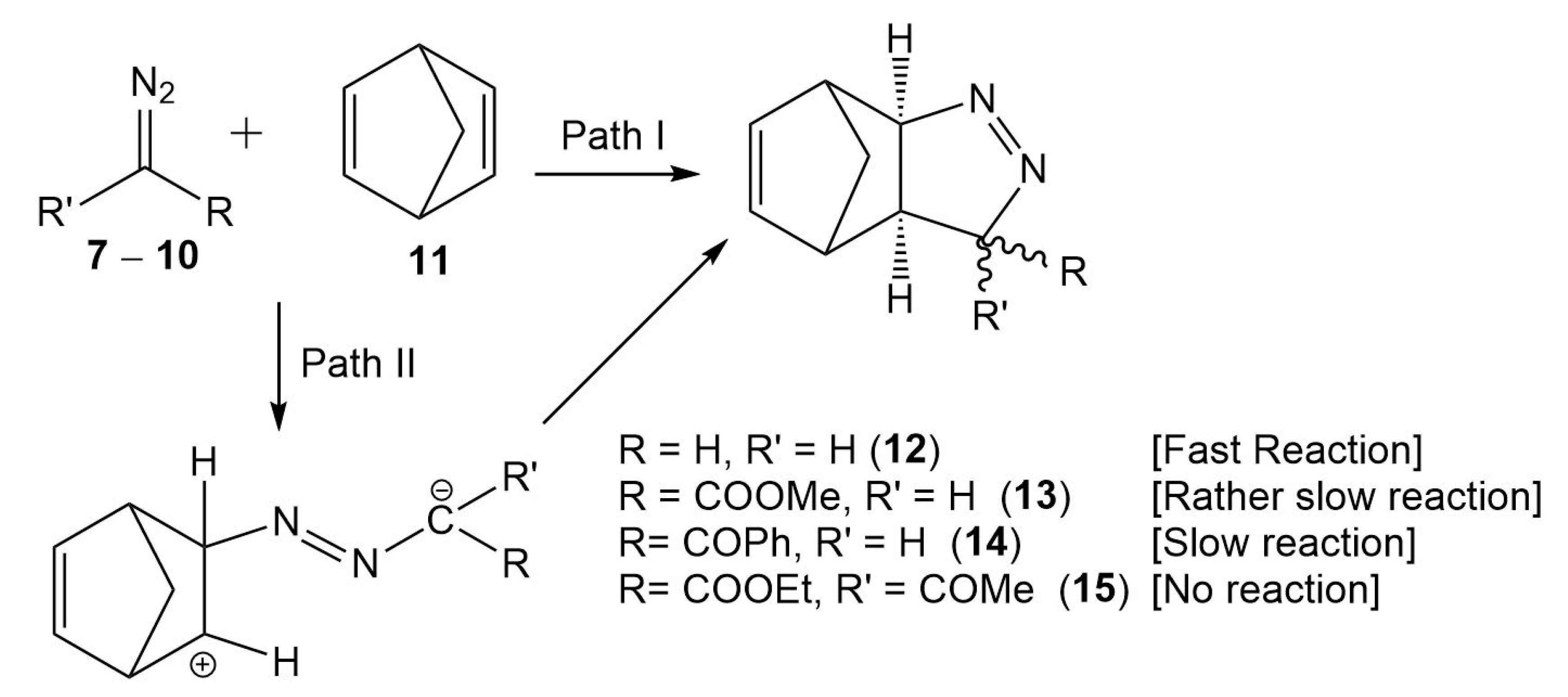
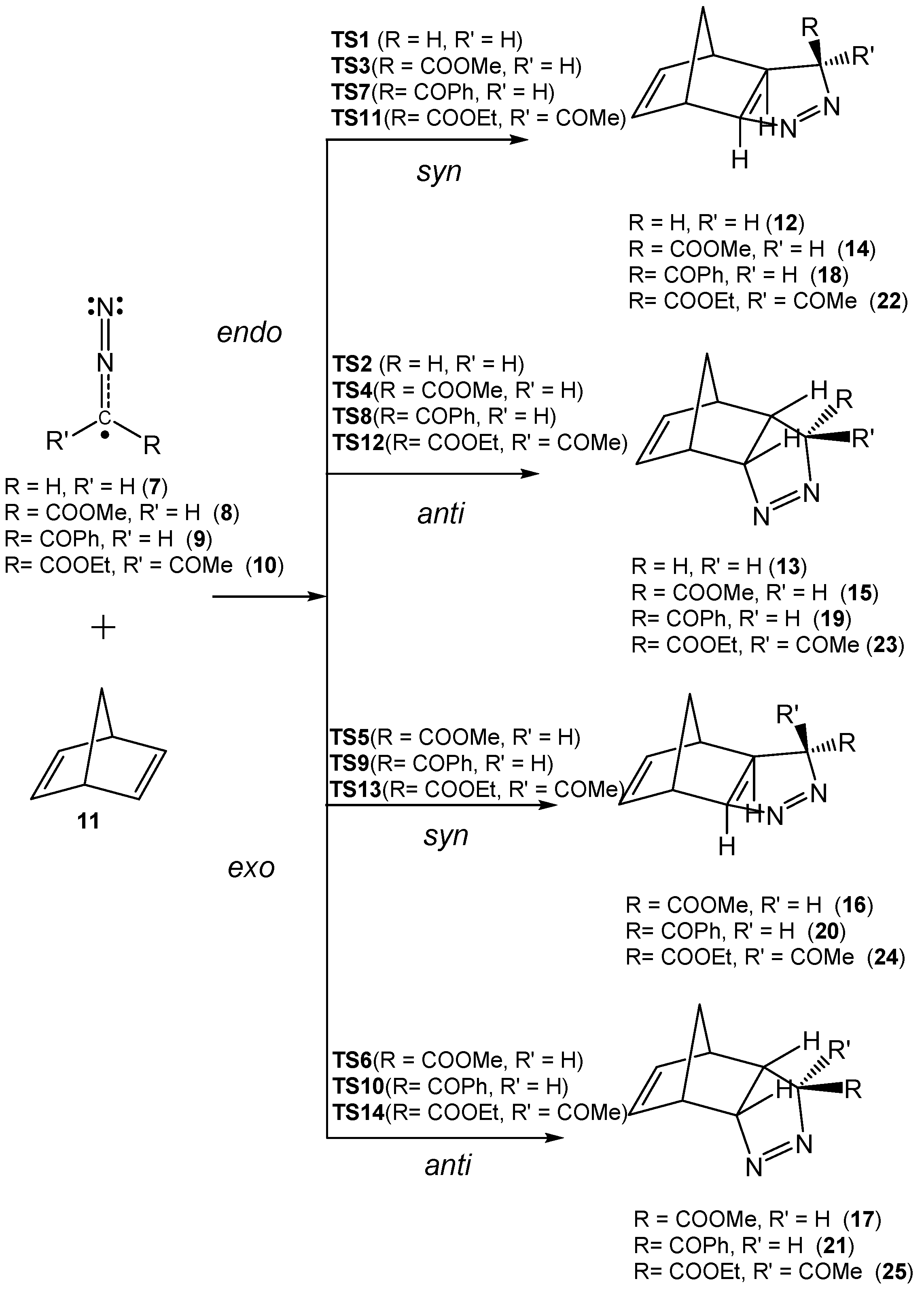
The substituent effects on the rates of the 32CA reactions between NBD and diazoalkanes (DAAs) were experimentally evidenced by Huisgen in 1963 to establish the accountability of the one-step mechanism for 32CA reactions. [22] Considering the 32CA reactions of substituted DAAs 7–10 with NBD 11, (Scheme 3) Huisgen compared the one-step mechanism (Path I, Scheme 3) and the two-step addition (Path II, Scheme 3) possibilities in 32CA reactions [22].
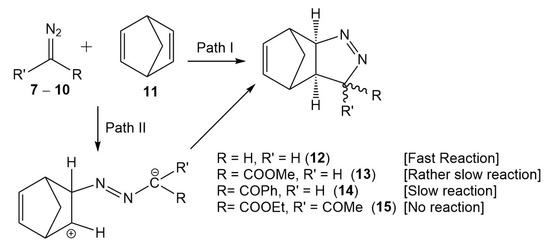
Scheme 3.
32CA reactions of substituted diazoalkanes (DAAs) 7–10 with NBD 11.
The addition tendency of simplest DAA 7 with NBD 11 was maximum along this series, while the 32CA reaction slowed down by introducing electron-withdrawing (EW) carbonyl substituents at the carbon of the DAA in 8 and 9, and finally, DAA 10, with two carbonyl substituents, was completely inactive. Since the nucleophilic additions of NBD to the terminal nitrogen of DAAs are well known [23], it was expected that DAAs act as an electrophilic species and NBD 11, with two electron rich C–C double bonds, as the nucleophilic one. Hence, EW substituents in DAA should enhance the addition tendency. However, this behavior was contrary to the experimental observation (Scheme 3), and consequently, it demands a detailed explanation for the bond formation process and a rationalization of the decrease in reaction rate experimentally observed.
The underlying theory adopted for explaining 32CA reactions was based on the Frontier Molecular Orbital [24] (FMO) concept for the last 50 years, until in 2016, Domingo proposed the Molecular Electron Density Theory [25,26] (MEDT) to recognize the decisive role of the changes in electron density on the molecular reactivity. The MEDT concept, which rejects any analysis based on molecular orbitals, has explained the experimental outcome of 32CA reactions, for example, the strain promotion [27,28], selectivities [29,30], substituent effects [31,32,33], catalysis [34,35], etc.
Within the MEDT framework, the three-atom-components (TACs) participating in 32CA reactions are classified into four types [36], which answers their reactivity in 32CA reactions. TACs with two pseudoradical centers participate in pdr-type 32CA reactions [37], which take place very easily. TACs with one pseudoradical center participate in pmr-type 32CA reactions [38], while TACs with a carbenoid center participate in cb-type 32CA reactions [39]. TACs showing the absence of pseudoradical or carbenoid centers are classified as the zwitterionic type and participate in zw-type 32CA reactions [29] associated with high energy barrier demanding overcome through appropriate electrophilic-nucleophilic activations [29]. Recent studies devoted to the participation of DAAs in 32CA reactions have evidenced that DAAs have a pseudoradical structure, thus participating in pmr-type 32CA reactions [28,40].
Herein, an MEDT study for the 32CA reactions of substituted DAAs 7–10 with NBD 11, experimentally studied by Huisgen [22] (Scheme 3), is performed at the MPWB1K/6-311G (d,p) computational level to explain the unexpected reactivity of these electrophilic DAAs. This MEDT report is shaped into eight sections: (i) In Section 3.1, a topological analysis of the electron localization function (ELF) [41,42] at the ground state electronic structures of the reagents is performed to structurally classify the DAAs and determine their reactivity; (ii) in Section 3.2, the global reactivity indices defined within the conceptual density functional theory [43,44] (CDFT) are discussed; (iii) in Section 3.3, the energy profiles of the feasible reaction paths and some of the properties of the corresponding stationary points are analyzed; (iv) in Section 3.4, a topological analysis of non-covalent interactions is performed to understand the origin of the stereoselectivity; (v) in Section 3.5, the molecular mechanisms of the 32CA reactions of DAAs 7 and 10 with NBD 11 are analyzed through a bonding evolution theory (BET) [45] study of the most favorable reaction paths; (vi) in Section 3.6, the topology of the ELF at the TSs is analyzed; (vii) in Section 3.7, the nature of bonding at the interatomic bonding regions is assessed through the Quantum Theory of Atoms in Molecules [46,47] (QTAIM) parameters proposed by Bader and coworkers; and finally, (viii) in Section 3.8, the origin of the deceleration with the EW substitution is analyzed.
2. Computational Methods
Optimization of the reactants, TSs, and products was done using Berny analytical gradient optimization method [48] at MPWB1K [49]/6-311G (d,p) [50]. This computational level has been recently used in studying 32CA reactions [27,28,30,31,32]. Frequency calculations were performed at the located stationary points to ensure that the TSs have one and only one imaginary frequency and all positive frequencies exist for the local minimum. The intrinsic reaction coordinate [51] (IRC) calculations using González-Schlegel integration method [52,53] verified the minimum energy reaction pathway connecting the reactants and the products. Solvent effects were studied by modeling the solvent acetonitrile using the polarizable continuum model [54,55] (PCM) with the self-consistent reaction field [56,57,58] method, and the stationary points were optimized at MPWB1K (PCM)/6-311G (d,p) level of theory. The thermodynamic parameters, i.e., the enthalpies, entropies, and Gibbs free energies, were calculated at 298.15 K and 1 atm in acetonitrile using the standard statistical thermodynamics [50].
Equations reviewed in reference 44 are used to calculate the CDFT indices, while the global electron density transfer [59] (GEDT) at the TSs was computed from the summation of charges at each fragment derived from natural population analysis [60,61] using the equation GEDT (f) = , where q denotes the natural atomic charges. The topological analysis of the ELF [41] and QTAIM [46,47] was performed using Multiwfn software [62]. The ELF localization domains (isovalue of 0.75 au) are represented from Paraview visualization software [63,64]. The calculations are performed using the Gaussian 03 package [65]. NCI analysis [66] was performed with the NCIPLOT4 program [67].
3. Results and Discussion
3.1. Analysis of the ELF Topology at the Ground State Electronic Structures of DAAs 7–10 and NBD 11
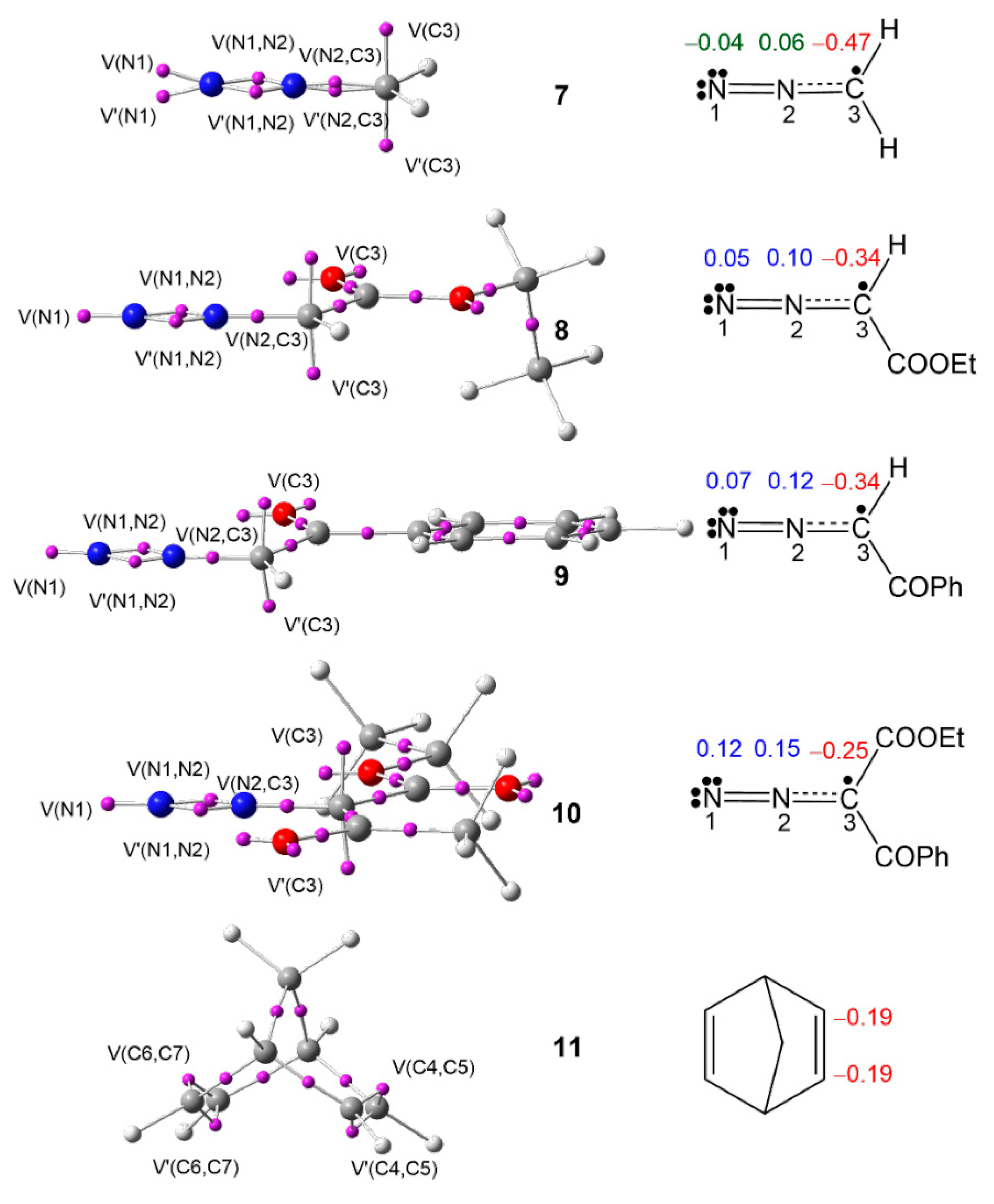
ELF [41,42] topology allows quantitative characterization of chemical regions of a molecule, which is used to classify the TACs, and then to correlate their electronic structure with their molecular reactivity within the MEDT framework. Proposed by Domingo in 2019 [36], this standard classification of TACs into pseudodiradical, pseudo(mono)radical, carbenoid, or zwitterionic type has characterized 32CA reactions into pmr-type, pdr-type, cb-type, and zw-type, respectively, based on the respective TAC participation [36]. Herein, the ELF topology of DAAs 7–10 and NBD 11 is studied to assess their electronic structures. The ELF valence basin populations are given in Table 1, while the ELF basin attractor positions are shown in Figure 1.

Table 1.
Electron localization function (ELF) valence basin populations, in average number of electrons e, at the MPWB1K/6-311G (d,p) optimized structures of DAAs 7–10 and NBD 11.
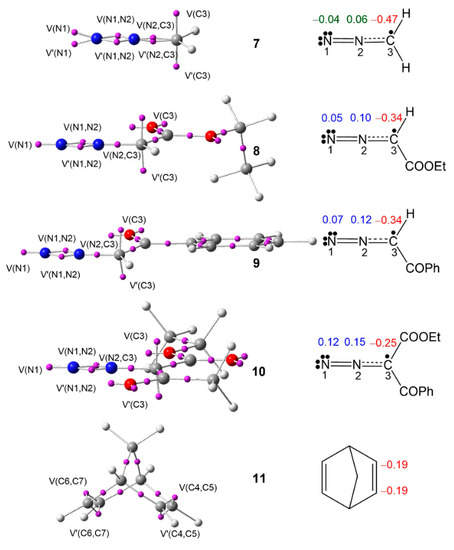
Figure 1.
MPWB1K/6-311G(d,p) ELF basin attractor positions of DAAs 7–10 and NBD 11 and the proposed Lewis-like structures together with the natural atomic charges in average number of electrons e. Negative, negligible, and positive charges are shown in red, green, and blue colors, respectively.
The ELF topological analysis of DAAs 7–10 shows the presence of two monosynaptic basins, V(N1) and V′(N1), in 7 and one V(N1) monosynaptic basin in 8–10, integrating a total population of 3.78 e (7), 3.61 e (8), 3.58 e (9), and 3.47 e (10), associated with the non-bonding electron density on N1 nitrogen; two disynaptic basins, V(N1,N2) and V′(N1, N2), integrating a total population of 3.74 e (7), 3.88 e (8), 3.90 e (9), and 3.97 e (10), associated with the N1–N2 double bond; and one V(C3,N2) disynaptic basin, integrating 3.01 e (7), 2.90 e (9), 2.88 e (9), and 2.82 e (10), associated with the C3–N2 overpopulated single bond, which shows the influence of substitution on the electronic distribution of the N1–N2–C3 moiety of these DAAs. Finally, the presence of two monosynaptic basins, V(C3) and V′(C3), integrating a total of 0.92 e (7), 0.92 e (8), 0.88 e (9), and 0.98 e (10), associated with a pseudoradical center at C3, classifies the DAAs 7–10 as pseudo(mono)radical TACs, thus suggesting their participation in pmr-type 32CA reactions [36].
ELF topology of NBD 11 shows the presence of two pairs of disynaptic basins, V(C4,C5) and V′(C4,C5), and V(C6,C7) and V′(C6,C7), integrating a total population of 3.48 e each pair, associated with the C4–C5 and C6–C7 double bonds of NBD 11.
The proposed Lewis-like structures, together with the natural atomic charges of compounds 7–11, are represented in Figure 1. The pseudoradical C3 carbon is negatively charged by −0.47 e (7), −0.34 e (8), −0.34 e (9), and −0.25 e (10), suggesting the influence of the EW substitution. The N1 and N2 nitrogen nuclei show negligible charges in simplest DAA 7, while the N2 nitrogen shows a charge slightly greater than 0.10 e at DAAs 8, 9, and 10. The charge distribution pattern in the DAAs 7–10 does not follow the commonly accepted 1,2-zwitterionic concept for DAAs.
3.2. Analysis of the CDFT Indices of DAAs 7–10 and NBD 11
The analysis of the reactivity indices based on CDFT [43,44] has become a useful tool for studying reactivity in polar reactions [44]. Therefore, an analysis of CDFT reactivity indices was performed to predict the reactivity of the reagents in these 32CA reactions. The CDFT indices were calculated at the B3LYP/6-31G (d) computational level since it was used to define the electrophilicity and nucleophilicity scales [43,68,69]. The electronic chemical potential μ, chemical hardness η, electrophilicity ω, and nucleophilicity N indices of compounds 7–11 are gathered in Table 2. The MPWB1K/6-311G (d,p) reactivity indices are gathered in Table S3 in Supplementary Material.

Table 2.
B3LYP/6-31G (d) electronic chemical potential μ, chemical hardness η, electrophilicity ω, and nucleophilicity N indices, in eV, of DAAs 7–10 and NBD 11.
The electronic chemical potentials μ of DAAs 7−10, μ = −3.63 (7), −4.27 (8), −4.20 (9), and −4.58 (10) eV, are lower than that of NBD 11, μ = −2.94 eV, suggesting that along a polar process the electron density will flux from NBD 11 to DAAs 7–10 via reverse electron density flux (REDF) reactions [70].
The chemical hardness η of DAAs 7–10, ranging from 4.70 to 4.87 eV, is also lower than that of NBD 11, 5.88 eV, which means that the DAAs are more prone to electron density deformation than NBD 11, i.e., they are softer. Interestingly, DAA 10, having more EW substituents, is chemically harder than the simplest DAA 7.
Within the electrophilicity scale [68], simplest DAA 7, ω = 1.40 eV, is classified as a moderate electrophile, while DAAs 8–10, ω > 1.85 eV, are classified as strong electrophiles. However, it is worth noting that among the strongly electrophilic DAAs, only DAA 10 would be electrophilically activated enough to work well experimentally, which is against the experimental findings. On the other hand, within the nucleophilicity scale [69], the simplest DAA 7, N = 3.13 eV, is classified as a strong nucleophile, while DAAs 8–10, N < 2.53 eV, are classified as moderate nucleophiles. As expected, the inclusion of EW groups at the C3 carbon of simplest DAA 7 increases the electrophilicity ω index and decreases the nucleophilicity N index of the DAA derivatives.
NBD 11 is classified as a marginal electrophile, ω = 0.74 eV, and a strong nucleophile, N = 3.23 eV. Both the strain caused by the bicyclic system and the presence of two C–C double bonds considerably increase the nucleophilicity N index of 11 with respect to that of ethylene 26, N = 1.87 eV.
The analysis of the CDFT indices predicts that along a polar process, DAAs 7–10 will behave as electrophilic species while NBD 11 as the nucleophilic one. However, the experimental outcome [22] revealed that the EW substitution in the DAA decreases its reactivity towards NBD 11. Along this series, the fastest 32CA reaction was that involving simplest DAA 7, while strongly electrophilic DAA 10 having two EW –COOMe and –COPh groups was completely inactive. In addition, except for the reaction involving DAA 10, CDFT predicts the other reactions to have a low-polar character as the DAAs are not sufficiently electrophilically activated. Thus, this CDFT analysis does not agree with the experimental results of this series of reactions.
A comparison of the B3LYP/6-31G (d) and MPWB1K/6-311G (d,p) reactivity indices shows that although they have different values, both computational levels give similar trends of reactivity.
3.3. Analysis of the Reaction Paths Associated with the 32CA Reactions between DAAs 7–10 and NBD 11
Due to the molecular symmetry of the simplest DAA 7 and NBD 11, two competitive stereoisomeric reaction paths are feasible for this 32CA reaction. They are related to the two approach modes of DAA 7 with respect to the two stereoisomeric faces of the C–C double bonds of NBD 11. These reaction paths are called syn and anti. For DAAs 8–10, which present a different substitution at the C3 carbon, two additional stereoisomeric reaction paths are feasible, the endo and the exo ones. Consequently, for the 32CA reactions involving DAAs 8–10, four competitive reaction paths were considered (see Scheme 4). For the 32CA reaction of simplest DAA 7, the syn and anti reaction paths lead to two diastereoisomeric pyrazolines 12 and 13, respectively, via TS1 and TS2. For the 32CA reactions involving substituted DAAs 8–10, four diastereoisomeric pyrazolines 14–17, 18–21, and 22–25 can be formed via TS3–TS6, TS7–TS10, and TS11–TS14, respectively (Scheme 4). The four 32CA reactions follow a one-step mechanism. Relative electronic energies, enthalpies, entropies, and Gibbs free energies of the TSs and adducts are given in Table 3.
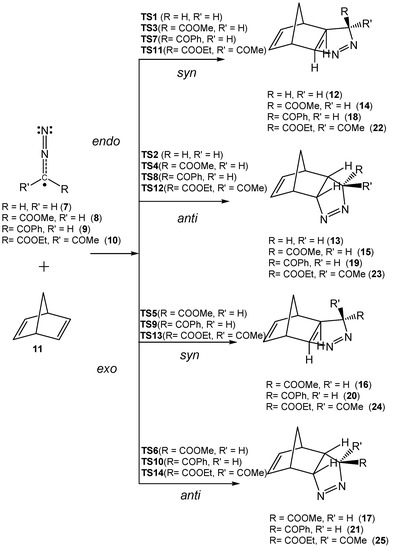
Scheme 4.
Studied reaction paths for the 32CA reactions of DAAs 7–10 with NBD 11.

Table 3.
MPWB1K/6-311G (d,p) relative energies (∆E, in kcal·mol−1), enthalpies (∆H, in kcal·mol−1), entropies (∆S, in cal·mol−1K−1), Gibbs free energies (∆G, in kcal·mol−1) and GEDT (in average number of electrons e) of TSs and pyrazolines for the 32CA reactions of DAAs 7–10 with NBD 11 (superscript ‘a’ refers to FEDF (forward electron density transfer) and superscript ‘b’ refers to REDF (reverse electron density transfer)).
From the energy results given in Table 3, a series of appealing conclusions can be obtained: (i) The gas phase activation energies associated with the energetically most favorable reaction paths of these 32CA reactions are found between 15.9 (TS1) and 23.3 (TS11) kcal·mol−1; (ii) the increase of the activation energies in the order TS1 (7) < TS3 (8) < TS10 (9) < TS11 (10) is in complete agreement with the experimental outcomes [22]; (iii) these reactions are strongly exothermic by more than 30 kcal·mol−1; (iv) inclusion of solvent effects increases the activation energies by between 0.3 (TS11) and 1.9 (TS7) kcal·mol−1, and increases the exothermic character of the reactions by between 2.0 (12) and 4.1 (22) kcal·mol−1. Formation of 18 is disfavored by 1.2 kcal·mol−1; (v) inclusion of the thermal corrections increases the activation enthalpies in acetonitrile by between 0.8 (TS3) and 1.1 (TS11) kcal·mol−1 and decreases the reaction enthalpies by between 3.6 (22) and 4.8 (12) kcal·mol−1; (vi) inclusion of entropies to enthalpies increases the activation Gibbs free energies in acetonitrile between 29.9 (TS1) and 43.0 (TS14) kcal·mol−1, and decreases the reaction Gibbs free energies between −16.2 (22) and −37.1 (12) kcal·mol−1; (vii) the strong exergonic character of these 32CA reactions makes them irreversible, and consequently, the products are obtained by kinetic control; (viii) the syn/anti facial stereoselectivity ranges from 0.7 (7) to 3.1 (8) kcal·mol−1. While DAAs 7, 8, and 10 prefer the syn attack, DAA 9 prefers the anti one; and (ix) these 32CA reactions present a low endo/exo stereoselectivity; lower than 0.8 kcal·mol−1. While the 32CA reactions of DAAs 7, 8, and 10 are endo selective, that of DAA 9 is exo selective.
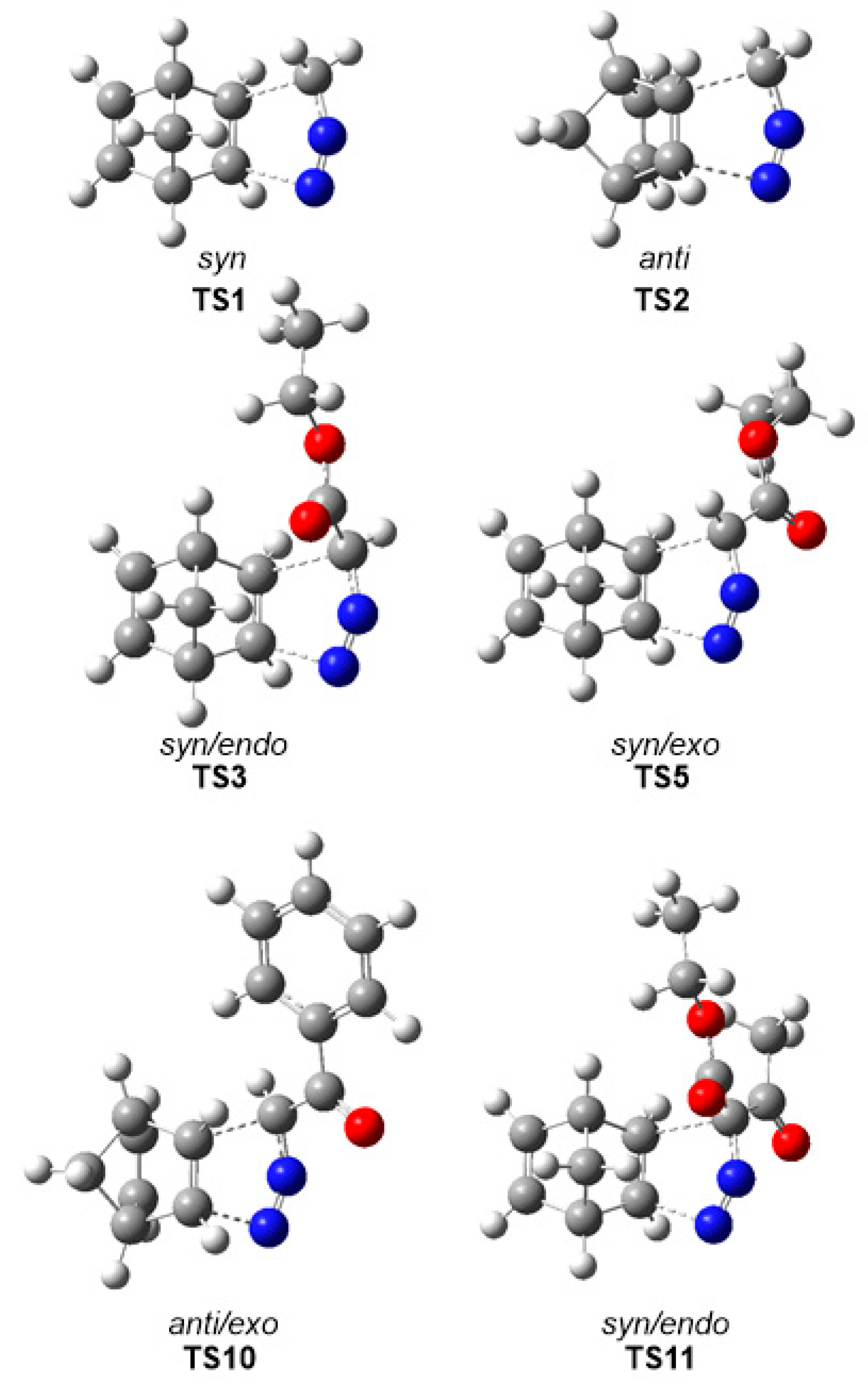
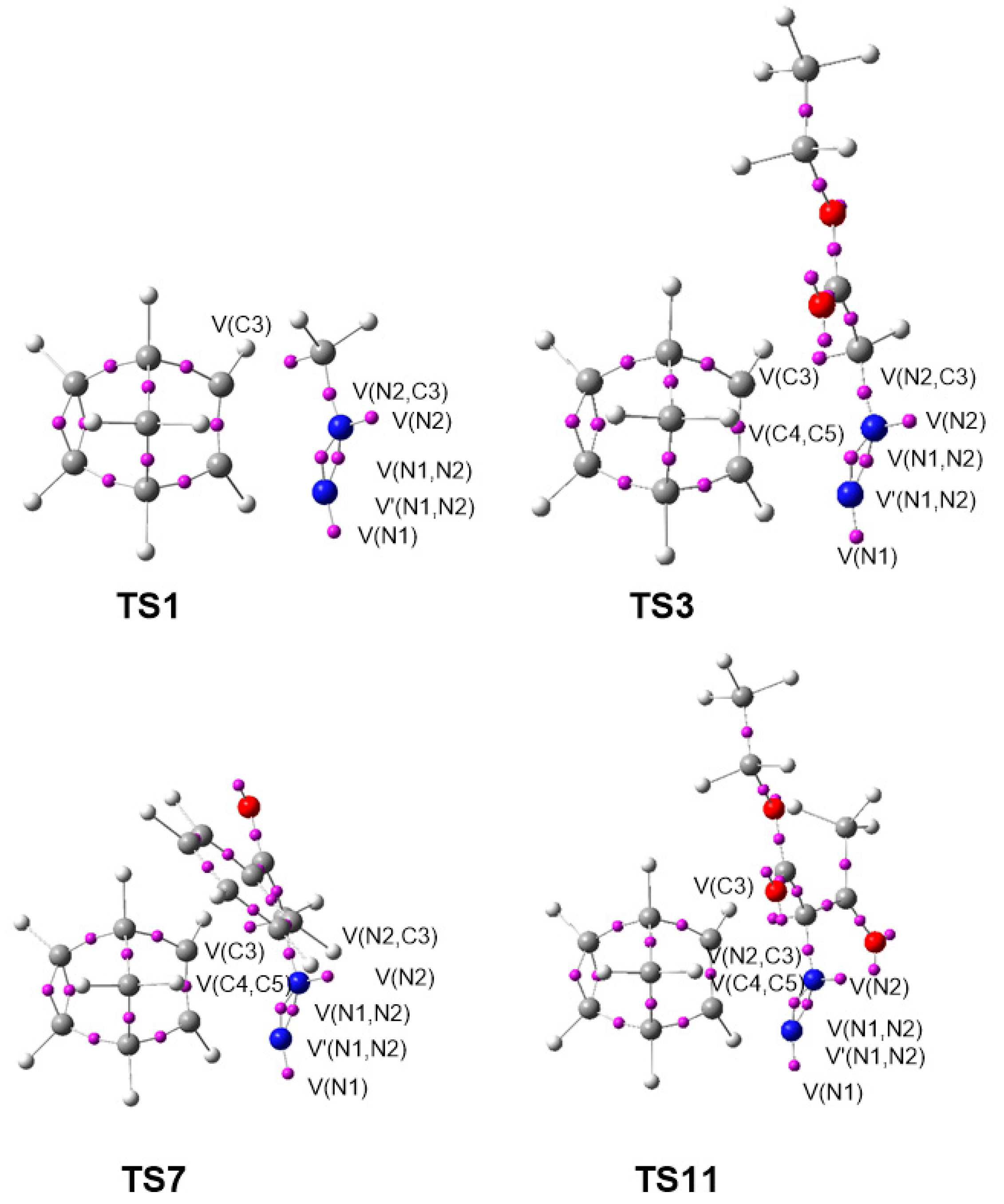
The gas phase optimized geometries of the more favorable TSs are given in Figure 2, while the distances between the C–C and C–N interacting nuclei are given in Table 4. Some appealing conclusions can be drawn for these geometrical parameters: (i) Considering that C–C bond formation begins slightly earlier than the C–N one, [36] these distances at TS1–TS10 indicate that, from a geometrical point of view, they correspond to hardly asynchronous TSs; (ii) a comparison of these distances at TS11–TS14 with respect to those at the other TSs shows that the TSs involving DAA 10 are clearly more asynchronous. At TS11–TS14, the C–N distances are shorter, while the C–C ones are longer, suggesting more delayed C–C bond formation but more advanced C–N bond formation; i.e., more asynchronous processes; (iii) as all these distances are greater than 2.0 Å in the gas phase and acetonitrile, and considering that the C–C and C–N single bond formation take place at the short range of 2.0–1.9 and 1.9–1.8 Å, respectively, [36] they indicate that formation of the new C–C and C–N single bonds has not started yet at any TS (see Section 3.6); (iv) inclusion of solvent effects in the optimizations does not considerably modify the gas phase geometries. Note that for the 32CA reactions of DAAs 7–9, these differences are lower than 0.02 Å.
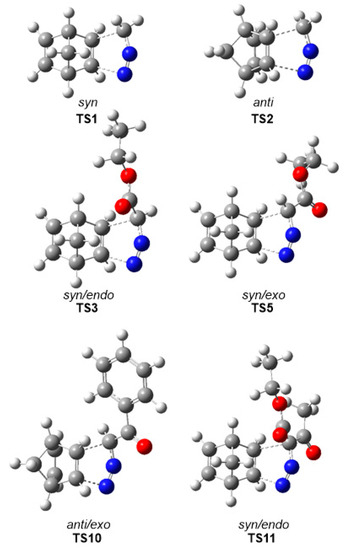
Figure 2.
MPWB1K/6-311G (d,p) optimized geometries of some selected TSs associated with the 32CA reactions of DAAs 7–10 with NBD 11.

Table 4.
C–C and C–N distances, in angstroms Å, at the TSs involved in the 32CA reactions of DAAs 7–10 with NBD 11, in the gas phase and in acetonitrile.
Finally, the GEDT at the TSs was calculated to assess the polarity of these 32CA reactions, and is given in Table 3. Reactions with GEDT values lower than 0.05 e correspond to non-polar processes, while values higher than 0.20 e correspond to polar processes. The 32CA reaction between simplest DAA 7 and NBD 11, with GEDT of 0.10 e (TS1) and 0.12 (TS2), in the gas phase and in acetonitrile, is predicted as a 32CA reaction with some polar character, with the electron density fluxing from DAA 7 to NBD 11. This GEDT analysis suggests that this 32CA reaction is classified as a forward electron density flux (FEDF) reaction [70]. Interestingly, this finding is against the analysis of the electronic chemical potential μ, electrophilicity ω, and nucleophilicity N indices of DAA 7 and NBD 11, which predict that the reaction would not work experimentally but, if so, it should be a reaction of reverse electron density flux (REDF) [70] (see Table 2 and Section 3.2).
For the 32CA reactions of DAAs 8–10 with NBD 11, the GEDT values are found between 0.02–0.05 e, predicting non-polar 32CA reactions, thus, being classified as reactions of null electron density flux (NEDF) [71]. This decrease in polarity is consistent with the increase in the energy barrier of the corresponding 32CA reactions. For the 32CA reaction of DAA 10 with NBD 11 in acetonitrile, which does not take place experimentally, the GEDT at the TSs is 0.08 e, showing a very low polar character. At this TS, the low electron density that fluxes from the NBD framework to the DAA ones, might permit to classify this 32CA reaction as a reaction of REDF [70]. However, note that this 32CA does not take place experimentally.
Interestingly, the 32CA reaction of simplest DAA 7, the least electrophilic DAA of the series, with NBD 11 presents the highest GEDT. In addition, the electron density fluxes from DAA 7 to NBD 11, against the analysis of the CDFT indices (see Section 3.2). Similar behavior was found along the pdr-type 32CA reaction between the simplest azomethine ylide and ethylene 26, GEDT = 0.10 e [37], which was expected to be a reaction of NEDF. Note that neither NBD 11 nor ethylene 26 have any tendency to act as electrophiles. It seems that the presence of a non-bonding electron density at the ends of the TACs, which accounts for their high nucleophilic character, is responsible for the local electron density transfer taking place from these terminal carbon and nitrogen atoms to the double bonds of NBD 11 and ethylene 26, a local phenomenon that cannot be anticipated by the analysis of the electronic chemical potential μ of the reagents.
3.4. NCI Analysis of the Preferred Anti/Exo Stereoselectivity in the 32CA Reaction of DAA 9 with NBD 11
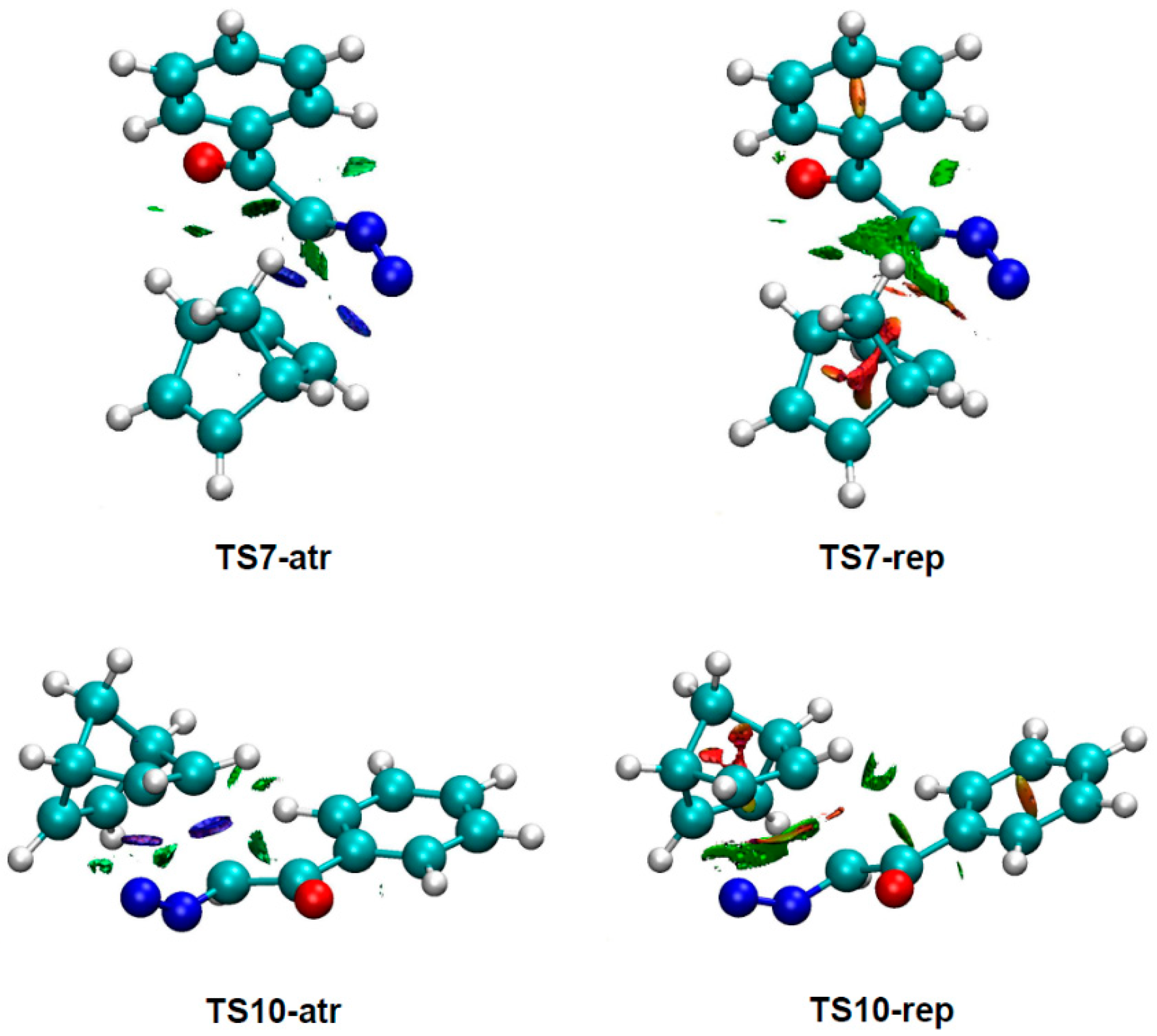
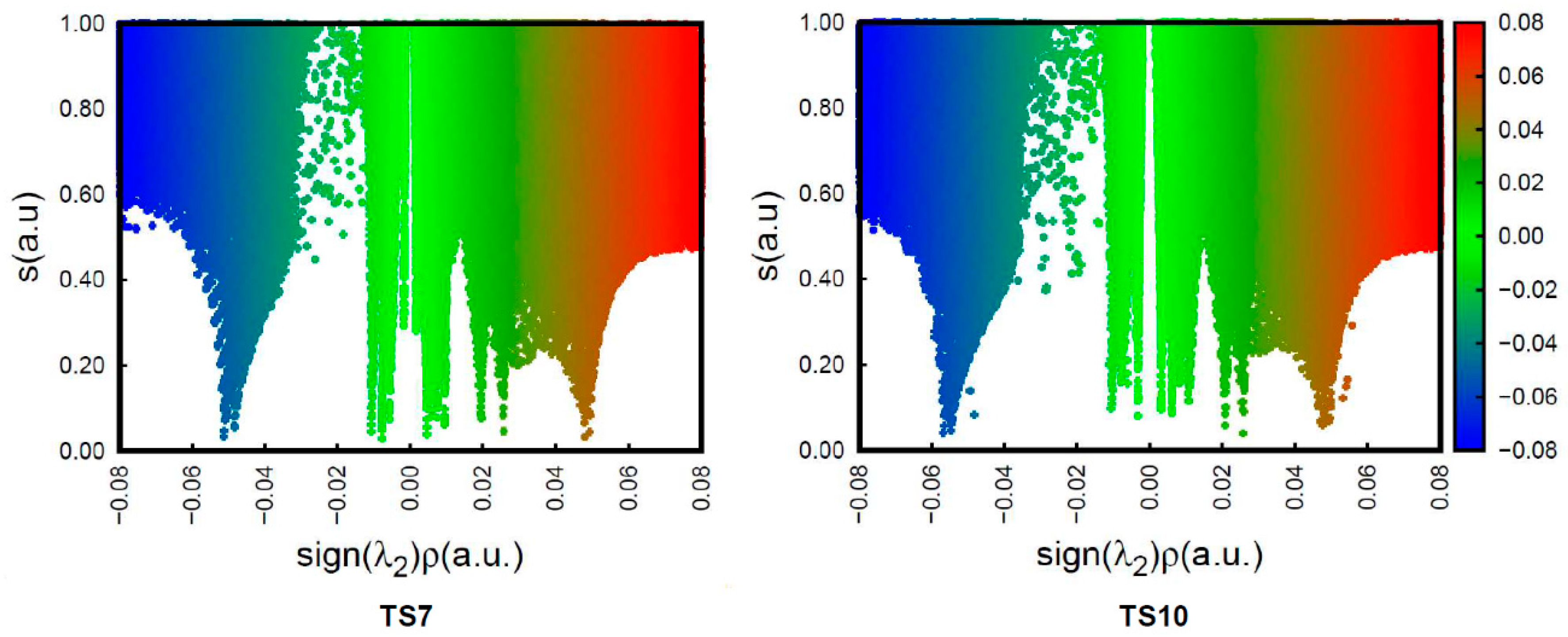
To understand the origin of the anti/exo stereoselectivity in the 32CA reaction of DAA 9 with NBD 11, a comparative topological analysis of the attractive and repulsive NCIs taking place at syn/endo TS7 and anti/exo TS10 was performed by means of the NCI approach. Note that the syn/endo stereoselectivity is preferred in every 32CA reaction except that involving DAA 9. The corresponding isosurfaces are represented in Figure 3, while the plots of the reduced density gradient s vs. sign(λ2) ρ(r) are given in Figure 4. A continuous color-coding scheme based on the second eigenvalue of the Hessian is used, where strong, attractive interactions are represented in blue, weak interactions in green, and strong repulsive interactions in red.
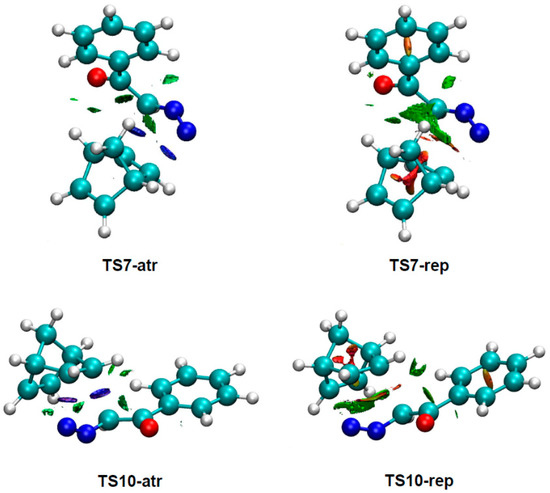
Figure 3.
NCI isosurfaces associated with the attractive and repulsive density overlap at TS7 and TS10. An isosurface value of 0.5 and a blue-green-red color scale from −0.05 < sign(λ2) ρ(r) < 0.05 a.u. were used.
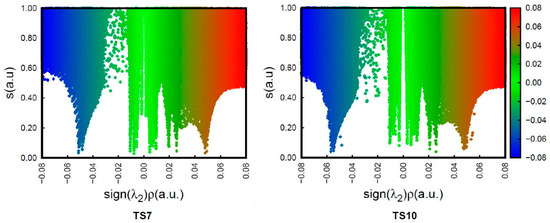
Figure 4.
Plot of the reduced density gradient s vs. sign(λ2) ρ for TS7 and TS10.
As can be observed in Figure 3, the surfaces associated with repulsive density overlap are greater than those associated with attractive interactions. This suggests that repulsive interactions have a greater role than the attractive ones in the preference for the anti/exo approach. A closer look reveals that considering only the surfaces associated with intermolecular interactions and skipping those associated with bond formation, the repulsive overlap spreads wider at TS7 than at TS10.
The plots of the reduced density gradient s vs. sign(λ2) ρ show that there are no qualitative differences between the density characteristics of the interactions (see Figure 4). The only noticeable change is that the peaks associated with the weak NCIs, both the attractive and the repulsive ones, reach higher values of s at TS10 than at TS7. This feature only indicates that these interactions are not associated with critical points in the electron density, [72] thus being only different in strength. Consequently, this analysis suggests that the weaker unfavorable interactions present at TS10 are responsible for the anti/exo stereoselectivity in the 32CA reaction between DAA 9 and NBD 11.
3.5. BET Study for the 32CA Reactions of DAAs 7 and 10 with NBD 11
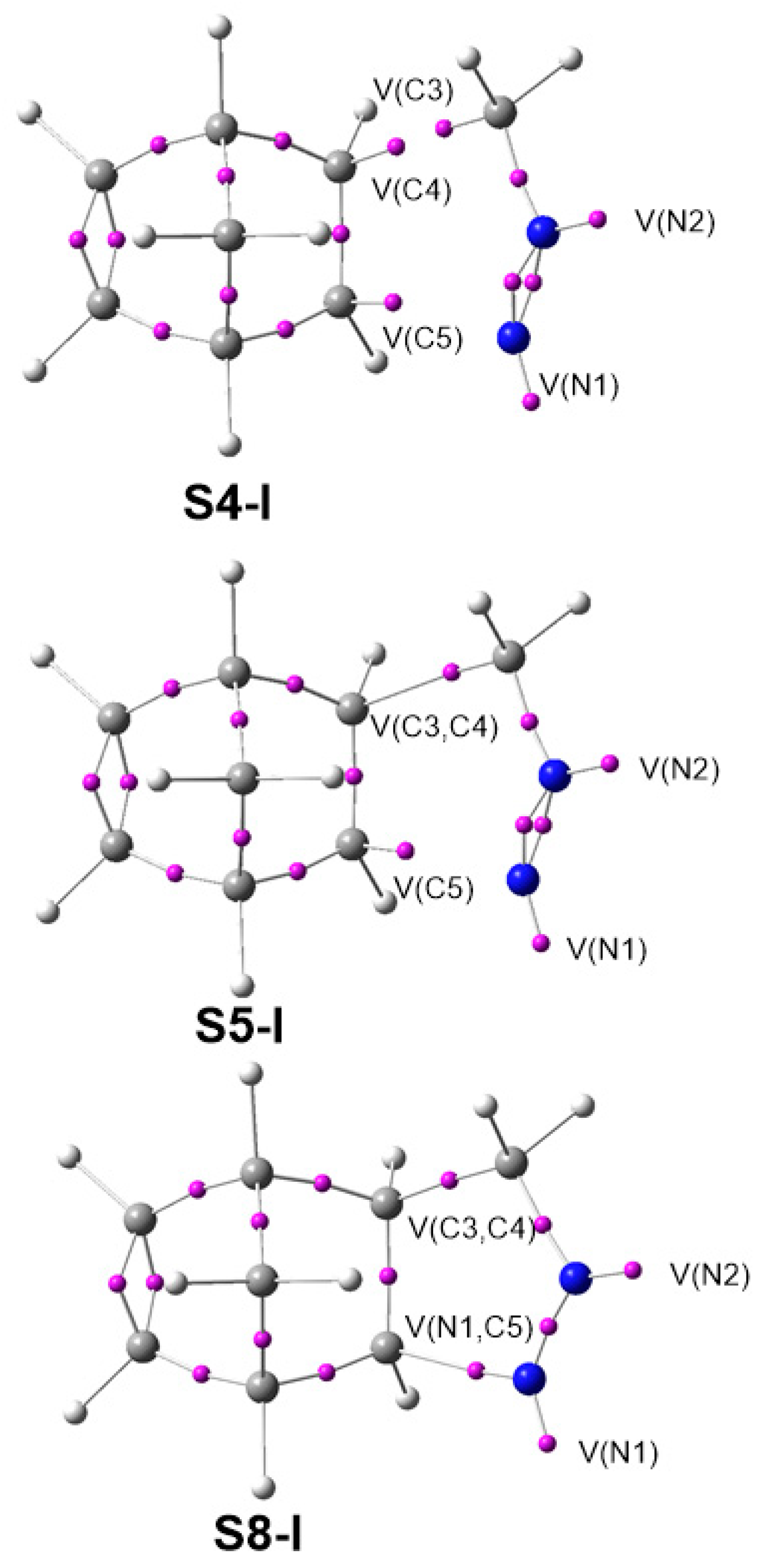
The BET has established the molecular mechanism of a great number of organic reactions by means of the analysis of the bonding changes along the reaction path, which is possible due to the straightforward connection between the electron density distribution and the chemical structure [42]. The detailed BET studies of the 32CA reactions of DAAs 7 and 10 with NBD 11 are given in the Supplementary Material in Section 1 and Section 2. Herein, only the most relevant conclusions are analyzed and compared. The ELF basin attractor positions of the structures involved in the bond formation are shown in Figure 5.
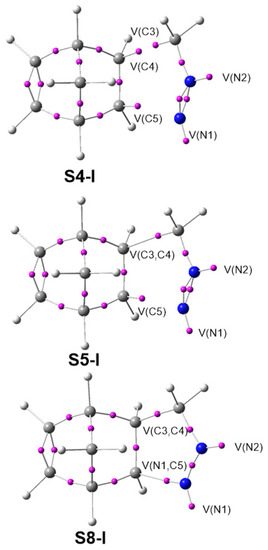
Figure 5.
ELF basin attractor positions of S4-I, S5-I and S8-I, involved in the formation of the C3–C4 and N1–C5 single bonds.
From the BET study of the 32CA reaction of simplest DAA 7 with NBD 11, we conclude that: (i) This 32CA reaction can be topologically divided into nine phases starting from structure S0-I and ending in pyrazoline 12; (ii) Phases I and II are characterized by the depopulation of the N1–N2, N2–C3 and C4–C5 bonding regions. These changes in electron density demand an energy cost (EC) of 15.4 kcal mol−1, which accounts for 97% of the activation energy needed to reach TS1, found in Phase III; (iii) the depopulation of these bonding regions creates the N2 nitrogen non-bonding electron density at structure S1-I, and the C4 and C5 pseudoradical centers at S3-I and S4-I, respectively (see S4-I in Figure 5); (v) formation of the first C3–C4 single bond takes place at S5-I, by the C-to-C coupling of the two C3 and C4 pseudoradical centers present in S4-I, at a C–C distance of 2.05 Å and with an initial population of 1.36 e (see S5-I in Figure 5). Note that the C3 pseudoradical center was already present at DAA 7; (vi) formation of the second N1–C5 single bond takes place at S8-I, by sharing part of the non-bonding electron density of the N1 nitrogen and that of the C5 pseudoradical center present in S7-I, at a C–N distance of 1.84 Å and with an initial population of 1.51 e (see S8-I in Figure 5); (vii) the formation of the second N1–C5 single bond begins when the first C3–C4 single bond formation has been completed by up to 93%, suggesting a two-stage one-step mechanism [73].
The BET study of the 32CA reaction of DAA 10 with NBD 11 draws some significant mechanistic conclusions: (i) This 32CA reaction can be divided into seven topological phases starting from S0-II and ending in pyrazoline 22; (ii) Phases I–III are characterized by the depopulation of the N1–N2, N2–C3 and C4–C5 bonding regions. These changes in electron density demand an EC of 20.9 kcal mol−1. This accounts for 90% of the activation energy needed to reach TS11, which is found in Phase IV; (iv) the depopulation of N1–N2 and N2–C3 bonding regions along Phase I creates the non-bonding electron density at N2 nitrogen at S1-II, while the depopulation of the C4–C5 bonding region along Phases I–III creates the C4 and C5 pseudoradical centers; (v) formation of the first C3–C4 single bond takes place at S5-II, by the C-to-C coupling of the two C3 and C4 pseudoradical centers present in S4-II, at a C–C distance of 2.11 Å, and with an initial population of 1.25 e. Note that the C3 pseudoradical center was already present at DAA 10; (vii) formation of the second N1–C5 single bond takes place at S6-II, by sharing part of the non-bonding electron density of the N1 nitrogen and that of the C5 pseudoradical center present in S5-I, at a C–N distance of 1.82 Å, and with an initial population of 1.38 e; (vii) the formation of second N1–C5 single bond begins when the first C3–C4 single bond formation has been completed by up to 70%, suggesting an asynchronous one-step mechanism.
From this comparative analysis of the two BET studies, we conclude: (i) These 32CA reactions follow an asynchronous one-step mechanism, the highest asynchronicity in the bond formation being observed for the most favorable 32CA reaction of simplest DAA 7 with NBD 11, which takes place via a two-stage one-step mechanism [73]. This finding confirms that any analysis based on the geometries of TSs, such as bond orders, is not suitable to assess the asynchronicity of a reaction; (ii) the initial phases of both 32CA reactions are related to the rupture of the N1–N2, N2–C3 and C4–C5 double bonds, demanded the formation of the N1, C3 and C5 non-bonding electron densities; (iii) while at the 32CA reaction involving simplest DAA 7 these bonding changes demand an EC of 15.4 kcal mol−1 with a GEDT of 0.10 e, at the 32CA involving DAA 10 these changes demand an EC of 20.9 kcal mol−1 with a GEDT of 0.01 e; and finally, (iv) formation of the C3–C4 single bond takes place before the N1–C5 bond formation, by coupling of two C3 and C4 pseudoradical centers. While the former is already present at the reagents, the latter is created along the reaction path. This behavior confirms the pmr-type mechanism [36].
3.6. ELF Study at the TSs Associated with the 32CA Reactions of DAAs 7–10 with NBD 11
A comparative topological analysis of the ELF of the TSs involved in the 32CA reactions of DAAs 7–10 with NBD 11 was performed. The ELF valence basin populations at TS1–TS14 associated with the 32CA reactions of DAAs 7–10 with NBD 11 are given in Table 5, while the ELF basin attractor positions of the syn/endo TSs, TS1, TS3, TS7, and TS11, are represented in Figure 6.

Table 5.
ELF valence basin populations, in average number of electrons e, at the MPWB1K/6-311G (d,p) optimized at the TSs associated with the 32CA reactions of DAAs 7–10 with NBD 11.
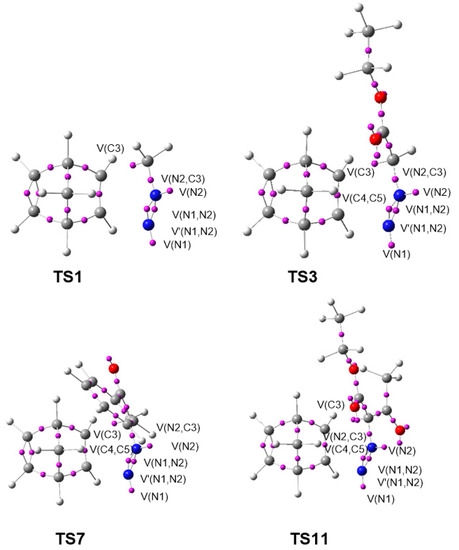
Figure 6.
ELF basin attractor positions at the most favorable endo/syn TSs, TS1, TS3, TS7, and TS11.
TS1–TS14 show the presence of a V(N2) monosynaptic basin integrating between 1.74 and 2.01 e, which is absent at the ground state electronic structures of the DAAs 7–10. The V(N1,N2) and V′(N1,N2) disynaptic basins are depopulated from 3.74 e at DAA 7 to 3.15 e at TS1 and 3.16 e at TS2, from 3.88 e at DAA 8 to 3.08–3.12 e at TS3–TS6, from 3.90 e at DAA 9 to 3.08–3.10 e at TS7–TS10, and from 3.97 e at DAA 10 to 3.01–3.07 e at TS11–TS14. On the other hand, the V(C3,N2) disynaptic basin integrating 3.01 e at DAA 7 is depopulated to 2.04 e at TS1 and 2.03 e at TS2, from 2.90 e at 8 to 2.00–2.04 e at TS3–TS6, from 2.88 e at 9 to 1.99–2.05 e at TS7–TS10, and from 2.82 e at 10 to 1.99–2.01 e at TS11–TS14. Thus, the N1–N2 and C3–N2 bonding regions are depopulated at the TSs to create the V(N2) monosynaptic basin associated with the N2 non-bonding electron density.
The V(C4,C5) and V′(C4,C5) disynaptic basins, integrating 3.48 e at NBD 11, are merged into one V(C4,C5) disynaptic basin, integrating 3.16–3.32 e at the TSs, due to the depopulation of that region.
As shown in Figure 6, the four TSs, TS1, TS3, TS7, and TS11, present an identical electronic structure. Only small differences in the basin populations are observed as a consequence of the different substitutions at the DAA framework (see Table 5).
The activation energies of the TSs is, therefore, associated with the EC for the formation of the N2 non-bonding electron density and the rupture of C4–C5 double bond of NBD 11. The TSs do not involve the formation of the new C3–C4 and N1–C5 single bonds, indicated by the absence of any V(C3,C4) and V(N1,C5) disynaptic basin. This is consistent with the distances between the C–C and N–C interacting centers, which are greater than 2 Å (see Figure 2) and the positive Laplacian of electron density at the bond critical points (BCPs) of the interatomic bonding regions at the TSs, discussed in Section 3.7.
3.7. Analysis of QTAIM Parameters at the TSs Associated with the 32CA Reactions of DAAs 7–10 with NBD 11
Bader and coworkers [46,47] proposed the QTAIM concept to reveal the nature of interatomic interactions. Accordingly, the total electron density ρ and the Laplacian of electron density are calculated at the BCPs CP1 and CP2 at the C3–C4 and N1–C5 bonding regions, respectively, and are given in Table 6. The total electron density accumulated at the BCPs is less than 0.1 a.u. and the Laplacian of electron density shows small positive values in TS1–TS14, suggesting rather non-covalent interactions. Thus, the formation of the new C–C and N–C covalent bonds has not started yet at the TSs, which is in agreement with the topological analysis of the ELF (see Section 3.6).

Table 6.
Total electron density, in average number of electrons e, ρ and Laplacian of electron density at the BCPs CP1 (C3–C4) and CP2 (N1–C5) of the interatomic bonding regions in TS1–TS14.
3.8. Analysis of the Origin of the Deceleration of These 32CA Reactions with the Increase of the EW Character of the Substituents Present in These DAA
Many MEDT studies of cycloaddition reactions have shown that the activation energies of non-polar and polar reactions are mainly associated with the EC demanded the depopulation, i.e., rupture, of the X–Y double bond regions involved in the reaction [36]. In polar reactions, this EC is decreased by the GEDT taking place along the reaction path [74]. The very low GEDT found at the TSs associated with the 32CA reactions of DAA 8–10, lower than 0.05 e (see Table 3), indicates that they have a non-polar character. Consequently, the corresponding activation energies can mainly be associated with the changes in electron density in the N1–N2–C3 and C4–C5 bonding regions. Analysis of the total population of the V(N1), V′(N1), V(N1,N2), V′(N1,N2), and V(C3,N2) valence basins of DAAs 7–10, 3.01 e 7, 2.90 e 8, 2.88 e 9, and 2.82 e 10, shows a continuous depopulation of the N1–N2–C3 bonding region of these TACs with the increase of the EW character of the substituents present at the C3 carbon (see Table 1).
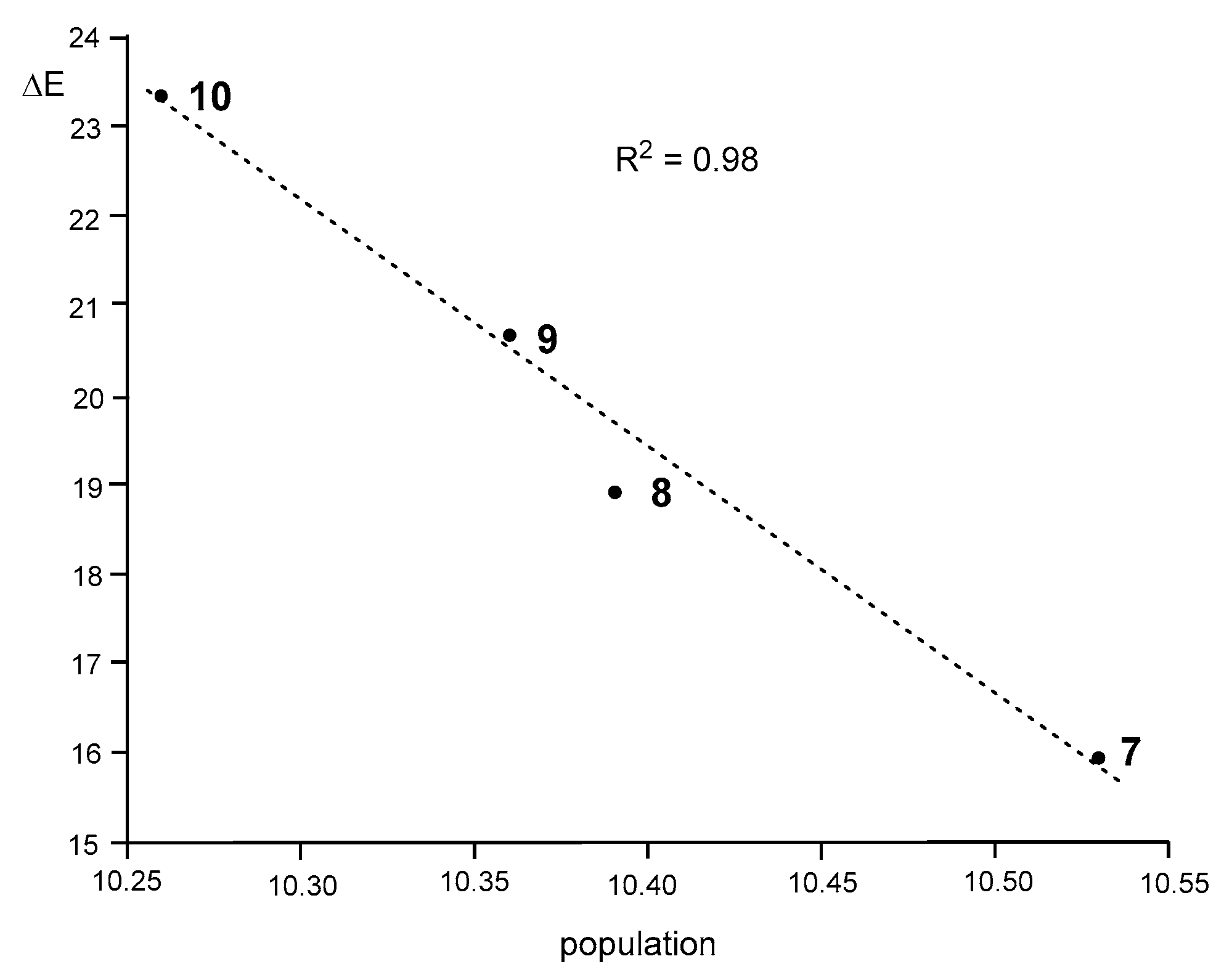
Interestingly, as Figure 7 shows, a very good correlation between the total populations of the V(N1), V′(N1), V(N1,N2), V′(N1,N2), and V(C3,N2) valence basins and the activation energies of these 32CA reactions can be established (R2 = 0.98).
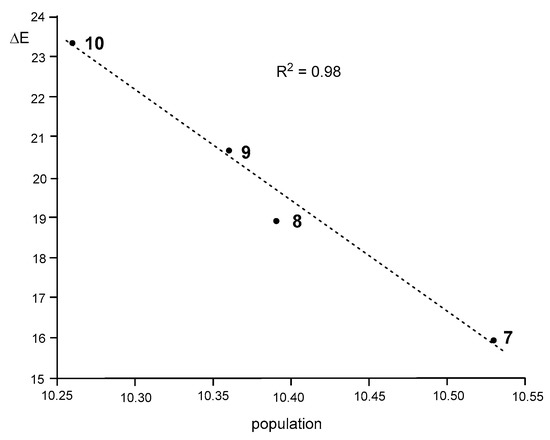
Figure 7.
A plot of the activation energies of the 32CA reactions between DAA 7–10 and NBD 11, ΔEact, in kcal·mol−1, vs. the total electron density population of the N1–N2–C3 bonding region of DAA 7–10, in average number of e.
As can be seen, the activation energies of this series of 32CA reactions linearly increase with the depopulation of the N1–N2–C3 bonding region, which could be understood as a drawback to using the electron density needed for the formation of the new bonds. Consequently, the lowest population found at the disubstituted DAA 10 provokes that the corresponding 32CA reaction presents the highest EC for the bonding changes, thus presenting the highest activation energy (see Section 3.3). Note that the chemical hardness of 10 is the highest (see Table 1). This finding justifies the observation that the 32CA reaction involving the most electrophilic species of this series does not take place experimentally.
4. Conclusions
The 32CA reactions of NBD 11 with simplest DAA 7 and three substituted DAAs 8–10 of increased electrophilic character, experimentally reported by Huisgen, [22] have been studied at MPWB1K/6-311G (d,p) level of theory within the MEDT framework.
Topological analysis of the ELF at the ground state electronic structures of DAAs 7–10 characterizes these TACs as pseudo(mono)radical ones, participating in pmr-type 32CA reactions.
Analysis of the CDFT indices predicts the strong nucleophilicity of NBD 11, while DAAs 7–10 are predicted to be electrophilic reagents. Contrary to the CDFT analysis, these pmr-type 32CA reactions experimentally show a decrease in reactivity with the introduction of EW substituents, the most electrophilic DAA 10 being completely inactive towards NBD 11.
These 32CA reactions are exergonic. The calculated activation enthalpies are found from 17.7 to 27.9 kcal mol−1 in acetonitrile. The 32CA reaction of simplest DAA 7, predicted as the least electrophilic DAA, with NBD 11, shows the lowest activation enthalpy, while the activation parameters increase progressively from the 32CA reaction with DAA 7 to that with DAA 10, in complete agreement with the experimental outcomes. The syn/endo attack is preferred over the anti/exo one in every case except DAA 9. An NCI analysis suggested that the weaker unfavorable interactions taking place at the corresponding anti/exo approach than at the syn/endo are responsible for that preference. Interestingly, the highest GEDT is found for the fastest 32CA reaction between DAA 7 and NBD 11, indicating a low polar FEDF process with electron density flux from DAA 7 to NBD 11, while the reaction with DAA 10, the most electrophilic species, is characterized as a NEDF process, contrary to the CDFT prediction.
BET study shows that the activation energies of the 32CA reactions of DAAs 7 and 10 with NBD 11 are mainly due to the rupture of the C4–C5 double bond of NBD 11 and the formation of non-bonding electron density at the N2 of the DAAs. The topological analysis of the ELF at the TSs and QTAIM parameters indicate that the formation of C3–C4 and N1–C5 covalent bonds has not started yet at any TS.
This MEDT study concludes that for the series of 32CA reactions of NBD 11 with substituted DAAs 7–10 of increased electrophilic character, the analysis based on CDFT indices does not account for the experimental outcomes. A good linear correlation between the populations of the N–N–C core of these DAAs and the activation energies associated with the corresponding pmr-type 32CA reactions is established, indicating that the depopulation of the N–N–C framework of the DAAs, considered an obstacle to the bond formation, is responsible for the experimentally found deceleration.
Supplementary Materials
The following are available online https://www.mdpi.com/2624-8549/3/1/6/s1.
Author Contributions
Conceptualization, L.R.D., M.R.-G. and N.A.; methodology, L.R.D., M.R.-G. and N.A.; software L.R.D., M.R.-G. and N.A.; validation, L.R.D., M.R.-G. and N.A.; formal analysis, L.R.D., M.R.-G. and N.A.; investigation L.R.D., M.R.-G. and N.A.; resources, L.R.D., M.R.-G. and N.A.; data curation, M.R.-G. and N.A.; writing—original draft preparation, M.R.-G. and N.A.; writing—review and editing, L.R.D., M.R.-G. and N.A..; visualization, L.R.D., M.R.-G. and N.A.; supervision, L.R.D.; project administration, L.R.D.; funding acquisition, L.R.D. All authors have read and agreed to the published version of the manuscript.
Funding
LRD is grateful to the Ministerio de Ciencias e Innovación (MICINN) of the Spanish Government for the project PID2019-110776GB-I00 (AEI/FEDER, UE). This project has also received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 846181 (MRG).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Flid, V.R.; Gringolts, M.L.; Shamsiev, R.S.; Finkelshtein, E.S. Norbornene, norbornadiene and their derivatives: Promising semi-products for organic synthesis and production of polymeric materials. Russ. Chem. Rev. 2018, 87, 1169. [Google Scholar] [CrossRef]
- Corey, E.J.; Shibasaki, M.; Nicolaou, K.C.; Malmsten, C.L.; Samuelsson, B. Simple, stereocontrolled total synthesis of a biologically active analog of the prostaglandin endoperoxides (PGH2, PGG2). Tetrahedron Lett. 1976, 17, 737–740. [Google Scholar] [CrossRef]
- Lee, M.; Ikeda, I.; Kawabe, T.; Mori, S.; Kanematsu, K. Enantioselective Total Synthesis of cis-Trikentrin B. J. Org. Chem. 1996, 61, 3406–3416. [Google Scholar] [CrossRef]
- Rosa, C.D.; Malafronte, A.; Auriemma, F.; Scoti, M.; Girolamo, R.D.; D’Alterio, M.C.; Ricci, G.; Zanchin, G.; Leone, G. Synthesis, chain conformation and crystal structure of poly(norbornadiene) having repeating 3,5-enchained nortricyclene units. Polym. Chem. 2019, 10, 4593–4603. [Google Scholar] [CrossRef]
- Petersen, A.U.; Hofmann, A.I.; Fillols, M.; Mansø, M.; Jevric, M.; Wang, Z.; Sumby, C.J.; Müller, C.; Moth-Poulsen, K. Solar Energy Storage by Molecular Norbornadiene–Quadricyclane Photoswitches: Polymer Film Devices. Adv. Sci. 2019, 6, 1900367. [Google Scholar] [CrossRef] [PubMed]
- Daştan, A.; Balci, M. High temperature bromination. Part 18: Bromination of benzonorbornadiene derivatives: Polybrominatedbenzonorbornenes and benzonorbornadienes. Tetrahedron 2005, 61, 5481–5488. [Google Scholar] [CrossRef]
- Horasan, N.; Kara, Y.; Azizoğlu, A.; Balci, M. Low and high temperature bromination of exocyclic dienes: High temperature bromination. Part 16. Tetrahedron 2003, 59, 3691–3699. [Google Scholar] [CrossRef]
- Altundas, R.; Dastan, A.; Ünaldi, N.S.; Güven, K.; Uzun, O.; Balci, M. The Di-π-methane Photorearrangement of 2,3-Disubstituted Benzobarrelenes and Benzonorbornadiene—Substituent Effects in Regioselectivity. Eur. J. Org. Chem. 2002, 526–533. [Google Scholar] [CrossRef]
- Nişancı, B.; Dalkılıç, E.; Güney, M.; Daştan, A. Synthesis and Diels–Alder cycloaddition reaction of norbornadiene and benzonorbornadiene dimers. Beilstein J. Org. Chem. 2009, 5, 39. [Google Scholar] [CrossRef]
- Khan, R.; Chen, J.; Fan, B. Versatile Catalytic Reactions of Norbornadiene Derivatives with Alkynes. Adv. Synth. Catal. 2020, 362, 1564. [Google Scholar] [CrossRef]
- Cristina, D.; Amici, M.D.; Micheli, C.D.; Gandolfi, R. Site selectivity in the reactions of 1,3-dipoles with norbornadiene derivatives. Tetrahedron 1981, 37, 1349–1357. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Roseblade, S.J.; Barrell, J.K.; Alexander, R. Highly stereoselective nitrone cycloaddition onto a chiral ketene equivalent: Asymmetric synthesis of cispentacin. Org. Lett. 2002, 4, 1227–1229. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, L.; Nino, A.D. Synthesis of isoxazolidines by 1, 3-dipolar cycloaddition: Recent advances. Targets Heterocycl. Syst. 2015, 19, 299–345. [Google Scholar]
- Rück-Braun, K.; Freysoldt, T.H.E.; Wierschem, F. 1,3-Dipolar cycloaddition on solid supports: Nitrone approach towards isoxazolidines and isoxazolines and subsequent transformations. Chem. Soc. Rev. 2005, 34, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Saeed, M.T.; Rahman, S.U. The isoxazolidines: A new class of corrosion inhibitors of mild steel in acidic medium. Corros. Sci. 2003, 45, 253–266. [Google Scholar] [CrossRef]
- Yip, C.; Handerson, S.; Tranmer, G.K.; Tam, W. Intramolecular 1,3-Dipolar Cycloadditions of Norbornadiene-Tethered Nitrile Oxides. J. Org. Chem. 2001, 66, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Tranmer, G.K.; Tam, W. Intramolecular 1,3-Dipolar Cycloadditions of Norbornadiene-Tethered Nitrones. J. Org. Chem. 2001, 66, 5113–5123. [Google Scholar] [CrossRef]
- Tajabadi, J.; Bakavoli, M.; Gholizadeh, M.; Eshghi, H.; Izadyar, M. The origin of regio- and stereoselectivity in the 1,3-dipolar cycloaddition of nitrile oxides with C1-substituted 7-oxabenzonorbornadienes, a DFT study. RSC Adv. 2015, 5, 38489–38498. [Google Scholar] [CrossRef]
- Adjieufack, A.I.; Ndassa, I.M.; Mbadcam, J.K.; Ríos-Gutiérrez, M.; Domingo, L.R. Steric interactions controlling the syn stereofacial selectivity in the [3 + 2] cycloaddition reaction between acetonitrile oxide and 7-oxanorborn-5-en-2-ones: A molecular electron density theory study. J. Phys. Org. Chem. 2017, 30, e3710. [Google Scholar] [CrossRef]
- Arhin, G.; Adams, A.H.; Opoku, E.; Tia, R.; Adei, E. 1,3-Dipolar cycloaddition reactions of selected 1,3-dipoles with 7-isopropylidenenorbornadiene and follow-up thermolytic cleavage: A computational study. J. Mol. Graph. Model. 2019, 92, 267–279. [Google Scholar] [CrossRef]
- Opoku, E.; Arhin, G.; Pipim, G.B.; Adams, A.H.; Tia, R.; Adei, E. Site-, enantio- and stereo-selectivities of the 1,3-dipolar cycloaddition reactions of oxanorbornadiene with C,N-disubstituted nitrones and dimethyl nitrilimines: A DFT mechanistic study. Theor. Chem. Acc. 2020, 139, 16. [Google Scholar] [CrossRef]
- Huisgen, R. Kinetics and mechanism of 1,3-dipolar cycloadditions. Angew. Chem., Int. Ed. 1963, 2, 633–696. [Google Scholar] [CrossRef]
- Huisgen, R. Altes und Neues über aliphatische Diazoverbindungen. Angew. Chem. 1955, 67, 439–463. [Google Scholar] [CrossRef]
- Fukui, K. Molecular Orbitals in Chemistry, Physics, and Biology; Academic Press: New York, NY, USA, 1964; p. 525. [Google Scholar]
- Domingo, L.R. Molecular electron density theory. Molecules. 2016, 21, 1319. [Google Scholar] [CrossRef]
- Domingo, L.R.; Acharjee, N. Molecular Electron Density Theory: A New Theoretical Outlook on Organic Chemistry. In Frontiers in Computational Chemistry; Ul-Haq, Z., Wilson, A.K., Eds.; Bentham and Science: Singapore, 2020; Volume 5, pp. 174–227. [Google Scholar] [CrossRef]
- Domingo, L.R.; Acharjee, N. Unravelling the strain-promoted [3 + 2] cycloaddition reactions of phenyl azide with cycloalkynes from the molecular electron density theory perspective. New J. Chem. 2020, 44, 13633–13643. [Google Scholar] [CrossRef]
- Domingo, L.R.; Acharjee, N. Unveiling the high reactivity of strained dibenzocyclooctyne in [3 + 2] cycloaddition reactions with diazoalkanes through the molecular electron density theory. J. Phys. Org. Chem. 2020, 33, e4100. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Molecular Electron Density Theory Study of the Reactivity and Selectivities in [3 + 2] Cycloaddition Reactions of C,N-Dialkyl Nitrones with Ethylene Derivatives. J. Org. Chem. 2018, 83, 2182–2197. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Acharjee, N. A Molecular Electron Density Theory Study of the Chemoselectivity, Regioselectivity, and Stereofacial Selectivity in the Synthesis of an Anticancer Spiroisoxazoline derived from α-Santonin. Molecules 2019, 24, 832. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Adjieufack, A.I.; Ndassa, I.M.; Nouhou, C.N.; Mbadcam, J.K. Molecular Electron Density Theory Study of Fused Regioselectivity in the Intramolecular [3 + 2] Cycloaddition Reaction of Nitrones. ChemistrySelect 2018, 3, 5412–5420. [Google Scholar] [CrossRef]
- Domingo, L.R.; Acharjee, N. [3 + 2] Cycloaddition Reaction of C-Phenyl-N-methyl Nitrone to Acyclic-Olefin-Bearing-Electron-Donating Substituent: A Molecular Electron Density Theory Study. Chemistryselect 2018, 3, 8373–8380. [Google Scholar] [CrossRef]
- Gutiérrez, M.R.; Nasri, L.; Nacereddine, A.K.; Djerourou, A.; Domingo, L.R. A molecular electron density theory study of the [3 + 2] cycloaddition reaction between an azomethine imine and electron deficient ethylenes. J. Phys. Org. Chem. 2018, 31, e3830. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Molecular Electron Density Theory Study of the Role of the Copper-Metallation of AzomethineYlides in [3 + 2] Cycloaddition Reactions. J. Org. Chem. 2018, 83, 10959–10973. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Acharjee, N. A molecular electron density theory study of the Grignard reagent mediated regioselective direct synthesis of 1,5-disubstituted-1,2,3-triazoles. J. Phys. Org. Chem. 2020, 33, e4062. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the mysteries of the [3 + 2] cycloaddition reactions. Eur. J. Org. Chem. 2019, 267–282. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the High Reactivity of the Azomethine Ylides in [3 + 2] Cycloaddition Reactions. Lett. Org. Chem. 2010, 7, 432–439. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. A Molecular Electron Density Theory Study of the Reactivity of Azomethine Imine in [3 + 2] Cycloaddition Reactions. Molecules. 2017, 22, 750. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R. The carbenoid-type reactivity of simplest nitrile imine from a molecular electron density theory perspective. Tetrahedron 2019, 75, 1961–1967. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Emamian, S. Understanding the domino reactions between 1-diazopropan-2-one and 1,1-dinitroethylene. A molecular electron density theory study of the [3 + 2] cycloaddition reactions of diazoalkanes with electron-deficient ethylenes. RSC Adv. 2017, 7, 15586–15595. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Krokidis, X.; Noury, S.; Silvi, B. Characterization of Elementary Chemical Processes by Catastrophe Theory. J. Phys. Chem. A 1997, 101, 7277–7282. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Bader, R.F.W.; Essén, H. The characterization of atomic interactions. J. Chem. Phys. 1984, 80, 1943–1960. [Google Scholar] [CrossRef]
- Schlegel, H.B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982, 3, 214–218. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Hybrid Meta Density Functional Theory Methods for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions: The MPW1B95 and MPWB1K Models and Comparative Assessments for Hydrogen Bonding and van der Waals Interactions. J. Phys. Chem. A 2004, 108, 6908–6918. [Google Scholar] [CrossRef]
- Hehre, W.J.; Radom, L.; Schleyer, P.V.R.; Pople, J. Ab initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher-order implicit algorithms. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef]
- Tomasi, J.; Persico, M. Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Simkin, B.I.A.; Sheikhet, I.I. Quantum Chemical and Statistical Theory of Solutions: A Computational Approach; Ellis Horwood: London, UK, 1995. [Google Scholar]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab Initio Study of Solvated Molecules: A New Implementation of the Polarizable Continuum Model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Ahrens, J.; Geveci, B.; Law, C. ParaView: An End-User Tool for Large Data Visualization; Visualization Handbook; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Ayachit, U. The ParaView Guide: A Parallel Visualization Application; Kitware: New York, NY, USA, 2015. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Boto, R.A.; Peccati, F.; Laplaza, R.; Quan, C.; Carbone, A.; Piquemal, J.P.; Maday, Y.; Contreras-García, J. NCIPLOT4: Fast, Robust, and Quantitative Analysis of Noncovalent Interactions. J. Chem. Theory Comput. 2020, 16, 4150–4158. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels–Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Jaramillo, P.; Domingo, L.R.; Chamorro, E.; Pérez, P. A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J. Mol. Struct. Theochem. 2008, 865, 68–72. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez., P. A molecular electron density theory study of the participation of tetrazines in aza-Diels–Alder reactions. RSC Adv. 2020, 10, 15394–15405. [Google Scholar] [CrossRef]
- Domingo, L.R.; Kula, K.; Ríos-Gutiérrez, M. Unveiling the Reactivity of Cyclic AzomethineYlides in [3 + 2] Cycloaddition Reactions within the Molecular Electron Density Theory. Eur. J. Org. Chem. 2020, 2020, 5938–5948. [Google Scholar] [CrossRef]
- Lane, J.R.; Contreras-García, J.; Piquemal, J.P.; Miller, B.; Kjaergaard, H.G. Are Bond Critical Points Really Critical for Hydrogen Bonding? J. Chem. Theory Comput. 2013, 9, 3263–3266. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Sáez, J.A.; Zaragozá, R.J.; Arnó, M. Understanding the Participation of Quadricyclane as Nucleophile in Polar [2σ + 2σ + 2π] Cycloadditions toward Electrophilic π Molecules. J. Org. Chem. 2008, 73, 8791–8799. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. How does the global electron density transfer diminish activation energies in polar cycloaddition reactions? A Molecular Electron Density Theory study. Tetrahedron 2017, 73, 1718–1724. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).