Advanced Nanoparticles in Combating Antibiotic Resistance: Current Innovations and Future Directions
Abstract
1. Introduction
2. Types of Nanoparticles Used Against Antibiotic-Resistant Bacteria
2.1. Metal and Metal Oxide Nanoparticles
2.2. Lipid-Based Nanoparticles
2.3. Polymer-Based Nanoparticles
2.4. Carbon-Based Nanoparticles
2.5. Composite Nanoparticles
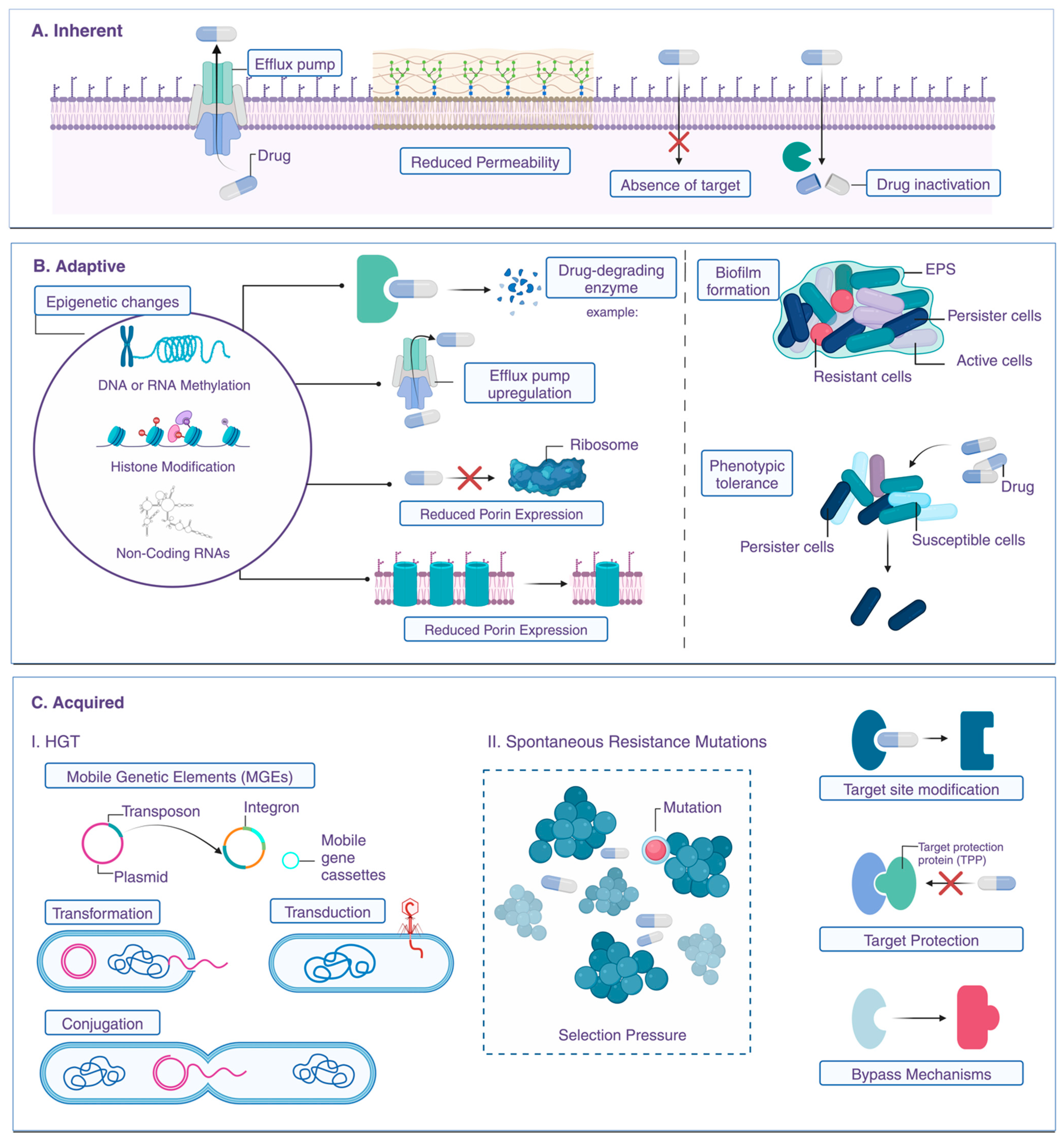
3. Mechanisms of Antibiotic Resistance
3.1. Enzyme Production (e.g., Beta-Lactamases)
3.2. Efflux Pumps and Reduced Permeability
3.3. Alterations in Target Sites
3.4. Biofilm Formation and Resistance
3.5. Quorum Sensing and Antibiotic Resistance
4. Challenges in Developing New Antibiotics
4.1. Rapid Resistance Development
4.2. Limited Pharmaceutical Innovation and Drug Pipelines
5. Mechanisms of Action of Nanoparticles Against Bacteria
6. Recent Advances and Case Studies
6.1. Case Studies of Successful Nanoparticle Applications
6.2. Notable Advances in Clinical Trials and Preclinical Studies
6.3. Nanoparticles as Antibacterial Agents in Medical Devices
7. Challenges and Limitations of Using Nanoparticles in Treating Resistant Bacteria
7.1. Potential Toxicity to Human Cells and Environmental Impact
7.2. Regulatory and Manufacturing Challenges
7.2.1. Ensuring Consistent Quality and Safety in Production
7.2.2. Factors Affecting the Efficient Production of Nanoparticles
7.2.3. Regulatory Approval Challenges
7.3. Cost and Accessibility of Nanotechnology-Based Treatments
Balancing Advanced Technology with Affordability and Scalability
8. Future Directions in Nanotechnology for Combating Antibiotic Resistance
8.1. Emerging Nanoparticle Designs New Materials and Hybrid Nanoparticles for More Effective Antibacterial Action
8.2. Personalized Nanomedicine Tailoring Nanoparticle Treatment to Individual Bacterial Infections and Patient Needs
8.3. Integration with Other Therapeutic Modalities Combining Nanoparticles with Phage Therapy, CRISPR, and Immunotherapy
8.3.1. Combining Nanoparticles with Phage Therapy
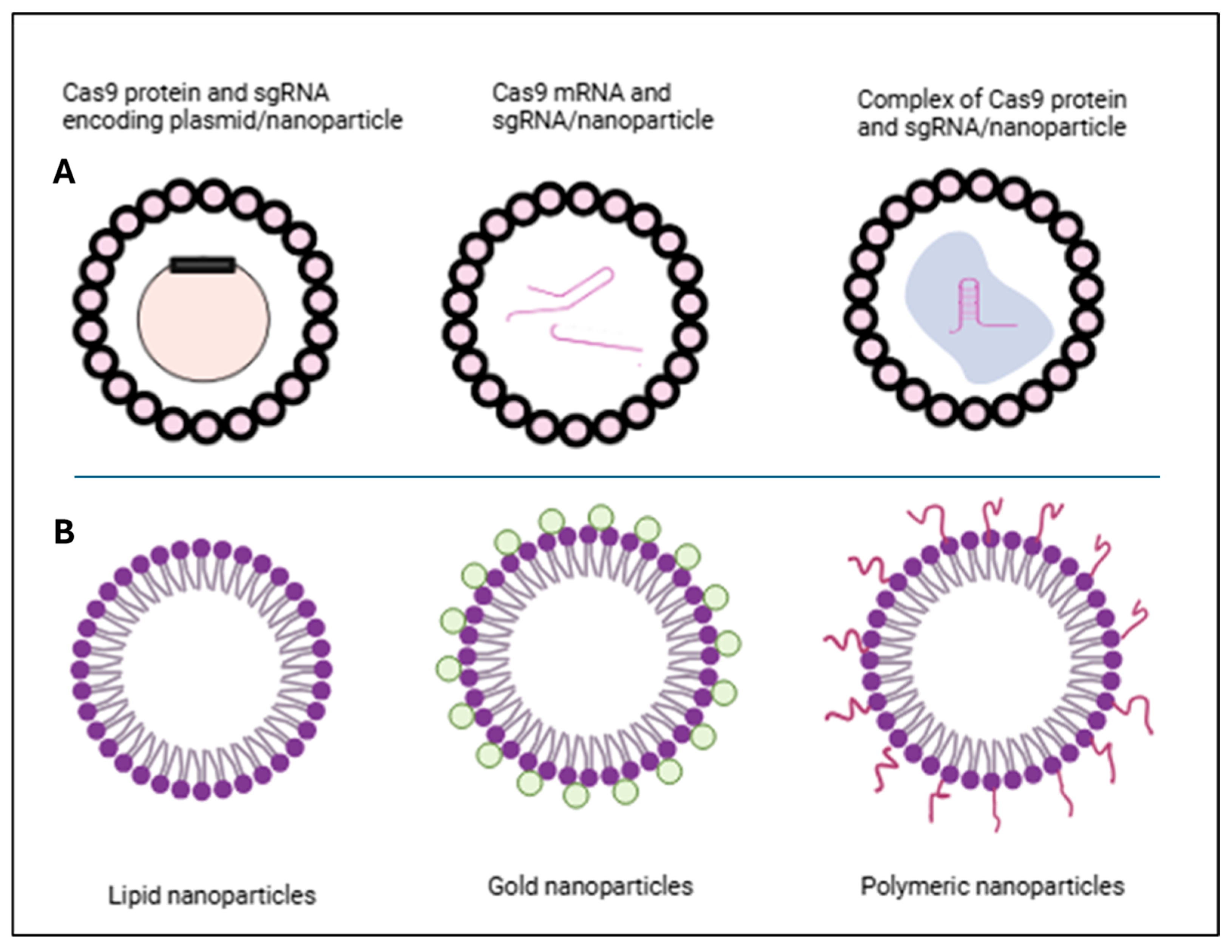
8.3.2. Combining Nanoparticles with CRISPR Therapy
8.3.3. Combining Nanoparticles with Immunotherapy
8.3.4. Combining Nanoparticles with Peptide
8.3.5. Integration of Nanotechnology and 3D Printing in Drug Delivery and Personalized Medicine
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ag | silver |
| AMR | antimicrobial resistance |
| AgNPs | silver nanoparticles |
| Au | gold |
| AuNPs | gold nanoparticles |
| CDC | Centers for Disease Control |
| CNTs | carbon nanotubes |
| CU | copper |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| DCs | dendritic cells |
| DNA | deoxyribonucleic acid |
| EPS | extracellular polymeric substance |
| ESBL | extended-spectrum beta-lactamases |
| Fe₂O₃ | hematite |
| FDA | Food and Drug Administration |
| GQDs | graphene quantum dots |
| GMPs | Good Manufacturing Practices |
| GO | graphene oxide |
| GLPs | Good Laboratory Practices |
| ICD | immunogenic cell death |
| LHNPs | liposome-templated hydrogel nanoparticles |
| LNPs | lipid nanoparticles |
| LNLS | lipid nanostructured lipid systems |
| MBLs | metallo-beta-lactamases |
| MDR | multidrug resistance |
| MIC | minimum inhibitory ccncentration |
| MOFs | metal–organic frameworks |
| MRI | magnetic resonance imaging |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MSNs | mesoporous silica nanoparticles |
| NCTR | National Center for Toxicological Research |
| NM | nanometers |
| NMDs | nanomedical devices |
| NPs | nanoparticles |
| PaβN | β-naphthylamide |
| PBPs | penicillin-binding proteins |
| PDA | polydopamine |
| PEG | polyethylene glycol |
| PEI | polyethyleneimine |
| PCL | polycaprolactone |
| PLGA | poly-lactic-co-glycolic acid |
| QS | quorum sensing |
| QSIs | quorum sensing inhibitors |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| SeNPs | selenium nanoparticles |
| SLNs | solid lipid nanoparticles |
| SORT | selective organ targeting |
| SPR | surface plasmon resonance |
| TiO2 NPs | titanium dioxide nanoparticles |
| UV | ultraviolet |
| WHO | World Health organization |
| ZN | zinc |
| ZnO NPs | zinc oxide nanoparticles |
References
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Devi, N.S.; Mythili, R.; Cherian, T.; Dineshkumar, R.; Sivaraman, G.K.; Jayakumar, R.; Prathaban, M.; Duraimurugan, M.; Chandrasekar, V.; Peijnenburg, W.J.G.M. Overview of antimicrobial resistance and mechanisms: The relative status of the past and current. Microbe 2024, 3, 100083. [Google Scholar] [CrossRef]
- Mittal, A.K.; Bhardwaj, R.; Mishra, P.; Rajput, S.K. Antimicrobials Misuse/Overuse: Adverse Effect, Mechanism, Challenges and Strategies to Combat Resistance. Open Biotechnol. J. 2020, 14, 107–112. [Google Scholar] [CrossRef]
- Abbas, A.; Barkhouse, A.; Hackenberger, D.; Wright, G.D. Antibiotic resistance: A key microbial survival mechanism that threatens public health. Cell Host Microbe 2024, 32, 837–851. [Google Scholar] [CrossRef]
- Okaiyeto, S.A.; Sutar, P.P.; Chen, C.; Ni, J.-B.; Wang, J.; Mujumdar, A.S.; Zhang, J.-S.; Xu, M.-Q.; Fang, X.-M.; Zhang, C.; et al. Antibiotic resistant bacteria in food systems: Current status, resistance mechanisms, and mitigation strategies. Agric. Commun. 2024, 2, 100027. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Ferraz, M.P. Antimicrobial Resistance: The Impact from and on Society According to One Health Approach. Societies 2024, 14, 187. [Google Scholar] [CrossRef]
- Sartelli, M.; Marini, C.P.; McNelis, J.; Coccolini, F.; Rizzo, C.; Labricciosa, F.M.; Petrone, P. Preventing and Controlling Healthcare-Associated Infections: The First Principle of Every Antimicrobial Stewardship Program in Hospital Settings. Antibiotics 2024, 13, 896. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Antimicrobial resistance: A concise update. Lancet Microbe 2024, 6, 100947. [Google Scholar] [CrossRef] [PubMed]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, Q.; Zhou, X.; Momeni, M.R. Antibiotic resistance and nanotechnology: A narrative review. Microb. Pathog. 2024, 193, 106741. [Google Scholar] [CrossRef] [PubMed]
- Kadeřábková, N.; Mahmood, A.J.S.; Mavridou, D.A.I. Antibiotic susceptibility testing using minimum inhibitory concentration (MIC) assays. npj Antimicrob. Resist. 2024, 2, 37. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef]
- Jin, S.-E.; Jin, H.-E. Antimicrobial Activity of Zinc Oxide Nano/Microparticles and Their Combinations against Pathogenic Microorganisms for Biomedical Applications: From Physicochemical Characteristics to Pharmacological Aspects. Nanomaterials 2021, 11, 263. [Google Scholar] [CrossRef]
- Wu, L.-P.; Wang, D.; Li, Z. Grand challenges in nanomedicine. Mater. Sci. Eng. C 2020, 106, 110302. [Google Scholar] [CrossRef]
- Fanciullino, R.; Ciccolini, J.; Milano, G. Challenges, expectations and limits for nanoparticles-based therapeutics in cancer: A focus on nano-albumin-bound drugs. Crit. Rev. Oncol./Hematol. 2013, 88, 504–513. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Nahar, D.; Mohite, P.; Lonkar, A.; Chidrawar, V.R.; Dodiya, R.; Uddin, M.J.; Singh, S.; Prajapati, B.G. An insight into new strategies and targets to combat antifungal resistance: A comprehensive review. Eur. J. Med. Chem. Rep. 2024, 10, 100120. [Google Scholar] [CrossRef]
- Alfutaimani, A.S.; Alharbi, N.K.; Alahmari, A.; Alqabbani, A.; Aldayel, A.M. Exploring the landscape of Lipid Nanoparticles (LNPs): A comprehensive review of LNPs types and biological sources of lipids. Int. J. Pharm. X 2024, 8, 100305. [Google Scholar] [CrossRef] [PubMed]
- Nazli, A.; He, D.L.; Liao, D.; Khan, M.Z.I.; Huang, C.; He, Y. Strategies and progresses for enhancing targeted antibiotic delivery. Adv. Drug Deliv. Rev. 2022, 189, 114502. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.; Kumar, A. Nanotechnology in Targeted Delivery of Antimicrobials and Overcoming Resistance. BioNanoScience 2024, 15, 20. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Li, X.; Liu, W. What happens when nanoparticles encounter bacterial antibiotic resistance? Sci. Total Environ. 2023, 876, 162856. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Finina, B.F.; Mersha, A.K. Nano-enabled antimicrobial thin films: Design and mechanism of action. RSC Adv. 2024, 14, 5290–5308. [Google Scholar] [CrossRef]
- Nawrot, R.; Barylski, J.; Nowicki, G.; Broniarczyk, J.; Buchwald, W.; Goździcka-Józefiak, A. Plant antimicrobial peptides. Folia Microbiol. 2014, 59, 181–196. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Rashed, Z.I.; Alharbi, A.A.; Alsharef, S.; Alkindy, T.T.; Alkhamali, A.; Albalawi, A.S.; Battah, B.; Donadu, M.G. Quorum Sensing Inhibitors: An Alternative Strategy to Win the Battle Against Multidrug-Resistant (MDR) Bacteria. Molecules 2024, 29, 3466. [Google Scholar] [CrossRef]
- Patel, K.; Panchal, R.; Sakariya, B.; Gevariya, M.; Raiyani, R.; Soni, R.; Goswami, D. Combatting antibiotic resistance by exploring the promise of Quorum Quenching in targeting bacterial virulence. Microbe 2025, 6, 100224. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Khan, M.E.; Mohammad, A.; Ali, W.; Imran, M.; Bashiri, A.H.; Zakri, W. 2—Properties of metal and metal oxides nanocomposites. In Nanocomposites-Advanced Materials for Energy and Environmental Aspects; Khan, M.E., Aslam, J., Verma, C., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 23–39. [Google Scholar]
- Akintelu, S.A.; Yao, B.; Folorunso, A.S. Bioremediation and pharmacological applications of gold nanoparticles synthesized from plant materials. Heliyon 2021, 7, e06591. [Google Scholar] [CrossRef] [PubMed]
- Ashhari, S.; Sehhat, E.; Ranjbar, Z. Nanomaterial-Based Antibacterial and Antiviral Thin Film Coatings. In Antibacterial and Antiviral Functional Materials, Volume 1; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2023; Volume 1458, pp. 203–250. [Google Scholar]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Magdy, G.; Aboelkassim, E.; Abd Elhaleem, S.M.; Belal, F. A comprehensive review on silver nanoparticles: Synthesis approaches, characterization techniques, and recent pharmaceutical, environmental, and antimicrobial applications. Microchem. J. 2024, 196, 109615. [Google Scholar] [CrossRef]
- Duman, H.; Eker, F.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties. Nanomaterials 2024, 14, 1527. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional Gold Nanoparticles as Potent Antimicrobial Agents against Multi-Drug-Resistant Bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef]
- Saed, M.; Ayivi, R.D.; Wei, J.; Obare, S.O. Gold nanoparticles antibacterial activity: Does the surface matter? Colloid. Interface Sci. Commun. 2024, 62, 100804. [Google Scholar] [CrossRef]
- Dey, S.; Mohanty, D.l.; Divya, N.; Bakshi, V.; Mohanty, A.; Rath, D.; Das, S.; Mondal, A.; Roy, S.; Sabui, R. A critical review on zinc oxide nanoparticles: Synthesis, properties and biomedical applications. Intell. Pharm. 2024, 3, 53–70. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Hayat, Z.; Zhang, D.-D.; Li, M.-Y.; Hu, S.; Wu, Q.; Cao, Y.-F.; Yuan, Y. Zinc Oxide Nanoparticles: Synthesis, Characterization, Modification, and Applications in Food and Agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci. 2022, 12, 141. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Holghoomi, R.; Shamsabadipour, A.; Maleki-baladi, R.; Rahdar, A.; Pandey, S. Copper nanoparticles from chemical, physical, and green synthesis to medicinal application: A review. Plant Nano Biol. 2024, 8, 100070. [Google Scholar] [CrossRef]
- Giannousi, K.; Pantazaki, A.; Dendrinou-Samara, C. Chapter 23—Copper-Based Nanoparticles as Antimicrobials. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 515–529. [Google Scholar]
- Manisekaran, R.; Rasu Chettiar, A.-D.; Marasamy, L.; Arthikala, M.K.; Kandasamy, G.; Campos, V.; Rathore, H. Copper, Zinc, and Titanium-Based Semiconductor Nanomaterials for Antimicrobial Coatings and Their Mechanisms. Nano Sel. 2024, e202400155. [Google Scholar] [CrossRef]
- Kaur, H.; Rauwel, P.; Rauwel, E. Chapter 6—Antimicrobial nanoparticles: Synthesis, mechanism of actions. In Antimicrobial Activity of Nanoparticles; Guisbiers, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 155–202. [Google Scholar]
- Yang, Y.; Liu, Y.; Song, L.; Cui, X.; Zhou, J.; Jin, G.; Boccaccini, A.R.; Virtanen, S. Iron oxide nanoparticle-based nanocomposites in biomedical application. Trends Biotechnol. 2023, 41, 1471–1487. [Google Scholar] [CrossRef]
- Nadeem, M.; Khan, R.; Shah, N.; Bangash, I.R.; Abbasi, B.H.; Hano, C.; Liu, C.; Ullah, S.; Hashmi, S.S.; Nadhman, A.; et al. A Review of Microbial Mediated Iron Nanoparticles (IONPs) and Its Biomedical Applications. Nanomaterials 2022, 12, 130. [Google Scholar] [CrossRef]
- Rathore, C.; Yadav, V.K.; Gacem, A.; AbdelRahim, S.K.; Verma, R.K.; Chundawat, R.S.; Gnanamoorthy, G.; Yadav, K.K.; Choudhary, N.; Sahoo, D.K.; et al. Microbial synthesis of titanium dioxide nanoparticles and their importance in wastewater treatment and antimicrobial activities: A review. Front. Microbiol. 2023, 14, 1270245. [Google Scholar] [CrossRef]
- Younis, A.B.; Haddad, Y.; Kosaristanova, L.; Smerkova, K. Titanium dioxide nanoparticles: Recent progress in antimicrobial applications. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1860. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Chen, Y.; Zhou, F.; Zhang, T.; Fan, X.; Chrzanowski, W.; Gillies, M.C.; Zhu, L. Recent advances and prospects for lipid-based nanoparticles as drug carriers in the treatment of human retinal diseases. Adv. Drug Deliv. Rev. 2023, 199, 114965. [Google Scholar] [CrossRef]
- Ghosh, R.; De, M. Liposome-Based Antibacterial Delivery: An Emergent Approach to Combat Bacterial Infections. ACS Omega 2023, 8, 35442–35451. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hasan, I.; Guo, B. Recent advances in nanoparticle-mediated antibacterial applications. Coord. Chem. Rev. 2023, 482, 215075. [Google Scholar] [CrossRef]
- Arabestani, M.R.; Bigham, A.; Kamarehei, F.; Dini, M.; Gorjikhah, F.; Shariati, A.; Hosseini, S.M. Solid lipid nanoparticles and their application in the treatment of bacterial infectious diseases. Biomed. Pharmacother. 2024, 174, 116433. [Google Scholar] [CrossRef]
- Nguyen, T.-T.-L.; Duong, V.-A. Solid Lipid Nanoparticles. Encyclopedia 2022, 2, 952–973. [Google Scholar] [CrossRef]
- Akanda, M.; Mithu, M.D.S.H.; Douroumis, D. Solid lipid nanoparticles: An effective lipid-based technology for cancer treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104709. [Google Scholar] [CrossRef]
- Pinilla, C.M.; Lopes, N.A.; Brandelli, A. Lipid-Based Nanostructures for the Delivery of Natural Antimicrobials. Molecules 2021, 26, 3587. [Google Scholar] [CrossRef]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies. Saudi Pharm. J. 2021, 29, 999–1012. [Google Scholar] [CrossRef]
- Shevalkar, G.; Vavia, P. Solidified nanostructured lipid carrier (S-NLC) for enhancing the oral bioavailability of ezetimibe. J. Drug Deliv. Sci. Technol. 2019, 53, 101211. [Google Scholar] [CrossRef]
- Perumal, S. Polymer Nanoparticles: Synthesis and Applications. Polymers 2022, 14, 5449. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, H.; Jangde, R.K. Current updated review on preparation of polymeric nanoparticles for drug delivery and biomedical applications. Next Nanotechnol. 2023, 2, 100013. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers 2024, 16, 2536. [Google Scholar] [CrossRef]
- Tarkistani, M.A.M.; Komalla, V.; Kayser, V. Recent Advances in the Use of Iron–Gold Hybrid Nanoparticles for Biomedical Applications. Nanomaterials 2021, 11, 1227. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, H.; Luo, Y.; Chen, Y.; Wang, M.; Wu, C.; Hu, P. Recent Applications of PLGA in Drug Delivery Systems. Polymers 2024, 16, 2606. [Google Scholar] [CrossRef]
- Gul, G.; Faller, R.; Ileri-Ercan, N. Polystyrene-modified carbon nanotubes: Promising carriers in targeted drug delivery. Biophys. J. 2022, 121, 4271–4279. [Google Scholar] [CrossRef]
- Haider, A.; Khan, S.; Iqbal, D.N.; Shrahili, M.; Haider, S.; Mohammad, K.; Mohammad, A.; Rizwan, M.; Kanwal, Q.; Mustafa, G. Advances in chitosan-based drug delivery systems: A comprehensive review for therapeutic applications. Eur. Polym. J. 2024, 210, 112983. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.; Pathan, A.; Nagaraj, S.; Kapare, H.; Giram, P.; Wavhale, R. Polycaprolactone and its derivatives for drug delivery. Polym. Adv. Technol. 2023, 34, 3296–3316. [Google Scholar] [CrossRef]
- Taghizadeh, F.; Heidari, M.; Mostafavi, S.; Mortazavi, S.M.; Haeri, A. A review of preparation methods and biomedical applications of poly(ε-caprolactone)-based novel formulations. J. Mater. Sci. 2024, 59, 10587–10622. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Ramalho, P.; Silva, A.P.; Teixeira-Santos, R.; Pina-Vaz, C.; Rodrigues, A.G. Polyethyleneimine and polyethyleneimine-based nanoparticles: Novel bacterial and yeast biofilm inhibitors. J. Med. Microbiol. 2014, 63, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, Z.M.; Guo, X.; Zhang, X. Synthesis and application of polyethyleneimine (PEI)-based composite/nanocomposite material for heavy metals removal from wastewater: A critical review. J. Hazard. Mater. Adv. 2022, 8, 100158. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Jin, W. Carbon nanomaterials: Synthesis, properties and applications in electrochemical sensors and energy conversion systems. Mater. Sci. Eng. B 2021, 272, 115341. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Y.; Lin, Y.; Wang, L.; Ma, R.; Zhang, Y.; Feng, X.; Wu, J.; Chen, L.; Shao, L. GO-based antibacterial composites: Application and design strategies. Adv. Drug Deliv. Rev. 2021, 178, 113967. [Google Scholar] [CrossRef]
- Rao, N.; Singh, R.; Bashambu, L. Carbon-based nanomaterials: Synthesis and prospective applications. Mater. Today Proc. 2021, 44, 608–614. [Google Scholar] [CrossRef]
- Quezada, C.P.; Congreve, R.C.; Kokkarachedu, V. Graphene Oxide: A Promising Nanomaterial for Antibacterial and Antiviral Applications. In Nanoparticles in Modern Antimicrobial and Antiviral Applications; Kokkarachedu, V., Sadiku, R., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 389–419. [Google Scholar]
- Ghazzy, A.; Naik, R.R.; Shakya, A.K. Metal–Polymer Nanocomposites: A Promising Approach to Antibacterial Materials. Polymers 2023, 15, 2167. [Google Scholar] [CrossRef]
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid polymer hybrid nanocarriers: Insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Colloid. Interface Sci. Commun. 2022, 46, 100570. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Alekshun, M.N.; Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef]
- Fontoura, I.; Veriato, T.S.; Raniero, L.J.; Castilho, M.L. Analysis of Capped Silver Nanoparticles Combined with Imipenem against Different Susceptibility Profiles of Klebsiella pneumoniae. Antibiotics 2023, 12, 535. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Bronstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Korany, S.M.; Sonbol, H.; Alhomaidi, E.A.; Alwakeel, S.S.; Elbaz, R.M. Myco-fabricated silver nanoparticle by novel soil fungi from Saudi Arabian desert and antimicrobial mechanism. Sci. Rep. 2024, 14, 15211. [Google Scholar] [CrossRef] [PubMed]
- Hochvaldova, L.; Posselt, G.; Wessler, S.; Kvitek, L.; Panacek, A. Implications of silver nanoparticles for H. pylori infection: Modulation of CagA function and signaling. Front. Cell. Infect. Microbiol. 2024, 14, 1419568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef]
- Zaman, W.; Manghwar, H. Nanoparticles and Their Biological Applications: Recent Advances in 2022–2023. Microorganisms 2024, 12, 489. [Google Scholar] [CrossRef]
- Caioni, G.; Reyes, C.P.; Laurenti, D.; Chiaradia, C.; Dainese, E.; Mattioli, R.; Di Risola, D.; Santavicca, E.; Francioso, A. Biochemistry and Future Perspectives of Antibiotic Resistance: An Eye on Active Natural Products. Antibiotics 2024, 13, 1071. [Google Scholar] [CrossRef]
- Namdar, N.; Nayeri Fasaei, B.; Shariati, P.; Joghataei, S.M.; Arpanaei, A. Mesoporous silica nanoparticles co-loaded with lysozyme and vancomycin for synergistic antimicrobial action. Sci. Rep. 2024, 14, 29242. [Google Scholar] [CrossRef]
- Muteeb, G. Nanotechnology-A Light of Hope for Combating Antibiotic Resistance. Microorganisms 2023, 11, 1489. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.I.R.; Kadu, P.; Bawane, P.; Nakhate, K.T.; Yele, S.; Ojha, S.; Goyal, S.N. Mechanisms of emerging resistance associated with non-antibiotic antimicrobial agents: A state-of-the-art review. J. Antibiot. 2023, 76, 629–641. [Google Scholar] [CrossRef]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef]
- Rajkhowa, S.; Hussain, S.Z.; Agarwal, M.; Zaheen, A.; Al-Hussain, S.A.; Zaki, M.E.A. Advancing Antibiotic-Resistant Microbe Combat: Nanocarrier-Based Systems in Combination Therapy Targeting Quorum Sensing. Pharmaceutics 2024, 16, 1160. [Google Scholar] [CrossRef]
- Nguyen, T.-K.; Duong, H.T.T.; Selvanayagam, R.; Boyer, C.; Barraud, N. Iron oxide nanoparticle-mediated hyperthermia stimulates dispersal in bacterial biofilms and enhances antibiotic efficacy. Sci. Rep. 2015, 5, 18385. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, S.; Chinnasamy, G.; Bhatnagar, S. Sustainable phyto-fabrication of silver nanoparticles using Gmelina arborea exhibit antimicrobial and biofilm inhibition activity. Sci. Rep. 2022, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Nageeb, W.M.; Adam, S.H.; Hashem, N.; Abdelsalam, N. In-vitro and In-silico evaluation of antimicrobial and antibiofilm effect of Neem oil and Calcium hydroxide nanoparticles against Mutans Streptococci and Enterococcus faecalis isolated from endodontic infections. Sci. Rep. 2024, 14, 26441. [Google Scholar] [CrossRef]
- Chen, K.; Najer, A.; Charchar, P.; Saunders, C.; Thanapongpibul, C.; Klöckner, A.; Chami, M.; Peeler, D.J.; Silva, I.; Panariello, L.; et al. Non-invasive in vivo sensing of bacterial implant infection using catalytically-optimised gold nanocluster-loaded liposomes for urinary readout. Nat. Commun. 2024, 15, 10321. [Google Scholar] [CrossRef]
- Bueloni, B.; Sanna, D.; Garribba, E.; Castro, G.R.; León, I.E.; Islan, G.A. Design of nalidixic acid-vanadium complex loaded into chitosan hybrid nanoparticles as smart strategy to inhibit bacterial growth and quorum sensing. Int. J. Biol. Macromol. 2020, 161, 1568–1580. [Google Scholar] [CrossRef]
- Owrang, M.; Gholami, A. Green-synthesized silver nanoparticles from Zataria multiflora as a promising strategy to target quorum sensing and biofilms in Pseudomonas aeruginosa. Heliyon 2024, 10, e38395. [Google Scholar] [CrossRef]
- Chen, H.; Hu, P.; Wang, Y.; Liu, H.; Zheng, J.; Huang, Z.; Zhang, X.; Liu, Y.; Zhou, T. From quorum sensing inhibition to antimicrobial defense: The dual role of eugenol-gold nanoparticles against carbapenem-resistant Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2025, 247, 114415. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Kishawy, A.T.Y.; Khater, S.I.; Khalifa, E.; Ismail, T.A.; Mohammed, H.A.; Elnahriry, S.S.; Tolba, H.A.; Sherief, W.R.I.A.; Farag, M.F.M.; et al. Interactive effects of dietary quercetin nanoparticles on growth, flesh antioxidant capacity and transcription of cytokines and Aeromonas hydrophila quorum sensing orchestrating genes in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2021, 119, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Raguvaran, K.; Kalpana, M.; Manimegalai, T.; Maheswaran, R. Larvicidal, antibacterial, antibiofilm, and anti-quorum sensing activities of silver nanoparticles biosynthesized from Streptomyces sclerotialus culture filtrate. Mater. Lett. 2022, 316, 132000. [Google Scholar] [CrossRef]
- Mercimek Takcı, H.A.; Takcı, D.K.; Icigen, S.; Ozkan, H.; Duman, S.E.; Huner, T. Manufacturing of Ag/Ag2O nanocomposites for anti-quorum sensing: A Safe and green approach. J. Cryst. Growth 2025, 652, 128041. [Google Scholar] [CrossRef]
- Cui, F.; Xi, L.; Wang, D.; Tan, X.; Li, J.; Li, T. Functional magnetic nanoparticles combined with molecular dynamics technology to screen quorum sensing inhibitors from natural substances: Accuracy, efficiency and high throughput. Sep. Purif. Technol. 2022, 300, 121932. [Google Scholar] [CrossRef]
- Rather, M.A.; Mandal, M. Attenuation of biofilm and quorum sensing regulated virulence factors of an opportunistic pathogen Pseudomonas aeruginosa by phytofabricated silver nanoparticles. Microb. Pathog. 2023, 185, 106433. [Google Scholar] [CrossRef]
- Fernandez-Billon, M.; Llambias-Cabot, A.E.; Jordana-Lluch, E.; Oliver, A.; Macia, M.D. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa biofilms. Biofilm 2023, 5, 100129. [Google Scholar] [CrossRef]
- Salama, S.A.; Essam, D.; Tagyan, A.I.; Farghali, A.A.; Khalil, E.M.; Abdelaleim, Y.F.; Hozzein, W.N.; Mubarak, M.; Nasr, F.A.; Eweis, A.A.; et al. Novel composite of nano zinc oxide and nano propolis as antibiotic for antibiotic-resistant bacteria: A promising approach. Sci. Rep. 2024, 14, 20894. [Google Scholar] [CrossRef]
- Lakshminarayanan, R.; Ye, E.; Young, D.J.; Li, Z.; Loh, X.J. Recent Advances in the Development of Antimicrobial Nanoparticles for Combating Resistant Pathogens. Adv. Health Mater. 2018, 7, e1701400. [Google Scholar] [CrossRef]
- de Oliveira, J.F.A.; Saito, A.; Bido, A.T.; Kobarg, J.; Stassen, H.K.; Cardoso, M.B. Defeating Bacterial Resistance and Preventing Mammalian Cells Toxicity Through Rational Design of Antibiotic-Functionalized Nanoparticles. Sci. Rep. 2017, 7, 1326. [Google Scholar] [CrossRef] [PubMed]
- Aloke, C.; Egwu, C.O.; Onisuru, O.O.; Otun, S.; Achilonu, I. Exploiting the therapeutic efficacy of nanoparticles in the treatment of multidrug-resistant bacteria: Excitements and pitfalls. J. Drug Deliv. Sci. Technol. 2025, 104, 106501. [Google Scholar] [CrossRef]
- Cobos, M.; De-La-Pinta, I.; Quindos, G.; Fernandez, M.J.; Fernandez, M.D. Synthesis, Physical, Mechanical and Antibacterial Properties of Nanocomposites Based on Poly(vinyl alcohol)/Graphene Oxide-Silver Nanoparticles. Polymers 2020, 12, 723. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Afridi, M.; Khan, S.; Afridi, R.; Ullah, F.; Majid, A.; Khan, A.; Ali, N. Combining antibiotics with silver nanoparticles: A potential treatment strategy against antimicrobial resistance. Main Group Chem. 2021, 21, 445–466. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Hochvaldová, L.; Panáček, D.; Válková, L.; Večeřová, R.; Kolář, M.; Prucek, R.; Kvítek, L.; Panáček, A. E. coli and S. aureus resist silver nanoparticles via an identical mechanism, but through different pathways. Commun. Biol. 2024, 7, 1552. [Google Scholar] [CrossRef]
- Girma, A. Alternative mechanisms of action of metallic nanoparticles to mitigate the global spread of antibiotic-resistant bacteria. Cell Surf. 2023, 10, 100112. [Google Scholar] [CrossRef] [PubMed]
- Romulo, A.; Anjani, V.S.; Wardana, A.A. Enhancing Antimicrobial Activity of Thyme Essential Oil Through Cellulose Nano Crystals-Stabilized Pickering Emulsions. Foods 2024, 13, 3706. [Google Scholar] [CrossRef]
- Radwan, I.T.; El-Sherbiny, I.M.; Selim, A.M.; Metwally, N.H. Design, synthesis of some novel coumarins and their nanoformulations into lipid-chitosan nanocapsule as unique antimicrobial agents. Sci. Rep. 2024, 14, 30598. [Google Scholar] [CrossRef]
- Cao, Y.-H.; Cai, W.-J.; He, X.-W.; Song, H.-L.; Gao, J.; Yang, Y.-L.; Zhou, J. A review of advances & potential of applying nanomaterials for biofilm inhibition. npj Clean Water 2024, 7, 131. [Google Scholar] [CrossRef]
- Su, Z.; Xu, D.; Hu, X.; Zhu, W.; Kong, L.; Qian, Z.; Mei, J.; Ma, R.; Shang, X.; Fan, W.; et al. Biodegradable oxygen-evolving metalloantibiotics for spatiotemporal sono-metalloimmunotherapy against orthopaedic biofilm infections. Nat. Commun. 2024, 15, 8058. [Google Scholar] [CrossRef] [PubMed]
- Omran, B.A.; Rabbee, M.F.; Abdel-Salam, M.O.; Baek, K.-H. Nanobiological synthesis of silver oxide-doped titanium oxide bionanocomposite targeting foodborne and phytopathogenic bacteria. Food Biosci. 2024, 61, 104790. [Google Scholar] [CrossRef]
- Chandoliya, R.; Sharma, S.; Sharma, V.; Joshi, R.; Sivanesan, I. Titanium Dioxide Nanoparticle: A Comprehensive Review on Synthesis, Applications and Toxicity. Plants 2024, 13, 2964. [Google Scholar] [CrossRef]
- Caldwell, M.; Hughes, M.; Wei, F.; Ngo, C.; Pascua, R.; Pugazhendhi, A.S.; Coathup, M.J. Promising applications of D-amino acids in periprosthetic joint infection. Bone Res. 2023, 11, 14. [Google Scholar] [CrossRef]
- Wu, G.-L.; Yen, C.-E.; Hsu, W.-C.; Yeh, M.-L. Incorporation of cerium oxide nanoparticles into the micro-arc oxidation layer promotes bone formation and achieves structural integrity in magnesium orthopedic implants. Acta Biomater. 2025, 191, 80–97. [Google Scholar] [CrossRef]
- Marino, A.; Augello, E.; Stracquadanio, S.; Bellanca, C.M.; Cosentino, F.; Spampinato, S.; Cantarella, G.; Bernardini, R.; Stefani, S.; Cacopardo, B.; et al. Unveiling the Secrets of Acinetobacter baumannii: Resistance, Current Treatments, and Future Innovations. Int. J. Mol. Sci. 2024, 25, 6814. [Google Scholar] [CrossRef]
- Yang, C.; Luo, Y.; Shen, H.; Ge, M.; Tang, J.; Wang, Q.; Lin, H.; Shi, J.; Zhang, X. Inorganic nanosheets facilitate humoral immunity against medical implant infections by modulating immune co-stimulatory pathways. Nat. Commun. 2022, 13, 4866. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Pourhajibagher, M.; Chiniforush, N.; Bahador, A. Propolis nanoparticle enhances the potency of antimicrobial photodynamic therapy against Streptococcus mutans in a synergistic manner. Sci. Rep. 2020, 10, 15560. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Arshad, A.; Alshehri, M.A.; Alhelaify, S.S.; Alharthy, O.M.; Shukry, M.; Sayed, S.M. Green synthesis of anethole-loaded zinc oxide nanoparticles enhances antibacterial strategies against pathogenic bacteria. Sci. Rep. 2024, 14, 24671. [Google Scholar] [CrossRef]
- Liu, H.; Xing, F.; Zhou, Y.; Yu, P.; Xu, J.; Luo, R.; Xiang, Z.; Maria Rommens, P.; Liu, M.; Ritz, U. Nanomaterials-based photothermal therapies for antibacterial applications. Mater. Des. 2023, 233, 112231. [Google Scholar] [CrossRef]
- Fan, X.-L.; Li, H.-Y.; Ye, W.-Y.; Zhao, M.-Q.; Huang, D.-n.; Fang, Y.; Zhou, B.-Q.; Ren, K.-F.; Ji, J.; Fu, G.-S. Magainin-modified polydopamine nanoparticles for photothermal killing of bacteria at low temperature. Colloids Surf. B Biointerfaces 2019, 183, 110423. [Google Scholar] [CrossRef]
- Ma, X.; Tian, Y.; Yang, R.; Wang, H.; Allahou, L.W.; Chang, J.; Williams, G.; Knowles, J.C.; Poma, A. Nanotechnology in healthcare, and its safety and environmental risks. J. Nanobiotechnol. 2024, 22, 715. [Google Scholar] [CrossRef] [PubMed]
- Shabatina, T.I.; Vernaya, O.I.; Melnikov, M.Y. Hybrid Nanosystems of Antibiotics with Metal Nanoparticles—Novel Antibacterial Agents. Molecules 2023, 28, 1603. [Google Scholar] [CrossRef] [PubMed]
- Puccetti, M.; Pariano, M.; Schoubben, A.; Giovagnoli, S.; Ricci, M. Biologics, theranostics, and personalized medicine in drug delivery systems. Pharmacol. Res. 2024, 201, 107086. [Google Scholar] [CrossRef]
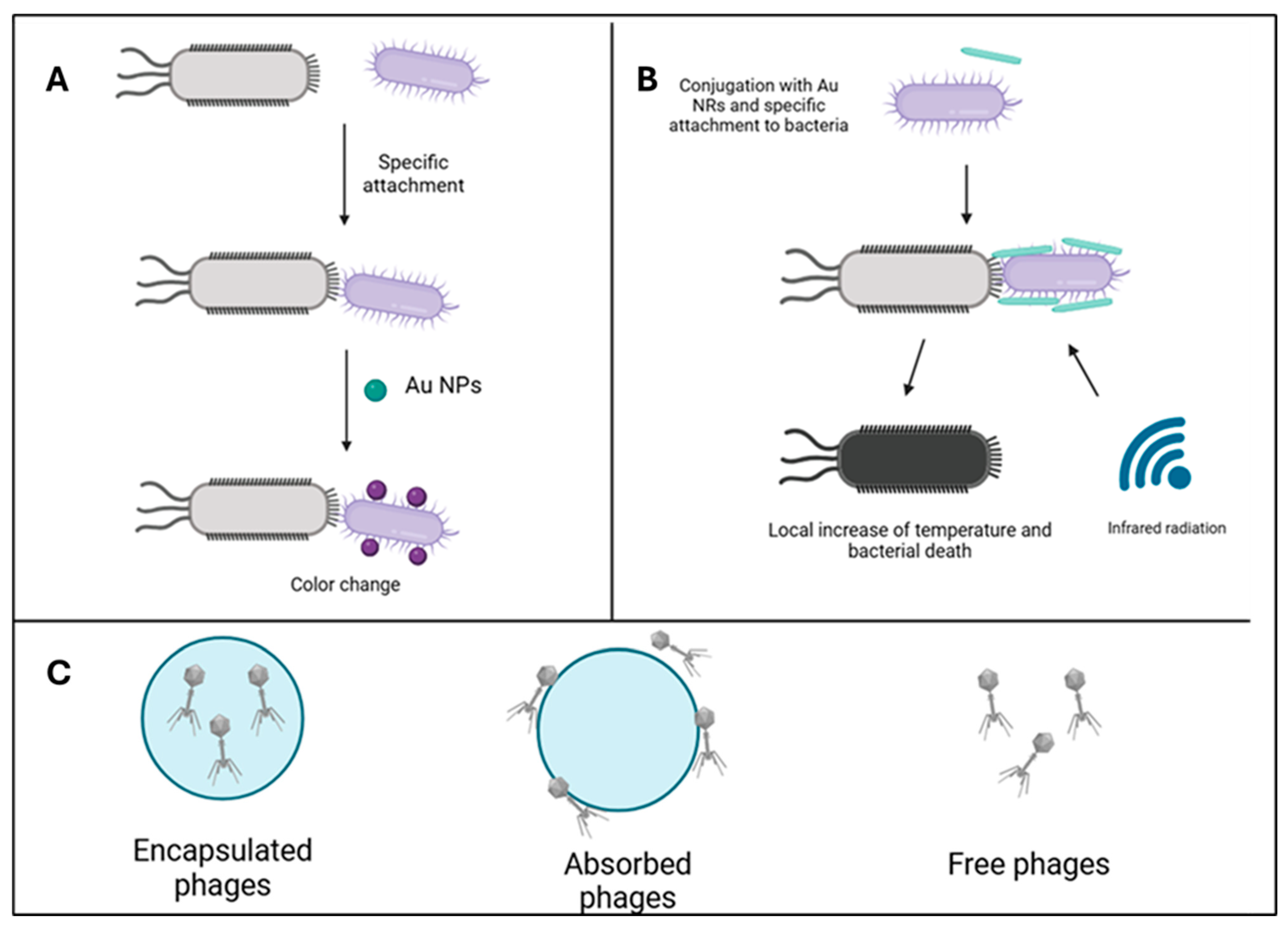
- Pardo-Freire, M.; Domingo-Calap, P. Phages and Nanotechnology: New Insights against Multidrug-Resistant Bacteria. BioDesign Res. 2023, 5, 0004. [Google Scholar] [CrossRef]
- Yang, D.; Ding, M.; Song, Y.; Hu, Y.; Xiu, W.; Yuwen, L.; Xie, Y.; Song, Y.; Shao, J.; Song, X.; et al. Nanotherapeutics with immunoregulatory functions for the treatment of bacterial infection. Biomater. Res. 2023, 27, 73. [Google Scholar] [CrossRef]
- Wan, F.; Draz, M.S.; Gu, M.; Yu, W.; Ruan, Z.; Luo, Q. Novel Strategy to Combat Antibiotic Resistance: A Sight into the Combination of CRISPR/Cas9 and Nanoparticles. Pharmaceutics 2021, 13, 352. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Xu, Y.; Chen, Y.; Zhang, W.; Liu, T.; Chen, G.; Wang, K. Phage-based delivery systems: Engineering, applications, and challenges in nanomedicines. J. Nanobiotechnol. 2024, 22, 365. [Google Scholar] [CrossRef]
- Fischetti, V.A. Development of Phage Lysins as Novel Therapeutics: A Historical Perspective. Viruses 2018, 10, 310. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, S.; Fu, Y.; Dong, N.; Bi, C.; Shan, A.; Shao, C. Advances in the delivery and application of antimicrobial peptide-based nanomaterials. Chem. Eng. J. 2024, 496, 154232. [Google Scholar] [CrossRef]
- Dai, J.; Ashrafizadeh, M.; Aref, A.R.; Sethi, G.; Ertas, Y.N. Peptide-functionalized, -assembled and -loaded nanoparticles in cancer therapy. Drug Discov. Today 2024, 29, 103981. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Wilson, R.L.; Castejon, A.M. Recent Advances in Peptide-Loaded PLGA Nanocarriers for Drug Delivery and Regenerative Medicine. Pharmaceuticals 2025, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlQurashi, D.M.; AlQurashi, T.F.; Alam, R.I.; Shaikh, S.; Tarkistani, M.A.M. Advanced Nanoparticles in Combating Antibiotic Resistance: Current Innovations and Future Directions. J. Nanotheranostics 2025, 6, 9. https://doi.org/10.3390/jnt6020009
AlQurashi DM, AlQurashi TF, Alam RI, Shaikh S, Tarkistani MAM. Advanced Nanoparticles in Combating Antibiotic Resistance: Current Innovations and Future Directions. Journal of Nanotheranostics. 2025; 6(2):9. https://doi.org/10.3390/jnt6020009
Chicago/Turabian StyleAlQurashi, Dana Mohammed, Tayf Fahad AlQurashi, Raneia Idrees Alam, Sumera Shaikh, and Mariam Abdulaziz M. Tarkistani. 2025. "Advanced Nanoparticles in Combating Antibiotic Resistance: Current Innovations and Future Directions" Journal of Nanotheranostics 6, no. 2: 9. https://doi.org/10.3390/jnt6020009
APA StyleAlQurashi, D. M., AlQurashi, T. F., Alam, R. I., Shaikh, S., & Tarkistani, M. A. M. (2025). Advanced Nanoparticles in Combating Antibiotic Resistance: Current Innovations and Future Directions. Journal of Nanotheranostics, 6(2), 9. https://doi.org/10.3390/jnt6020009





