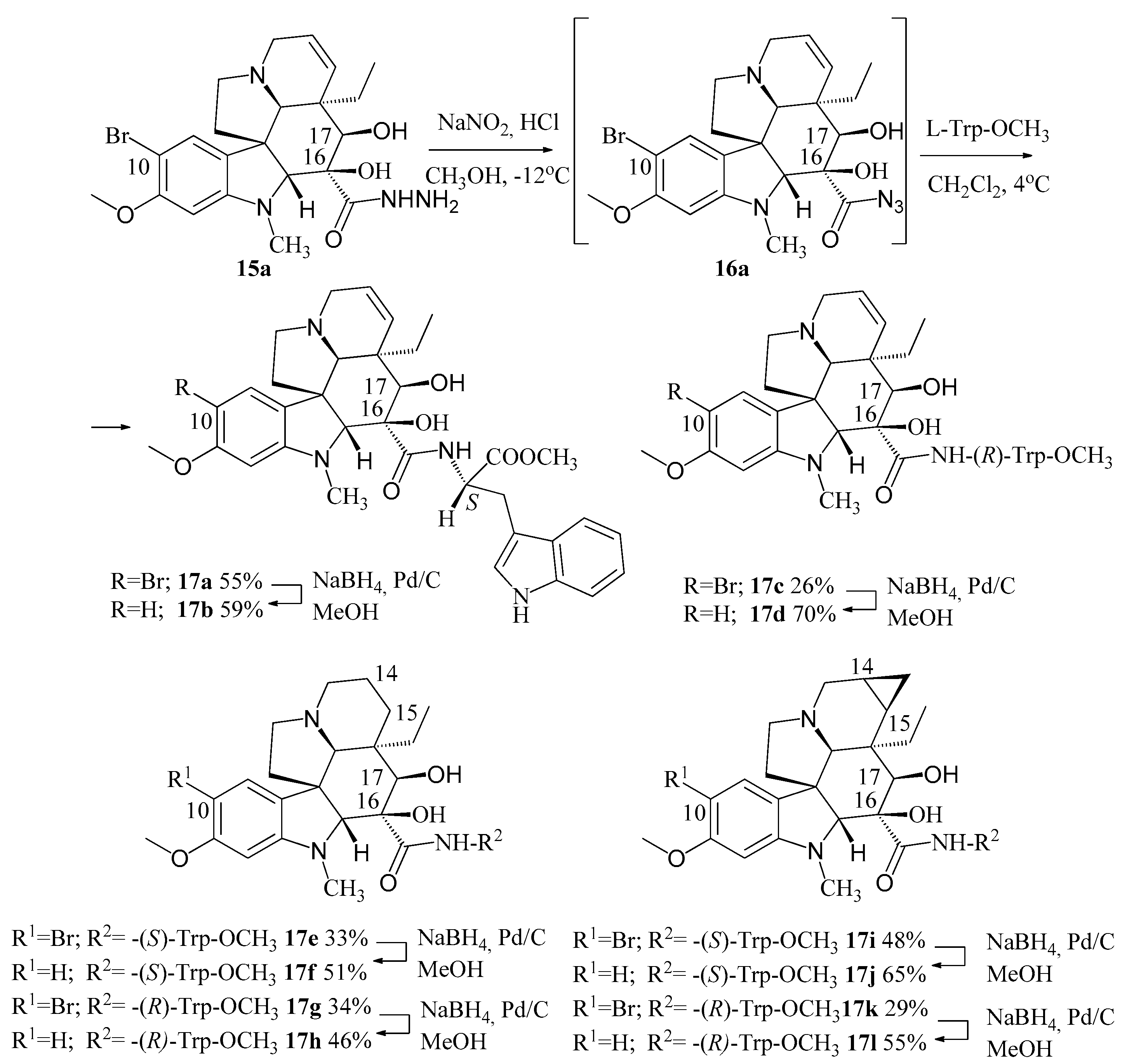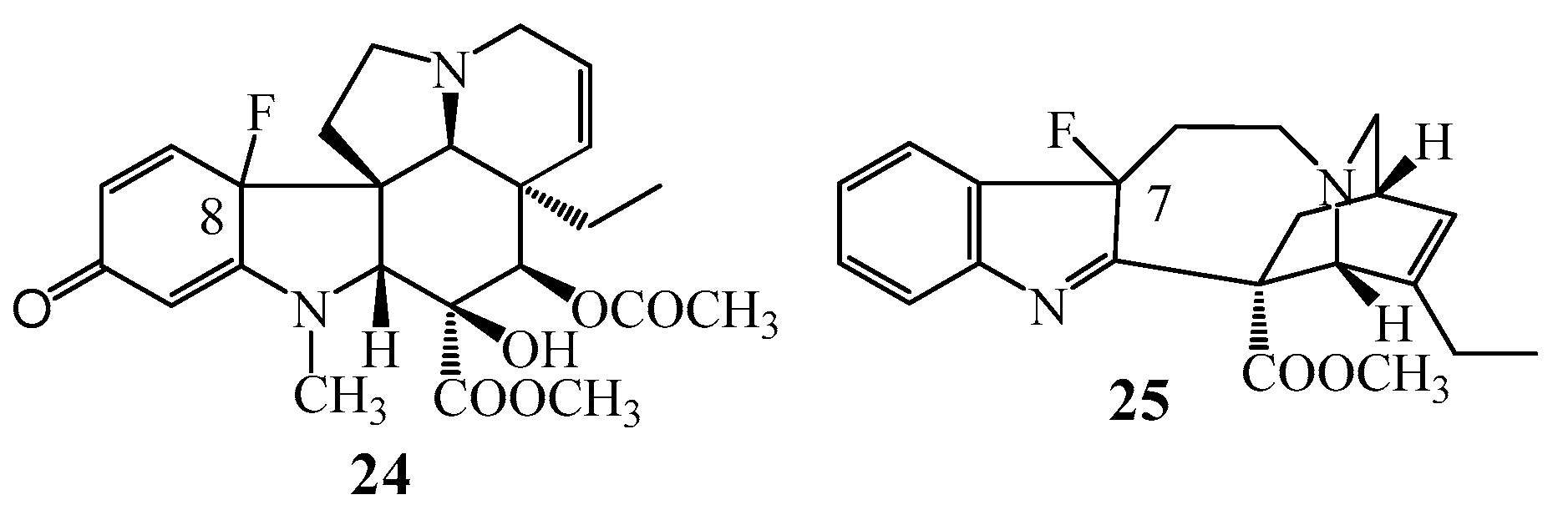Abstract
The antitumor indole–indoline alkaloids of the evergreen Catharanthus roseus—namely vinblastine and vincristine—are widely used in chemotherapy of cancer. Many efforts were made to synthesize more efficient derivatives with less side-effect. The 14,15-cyclopropane derivative of vinblastine was synthesized successfully by a five-step procedure starting from vindoline. Vincristine, vinorelbine and several derivatives condensed with a cyclopropane ring were synthesized. Various hybrid molecules were prepared by the coupling reaction of vindoline and methyl ester of tryptophan, which were conjugated by carrier peptides of octaarginine. Studying the halogenation reactions of vindoline and catharanthine some fluorine derivatives were obtained which showed promising antitumor activity on various tumor types. The synthesis of the Aspidospermane alkaloid bannucine and 5′-epibannucine were carried out using N-acyliminium intermediates. The same intermediate was also applied in the first synthesis of sessiline. The research group have synthesized of flavonoid alkaloids: dracocephins A and B. Further three flavonoid alkaloids, namely 8-(2”-pyrrolidinon-5′′-yl)quercetin, 6-(2′′-pyrrolidinon-5′′-yl)-(−)- and 8-(2′′-pyrrolidinon-5′′-yl)-(−)-epicatechin were prepared by acid-catalyzed regioselective Mannich reaction starting from the corresponding flavonoid precursor. Vindoline was also coupled to synthetic pharmacophores, such as triphenylphosphine and various N-heterocycles. Some of these hybrid molecules showed significant antitumor activity. Furthermore, 7-OH and 7-NH modified flavonoid derivatives were synthesized by a regioselective alkylation followed by Smiles rearrangement and hydrolysis.
1. Introduction, Therapy and Previous Results
Studies dealing with natural organic compounds of biologic activity can be divided into four main groups: (i) the first is the isolation of the molecule in question from the given plant, (ii) the investigation of the biosynthetic pathways, (iii) elaboration of the total synthesis for preparing the biologic effective structure and (iv) synthesis of new derivatives by modification of the original structure for obtaining more efficient, more selective and less toxic molecules.
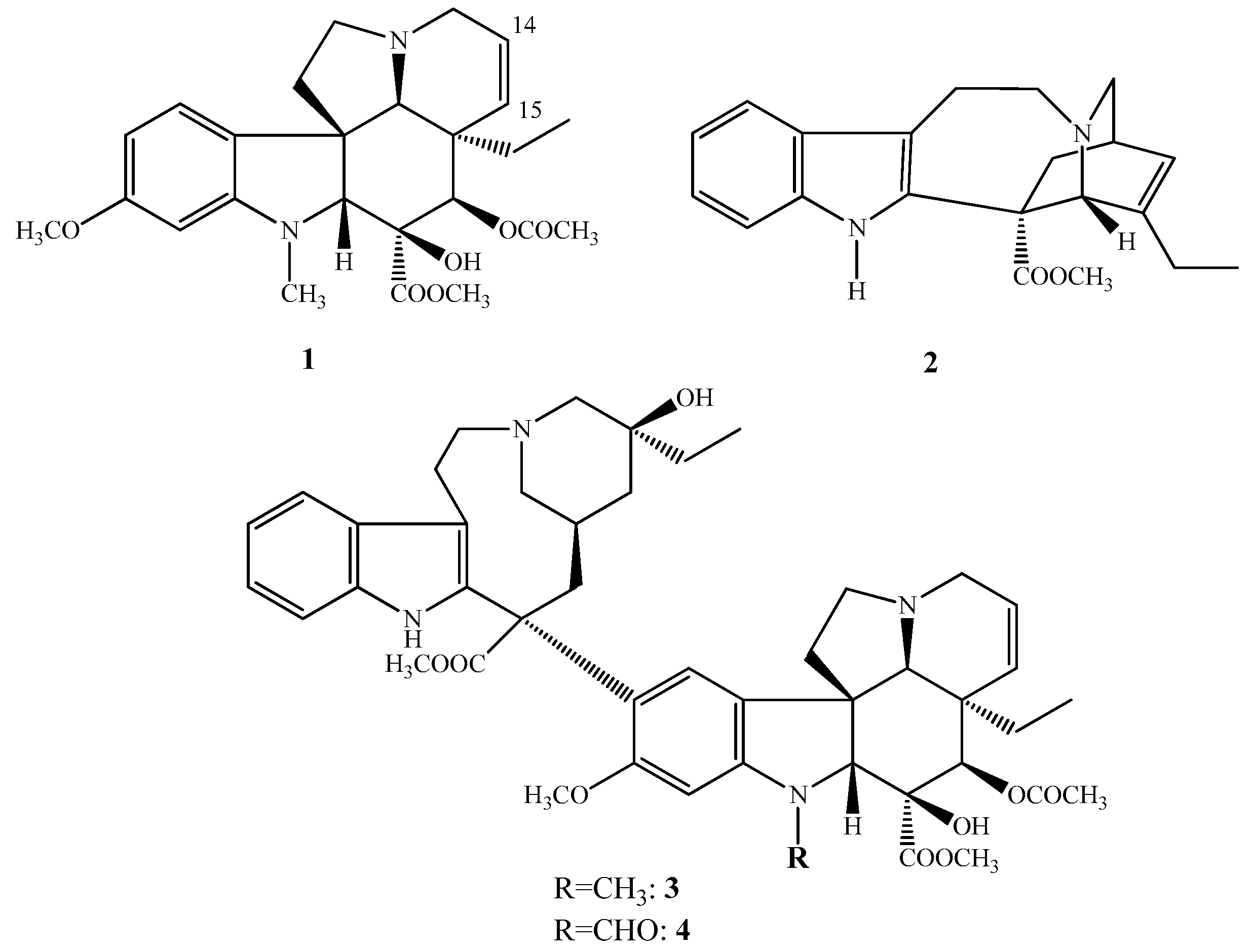
(−)-Vindoline (1) and (+)-catharanthine (2) are Vinca alkaloids containing an indole skeleton, coupled together forming the dimer alkaloids (+)-vinblastine (3) and (+)-vincristine (4). Vincristine (4) differs from vinblastine (3) in position 1; vincristine (4) contains on the indole nitrogen atom a formyl group (Figure 1) instead of a methyl group as is in the vinblastine (3). These compounds can be classified as Vinca alkaloids, which were isolated first in the 1950s from the periwinkle Catharanthus roseus being native to Madagascar. These compounds are antitumor agents and are used in anticancer therapy. During cell division they act as inhibitors of tubulin polymerization thus blocking the formation of mitotic spindle. In the cancer cells they also inhibit the DNS repairing mechanism and the synthesis of RNS, inhibiting the DNS-dependent RNS polymerase. In anticancer therapy, these types of compounds are used especially against leukemia and lymphoma.
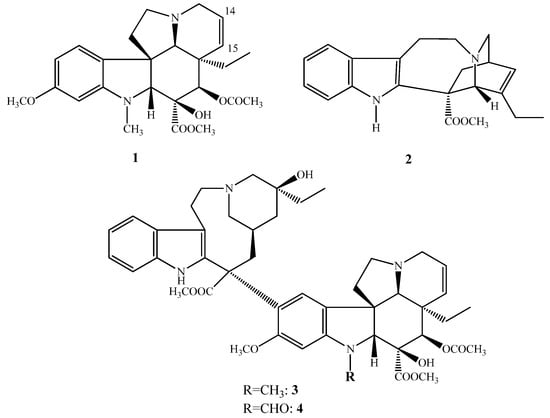
Figure 1.
(1–4) Structure of the most important natural Vinca alkaloids.
In the literature, there are many publication in connection of chemical and biologic feature of vinblastine (3) and vincristine (4) as well as synthesis of their new derivatives having important biologic activity [1,2,3]. Moreover, in the course of our research work, the purpose was not only the synthesis of new vinblastine (3) and vincristine (4) derivatives, but also the extensive investigation of the chemistry of their monomers vindoline (1) and catharanthine (2). In 2015, a detailed review was published presenting the most important results obtained by our research group in this area [4].
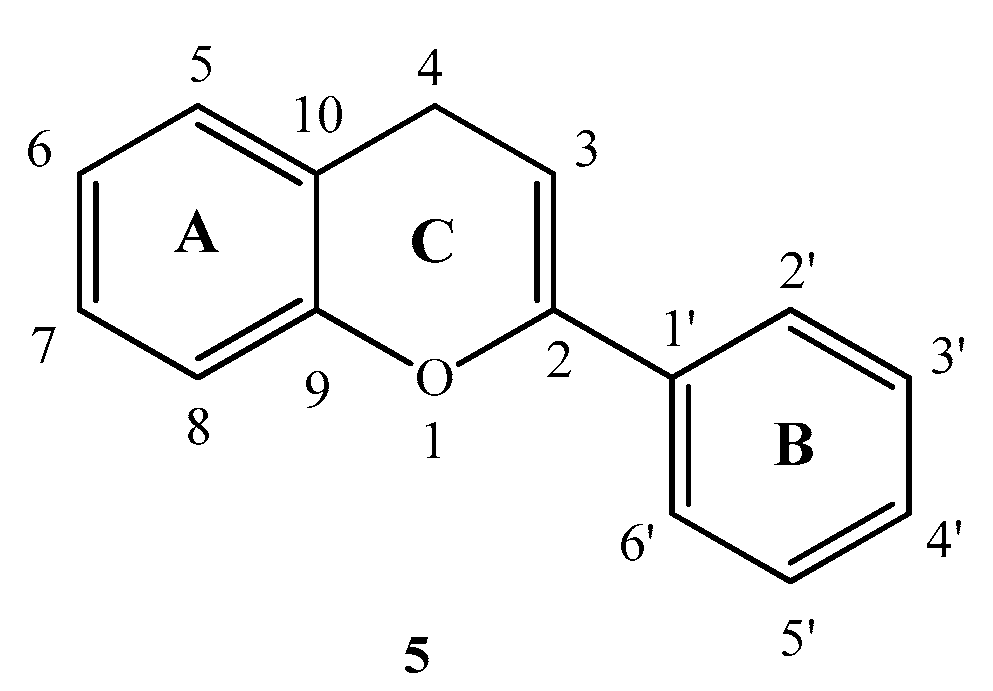
Flavonoids are secondary plant metabolites containing numerous low-molecular-weight members [5,6]. Their general structure consists of a 15-carbon skeleton with a heterocyclic (pyran) ring (C) between two phenyl rings (A and B) (Figure 2). Flavonoids have a broad-spectrum of biologic activity including anti-inflammatory [7,8], antiviral [8,9,10], antioxidant [11,12] and antitumor [5,8] effects. Flavonoids have been found to be able to dock into ATP binding sites using the adenosine like part at position 4 and 5 [13].
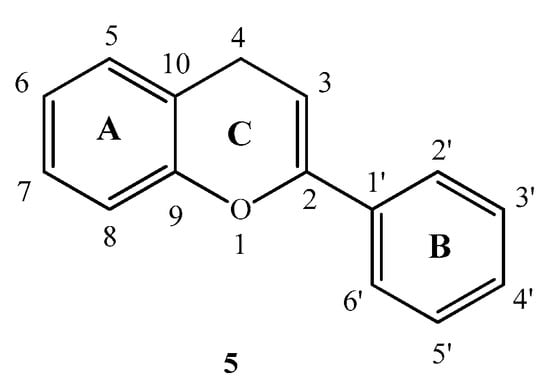
Figure 2.
Basic structure of flavonoids.
2. Research on Vinca Alkaloids
2.1. Derivatives Condensed the Three-Membered Rings
It was observed recently that the saturation of the carbon–carbon double bond in position 14,15 of vinblastine (3) by catalytic hydrogenation decreased the biologic effect almost with two orders of magnitude [14]. Considering the drastic change in the biologic activity of this rather large molecule was caused by a minor structural modification; it can be concluded that this C=C double bond has an important role in the biologic effect. Since that, these compounds can be seen appropriate for cyclopropanation, the question is coming up, how the biologic activities change in the case of replacing this double bond with cyclopropane ring having a similar electron structure. This was the reason to propose the synthesis of new vinblastine derivatives condensed with cyclopropane ring in position 14,15.
Cyclopropane skeletons can be found in several molecules—as well as in natural organic compounds in a condensed form or as substituents [15]. Nevertheless, the cyclopropane ring has specific properties thanks to its unique structure. Based on the NMR spectra of the different cyclopropane derivatives, it can be concluded that the C–H bond in the cyclopropane ring has larger s-character than in other hydrocarbons. This is the reason, however, that the C–C bonds have larger p-character. It was calculated that the s-character of these C–C bonds is not more than 17%, so that it corresponds to the special sp5 hybrid state. This is supported by the C–C coupling constants measured in the 13C-NMR spectra of cyclopropane derivatives [16].
The 1H-NMR shifts of some cyclopropane derivatives are also interesting [17,18]. It can be observed that the chemical shift is slightly increased in the case of the possibility of conjugation, however, development of an aromatic character causes an enormous increase in the chemical shift, e.g., the value of cyclopentadiene-spiro-cyclopropane changes almost fourfold compared to the saturated cyclopentane-spiro-cyclopropane. Thus, one of cyclopropane ring can give two electrons to form the aromatic delocalized electron sextet.
X-ray crystallographic investigations confirmed that the C–C bonds in cyclopropane are shorter than usual in the normally saturated analogs and show slight diffraction because of the large strain of the cyclopropane ring [19]. Therefore, the classical valence theories in the case of cyclopropane can be hardly used. Moreover, how it changes the biologic activity?
Many methods can be offered for the preparation of cyclopropane derivatives. Of these, the classic Simmons–Smith reaction exceeds, in which the carbene unit is given by diethyl zinc [20].
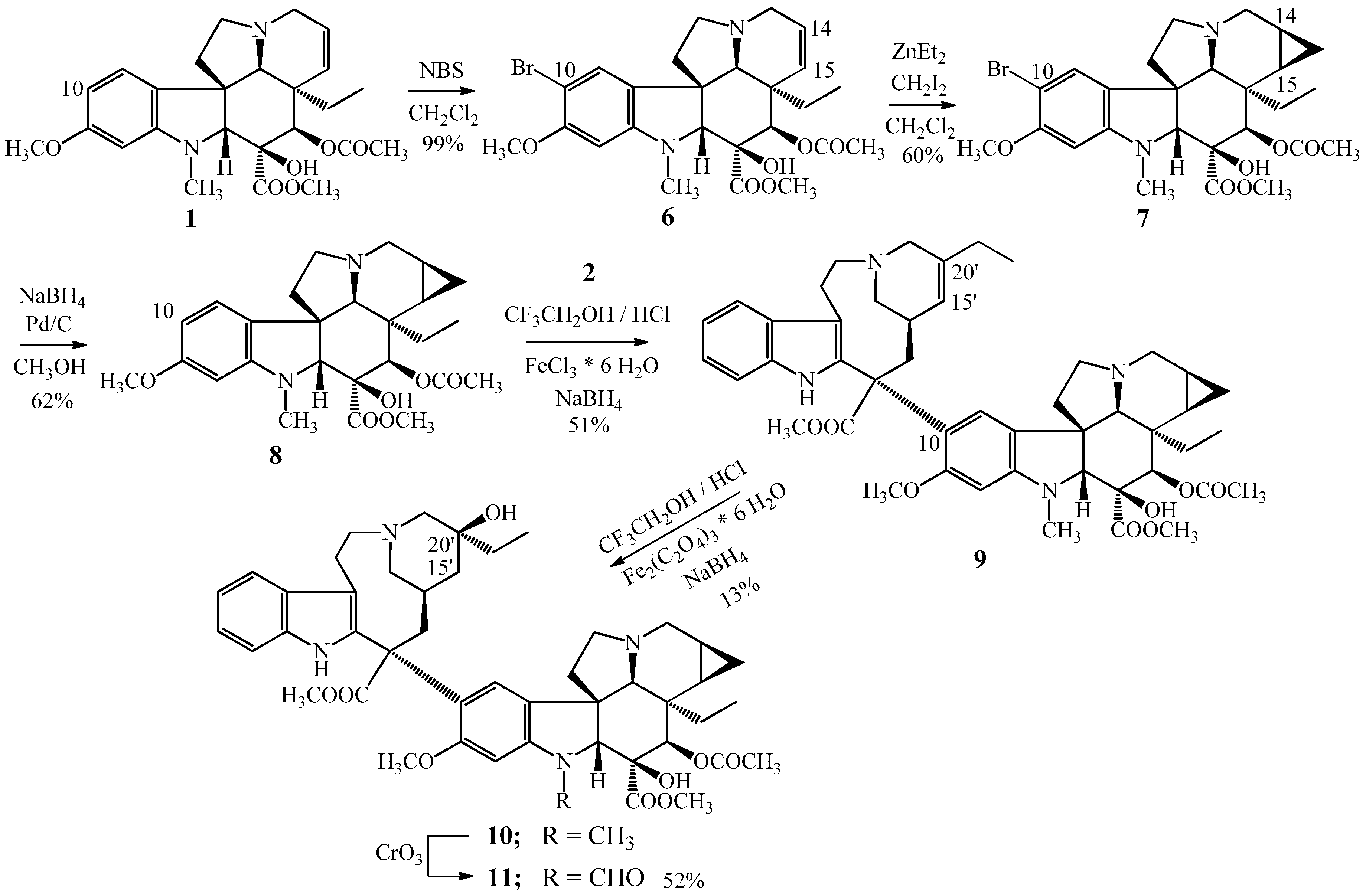
At first, the direct cyclopropanation of vinblastine (3) was tried. However, it failed using known procedures. Then the monomer vindoline (1) was treated with diethylzinc and diiodomethane, protecting the position 10 of vindoline (1) with a bromine substituent for avoiding the dimerization reaction taking place through the diiodomethane. Hence, thus, we succeeded in the synthesis of the desired 14,15-cyclopropanovinblastine (10) in an indirect way by a 5-step synthesis [4,21,22,23].
In the first step vindoline (1) was brominated by N-bromosuccinimide in the position 10 (Figure 3) then in the second reaction step, the cyclopropane ring was formed into the position 14,15. Following the bromo atom was hydrogenated from C-10 by sodium borohydride in the presence of a palladium catalyst on charcoal. Then the obtained 14,15-cyclopropanovindoline (8) was coupled with catharanthine (2) resulting in the 14,15-cyclopropanoanhydrovinblastine (9) [21,24]. The last step was the hydration of the 15′-20′ carbon–carbon double bond obtaining the 14,15-cyclopropanovinblastine (10). The 14,15-cyclopropanovincristine (11) was prepared by the CrO3 oxidation of cyclopropane derivative (10) of vinblastine (3) [21,25].
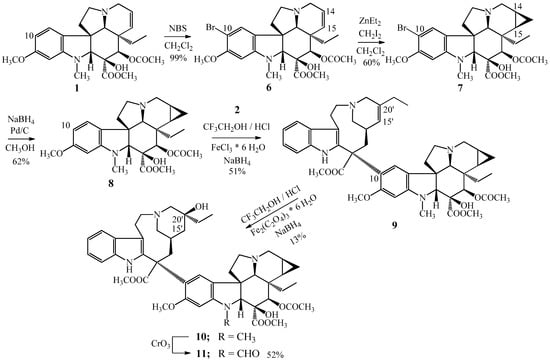
Figure 3.
Synthesis of (10) 14,15-cyclopropanovinblastine and (11) 14,15-cyclopropanovincristine.
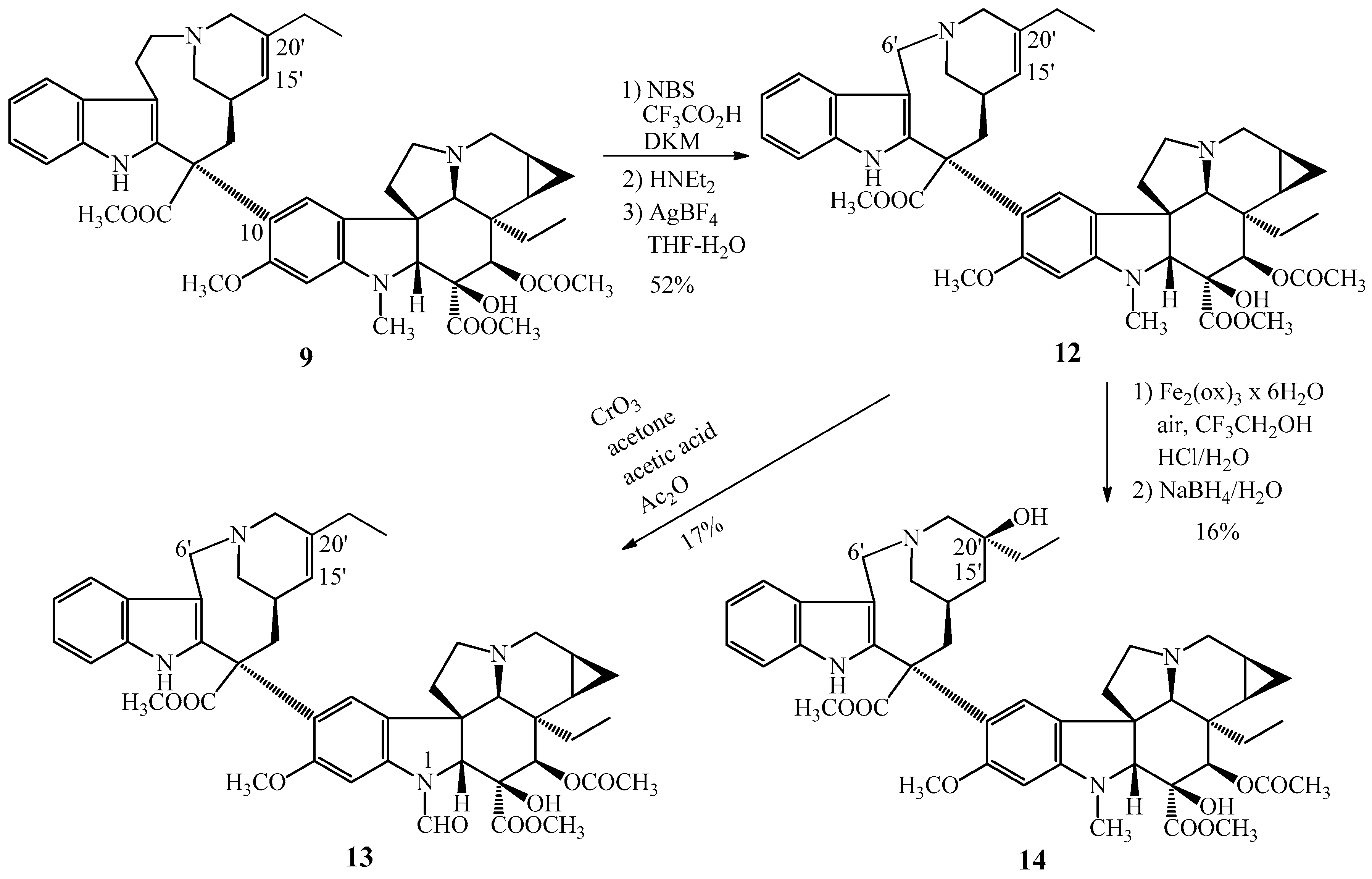
On the base of our results, further dimer alkaloids condensed with cyclopropane ring were synthesized [22]. The cyclopropane derivative (12) of vinorelbine was obtained from the ring contraction reaction of 14,15-cyclopropanoanhydrovinblastine (9) (Figure 4).
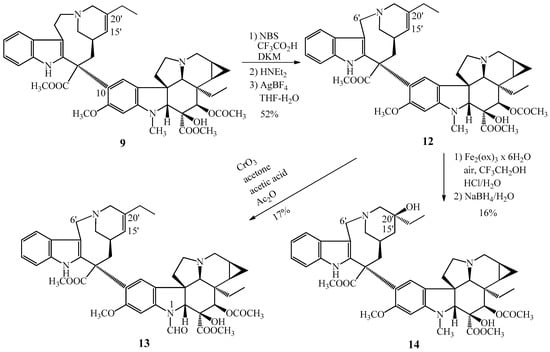
Figure 4.
Synthesized semisynthetic Vinca alkaloids condensed with a cyclopropane ring. (12) 14,15-cyclopropanovinorelbine and (13–14) two other derivatives of 12.
Synthesis of 14,15-cyclopropanovinorelbine (12) gave the possibility to prepare two further cyclopropanovinorelbine derivatives, the 1-N-formyl-14,15-cyclopropanovinorelbine (13) and the 5′-demethylene–vinblastine cyclopropane derivative (14). The 13 N-formyl derivative was obtained by CrO3 oxidation reaction of cyclopropanovinorelbine (12), compound 14 was formed by the hydration of the 15′-20′ carbon–carbon double bond also of 12.
Dimer alkaloids condensed with a cyclopropane ring synthesized by us were investigated by the National Institute of Health (NIH, Bethesda, MD, USA) on 60 different cell lines of 9 different tumor types.
The cytostatic activity of compounds 14,15-cyclopropanovinblastine (10) and 14,15-cyclopropanovincristine (11) slightly differs from vinblastine (3) and vincristine (4) used in therapy as anticancer medicines; the cyclopropane analogs are more effective inhibitors for the cell proliferation, respectively and destroy the tumorous cells with more efficiency. The 14,15-cyclopropanovinblastine (10) shows excellent anticancer activity in the case of leukemia, non-small cell lung cancer, colon cancer, melanoma and breast cancer, the vincristine derivative (11) is outstanding against colon cancer, melanoma, ovarian cancer and prostate cancer. From the vinorelbine derivatives, the 14,15-cyclopropanovinorelbine (12) has the most significant effect in the case of non-small cell lung cancer, colon cancer, central nervous system cancers, melanoma and breast cancer.
The 1-N-formyl-14,15-cyclopropanovinorelbine (13) shows an important activity and significant selectivity on COLO-205 colon cancer cell line [23].
2.2. Vinca Hybrid Molecules Containing Amino Acid Esters
In the last few years, there was great interest in the hybrid molecules. Among anticancer compounds, some hybrid molecules were built by coupling an antiproliferative structural part with another pharmacophore. From our compounds at first vindoline (1) was coupled with amino acid esters [26].
10-bromovindoline (6) was treated with hydrazine resulting in 15a hydrazide derivative and the latter was coupled with (l)- and (d)-tryptophan methyl ester, respectively, by means of azide coupling method known in peptide chemistry (Figure 5). Different derivatives of vindoline (1) were used: 14,15-dihydro-, 14,15-cyclopropanovindoline, moreover, the derivatives not containing a bromo substituent at position 10 were also synthesized. The cytostatic activity of the prepared compounds (17a–l) was investigated on HL-60 leukemia cells. It was established, that the most effective molecule was compound 17f, which has hydrogen atom at C–10 and is coupled with (l)-tryptophan methyl ester at position 16. Derivatives of vinblastine (3) coupled also with (l)-tryptophan methyl ester, conjugated with N-terminal of octaarginine carrier peptide after hydrolysis of the ester to the corresponding carboxylic acid showed substantially larger activity [27].
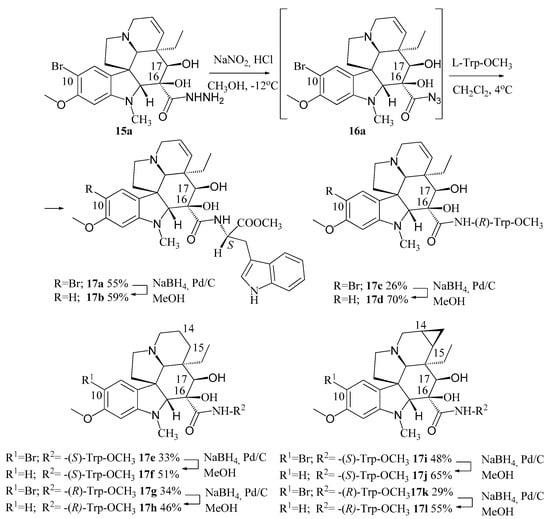
Figure 5.
Synthesis of different vindoline derivatives (17a–l) coupled with (l)- and (d)-tryptophan methyl ester in position 16.
2.3. Vinca Hybrid Molecules Containing Steroid Vectors
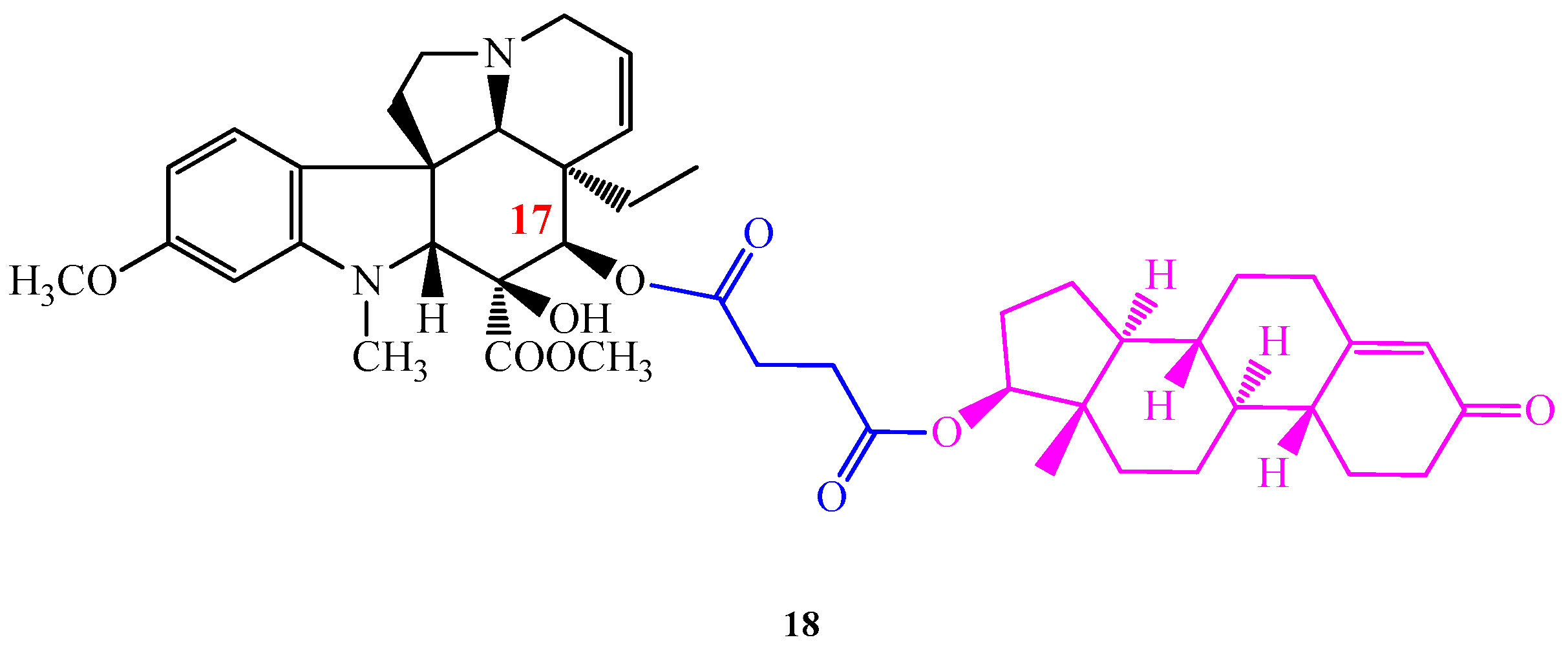
The conjugation of Vinca alkaloids with (l)- and (d)-tryptophan methyl ester proved that the cytotoxic effect of vindoline (1) can be promoted by coupling this monomer with suitable pharmacophores. As a continuation of our work we aimed to synthesize vindoline (1) steroid hybrids presuming that the lipophilic steroid vector can facilitate Vinca alkaloids to pass through the cell membranes and reach higher bioavailability. Accordingly, 5α-dihydrotestosterone and 19-nortestosterone were connected to vindoline (1) in positions 10 and 17 through succinate linkers. From the successfully synthesized four new hybrid derivatives, the most efficient one was compound 18, where 19-nortestosterone was coupled with vindoline (1) in position 17 (Figure 6) [28]. According to the in vitro biologic evaluations of NIH (USA), compound 18 displayed increased cell growth inhibition than vindoline (1), furthermore, it showed a higher antitumor effect on several cell lines even than vinblastine (3) sulfate used in therapy.
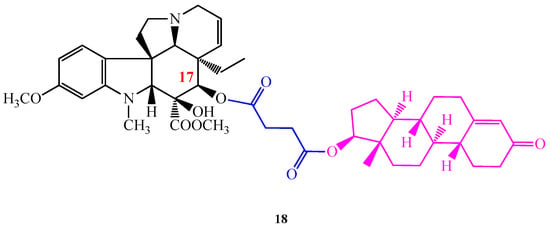
Figure 6.
(18) 17-desacetylvindoline-19-nortestosterone hemisuccinate hybrid molecule found to have an outstanding antiproliferative activity.
2.4. Vinca Hybrid Molecules Containing Synthetic Pharmacophores
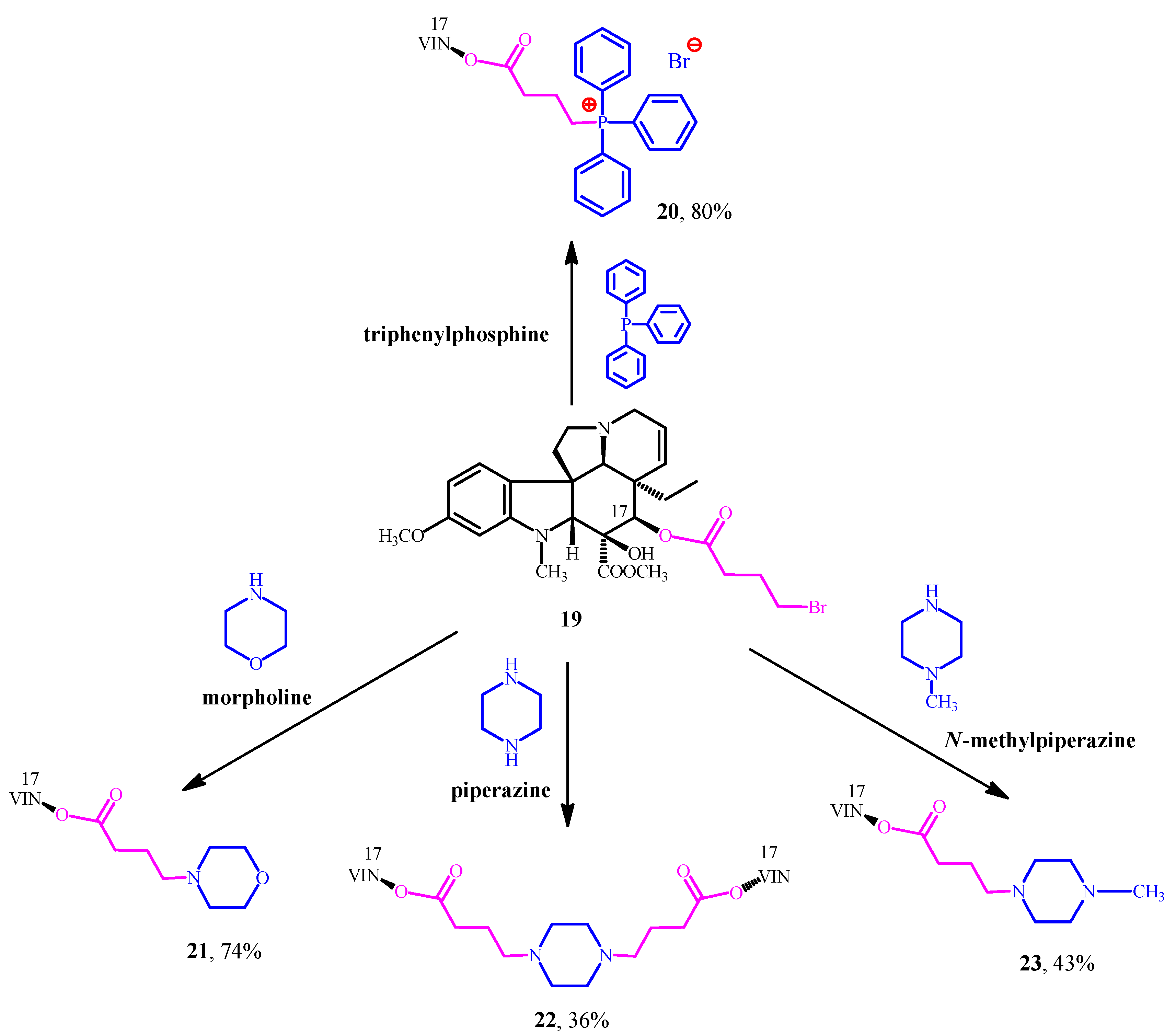
After the encouraging results derived from the conjugation of natural pharmacophores (amino acid esters and steroids) and Vinca alkaloids, our next research project was to combine vindoline (1) with well-known synthetic pharmacophores for example triphenylphosphine and certain N-heterocycles such as morpholine, piperazine and N-methylpiperazine. These moieties are widely used pharmacophores in modern drug discovery and proved to be efficient structural units of several drugs on market [29,30]. Our main purpose was still the same: producing new vindoline hybrid molecules which could have potential anticancer activity. First, vindoline (1) was O-acylated with 4-bromobutyric acid, after hydrolysis in position 17. After this step, the linker-containing vindoline derivative (19) was successfully coupled with the mentioned synthetic pharmacophores to give the expected hybrid molecules (20–23) (Figure 7) [31,32,33].
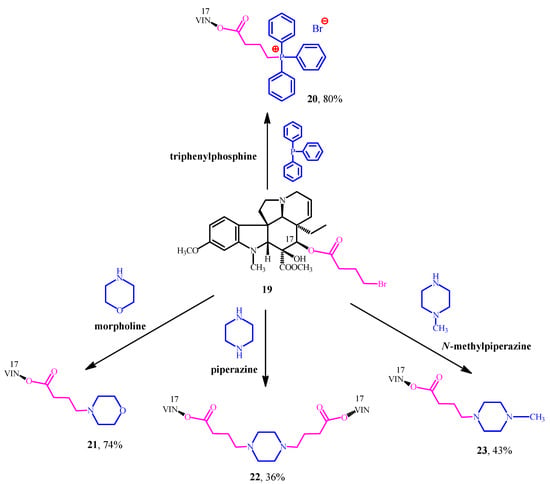
Figure 7.
(20–23) Synthesized Vinca alkaloid hybrids by the conjugation of (1) vindoline and the chosen synthetic pharmacophores.
The new compounds (20–23) were tested on 60 different human tumor cell lines in vitro (NIH, Bethesda, MD, USA). The results showed that the linker, the morpholine, and the N-methylpiperazine moieties could not improve the anticancer activity of vindoline (1). However, the 20 phosphonium-salt and the 22-vindoline dimer (containing piperazine) represented outstanding cytotoxic activities. These derivatives (20, 22) were more potent than vinblastine (3) sulfate itself on several cell lines. The anticancer investigation (NIH, USA) of these compounds (19–23) confirmed our hypothesis suggesting vindoline (1) can become an effective anticancer drug by conjugating it with suitable pharmacophores.
2.5. Halogenation Reactions of VINCA Alkaloids
The mechanism of the cyclopropanation reaction of vinblastine (3) presented in chapter 2.1. was investigated and established the reason for forming the N-methyl quaternary salt of vinblastine instead of cyclopropanation in the course of Simmons–Smith reaction [34].
Halogenation reactions of monomer indole alkaloids vindoline (1) and catharanthine (2) were studied in detail, first of all the introduction of fluorine atom was investigated (Figure 8). In both cases the reactions resulted in unexpected products. Thus, in the case of vindoline (1) the fluoro containing quinone derivative (24) was formed and, in the case of catharanthine (2) a fluoro substituted indolenine (25) could be isolated [35].
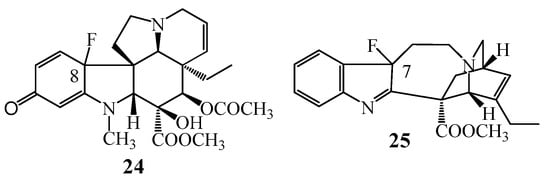
Figure 8.
(24–25) Halogenated products obtained in the fluorination reactions of (1) vindoline and catharanthine (2).
3. Vindoline and Flavone Derivatives Containing 2-Pyrrolidone Ring
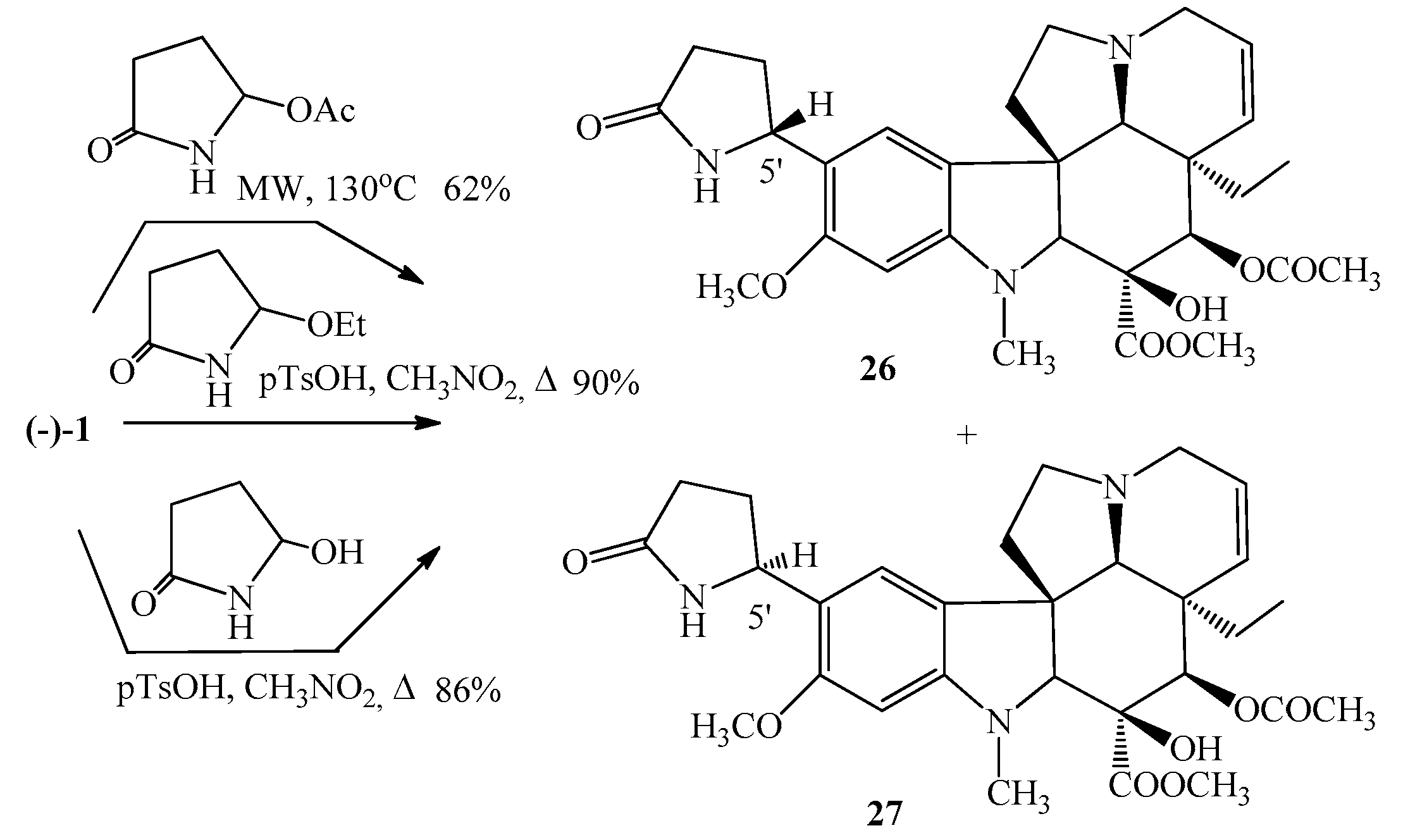
Our other important research area was the chemistry of Aspidospermane alkaloids. A detailed review was published in 2008 by us presenting the synthesis and derivatives of these types of alkaloids [36]. Then the first synthesis of bannucine (26) and 5′-epibannucine (27) was elaborated [37]. This alkaloid was first isolated in 1986 from the leaves of Catharanthus roseus by Atta-Ur-Rahman and coworkers [38]. Bannucine (26) is a derivative of vindoline (1) having a 2-pyrrolidone ring in position 10. The key step of the procedure appears the reaction between the natural (−)-vindoline (1) and a cyclic N-acyliminium intermediate. As a result of the reaction not only the bannucine (26), but also its 5′-epimeric isomer (27) was formed in an approximate ratio of 2:3 (Figure 9). The isomers were separated, and their structure was identified also by X-ray crystallography. The pure epimers were investigated on 57 cell lines of different types of cancer, however, important anticancer activity was not found [36].
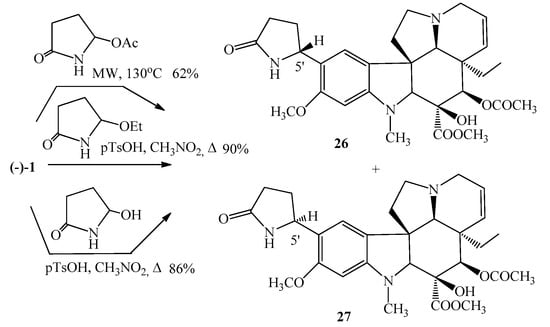
Figure 9.
(26) Synthesis of bannucine and (27) its epimer 5′-epibannucine with different methods.
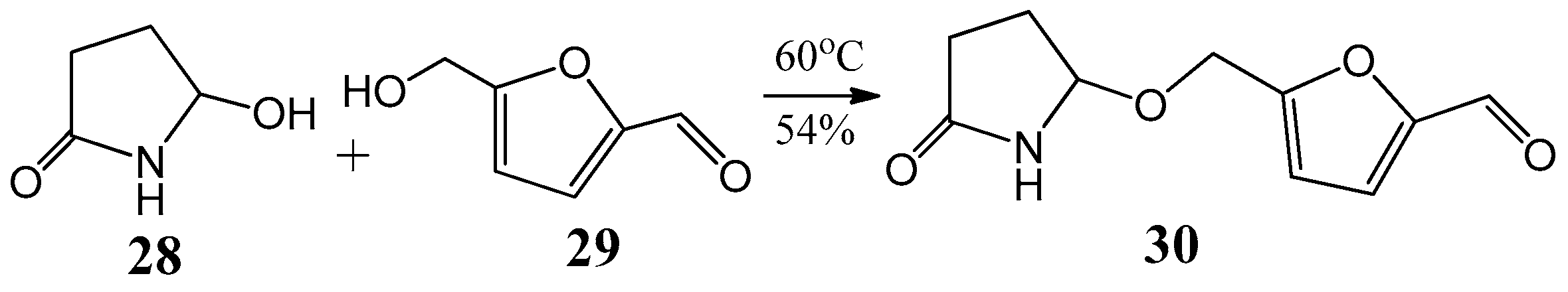
The first synthesis of sessiline (30) was achieved by applying the experiences gained in the field of acylaminocarbinols [39]. The alkaloid sessiline (30) was isolated from the fruits of Acanthopanas sessiliflorus in 2002 [40]. The molecule consists of two 5-membered heterocycles coupling through an acylaminocarbinol ether-type bond (Figure 10). Sessiline (30) was prepared by reaction of 5-hydroxypyrrolidin-2-one (28) with 5-hydroxymethyl-furfural (29) at 60 °C with 54% yield [39].
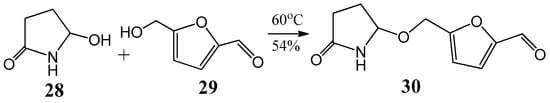
Figure 10.
An alternative method to synthesize (30) sessiline from (28) 5-hydroxypyrrolidin-2-one and (29) 5-hydroxymethyl-furfural.
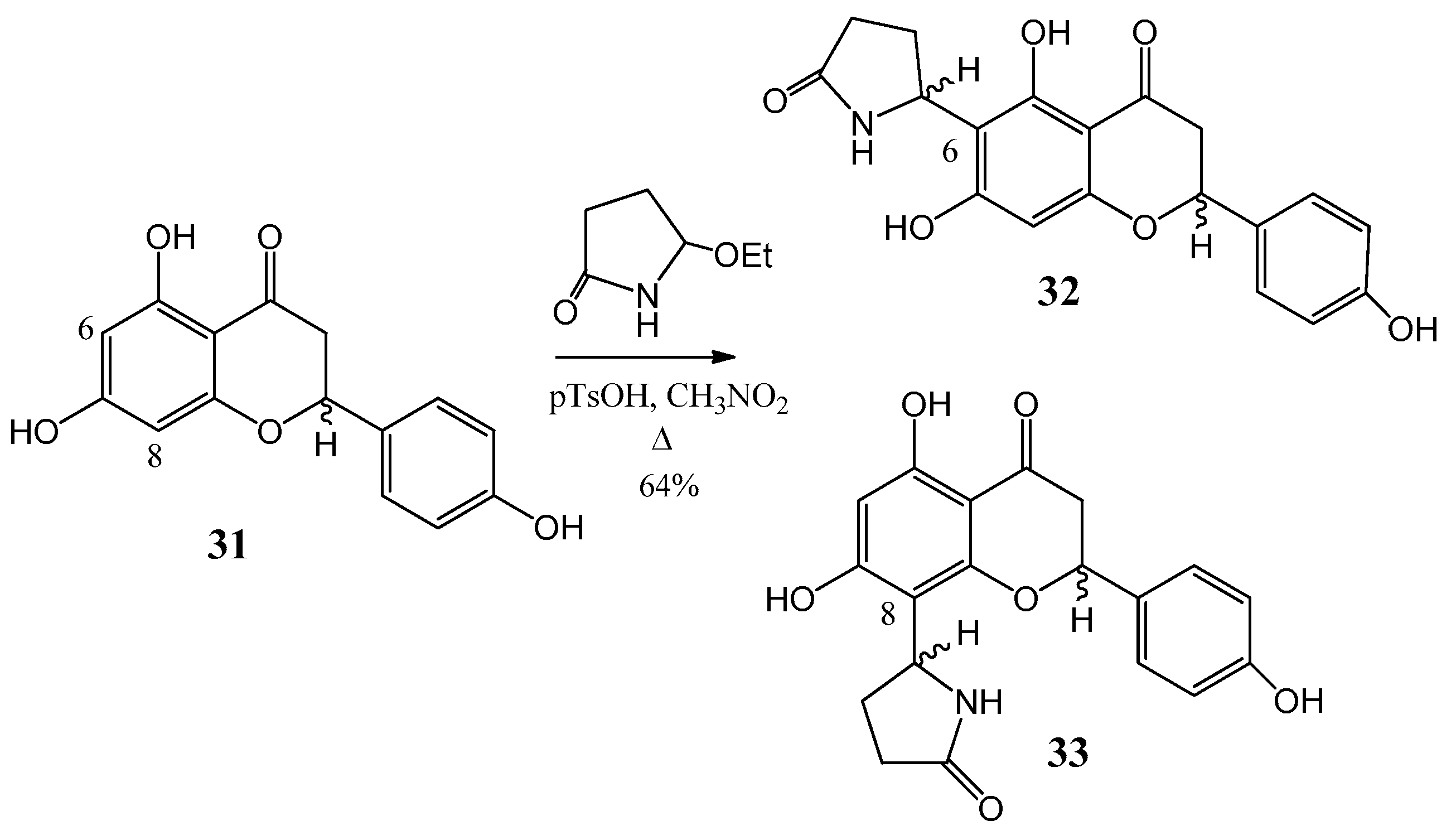
Previously in our department, serious research was followed on the flavonoid chemistry. On the base of these results, the synthesis of flavones coupled with a heterocyclic ring was investigated. dracocephin A (32) and its regioisomer B (33) as a mixture of four stereoisomers were isolated in China from Dracocephalum rupestre in 2008 by Ren and coworkers [41]. A mixture of dracocephin A (32) and B (33) in a ratio of 43:57 was prepared in a one-step reaction from (±)-naringenin (31) and separated by analytical chiral HPLC, the absolute configuration was identified by HPLC–ECD measurements and by using TDDFT–ECD (Time-dependent density functional theory electronic circular dichroism) calculations (Figure 11). The physicochemical parameters and biologic activities of the compounds were also studied.
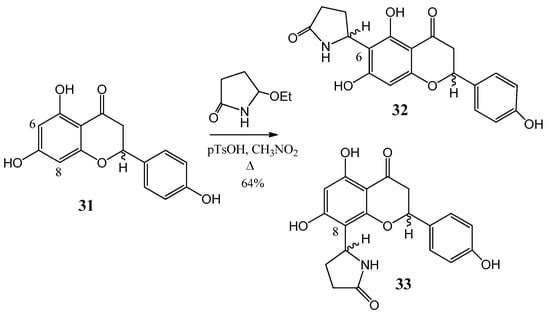
Figure 11.
Synthesis of dracocephin (32) A and (33) B.
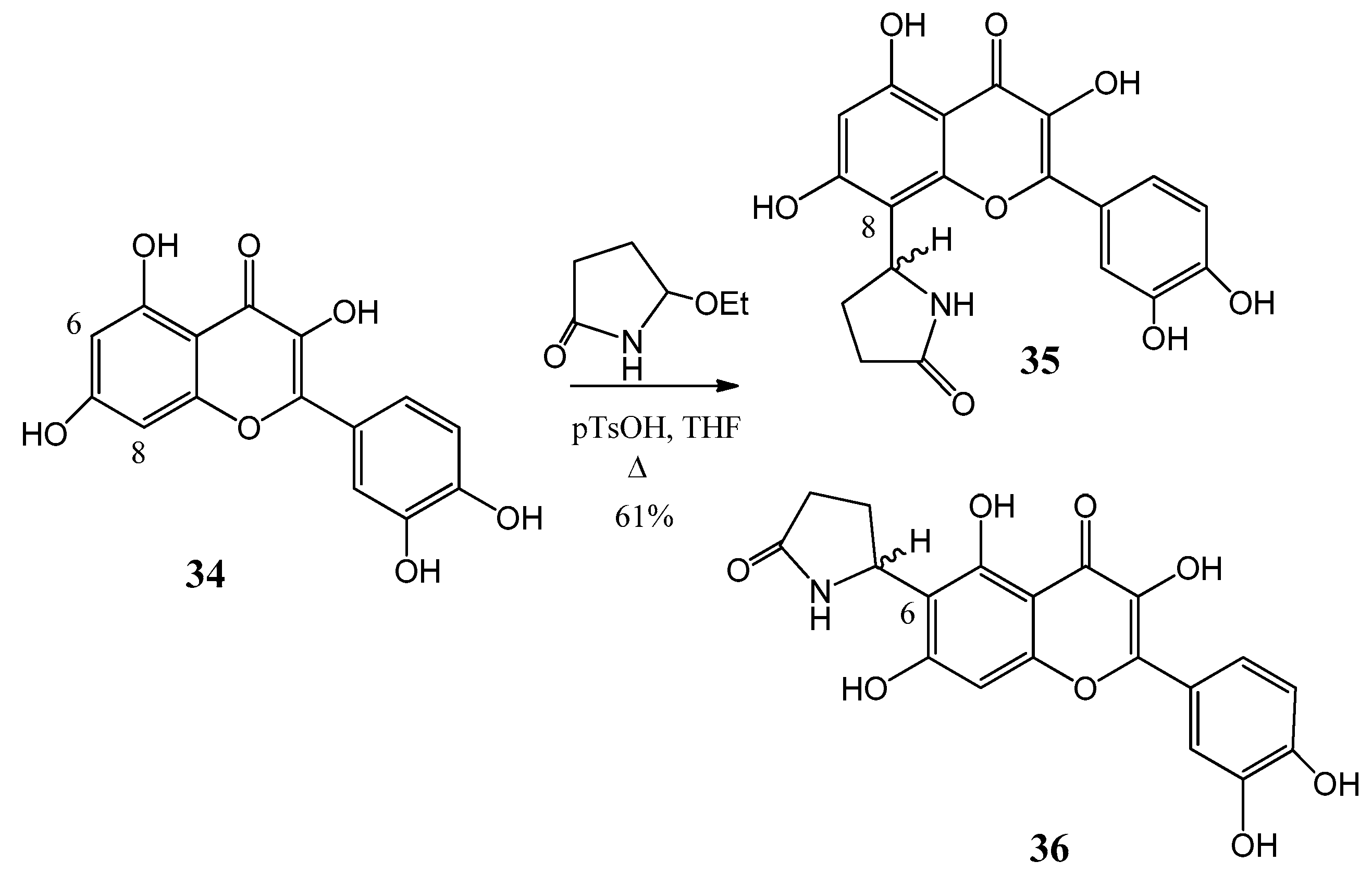
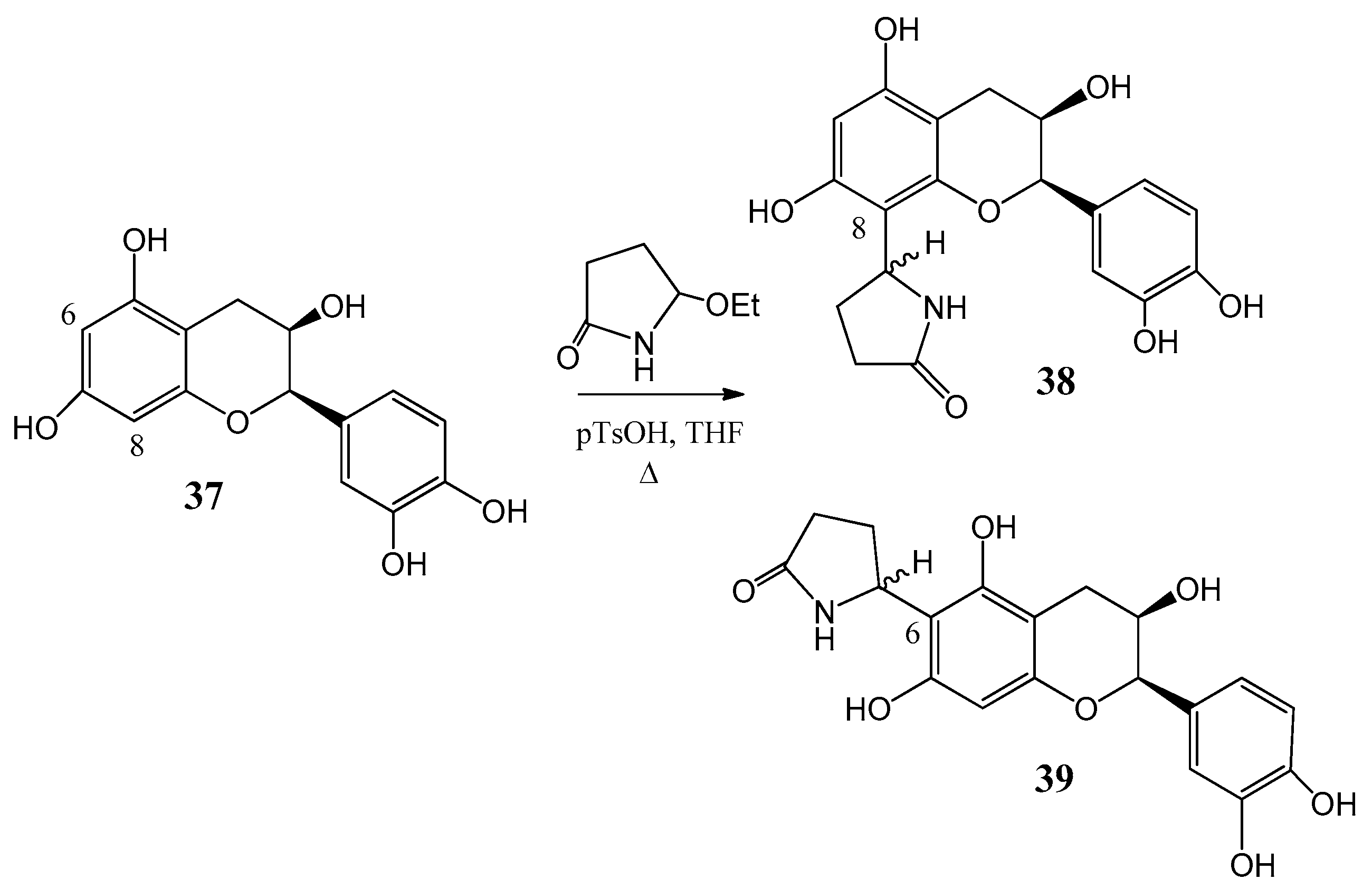
Further flavone alkaloids were also synthesized: 8-(2′′-pyrrolidinon-5′′-il)-quercetin (35) and (−)-epicatechin (37) with the same substituent in position eight (38) and in position six (39), respectively [42,43]. Starting from quercetin (34) compound 35 and its C-6 (36) isomer were obtained in a ratio of 94:6 (Figure 12). By a similar procedure, epicatechin (37) yielded a mixture of 38 and 39 in a 87:13 ratio (Figure 13). These derivatives were prepared by acid-catalyzed regioselective Mannich reaction in which the in situ generated N-acyliminium ion started an electrophile attack to the aromatic A ring of the corresponding flavonoid precursors.
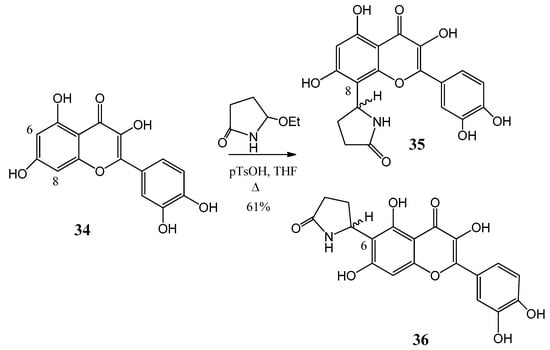
Figure 12.
Synthesis of compound 35 and its (36) C-6 isomer from (34) quercetin.
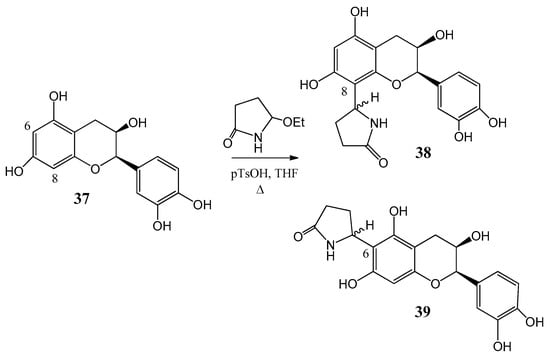
Figure 13.
Synthesis of compound 38 and 39 from (37) epicatechin by an acid-catalyzed regioselective Mannich reaction.
4. 7-OH and 7-NH Modified Flavonoid Derivatives
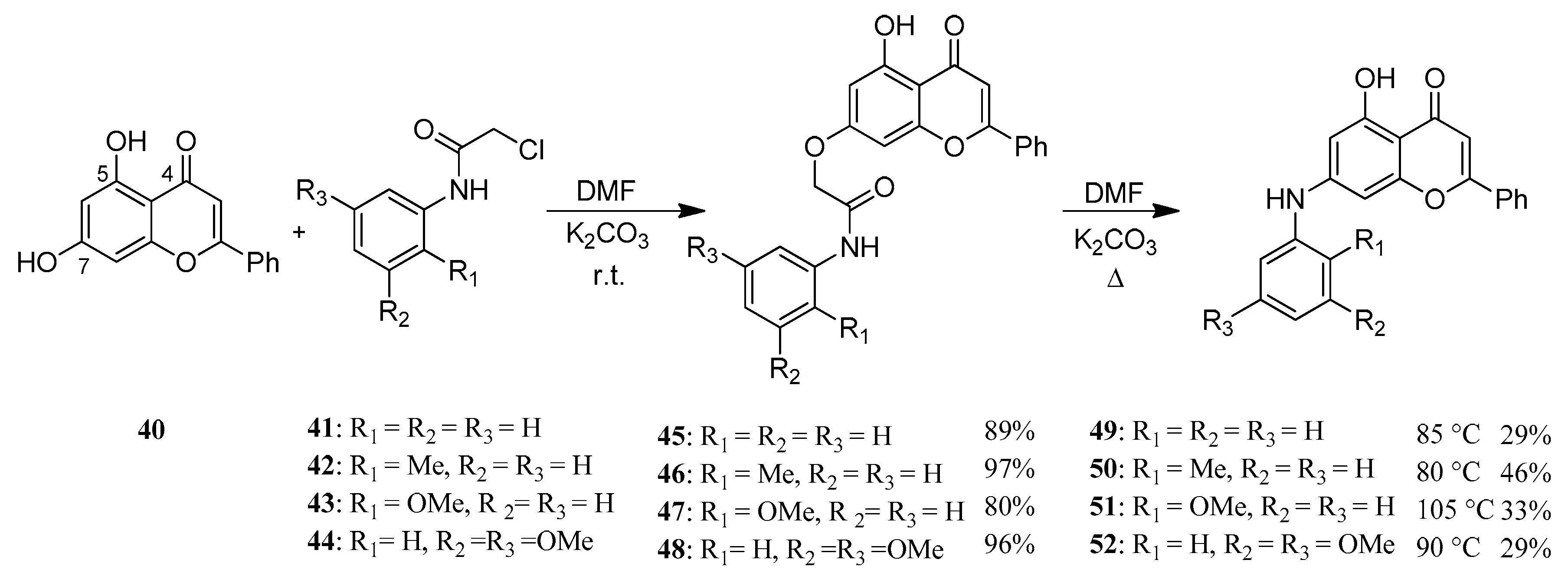
Chrysin (40)—also known as 5,7-dihydroxyflavone—can be found among others in Passiflora caerulea [44,45], honey and propolis. Like several flavonoids, it has a number of biologic effects, of which it is important to mention its anticancer activity both as a chemopreventive and as a chemotherapeutic agent [46]. The driving force of our work was that several 7-O-modified derivatives had been found to be effective against different malignant tumor cells [47,48]. Therefore, we synthesized several new aryloxy acetamide derivatives (45–48) (Figure 13). These products were rearranged to a biphenyl amine structure via Smiles rearrangement and hydrolysis to obtain a new series of 7-aminoflavone derivatives (49–52) (Figure 14) [49].
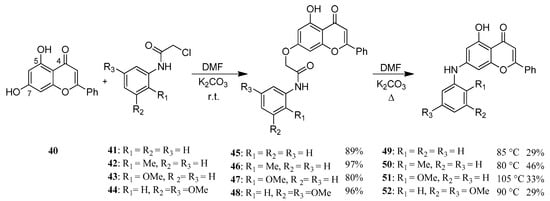
Figure 14.
Synthesis of 7-OH and 7-NH modified flavonoid derivatives.
The products (45–52) were tested on 60 different human tumor cell lines in vitro (NIH, MD, USA) and three of the hydrolyzed Smiles products (50–52) showed prominent antitumor effect on several cell lines [49].
Author Contributions
S.M., A.K., C.S.F., H.B., V.I., P.K. and L.H. wrote the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brossi, A.; Suffness, M. The Alkaloids; Academic Press Inc.: New York, NY, USA, 1990; Volume 37, pp. 1–240. [Google Scholar]
- Bölcskei, H.; Szabó, L.; Szántay, C. Synthesis of Vinblastine Derivatives. Front. Nat. Prod. Chem. 2005, 1, 43–49. [Google Scholar] [CrossRef]
- Keglevich, P.; Hazai, L.; Kalaus, G.; Szántay, C. Modifications on the Basic Skeletons of Vinblastine and Vincristine. Molecules 2012, 17, 5893–5914. [Google Scholar] [CrossRef] [PubMed]
- Keglevich, P.; Hazai, L.; Kalaus, G.; Szántay, C. Új, daganatellenes hatású, ciklopropángyűrűt tartalmazó vinblasztinszármazékok előállítása. Magy. Kém. F. Kém. Közlemények 2015, 121, 136–141. [Google Scholar]
- Raffa, D.; Maggio, B.; Riamondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef]
- Midelton, E., Jr.; Kandswami, C. The Flavonoids—Advences in Research Since 1986; Harborne, J.B., Ed.; Chapman and Hall: Cambridge, UK, 1993; pp. 619–652. ISBN 978-1-4899-2915-0. [Google Scholar]
- Read, M.A. Flavonoids: Naturally occurring anti-inflammatory agents. Am. J. Pathol. 1995, 147, 235–237. [Google Scholar]
- Mani, R.; Natesan, V. Chrysin: Sorurces, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, F.; Serrao, E.; Cheng, H.; Sanches, T.W.; Yang, L.; Nemati, N.; Zheng, Y.; Wang, H.; Long, Y. Design and discovery of flavonoid-based HIV-1 integrase inhibitors targeting both the active site and the interaction with LEDGF/p75. Bioorg. Med. Chem. 2014, 22, 3146–3158. [Google Scholar] [CrossRef]
- Brinkworth, R.I.; Stoermer, M.J.; Fairlie, D.P. Flavones are inhibitors of HIV-1 proteinase. Biochem. Biopshys. Res. Commun. 1992, 188, 631–637. [Google Scholar] [CrossRef]
- Catapano, A.L. Antioxidant effect of flavonoids. Angiology 1997, 48, 39–44. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yang, Z.S.; Wen, C.C.; Chang, Y.S.; Wang, B.C.; Hsiao, C.A. Evaluation of the structure-activity relationship of flavonoids as antioxidants and toxicants of zebrafish larvae. Food Chem. 2012, 134, 717–724. [Google Scholar] [CrossRef]
- Kim, M.K.; Choo, H.; Chong, Y. Water-Soluble and Cleavable Quercetin–Amino Acid Conjugates as Safe Modulators for P-Glycoprotein-Based Multidrug Resistance. J. Med. Chem. 2014, 57, 7216–7233. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.L.; Beer, M.D.C.T.; McIntyre, R.W. Biological effects of dihydrovinblastine. Cancer 1967, 20, 885–890. [Google Scholar] [CrossRef]
- Keglevich, P.; Keglevich, A.; Hazai, L.; Kalaus, G.; Szántay, C. Natural Compounds Containing a Condensed Cyclopropane Ring. Natural and Synthetic Aspects. Curr. Org. Chem. 2014, 18, 2037–2042. [Google Scholar] [CrossRef]
- Weigert, F.J.; Roberts, J.D.J. Nuclear magnetic resonance spectroscopy. Carbon-carbon coupling in cyclopropane derivatives. Am. Chem. Soc. 1967, 89, 5962–5963. [Google Scholar] [CrossRef]
- Proton Chemical Shifts. Available online: https://www.chem.wisc.edu/areas/reich/nmr/h-data/hdata.htm (accessed on 19 June 2020).
- Clark, E.A.; Fiato, R.A. Aromaticity via cyclopropyl conjugation. Electronic structure of spiro[2.4]hepta-4,6-diene. J. Am. Chem. Soc. 1970, 92, 4736–4738. [Google Scholar] [CrossRef]
- Wiberg, K.B. Bent Bonds in Organic Compounds. Acc. Chem. Res. 1996, 29, 229–234. [Google Scholar] [CrossRef]
- Simmons, H.E.; Smith, R.D. A New Synthesis of Cyclopropanes from Olefins. J. Am. Chem. Soc. 1958, 80, 5323–5324. [Google Scholar] [CrossRef]
- Keglevich, P.; Hazai, L.; Dubrovay, Z.; Dékány, M.; Szántay, C., Jr.; Kalaus, G.; Szántay, C. Bisindole Alkaloids Condensed with a Cyclopropane Ring, Part 1. 14,15-Cyclopropanovinblastine and -vincristine. Heterocycles 2014, 89, 653–668. [Google Scholar] [CrossRef]
- Keglevich, P.; Hazai, L.; Dubrovay, Z.; Sánta, Z.; Dékány, M.; Szántay, C., Jr.; Kalaus, G.; Szántay, C. Bisindole Alkaloids Condensed with a Cyclopropane Ring, Part 2. Cyclopropano-vinorelbine and Its Derivatives. Heterocycles 2015, 90, 316–326. [Google Scholar] [CrossRef]
- Keglevich, P.; Hazai, L.; Kalaus, G.; Szántay, C. Cyclopropanation of Some Alkaloids. Period. Politech. Chem. Eng. 2015, 59, 3–15. [Google Scholar] [CrossRef]
- Vukovic, J.; Goodbody, A.E.; Kutney, J.P.; Misawa, M. Production of 3’, 4’-anhydrovinblastine: A unique chemical synthesis. Tetrahedron 1988, 44, 325–331. [Google Scholar] [CrossRef]
- Jovanovics, K.; Szász, K.; Fekete, G.; Bittner, E.; Dezséri, E.; Éles, J. Chromic Acid Oxidation of Vinblastine Sulfate to Form Vincristine. U.S. Patent US3899493A, 12 August 1975. [Google Scholar]
- Keglevich, P.; Hazai, L.; Gorka-Kereskényi, Á.; Péter, L.; Gyenese, J.; Lengyel, Z.; Kalaus, G.; Orbán, E.; Bánóczi, Z.; Szántay, C., Jr.; et al. Synthesis and in vitro Antitumor Effect of New Vindoline Derivatives Coupled with Amino Acid Esters. Heterocycles 2013, 87, 2299–2317. [Google Scholar] [CrossRef]
- Bánóczi, Z.; Gorka-Kereskényi, Á.; Reményi, J.; Orbán, E.; Hazai, L.; Tőkési, N.; Oláh, J.; Ovádi, J.; Béni, Z.; Háda, V.; et al. Synthesis and in Vitro Antitumor Effect of Vinblastine Derivative-Oligoarginine Conjugates. Bioconjugate Chem. 2010, 21, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Keglevich, A.; Zsiros, V.; Keglevich, P.; Szigetvári, Á.; Dékány, M.; Szántay, C., Jr.; Mernyák, E.; Wölfling, J.; Hazai, L. Synthesis and in vitro Antitumor Effect of New Vindoline-Steroid Hybrids. Curr. Org. Chem. 2019, 23, 959–967. [Google Scholar] [CrossRef]
- Tsepaeva, O.V.; Nemtarev, A.V.; Abdullin, T.I.; Grigor’eva, L.R.; Kuznetsova, E.V.; Akhmadishina, R.A.; Ziganshina, L.E.; Cong, H.H.; Mironov, V.F. Design, Synthesis, and Cancer Cell Growth Inhibitory Activity of Triphenylphosphonium Derivatives of the Triterpenoid Betulin. J. Nat. Prod. 2017, 80, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghorbani, M.; Bushra, B.A.; Zabiulla, S.; Mamatha, S.V.; Ara Khanum, S. Piperazine and morpholine: Synthetic preview and pharmaceutical applications. J. Chem. Pharm. Res. 2015, 7, 281–301. [Google Scholar] [CrossRef]
- Keglevich, A.; Szigetvári, Á.; Dékány, M.; Szántay, C., Jr.; Keglevich, P.; Hazai, L. Synthesis of vinca alkaloid–triphenylphosphine derivatives having potential antitumor effect. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 606–609. [Google Scholar] [CrossRef]
- Keglevich, A.; Szigetvári, Á.; Dékány, M.; Szántay, C., Jr.; Keglevich, P.; Hazai, L. Synthesis and in vitro Antitumor Effect of New Vindoline Derivatives Coupled with Triphenylphosphine. Curr. Org. Chem. 2019, 23, 852–858. [Google Scholar] [CrossRef]
- Keglevich, A.; Dányi, L.; Rieder, A.; Horváth, D.; Szigetvári, Á.; Dékány, M.; Szántay, C., Jr.; Latif, A.D.; Hunyadi, A.; Zupkó, I.; et al. Synthesis and Cytotoxic Activity of New Vindoline Derivatives Coupled to Natural and Synthetic Pharmacophores. Molecules 2020, 25, 1010. [Google Scholar] [CrossRef]
- Keglevich, P.; Ábrányi-Balogh, P.; Szigetvári, Á.; Szántay, C., Jr.; Szántay, C.; Hazai, L. Studies on the mechanism of quaternization of the catharanthine part of vinblastine and vincristine. Tetrahedron Lett. 2016, 57, 1672–1677. [Google Scholar] [CrossRef]
- Keglevich, A.; Hegedűs, L.; Péter, L.; Gyenese, J.; Szántay, C., Jr.; Dubrovay, Z.; Dékány, M.; Szigetvári, Á.; Martins, A.; Molnár, J.; et al. Anomalous products in the halogenation reactions of Vinca alkaloids. Curr. Org. Chem. 2016, 20, 2639–2646. [Google Scholar] [CrossRef]
- Novák, L.; Tóth, F.; Kalaus, G. Kutatások az MTA-BME Alkaloidkémiai Kutatócsoportban. Magy. Kém. Folyóirat 2008, 114, 88–94. [Google Scholar]
- Ilkei, V.; Bana, P.; Tóth, F.; Palló, A.; Holczbauer, T.; Czugler, M.; Sánta, Z.; Dékány, M.; Szigetvári, Á.; Hazai, L.; et al. A simple synthesis of bannucine and 5′-epibannucine from (−)-vindoline. Tetrahedron 2015, 71, 9579–9586. [Google Scholar] [CrossRef]
- Ali, I.; Chaudhary, M.I. Bannucine—A new dihydroindole alkaloid from Catharanthus roseus (L) G. Don. J. Chem. Soc. Perkin Trans. 1 1986, 923–926. [Google Scholar] [CrossRef]
- Ilkei, V.; Faragó, K.; Sánta, Z.; Dékány, M.; Hazai, L.; Szántay, C., Jr.; Szántay, C.; Kalaus, G. The First Synthesis of Sessiline. Int. J. Org. Chem. 2014, 4, 309–313. [Google Scholar] [CrossRef][Green Version]
- Lee, S.; Ji, J.; Shin, K.H.; Kim, B.-K. Composition and antimicrobial activity of the essential oil of Achillea multifida. Planta Medica 2002, 68, 939–941. [Google Scholar] [CrossRef]
- Ren, D.-M.; Guo, H.-F.; Yu, W.-T.; Wang, S.-Q.; Ji, M.; Lou, H.-X. Stereochemistry of flavonoidal alkaloids from Dracocephalum rupestre. Phytochemistry 2008, 69, 1425–1433. [Google Scholar] [CrossRef]
- Ilkei, V.; Spaits, A.; Prechl, A.; Szigetvári, Á.; Béni, Z.; Dékány, M.; Szántay, C., Jr.; Müller, J.; Könczöl, Á.; Szappanos, Á.; et al. Biomimetic synthesis and HPLC–ECD analysis of the isomers of dracocephins A and B. Beilstein J. Org. Chem. 2016, 12, 2523–2534. [Google Scholar] [CrossRef]
- Ilkei, V.; Spaits, A.; Prechl, A.; Müller, J.; Könczöl, Á.; Lévai, S.; Riethmüller, E.; Szigetvári, Á.; Béni, Z.; Dékány, M.; et al. C8-selective biomimetic transformation of 5, 7-dihydroxylated flavonoids by an acid-catalysed phenolic Mannich reaction: Synthesis of flavonoid alkaloids with quercetin and (–)-epicatechin skeletons. Tetrahedron 2017, 73, 1503–1510. [Google Scholar] [CrossRef]
- Medina, J.H.; Paladini, A.C.; Wolfman, C.; de Stein, M.L.; Calvo, D.; Diaz, L.E.; Peña, C. Chrysin (5,7-di-OH-flavone), a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant properties. Biochem. Pharmacol. 1990, 40, 2227–2231. [Google Scholar] [CrossRef]
- Dhawan, K.; Dhawan, S.; Sharma, A. Passiflora: A review update. J. Ethnopharmacol. 2004, 94, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ksaka, E.R.; Bodduluru, L.N.; Madana, R.M.; Athira, K.V.; Gogoi, R.; Barua, C.C. Chemopreventive and therapeutic potetntial of chrysin in cancer: Mechanistic perspectives. Toxicol. Lett. 2015, 233, 214–225. [Google Scholar] [CrossRef]
- Choe, H.; Kim, J.; Hong, S. Structure-based design of flavone-based inhibitors of wild-type and T315I mutant of ABL. Bioorg. Med. Chem. Lett. 2013, 23, 4324–4327. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Ma, J.; He, J.; Zheng, X.; Lei, X.; Jiang, G.; Zhao, Z.; Pan, X. Synthesis of new 7-O-modified chrysin derivatives and their anti-proliferative and apoptotic effects on human gastric carcinoma MGC-803 cells. Chem. Res. Chin. Univ. 2014, 30, 925–930. [Google Scholar] [CrossRef]
- Mayer, S.; Keglevich, P.; Ábrányi-Balogh, P.; Szigetvári, Á.; Dékány, M.; Szántay, C., Jr.; Hazai, L. Synthesis and In Vitro Anticancer Evaluation of Novel Chrysin and 7-Aminochrysin Derivatives. Molecules 2020, 25, 888. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).