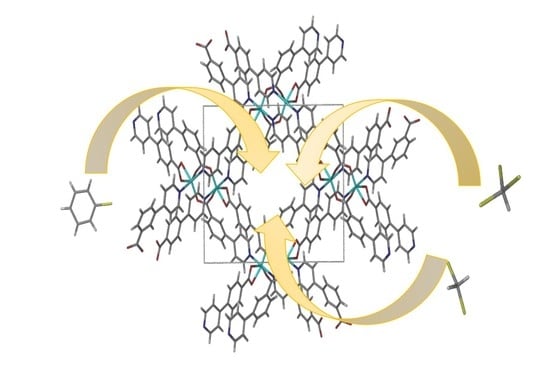
Solvatochromism and Selective Sorption of Volatile Organic Solvents in Pyridylbenzoate Metal-Organic Frameworks
Abstract
1. Introduction
2. Materials and Methods
2.1. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
2.2. Infrared Spectroscopy
2.3. Nuclear Magnetic Resonance (NMR)
2.4. Powder X-ray Diffraction (PXRD)
2.5. Crystal Structure Determination
3. Results and Discussion
3.1. Sorption of VOCs by Activated MOFs
3.2. Solvatochromism
3.3. Kinetics of Desorption from 1 and 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lewandowski, D.A. Design of Thermal Oxidation Systems for Volatile Organic Compounds; CRC Press LLC: Boca Raton, FL, USA, 2000. [Google Scholar]
- Martin, L.; Ognier, S.; Gasthauer, E.; Cavadias, S.; Dresvin, S.; Amouroux, J. Destruction of Highly Diluted Volatile Organic Components (VOCs) in Air by Dielectric Barrier Discharge and Mineral Bed Adsorption. Energy Fuels 2008, 22, 576–582. [Google Scholar] [CrossRef]
- Hinojosa-Reyes, M.; Arriaga, S.; Diaz-Torres, L.A.; Rodríguez-González, V. Gas-Phase Photocatalytic Decomposition of Ethylbenzene over Perlite Granules Coated with Indium Doped TiO2. Chem. Eng. J. 2013, 224, 106–113. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, S.; Yang, Y.; Li, X.; Li, J.; Li, Z. Competitive Adsorption and Selectivity of Benzene and Water Vapor on the Microporous Metal Organic Frameworks (HKUST-1). Chem. Eng. J. 2015, 259, 79–89. [Google Scholar] [CrossRef]
- Braek, A.M.; Almehaideb, R.A.; Darwish, N.; Hughes, R. Optimization of Process Parameters for Glycol Unit to Mitigate the Emission of BTEX/VOCs. Process Saf. Environ. Prot. 2001, 79, 218–232. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kim, H.J. The Effect of Temperature on VOCs and Carbonyl Compounds Emission from Wooden Flooring by Thermal Extractor Test Method. Build. Environ. 2012, 53, 95–99. [Google Scholar] [CrossRef]
- Yang, K.; Sun, Q.; Xue, F.; Lin, D. Adsorption of Volatile Organic Compounds by Metal–Organic Frameworks MIL-101: Influence of Molecular Size and Shape. J. Hazard. Mater. 2011, 195, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Li, Q.; Li, Y.; Liu, Y.; Li, A.; Zhang, Q. Adsorption Characteristics of Benzene-Chlorobenzene Vapor on Hypercrosslinked Polystyrene Adsorbent and a Pilot-Scale Application Study. Chem. Eng. J. 2010, 160, 723–728. [Google Scholar] [CrossRef]
- Bacchi, A.; Bourne, S.; Cantoni, G.; Cavallone, S.A.M.; Mazza, S.; Mehlana, G.; Pelagatti, P.; Righi, L. Reversible Guest Removal and Selective Guest Exchange with a Covalent Dinuclear Wheel-and-Axle Metallorganic Host Constituted by Half-Sandwich Ru(II) Wheels Connected by a Linear Diphosphine Axle. Cryst. Growth Des. 2015, 15, 1876–1888. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive Removal of Hazardous Materials Using Metal-Organic Frameworks (MOFs): A Review. J. Hazard. Mater. 2013, 244–245, 444–456. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, Y.; Zhang, X.; Tian, F.; Zu, Z. Zeolites Developed from Mixed Alkali Modified Coal Fly Ash for Adsorption of Volatile Organic Compounds. Mater. Lett. 2014, 119, 140–142. [Google Scholar] [CrossRef]
- Jhung, S.H.; Lee, J.H.; Yoon, J.W.; Serre, C.; Férey, G.; Chang, J.S. Microwave Synthesis of Chromium Terephthalate MIL-101 and Its Benzene Sorption Ability. Adv. Mater. 2007, 19, 121–124. [Google Scholar] [CrossRef]
- Kobalz, M.; Lincke, J.; Kobalz, K.; Erhart, O.; Bergmann, J.; Lässig, D.; Lange, M.; Möllmer, J.; Gläser, R.; Staudt, R.; et al. Paddle Wheel Based Triazolyl Isophthalate MOFs: Impact of Linker Modification on Crystal Structure and Gas Sorption Properties. Inorg. Chem. 2016, 55, 3030–3039. [Google Scholar] [CrossRef] [PubMed]
- Lincke, J.; Lässig, D.; Kobalz, M.; Bergmann, J.; Handke, M.; Möllmer, J.; Lange, M.; Roth, C.; Möller, A.; Staudt, R.; et al. An Isomorphous Series of Cubic, Copper-Based Triazolyl Isophthalate MOFs: Linker Substitution and Adsorption Properties. Inorg. Chem. 2012, 51, 7579–7586. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Vimont, A.; Daturi, M.; Hamon, L.; De Weireld, G.; Chang, J.-S.; Hong, D.-Y.; Hwang, Y.K.; et al. High Uptakes of CO2 and CH4 in Mesoporous Metal–Organic Frameworks MIL-100 and MIL-101. Langmuir 2008, 24, 7245–7250. [Google Scholar] [CrossRef] [PubMed]
- Junghans, U.; Kobalz, M.; Erhart, O.; Preißler, H.; Lincke, J.; Möllmer, J.; Krautscheid, H.; Gläser, R. A Series of Robust Copper-Based Triazolyl Isophthalate Mofs: Impact of Linker Functionalization on Gas Sorption and Catalytic Activity. Materials (Basel) 2017, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Hu, Z.; Yang, W.; Cao, D. Lithium Doping on Metal-Organic Frameworks for Enhancing H2 Storage. Int. J. Hydrogen Energy 2012, 37, 946–950. [Google Scholar] [CrossRef]
- Lv, X.; Shi, L.; Li, K.; Li, B.; Li, H. An Unusual Porous Cationic Metal – Organic Framework Fast and Highly Efficient Dichromate Trapping through a Single-Crystal to Single-Crystal Process. Chem. Commun. 2017, 53, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Mehlana, G.; Bourne, S.; Ramon, G. The Role of C–H⋯π Interactions in Modulating the Breathing Amplitude of a 2D Square Lattice Net: Alcohol Sorption Studies. CrystEngComm 2014, 16, 8160. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Ahmed, I.; Jhung, S.H. Remarkable Adsorbent for Phenol Removal from Fuel: Functionalized Metal–Organic Framework. Fuel 2016, 174, 43–48. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.Z.; Zhang, D.S.; Zhu, B.; Li, J.R. A Hydrothermally Stable Zn(II)-Based Metal-Organic Framework: Structural Modulation and Gas Adsorption. Dalton Trans. 2015, 44, 15697–15702. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-Y.; Wang, C.-J.; Chen, S.; Dai, H.-Y. A Terbium(III) Organic Framework as a Fluorescent Probe for Selectively Sensing of Organic Small Molecules and Metal Ions Especially Nitrobenzene and Fe3+. J. Coord. Chem. 2017, 70, 3996–4007. [Google Scholar] [CrossRef]
- Mahata, P.; Mondal, S.K.; Singha, D.K.; Majee, P. Luminescent Rare-Earth-Based MOFs as Optical Sensors. Dalton Trans. 2017, 46, 301–328. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, G.-P.; Zhao, Y.; Wu, W.-P.; Liu, B.; Wang, Y.-Y. Three New Solvent-Directed Cd(II)-Based MOFs with Unique Luminescent Properties and Highly Selective Sensors for Cu(2+) Cations and Nitrobenzene. Dalton Trans. 2015, 44, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical Sensors Definitions and Classification. Int. Union Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Zhou, H.L.; Zhang, Y.B.; Zhang, J.P.; Chen, X.M. Supramolecular-Jack-like Guest in Ultramicroporous Crystal for Exceptional Thermal Expansion Behaviour. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dzesse T, C.N.; Nfor, E.N.; Bourne, S.A. Vapor Sorption and Solvatochromism in a Metal-Organic Framework of an Asymmetric Pyridylcarboxylate. Cryst. Growth Des. 2018, 18, 416–423. [Google Scholar] [CrossRef]
- Mehlana, G.; Ramon, G.; Bourne, S.A. Methanol Mediated Crystal Transformations in a Solvatochromic Metal Organic Framework Constructed from Co(II) and 4-(4-Pyridyl) Benzoate. CrystEngComm 2013, 15, 9521–9529. [Google Scholar] [CrossRef][Green Version]
- Davies, K.; Bourne, S.A.; Öhrström, L.; Oliver, C.L. Anionic Zinc-Trimesic Acid MOFs with Unusual Topologies: Reversible Hydration Studies. Dalton Trans. 2010, 39, 2869–2874. [Google Scholar] [CrossRef]
- Lässig, D.; Lincke, J.; Moellmer, J.; Reichenbach, C.; Moeller, A.; Gläser, R.; Kalies, G.; Cychosz, K.A.; Thommes, M.; Staudt, R.; et al. A Microporous Copper Metal-Organic Framework with High H2 and CO2 Adsorption Capacity at Ambient Pressure. Angew. Chem. Int. Ed. 2011, 50, 10344–10348. [Google Scholar] [CrossRef]
- Kubo, M.; Ushiyama, H.; Shimojima, A.; Okubo, T. Investigation on Specific Adsorption of Hydrogen on Lithium-Doped Mesoporous Silica. Adsorption 2011, 17, 211–218. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Version 2.05; University of Göttingen: Göttingen, Germany, 2007. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.J. X-Seed—A software tool for supramolecular crystallography. J. Supramol. Chem. 2001, 1, 189–191. [Google Scholar] [CrossRef]
- Wang, Z.; Richter, S.M.; Rozema, M.J.; Schellinger, A.; Smith, K.; Napolitano, J.G. Potential Safety Hazards Associated with Using Acetonitrile and a Strong Aqueous Base. Org. Process Res. Dev. 2017, 21, 1501–1508. [Google Scholar] [CrossRef]
- Bennett, J.A.; Parlett, C.M.A.; Isaacs, M.A.; Durndell, L.J.; Olivi, L.; Lee, A.F.; Wilson, K. Acetic Acid Ketonization over Fe3O4/SiO2 for Pyrolysis Bio-Oil Upgrading. ChemCatChem 2017, 9, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Lukevics, E.; Stonkus, V.; Liepina, I.; Edolfa, K.; Jansone, D.; Leite, L.; Lukevics, E. Theoretical Study of the Ketonization Reaction Mechanism of Acetic Acid. Latvian J. Chem. 2009, 1, 61–67. [Google Scholar]
- Mehlana, G.; Bourne, S.A.; Ramon, G. A New Class of Thermo- and Solvatochromic Metal–Organic Frameworks Based on 4-(Pyridin-4-Yl)Benzoic Acid. Dalton Trans. 2012, 41, 4224. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent Metal–Organic Frameworks for Chemical Sensing and Explosive Detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Xu, J.; Cao, D.; Chang, Z.; Bu, X.H. A Rigid Nested Metal–Organic Framework Featuring a Thermoresponsive Gating Effect Dominated by Counterions. Angew. Chem. Int. Ed. 2016, 55, 15027–15030. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; Mccabe, P.; Pidcock, E.; Rodriguez-monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Hwang, Y.K.; Hong, D.Y.; Chang, J.S.; Jhung, S.H.; Seo, Y.K.; Kim, J.; Vimont, A.; Daturi, M.; Serre, C.; Férey, G. Amine Grafting on Coordinatively Unsaturated Metal Centers of MOFs: Consequences for Catalysis and Metal Encapsulation. Angew. Chem. Int. Ed. 2008, 47, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Prodi, L.; Bolletta, F.; Montalti, M.; Zaccheroni, N. Luminescent Chemosensors for Transition Metal Ions. Coord. Chem. Rev. 2000, 205, 59–83. [Google Scholar] [CrossRef]
- Britt, D.; Tranchemontagne, D.; Yaghi, O.M. Metal-Organic Frameworks with High Capacity and Selectivity for Harmful Gases. Proc. Natl. Acad. Sci. USA 2008, 105, 11623–11627. [Google Scholar] [CrossRef] [PubMed]
- Dybtsev, D.N.; Chun, H.; Kim, K. Rigid and Flexible: A Highly Porous Metal–Organic Framework with Unusual Guest-Dependent Dynamic Behavior. Angew. Chem. Int. Ed. 2004, 116, 5143–5146. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Kim, J.; Ahn, W. Liquid Phase Adsorption of Selected Chloroaromatic Compounds over Metal Organic Frameworks. Mater. Res. Bull. 2013, 48, 4499–4505. [Google Scholar] [CrossRef]
- Mehlana, G.; Bourne, S.A.; Ramon, G.; Öhrström, L. Concomitant Metal Organic Frameworks of Cobalt(II) and 3-(4-Pyridyl) Benzoate: Optimized Synthetic Conditions of Solvatochromic and Thermochromic Systems. Cryst. Growth Des. 2013, 13, 633–644. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Khuong, T.; Ramsahye, N.A.; Trens, P.; Tanchoux, N.; Serre, C.; Fajula, F.; Férey, G. Microporous and Mesoporous Materials Adsorption of C5–C9 Hydrocarbons in Microporous MOFs MIL-100 ( Cr ) and MIL-101 ( Cr ): A Manometric Study. Microporous Mesoporous Mater. 2010, 134, 134–140. [Google Scholar]
- Xian, S.; Yu, Y.; Xiao, J.; Zhang, Z.; Xia, Q.; Wang, H.; Li, Z. RSC Advances Competitive Adsorption of Water Vapor with VOCs Dichloroethane, Ethyl Acetate and Benzene on MIL-101(Cr) in Humid Atmosphere. RSC Adv. 2015, 5, 1827–1834. [Google Scholar] [CrossRef]
- Costa, C.; Dzikh, I.P.; Lopes, M.; Lemos, F. Activity–Acidity Relationship in Zeolite ZSM-5. Application of Bronsted-Type Equations. Mol. Catal. A 2000, 154, 193–201. [Google Scholar] [CrossRef]
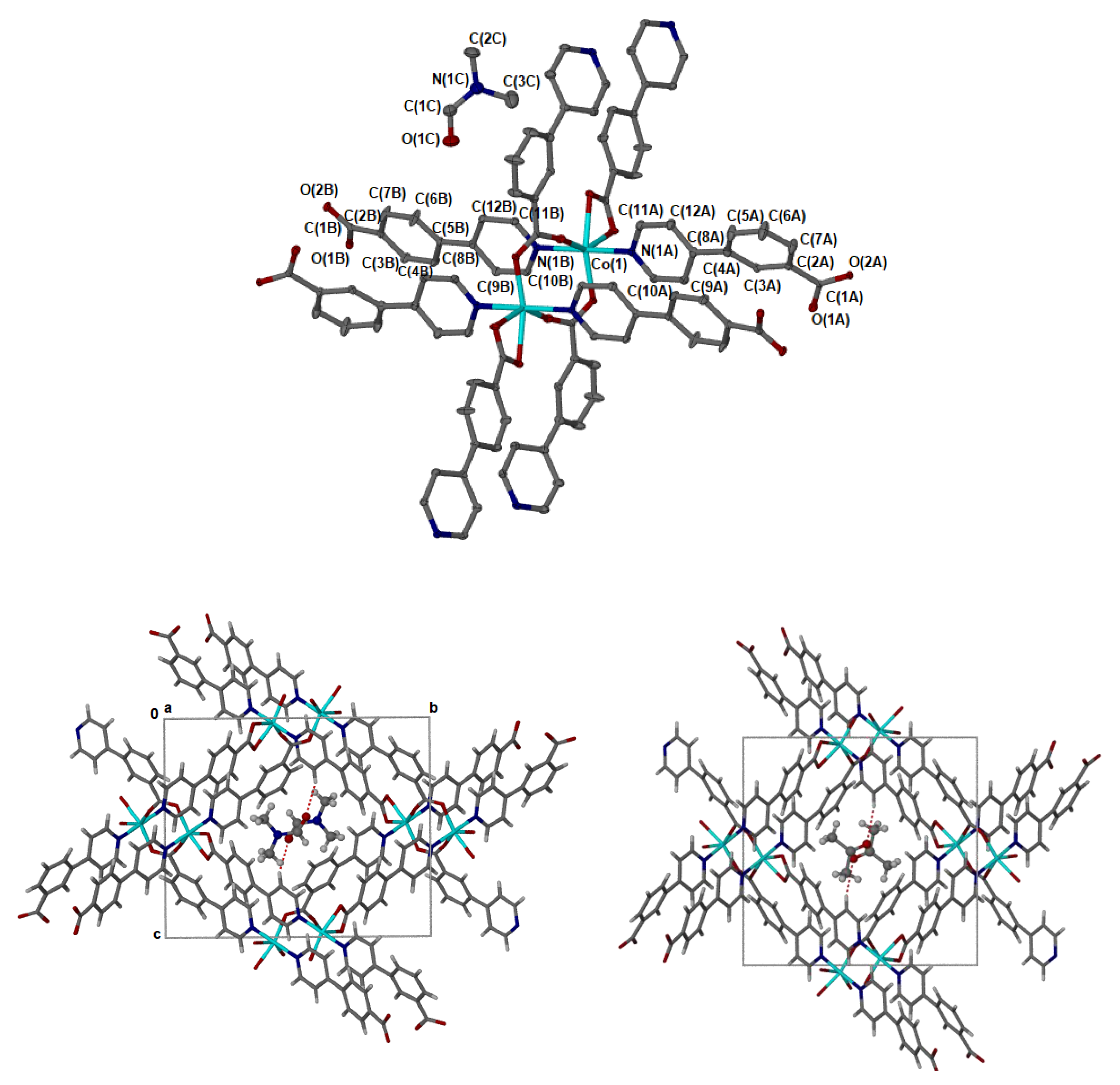
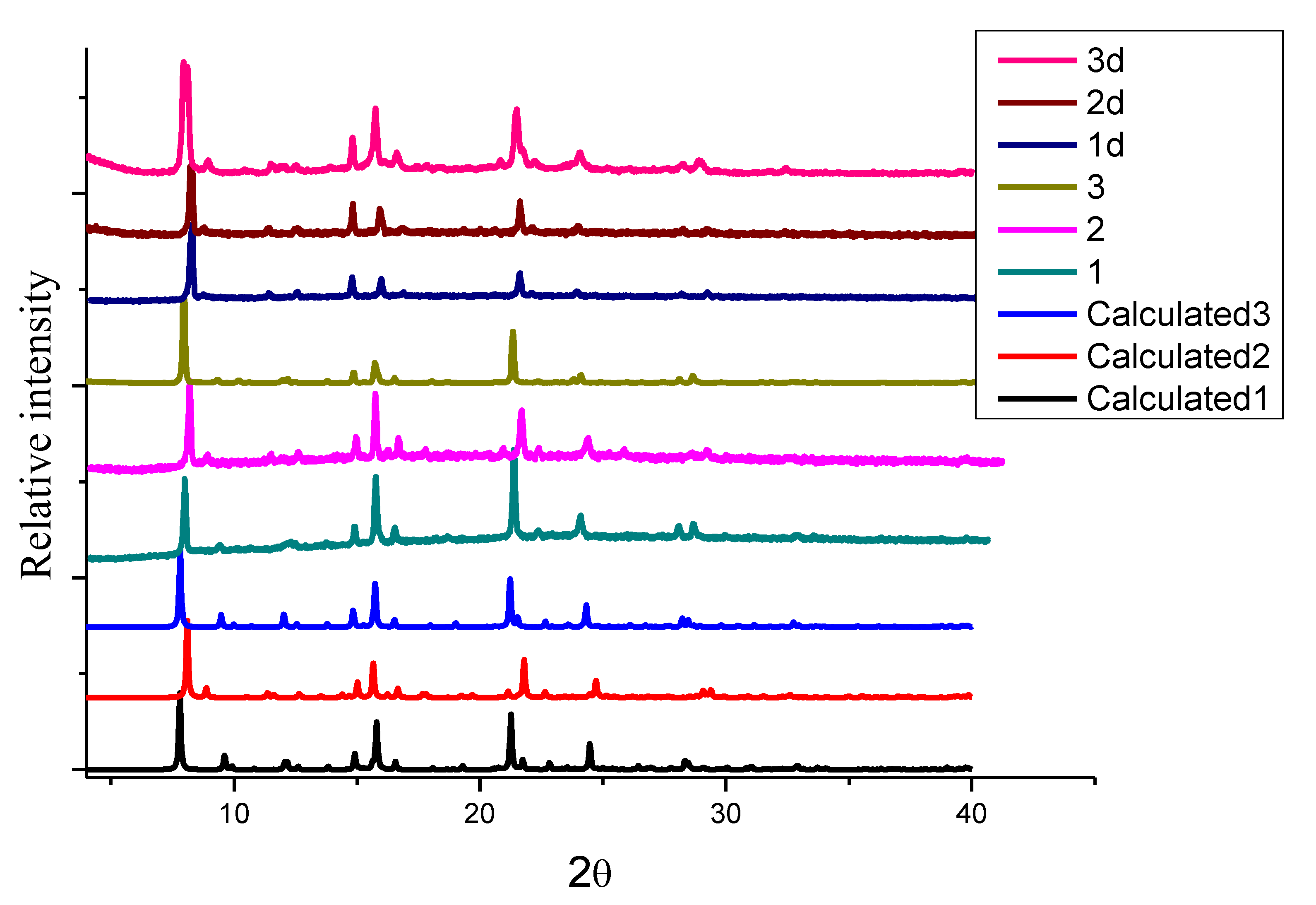
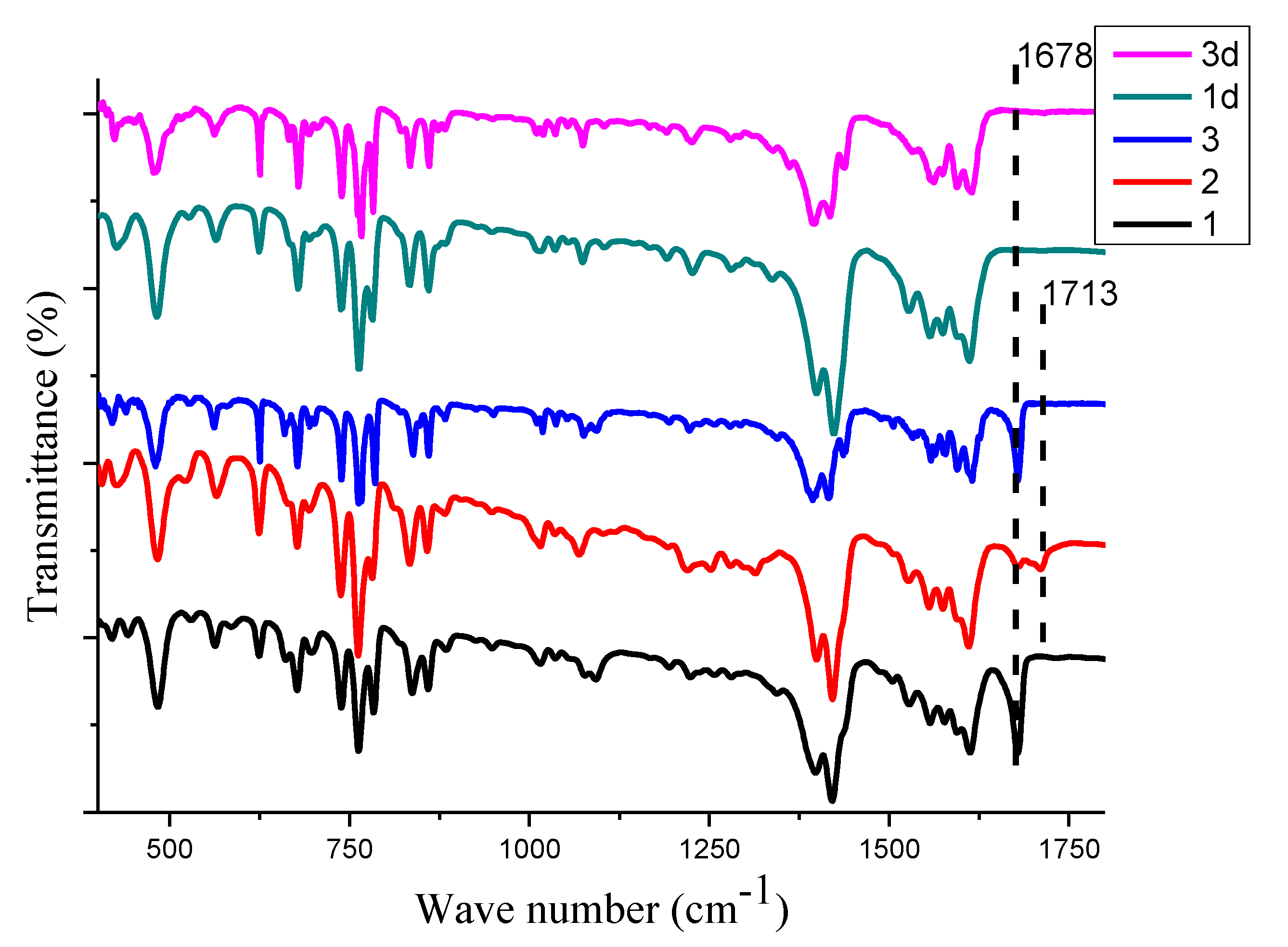

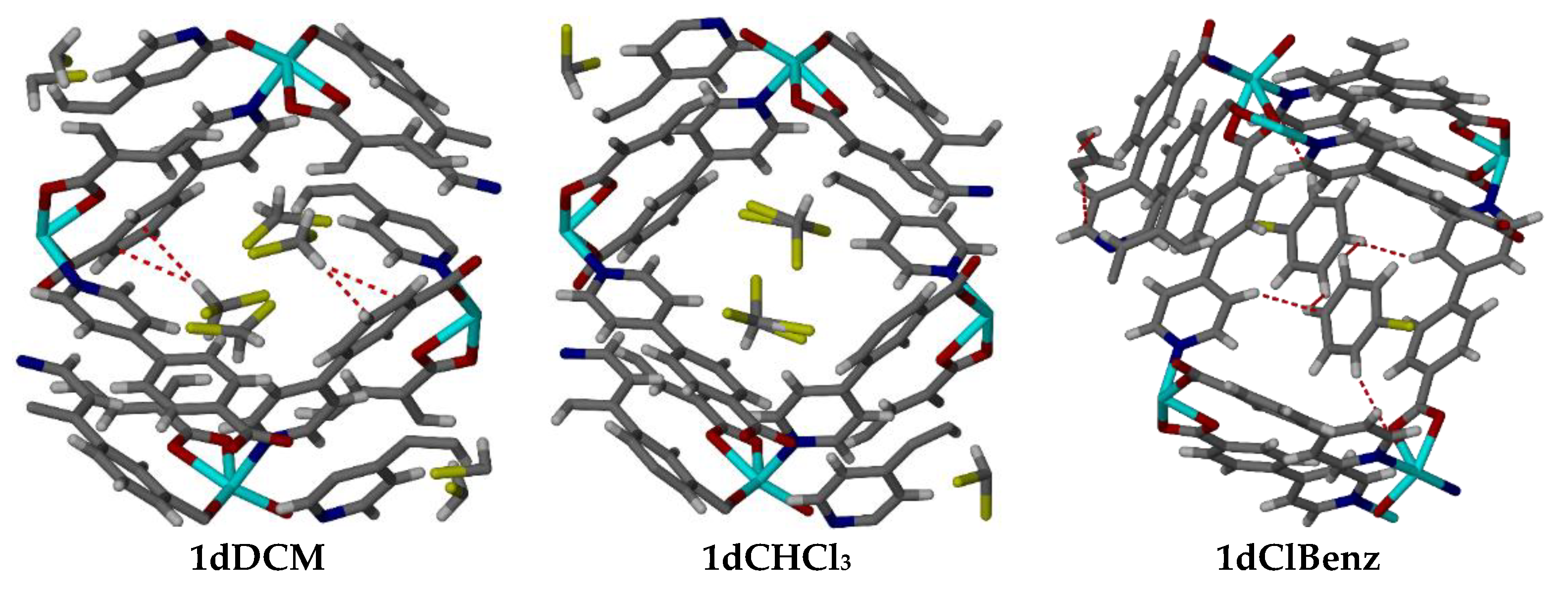
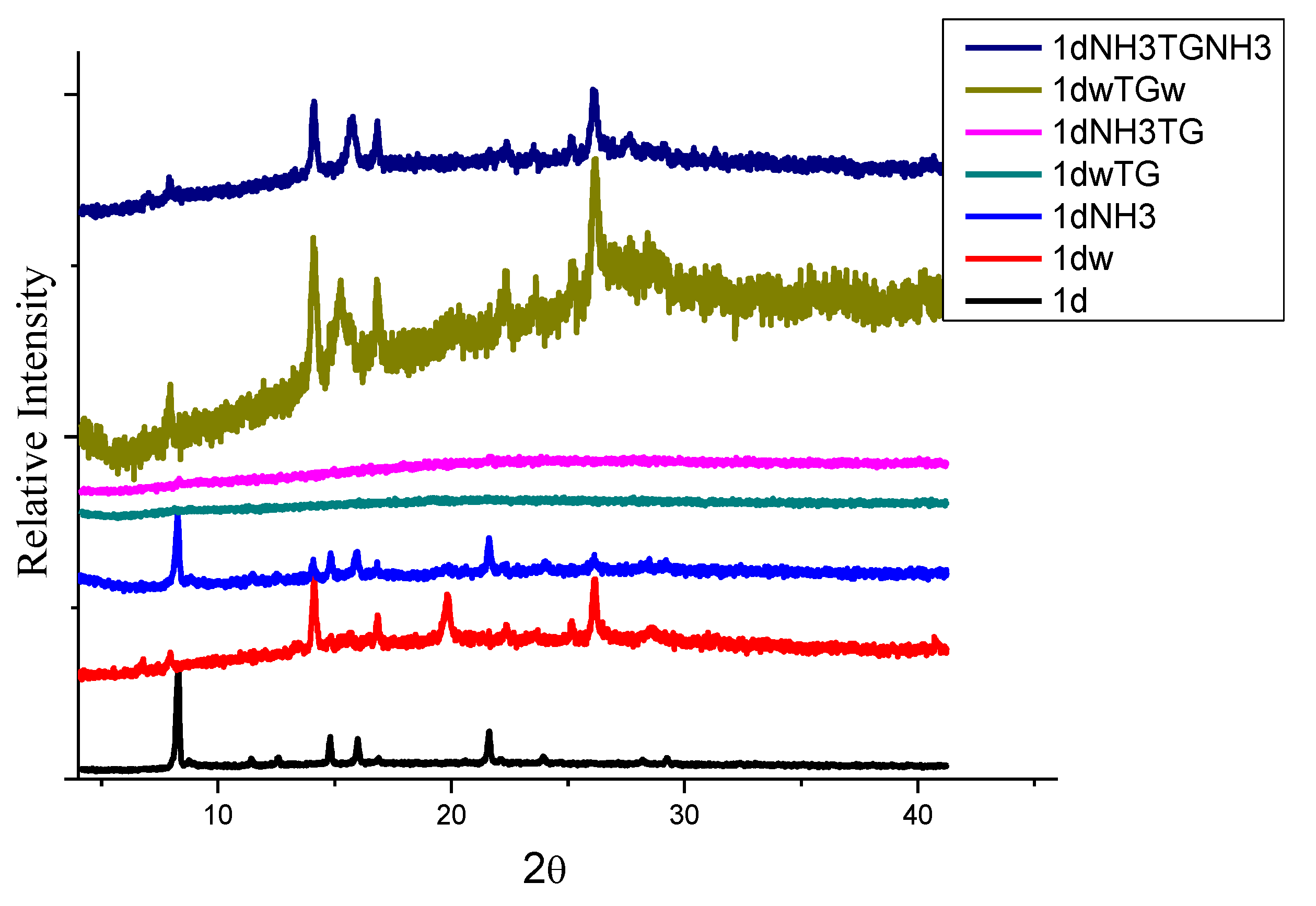
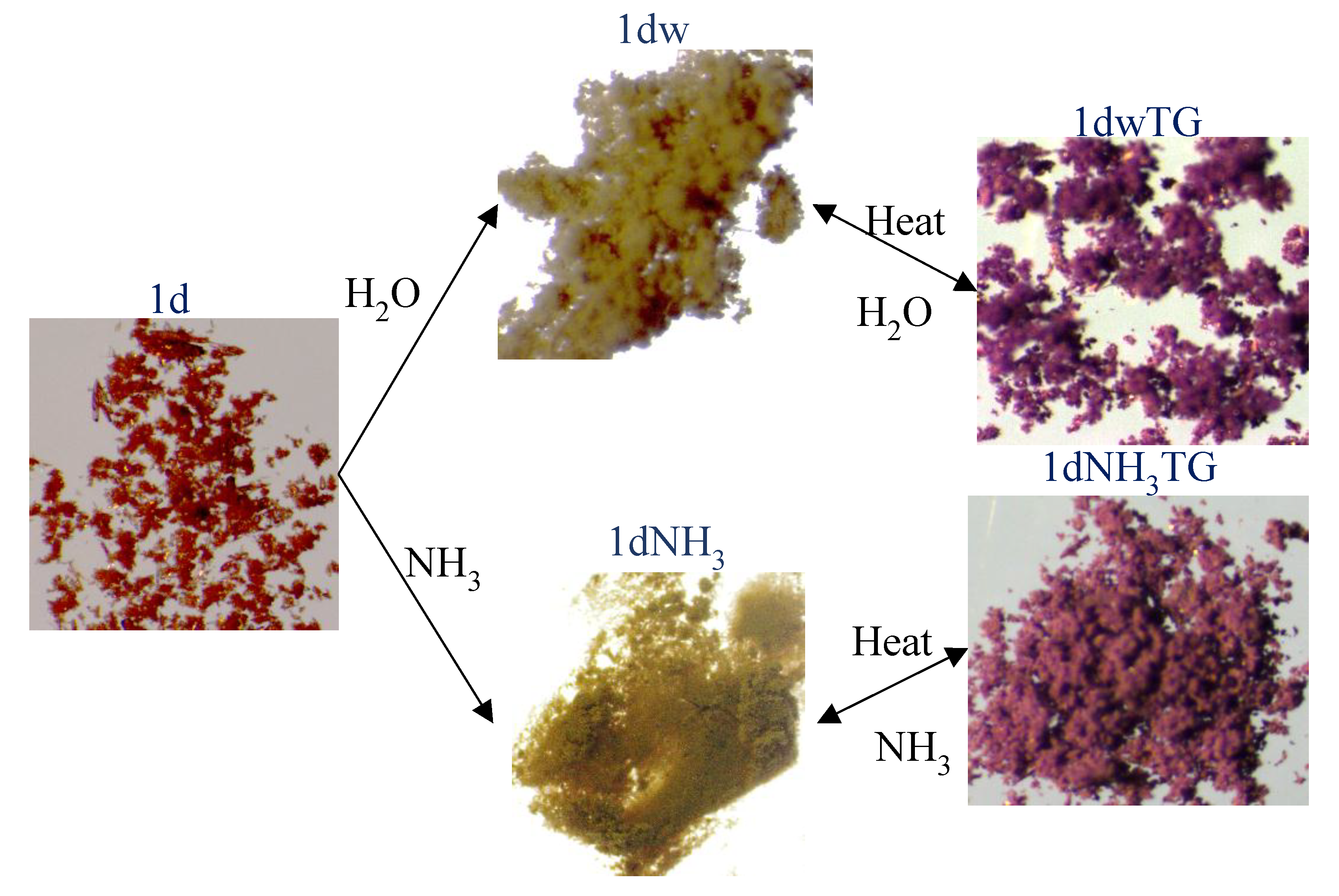
| Metal Salt | Ligands | Solvent System | Conditions | |
|---|---|---|---|---|
| 1 | CoCl2·6H2O (6 mg, 0.03 mmol) | 34pba/44pba (10 mg, 0.050 mmol each) | DMF(6 mL)/Ethanol (2 mL) | 120 °C for 3 days |
| 2 | CoCl2·6H2O (6 mg, 0.03 mmol) | 34pba/44pba (10 mg, 0.050 mmol each) | Acetonitrile(6 mL)/water (2 mL) | 120 °C for 3 days |
| 3 | Zn(NO3)·6H2O (30 mg, 0.13 mmol) | 34pba/44pba (40 mg, 0.20 mmol each) | DMF(6 mL)/Ethanol (2 mL) | 120 °C for 3 days |
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Formula | C27H23CoN3O5 | C27H22CoN2O5 | C27H23N3O5Zn |
| Mass (g·mol−1) | 528.41 | 513.39 | 534.85 |
| Crystal size (mm3) | 0.080 × 0.10 × 0.11 | 0.030 × 0.060 × 0.090 | 0.030 × 0.030 × 0.090 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c | P21/c |
| a (Å) | 9.203(2) | 10.068(4) | 9.339(1) |
| b (Å) | 17.823(4) | 15.632(5) | 17.678(3) |
| c (Å) | 14.718(3) | 15.399(5) | 14.735(2) |
| β (°) | 92.75(3) | 98.588(7) | 93.189(5) |
| V (Å3) | 2411.3(8) | 2396.4(1) | 2428.84(7) |
| T (K) | 100(2) | 100(2) | 173(2) |
| Z | 4 | 4 | 4 |
| Dc (g·cm−3) | 1.456 | 1.423 | 1.463 |
| μ(Mo−Kα) (mm−1) | 0.756 | 0.757 | 1.055 |
| F(000) | 1092 | 1060 | 1104 |
| Range scanned, θ (°) | 1.80–28.34 | 1.87–25.09 | 1.80–27.58 |
| No. reflections collected | 22,928 | 18,219 | 22,013 |
| No. unique reflection | 5981 | 4250 | 5584 |
| No. reflections with I ≥ 2σ(I) | 4089 | 2860 | 3713 |
| Parameters/restraints | 327/0 | 318/0 | 327/0 |
| Goodness of fit, S | 1.034 | 1.024 | 1.006 |
| Final R indices (I ≥ 2σ(I)) | 0.0859 | 0.0899 | 0.0867 |
| Final wR2 (all data) | 0.1198 | 0.1248 | 0.1107 |
| Min, max e− density (e Å−3) | 0.414, −0.417 | 0.653, −0.455 | 0.421, −0.443 |
| 1d | Mole Ratio of VOCs in 1d a | Selectivity (Major Component) |
| DCM/Chloroform | 1:1 | none |
| DCM/Chlorobenzene | 8.3:1 | DCM |
| Chloroform/Chlorobenzene | 10:1 | Chloroform |
| 3d | Mole Ratio of VOCs in 3d | Selectivity (Major Component) |
| DCM/Chloroform | 1.3:1 | DCM |
| DCM/Chlorobenzene | 1:0 | DCM |
| Chloroform/Chlorobenzene | 3:1 | Chloroform |
| VOC | Experimental Mass Loss, TGA (%) | Temperature Range of Mass Loss (°C) | Loading Capacity, Lc (x in Proposed Formula: {[M(34pba)(44pba)]·x Solvent}n) | MLc | % Loading Capacity |
|---|---|---|---|---|---|
| 1d | |||||
| DCM | 14.0 | 60–154 | 0.9 | 1.3 | 69 |
| CHCl3 | 17.1 | 118–285 | 0.8 | 1.0 | 80 |
| ClBenz | 13.0 | 87–264 | 0.6 | 0.8 | 75 |
| H2O | 15.4 | 60–134 | 4.6 | 4.6 | 100 |
| NH3 | 12.9 | 60–150 | 4.0 | 3.5 | 114 |
| MeNH2 | 26.1 | 30–220 | 5.2 | 1.9 | 273 |
| PropNH2 | 33.4 | 30–220 | 3.9 | 1.0 | 390 |
| ButNH2 | 31.0 | 30–220 | 2.8 | 0.8 | 350 |
| BzNH2 | 52.0 | 65–260 | 4.6 | 0.8 | 575 |
| PhEtNH2 | 9.7 | 170–310 | 0.4 | 0.7 | 57 |
| 3d | |||||
| DCM | 11.0 | 88–220 | 0.7 | 1.4 | 50 |
| CHCl3 | 13.3 | 110–232 | 0.6 | 1.1 | 55 |
| ClBenz | 11.0 | 61–252 | 0.5 | 0.8 | 63 |
| H2O | 12.9 | 73–155 | 3.8 | 4.8 | 79 |
| NH3 | 12.5 | 59–127 | 3.9 | 3.6 | 108 |
| MeNH2 | 18.2 | 30–280 | 3.3 | 1.9 | 174 |
| PropNH2 | 18.4 | 30–263 | 1.8 | 1.0 | 180 |
| ButNH2 | 29.2 | 50–290 | 2.6 | 0.9 | 289 |
| BzNH2 | 36.0 | 88–290 | 2.4 | 0.8 | 300 |
| PhEtNH2 | 8.4 | 77–290 | 0.3 | 0.7 | 43 |
| Mass Loss (%) | Ea (kJ mol−1) | |||||
|---|---|---|---|---|---|---|
| DMF from 1d | DMF from 3d | H2O from 1dW | H2O from 3dW | NH3 from 1dNH3 | NH3 from 3dNH3 | |
| 20 | 74.77 | 68.77 | 77.3 | 64.78 | 65. 10 | 58.46 |
| 40 | 75.31 | 66.50 | 72.59 | 57.35 | 67.8 | 59.39 |
| 60 | 72.77 | 70.57 | 75.24 | 65.23 | 68.61 | 62.01 |
| 80 | 77.30 | 64.08 | 74.75 | 68.38 | 68.77 | 62.01 |
| Mean | 75.04 ± 1.68 | 67.48 ± 2.81 | 74.97 ± 1.93 | 63.94 ± 4.67 | 67.57 ± 1.70 | 60.47 ± 1.82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndamyabera, C.A.; Zacharias, S.C.; Oliver, C.L.; Bourne, S.A. Solvatochromism and Selective Sorption of Volatile Organic Solvents in Pyridylbenzoate Metal-Organic Frameworks. Chemistry 2019, 1, 111-125. https://doi.org/10.3390/chemistry1010009
Ndamyabera CA, Zacharias SC, Oliver CL, Bourne SA. Solvatochromism and Selective Sorption of Volatile Organic Solvents in Pyridylbenzoate Metal-Organic Frameworks. Chemistry. 2019; 1(1):111-125. https://doi.org/10.3390/chemistry1010009
Chicago/Turabian StyleNdamyabera, Christophe A., Savannah C. Zacharias, Clive L. Oliver, and Susan A. Bourne. 2019. "Solvatochromism and Selective Sorption of Volatile Organic Solvents in Pyridylbenzoate Metal-Organic Frameworks" Chemistry 1, no. 1: 111-125. https://doi.org/10.3390/chemistry1010009
APA StyleNdamyabera, C. A., Zacharias, S. C., Oliver, C. L., & Bourne, S. A. (2019). Solvatochromism and Selective Sorption of Volatile Organic Solvents in Pyridylbenzoate Metal-Organic Frameworks. Chemistry, 1(1), 111-125. https://doi.org/10.3390/chemistry1010009