Neutral and Cationic Chelidonate Coordination Polymers with N,N′-Bridging Ligands
Abstract
1. Introduction
2. Results and Discussion
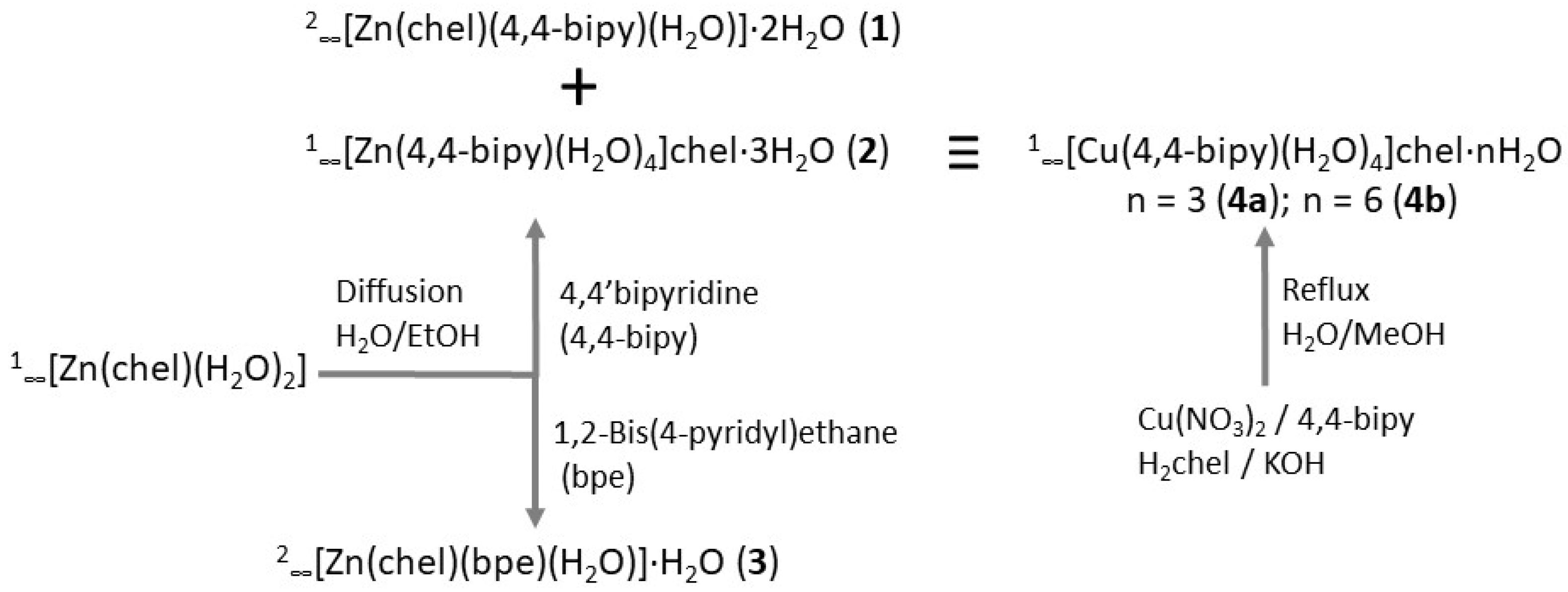
2.1. Preparation and Spectroscopic Characterisation of Complexes
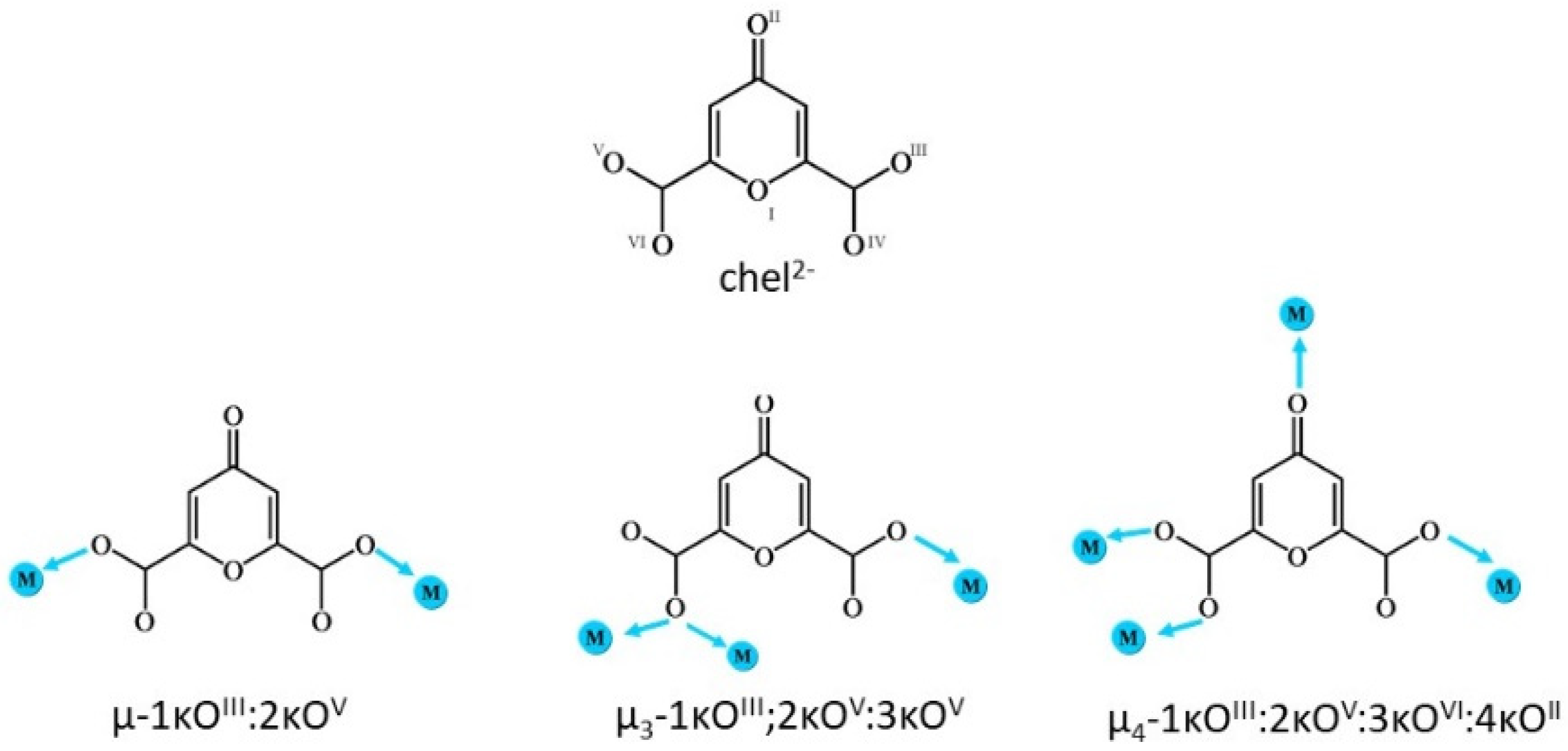
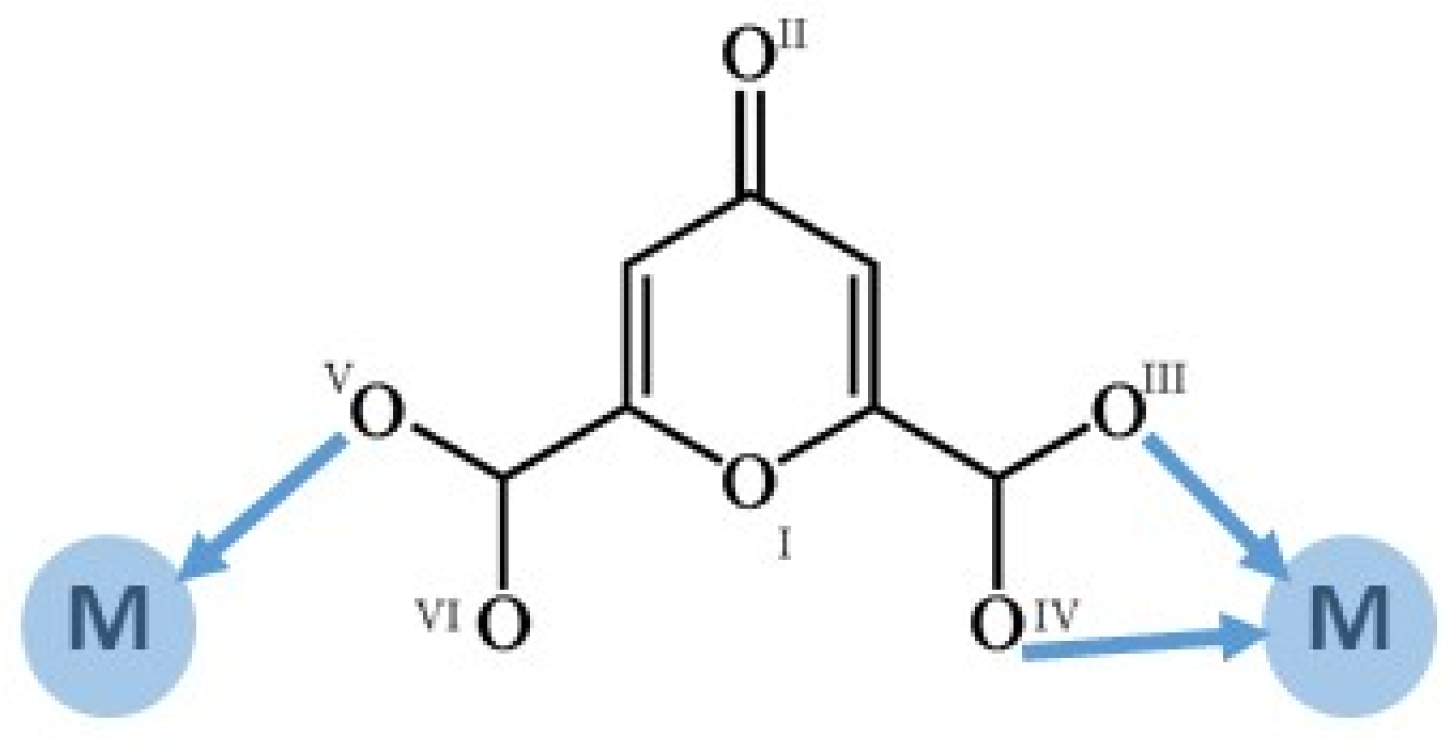
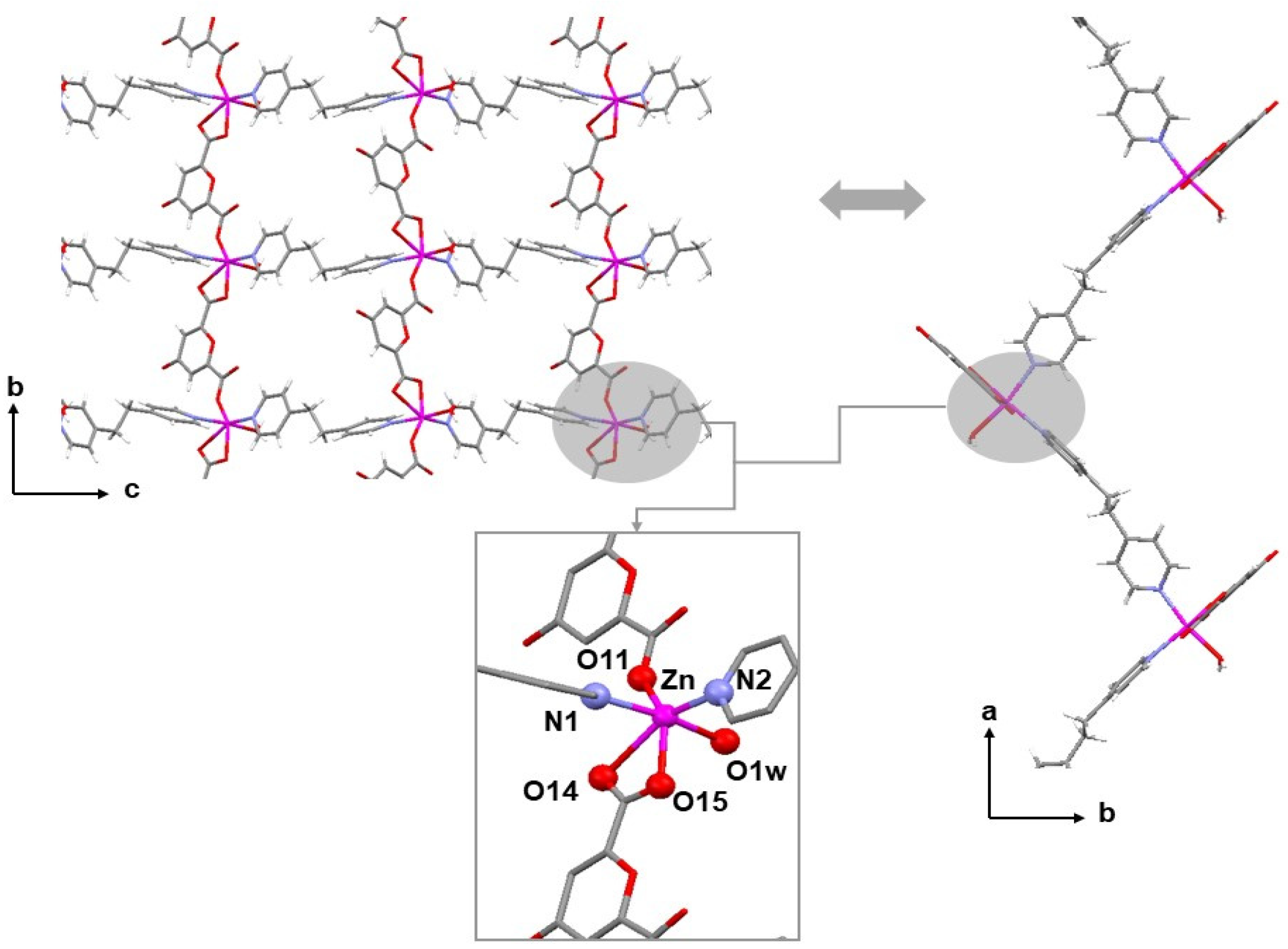
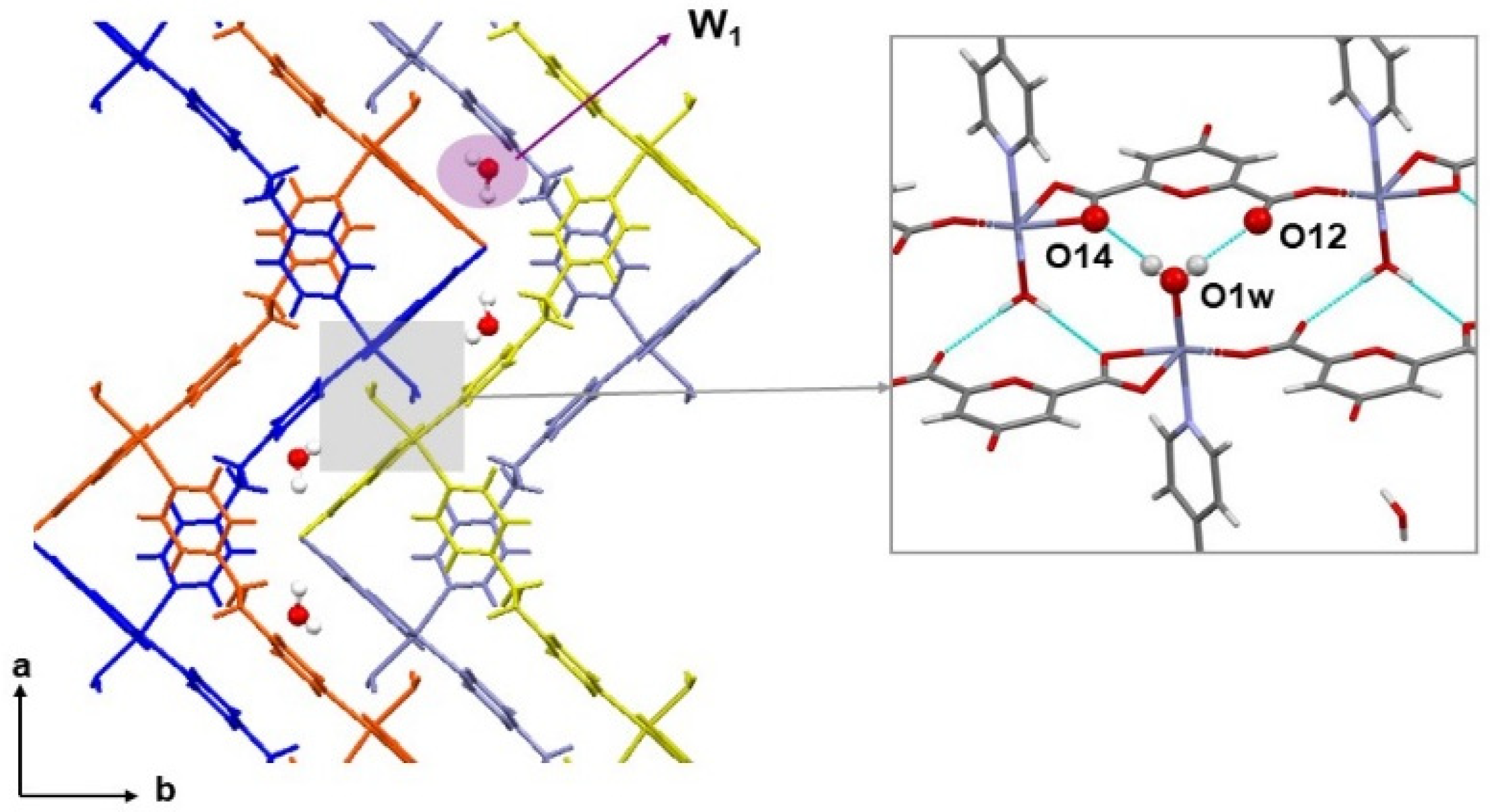
2.2. Structural Studies
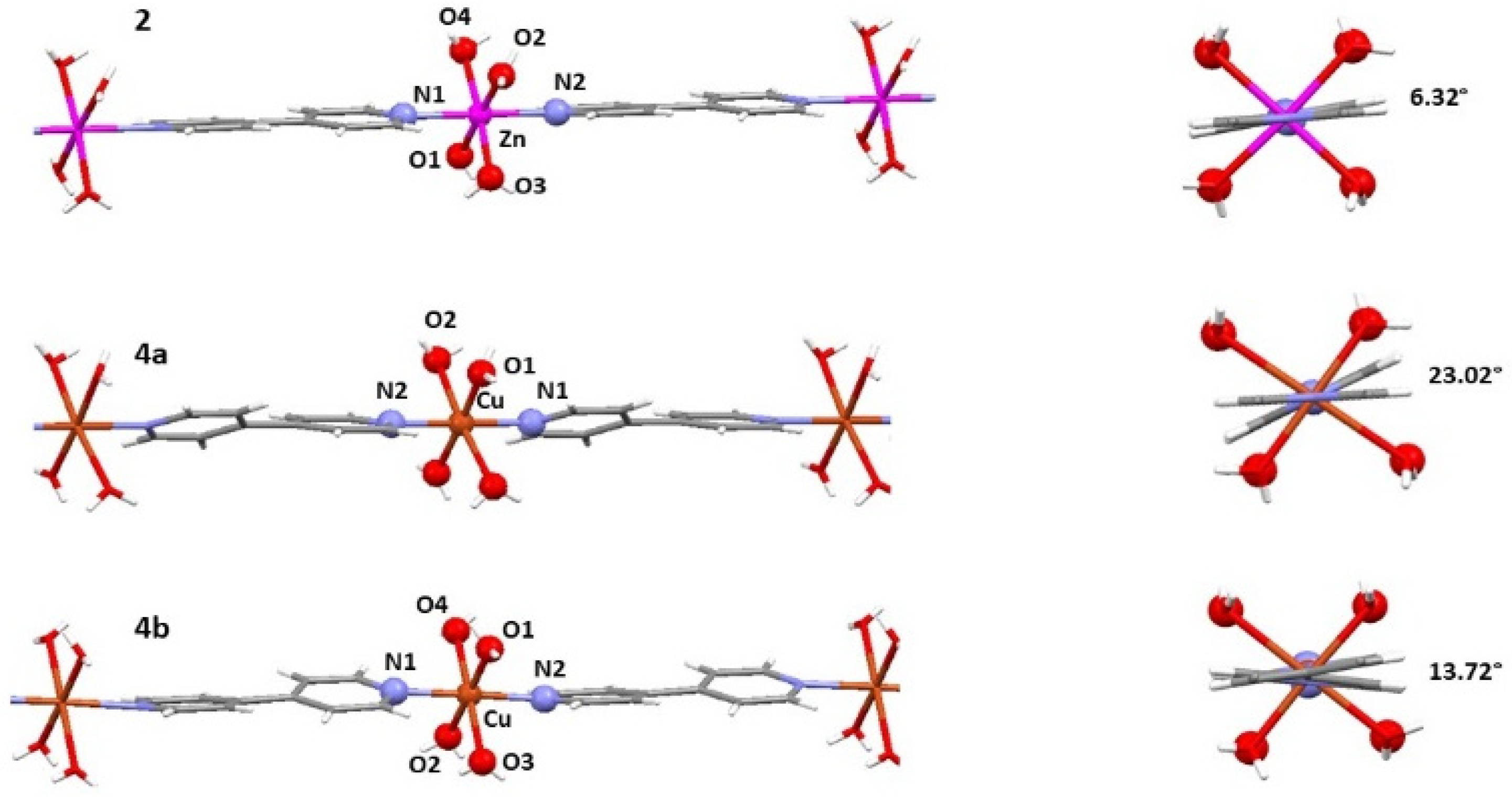
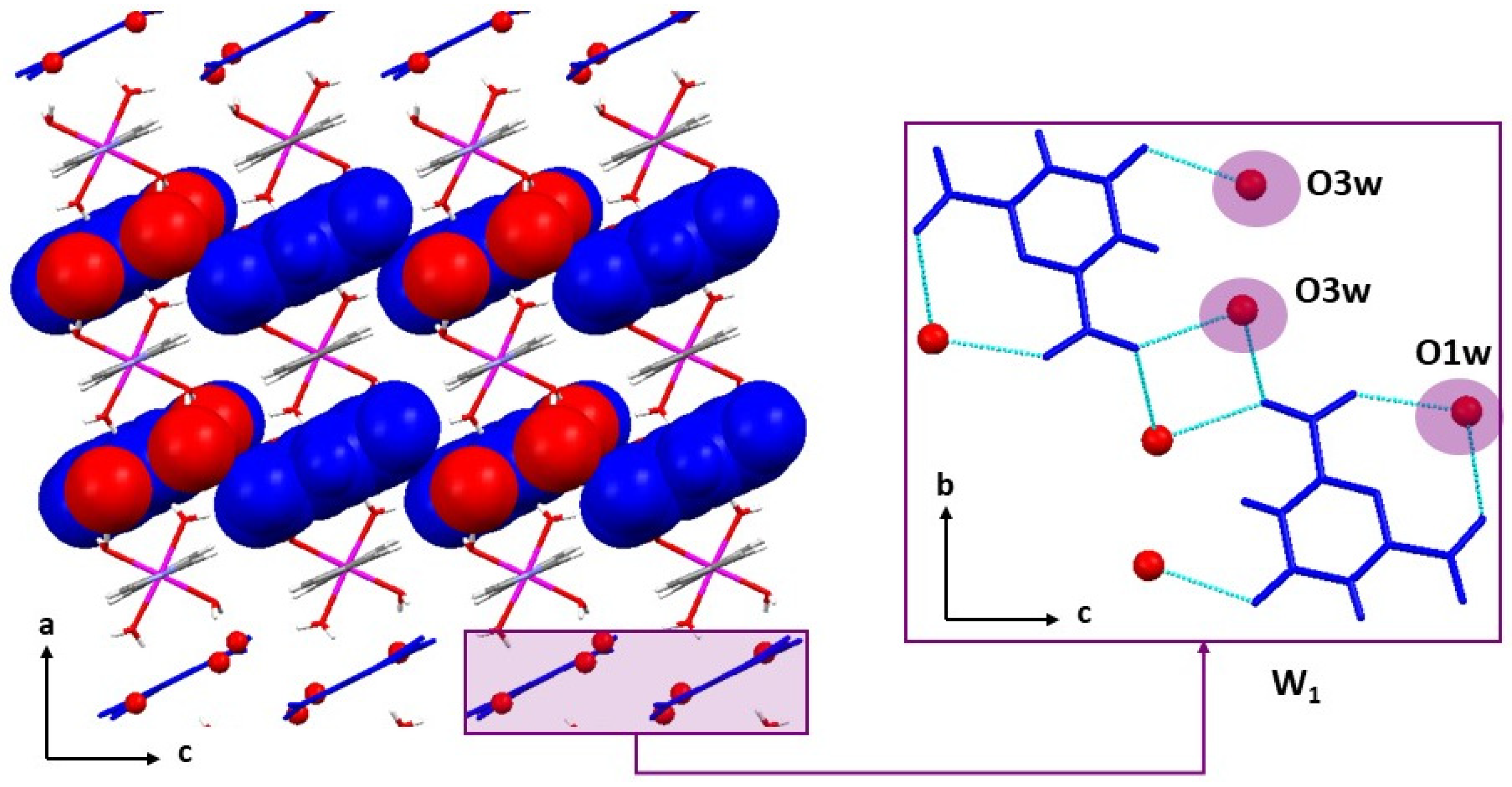
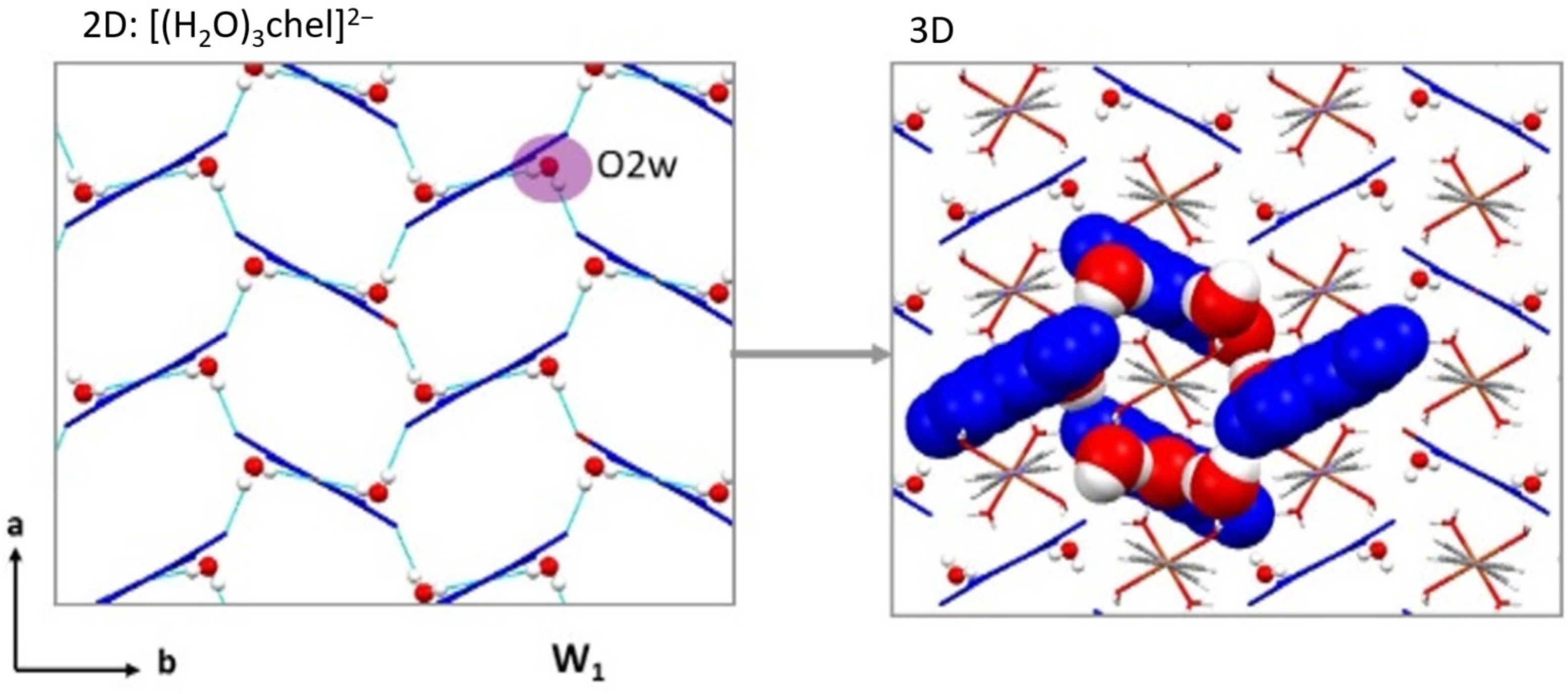
2.3. Crystal Structures of the Cationic Polymers 2, 4a and 4b
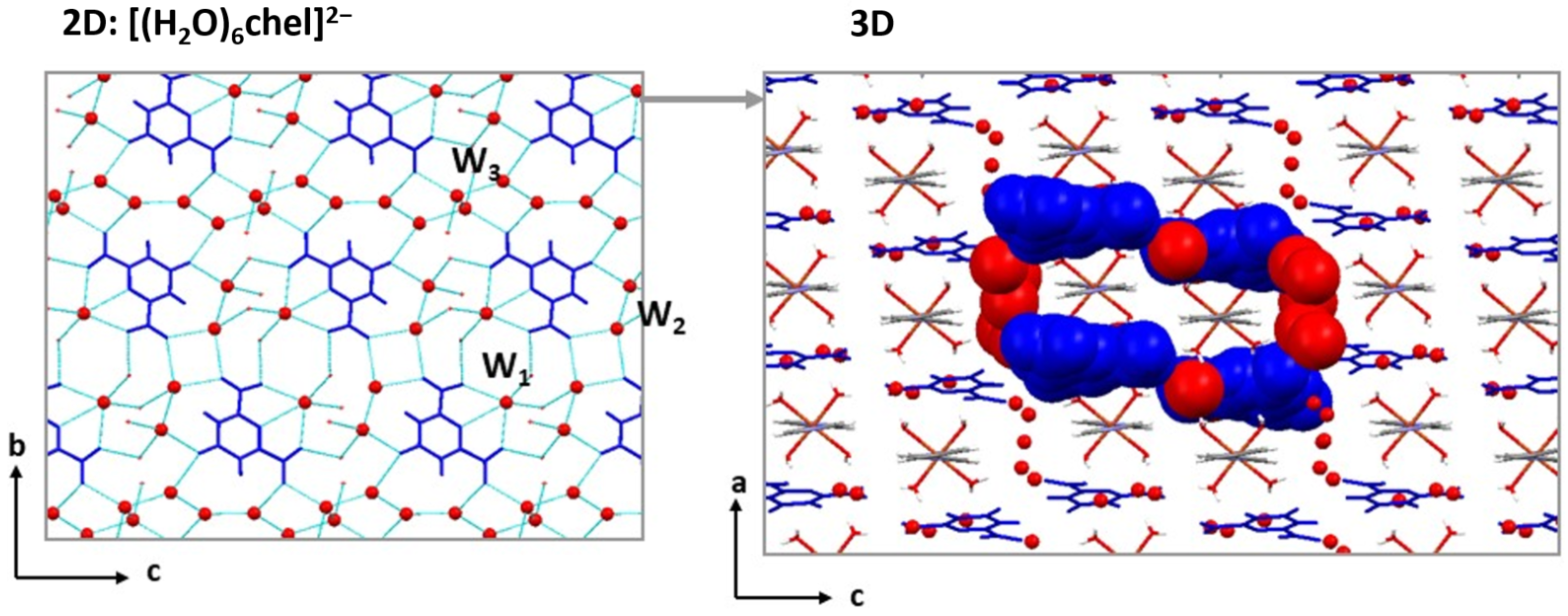
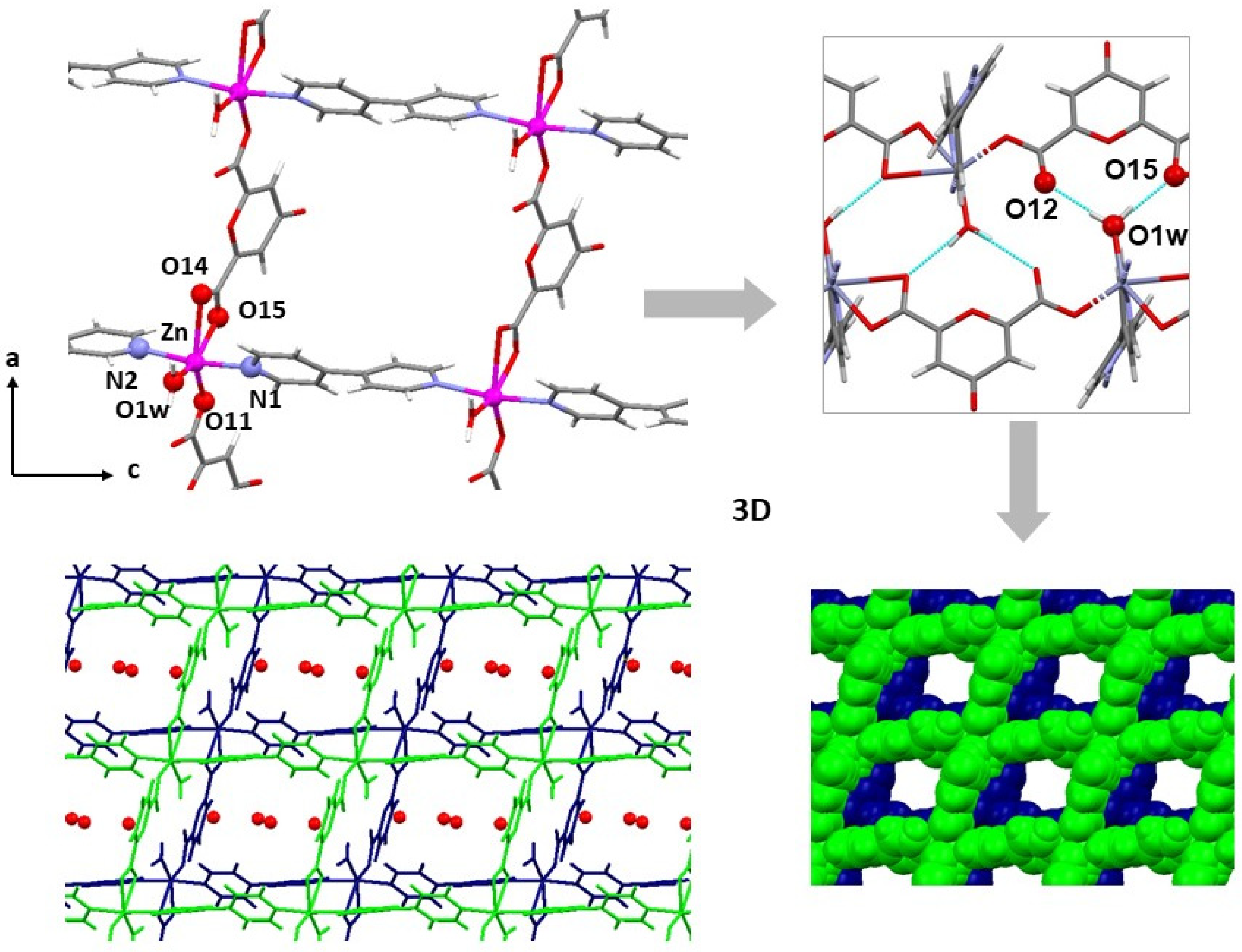
2.4. Crystal Structures of Neutral Polymers 1 and 3
3. Experimental
3.1. Materials and Physical Measurements
3.2. Synthesis of the Precursor 1∞[Zn(chel)(H2O)2]
3.3. Synthesis of the Complexes
3.3.1. 2∞[Zn(chel)(4,4-bipy)(H2O)]·2H2O (1)
3.3.2. 1∞[Zn(4,4-bipy)(H2O)4]chel·3H2O (2)
3.3.3. 2∞[Zn(chel)(bpe)(H2O)]·H2O (3)
3.3.4. 1∞[Cu(4,4-bipy)(H2O)4]chel·3H2O (4a) and 1∞[Cu(4,4-bipy)(H2O)4]chel·6H2O (4b)
3.4. Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kumar Singh, D.; Gulati, K.; Ray, A. Effects of chelidonic acid, a secondary plant metabolite, on mast cell degranulation and adaptive immunity in rats. Int. Immunopharmacol. 2016, 40, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Kim, S.-J.; Kim, M.-C.; Jeon, Y.-D.; Um, J.-Y.; Hong, S.-H. The Therapeutic Effect of Chelidonic Acid on Ulcerative Colitis. Biol. Pharm. Bull. 2012, 35, 666–671. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Yang, S.-Y.; Kim, H.-Y.; Kim, N.-R.; Jang, J.-B.; Kim, H.-M. Chelidonic acid evokes antidepressant-like effect through the up-regulation of BDNF in forced swimming test. Exp. Biol. Med. 2016, 241, 1559–1567. [Google Scholar] [CrossRef]
- Zhou, X.-X.; Liu, M.-S.; Lin, X.-M.; Fang, H.-C.; Chen, J.-Q.; Yang, D.-Q.; Cai, Y.-P. Construction of three low dimensional Zn(II) complexes based on different organic-carboxylic acids. Inorg. Chim. Acta 2009, 362, 1441–1447. [Google Scholar] [CrossRef]
- Yasodha, V.; Govindarajan, S.; Lowb, J.N.; Glidewell, C. Cationic, neutral and anionic metal(II) complexes derived from 4-oxo-4Hpyran-2,6-dicarboxylic acid (chelidonic acid). Acta Cryst. 2007, 63, m207–m215. [Google Scholar]
- Fainerman-Melnikova, M.; Clegg, J.K.; Pakchung, A.A.H.; Jensen, P.; Codd, R. Structural diversity of complexes between Cu(II) or Ni(II) and endocyclic oxygen- or nitrogen-containing ligands: Synthesis, X-ray structure determinations and circular dichroism spectra. Cryst. Eng. Comm. 2010, 12, 4217–4225. [Google Scholar] [CrossRef]
- Fang, M.; Chang, L.; Liu, X.; Zhao, B.; Zuo, Y.; Chen, Z. Fabrication and Properties of Eight Novel Lanthanide−Organic Frameworks Based on 4-Hydroxypyran-2,6-dicarboxylate and 4-Hydroxypyridine-2,6-dicarboxylate. Cryst. Growth Des. 2009, 9, 4006–4016. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Zhang, S.-Y.; Li, Y.; Niu, Z.; Shi, W.; Cheng, P. Systematic investigation of the lanthanide coordination polymers with γ-pyrone-2,6-dicarboxylic acid. Cryst. Eng. Comm. 2010, 12, 1809–1815. [Google Scholar] [CrossRef]
- Qu, B.-T.; Lai, J.-C.; Liu, S.; Liu, F.; Gao, Y.-D.; You, X.-Z. Cu- and Ag-Based Metal−Organic Frameworks with 4-Pyranone-2,6-dicarboxylic Acid: Syntheses, Crystal Structures, and Dielectric Properties. Cryst. Growth Des. 2015, 15, 1707–1713. [Google Scholar] [CrossRef]
- Yasodha, V.; Govindarajan, S.; Starosta, W.; Leciejewicz, J. New Metal–Organic Framework Solids Built from Barium and Isoelectronic Chelidamic and Chelidonic Acids. J. Chem. Crystallogr. 2011, 41, 1988–1997. [Google Scholar] [CrossRef]
- Eubank, J.F.; Kravtsov, V.C.; Eddaoudi, M. Synthesis of Organic Photodimeric Cage Molecules Based on Cycloaddition via Metal−Ligand Directed Assembly. J. Am. Chem. Soc. 2007, 129, 5820–5821. [Google Scholar] [CrossRef] [PubMed]
- Lago, A.B.; Carballo, R.; Fernández-Hermida, N.; Rodríguez-Hermida, S.; Vázquez-López, E.M. Coordination polymers with chelidonate (4-oxo-4H-pyran-2,6-dicarboxylate) anions and dmso: [Zn(chel)(dmso)2] and linkage isomers of [Co(chel)(dmso)(OH2)3]·H2O. J. Mol. Struct. 2011, 1003, 121–128. [Google Scholar] [CrossRef]
- Lago, A.B.; Carballo, R.; Fernández-Hermida, N.; Vázquez-López, E.M. Mononuclear discrete complexes and coordination polymers based on metal(II) chelidonate complexes with aromatic N,N-chelating ligands. CrystEngComm. 2011, 13, 941–951. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Proserpio, D.M.; Sironi, A. Extended networks via hydrogen bond cross-linkages of [M(bipy)] (M = Zn2+ or Fe2+; bipy = 4,4′-bipyridyl) linear co-ordination polymers. J. Chem. Soc. Dalton Trans. 1997, 1801–1804. [Google Scholar] [CrossRef]
- Lian, Z.-X.; Cai, J.; Chen, C.-H. A series of metal-organic frameworks constructed with arenesulfonates and 4,4′-bipy ligands. Polyhedron 2007, 26, 2647–2654. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.-G. Syntheses, structures, and supra-molecular assembles of zinc 4-sulfobenzoate complexes with chelating and/or bridging ligands. J. Mol. Struct. 2009, 931, 87–93. [Google Scholar] [CrossRef]
- Xiong, W.; Su, Y.; Zhang, Z.; Chen, Z.; Liang, F.; Wang, L. Synthesis and Crystal Structures of Two Metal Complexes Formed in the Solvothermal Decomposition Reactions of N-Carboxyphenylenesulfonyl-S-Carboxymethyl-l-Cysteine. J. Chem. Cryst. 2011, 41, 1510–1514. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, C.; Li, G.; Zhang, H.; Hou, H.; Li, L.; Wu, Q.; Zhu, Y.; Wang, E. New types of the flexible self-assembled metal–organic coordination polymers constructed by aliphatic dicarboxylates and rigid bidentate nitrogen ligands. Dalton Trans. 2004, 22, 3918–3925. [Google Scholar] [CrossRef]
- García, H.C.; Diniz, R.; Yoshida, M.I.; de Oliveira, L.F.C. Synthesis, structural studies and vibrational spectroscopy of Fe2+ and Zn2+ complexes containing 4,4′-bipyridine and barbiturate anion. J. Mol. Struct. 2010, 978, 79–85. [Google Scholar] [CrossRef]
- Colinas, R.; Rojas-Andrade, M.D.; Chakraborty, I.; Oliver, S.R.J. Two structurally diverse Zn-based coordination polymers with excellent antibacterial activity. CrystEngComm. 2018, 20, 3353–3362. [Google Scholar] [CrossRef]
- CSD Web Interface–Intuitive, Cross-Platform, Web-Based access to CSD Data; Cambridge Crystallographic Data Centre: Cambridge, UK, 2017.
- Wang, Y.; Feng, L.; Li, Y.; Hu, C.; Wang, E.; Hu, N.; Jia, H. Novel hydrogen-bonded three-dimensional networks encapsulating one-dimensional covalent chains: [M(4,4′-bipy)(H2O)4](4-abs)2.nH2O (4,4′-bipy = 4,4′-bipyridine; 4-abs = 4-aminobenzenesulfonate) (M = Co, n = 1; M = Mn, n = 2). Inorg. Chem. 2002, 41, 6351–6357. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.C.; Ghosh, A.K.; Zangrando, E.; Ray Chaudhuri, N. 3D supramolecular networks of Co(II)/Fe(II) using the croconate dianion and a bipyridyl spacer: Synthesis, crystal structure and thermal study. Polyhedron 2007, 26, 1105–1112. [Google Scholar] [CrossRef]
- Elahi, S.M.; Chand, M.; Deng, W.-H.; Pal, A.; Das, M.C. Polycarboxylate-Templated Coordination Polymers: Role of Templates for Superprotonic Conductivities of up to 10−1 S cm−1. Angew. Chem. Int. Ed. Engl. 2018, 57, 6662–6666. [Google Scholar] [CrossRef]
- Wang, X.-L.; Qin, C.; Wang, E.-B. Polythreading of Infinite 1D Chains into Different Structural Motifs: Two Poly(pseudo-rotaxane) Architectures Constructed by Concomitant Coordinative and Hydrogen Bonds. Cryst. Growth Des. 2006, 6, 439–443. [Google Scholar] [CrossRef]
- Kanoo, P.; Gurunatha, K.L.; Maji, T.K. Temperature-Controlled Synthesis of Metal-Organic Coordination Polymers: Crystal Structure, Supramolecular Isomerism, and Porous Property. Cryst. Growth Des. 2009, 9, 4147–4156. [Google Scholar] [CrossRef]
- García, H.C.; Diniz, R.; Yoshidab, M.I.; de Oliveira, L.F.C. An intriguing hydrogen bond arrangement of polymeric 1D chains of 4,4′-bipyridine coordinated to Co2+, Ni2+, Cu2+ and Zn2+ ions having barbiturate as counterions in a 3D network. CrystEng Comm. 2009, 11, 881–888. [Google Scholar] [CrossRef]
- Fei, H.; Oliver, S.R.J. Two cationic metal–organic frameworks based on cadmium and α,ω-alkanedisulfonate anions and their photoluminescent properties. Dalton Trans. 2010, 39, 11193–11200. [Google Scholar] [CrossRef]
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O- and N-donors: What they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Carballo, R.; Covelo, B.; García-Martínez, E.; Lago, A.B.; Vázquez-López, E.M. Role of inorganic and organic anions in the formation of metallosupramolecular assemblies of silver(I) coordination polymers with the twisted ligand bis (4-pyridylthio) methane. Polyhedron 2009, 5, 923–932. [Google Scholar] [CrossRef]
- Kitaigorodskii, A.I. Molecular Crystals and Molecules; Academic Press: New York, NY, USA, 1973. [Google Scholar]
- Siemens SAINT. Version 4, Software Reference Manual; Siemens Analytical X-Ray Systems, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXS-97 and SHELXL-97, Program for Crystal Structure Solution and Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Cryst. 1983, 39, 876–881. [Google Scholar] [CrossRef]
- Bruno, J.; Cole, J.C.; Edgington, P.R.; Kessler, M.K.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. MERCURY. New Software for Visualizing Crystal Structures. Acta Cryst. 2002, B58, 389. [Google Scholar] [CrossRef] [PubMed]
| 2 | 4a | 4b | |
|---|---|---|---|
| M–OH2 | 2.088(2)–2.105(2) | 1.952(2)–2.417(2) | 2.028(2)–2.291(2) |
| M–N | 2.174(2) 2.195(2) | 2.049(3) 2.031(3) | 2.093(3) |
| cis angles | 87.15(9)–93.49(9) | 87.30(7)–92.70(7) | 88.26(9)–91.70(9) |
| trans angles | 174.83(10)–178.58(8) | 174.59(15)–180.0 | 177.21(9)–179.90(10) |
| 1 | 3 | |
|---|---|---|
| Zn–O(1W) | 2.032(3) | 2.158(2) |
| Zn–Ochel | 2.015(3) 2.539(3) 2.130(3) | 1.978(2) 2.557(2) 2.085(2) |
| Zn–N | 2.151(4) 2.157(3) | 2.147(2) 2.084(2) |
| cis angles | 82.27(12)–116.83(12)° | 88.37(8)–97.42(8) |
| trans angles | 149.06(11)–169.87(14)° | 138.00(8)–169.69(9)° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carballo, R.; Lago, A.B.; Pino-Cuevas, A.; Gómez-Paz, O.; Fernández-Hermida, N.; Vázquez-López, E.M. Neutral and Cationic Chelidonate Coordination Polymers with N,N′-Bridging Ligands. Chemistry 2021, 3, 256-268. https://doi.org/10.3390/chemistry3010019
Carballo R, Lago AB, Pino-Cuevas A, Gómez-Paz O, Fernández-Hermida N, Vázquez-López EM. Neutral and Cationic Chelidonate Coordination Polymers with N,N′-Bridging Ligands. Chemistry. 2021; 3(1):256-268. https://doi.org/10.3390/chemistry3010019
Chicago/Turabian StyleCarballo, Rosa, Ana Belén Lago, Arantxa Pino-Cuevas, Olaya Gómez-Paz, Nuria Fernández-Hermida, and Ezequiel M. Vázquez-López. 2021. "Neutral and Cationic Chelidonate Coordination Polymers with N,N′-Bridging Ligands" Chemistry 3, no. 1: 256-268. https://doi.org/10.3390/chemistry3010019
APA StyleCarballo, R., Lago, A. B., Pino-Cuevas, A., Gómez-Paz, O., Fernández-Hermida, N., & Vázquez-López, E. M. (2021). Neutral and Cationic Chelidonate Coordination Polymers with N,N′-Bridging Ligands. Chemistry, 3(1), 256-268. https://doi.org/10.3390/chemistry3010019






