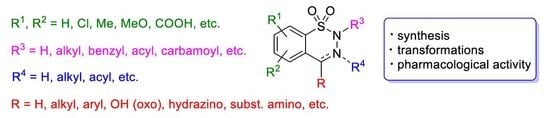
Synthesis and Chemistry of 1,2,3-Benzothiadiazine 1,1-Dioxide Derivatives: A Comprehensive Overview
Abstract
1. Introduction
2. Synthesis and Reactions of 4-Unsubstituted, 4-Aryl and 4-Alkyl Derivatives
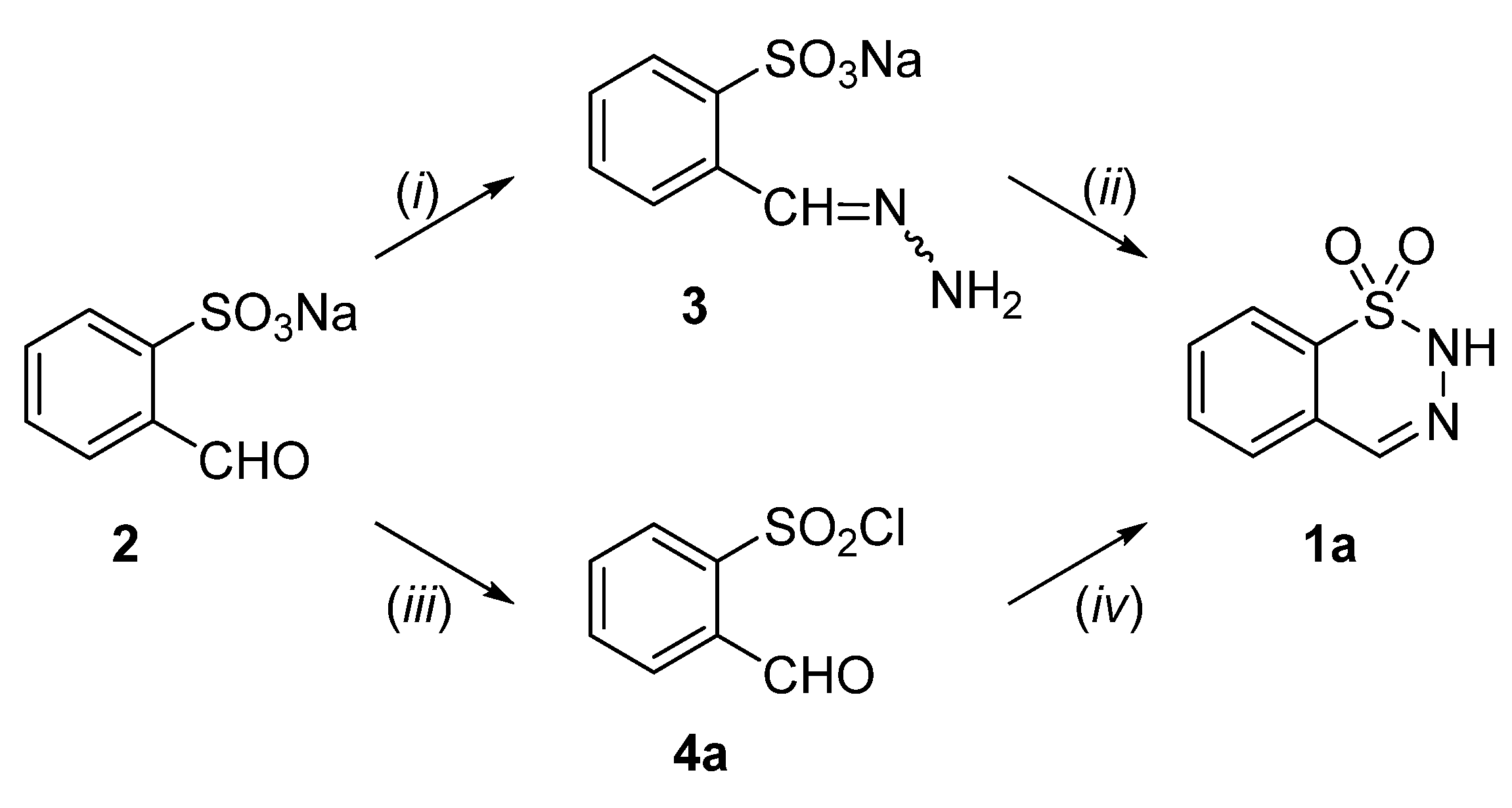
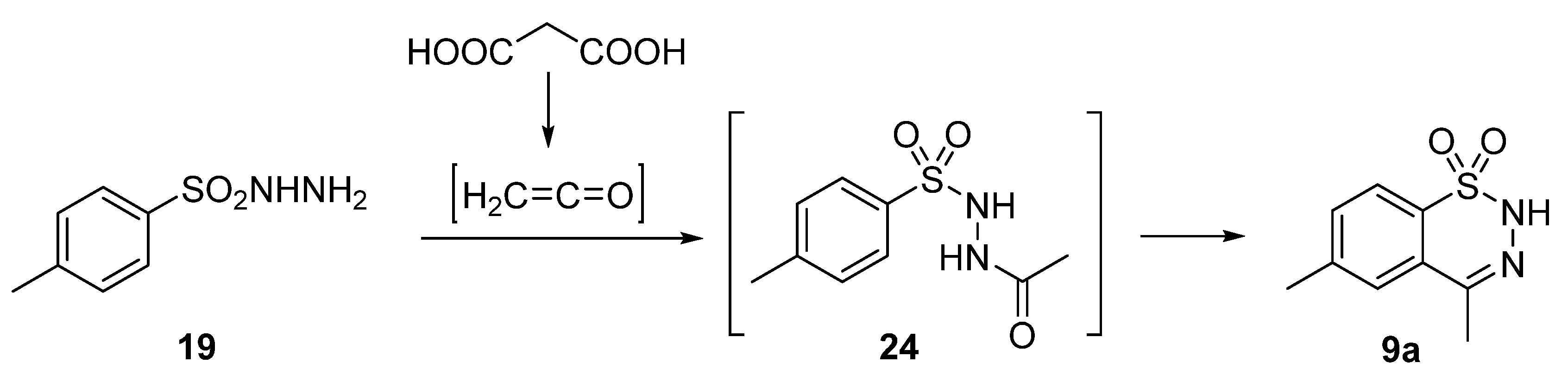
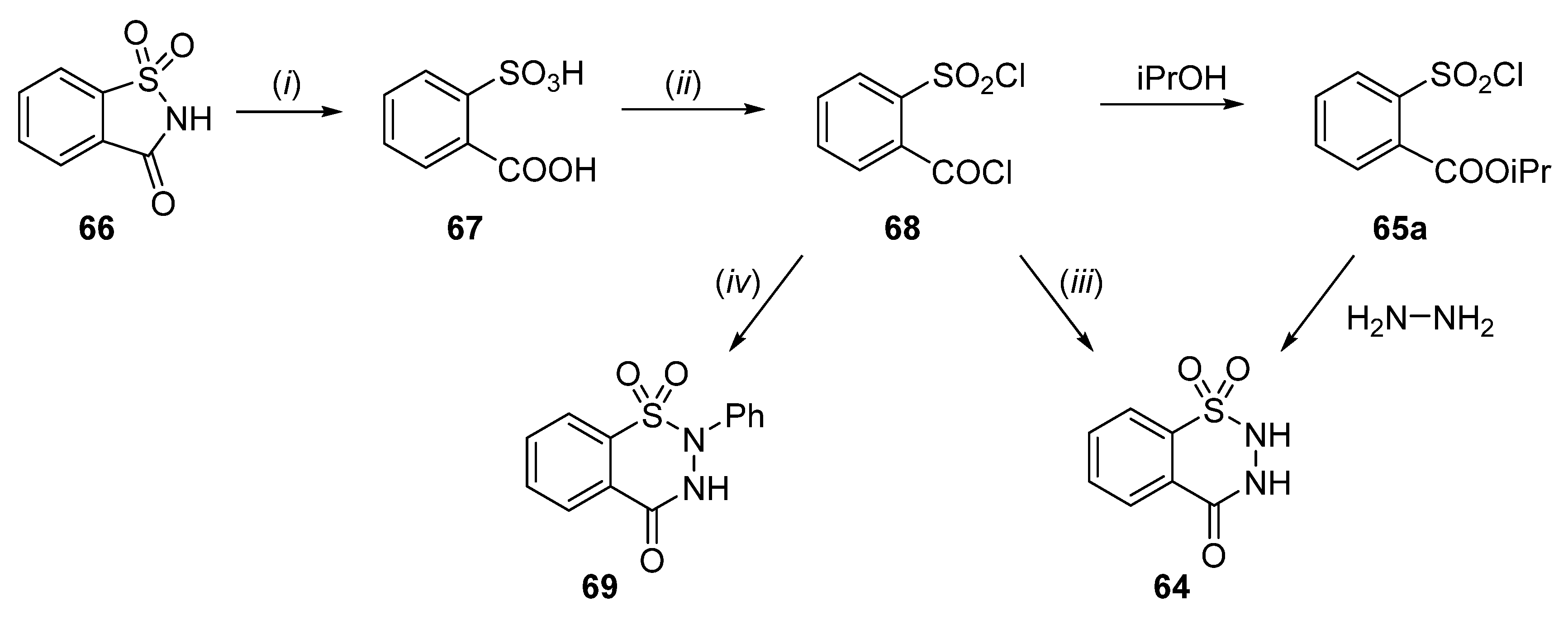
2.1. Synthesis of 4-Unsubstituted, 4-Aryl and 4-Alkyl Derivatives
2.2. Reactions of 4-Unsubstituted, 4-Aryl and 4-Alkyl Derivatives
2.2.1. Alkylations
2.2.2. Acylations and Carbamoylations
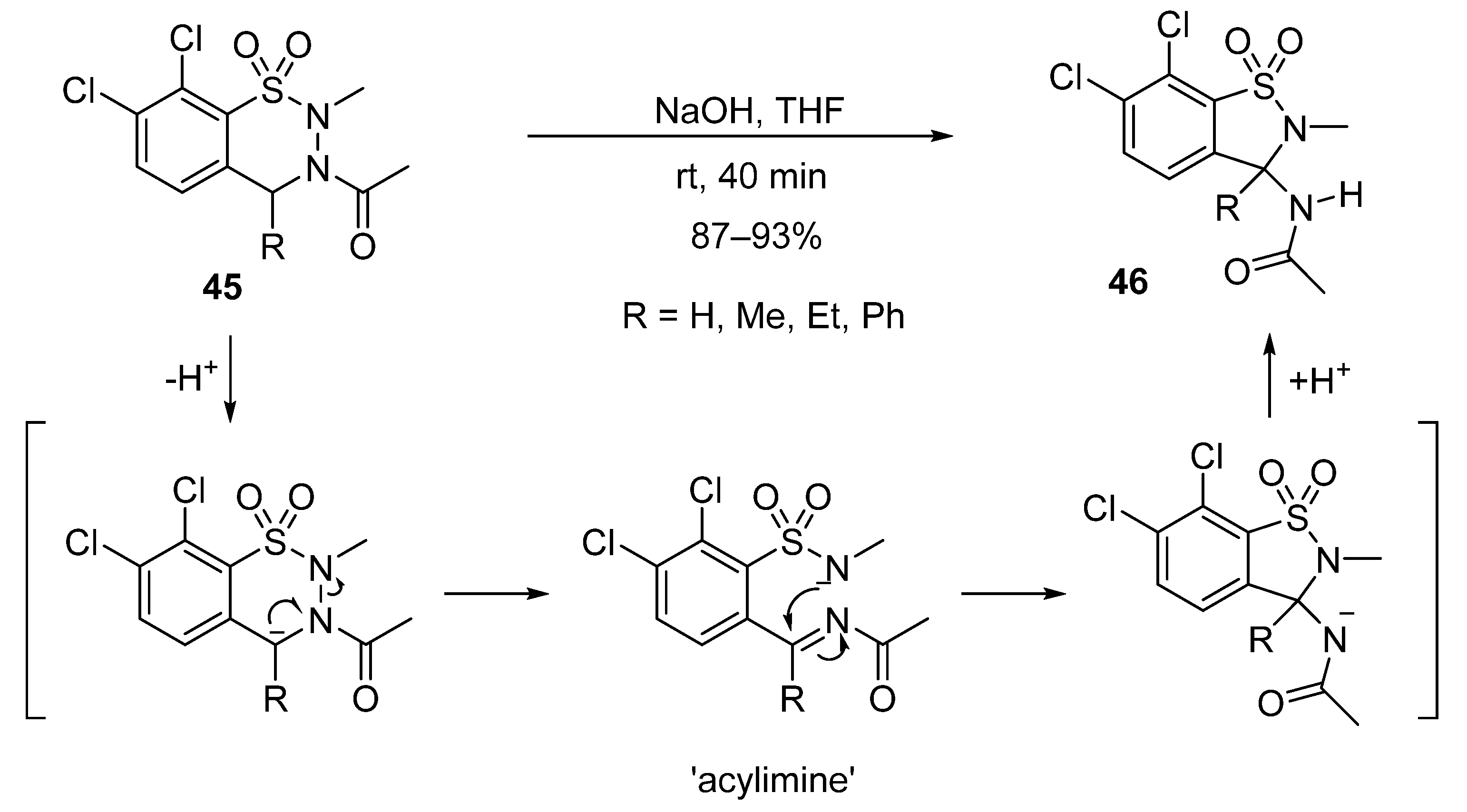
2.2.3. Reductions of the C=N Double Bond and Subsequent Alkylations and Acylations
2.2.4. Lithiation of 7,8-Dichloro- and 8-Chloro-7-methoxy-2H-1,2,3-benzothiadiazine 1,1-dioxides
2.2.5. Ring Contraction
2.2.6. Chlorination and Thermolysis of 2H-1,2,3-Benzothiadiazine 1,1-dioxide (1a)
3. Synthesis and Transformations of 4-Hydrazino-2H-1,2,3-benzothiadiazine 1,1-dioxides
4. Synthesis and Transformations of 1,2,3,4-Tetrahydro-1,2,3-benzothiadiazine-1,1,4-triones
5. Synthesis and Transformations of 4-Amino-2H-1,2,3-benzothiadiazine 1,1-dioxides
6. Conclusions
Funding
Conflicts of Interest
References
- Ernst, M.E.; Grimm, R.H., Jr. Thiazide Diuretics: 50 Years and Beyond. Curr. Hypertens. Rev. 2008, 4, 256–265. [Google Scholar] [CrossRef]
- Duarte, J.D.; Cooper-DeHoff, R.M. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics. Expert Rev. Cardiovasc. Ther. 2010, 8, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.W.; Traverso, J.J.; Sullivan, H.R.; Marshall, F.J.; Diuretics, V. 3,4-Dihydro-1,2,4-benzothiadiazine 1,1-Dioxides. J. Org. Chem. 1961, 26, 2814–2818. [Google Scholar] [CrossRef]
- Doyle, M.E.; Egan, J.M. Pharmacological Agents That Directly Modulate Insulin Secretion. Pharmacol. Rev. 2003, 55, 105–131. [Google Scholar] [CrossRef]
- Schroeder, H.A. The Effect of 1-Hydrasinophthalasine in Hypertension. Circulation 1952, 5, 28–37. [Google Scholar] [CrossRef]
- Cohn, J.N.; McInnes, G.T.; Shepherd, A.M. Direct-Acting Vasodilators. J. Clin. Hypertens. 2011, 13, 690–692. [Google Scholar] [CrossRef]
- Menear, K.A.; Adcock, C.; Boulter, R.; Cockcroft, X.L.; Copsey, L.; Cranston, A.; Dillon, K.J.; Drzewiecki, J.; Garman, S.; Gomez, S.; et al. 4-[3-(4-Cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: A Novel Bioavailable Inhibitor of Poly(ADP-ribose) Polymerase-1. J. Med. Chem. 2008, 51, 6581–6591. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Mylari, B.L.; Larson, E.R.; Beyer, T.A.; Zembrowski, W.J.; Aldinger, C.E.; Dee, M.F.; Siegel, T.W.; Singleton, D.H. Novel, potent aldose reductase inhibitors: 3,4-dihydro-4-oxo-3-[[5-(trifluoromethyl)-2-benzothiazolyl]methyl]-1-phthalazineacetic acid (zopolrestat) and congeners. J. Med. Chem. 1991, 34, 108–122. [Google Scholar] [CrossRef]
- Zhai, J.; Zhang, H.; Zhang, L.; Zhao, Y.; Chen, S.; Chen, Y.; Peng, X.; Li, Q.; Yuan, M.; Hu, X. Zopolrestat as a Human Glyoxalase I Inhibitor and its Structural Basis. ChemMedChem 2013, 8, 1462–1464. [Google Scholar] [CrossRef]
- McNeely, W.; Wiseman, L.R. Intranasal azelastine. A review of its efficacy in the management of allergic rhinitis. Drugs 1998, 56, 91–114. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.; Zieglmayer, U.P. Azelastine nasal spray for the treatment of allergic and nonallergic rhinitis. Expert Rev. Clin. Immunol. 2009, 5, 659–669. [Google Scholar] [CrossRef] [PubMed]
- King, J.F.; Hawson, A.; Deaken, D.M.; Komery, J. Synthesis and chlorinolysis of 2H-1,2,3-benzothiadiazine 1,1-dioxide. Chem. Commun. 1969, 1, 33–34. [Google Scholar] [CrossRef]
- King, J.F.; Huston, B.L.; Hawson, A.; Komery, J.; Deaken, D.M.; Harding, D.R.K. Synthesis and Thermolysis of 2H-1,2,3-Benzothiadiazine 1,1-Dioxide and 2,1-Benzoxathiin-3-one 1,1-Dioxide. Can. J. Chem. 1971, 49, 936–942. [Google Scholar] [CrossRef]
- J. R. Geigy & Co. Representation the Benzaldehyde-o-sulfonic Acid. German Patent Application DE88952, 25 February 1896. [Google Scholar]
- Chemische Fabrik vorm. Sandoz. Method for the Preparation of Sulfonic Acid of Benzaldehyde from Toluenesulfonic Acid. German Patent Application DE154528, 27 April 1902. [Google Scholar]
- Wright, J.B.; Kalamazoo, M. Upjohn Co. 2H-1,2,3-Benzothiadiazine 1,1-dioxides. Chem. Abstr. 1969, 70, 57914. [Google Scholar]
- Wright, J.B. The preparation of 2H-1,2,3-benzothiadiazine-1,1-dioxides, 11H-11a-dihydrobenzimidazo[1,2-b][1,2]benzisothiazole-5,5-dioxides, 6H-dibenzo[c,g][1,2,5] thiadiazocine-5,5-dioxides and 5H-dibenzo[c,g][1,2,6]thiadiazocine-6,6-dioxides. J. Heterocycl. Chem. 1968, 5, 453–459. [Google Scholar] [CrossRef]
- Wright, J.B.; Kalamazoo, M. Upjohn Co. 2H-1,2,3-Benzothiadiazine 1,1-dioxides. Chem. Abstr. 1969, 70, 28960. [Google Scholar]
- Nielsen, O.B.T.; Nielsen, C.K.; Feit, P.W. Aminobenzoic acid diuretics. 5. 3-Amino-4-arylmethyl-5-sulfamylbenzoic acid derivatives and related compounds. J. Med. Chem. 1973, 16, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Porcs-Makkay, M.; Lukács, G.; Pandur, A.; Simig, G.; Volk, B. Synthesis of 4-unsubstituted 2H-1,2,3-benzothiadiazine 1,1-dioxides via ortho lithiation of protected benzaldehyde derivatives. Tetrahedron 2014, 70, 286–293. [Google Scholar] [CrossRef]
- Lukács, G.; Porcs-Makkay, M.; Simig, G. Lithiation of 2-Aryl-2-(chloroaryl)-1,3-dioxolanes and Its Application in the Synthesis of New ortho-Functionalized Benzophenone Derivatives. Eur. J. Org. Chem. 2004, 20, 4130–4140. [Google Scholar] [CrossRef]
- Lukács, G.; Porcs-Makkay, M.; Simig, G. Lithiation of 2-(chloroaryl)-2-methyl-1,3-dioxolanes and application in synthesis of new ortho-functionalized acetophenone derivatives. Tetrahedron Lett. 2003, 44, 3211–3214. [Google Scholar] [CrossRef]
- Gyűjtő, I.; Porcs-Makkay, M.; Lukács, G.; Pusztai, G.; Garádi, Z.; Tóth, G.; Nyulasi, B.; Simig, G.; Volk, B. Synthesis of 4-methyl-2H-1,2,3-benzothiadiazine 1,1-dioxides and their further transformation via alkylation and reduction steps. Synth. Commun. 2019, 49, 3475–3485. [Google Scholar] [CrossRef]
- Lukács, G.; Porcs-Makkay, M.; Komáromi, A.; Simig, G. Microwave assisted synthesis of benzophenone and acetophenone ethylene ketals. Arkivoc 2008, iii, 17–24. [Google Scholar] [CrossRef]
- Porcs-Makkay, M.; Lukács, G.; Kapus, G.; Gacsályi, I.; Simig, G.; Lévay, G.; Mezei, T.; Végh, M.; Kertész, S.; Barkóczy, J.; et al. Egis Gyógyszergyár Nyrt.: Preparation of benzo[1,2,3]thiadiazine Derivatives for Treatment of CNS Disorders. Chem. Abstr. 2008, 148, 262627. [Google Scholar]
- Porcs-Makkay, M.; Gyűjtő, I.; Lukács, G.; Komáromi, A.; Tóth, G.; Garádi, Z.; Simig, G.; Volk, B. Synthesis, Alkylation and Reduction of 4-Aryl-2H-1,2,3-benzothiadiazine 1,1-dioxides. ChemistrySelect 2019, 4, 8295–8300. [Google Scholar] [CrossRef]
- Kacem, Y.; Hassine, B.B. A new procedure for the synthesis of 4-substituted-2H-1,2,3-benzothiadiazine 1,1-dioxides via directed ortho-lithiation of N1-arylsulfonylhydrazonates. Tetrahedron Lett. 2013, 54, 4023–4025. [Google Scholar] [CrossRef]
- Chandra, K.; Naoum, J.N.; Roy, T.K.; Gilon, C.; Gerber, R.B.; Friedler, A. Mechanistic studies of malonic acid-mediated in situ acylation. Biopolymers 2015, 104, 495–505. [Google Scholar] [CrossRef]
- Porcs-Makkay, M.; Lukács, G.; Kapus, G.; Gacsályi, I.; Simig, G.; Lévay, G.; Mezei, T.; Végh, M.; Kertész, S.; Barkóczy, J.; et al. Egis Gyógyszergyár Nyrt.: 3,4-Dihydrobenzo[1,2,3]thiadiazine-1,1-dioxide Derivatives, Process for Preparation Thereof, Medicaments Containing Said Derivatives and Their Use in Treating CNS disorders. Chem. Abstr. 2008, 148, 262626. [Google Scholar]
- Porcs-Makkay, M.; Kapiller-Dezsöfi, R.; Párkányi, L.; Pandur, A.; Simig, G.; Volk, B. Alkylation of 2H-1,2,3-benzothiadiazine 1,1-dioxides. Formation of a new family of mesoionic compounds. Tetrahedron 2014, 70, 2169–2174. [Google Scholar] [CrossRef]
- Aoki, K.; Sasatake, K.; Kimura, T.; Hatakeyama, N. Kureha Chemical Industry Co., Ltd.: Benzothiadiazine Dioxides as Bactericides and Fungicides. Chem. Abstr. 1975, 82, 165882. [Google Scholar]
- Aoki, K.; Sasatake, K.; Kimura, T.; Hataheyama, N. Kureha Chemical Industry Co., Ltd.: 2-Substituted-1,2,3-benzothiadiazine 1,1-dioxides as Fungicides. Chem. Abstr. 1975, 82, 120070. [Google Scholar]
- Ratcliffe, R.W.; Waddell, S.T.; Morgan, J.D., II; Blizzard, T.A. Merck and Co. Inc.: Preparation of Heterocyclic Substituted Carbapenem Antibacterials. Chem. Abstr. 1999, 132, 64104. [Google Scholar]
- Aoki, K.; Kimura, T.; Satake, K.; Yamazaki, S. Kureha Chemical Industry Co., Ltd.: 2-(N-Alkylcarbamoyl)-1,2,3-benzothiadiazine 1,1-dioxide for Rice Blast Control. Chem. Abstr. 1974, 80, 141813. [Google Scholar]
- Aoki, K.; Sasatake, K.; Kimura, T.; Yamazaki, S. Kureha Chemical Industry Co., Ltd.: Benzothiadiazine Dioxides as Bactericides and Fungicides. Chem. Abstr. 1975, 82, 150500. [Google Scholar]
- Aoki, K.; Sasatake, K.; Kimura, T.; Yamazaki, S. Kureha Chemical Industry Co., Ltd.: Benzothiadiazine Dioxides as Bactericides and Fungicides. Chem. Abstr. 1975, 82, 150499. [Google Scholar]
- Porcs-Makkay, M.; Pandur, A.; Simig, G.; Volk, B. Consecutive alkylation–reduction reactions of 2H-1,2,3-benzothiadiazine 1,1-dioxide derivatives. Synthesis of 2-alkyl-, 3-alkyl-, and 2,3-dialkyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxides. Tetrahedron 2015, 71, 44–50. [Google Scholar] [CrossRef]
- Porcs-Makkay, M.; Gyűjtő, I.; Simig, G.; Volk, B. Synthesis and base-mediated rearrangement of 3-acetyl-2-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxides. Tetrahedron 2016, 72, 8463–8469. [Google Scholar] [CrossRef]
- King, J.F.; Hawson, A.; Huston, B.L.; Danks, L.J.; Komery, J. Chlorination of Heterocyclic and Acyclic Sulfonhydrazones. Can. J. Chem. 1971, 49, 943–955. [Google Scholar] [CrossRef]
- Schrader, E. Über Hydrazide und Azide von Sulfocarbonsäuren III. Die Einwirkung von Hydrazin auf o-Cyanbenzolsulfochlorid. J. Prakt. Chem. 1917, 96, 180–185. [Google Scholar] [CrossRef][Green Version]
- Robertson, J.E.; Biel, J.H. Colgate-Palmolive Co.: 4-Hydrazino-1,2,3-benzothiadiazine 1,1-dioxides. Chem. Abstr. 1965, 62, 9171. [Google Scholar]
- Goudal, M.; Goudal, A.; Vernadeau, P.; Vernadeau, J. 1-Hydrazino-4-thiaphthalazine 4,4-dioxide. Chem. Abstr. 1964, 60, 45789. [Google Scholar]
- CIBA Ltd. 1,1-Dioxides of 4-hydrazino-7-substituted-2H-1,2,3-benzothiadiazines. Chem. Abstr. 1962, 57, 62827. [Google Scholar]
- Severs, W.B.; Kinnard, W.J.; Buckley, J.P. Evaluation of certain hypotensive agents VII. Tetramethylpiperidine and benzothiadiazinate derivatives. J. Pharm. Sci. 1965, 54, 1025–1029. [Google Scholar] [CrossRef]
- Schmidt, P.; Eichenberger, K.; Wilhelm, M. Heilmittelchemische Studien in der heterocyclischen Reihe. 31. Mitteilung. Benzo-1,2,3-thiadiazine mit hypotensiver und diuretischer Wirkung. Helv. Chim. Acta 1962, 45, 996–999. [Google Scholar] [CrossRef]
- Edlin, A.I.; Kinnard, W.J.; Vogin, E.E.; Buckley, J.P. Hypotensive activity of two benzothiadiazine derivatives. J. Pharm. Sci. 1965, 54, 20–24. [Google Scholar] [CrossRef]
- CIBA Ltd. 1-Heterobicyclo-4-piperazinoalkanopyrazoles. Chem. Abstr. 1969, 70, 57898. [Google Scholar]
- Arya, V.P.; Grewal, R.S.; Kaul, C.L.; Ghate, S.P.; Mehta, D.V.; George, T. Antihypertensive Agents II: Synthesis and Hypotensive Activity of Certain 1,4,5-Trisubstituted Pyrazoles. J. Pharm. Sci. 1969, 58, 432–440. [Google Scholar] [CrossRef]
- Robertson, J.E.; Dusterhoft, D.A.; Mitchell, T.F., Jr. Diuretics. 6-Substituted 3-Ketoalkyl-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-Dioxides and Related Anils, Oximes, and Hydrazones. J. Med. Chem. 1965, 8, 90–95. [Google Scholar] [CrossRef]
- Loev, B.; Kormendy, M. 2-Sulfobenzoic Acid Esters. I. 2-Sulfamyl Derivatives. J. Org. Chem. 1962, 27, 1703–1709. [Google Scholar] [CrossRef]
- Ramana, P.V.; Reddy, A.R. Synthesis of a few cyclothiadiazanones and aminosulfonyl benzamides from saccharin. J. Sulfur Chem. 2010, 31, 71–81. [Google Scholar] [CrossRef]
- Bennani, Y.; Tumey, L.N.; Gleason, E.A.; Robarge, M.J. Athersys, Inc.: Indole acetic Acids Exhibiting CRTH2 Receptor Antagonism and Uses Thereof. Chem. Abstr. 2006, 144, 350540. [Google Scholar]
- Cunsheng, L.; Mei, Z.; Chengxin, Z.; Qing, L.; Yu, S.; Baohua, H.; Yinbo, Z. CECEP Valiant Co., Ltd.: Chinese Patent Application CN108640932, 12 October 2018. Chem. Abstr. 2018, 170, 18122. [Google Scholar]
- Nabar, U.V.; Mayadeo, M.S.; Deodhar, K.D. Reactions of 1,2-benzisothiazol-3-one and 3-thione 1,1-dioxide-N-acetic esters with hydrazine: Unusual formation of 4-(substituted amino)-1,2,3-benzothiadiazine 1,1-dioxides. Indian J. Chem. B 1988, 27B, 109–111. [Google Scholar] [CrossRef]
- Sadana, G.S.; Pradhan, N.S.; Deodhar, K.D. Antibacterial activity and syntheses of 4-(substituted benzylamino)-1,2,3-benzothiadiazine 1,1-dioxides. Indian J. Chem. B 1990, 29B, 598–599. [Google Scholar] [CrossRef]
- Kelley, M.J.; Nakagawa, K.; Dent, B.R. United States Dept. of Health and Human Services: Cyclin-Dependent Kinase (cdk)4 Inhibitors and Their Use for Treating Cancer. Chem. Abstr. 1998, 130, 483. [Google Scholar]
- Kubo, A.; Nakagawa, K.; Varma, R.K.; Conrad, N.K.; Cheng, J.Q.; Lee, W.C.; Testa, J.R.; Johnson, B.E.; Kaye, F.J.; Kelley, M.J. The p16 Status of Tumor Cell Lines Identifies Small Molecule Inhibitors Specific for Cyclin-dependent Kinase 4. Clin. Cancer Res. 1999, 5, 4279–4286. [Google Scholar] [PubMed]
- Sadana, G.S.; Pradhan, N.S.; Deodhar, K.D. Potential antimicrobial agents—synthesis of 2,4-disubstituted-1,2,3-benzothiadiazine-1,1-dioxides. Indian Drugs 1991, 28, 259–261. [Google Scholar]



























© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyűjtő, I.; Simig, G.; Porcs-Makkay, M.; Volk, B. Synthesis and Chemistry of 1,2,3-Benzothiadiazine 1,1-Dioxide Derivatives: A Comprehensive Overview. Chemistry 2020, 2, 674-690. https://doi.org/10.3390/chemistry2030043
Gyűjtő I, Simig G, Porcs-Makkay M, Volk B. Synthesis and Chemistry of 1,2,3-Benzothiadiazine 1,1-Dioxide Derivatives: A Comprehensive Overview. Chemistry. 2020; 2(3):674-690. https://doi.org/10.3390/chemistry2030043
Chicago/Turabian StyleGyűjtő, Imre, Gyula Simig, Márta Porcs-Makkay, and Balázs Volk. 2020. "Synthesis and Chemistry of 1,2,3-Benzothiadiazine 1,1-Dioxide Derivatives: A Comprehensive Overview" Chemistry 2, no. 3: 674-690. https://doi.org/10.3390/chemistry2030043
APA StyleGyűjtő, I., Simig, G., Porcs-Makkay, M., & Volk, B. (2020). Synthesis and Chemistry of 1,2,3-Benzothiadiazine 1,1-Dioxide Derivatives: A Comprehensive Overview. Chemistry, 2(3), 674-690. https://doi.org/10.3390/chemistry2030043