Abstract
Nanotheranostics—where nanoscale materials serve both diagnostic and therapeutic functions—are rapidly transforming gene therapy by tackling critical delivery challenges. This review explores the design and engineering of various nanoparticle systems (lipid-based, polymeric, inorganic, and hybrid) to enhance stability, targeting, and endosomal escape of genetic payloads. We discuss how real-time imaging capabilities integrated into these platforms enable precise localization and controlled release of genes, improving treatment efficacy while reducing off-target effects. Key strategies to overcome delivery barriers (such as proton sponge effect and photothermal disruption) and to achieve nuclear localization are highlighted, along with recent advances in stimuli-responsive systems that facilitate spatiotemporal control of gene expression. Clinical trials and preclinical studies demonstrate the expanding role of nanotheranostics in managing cancer, inherited disorders, and cardiovascular and neurological diseases. We further address regulatory and manufacturing hurdles that must be overcome for the widespread clinical adoption of nanoparticle-based gene therapies. By synthesizing recent progress and ongoing challenges, this review underscores the transformative potential of nanotheranostics for effective, targeted, and image-guided gene delivery.
1. Introduction
Gene therapy is a revolutionary technique that involves the manipulation or modification of gene expression. This technology has become one of the most significant in the modern era by enabling the treatment of otherwise incurable diseases with conventional medications [1,2]. Gene therapy can be applied to silence a gene, replace a defective gene with a functional one, suppress a gene, or edit genes directly, as shown in Figure 1 [3,4]. One of the most prominent approaches in recent years is gene editing, particularly since Jennifer Doudna and Emmanuelle Charpentier revolutionized molecular biology with the development of the CRISPR-Cas9 genetic editing tool [5]. This advancement has made gene editing more accessible and cost-effective compared to other technologies, such as transcription activator-like effector nucleases (TALEN) and zinc finger nucleases (ZFNs) [1,6].
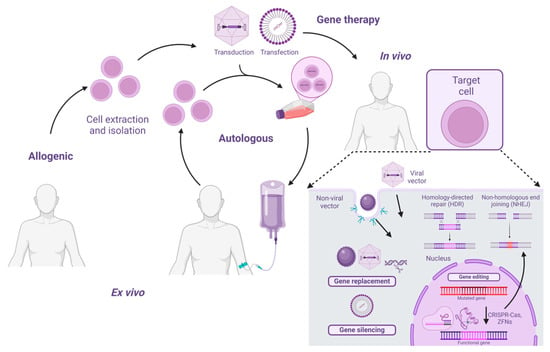
Figure 1.
Overview of gene therapy. Gene therapy can be classified as allogenic, where cells from a donor (someone other than the recipient) are used for the treatment, or autologous, where the patient’s own cells are utilized. Additionally, gene therapy can be either ex vivo, where cells are isolated, extracted, and then treated with the gene therapy outside the body, or in vivo, where the gene therapy is applied directly to the patient.
Gene therapy made significant progress in 1990 when William French Anderson developed a protocol to treat adenosine deaminase (ADA) deficiency using T cells modified with a recombinant retrovirus [1,7]. However, setbacks occurred in 1999 due to adverse events. A turning point came in 2012 with the EMA’s approval of alipogene tiparvovec for lipoprotein lipase deficiency and the use of CAR-T therapy for acute lymphoblastic leukemia (ALL) [8]. That same year, CRISPR-Cas9 was applied in various fields, solidifying gene editing as a key tool [9,10]. The FDA’s 2017 approval of Luxturna for Leber congenital amaurosis type 2 (LCA2) further integrated gene therapy into clinical practice [11,12].
The advancements in gene therapy have been significantly supported by molecular biology techniques, particularly nucleic acid-based methodologies. Among these, polymerase chain reaction (PCR) has emerged as a crucial tool for the amplification and detection of specific DNA sequences, playing a fundamental role in assessing gene therapy efficacy, biodistribution, and transgene expression.
A key component of PCR is the use of primers, short oligonucleotide sequences that guide DNA amplification. The gold standard for oligonucleotide synthesis is phosphoramidite chemistry, where nucleoside phosphoramidite reacts with the 5′-OH terminal group of the growing oligonucleotide [13]. However, traditional methods face challenges such as low yield, high costs, and limited sustainability [14]. To overcome these limitations, biocatalytic and chemoenzymatic strategies are emerging as promising alternatives to enhance synthesis efficiency [14].
Beyond primer synthesis, PCR itself serves as a widely used quantitative method in gene therapy due to its standardization, which allows for comparisons in terms of efficiency, assembly, purification, and experimental and therapeutic dosing [15]. Quantitative PCR (qPCR) is also employed, as it enables real-time measurement of amplification products through the fluorescence signal emitted by DNA-intercalating agents [15]. The advantages of qPCR include its sensitivity, specificity, and ease of implementation. However, it is not entirely precise, robust, or efficient, as its performance depends on the design of primer pairs, the presence of inhibitors, and the formation of secondary structures in the template [15].
To overcome these limitations, microfluidic systems have facilitated the development of new devices for both endpoint and real-time PCR [16]. Digital PCR (dPCR) partitions the PCR sample into thousands of subsamples, where each contains either a single or no DNA molecule [16]. dPCR is microfluidics-based and can be categorized into droplet-based dPCR (ddPCR) and chip-based dPCR (cdPCR) [16]. These platforms are essential in gene therapy for assessing biodistribution, shedding, and transgene expression [17]. In biodistribution studies, they are used to measure the presence of the gene therapy product in both target and non-target tissues [17]. In shedding analysis, they help quantify the release of virus-based gene therapy products from the patient through excreta and secretions. In transgene expression studies, they measure the expression levels of the transgene delivered by the gene therapy product in both target and non-target tissues [17].
Beyond these tools, the successful application of gene therapy relies on the selection of an appropriate delivery method. Currently, gene therapy strategies can be categorized into viral and non-viral vehicles [18]. These therapies can be administered ex vivo, where cells are extracted from the patient, modified, and reintroduced, or in vivo, where genetic material is directly transferred into target cells [19]. Most FDA-approved gene therapies are in vivo and use viral vectors [12]. Gene therapy has been primarily applied to treat inherited blood cell disorders, such as hemoglobinopathies, innate immune errors (IEIs), and lysosomal storage diseases [20]. Additionally, gene therapy has found applications in immunotherapy, particularly in oncology, to redirect immune cells, especially T cells, using T cell receptors (TCRs) and chimeric antigen receptors (CARs) to target tumors [12,21].
Most gene therapies have relied on recombinant adeno-associated virus (AAV) vectors due to their efficiency and low immunogenicity. AAV-based therapies approved by the FDA include Luxturna™, Zolgensma™, and Hemgenix™ [12].
In addition to traditional viral vectors, non-viral delivery systems based on nanomaterials have gained popularity for gene delivery due to their low immunogenicity, small size, and high efficiency. A particularly innovative subset of these systems is nanotheranostics, which are defined by their ability to integrate both therapeutic and diagnostic functions within a single platform. In this context, “theranostics” refers to the convergence of therapy and diagnosis: the same nanosystem is engineered not only to deliver therapeutic agents—such as genetic material for gene delivery—but also to provide diagnostic capabilities, such as real-time imaging and tracking of biodistribution [22,23]. Here, “nano” refers to materials with dimensions typically ranging from 1 to 100 nm. These multifunctional platforms are often designed to respond to external stimuli (thermal, enzymatic, pH, or light), which trigger the release of their therapeutic payload at specific sites while simultaneously enabling diagnostic imaging. By incorporating these dual functions, nanotheranostics create a feedback loop where the diagnostic data directly informs and optimizes the therapeutic intervention, thus embodying the core principle of integrated gene therapy and diagnostic monitoring [24,25]. Common nanomaterials used in these systems include carbon nanotubes, nanoliposomes, dendrimers, polymeric micelles, and magnetic nanomaterials [26], all selected for their ability to support both therapeutic and diagnostic roles.
Nanotheranostics have been applied in the treatment of various diseases, including cancer, neurodegenerative diseases, diabetes, hormonal deficiencies, liver diseases, respiratory diseases, and ocular disorders [23]. Nanotheranostics refers to the design and engineering of nanoscale materials that simultaneously enable diagnosis (through imaging or detection capabilities) and therapy (delivering drugs, genetic material, or other treatments) [27]. In the context of gene therapy, nanotheranostics offers the unique advantage of monitoring vector localization and therapeutic effectiveness in real time, thereby improving treatment precision and reducing unintended effects on healthy tissues [28]. By combining imaging agents (e.g., fluorescent dyes, magnetic nanoparticles, or PET tracers) with gene-delivery vehicles (e.g., liposomes or polymeric nanoparticles), clinicians can track the biodistribution, endosomal escape, and gene expression within targeted tissues [28,29,30]. This synergy is especially important for genetic interventions that require localized, controllable release over a defined period.
In this review, we aim to provide a comprehensive examination of how nanotheranostic platforms are redefining gene therapy delivery. Specifically, we dissect the recent advances in nanoparticle engineering for efficient endosomal escape and nuclear localization of genetic cargo, discuss the critical role of imaging-guided delivery in achieving precise spatiotemporal control, and summarize the current landscape of clinical and preclinical trials. By focusing on both established and emerging gene-editing tools, such as CRISPR/Cas9, and the evolving regulatory framework, our review highlights the unique challenges and unparalleled potential of nanotheranostics to revolutionize gene therapy. In the following sections, we present the fundamental characteristics of nanotheranostics, review key barriers and solutions for gene delivery, and explore major therapeutic applications in oncology, neurology, and cardiology.
2. Fundamentals of Nanotheranostics
Nanocarriers are the central component of nanotheranostics, as shown in Figure 2, and they possess customizable physicochemical properties that enable them to overcome barriers present during cellular internalization and delivery to target tissues [31]. These nanotheranostic platforms can be lipidic, polymeric, metallic, or hybrid [32]. Due to their chemical versatility, nanotheranostic platforms can be used for gene delivery. However, gene delivery systems must meet several key properties to ensure their effectiveness in clinical applications. These properties are essential for ensuring that gene therapy is not only efficient but also safe and sustainable. Biocompatibility, stability, controlled release, and targeting capabilities are among the most crucial factors for the success of gene delivery platforms.
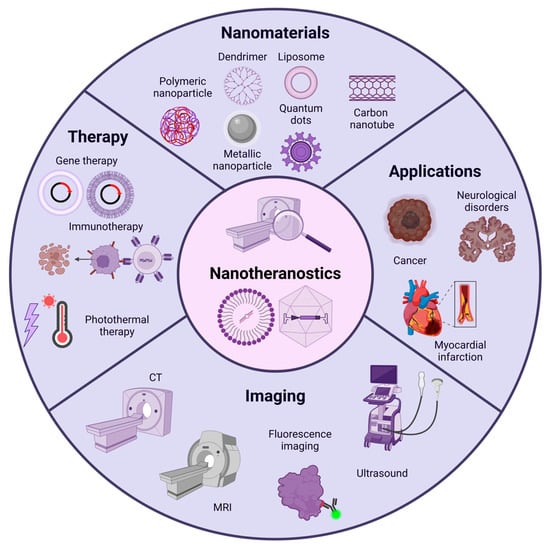
Figure 2.
Overview of nanotheranostics. Nanotheranostics are composed of nanomaterials such as liposomes, polymeric nanoparticles, metallic nanoparticles, carbon nanotubes, and quantum dots. These nanomaterials serve therapeutic purposes by enabling the delivery of various agents, including gene therapy. Additionally, they can be modified for visualization using diagnostic imaging equipment, allowing real-time monitoring of the therapy and validating its presence in the target tissue. Nanotheranostics have broad applications in fields such as cancer, cardiovascular diseases, neurodegenerative disorders, and more.
2.1. Nanotheranostic Platforms
Nanocarriers have emerged as powerful tools [33,34] for overcoming the challenges associated with nucleic acid delivery, combining protection and stability outside target cells with the ability to degrade and release intact nucleic acids upon internalization. To achieve these seemingly incompatible requirements, a wide variety of cationic formulation materials, including natural and synthetic lipids and polymers have been explored [35,36,37]. These materials facilitate the interaction of nucleic acids into nanoparticles through electrostatic interactions, resulting in structures optimized for cellular uptake. With their customizable physicochemical properties, nanomaterials provide versatility in addressing systemic and intracellular barriers such as rapid degradation, renal clearance, and poor cellular uptake [38]. This adaptability has led to the development of diverse nanocarrier systems, including lipid-based nanoparticles, polymeric nanoparticles, and inorganic nanoparticles, each offering unique advantages for efficient nucleic acid delivery [38].
However, nanoparticle-based delivery systems face significant challenges in protecting genetic payloads from enzymatic degradation and premature clearance. To enhance stability, lipid nanoparticles (LNPs) and polymeric carriers such as polyethyleneimine (PEI), poly(lactic-co-glycolic acid) (PLGA), and chitosan encapsulate nucleic acids, shielding them from degradation while facilitating controlled release [39]. PEGylation further extends circulation time by reducing opsonization, while covalent crosslinking strategies improve structural integrity [40,41]. The inclusion of ionizable lipids also stabilizes RNA-based therapies by modulating charge interactions, ensuring efficient cellular uptake [42]. Additionally, biodegradable cationic polymers, such as polylactic acid (PLA), PLGA, and poly(ε-caprolactone) (PCL), offer lower cytotoxicity and improved transgene release compared to non-degradable cationic polymers like PEI and poly-l-lysine (PLL) [39]. The development of copolymers, such as PLA-PEI and PLA-PEG-PLA, has further enhanced stability and reduced PEI-related toxicity, improving gene delivery efficacy [43].
Targeting strategies are essential to maximize therapeutic efficacy and minimize off-target effects. Ligand-functionalized nanoparticles, decorated with aptamers, monoclonal antibodies, or peptides [44], enable receptor-mediated endocytosis for selective delivery [45,46]. Additionally, stimuli-responsive nanoparticles engineered with pH-sensitive or redox-responsive coatings enable controlled activation and site-specific cargo release, improving localization within diseased tissues [47,48]. Magnetic nanoparticles, such as Fe3O4 and γ-Fe2O3, have also been explored for gene delivery due to their superparamagnetic properties. These nanoparticles can be directed to specific sites using external magnetic fields [49], enhancing transfection efficiency while maintaining excellent biological safety [39].
Following cellular uptake, efficient endosomal escape is critical for therapeutic action. The proton sponge effect, employed by polymers like PEI and poly(beta-amino esters) (PBAEs), induces osmotic swelling, leading to endosomal rupture [50]. pH-sensitive lipids, such as DLin-MC3-DMA, destabilize endosomal membranes under acidic conditions, releasing genetic cargo into the cytosol [51]. Additionally, fusogenic peptides like GALA, HA2, and melittin facilitate membrane disruption, further enhancing cytoplasmic delivery [52,53].
2.1.1. Lipid-Based Nanoparticles
Lipid-based nanoparticles (LBNPs) have emerged as promising carriers for nucleic acid delivery due to their high biocompatibility, low immunogenicity, stability, ease of preparation, and high loading efficiency, as shown in Table 1 [54]. LBNPs can be categorized into four types based on their structure and composition: (I) liposomes, (II) LNPs, (III) lipid nanoemulsions (LNEs), and (IV) solid lipid nanoparticles (SLNs), as shown in Figure 3 [54]. In general, LBNPs are spherical nano-sized particles composed of various lipids, such as ionizable lipids, phospholipids, cholesterol, and solid or liquid lipids [55].
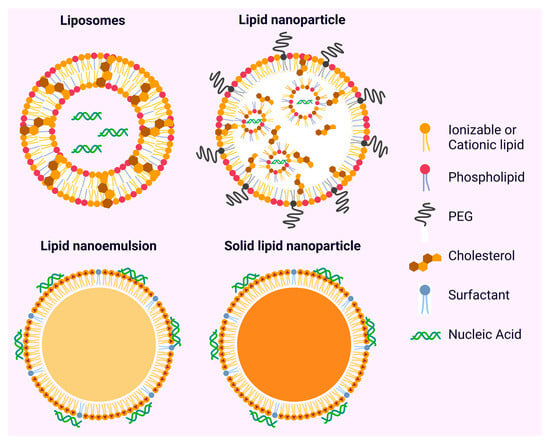
Figure 3.
Types of lipid-based nanoparticles (LBNPs). Liposomes are composed of phospholipid bilayers that enclose aqueous cores. Lipid nanoparticles (LNPs), unlike liposomes, do not have a bilayer but instead form nanostructures using ionizable lipids. Lipid nanoemulsions (LNEs) consist of oil-in-water droplets that are stabilized by phospholipids and emulsifiers. Solid lipid nanoparticles (SLNs) feature solid lipid cores that are stabilized by surfactant monolayers.
Liposomes, which are formed by phospholipid bilayers enclosing aqueous cores, can encapsulate hydrophilic nucleic acids or embed hydrophobic drugs within their membranes [56]. Their composition can be modified to alter size, charge, and functionality, with cationic liposomes being particularly effective due to their electrostatic interactions with nucleic acids [57]. LNPs, unlike liposomes, lack a bilayer and instead form nanostructures with ionizable lipids that respond to pH changes, facilitating nucleic acid encapsulation, stability, and endosomal escape [58]. LNPs have demonstrated clinical success in mRNA delivery, notably in vaccines like BNT162b2 targeting SARS-CoV-2 [59]. LNEs are oil-in-water droplets stabilized by phospholipids and emulsifiers. These structures use cationic lipids to adsorb nucleic acids electrostatically on their surface, while the oil phase protects the nucleic acids from degradation [60]. Additionally, LNEs can co-deliver lipophilic drugs, as exemplified by GEMCOVAC-19 [61]. SLNs feature solid lipid cores stabilized by surfactant monolayers. SLNs embed lipophilic drugs and load negatively charged nucleic acids onto their surface using cationic lipids. These nanoparticles offer stability, drug protection, and sustained release, although their nucleic acid loading capacity is somewhat limited [62].
In 2018, the first FDA approval of an LBNP-based therapy was granted for a siRNA encapsulated in lipid nanoparticles to target hepatocytes for the treatment of polyneuropathy in hereditary TTR-mediated amyloidosis (hATTR) [63]. Currently, 46 interventional clinical trials are active using LBNPs, primarily for the treatment of genetic diseases and vaccine development [64].

Table 1.
Comparison of lipid-based approaches: advantages and disadvantages.
Table 1.
Comparison of lipid-based approaches: advantages and disadvantages.
| Type of LBNP | Structure | Advantages | Disadvantages |
|---|---|---|---|
| Liposomes | Phospholipid bilayers enclosing aqueous cores |
|
|
| Lipid Nanoparticles (LNPs) | Ionizable lipid structures lacking bilayers |
|
|
| Lipid Nanoemulsions (LNEs) | Oil-in-water droplets stabilized by phospholipids and emulsifiers |
|
|
| Solid Lipid Nanoparticles (SLNs) | Solid lipid cores stabilized by surfactant monolayers |
|
|
2.1.2. Micelles
Micelles are formed through the dynamic aggregation of amphiphilic molecules, driven by the hydrophobic effect in aqueous solutions and hydrogen bond formation [72]. Typically, micelles consist of a nonpolar segment known as the hydrophobic tail and a polar segment called the head [72]. As the concentration of amphiphilic molecules increases, the critical micelle concentration (CMC) rises, leading to the self-assembly of surfactant molecules into micellar aggregates [72].
Micelles can be prepared using various techniques, such as simple dissolution, W/O emulsion, solvent evaporation, lyophilization, or dialysis [72]. In direct dissolution, highly water-soluble copolymers are used, whereas in lyophilization, cosolvents with high vapor pressure, such as dimethylacetamide and tert-butanol, are employed to generate stable micelles with a prolonged shelf life [72].
There are different types of micelles, including reverse micelles, regular micelles, and unimolecular micelles [72]. They can be broadly categorized into two groups: surfactant-based micelles (low molecular weight) and polymeric micelles (PMs) [72]. PMs are distinguished by their core–shell configuration, which allows for the encapsulation of therapeutic agents such as drugs or nucleic acids within the hydrophobic core [72]. The hydrophilic shell enhances solubility in aqueous media, provides stability, and serves as a protective interface [72].
To prevent immune system clearance, micelles can be coated or conjugated with cell-penetrating peptides [73], typically consisting of fewer than 15 amino acids [74]. In one study, micelles were coated with a hybrid membrane composed of erythrocyte and murine breast cancer cell membranes to enhance circulation time and retention in the target tissue [74]. Additionally, cholesterol and sphingolipids can be incorporated to facilitate anchoring in lipid raft-rich regions of the plasma membrane [74].
In biomedical applications, micelles have been widely studied for nucleic acid delivery, such as siRNA and shRNA, in cancer treatments [72]. Hybrid polymeric micelles have been designed for siRNA delivery in lung cancer using conjugations of linoleic acid with PEI and mPEG. Similarly, polymeric micelles composed of Pluronic F127 and polyplexes have been used for AKT2-siRNA delivery in breast cancer treatment [72].
In the field of theranostics, micelles can be functionalized with imaging agents to monitor the target tissue and assess treatment efficacy [72]. By loading contrast agents, they can facilitate both diagnosis and visualization of the biodistribution of therapeutic nanocarriers. One example is the use of gadolinium (Gd) chelates, such as diethylenetriaminepentaacetic acid (DTPA), for MRI imaging [72]. In one study, an anticancer therapeutic agent was encapsulated in a polymeric micelle system, increasing tumor contrast following intrahepatic administration [72]. However, the undesired accumulation of these agents in organs such as the liver and spleen remains a challenge that must be addressed in the design of these systems [72].
Among the various types of micelles, PMs can be designed with tissue-targeting capabilities and sensitivity to chemical or physical stimuli, making them promising nanocarriers for the efficient delivery of drugs and nucleic acids [75]. PMs and polyelectrolyte complex micelles (PICMs) are core–shell nanostructures ranging in size from 10 to 100 nm [75]. Their formation typically involves polymers that facilitate the development of a hydrophobic or ion-containing core, such as poly(d,l-lactide) (PLA), poly(glycolic acid) (PGA), poly(ε-caprolactone) (PCL), poly(trimethylene carbonate) (PTMC), and poly(propylene oxide) (PPO), all of which are FDA-approved for biomedical applications [75]. Additionally, poly (amino acids), including poly(α,β-aspartic acid) (PAsp), poly(β-benzyl-l-aspartate) (PBLA), and poly(l-lysine) (PLys), are utilized due to their ability to facilitate drug conjugation and nucleic acid incorporation into the micelle core [75].
PMs offer several advantages, including low toxicity, deep tissue penetration, targeting capability, ease of synthesis, and prolonged circulation time [75]. However, they also present limitations, such as potential cargo leakage-induced destabilization [75]. Additionally, the small size of micelles restricts the amount of cargo that can be incorporated into the core. Increasing the cargo load results in micelle enlargement, which, in turn, raises the likelihood of aggregation [75].
PMs can be modified with targeting functional groups to enhance their delivery efficiency to specific tissues [74]. These modifications allow functional groups to interact with target tissues through covalent or non-covalent interactions and to respond to specific stimuli in the tissue microenvironment, such as pH or reactive oxygen species (ROS) [74,75]. Additionally, ligands can be conjugated to PMs for recognition by receptors in the target tissue. Surface modification of micelles also facilitates their cellular integration [74]. An example of electrostatic interactions for tissue targeting includes positively charged micelles, which can be directed toward negatively charged mucosal membranes in the intestine, stomach, cornea, and nose. Another example is the use of folic acid (FA) for delivering anticancer agents [74]. Cancer cells create an acidic microenvironment that protonates FA, giving it a positive charge and enhancing its interaction with negatively charged cell membranes [74]. Among covalent interactions that allow greater specificity, functional groups such as maleimide, thiol, and catechol facilitate active targeting [74,75]. For instance, micellar nanocarriers have been modified with PBA to specifically target mucin-rich glycan layers in the eye and cancer cells overexpressing sialic acid (SA) [74].
Regarding micelle sensitivity to other stimuli, micellar systems can detect and respond to external factors such as light, temperature, and electrochemical activation, as well as microenvironmental cues like pH, redox imbalance, and enzymes to trigger the release of therapeutic agents, including gene therapy [74]. Micelles can also be designed to respond to ROS, such as superoxide (O2−), hydroxyl radicals (•OH), hydroperoxyl radicals (RO2−), hypochlorite ions (OCl−), hydrogen peroxide (H2O2), singlet oxygen (1O2), and ozone (O3), which are typically generated during mitochondrial dysfunction or chronic inflammation. ROS-responsive micelles can incorporate ROS-sensitive units such as thioketal (TK), peroxalate ester, thioether, phenylboronic ester, diselenide, and polypeptides [74]. For example, TK linkages undergo oxidative cleavage in response to ROS [74].
2.1.3. Polymeric-Based Nanoparticles
Polymers play a crucial role in various fields, from food packaging and textile manufacturing to advanced biomedical applications [76,77]. Their adaptability and diverse properties make them essential in developing novel materials. In biomedical engineering, polymeric nanoparticles (PNPs) are increasingly being explored for gene therapy and drug delivery, owing to their versatility, biocompatibility, and ability to encapsulate a wide range of therapeutic agents [78]. These nanoparticles offer controlled release and targeted delivery, which are vital for improving the efficacy and reducing the side effects of treatments. In gene therapy, polymeric carriers, particularly cationic polymers, have gained attention due to their ability to condense nucleic acids into compact, stable structures for effective delivery to target cells [79]. By forming stable polyplexes with nucleic acids, PNPs can shield their negative charge, enabling transport through the bloodstream and eventual delivery to target cells [80,81]. However, achieving a balance between stability and disassembly is crucial. Stable polyplexes must protect the genetic cargo during circulation but also release it within the cell to enable therapeutic action [82]. Strategies to improve stability include covalent cross-linking of the nanoparticle core and surface shielding with PEGylation, which minimizes interactions with serum proteins and reduces immune recognition. Furthermore, surface modifications with ligands can enhance targeting efficiency by promoting receptor-specific cellular uptake [83]. The size and charge of PNPs also play critical roles in their pharmacokinetics and biodistribution. Small particles (<6 nm) are rapidly cleared by the kidneys, whereas larger particles (up to 400 nm) exploit the enhanced permeability and retention (EPR) effect to accumulate in highly vascularized tissues, such as tumors [84,85]. This phenomenon has been extensively studied in cancer therapy and can be applied for targeted gene delivery. To address more complex delivery requirements, such as crossing the blood–brain barrier (BBB), polymeric systems can incorporate receptor-specific ligands that enable efficient cellular uptake. Additionally, combining multiple ligands on the nanoparticle surface can facilitate sequential delivery processes, such as endothelial barrier crossing and subsequent cellular internalization [86].
Although many gene delivery strategies demonstrate promising efficiencies in vitro, where conditions are controlled and tumor microenvironments (TMEs) are simplified, translating these findings to clinical settings has proven difficult. In human patients, the EPR effect can vary widely, immune responses may be unpredictable, and off-target uptake by healthy tissues remains a concern [38,87,88]. As a result, while the design principles, as shown in Figure 4, outlined here form the foundation of successful gene delivery, further optimization and robust in vivo validation are essential to ensure therapeutic efficacy and safety.
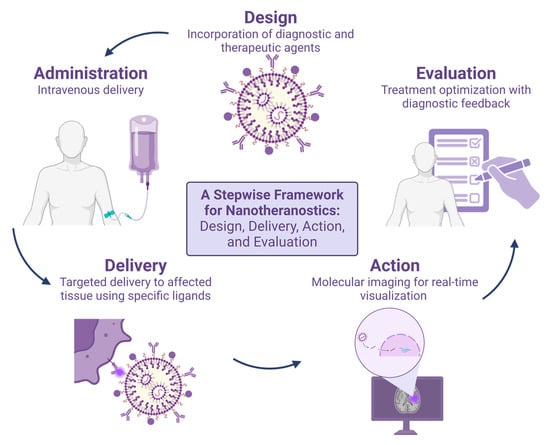
Figure 4.
A Stepwise Framework for Nanotheranostics: Design, Delivery, Action, and Evaluation. In the design stage, agents are incorporated to provide both therapeutic and diagnostic effects. These agents are then administered to the patient. Once internalized, they are delivered to the target tissue, potentially through specific ligands. Finally, real-time visualization can be performed using medical imaging equipment to confirm that the nanotheranostic has reached the target tissue and is detectable.
Cationic polymers such as PEI, PLGA, and chitosan are widely used to form complexes with negatively charged nucleic acids [89]. These complexes protect the nucleic acids from degradation by nucleases, facilitate cellular uptake, and promote endosomal escape, making them promising candidates for gene delivery applications [90]. Upon cellular uptake via endocytosis, polyplexes are entrapped in endosomes, which undergo a maturation process characterized by a gradual decrease in pH. Early endosomes typically maintain a pH of around 6.5–6.8, which then drops to approximately 5.0–5.5 as the endosome matures into a late endosome or lysosome [91,92]. Cationic polymers like PEI exploit these pH changes through mechanisms such as the “proton sponge effect” [93]. In this process, the high buffering capacity of these polymers at acidic pH levels leads to the influx of protons (H+) and accompanying chloride ions (Cl−) into the endosome. This ion influx causes osmotic swelling and ultimately disrupts the endosomal membrane, releasing the polyplex cargo into the cytoplasm [94]. Additionally, the positive charge of the polymers at lower pH levels enhances their interaction with the negatively charged endosomal membrane, further destabilizing the membrane structure and promoting escape. These properties make polyplexes particularly well-suited for gene delivery, as they can efficiently bypass one of the major barriers to intracellular delivery—endosomal entrapment [94].
Many approved polymeric nanoparticle-based therapeutics, as well as those currently in clinical trials, are primarily focused on drug delivery, particularly in oncology. Notable examples include clinical trials with IDs NCT02064829 and NCT03618355 [95,96,97,98,99,100]. One prominent category of PNPs includes polymer–protein conjugates, where therapeutic proteins are covalently linked to polymers to improve their stability and efficacy. For example, PEG (polyethylene glycol)–arginine deaminase is undergoing Phase I trials for hepatocellular carcinoma [101]. Polymer–drug conjugates represent another major area of advancement. These systems covalently attach chemotherapeutics to polymer backbones, often allowing for sustained and targeted drug release. Polyglutamate–paclitaxel has reached Phase III trials for non-small-cell lung cancer [102].
2.1.4. Inorganic Nanoparticles
Inorganic nanomaterials are particularly valued for their versatile functionalization capabilities, unique electrical and optical characteristics, biocompatibility, and relatively low cytotoxicity [103]. Common examples include gold, silver, calcium phosphate, graphene oxide, quantum dots, and magnetic nanoparticles [104] such as iron oxides.
Gold-based nanomaterials stand out due to their highly modifiable surfaces, which facilitate direct DNA complexation. To load the DNA into gold nanoparticles, a direct conjugation based on thiolated (-SH) molecules is formed. This interaction is partially covalent and mostly electrostatic [105]. For instance, pH-sensitive DNA-gold nanocarriers have been developed to deliver small interfering RNA (siRNA) targeting polo-like kinase 1 (PLK1), a critical enzyme for genomic stability and mitosis [106]. Additionally, photothermal ablation, wherein laser-induced heat destroys target cells, is enhanced by compact gold nanoparticle aggregates, minimizing collateral damage to surrounding tissues [106,107]. AuroLase was developed for the ablation of prostate tumors by Nanospectra Biosciences [108].
Graphene oxide (GO), a carbon-based nanomaterial, offers high drug-loading efficiency, due to non-covalent interactions between aromatic rings, and controlled release capabilities [109]. Additionally, its protective properties shield nucleotides from enzymatic degradation. Reduced GO functionalized with PEG and PEI (PEG-BPEI-rGO) has demonstrated stimuli-responsive delivery under near-infrared light, achieving photothermal transfection through localized temperature increases [110]. Furthermore, GO complexes have been used to co-deliver microRNA-21 and chemotherapeutic agents [111]. However, care must be taken with GO due to potential pulmonary toxicity upon inhalation and induction of inflammatory responses in some animal models [112,113,114]. Thorough purification, controlled dosing, and functionalization [115] (e.g., PEG, polymer coatings) can mitigate these risks and are pivotal for safety in vivo applications.
Quantum dots (QDs) are nanoscale semiconductor crystals with tunable optical and electrical properties. They are characterized by their unique physicochemical properties, such as their size, high stability, and low toxicity [116,117]. This approach uses the unique features of the vasculature in certain regions, such as tumors or inflamed tissues, to enable preferential accumulation of nanoparticles. Unlike active targeting, which requires the functionalization of nanoparticle surfaces with ligands that bind to specific receptors, passive targeting relies on non-specific factors like the structure of blood vessels and the ability of nanoparticles to penetrate and accumulate in tissues [118].
Magnetic nanoparticles, particularly those composed of iron oxides, combine therapeutic and diagnostic capabilities. Their magnetic properties enable precise targeting and tracking, as well as applications in magnetic resonance imaging (MRI) [119]. Polyeth-yleneimine-functionalized MNPs have been shown to enhance gene delivery under the influence of magnetic fields, prolonging gene expression and allowing In vivo tracking [120]. Superparamagnetic iron oxide nanoparticles (SPIONs) have also been explored for targeted gene delivery, leveraging their magnetic responsiveness to minimize off-target effects. Recent advancements include the development of bioreducible polymer-coated SPIONs, which enable siRNA delivery with reduced cytotoxicity, offering a promising platform for theranostic applications [121].
2.1.5. Hybrid Systems and Multifunctional Platforms
Hybrid systems in nanomedicine represent a powerful approach to enhancing gene de-livery and overcoming the inherent limitations of single-material platforms. These nano systems merge distinct material types—such as lipids, polymers, and inorganic nanoparticles—into architectures that use the complementary strengths of their com-ponents [122].
Hybrid architectures in gene delivery commonly adopt specific forms to optimize their physical and functional properties. For example, lipid-inorganic hybrid systems, such as nanoparticle-functionalized liposomes, integrate the loading and delivery efficiency of liposomes with the targeting and transfection properties of inorganic nanoparticles [123]. Cell membrane-encapsulated nanoparticles provide serum stability and biospecific targeting by mimicking natural cell interfaces, with the inorganic core [124]. Another configuration includes metal nanoparticles bound to liposome surfaces, enabling stimulus-responsive release of encapsulated therapeutic agents [125].
Polymer-based hybrids extend these capabilities by incorporating polymers or dendrimers as stabilizing shells around inorganic cores, forming core-shell architectures [126]. Alternatively, layer-by-layer assembly techniques sequentially coat metallic nanoparticles with polymers and therapeutics, creating platforms with tunable release profiles [127]. In gene therapy, hybrid systems have significantly advanced nonviral delivery strategies. Liposome-based hybrids use the properties of liposomal systems which address gene transfection [128] challenges by offering a positively charged sur-face and a lipid shell, which mitigate charge repulsion and facilitate membrane fusion for effective gene delivery. Gold-functionalized liposomes have been shown to enhance gene transfection by improving cellular uptake and avoiding lysosomal degradation. For example, gold-liposome hybrids delivering siRNA have demonstrated superior knockdown efficiency of oncogenes in cancer models [129]. These systems also support the co-delivery of therapeutic agents like CRISPR/Cas9 components, enabling precise gene editing [130].
Polymer-inorganic hybrids similarly show promise in gene delivery applications. Cationic polymers such as polyethyleneimine (PEI) or poly-L-lysine (PLL) are often combined with gold nanoparticles (AuNPs) to facilitate nucleic acid delivery. Core-shell gold-polymer hybrids have been employed to treat various cancers, including prostate, breast, and liver, by improving transfection efficiency and enabling targeted delivery [131]. Beyond gold, other inorganic materials such as iron oxide nanoparticles (IONPs), mesoporous silica nanoparticles (MSNs), and calcium phosphate (CaP) have been integrated into hybrid systems to deliver siRNA, plasmid DNA or microRNA for cancer therapy and antiviral applications [132].
2.2. Key Properties for Gene Delivery
Gene delivery systems must meet several key properties to ensure their effective use in clinical applications. These properties are integral for ensuring that gene therapy is not only efficient but also safe and long-lasting. Biocompatibility, stability, controlled re-lease, and targeting capabilities are among the most crucial factors for the success of gene delivery platforms [133].
Biocompatibility is one of the foundational requirements for gene delivery systems, as any material used in vivo must not induce harmful reactions in the body. For nanoparticles to be suitable for clinical use, they must be non-toxic, not provoke significant immune responses, and should be biodegradable or cleared from the body efficiently [134]. Strategies to enhance biocompatibility include surface modification, such as PEGylation, which introduces hydrophilic polyethylene glycol chains to shield nanoparticles from immune recognition and reduce protein adsorption [83]. Additionally, zwitterionic coatings, which create neutral surfaces, can minimize nonspecific interactions with biological molecules, further improving biocompatibility [135]. The use of biodegradable materials, such as certain polymers and lipids, also ensures that the nanoparticles degrade into harmless byproducts after fulfilling their gene delivery function, mitigating the risk of long-term accumulation [136]. To evaluate these aspects, standard assays such as cytotoxicity tests and in vivo compatibility studies are employed, assessing the potential harm to cells and tissues.
Stability and controlled release are essential properties for ensuring that gene delivery systems remain effective in the dynamic and complex biological environment. Maintaining nanoparticle stability is challenging due to factors such as changes in pH, enzymatic degradation, and ionic strength, all of which can lead to premature degradation or release of the therapeutic payload [137]. Nanoparticle design, as shown in Table 2, plays a crucial role in ensuring stability, with core-shell structures and crosslinked networks offering significant advantages. Core-shell systems can protect sensitive genetic material from degradation, while crosslinked polymers can provide structural integrity under varying environmental conditions [138]. Controlled release mechanisms further enhance the therapeutic potential of these systems. Triggered release, where the payload is released in response to specific stimuli such as pH changes, temperature fluctuations, or enzymatic activity, allows for precise control over when and where the gene therapy is active [130]. Additionally, sustained release mechanisms ensure prolonged therapeutic effects, providing long-term benefits without the need for repeated administration [139].
Targeting capabilities are another critical property that distinguishes highly effective gene delivery systems. There are two primary strategies for targeting: passive and active. Passive targeting in the context of gene therapy refers to the natural tendency of nanoparticles (NPs) to accumulate in specific tissues or organs based on their inherent physiological or pathological characteristics, without the need for specific targeting agents [117]. This approach uses the unique features of the vasculature in certain regions, such as tumors or inflamed tissues, to enable preferential accumulation of nanoparticles. Unlike active targeting, which requires the functionalization of nanoparticle surfaces with ligands that bind to specific receptors, passive targeting relies on non-specific factors like the structure of blood vessels and the ability of nanoparticles to penetrate and accumulate in tissues [118].
Passive targeting relies primarily on the EPR effect, which exploits the abnormal vasculature found in tumors and inflamed tissues. In these conditions, blood vessels are leaky, allowing nanoparticles to accumulate more readily in the target area [140]. However, the effectiveness of passive targeting can be limited by factors such as the heterogeneity in tumor vasculature, which results in uneven distribution and accumulation of the NPs. Additionally, the presence of physiological barriers like the extracellular matrix can impede the efficient uptake into target cells, reducing the overall efficacy of gene delivery [141].
Active targeting, in contrast, involves functionalizing the surface of NPs with specific ligands, such as antibodies, peptides, or aptamers, that bind to receptors or antigens overexpressed on the target cells. This strategy significantly enhances the specificity of gene delivery, as it enables precise recognition and binding to the target site [142]. Antibody-conjugated NPs, for instance, can bind to tumor-specific antigens, facilitating more effective targeting of cancer cells [143]. Similarly, peptides and aptamers can be used to target receptors that are overexpressed on other disease-related cells, such as those involved in neurological or cardiovascular conditions. The surface modification of NPs with such targeting ligands can be achieved through various strategies, including covalent attachment via cross-linkers or the use of self-assembling peptides [144]. By adjusting the surface properties of the nanoparticles, including their charge, size, and the density of targeting ligands, it is possible to optimize cellular uptake and improve the efficiency of gene delivery.

Table 2.
Comparison of nanoparticle systems for gene therapy.
Table 2.
Comparison of nanoparticle systems for gene therapy.
| Feature | Lipid-Based Nanoparticles | Polymeric Nanoparticles | Inorganic Nanoparticles | Hybrid Nanoparticles |
|---|---|---|---|---|
| Key Delivery Mechanisms | Endocytosis, endosomal escape (pH-dependent), membrane fusion [145,146] | Endocytosis, proton sponge effect (cationic polymers) [82,147,148,149] | Endocytosis [150,151] | Combination of mechanisms from constituent materials [152,153,154,155,156,157,158,159,160,161,162,163] |
| Key Material Properties | Self-assembling lipids, ionizable lipids, PEGylated lipids [156,157] | Variety of synthetic and natural polymers, biodegradable options [82,147] | Metals, metal oxides, silica, carbon-based materials [150,151,152,153] | Combination of organic and inorganic materials [152,153,154,155,156,157,158,159,160,161,162,163] |
| Major Advantages | Effective for RNA delivery, scalable production, modifiable [156] | Versatile, biodegradable, safe, high loading capacity [82,147] | High stability, potential for multimodal applications [150,151,152,153] | Synergistic properties, enhanced biocompatibility and efficacy [152,153,154,155,156,157,158,159,160,161,162,163] |
| Major Limitations | Potential toxicity, endosomal escape can be inefficient [158,159] | Lower transfection efficiency than viral vectors, potential toxicity [160] | Potential toxicity, lower transfection efficiency, aggregation [151,153] | Complexity in design, potential for component-specific limitations [161] |
| Influence of Size/Charge/Ligands | Critical for uptake, stability, and targeted delivery [162] | Critical for uptake, DNA complexation, and targeted delivery [148] | Critical for uptake, biodistribution, and targeted delivery [154] | Critical for overall effectiveness, tunable by composition [163] |
A comparison between active and passive targeting reveals significant differences in efficiency and specificity. Passive targeting via the EPR effect is generally less specific and more dependent on the physiological conditions of the target tissue, which may limit its application in more complex or heterogeneous diseases [84]. In contrast, active targeting offers superior specificity by directing nanoparticles precisely to the desired target cells, minimizing off-target effects and improving therapeutic outcomes. However, active targeting can be more complex and costly, requiring the careful design and attachment of ligands, as well as ensuring that the ligands maintain their targeting ability in the biological environment [118].
2.3. Imaging-Guided Delivery
Imaging-guided delivery is crucial for ensuring precise gene therapy. Incorporating imaging agents into nanoparticles allows for real-time tracking of vector biodistribution, optimizing dosages, and verifying delivery accuracy. Techniques like fluorescence microscopy, radiolabeling, and live-cell imaging systems enable dynamic observation of nanoparticles, with methods such as TIRF and confocal microscopy providing insights into nanoparticle uptake and intracellular transport.
Advanced imaging techniques like Stimulated Emission Depletion (STED) microscopy and Stochastic Optical Reconstruction Microscopy (STORM) offer nanometer-scale resolution. Hybrid nanoparticles, including gold or iron oxide combined with liposomes or polymers, facilitate both therapeutic delivery and tracking. Imaging technologies like MRI, fluorescence imaging, and Positron Emission Tomography (PET) play vital roles in monitoring biodistribution, tissue penetration, and therapeutic efficacy, especially when combined in multimodal systems for enhanced diagnostic accuracy.
2.3.1. Real-Time Tracking of Vectors
Real-time tracking of gene delivery vectors has emerged as a pivotal strategy for ensuring precise and efficient therapeutic delivery. Incorporating imaging agents into nanoparticles provides continuous monitoring, allowing researchers to verify vector biodistribution, optimize dosages, and ensure delivery accuracy. Techniques such as fluorescence microscopy, radiolabeling, and advanced live-cell imaging systems enable dynamic observation of nanomaterials within biological systems [36,164].
Temporal analytical methods have advanced significantly. For instance, fluorescence microscopy with high temporal and spatial resolution enables single particle tracking of nanoparticles such as carbon nanotubes, quantum dots, and polystyrene NPs [164]. Techniques like total internal reflection fluorescence (TIRF) microscopy and laser scanning confocal microscopy provide real-time visualization of nanoparticle uptake and intracellular transport, unveiling complex interactions at the molecular level [165]. The development of super-resolution imaging modalities, including STED and STORM, allows researchers to observe cellular processes at nanometer-scale resolution [166,167].
Nanoparticles designed for real-time imaging have demonstrated potential in diverse applications. For example, hybrid systems combining AuNPs or IONPs with liposomes or polymers enable the simultaneous delivery of therapeutic agents and real-time tracking [168]. A silica nanoparticle is under investigation for the real-time intraoperative mapping of nodal metastases in various cancers, including breast, colorectal, and head and neck melanomas [169].
2.3.2. Imaging Modalities
Imaging technologies form the cornerstone of nanotheranostics, providing crucial insights into vector behavior and therapeutic efficacy. MRI offers non-invasive, high-resolution imaging with excellent soft-tissue contrast [170]. Nanoparticles such as iron oxide variants serve as MRI-active agents, enabling precise localization of therapeutic vectors. MRI’s ability to monitor biodistribution and tissue penetration makes it invaluable for longitudinal studies [171,172]. Fluorescence imaging, using quantum dots and organic dyes, offers real-time visualization of nanoparticles due to their tunable optical properties. This technique enables tracking of multiple biomarkers simultaneously, providing a comprehensive view of therapeutic delivery [173].
PET represents a highly sensitive modality for tracking nanoparticles labeled with radiotracers. PET imaging enables quantification of vector distribution and accumulation in targeted tissues. Dual-mode systems integrating PET with other modalities, such as MRI or fluorescence imaging, combine the strengths of each technique. Such multimodal imaging platforms enhance diagnostic accuracy and offer a holistic view of therapeutic processes [174].
Each imaging modality presents distinct trade-offs. MRI provides superior soft-tissue contrast but relatively lower sensitivity compared to fluorescence, while PET offers high sensitivity but lower spatial resolution. Fluorescence imaging achieves real-time visualization at subcellular resolution but has limited tissue penetration depth. Hence, multimodal systems that integrate MRI with fluorescence or PET can capture both anatomical and functional data, significantly enhancing the precision of gene delivery. By leveraging these complementary imaging techniques, researchers can precisely localize nanoparticle-based gene vectors, monitor their accumulation in target tissues, and assess transfection efficiency in real time, thereby refining the safety and efficacy profile of advanced gene therapies.
3. Enhancing Gene Delivery with Nanotheranostics
Gene therapy has emerged as a crucial strategy for treating diseases caused by dysregulated gene expression, including cancer, infections, and hereditary disorders [175]. This approach involves the delivery of exogenous nucleic acids—such as DNA, siRNA, mRNA, and miRNA—to regulate gene expression effectively.
However, its success depends on the precise and targeted delivery of these molecules while overcoming physiological barriers. To address this challenge, nanotheranostic platforms have gained significant attention for gene delivery. These systems facilitate the spatial and temporal localization of genetic material while integrating imaging capabilities for real-time disease diagnosis and treatment monitoring.
Extensive research has explored various nanotheranostic agents, with particular emphasis on AuNPs, IONPs, QDs, and upconversion nanoparticles (UCNPs), among others.
The integration of nanotheranostic agents plays a crucial role in enhancing the precision and control of gene delivery. These agents respond to various stimuli, enabling the spatial and temporal localization of gene therapy. The incorporation of imaging modalities such as PET, MRI, and fluorescence allows for real-time tracking of nanoparticle distribution, ensuring precise delivery at the intended site of action. Additionally, imaging provides essential feedback on biodistribution, gene transfection, and expression, facilitating real-time adjustments in localization and dosage to optimize therapeutic outcomes. For instance, magnetic nanoparticles detectable via MRI [176] and gold nanoparticles, which serve as effective contrast agents in X-ray imaging due to their high atomic number (Z = 79) and strong X-ray attenuation [177], exemplify the potential of nanotheranostics in medical imaging.
Beyond imaging, external stimuli such as light, temperature, and magnetic fields enable the controlled activation of nanotheranostic agents, precisely regulating their passage across cellular barriers and the release of therapeutic molecules at targeted intracellular sites. This level of control minimizes off-target effects, enhances therapeutic efficacy, and improves patient safety.
For example, ultrasound-sensitive microbubbles release their payload into the intracellular environment upon stimulation [178], while magnetic nanoparticles can be selectively internalized into target tissues through the guidance of magnetic fields, ensuring precise localization [179].
3.1. Overcoming Delivery Barriers
The implementation of nanotheranostics for targeted disease treatment faces several challenges, particularly in achieving the precise intracellular delivery of genetic material. Over the years, key barriers have been identified, including the need for accurate localization within specific cellular compartments, as shown in Figure 5. Among the most significant obstacles are endosomal escape and nuclear delivery, both of which have been extensively studied to develop effective strategies for overcoming these limitations.
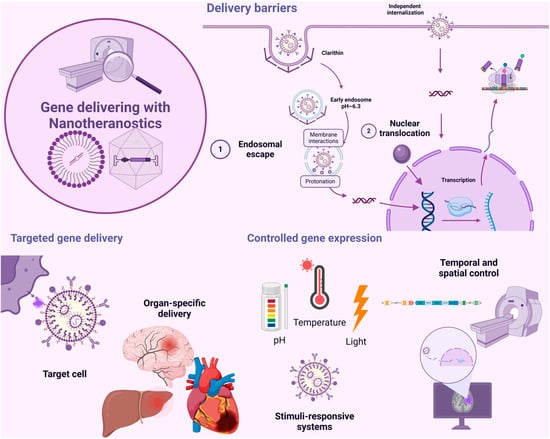
Figure 5.
Gene delivery with nanotheranostics: delivery barriers, targeted gene delivery, and controlled gene expression. During gene delivery facilitated by nanotheranostics, certain barriers may arise, such as endosomal escape and nuclear translocation. Additionally, the delivery must be specific to the target tissue, which requires considering strategies like passive targeting and active targeting. Finally, controlled gene expression is crucial, and systems that respond to external stimuli with spatiotemporal control can be utilized for this purpose.
3.1.1. Endosomal Escape Strategies
Endosomal escape is a critical step in the delivery of genetic material via nanotheranostic agents, as these agents typically enter cells through pinocytosis. This process is classified into four subtypes: macropinocytosis (an actin-driven endocytic mechanism), clathrin-mediated endocytosis, caveolin-mediated endocytosis, and clathrin- and caveolin-independent endocytosis [180].
During pinocytosis, extracellular contents are internalized through the formation of endosomes, which transport nanotheranostic agents toward lysosomal degradation. Therefore, ensuring endosomal escape into the cytoplasm before lysosomal breakdown of the delivered genetic material is essential for effective gene therapy [50].
Proton Sponge Effect
Various strategies have been explored to achieve endosomal escape, depending on the functionalization of nanotheranostic agents used for genetic material delivery. One of the primary mechanisms involves pH-mediated processes, with the proton sponge effect playing a key role.
The proton sponge effect is one of the most extensively studied strategies for overcoming the endosomal barrier in gene delivery. This mechanism prevents theranostic agents from following the endosomal pathway to lysosomes, where they would otherwise be degraded. By inducing endosomal membrane lysis before degradation occurs, the proton sponge effect facilitates the efficient release of gene therapy into the cytoplasm [181]. Once in the cytoplasm, the therapeutic molecules can either act directly or migrate to the nucleus to exert their effects on gene expression.
The proton sponge effect exploits the low pH environment inside endosomes, which ranges from 6.0 to 6.5 in early and late endosomes, in contrast to the extracellular milieu (pH 7.4) and cytoplasm (pH 7.2) [182]. This acidic environment is created by proton influx through ATPase channels in the endosomal membrane [183].
When endosomal components possess a high cationic capacity, they sequester protons, maintaining pH balance through a buffering effect. These buffering triggers endosomal ion channels to transport chloride ions, leading to an osmotic effect that drives an excessive influx of water into the endosome. As the intraluminal volume increases by approximately 5%, the endosome ruptures, releasing its contents into the cytoplasm [181].
To activate this endosomal escape pathway, nanotheranostic agents are often functionalized with polyamines. Among them, PEI is the most widely used for facilitating the proton sponge effect. In its liquid state, PEI consists of approximately 30% primary, 40% secondary, and 30% tertiary amines, all of which exhibit high protonation capacity within endosomes [181]. PEI has been applied to functionalize various nanotheranostic agents for gene delivery, including IONPs for DNA delivery [184], QDs for siRNA delivery [185], and AuNPs for siRNA delivery [186], among others.
In addition to PEI, other buffering molecules have been used for nanotheranostic functionalization to enhance endosomal escape, including Chloroquine, dendrimers, Poly (propylacrylic acid) (PPAA), Poly(amidoamine)s (PAAs), chitosan [187], and Poly(2-(dimethylamino) ethyl methacrylate) (PDMAEMA) [183].
Additionally, Poly (dimethyldiallylammonium chloride) (PDDA) has been used to coat MSNs for plasmid transport, achieving successful endosomal escape [188].
While widely studied in vitro, clinical translation of proton sponge-based polymers like PEI has been hindered by cytotoxicity [189]. Nevertheless, derivatives and low-molecular-weight modifications are in preclinical studies for tumor-targeted gene delivery [189,190,191].
Photochemical Internalization
Photosensitizers (PSs) are typically amphiphilic molecules capable of embedding into endocytic membranes [192]. Upon light exposure, they undergo activation, triggering chemical or physical changes associated with energy level transitions. Upon absorbing light, PS molecules transition to an excited singlet state (1P*), from which they can dissipate energy as heat or fluorescence or undergo intersystem crossing (ISC) to convert into an excited triplet state (3P*) [187].
In the triplet state, PS molecules can release energy as heat or phosphorescence or transfer energy to a target molecule or molecular oxygen via two photochemical pathways. In one pathway, reactive oxygen species (ROS) are generated through electron or hydrogen transfer between the PS and the target molecule. In the other, energy transfer from the PS to molecular oxygen produces singlet oxygen, which induces oxidative damage to endocytic membranes, facilitating vesicle disruption [187].
Commonly used compounds for PCI include disulfonated meso-tetraphenylporphine (TPPS2), disulfonated aluminum phthalocyanine (AlPcS2), dendrimer phthalocyanine (DPc), 5,10,15-tri(4-acetamidophenyl)-20-mono(4-carboxyl-phenyl) porphyrin (TAMCPP), and tetra(4-sulfonatophenyl) porphyrin (TPPS4) [193,194,195,196].
Researchers have investigated loading siRNA and the PS hypocrellin A (HA) into MSNP pores [197], subsequently coating these complexes with a PEG polymer via a photocleavable ONB-based linker. Upon near-infrared (NIR) radiation exposure, the UCNPs emitted ultraviolet (UV) light, triggering the degradation of the photocleavable linker and siRNA release. This process also activated HA, generating ROS that disrupted the endosomal membrane [198].
Photothermal Internalization
This technique employs a photothermal transduction agent (PTA) to convert light into heat, increasing the temperature in a controlled and localized manner. This temperature rise disrupts endocytic vesicles, promoting efficient endosomal escape [187]. Nanotheranostic formulations incorporate a photothermal agent (PTA) to enable light irradiation-induced heating. The localized temperature increase disrupts endocytic vesicles, promoting efficient endosomal escape and ensuring the successful delivery of gene therapy past the cellular barrier [187].
Photothermal-induced endosomal escape occurs via two primary mechanisms. In the first, heat generated upon light activation directly destabilizes the endosomal membrane. In the second, heat creates a vapor layer around the PTA, leading to bubble formation. The subsequent collapse of these bubbles induces mechanical disruption of the endocytic membrane [187].
Gold nanoshells have been widely explored as effective PTAs. Functionalization with pyrogallol 2-aminoethane has facilitated the delivery of siRNA for gene silencing, demonstrating promising results in enhancing endosomal escape through a photothermal mechanism following NIR irradiation [199].
Membrane Translocation or Destabilization-Mediated
This mechanism induces the formation of pores in the endosomal membrane through the cationic functionalization of nanotheranostic agents. Cationic molecules interact with anionic components on the outer surface of the endosomal membrane, triggering a structural rearrangement known as “flip-flop”. This rearrangement leads to the formation of small pores in the membrane, allowing nanoparticles to escape into the cytoplasm [181].
Iron oxide nanoparticles have been functionalized with poly-arginine, a highly cationic polymer, which has been shown to facilitate siRNA delivery. This approach enables effective endosomal escape via a membrane destabilization-mediated mechanism [200].
Membrane Fusion-Mediated
The Membrane Fusion-mediated technique involves the use of fusogenic lipids or amphiphilic molecules (FLAMs) [201,202] to encapsulate gene-delivering nanotheranostic agents. Once inside the endosomes, these agents leverage the acidic environment, allowing protonated FLAMs to fuse with the endosomal membrane and release their contents into the cytoplasm. The fusion is initiated by a conformational change in FLAMs under acidic conditions, leading to interactions between the zwitterionic luminal lipids of the endosome and the protonated FLAMs. This interaction promotes membrane fusion and the subsequent release of the FLAMs’ cargo into the cytosol [187,203].
This mechanism has been applied in magnetoliposome formulations for the delivery of SLP2 shRNA plasmids, where IONPs were encapsulated within liposomes for glioblastoma treatment. In this case, the liposomes act as FLAMs, capable of fusing with the endosomal membrane and releasing the encapsulated nanotheranostic agents and plasmids [204].
Overall, these methods have shown promising results in preclinical models. However, real-world effectiveness depends heavily on the route of administration, TME, and safety constraints. For instance, photothermal or photochemical approaches require external energy sources and may have limited penetration depths in human tissues [205]. Future research must address these practical concerns to realize the full potential of endosomal escape strategies in clinical gene therapy.
3.1.2. Improving Nuclear Translocation
The nucleus is one of the most intriguing organelles for gene delivery, especially considering the wide range of diseases caused by genetic information errors, such as cancer, heart failure, and other conditions. However, achieving the effective delivery of specific genes into the nucleus by nanotheranostic agents remains a significant challenge due to the high selectivity of substances that can correctly enter the nucleus.
The nuclear envelope consists of two lipid bilayers: an inner and an outer membrane, separated by a perinuclear space. Within the envelope, small openings called nuclear pores are formed by the nuclear pore complex (NPC) [206]. The NPC is structured into three primary layers arranged perpendicular to its transport axis: the luminal ring, the core scaffold, and the FG nucleoporins (FG Nups). The luminal ring, composed of transmembrane proteins like Pom121, anchors the NPC to the nuclear envelope. The core scaffold provides structural integrity and connects the FG Nups to the envelope. The FG Nups, made up of 200–250 intrinsically disordered polypeptides, form a selective permeability barrier that permits ions and small molecules to pass while preventing the movement of larger molecules [207].
The nuclear pore complex allows the passage of very small substances, with a diameter of 9 nm, via passive transport [181]. However, for larger particles, such as many gene-delivering nanotheranostic agents, transport occurs through facilitated diffusion. Facilitated diffusion operates by tagging cargo destined for nuclear transport with nuclear localization signals (NLSs), which are recognized by importin α in the cytoplasm. Importin α binds to the cargo and links it to importin β, a nuclear transport receptor responsible for guiding the complex through the nuclear pore [206,208]. Once inside the nucleus, Ran-GTP binds to importin β, inducing a conformational change that releases the cargo-importin α complex. Importin β then returns to the cytoplasm independently, while importin α is exported via exportin CAS [181].
Nuclear Localization Signals (NLSs)
Numerous strategies incorporate nuclear localization signals (NLS) or transport carriers that leverage the cell’s intrinsic transport mechanisms, embedded within the chemical structure of nanotheranostic agents. This ensures efficient gene delivery to the nucleus for transcription. Importin α plays a key role in the nuclear import of proteins containing a classical NLS. Its central region consists of ten consecutive arginine-rich motifs (ARM), which form a binding domain for the NLS [209]. Structural studies have identified two distinct NLS-binding sites within this region. The first site, located between ARM motifs 1–4, interacts directly with the amino acid sequences of monopartite NLS, as well as the longer sequences in bipartite NLS. The second site, within motifs 7–8, specifically binds to the shorter sequence found in bipartite NLS [207].
Classic nuclear localization signals (CNLS) typically consist of one (monopartite) or two (bipartite) clusters of basic residues [210]. Monopartite NLS, such as those in the large T antigen of the SV40 virus, the large T antigen of polyomavirus, hepatitis D virus antigen, murine p53 protein, NF-κB p50, NF-κB p65, and human c-myc, usually contain a single group of four or five basic residues. In contrast, bipartite NLS, such as those in Xenopus nucleoplasmin (KRPAATKKAGQAKKKKLD), rat glucocorticoid receptor (YRKCLQAGMNLEARKTKKKIKGIQQATA), and RCC1 (MSPKRIAKRRSPPADAIPKSKKVKVSHR), feature two distinct groups of basic residues [207]. Mesoporous silica nanoparticles (MSNs) have been functionalized with the NLS (PKKKRKV) for the nuclear delivery of plasmids [188].
The Transactivator of Transcription (TAT) peptide (GRKKRRQRRRAPQN), derived from the human immunodeficiency virus type 1 (HIV-1), has shown exceptional ability to facilitate the delivery of various cargos into cells and the nucleus. The TAT peptide contains overlapping regions responsible for cellular localization, including a classical nuclear localization signal (cNLS) (GRKKRR) and a cell-penetrating peptide (CPP) signal (GRKKRRQRRRAPQN). These distinct regions within TAT are essential for the efficient transport of cargos across cellular barriers and their subsequent entry into the nucleus [207]. The TAT peptide has been studied in AuNPs, where it was functionalized with PEI for gene delivery to stem cells and for targeting the nucleus for pDNA entry [211]. It has also been employed in co-treatment strategies using AuNPs, including photodynamic therapy and pDNA transport [212].
Glyco-Dependent Nuclear Import
Another approach to enhance nuclear entry involves the recognition of glycosylated proteins. Lectins facilitate this process by interacting with sugar moieties on the proteins [213,214]. This mechanism operates independently of the number of NLS-containing proteins in the cytoplasm. Consequently, glycosylated nanotheranostic agents have been studied for their ability to enter the nucleus via lectin-mediated gene delivery [215]. For example, glycopolymer-stabilized gold nanoparticles have been used for gene delivery, achieving high nuclear targeting efficiency [216].
Nuclear Receptors-Based Import
Nuclear receptors are a group of transcription factors with a high negative charge, which, like importins, facilitate their transport through the positively charged NPCs. These receptors, including estrogen receptors, glucocorticoid receptors, and others, play a vital role in the physiological processes of acquiring necessary substances for the nucleus [215].
For example, PEI-gold nanoparticles modified with dexamethasone have been used to enhance gene transfection efficiency. The presence of the dexamethasone ligand enabled nuclear entry via the nuclear glucocorticoid receptor [217].
Direct Interaction with NPC
Another mechanism of nuclear entry involves direct interaction with the NPC. This can be achieved using cationic polymers such as PEI and fusogenic lipids and peptides [215]. The functionalization of AuNPs with PEI has been explored, demonstrating successful nuclear entry driven solely by the action of PEI [218]. Additionally, PEI has been utilized as a nuclear targeting agent in IONPs for DNA delivery to the nucleus, achieving precise targeting exclusively through PEI’s action [219].
While multiple nuclear targeting strategies (e.g., TAT peptide-based translocation) show high transfection efficiency in vitro, translation to clinical gene therapy remains limited. Issues such as immunogenicity of viral- or peptide-derived signals, potential unintended gene activation, and scalability of manufacturing must be resolved before these techniques see widespread adoption in human trials [220,221,222].
3.2. Targeted Gene Delivery
The use of nanotheranostic agents in targeted gene delivery represents one of the most promising applications of nanobiotechnology in theranostics. The ability to track nanoparticles in a spatiotemporal manner and pinpoint the exact location of a gene therapy intervention in the body is a key motivator behind the advancement of theranostics. Precise targeting, as shown in Figure 6, is crucial in various contexts, not only for accurately treating diseases but also for serving as a diagnostic tool to visualize disease sites.
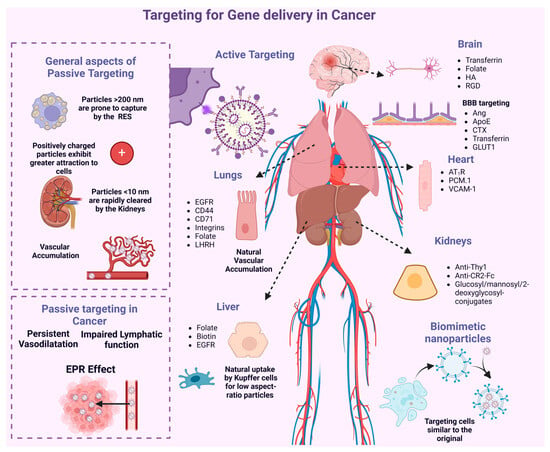
Figure 6.
Passive and active targeting for gene delivery in cancer. In the passive targeting strategy, the properties of tumor tissues and the inherent characteristics of nanotheranostic agents are exploited. Tumor sites exhibit higher vascular permeability compared to normal tissues, along with a compromised lymphatic drainage system, leading to increased retention of particles in these areas. In the active targeting strategy, ligands are employed to specifically bind to overexpressed membrane receptors on cancer cells.
3.2.1. Tumor-Targeted Systems
One of the key applications of targeted gene therapy is in cancer treatment. The primary objective is to selectively target cancerous cells while preserving the integrity of healthy, non-cancerous cells, thereby minimizing risks to the well-being of patients undergoing the treatment.
Passive Targeting
Passive targeting is a well-established approach for targeting cancer cells within a patient’s system. A key mechanism behind this method is the EPR effect. Tumor sites exhibit higher vascular permeability compared to normal tissues, in addition to a compromised lymphatic drainage system, which results in increased retention of particles within these areas. The microvasculature in tumors lacks smooth muscle cells, causing persistent vasodilation and disrupting the normal regulation of blood flow. These abnormal vessels impede fluid and solute transport, which can be exploited to enhance the EPR effect further [140].
The EPR effect is also influenced by vasogenic mediators such as bradykinin, nitric oxide (NO), peroxynitrite, matrix metalloproteinases (MMPs), vascular endothelial growth factor (VEGF), and prostaglandins (PGs). Regulating these mediators may optimize the EPR effect in tumor sites, helping nanotheranostic agents to accumulate in the tumor tissues [140].
In this strategy, both the properties of tumor tissues and the inherent characteristics of nanotheranostic agents are utilized. For instance, agents are designed to exploit the EPR effect, typically by selecting an appropriate size range to avoid renal clearance. Studies show that macromolecules with a molecular weight above the renal threshold (~40 kDa) tend to preferentially accumulate in neoplastic tissues after intravenous administration [140].
However, passive targeting alone is not ideal, as many non-cancerous tissues also possess high vascular density, and some tumors exhibit poor vascularization. Moreover, the mere accumulation of nanotheranostic agents in tumor regions does not guarantee their ability to reach the tumor cells. As such, passive targeting should be combined with active targeting, which utilizes ligands that specifically bind to overexpressed receptors on tumor cells [140].
Active Targeting
Other strategies, such as active targeting, have been explored, where ligands are used to specifically bind to overexpressed membrane receptors on cancer cells. By understanding the biological characteristics of the target cells, various types of ligands or molecules, such as peptides, antibodies, proteins, polysaccharides, nucleic acids, and receptors, can be conjugated to the surface of the carrier particles to improve the target-to-non-target ratio [37].
Active targeting can be classified into two types [223]: First, receptor-mediated endocytosis, where drug-loaded nanocarriers are coated with specific ligands to target receptors on tumor lesions for binding and subsequent cellular uptake. Second, stimuli-responsive intracellular drug delivery, which relies on subtle changes in the microenvironment of the pathological area to trigger the release of the therapeutic payload [223].
Cancer Cell Surface Targeting Strategy
There are multiple receptors that are overexpressed in certain cancer cells. Each of these receptors has its own targeting moieties. Many of these targeting moieties can serve as ligands in a nanotheranostic agent transporting genes, thereby facilitating the active targeting of genes to specific cancer cells.
Among the most used ligands is folic acid, which plays a vital role in the synthesis of nitrogenous bases [37]. With its ability to target the folate receptor, a glycosylphosphatidylinositol-anchored protein that binds folic acid and folate, this ligand has been instrumental in tumor diagnosis. The folate receptor is exclusively expressed in healthy kidney tissues exposed to blood, making it particularly valuable for imaging diagnostics [224]. Additionally, folic acid has been employed in gene delivery systems using nanotheranostic agents, such as AuNPs coated with lipids and folic acid, for cancer detection and DNA delivery [225].
Another well-known ligand is Transferrin, a glycoprotein that plays a critical role in managing the body’s Fe (III) pool. Due to the immense iron requirements of rapidly growing cancer cells, transferrin is highly overexpressed in such cells [37]. Studies have explored the use of magnetic nanoparticles conjugated with PEI for pDNA delivery into cancer cells [226].
The epidermal growth factor receptor (EGFR), a transmembrane protein involved in cellular growth, angiogenesis, invasion, and metastasis, has also been investigated [37]. EGFR-targeted strategies include delivering the tumor suppressor gene p53 for ovarian cancer treatment using AuNPs. These approaches have demonstrated high in vivo effectiveness following intraperitoneal administration [227].
Lastly, the human epidermal growth factor receptor 2 (HER2) belongs to a receptor family that regulates cell growth, survival, and differentiation [37]. HER2-targeting ligands, such as trastuzumab, have been applied with mesoporous silica nanoparticles for the delivery of siRNA in breast cancer treatment, showing promising results [227].
Tumor Microenvironment
Leveraging the characteristics of the TME is a viable strategy for enhancing the targeted delivery of nanotheranostic agents for gene therapy. One critical factor to consider is the hypoxic conditions prevalent in tumor regions, which arise from the immature and poorly functioning vasculature. As solid tumors grow, their oxygen demand rises significantly. However, the newly formed blood vessels are often defective, exhibiting poor perfusion and increased permeability. This, combined with the rapid proliferation of tumor cells, depletes the available oxygen, creating a hypoxic microenvironment that can be exploited for precise therapeutic targeting [37]. Gene delivery strategies using bionanotechnology have been developed based on interactions with hypoxia-sensitive compounds, such as 2-nitroimidazole or azobenzene, for the controlled release of oligonucleotides [203].
Another key factor in the TME is the acidity of tumor tissues [228]. Tumors exhibit high acidity due to a metabolic shift known as the Warburg effect, where cancer cells favor glycolysis over oxidative phosphorylation, even in the presence of oxygen. This process generates an excess of lactic acid and protons (H+). To maintain intracellular pH stability, cancer cells expel these protons into the extracellular space, leading to an acidic TME. This acidic environment contributes to drug resistance, disrupts the tumor cell cycle, and suppresses immune responses, presenting both challenges and opportunities for targeted therapeutic strategies [37].
Similar to hypoxia-based gene delivery strategies, achieving pH-responsive nanotheranostic agents involves incorporating pH-sensitive functional groups or acid-labile chemical bonds [37]. For example, magnetic silica nanoparticles assembled with multilayers of alginate/chitosan polyelectrolytes have been explored. These nanoparticles, containing carboxyl groups in alginate and amino groups in chitosan, exhibit high sensitivity to structural changes triggered by pH variations. This property enables the controlled release of shRNA in response to pH changes in cancer cells [229].
3.2.2. Organ-Specific Delivery
A highly valuable application of localized gene delivery systems is targeting specific organs to provide treatment exclusively for the affected area while minimizing impact on other parts of the body. The success of precise delivery for nanotheranostic agents largely depends on the chosen method of administration. Commonly used routes include inhalation, which facilitates efficient transport to the lungs; oral administration; and intranasal delivery, often employed to bypass the BBB and directly target the brain. Intravenous delivery is another widely used approach for both systemic and localized targeting [230].
An example highlighting the significance of the administration route is the reported behavior of glyconanoparticles, which accumulate in different sites depending on the method of delivery. Studies have shown that when administered via intravenous injection, these nanoparticles predominantly accumulate in the lungs. In contrast, when delivered intraperitoneally, they tend to accumulate in the spleen, liver, and brain [231].
However, selecting the appropriate administration route alone is insufficient. Similar to tumor targeting, various strategies have been developed to ensure precise delivery to specific organs, utilizing either passive or active targeting methods.
Passive Targeting
Passive targeting exploits the natural physiological characteristics of certain organs to enhance the accumulation of nanotheranostic agents. For instance, nanoparticles are naturally sequestered by the liver due to their fenestrated endothelium and resident macrophages (Kupffer cells) [232]. Likewise, intravenously administered nanotheranostic agents tend to accumulate in the lungs due to their extensive vascularization [233].
Nanoparticle size plays a pivotal role in determining their biodistribution. Particles smaller than 10 nm are rapidly filtered by the kidneys, whereas those exceeding 200 nm are more likely to be recognized by the RES, leading to their removal and accumulation in organs such as the liver and spleen [37,234]. Studies on muscle-targeted drug delivery demonstrated that PEG-grafted copolymers ranging from 11 to 32 nm improved permeability in Duchenne muscular dystrophy mice [234]. For targeting other organs, nanoparticles sized between 50 and 200 nm are generally optimal, as they enable prolonged circulation and targeted accumulation in specific tissues [234]. For instance, PLGA nanoparticles approximately 120 nm in size have shown enhanced delivery to the lungs and bone marrow [234].
The shape of nanoparticles significantly influences their efficiency in cellular internalization and circulation [235]. High-aspect-ratio nanoparticles, such as rod- or disk-shaped particles, are internalized more efficiently than those with a low aspect ratio. Shape also affects opsonization, influencing circulation time. Rod-shaped nanoparticles exhibit reduced phagocytosis and prolonged blood circulation compared to spherical ones, leading to lower liver accumulation due to their ability to evade Kupffer cells. Similarly, disk-shaped nanoparticles have extended circulation times and tend to accumulate in the lungs and heart rather than in the liver. In contrast, spherical, elliptical, and cylindrical nanoparticles show a higher tendency to concentrate in the liver, while irregularly shaped nanoparticles preferentially accumulate in the spleen [234].
Nanoparticle stiffness also affects clearance rates. Stiffer nanoparticles are more likely to be engulfed by macrophages, resulting in faster removal from circulation [236,237]. Conversely, nanoparticles with lower stiffness tend to remain in the bloodstream for longer periods. Additionally, surface charge plays a role in cellular uptake. Positively charged nanoparticles are more efficiently internalized by non-phagocytic cells due to their electrostatic attraction to negatively charged cell membranes [234].
Active Targeting
Similarly to tumor treatments, active targeting strategies have been developed for specific organs. For instance, liver targeting can be achieved using ligands such as galactose or mannose to bind to glycoprotein receptors, or folate, as a high expression of the folate receptor has been reported in hepatocytes [238]. Similarly, asialoglycoprotein receptors, low-density lipoprotein receptors, Ganglioside GM1 cell surface ligands, EGFR receptors, and others have been explored [239].
Several nanotheranostic agents with active liver targeting have also been studied for gene delivery. Studies have been conducted using SPIONs with ligands targeting the EGFR receptor to transport Human VEGF siRNA to the liver [240]. Similarly, SPIONs functionalized with PEG and PEI have been explored for the delivery of Survivin siRNA to the liver [241]. On the other hand, the behavior of mesoporous silica nanoparticles within liposomes has been investigated, using SP94 peptides as targeting molecules for liver-specific delivery of siRNA [242].
Analogous to the nervous tissue, particularly the brain, studies have focused on using ligands for active targeting in gene delivery. The primary goal is to enable crossing the BBB, where targeting techniques can be employed using ligands such as the GLUT1 receptor, which is overexpressed in the BBB, and others like Angiopep-2 (Ang), Apolipoprotein E (ApoE), CTX, and transferrin [243,244]. For example, gold liposomes functionalized with ApoE were used to target and enhance BBB crossing for delivering siRNA to treat glioma [244].
In addition to targeting the BBB, ligands have also been used for neuron-specific targeting, such as RGD, Transferrin, Folate, and hyaluronic acid (HA) [243,245]. For instance, QDs modified with RGD have been employed to transport siRNA, leveraging the RGD peptide’s ability to accumulate within glioma tissue [245].
Regarding the lungs, targeting receptors like LHRH, EGFR, CD44, CD71, integrins, and folate has been explored [246]. For example, MSNs were developed for the targeted delivery of siRNA using the LHRH ligand to target lung cells [247].
In cardiac tissue, significant attention has been given to the angiotensin II type 1 receptor (AT1R), which is highly expressed on the membranes of cardiomyocytes, particularly in ischemic tissue. Other targets include PCM-1 and vascular cell adhesion molecule-1 (VCAM-1) [248]. For example, Fe3O4 cores with a silica shell were modified with antibodies targeting CD63 antigens on myosin light chain surface markers in injured myocardium [248].
For kidney targeting, ligands such as Anti-Thy1, Anti-CR2-Fc, and glucosyl/mannosyl/2-deoxyglycosyl conjugates have been utilized [249].
Another well-known strategy is the use of biomimetic nanoparticles for gene delivery. This approach combines biological vectors with gene-carrying nanoparticles to camouflage the presence of nanoparticles and enhance their targeted delivery to specific sites that mimic the selected biological vectors. These vectors include cell membranes, extracellular vesicles (e.g., exosomes), and engineered particles inspired by viruses [250]. For instance, VCAM-1, a receptor in cardiac tissue that macrophages can target, was used in a study where nanoparticles were encapsulated within macrophage membranes to enhance targeting [248].
External Stimuli-Responsive Systems
It has also been demonstrated that the use of external stimuli can facilitate the redirection of nanotheranostic delivery agents. Of particular interest is the application of magnetofection, a technique that uses magnetic fields to guide magnetic nanotheranostic agents to specific target areas. Several studies have explored the use of magnetic stimuli for gene delivery to specific organs, enabling highly localized treatments. For instance, the use of magnetic nanoparticles to transport the CRISPR-Cas9 system across the BBB has been demonstrated when the nanoparticles were sized between 25 ± 5 nm [251].
Additionally, the influence of a magnetic field was applied for six hours to magnetite nanoparticles functionalized with chitosan for DNA delivery. Two magnetic field configurations were tested: one directed toward the heart and the other toward the kidney. Both configurations successfully guided the nanoparticles and genetic material to their respective targets [252].
3.3. Controlled Gene Expression
Controlled gene expression, as shown in Figure 7, is a key objective in nanotheranostic platforms for gene delivery. Achieving spatiotemporal regulation of gene expression can be facilitated by applying external stimuli to nanoparticles once they have reached the target site at the desired time, guided by diagnostic imaging provided by nanotheranostic agents.
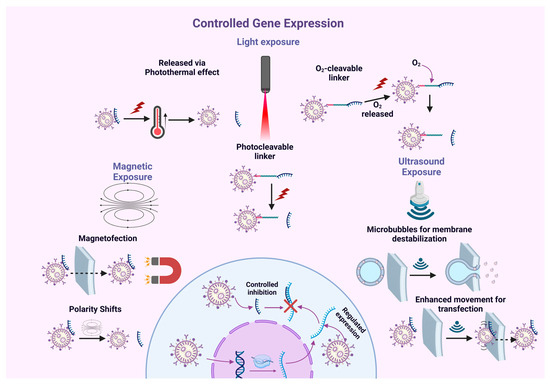
Figure 7.
Controlled gene expression. There are various techniques to control gene expression, including light, magnetic fields, and ultrasound. Light is the most extensively studied exogenous factor for controlled gene expression following delivery via nanotheranostic agents. Many of these agents are designed to respond to low-wavelength light, such as UV light, which carries high energy. Magnetic fields not only help direct nanoparticles to specific tissues but also serve as a physical stimulus for controlling gene expression through nanotheranostic agents. Ultrasound has emerged as a highly effective method for achieving spatiotemporal control over oligonucleotide release. In particular, air-filled microbubbles have drawn considerable attention due to their ability to encapsulate molecules within their shell and be destroyed by ultrasound-induced acoustic cavitation. This process destabilizes the membrane, alters its permeability, or even causes the microbubbles to collapse, releasing their contents.
Various external stimuli have been explored in the literature to enable precise and controlled gene expression through these advanced platforms, including light-, ultrasound-, and magnetic-responsive nanotheranostic agents. These strategies typically rely either on the intrinsic properties of the nanotheranostic agents or on engineered modifications. For instance, some nanoparticles undergo structural changes in response to external stimuli, such as gold nanoparticles, which exhibit localized surface plasmon resonance when exposed to NIR light. This photothermal effect facilitates gene delivery [253]. Similarly, IONPs respond to magnetic fields, enabling magnetofection, a technique that allows for the controlled intracellular delivery of genetic material [179].
Alternatively, engineered modifications can be designed to make nanoparticles interact with external stimuli through specific chemical bonds. For example, light-cleavable molecules release genetic material upon exposure to light, providing a highly controlled mechanism for gene delivery [203].
The choice of strategy depends on the type of external stimulus applied, as detailed in the following sections.
3.3.1. Light-Responsive Gene Expression
Light is the most extensively studied exogenous factor for controlled gene expression following delivery via nanotheranostic agents. Many of these agents are designed to respond to light at low wavelengths, such as UV light, which carries high energy. However, UV light is heavily absorbed by tissues and has been reported to cause damage. Consequently, light with higher wavelengths, such as NIR light, is more commonly used to induce gene responses. NIR light is highly effective at penetrating deep into biological tissues, facilitating easier access to target tissues of interest [203].
Among the nanotheranostic agents tested, gold stands out due to its remarkable ability to interact across a broad NIR spectrum and convert light energy into heat for controlled gene release. One study explored a gold-based structure incorporating a Prussian blue analogue, a photothermal material, as the nucleation point, surrounded by gold in a floral configuration to increase surface area. This nanostructure was coated with liposomes and demonstrated high siRNA release into the cytoplasm upon NIR exposure, which increased liposome permeability through the photothermal effect, enabling controllable RNA cleavage [254].
To facilitate nucleic acid release in response to NIR irradiation, nanotheranostic agents typically functionalize with light-cleavable molecules, such as nitrobenzyl or azobenzene. Upon NIR exposure, these molecules undergo structural changes, triggering the release of the genomic material [203].
For example, azobenzene has been studied for siRNA release in a spherical nanostructure, where it is bound to the nucleation point of UCNPs via azobenzene. Upon NIR excitation, these nanoparticles emit UV spectra, facilitating the trans-to-cis photoisomerization of azobenzene and enabling siRNA release. This mechanism allows for spatiotemporal control of gene silencing [255].
Similarly, 2-nitrobenzyl has been tested in a system where silanized UCNPs were externally functionalized with 2-nitrobenzyl, forming a spherical structure. SiRNA particles interacted with the structure via electrostatic interactions with 2-nitrobenzyl. Upon NIR irradiation and subsequent upconversion to UV, controlled siRNA release into the extracellular space was achieved, enabling precise control of gene expression [256]. UCNPs conjugated to Cas9 via a covalent 2-nitrobenzyl linker have also been used to enable spatiotemporal control of gene editing through NIR irradiation, resulting in a reduction in tumor size in mice [257].
Another structure explored involves a PS as the nucleation point, surrounded by siRNA and antisense oligonucleotides (ASOs) for mRNA cleavage [258], within a protective spherical structure that prevents nucleotide degradation. This system operates via an O2-cleavable linker connecting the siRNA and ASOs, which are part of a protective structure. Upon NIR excitation of the PS, a slight increase in O2 concentration within the endosome occurs, interacting with the O2-cleavable linker and triggering the release of the oligonucleotides and their subsequent cleavage of mRNA [259].
3.3.2. Magnetic-Responsive Gene Expression
Magnetic fields not only have the capacity to direct nanoparticles to specific tissues, but they have also been identified as a physical stimulus for controlling gene expression through nanotheranostic agents. It has been shown that magnetic fields can disrupt the binding between oligonucleotides and magnetic nanoparticles, inducing changes in surface polarization that ultimately lead to controllable gene expression [251]. This effect was observed with the ferromagnetic nanomaterial BaTiO3@CoFe2O4, which delivered CRISPR-Cas9/gRNA across the BBB and released the CRISPR-Cas9/gRNA in a controlled manner upon exposure to a magnetic field [260].
Magnetofection is a well-established technique for achieving spatiotemporal control of gene expression. This method involves applying a magnetic field to guide nanoparticles carrying oligonucleotides toward target cells along the field’s direction. It has been successfully implemented both In vitro and In vivo, serving as an external stimulus to enable the precise delivery of nucleotides into the cytoplasm. Once inside, the nucleotides can undergo expression or perform their intended function, providing a controlled and efficient approach to gene delivery.
3.3.3. Ultrasound-Responsive Gene Expression
Ultrasound has emerged as an effective technique [261] for achieving spatiotemporal control over the release of oligonucleotides. Specifically, air-containing microbubbles have gained significant interest due to their ability to encapsulate molecules within their shell and be destroyed by ultrasound-induced acoustic cavitation. This process causes membrane destabilization, altering its permeability or even leading to the collapse of the microbubbles, thereby releasing their contents. These microbubbles have been extensively studied as agents with high acoustic impedance for diagnostic purposes, especially in ultrasound imaging.
Mesoporous silica nanoparticles loaded with pDNA inside microbubbles have been explored. The mechanism involves the magnetic guidance of the nanostructures, taking advantage of the magnetic properties of silica. This allows for the use of magnetofection to direct the nanoparticles to the target cells. Once proper targeting is achieved, the microbubbles release their contents upon ultrasound exposure, ensuring accurate spatiotemporal targeting [178].
Another fascinating approach involving ultrasound stimulation is the use of nanomotors—nanoparticles designed to harness ultrasound waves as an energy source for controlled movement. When these nanomotors come into contact with the cellular membrane and are activated by ultrasound, their rapid motion allows for efficient and non-destructive membrane penetration. This process offers a precise method for intracellular delivery, making them ideal carriers for the release of therapeutic payloads, such as oligonucleotides, directly into the intracellular environment [251].
A notable example of this approach involves the delivery of CRISPR/Cas9 systems. In this study, Cas9/sgRNA complexes were attached to the surface of gold nanowires (AuNWs) via chemical bonds. The gold nanowires exhibited active movement in response to ultrasound stimulation, allowing for their controlled internalization into the cytoplasm. This innovative use of ultrasound-driven nanomotors presents a promising, targeted strategy for gene editing and intracellular delivery [262].
Despite promising preclinical data, external-stimuli gene expression control remains largely experimental. Further advances in imaging guidance, device miniaturization, and safe energy delivery are needed to ensure both efficacy and patient comfort in clinical settings.
4. Emerging Applications in Gene Therapy
As expressed earlier, traditional approaches to modifying genetic material are classified based on where the modification takes place [263]. Ex vivo gene therapy involves removing cells from the body, modifying them externally, and then reintroducing them into the individual. In vivo gene therapy aims to make systemic changes, while in situ gene therapy targets specific tissues or cells for editing.
These advancements have significantly influenced four main research areas in recent years, as shown in Figure 8. First, improvements in the diagnosis of rare diseases through genome sequencing [264] have enabled the identification of variants associated with diseases or patterns that could guide the development of therapeutic strategies. Second, the combined use of biomolecular properties for both diagnosis and treatment—often through imaging technologies, referred to as theranostics [265] has opened new avenues for managing various conditions. Third, novel treatments have emerged for previously incurable diseases, such as diabetes [266], cancer [267], and sickle cell disease, as shown in Figure 9 [268].
Figure 8.
Number of gene therapies approved by the FDA from 2011 to 2024. Regarding the European Medicines Agency, these numbers include tissue-engineered medicines, which use modified cells or tissues to regenerate or replace human tissue; somatic-cell therapy medicines, which involve cells or tissues whose biological characteristics have been altered and are not intended for the same function in the body; and gene therapy medicines, which insert recombinant genes into the body.
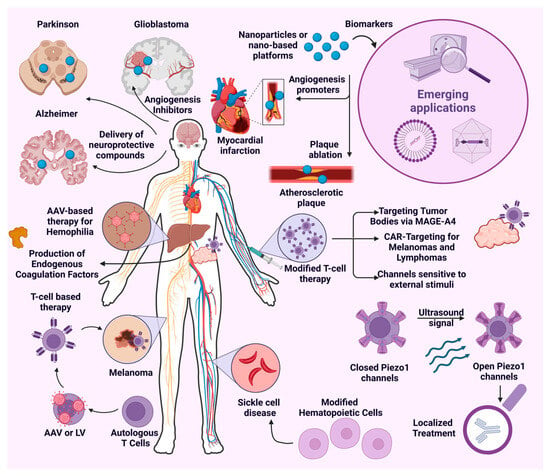
Figure 9.
Emerging applications: cancer, neurological disorders, and cardiovascular diseases. Cancer theranostics combine imaging, tumor targeting, and therapy. Photosensitizers with both fluorescence emission and phototoxicity are emerging as key agents. In neuro-oncology, theranostics have addressed brain tumors like gliomas and meningiomas. For neurodegenerative disorders, quantum dots are gaining attention for tracking amyloid-β peptide formation in Alzheimer’s disease, enabling in vivo fluorescence imaging. In cardiovascular diseases, theranostics are being explored for conditions such as atherosclerotic plaques, myocardial infarctions, aneurysms, and ischemic heart disease.
Notably, the FDA has approved several cellular and gene therapies in recent years. Despite these advancements, a significant portion of the scientific community acknowledges that gene therapies still face major challenges before becoming viable alternatives to conventional approaches [269].
Historically, the first challenge has been identifying disease-causing mutations. Even with current technologies capable of sequencing entire genomes at an approximate cost of USD 1000 [270], this remains a critical hurdle. Furthermore, the cost and effort required for developing a single therapy are immense. For instance, RETHYMIC—the first FDA-approved allogeneic processed thymus tissue therapy—underwent clinical trials spanning nearly three decades (1993–2020) [271]. Infections following thymus placement were a leading cause of mortality in these trials, underscoring significant safety concerns.
Safety remains a major issue, as studies have revealed side effects such as triggered immune responses leading to secondary conditions [272], renal toxicity, thrombocytopenia, and other adverse effects observed in both human and animal models [273]. These findings suggest that, despite considerable progress, much remains to be understood about the pathophysiology and organismal reactions to various therapeutic approaches [274].
4.1. Cancer in Gene Therapy
Conventional cancer gene therapy, while significant, often lacks real-time tracking capabilities. Nanotheranostics, however, enables simultaneous imaging of nanoparticle localization and targeted gene silencing within tumor tissues, significantly enhancing therapeutic precision.
Cancer is considered one of the major global health problems, accounting for at least one-sixth of total deaths worldwide. Conventional treatments, such as chemotherapy, radiotherapy, and surgery, aim to remove or prevent uncontrolled cell growth characteristic of this disease [275,276]. Despite these traditional approaches, significant efforts have been made to develop alternatives.
Over the past four years, the FDA has approved three innovative therapies based on autologous modified T cells for treating melanoma. These include idecabtagene vicleucel [277], ciltacabtagene autoleucel [278], and lifileucel [279]. These therapies utilize a viral vector to modify T cells, enabling them to express anti-CD19 CARs that specifically target antigens found exclusively in tumors. This selectivity minimizes toxicity [277]. This approach, commonly known as CAR T-cell therapy, represents a significant milestone in cancer treatment. In fact, this approach allows for monitoring treatment progress with commonly used techniques such as Computed Tomography or Positron Emission Tomography [280].
Despite the success of CAR T-cell therapy, other strategies are being explored, including modifying different immune cells, such as natural killer (NK) cells. NK cells are particularly advantageous as they can be sourced from healthy donors, eliminating the need for autologous cells [281]. For instance, lifileucel has been proposed as a potential treatment for metastatic non–small cell lung cancer, achieving an objective response rate of 21.4% in tumors with chemical profiles typically resistant to immunotherapy, such as PD-L1–negative and STK11-mutated tumors [282].
Similar attempts have been made with large B-cell non-Hodgkin lymphomas. Initially, CAR T therapy showed promising results in clinical trials for treating lymphomas [283], leading to the rapid approval of therapies. Axicabtagene ciloleucel [284] achieved an objective response rate of 82% and an overall survival rate at 18 months of 52%. Comparable outcomes were observed with lisocabtagene maraleucel [285] and tisagenlecleucel [286]. However, poor outcomes have also been reported, with some patients showing an absence of response, yielding a 12-month overall survival rate of 35% [283]. Additionally, the treatment has been associated with the development of neutropenia, anemia, and thrombocytopenia [284,285], highlighting the need for further studies to understand mechanisms of resistance to CAR T cell therapy [287]. Consequently, this treatment is generally recommended as a second-line therapy [288] due to the associated risks.
Due to these side effects, the scope of newly developed therapies can be limited to unresectable cases. For instance, afovirsen autoleucel is recommended for metastatic synovial sarcoma in patients previously treated with chemotherapy [289]. In this case, T cells are engineered to recognize melanoma-associated antigen 4 (MAGE-A4), which is expressed in many tumors. It is anticipated that such approaches could be extended to various solid tumors, including ovarian and head and neck cancers [290]. However, clinical trials have shown a high incidence of hematologic toxicities and cytokine release syndrome in treated patients [291,292]. In fact, it has been recommended that treatment coupled with imaging, beyond monitoring treatment progress, can track side effects and allow for handling them effectively [293].
Immunotherapies have gained popularity and demonstrated relative success, but alternative approaches have been explored in the literature. Tumor suppressor gene activation targets well-known genes such as Rb, p53, CDK inhibitors, and BRCA-1/2, which prevent uncontrolled cell proliferation by triggering apoptosis or interrupting the cell cycle [294]. Among these, p53 is the most extensively studied due to its role in DNA damage detection and cell apoptosis; its mutation is the most frequently observed in TP53 across human cancers [295]. Gendicine, approved by the Chinese State Food and Drug Administration in 2003, was the first gene therapy for cancer [276]. Combining Gendicine adenovirus with chemotherapy has been suggested to significantly enhance treatment effectiveness, although this hypothesis and administration strategies for different cancer types remain under debate and investigation [296].
Another approach involves inhibiting oncogene activation, targeting genes that are typically downregulated but become activated under oncogenic conditions. Recent studies indicate that using iRNA to target such genes can significantly slow cancer progression [297,298]. Similarly, reducing natural antisense transcripts has been shown to decrease oncogene expression [299]. While the underlying mechanisms remain unclear, this area requires extensive research to understand and utilize it therapeutically. Additionally, angiogenesis is critical for cancer tissue proliferation as it provides the necessary nutrients for survival. Antiangiogenic therapies have been developed to inhibit angiogenic inducers like angiopoietin or employ inhibitors such as endostatin [267]. Researchers continue to search for new anticancer agents with improved activity and selectivity for testing in cancer cell lines [300,301].
Finally, suicide gene therapy delivers pharmacological treatments directly to cancer cells by administering a toxic chemotherapeutic agent as a prodrug, along with genes encoding enzymes needed for its activation in tumoral cells [267]. The primary challenge for this approach is identifying suitable gene carriers. For instance, a luminal breast cancer organoid model was proposed using graphene oxide quantum dots as carriers, coupled with an aptamer targeting MUC1, a gene overexpressed in breast cancer. This strategy selectively targets cancer cells [302]. Similarly, GSH-responsive morphology-transformable enantiomeric peptide assemblies have been proposed as carriers for both a suicide gene and the prodrug paclitaxel in ovarian cancer models. These assemblies also incorporate a transcription factor with a Renilla luciferase signal to monitor tumor growth and peptide targeting [303,304]. Imaging techniques developed for such applications have facilitated theragnostics, combining therapy and diagnosis to track anticancer drug effectiveness and enable early-stage cancer diagnosis and treatment [305,306].
Cancer Theranostics
In fact, cancer theranostics typically involve imaging, tumor targeting, and therapy. PSs capable of both fluorescence emission and phototoxicity have emerged as valuable theranostic agents [307]. These agents can act through photothermal therapy (PTT) or PDT, utilizing heat and ROS, respectively, to eliminate cancer cells. PTT is particularly promising due to its high specificity, minimal invasiveness, and low side effects. It employs near-infrared light to elevate tumoral temperature, leading to cancer cell death [307]. Beyond hyperthermia, PTT has been shown to accelerate glutathione consumption, thereby enhancing its anticancer effects [308].
While early PDT faced challenges related to light penetration and tumor hypoxia, these obstacles have been addressed through two-stage photodynamic therapy, which separates photosensitization from singlet oxygen delivery [309]. Current PDT approaches employ nanotheranostic carriers tailored for efficient singlet oxygen production upon light irradiation [310]. Moreover, combining these therapies with immunotherapies has shown promising outcomes in treating cancers such as esophageal cancer [311].
Traditional imaging technologies like magnetic resonance imaging (MRI), computed tomography (CT) scans, ultrasound, and X-rays lack the precision to detect specific cancer cell surface markers [312]. This limitation underscores the urgent need for advanced imaging techniques to significantly impact cancer diagnosis and treatment [306]. Nonetheless, efforts have been made to adapt existing technologies. Ultrasound-sensitive biomaterials, such as microbubbles, have been explored for cancer theranostics. These gas-filled structures, stabilized by proteins, polymers, or lipids, are non-toxic and non-immunogenic. Traditionally used as contrast agents, microbubbles are now being investigated for theranostic applications via cavitation effects [107].
The integration of genetic engineering with ultrasound technology, termed “sonogenetics”, is another innovative approach. For instance, controllable CAR-T cells have been engineered to respond to ultrasound stimulation through Piezo1 channels expressed on their surface. Upon ultrasound exposure, these channels open, allowing calcium ion influx and CAR-T cell activation, thereby enhancing selectivity. Furthermore, cationic microbubbles have been introduced that respond to ultrasound stimuli improving gene transfection in focalized areas, delivering AKT2 protein for tumor treatment and further allowing monitoring of tumoral size [313].
Nanoscale metal–organic frameworks (nMOFs) have also garnered attention in oncology. These frameworks can be activated by X-rays to increase local radiation doses and promote ROS generation, ultimately enhancing radiotherapeutic efficacy. Studies using iron ion-based nMOFs have demonstrated persistent oxidative stress in both In vivo and In vitro models [313,314].
MRI remains a cornerstone in tracking drug carriers In vivo and obtaining detailed structural and functional information about tumor regions [315]. In theranostics, MRI-guided therapies target cancer cells overexpressing specific receptors [316]. For example, mesoporous silica nanoparticles coupled with redox-responsive doxorubicin (DOX) prodrugs have been developed to enhance active targeting and endocytosis via magnetic targeting [317]. These delivery systems can be engineered to respond to various stimuli, including pH, enzymes, ultrasound, and magnetic fields, either individually or in combination [318].
4.2. Neurological Disorders
Treating nervous system disorders remains a significant challenge due to the intricate nature of the central nervous system (CNS), the BBB, and the inherently slow regenerative capacity of nerve tissue. The BBB is a natural barrier that severely restricts the passage of biomolecules, complicating therapeutic approaches targeting the brain [319]. Studies suggest that the cavitation effect may facilitate BBB penetration [320]. However, large, lipophilic molecules often require invasive methods, such as intracerebroventricular injections or osmotic mini pumps, to reach therapeutic targets. This makes the application of gene therapy particularly challenging in this context.
Among the commonly used viral vectors in the CNS are adeno-associated viruses (AAVs) [319], herpes simplex virus-1 [321], and lentiviruses [322]. However, concerns regarding genome integration, antigenicity, and tumorigenicity have limited their therapeutic potential. Despite these concerns, adenoviruses have demonstrated high effectiveness in neurological contexts. Notably, in 2024, the FDA approved onasemnogene abeparvovec-xioi, an AAV-based therapy for spinal muscular atrophy (SMA) targeting the SMN1 gene [323]. This therapy utilizes intravenous infusion to promote functional SMN protein in motor neurons. To date, over 20 clinical trials have explored AAV-based treatments for neurological disorders [324].
Alzheimer’s disease (AD), the most prevalent progressive neurological disorder worldwide, is characterized by the accumulation of amyloid plaques in the brain [325]. Nerve growth factor (NGF), known for its neuroprotective effects, has been explored in rodent AD models. Neural stem cells modified to express NGF restored cognitive function in these models [326]. Another approach utilized antisense overexpression to knock down miRNA-937 in mesenchymal stem cells, which, when introduced into the hypothalamus, improved cognitive outcomes [327].
Parkinson’s disease (PD) is another major neurodegenerative disorder, marked by the progressive loss of dopaminergic neurons in the substantia nigra [328]. Therapeutic strategies include symptomatic and neurorestorative approaches. The latter often involves neurotrophic factors, such as neurturin or GDNF, delivered through In vitro [328] or In vivo methods [329]. Transplantation of fetal neural stem cells treated ex vivo with GDNF has shown significant dopaminergic neuron protection in MPTP-administered mice [330].
Similar progress has been made in other disorders. For amyotrophic lateral sclerosis, neuroprotective genes like GDNF have shown promise [331]. In epilepsy, restoring neuropeptide balance through CG01, NPY, and Y2 genes has demonstrated therapeutic potential [332]. Huntington’s disease models have benefited from neural stem cells expressing GDNF or BDNF [333]. Clinical trials include sustained transgene expression for dopamine production in PD [334,335]. However, while ex vivo gene therapy shows promise, further research is required to better understand its effects on astrocytes and microglia [336].
Neurological Disorders Theranostics
Similarly to cancer developments, in recent years, nanoparticles have been proposed for theranostic applications, such as magnetic particles, gold nanoparticles, dendrimers, and carbon nanomaterials [320]. However, their use can be limited, as the liver clears them rapidly from the body’s circulation. This means modifications are a must in order to achieve the desired surface properties, extend their half-life and circulation time, and facilitate their ability to cross the BBB to reach desired cellular populations [337]. Thus, efforts are being made towards creating nanoparticles of appropriate size, responsive to stimuli, stable in physiological conditions, and biocompatible, which are particularly relevant in current neurological theranostics proposals [338].
Current trends are primarily focused on neuro-oncological disorders, cerebrovascular disorders, and neurodegenerative disorders. In the field of neuro-oncological disorders, a wide range of brain tumors have been addressed, including gliomas [339], meningiomas [340], and others [320]. AuNPs functionalized with monoclonal antibodies targeting the EGFR have had promising results in recognizing and binding to EGFR in glioma cells, helping to provide accurate diagnosis and serving as a vehicle to deliver pharmacological agents [341]. Furthermore, approaches targeting tumoral angiogenesis to inhibit neovascularization using monoclonal antibodies have been proposed [342,343], but some authors consider inadequate medication transport to the brain to limit further development, suggesting that novel medicines still need to be found [343]. Other popular approaches are mesoporous silica nanoparticles, whose structure is suitable to carry therapeutic and imaging agents [344], gadolinium-based nanoparticles that have been functionalized to increase MRI contrast [345], and PEGylated liposomes, which are known for efficiently crossing the BBB [346].
Cerebrovascular disorders refer to the different conditions that affect cerebral circulation. Some approaches have proposed targeting cerebral amyloid angiopathy, where the deposition of proteins within the cerebral vasculature walls leads to recurrent vascular inflammation and hemorrhagic strokes. Using polymeric nanocores, Magnevist (an MRI contrast agent), anti-inflammatory, and anti-amyloid antigens were loaded, resulting in promising results [347]. However, concerns regarding systemic side effects have been suggested [320]. Furthermore, the use of the aggregation-induced emission phenomenon in the theranostic field has exhibited interesting results, as it has allowed for the identification of lipid-rich tissues relevant for some diseases like atherosclerosis [348] and, when coupled with gadolinium, it offers a promising imaging technique for obtaining detailed information about the cerebral vasculature [349]. Finally, another popular approach is the use of exosomes, as they can easily penetrate the BBB. They have been used to carry therapeutic RNA [350] and to stimulate angiogenesis by using mesenchymal cell-derived exosomes [351,352]. Furthermore, recent approaches have used magnetic resonance imaging for real-time monitoring using MRI of gene therapy perfusion to accurately define the distribution of infused vectors, in particular using viral vectors carrying therapeutic genes [353]. This approach is, in fact, being used by clinical trials (ID: NCT02852213).
Finally, in the field of neurodegenerative disorders, quantum dots are gaining relevance in AD studies, as they have been proposed as an effective way to track and observe amyloid-β peptide formation in animal models using fluorescence, which allows for In vivo imaging [354]. Moreover, ceria nanoparticles change their oxidation state in the presence of ROS, which has led to the hypothesis that selectively targeting them to mitochondria could have promising results for oxidative stress-related disorders like AD and PD [355]. In fact, nanoparticle approaches to deliver neuroprotective compounds have improved dopamine levels in PD models [356] and modulated harmful proteins like α-synuclein [357]. In epilepsy, pH-sensitive nanoparticles have proven to reduce the severity of attacks in animal models [358].
Despite significant progress, challenges remain in integrating advanced imaging modalities with the development of highly specific and sensitive theranostic agents. Multifunctional nanoparticles designed to target specific cellular populations and respond to pathological stimuli hold promise for overcoming these hurdles. Addressing systemic clearance, side effects, and BBB penetration will be essential for realizing the potential of nanoparticles in neurological theranostics.
4.3. Cardiovascular Applications
Gene therapy for cardiac diseases has been considered for several conditions. For instance, RNA interference (iRNA) has been proposed for the treatment of hyperlipidemia [359] and cardiac amyloidosis by reducing levels of damaging proteins [360]. However, despite initial enthusiasm, many cardiovascular applications face significant challenges before being ready for clinical use. Among the most prevalent issues is the insufficient understanding of pathophysiological mechanisms inherent to many of these conditions, which leads to poor strategy selection and design [359,360].
Nevertheless, the first FDA-approved gene therapy, Glybera, indicated for the treatment of severe lipoprotein lipase deficiency, demonstrated success [361]. This AAV-based therapy adds episomal copies of the LPL gene, improving or even restoring metabolic function in treated muscles [362]. A similar strategy was used with delandistrogene moxeparvovec-rokl for the treatment of Duchenne muscular dystrophy [363]. However, despite FDA approval, recent research has pointed out that this therapy may have higher immunogenic potential [363].
Gene therapy research for cardiovascular diseases has primarily focused on four main conditions, using mostly AAV-based therapies for gene replacement. Arrhythmogenic cardiomyopathy, caused by a defect in the proteins that connect myocytes, results in cell death [364]. Three clinical trials have FDA approval to move into phase 1 trials using AAV-based therapy to modify the PKP2 gene [365]. Similar approaches are being developed for Duchenne muscular dystrophy, aiming to replace the DMD gene. Interestingly, some researchers have proposed myostatin inhibitors to increase muscle mass, though it is still debated whether this approach can truly improve muscle function [366]. Gene replacement using AAV-based therapies targeting TTR, FXN, GAA, GLA, and LAMP2 genes is being researched for Transthyretin amyloidosis, Friedreich’s ataxia, Pompe disease, Fabry disease, and Danon disease, respectively [367].
Despite the predominance of AAV-based approaches, some researchers believe nanoparticles and nanotechnology hold promise for cardiovascular disease diagnosis and imaging, particularly in three key areas of advancement [368]. In targeted drug delivery, nanoparticles incorporated into scaffold materials have been proposed to release growth factors or other molecules that promote neovascularization and tissue regeneration [369]. Other nano-delivery systems studied include liposomes, dendrimers, and micelles [35], which are closely related to molecular imaging and potential cardiovascular theranostics. For instance, in atherosclerosis, nano-based platforms have been proposed, similar to those used in cancer, as well as amine-functionalized nanoparticles labeled with fluorescent agents to promote macrophage ablation of atherosclerotic plaques [35]. Similar approaches have been suggested for aneurysms, where nano-based platforms could encapsulate drugs to manage aneurysms by targeting matrix regulation, potentially delaying the growth and progression of this condition [370].
In tissue engineering, the development of nanofibers crafted from biocompatible materials has emulated natural heart tissue architecture [371], which could transform the way heart diseases are studied and treated. Furthermore, the FDA recently approved a novel acellular tissue-engineered vessel designed for treating arterial injury when urgent revascularization is needed. This is the first FDA-approved acellular engineered tissue [372].
Cardiovascular Theranostics
As mentioned earlier, theranostics in cardiovascular diseases have been proposed for treating a variety of conditions, including blood vessel blockages such as atherosclerotic plaques, myocardial infarctions, aneurysms, ischemic heart disease, and, to a lesser extent, valvular heart disease [35,373]. In fact, some imaging techniques have been proposed as a potential solution to the transgene expression monitoring issue, as they can allow tracking of therapy consequences, like using myocardial perfusion imaging by positron emission tomography in an AVV-based therapy that targets angiogenetic genes [374]. An aneurysm, for instance, refers to the enlargement of an artery due to the progressive weakening of the arterial wall, and it can be classified as aortic, intracranial, peripheral, or thoracic aortic, depending on its location. Note that access challenges to different body areas vary greatly, making this a crucial design parameter [375]. Thus, nano-based platforms have been proposed for early diagnosis and pharmaceutical delivery to slow aneurysm progression towards a specific location. For example, in the treatment of abdominal aortic aneurysms caused by matrix metalloproteinase enzymes, doxycycline is used. However, a side effect of this pharmaceutical agent on elastin can further weaken the aortic wall. Therefore, a nano-based platform was designed to bind elastin by encapsulating superparamagnetic iron oxide nanoparticles and gradually releasing doxycycline, reducing side effects [370]. Nevertheless, recent reports on Chemokine Receptor 2 using radiotracers and positron emission tomography/computed tomography allowed for tracking the formation and progression of abdominal aortic aneurysms [376], meaning much can be done for further integrating treatments and diagnosis for cardiovascular applications.
Another relevant disease addressed in the literature from a theranostics point of view is myocardial infarction, which is caused by the obstruction of coronary arteries, resulting in ischemia and the loss of cardiomyocytes, vascular cells, and interstitial cells [377]. Traditional bioimaging techniques use nanomaterials with desired physicochemical and optical properties that make them suitable as contrast agents. However, they can be further engineered to extend their functionality, such as albumin nanocomposites in conjugation with manganese dioxide. This novel nanocomposite has been shown to increase relaxation in myocardial infarction. Macrophages then take up the nanocomposites, releasing Mn ions that work as biomarkers for bioimaging [378]. Another approach involves triggering glutathione levels by using carbon dots, as myocardial ischemia produces hypoxia, which increases ROS production and consequently reduces antioxidant levels in the affected tissue. These biosensors can ultimately be used for the early detection of myocardial infarction [379]. Similarly, gold nanoparticles have shown the ability to maintain cardiomyocyte architecture during myocardial infarction, having a cardioprotective effect by reducing isoproterenol-induced myocardial injury, in addition to being a potential contrast agent for bioimaging modalities [380].
Angiogenesis and regeneration have also been extensively studied for a wide range of purposes [267,351,352]. In the context of cardiovascular diseases, the main goal is to restore blood supply to ischemic tissues. This has been achieved using stem cell-based gene therapy [381] and by directly intervening with angiogenic proteins [35]. However, direct intervention has advantages such as tunability and localized delivery, even with systemic administration. Hence, different approaches have been tried, such as blocking SPRED1, which is known to inhibit angiogenic factors, while loading microRNA-126 to stimulate it [382], or inducing angiogenesis by modulating the physiological level of ROS and activating endothelial NO synthesis with graphene oxide and reduced graphene oxide nanoparticles [383].
Despite these advances, the main challenge these approaches face is that they only delay the progression of pathophysiological conditions while potentially causing systemic side effects. This leads to another major problem: the usage of these platforms is limited to laboratory setups or the preliminary stages of clinical settings, meaning further research must be done to successfully apply these technologies to patients.
4.4. Other Genetic Therapy Approaches
Finally, other genetic therapies for rare diseases have also been studied extensively. In addition to the diseases discussed earlier, significant advances have been made in studying hemophilia A and B. Hemophilia is the most prevalent coagulation disorder, with the A and B subtypes caused by a deficiency in clotting factors VIII and IX, respectively [384]. In fact, valoctocogene roxaparvovec-rvox is an AAV-based therapy driven by a liver-selective promoter, indicated for severe cases of hemophilia A, and provides endogenous factor VIII production [385]. Similarly, etranacogene dezaparvovec-drlb [386] and fidanacogene elaparvovec-dzkt [387] have proven superior to other treatments, such as prophylaxis. However, after 15 months, the mean factor IX activity was reduced by 26.9%, indicating that further improvements are required.
Type 1 diabetes has also been extensively studied. In fact, in 2023, donislecel was approved as a potential treatment for this disease, involving the infusion of allogeneic pancreatic cells through a hepatic portal vein [388]. This therapy achieved endogenous production of insulin by generating islet cells, becoming the first cell therapy approved for type 1 diabetes. Furthermore, other approaches have been proposed, such as “smart” insulin, an insulin analog intended to mimic the body’s natural insulin secretion [389]. This concept was initially proposed assuming that the analog would behave as a controller [390], and it is currently available on the market. Other approaches involve stem cell therapy for the generation of insulin-producing cells [391,392]. However, these approaches, which could potentially treat both type 1 and type 2 diabetes, are still under development.
Some successful approaches that did not use AAV-based vectors include beremagene geperpavec and betibeglogene autotemcel. In fact, beremagene geperpavec was designed for use in wounds in patients with a collagen type VII mutation (Dystrophic Epidermolysis Bullosa), a condition where healing is an arduous process [393]. In this case, an HSV-1 vector is applied dermally to the wound and contains copies of the human COL7A1 gene, which restores COL7 protein functionality [394]. On the other hand, betibeglogene autotemcel was designed for patients with β-Thalassemia, a rare condition that reduces the body’s production of hemoglobin, containing autologous CD34+ hematopoietic stem cells [395].
Gene therapy has reached many diseases, focusing on different sections with fresh perspectives and hopes, aiming to propose solutions for previously unsolvable conditions. Recently, an intraocular suspension for subretinal injection was approved for patients with retinal dystrophy associated with a biallelic RPE65 mutation [396,397]. This AAV-based therapy, called voretigene neparvovec-rzyl, is a clear example of the versatility and progress of gene therapy today.
5. Conclusions, Challenges, and Future Directions
The clinical translation of nanomedicines faces regulatory challenges due to the lack of global standards for their characterization, complicating the assessment of their safety, efficacy, and reproducibility. Large-scale production requires maintaining uniformity in critical parameters such as size, charge, and stability, which are difficult to standardize. Additionally, the absence of a unified regulatory framework delays approval and adoption. The safety of nanomaterials remains a concern, as their small size allows them to interact with cells and organs, inducing oxidative stress, organelle damage, and immune responses. Uncertainties also persist regarding their long-term toxicity and environmental impact. Strategies such as encapsulation [398,399], real-time monitoring, and green nanotechnology aim to mitigate these risks.
5.1. Clinical Translation Hurdles
Currently, approximately 100 nanomedicines have been approved by regulatory agencies worldwide, with additional candidates undergoing advanced preclinical and clinical evaluations [400]. The clinical translation of nanocarriers faces a complex and demanding regulatory landscape. Nanocarriers, due to their unique properties, must meet rigorous safety and efficacy requirements before gaining approval. Precise characterization of parameters such as particle size, surface charge, shape, and stability are critical, as these influence their biological interactions, including biodistribution, clearance, and therapeutic performance. However, the lack of universally accepted standards for nanoparticle characterization introduces variability in data between studies, complicating regulatory assessments. This inconsistency not only delays approval processes but also hinders the reproducibility of results across laboratories and institutions [400].
The regulation of nanomedicines remains an evolving challenge due to the lack of established global standards and regulatory frameworks. Despite the approval of numerous nanotechnology-based medical products, there is no universal consensus on their classification and safety evaluation, which complicates the regulatory process [401]. Nanomedicines present distinct pharmacodynamic (PD) and pharmacokinetic (PK) profiles compared to conventional drug molecules, necessitating tailored regulatory approaches. Additionally, their physicochemical properties influence their biodistribution, interactions with biological membranes, and overall therapeutic efficacy, which must be carefully assessed by regulators [402]. The absence of a unified definition and classification for nanomedicines further contributes to regulatory confusion, as different countries apply varying criteria for approval [399]. As a result, the regulatory pathway for these products is inconsistent, delaying market access and the widespread adoption of nanomedicine-based therapies. Current In vitro and pre-clinical toxicological studies often fall short in replicating the complexities of In vivo environments, making it difficult to accurately predict the behavior and safety of nanomedicines [403]. Challenges also arise in assessing their systemic biodistribution and long-term fate within the body, as nanomaterials may behave differently in living organisms compared to isolated conditions. Additionally, the potential environmental impact of nanomedicines, including their persistence and toxicity in ecosystems, remains an underexplored concern that adds another layer of complexity to their regulation and approval [404].
The transition from laboratory-scale production to large-scale manufacturing is one of the most challenging aspects of nanomedicine development. Maintaining consistency in properties such as particle size, shape, charge, composition and encapsulation efficiency across production batches is critical for ensuring their safety and therapeutic efficacy. Variability in these parameters can compromise the reliability of the therapy and increase the difficulty of meeting regulatory requirements as well as Good Manufacturing Practices (GMP) [399].
While various production methods are employed, each comes with its own set of challenges in terms of scalability and control. Additionally, implementing Quality-by-Design (QbD) strategies, such as identifying Critical Quality Attributes (CQAs) and conducting risk assessments, is crucial for minimizing defects and ensuring that the final product meets the required safety and efficacy standards [405,406]. The use of Process Analytical Technology (PAT) for real-time monitoring is becoming increasingly important to maintain robust manufacturing processes and facilitate continuous improvements [407]. Despite these advancements, scaling nanomedicines for clinical use remains a significant barrier, compounded by the lack of globally standardized quality management systems (QMS) tailored to the unique needs of nanomedicine production.
Emerging technologies, such as microfluidic systems [408,409,410], offer promising solutions by enabling precise control over production conditions, which helps to achieve uniform particle characteristics. Additionally, advanced monitoring and quality control systems can improve the reproducibility and safety of the final product [411].
5.2. Addressing Safety Concerns
The use of NPs in medical, industrial, and environmental applications has grown significantly in recent years, but this rapid advancement has also highlighted numerous safety concerns. The unique properties of NPs—such as their small size, high reactivity, and ability to interact at the molecular level—make them highly effective in various fields. However, these same properties pose potential risks to human health and the environment, necessitating thorough safety evaluations [412]. While the benefits of NPs are well-established, understanding their toxicological effects remains a significant challenge. NPs can enter the human body through inhalation, ingestion, or skin contact, and subsequently translocate to critical organs such as the liver, lungs, heart, and brain. Their small size allows them to penetrate cellular membranes and interact directly with intracellular components. Key mechanisms of NP-induced toxicity include oxidative stress, organelle damage, and cellular and systemic effects. Oxidative stress involves the generation of ROS, which leads to lipid peroxidation, protein denaturation, and DNA damage. Organelle damage occurs when NPs translocate to cellular organelles like mitochondria, disrupting their functions and potentially contributing to chronic conditions such as asthma or cancer. Additionally, interactions between NPs and proteins or genetic material can provoke immune responses, neoantigen formation, or even organ enlargement and dysfunction [413].
The most reported effects of NPs include decreased cell viability and cell death [411,412]. Reactive oxygen species generation is a frequent observation, particularly with exposure to carbon nanotubes (CNTs), ZnO, SiO2, and TiO2 nanoparticles [413]. Furthermore, dose-dependent oxidative stress has been noted in biomarkers, with significant impacts observed for SiO2, ZnO, Fe3O4, CeO2, and CuO. DNA damage is another widely reported effect, primarily associated with ZnO and multi-walled carbon nanotubes (MWCNTs) [395]. These findings emphasize the need for further research to elucidate the mechanisms underlying NP-induced toxicity and their long-term implications for human health.
Nanomaterials possess unique properties that, if not adequately managed, can result in significant risks [414]. Agglomeration or aggregation of nanoparticles, caused by weakly bound or fused particles, compromises structural integrity, leading to poor corrosion resistance, high solubility, and phase changes that challenge long-term stability [415]. The reactivity or charge of nanoparticles, influenced by functionalization or degradative reactions, impacts their bioavailability and specific functionality, with critical chemical species and charge-related groups playing a role [416]. Impurities inherent to the high reactivity of nanoparticles necessitate encapsulation to stabilize reactive entities, especially in solution-based synthesis. Residual contaminants, such as sulfur impurities in iron oxide nanoparticles or metal impurities in carbon nanotubes, further highlight the risks associated with nanoparticles [417]. Particle size also plays a crucial role, as smaller particles aggregate more slowly but require encapsulation to maintain stability [417]. Additionally, the lack of established policies for recycling and disposing of nanomaterials increases uncertainties regarding their environmental and health impacts, particularly as experimental data on long-term exposure and toxicity remain insufficient [413].
Risk mitigation strategies are essential to address the safety concerns associated with NPs while enabling their continued use in diverse applications. One critical approach is the thorough characterization and encapsulation of NPs, as understanding their size, shape, and surface properties is vital for predicting interactions with biological and environmental systems [412]. Encapsulation techniques can enhance NP stability, minimize reactivity, and reduce their potential toxicity [418]. Additionally, standardized toxicity assessments are necessary to evaluate NP safety reliably. These protocols should account for key variables such as aggregation, charge, dissolution, and environmental persistence, enabling a more comprehensive understanding of their risks [418]. Establishing robust regulations for the recycling and disposal of nanomaterials is another priority, as improper handling can lead to unintended releases that harm ecosystems. The emergence of green nanotechnology offers a promising solution by promoting the design of less toxic, biodegradable nanoparticles that reduce environmental impact [419].
5.3. Emerging Trends and Technologies
Emerging imaging technologies enhance theranostics by improving nanoparticle visualization and diagnostic precision. Multimodal imaging (e.g., PET-MRI) and super-resolution methods (e.g., STORM, PALM) overcome resolution limits, while quantum dots, upconversion nanocrystals, and iron oxide nanoparticles offer high stability and functionality. AI-driven analysis further optimizes imaging.
Nanotheranostics also enhances CRISPR/Cas9 delivery, improving efficiency and reducing off-target effects. Lipid nanoparticles (LNPs) and alternative carriers protect genetic material and enable real-time monitoring. Clinical trials, such as the Pfizer-BioNTech vaccine, highlight nanotechnology’s medical impact, advancing precision medicine and next-generation therapies.
5.4. Advances in Imaging Modalities for Theranostics
The integration of advanced imaging modalities into theranostics represents a transformative step toward precision medicine. Traditional techniques, such as MRI, CT, PET, and optical imaging, have long been employed for diagnostics and therapeutic monitoring. However, these methods face inherent limitations in resolution, sensitivity, and specificity when adapted to nanoscale interventions. Emerging imaging technologies bridge this gap, offering higher precision and functionality. Multimodal imaging, which combines the strengths of multiple imaging techniques (e.g., PET-MRI), has emerged as a powerful tool for visualizing nanoparticles In vivo with enhanced resolution and diagnostic accuracy [174]. Similarly, super-resolution imaging methods such as STORM and photoactivated localization microscopy (PALM) have enabled visualization at the nanoscale, significantly advancing the understanding of nanoparticle behavior in biological systems [420,421].
The role of nanoparticles as functional imaging agents is also pivotal. Fluorescent proteins and organic dyes have long been utilized in microscopy due to their small size and compatibility with biological samples, making them ideal for imaging cellular structures. However, the limitations of these traditional probes, particularly in terms of photostability and brightness, have posed challenges for long-term tracking of single molecules and real-time super-resolution imaging of subcellular structures. Conventional molecular dyes and fluorescent proteins often exhibit rapid photobleaching, dim emissions, and limited photochemical switching, hindering their application in more advanced imaging techniques [421]. To address these challenges, recent advancements in material science have led to the development of luminescent NPs, such as QDs, upconversion nanocrystals (UCNCs), polymer dots (PDots), and CDs [422,423].
For example, QDs, the first generation of inorganic fluorescent nanoparticles, feature narrow-band emissions that can be precisely tuned by adjusting their size, making them ideal for multicolor imaging and super-resolution microscopy. QDs also exhibit high luminescent quantum yields and resistance to photobleaching, allowing for extended imaging times without a loss in brightness [424]. UCNPs, on the other hand, are a novel class of probes that use multiphoton emission to convert near-infrared photons into visible and ultraviolet light. This property enables background-free imaging with exceptional photostability, making them well-suited for super-resolution imaging [425]. Fluorescent CDs exhibit excellent optical properties, with emission wavelengths spanning from UV to NIR, and they can be doped with elements like Gd3+ or Mn2+ to integrate both fluorescence and magnetic resonance (MR) imaging modalities, creating multi-modal imaging probes that overcome the limitations of single-modal imaging techniques. They combine the high sensitivity of fluorescence with the high spatial resolution of MR imaging [426].
Another class of nanoparticles that has garnered significant attention in imaging and therapeutic applications are IONPs. Unlike fluorescent nanoparticles, which rely on light emission for imaging, IONPs exploit their magnetic properties to enable highly sensitive imaging techniques, such as magnetic resonance imaging (MRI) [427]. Dual-purpose nanoparticles, which integrate imaging and therapeutic capabilities, exemplify the future of theranostics [421]. These advancements are further complemented by artificial intelligence (AI)-driven image analysis, which facilitates the interpretation of complex imaging datasets and enhances diagnostic reliability [428].
5.5. Synergy Between Nanotheranostics and Emerging Gene-Editing Technologies
The convergence of nanotheranostics and gene-editing technologies, such as CRISPR/Cas9, represents a promising frontier in biomedical innovation. NPs serve as highly effective delivery vehicles for gene editors, addressing challenges such as poor cellular uptake, off-target effects, and immune responses [429]. LNPs have emerged as the gold standard for delivering RNA-based gene editors due to their high biocompatibility and efficient encapsulation [430]. Polymer-based nanoparticles and exosome-like carriers are also being explored for their ability to traverse biological barriers and target specific tissues. Additionally, PEG coatings, as discussed in previous chapters, prevent nonspecific interactions between gene-loaded nanoparticles and plasma components [429]. Moreover, the primary role of nanoparticles in nucleic acid delivery is their ability to protect genetic material from enzymatic degradation. Encapsulation or electrostatic binding by nanoparticles shields nucleic acids from DNase and RNase activity, ensuring their integrity and functionality [429].
The transformative potential of nanotechnology is underscored by several clinical trials demonstrating its efficacy across diverse medical applications. For instance, the Phase III trial of Comirnaty, the Pfizer-BioNTech COVID-19 vaccine, achieved a remarkable 95% efficacy in preventing symptomatic COVID-19, highlighting the critical role of LNP technology in stabilizing and delivering mRNA for rapid vaccine development during pandemics [412]. Similarly, the Phase III trial of Vyxeos, a nanotechnology-based therapy for acute myeloid leukemia (AML), demonstrated a 31% improvement in overall survival compared to traditional treatments, offering hope for older AML patients [431].
Beyond delivery, nanotheranostics play a crucial role in real-time monitoring of gene-editing efficacy [169]. Imaging-guided delivery systems, equipped with fluorescent or radiolabeled nanoparticles, enable visualization of DNA repair, RNA expression, or other molecular changes post-editing. This dual functionality facilitates both precise targeting and non-invasive monitoring, enhancing the overall safety and efficacy of gene-editing therapies.
The integration of nanotechnology-based CRISPR/Cas delivery systems holds significant promise as a powerful therapeutic strategy. To ensure success in clinical applications, a thorough understanding of both therapeutic targets and delivery systems is crucial. Targeting specific lesion sites with high precision allows CRISPR/Cas editing to be performed without unintended damage to non-target organs or cells. Furthermore, addressing multiple targets may enhance therapeutic efficacy [28]. A safe, efficient, and specific delivery system is essential for achieving practical CRISPR/Cas editing, which requires transient expression, biocompatible nanoparticles, and effective encapsulation. Enhancing the system’s efficiency through CRISPR/Cas optimization and improving delivery methods—such as rapid endosome escape and efficient nuclear localization—will be key to maximizing therapeutic outcomes. As the next generation of CRISPR/Cas-based systems develops, this approach has the potential to revolutionize diagnostics and treatments [28].
Author Contributions
P.G.-S., C.F.R., M.C.M., S.C. and A.M.-M. wrote the original draft. L.H.R. and J.C.C. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors acknowledge the Department of Biomedical Engineering and the Department of Chemical and Food Engineering of the Universidad de Los Andes for their support in the development of this review. During the preparation of this manuscript, the author utilized an AI-powered tool (ChatGPT) to improve the clarity and readability of the manuscript and ensure the logical flow of ideas. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Li, X.; Le, Y.; Zhang, Z.; Nian, X.; Liu, B.; Yang, X. Viral Vector-Based Gene Therapy. Int. J. Mol. Sci. 2023, 24, 7736. [Google Scholar] [CrossRef] [PubMed]
- Swingle, K.L.; Hamilton, A.G.; Safford, H.C.; Geisler, H.C.; Thatte, A.S.; Palanki, R.; Murray, A.M.; Han, E.L.; Mukalel, A.J.; Han, X.; et al. Placenta-Tropic VEGF MRNA Lipid Nanoparticles Ameliorate Murine Pre-Eclampsia. Nature 2025, 637, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Reinisch, A. Gene Therapy: Principles, Challenges and Use in Clinical Practice. Wien. Klin. Wochenschr. 2024. [Google Scholar] [CrossRef]
- Papanikolaou, E.; Bosio, A. The Promise and the Hope of Gene Therapy. Front. Genome Ed. 2021, 3, 618346. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The New Frontier of Genome Engineering with CRISPR-Cas9. Science (1979) 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of Genome Editing Technology in the Targeted Therapy of Human Diseases: Mechanisms, Advances and Prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Nienhuis, A.W. Development of Gene Therapy for Blood Disorders. Blood 2008, 111, 4431–4444. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Manoochehri, H.; Dama, P. Use of CAR T-Cell for Acute Lymphoblastic Leukemia (ALL) Treatment: A Review Study. Cancer Gene Ther. 2022, 29, 1080–1096. [Google Scholar] [CrossRef]
- Mohammadian Gol, T.; Zahedipour, F.; Trosien, P.; Ureña-Bailén, G.; Kim, M.; Antony, J.S.; Mezger, M. Gene Therapy in Pediatrics–Clinical Studies and Approved Drugs (as of 2023). Life Sci. 2024, 348, 122685. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.; Yu, T. CRISPR/Cas9 Therapeutics: Progress and Prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef]
- Darrow, J.J. Luxturna: FDA Documents Reveal the Value of a Costly Gene Therapy. Drug Discov. Today 2019, 24, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Kohn, D.B.; Chen, Y.Y.; Spencer, M.J. Successes and Challenges in Clinical Gene Therapy. Gene Ther. 2023, 30, 738–746. [Google Scholar] [CrossRef]
- Sandahl, A.F.; Nguyen, T.J.D.; Hansen, R.A.; Johansen, M.B.; Skrydstrup, T.; Gothelf, K.V. On-Demand Synthesis of Phosphoramidites. Nat. Commun. 2021, 12, 2760. [Google Scholar] [CrossRef]
- Bizat, P.N.; Sabat, N.; Hollenstein, M. Recent Advances in Biocatalytic and Chemoenzymatic Synthesis of Oligonucleotides. ChemBioChem 2025, e202400987. [Google Scholar] [CrossRef]
- Blay, E.; Hardyman, E.; Morovic, W. PCR-Based Analytics of Gene Therapies Using Adeno-Associated Virus Vectors: Considerations for CGMP Method Development. Mol. Ther. Methods Clin. Dev. 2023, 31, 101132. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, H.; Xu, Y.; Laššáková, S.; Korabečná, M.; Neužil, P. PCR Past, Present and Future. Biotechniques 2020, 69, 317–325. [Google Scholar] [CrossRef]
- Hays, A.; Wissel, M.; Colletti, K.; Soon, R.; Azadeh, M.; Smith, J.; Doddareddy, R.; Chalfant, M.; Adamowicz, W.; Ramaswamy, S.S.; et al. Recommendations for Method Development and Validation of QPCR and DPCR Assays in Support of Cell and Gene Therapy Drug Development. AAPS J. 2024, 26, 24. [Google Scholar] [CrossRef]
- Bhatia, R.; Singh, A.; Singh, S.; Navneesh; Rawal, R.K. Emerging Trends in Nano-Carrier Based Gene Delivery Systems for Targeted Cancer Therapy. J. Drug Deliv. Sci. Technol. 2024, 95, 105546. [Google Scholar] [CrossRef]
- Volodina, O.; Smirnikhina, S. The Future of Gene Therapy: A Review of In Vivo and Ex Vivo Delivery Methods for Genome Editing-Based Therapies. Mol. Biotechnol. 2025, 67, 425–437. [Google Scholar] [CrossRef]
- Hwu, W.-L. Gene Therapy for Ultrarare Diseases: A Geneticist’s Perspective. J. Biomed. Sci. 2024, 31, 79. [Google Scholar] [CrossRef]
- Tao, Z.; Chyra, Z.; Kotulová, J.; Celichowski, P.; Mihályová, J.; Charvátová, S.; Hájek, R. Impact of T Cell Characteristics on CAR-T Cell Therapy in Hematological Malignancies. Blood Cancer J. 2024, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Khadela, A.; Shah, Y.; Postwala, H.; Balar, P.; Vora, L. Current Status of Cancer Nanotheranostics: Emerging Strategies for Cancer Management. Nanotheranostics 2023, 7, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Akpa, P.A.; Peter, I.E.; Onwuka, A.M.; Obi, B.C.; Akunne, M.O.; Nworu, C.S.; Ejikeme, P.M.; Akunne, T.C.; Attama, A.A.; Akah, P.A. Nanotheranostics: Platforms, Current Applications, and Mechanisms of Targeting in Breast and Prostate Cancers. J. Nanotheranostics 2023, 4, 346–383. [Google Scholar] [CrossRef]
- Ghosh, S.; Kitture, R. Nanotheranostics. In Nanobiotechnology in Diagnosis, Drug Delivery, and Treatment; Wiley & Sons: New York, NY, USA, 2020; pp. 63–94. [Google Scholar]
- Yang, F.; Lv, J.; Ma, W.; Yang, Y.; Hu, X.; Yang, Z. Engineering Sonosensitizer-Derived Nanotheranostics for Augmented Sonodynamic Therapy. Small 2024, 20, 2402669. [Google Scholar] [CrossRef]
- Ortegón, S.; Peñaranda, P.A.; Rodríguez, C.F.; Noguera, M.J.; Florez, S.L.; Cruz, J.C.; Rivas, R.E.; Osma, J.F. Magnetic Torus Microreactor as a Novel Device for Sample Treatment via Solid-Phase Microextraction Coupled to Graphite Furnace Atomic Absorption Spectroscopy: A Route for Arsenic Pre-Concentration. Molecules 2022, 27, 6198. [Google Scholar] [CrossRef]
- Gupta, D.; Roy, P.; Sharma, R.; Kasana, R.; Rathore, P.; Gupta, T.K. Recent Nanotheranostic Approaches in Cancer Research. Clin. Exp. Med. 2024, 24, 8. [Google Scholar] [CrossRef]
- Kong, H.; Ju, E.; Yi, K.; Xu, W.; Lao, Y.; Cheng, D.; Zhang, Q.; Tao, Y.; Li, M.; Ding, J. Advanced Nanotheranostics of CRISPR/Cas for Viral Hepatitis and Hepatocellular Carcinoma. Adv. Sci. 2021, 8, 2102051. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Pombo, I.; Raposo, L.; Pedrosa, P.; Fernandes, A.R.; Baptista, P.V. Nanotheranostics Targeting the Tumor Microenvironment. Front. Bioeng. Biotechnol. 2019, 7, 197. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Benival, D.; Khunt, D.; Prajapati, B.G. Achieving Endo/Lysosomal Escape Using Smart Nanosystems for Efficient Cellular Delivery. Molecules 2024, 29, 3131. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart Nanocarrier-Based Drug Delivery Systems for Cancer Therapy and Toxicity Studies: A Review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Ladju, R.B.; Ulhaq, Z.S.; Soraya, G.V. Nanotheranostics: A Powerful next-Generation Solution to Tackle Hepatocellular Carcinoma. World J. Gastroenterol. 2022, 28, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.F.; Guzmán-Sastoque, P.; Muñoz-Camargo, C.; Reyes, L.H.; Osma, J.F.; Cruz, J.C. Enhancing Magnetic Micro- and Nanoparticle Separation with a Cost-Effective Microfluidic Device Fabricated by Laser Ablation of PMMA. Micromachines 2024, 15, 1057. [Google Scholar] [CrossRef]
- Orjuela-Garzón, I.C.; Rodríguez, C.F.; Cruz, J.C.; Briceño, J.C. Design, Characterization, and Evaluation of Textile Systems and Coatings for Sports Use: Applications in the Design of High-Thermal Comfort Wearables. ACS Omega 2024, 9, 49143–49162. [Google Scholar] [CrossRef] [PubMed]
- Pala, R.; Pattnaik, S.; Busi, S.; Nauli, S.M. Nanomaterials as Novel Cardiovascular Theranostics. Pharmaceutics 2021, 13, 348. [Google Scholar] [CrossRef]
- Pijeira, M.S.O.; Viltres, H.; Kozempel, J.; Sakmár, M.; Vlk, M.; İlem-Özdemir, D.; Ekinci, M.; Srinivasan, S.; Rajabzadeh, A.R.; Ricci-Junior, E.; et al. Radiolabeled Nanomaterials for Biomedical Applications: Radiopharmacy in the Era of Nanotechnology. EJNMMI Radiopharm. Chem. 2022, 7, 8. [Google Scholar] [CrossRef]
- Dutta, B.; Barick, K.C.; Hassan, P.A. Recent Advances in Active Targeting of Nanomaterials for Anticancer Drug Delivery. Adv. Colloid. Interface Sci. 2021, 296, 102509. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Afrouz, M.; Ahmadi-Nouraldinvand, F.; Amani, A.; Zahedian, H.; Elias, S.G.; Arabnejad, F.; Yaghoubi, H.; Farshad, O.; Farazi, N.; Jalali, A.; et al. Preparation and Characterization of Magnetic PEG-PEI-PLA-PEI-PEG/Fe3O4-PCL/DNA Micelles for Gene Delivery into MCF-7 Cells. J. Drug Deliv. Sci. Technol. 2023, 79, 104016. [Google Scholar] [CrossRef]
- Amani, A.; Alizadeh, M.R.; Yaghoubi, H.; Ebrahimi, H.A. Design and Fabrication of Novel Multi-Targeted Magnetic Nanoparticles for Gene Delivery to Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102151. [Google Scholar] [CrossRef]
- Saeed, R.M.; Dmour, I.; Taha, M.O. Stable Chitosan-Based Nanoparticles Using Polyphosphoric Acid or Hexametaphosphate for Tandem Ionotropic/Covalent Crosslinking and Subsequent Investigation as Novel Vehicles for Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 4. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, L.; Wang, X.; Jin, H. RNA-Based Therapeutics: An Overview and Prospectus. Cell Death Dis. 2022, 13, 644. [Google Scholar] [CrossRef] [PubMed]
- Amani, A.; Kabiri, T.; Shafiee, S.; Hamidi, A. Preparation and Characterization of PLA-PEG-PLA/PEI/DNA Nanoparticles for Improvement of Transfection Efficiency and Controlled Release of DNA in Gene Delivery Systems. Iran. J. Pharm. Res. 2019, 18, 125–141. [Google Scholar] [PubMed]
- Rodríguez, C.F.; Quezada, V.; Andrade-Pérez, V.; Reyes, G.; Vargas, M.C.; Reyes, L.H.; Cruz, J.C. Classical and Emerging Approximations for the Screening of Antimicrobial Peptide Libraries. In Antimicrobial Peptides; Elsevier: Amsterdam, The Netherlands, 2025; pp. 195–232. [Google Scholar]
- Eloy, J.O.; Petrilli, R.; Lee, R.J. Targeting of Drug Nanocarriers. In Nanocarriers for Drug Delivery; Springer: Cham, Switzerland, 2021; pp. 107–126. [Google Scholar]
- Narwade, M.; Shaikh, A.; Gajbhiye, K.R.; Kesharwani, P.; Gajbhiye, V. Advanced Cancer Targeting Using Aptamer Functionalized Nanocarriers for Site-Specific Cargo Delivery. Biomater. Res. 2023, 27, 42. [Google Scholar] [CrossRef]
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-Responsive Nanomaterials for Application in Antitumor Therapy and Drug Delivery. Pharmaceutics 2020, 12, 630. [Google Scholar] [CrossRef]
- Yan, Y.; Ding, H. PH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials 2020, 10, 1613. [Google Scholar] [CrossRef]
- Mantilla-Orozco, A.; Rodriguez, C.F.; Quiroz, I.; Bermudez, J.S.; Forero, D.F.; Monsalve, M.C.; Muñoz-Camargo, C.; Reyes, L.H.; Osma, J.F.; Quezada, V.; et al. Modeling and Simulation of Magnetoliposome Formation by Encapsulation of Core-Shell, Magnetite-Chitosan Nanoparticles in Liposomes Enabled by a Low-Cost Microfluidic System. Biol. Life Sci. Forum 2022, 20, 29. [Google Scholar] [CrossRef]
- Qiu, C.; Xia, F.; Zhang, J.; Shi, Q.; Meng, Y.; Wang, C.; Pang, H.; Gu, L.; Xu, C.; Guo, Q.; et al. Advanced Strategies for Overcoming Endosomal/Lysosomal Barrier in Nanodrug Delivery. Research 2023, 6, 0148. [Google Scholar] [CrossRef]
- Sun, D.; Lu, Z.-R. Structure and Function of Cationic and Ionizable Lipids for Nucleic Acid Delivery. Pharm. Res. 2023, 40, 27–46. [Google Scholar] [CrossRef]
- Neundorf, I.; Rennert, R.; Hoyer, J.; Schramm, F.; Löbner, K.; Kitanovic, I.; Wölfl, S. Fusion of a Short HA2-Derived Peptide Sequence to Cell-Penetrating Peptides Improves Cytosolic Uptake, but Enhances Cytotoxic Activity. Pharmaceuticals 2009, 2, 49–65. [Google Scholar] [CrossRef]
- Tarvirdipour, S.; Skowicki, M.; Schoenenberger, C.-A.; Palivan, C.G. Peptide-Assisted Nucleic Acid Delivery Systems on the Rise. Int. J. Mol. Sci. 2021, 22, 9092. [Google Scholar] [CrossRef]
- Hu, M.; Li, X.; You, Z.; Cai, R.; Chen, C. Physiological Barriers and Strategies of Lipid-Based Nanoparticles for Nucleic Acid Drug Delivery. Adv. Mater. 2024, 36, 202303266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, Y.; Zhang, J.; Kuo, J.C.-T.; Zhang, Z.; Xie, H.; Zhu, J.; Liu, T. Modification of Lipid-Based Nanoparticles: An Efficient Delivery System for Nucleic Acid-Based Immunotherapy. Molecules 2022, 27, 1943. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome Composition in Drug Delivery Design, Synthesis, Characterization, and Clinical Application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef]
- Eygeris, Y.; Patel, S.; Jozic, A.; Sahay, G. Deconvoluting Lipid Nanoparticle Structure for Messenger RNA Delivery. Nano Lett. 2020, 20, 4543–4549. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 MRNA Vaccine Development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Tiwari, A.; Gulbake, A.S.; Kumar, P. Lipid Nanoparticles and Nanoemulsions Exploited in the Diagnosis and Treatment of Infectious Diseases. In Nanotheranostics for Treatment and Diagnosis of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2022; pp. 229–273. [Google Scholar]
- Saraf, A.; Gurjar, R.; Kaviraj, S.; Kulkarni, A.; Kumar, D.; Kulkarni, R.; Virkar, R.; Krishnan, J.; Yadav, A.; Baranwal, E.; et al. An Omicron-Specific, Self-Amplifying MRNA Booster Vaccine for COVID-19: A Phase 2/3 Randomized Trial. Nat. Med. 2024, 30, 1363–1372. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R. Solid Lipid Nanoparticles: A Modern Formulation Approach in Drug Delivery System. Indian. J. Pharm. Sci. 2009, 71, 349. [Google Scholar] [CrossRef]
- Hoy, S.M. Patisiran: First Global Approval. Drugs 2018, 78, 1625–1631. [Google Scholar] [CrossRef]
- ClinicalTrials.gov Lipid-Based Nanoparticles in Drug Delivery. Available online: https://clinicaltrials.gov/search?term=lipid%20based%20nanoparticles&aggFilters=studyType:int (accessed on 30 January 2025).
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and Innovation in the Manufacturing Process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Wagner, A.; Vorauer-Uhl, K. Liposome Technology for Industrial Purposes. J. Drug Deliv. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An Advanced Mode of Drug Delivery System. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, K.; Zimmer, A. Drug Delivery and Drug Targeting with Parenteral Lipid Nanoemulsions—A Review. J. Control. Release 2016, 223, 85–98. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A Brief Review on Solid Lipid Nanoparticles: Part and Parcel of Contemporary Drug Delivery Systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Gastaldi, L.; Battaglia, L.; Peira, E.; Chirio, D.; Muntoni, E.; Solazzi, I.; Gallarate, M.; Dosio, F. Solid Lipid Nanoparticles as Vehicles of Drugs to the Brain: Current State of the Art. Eur. J. Pharm. Biopharm. 2014, 87, 433–444. [Google Scholar] [CrossRef]
- Araujo, V.H.S.; Delello Di Filippo, L.; Duarte, J.L.; Spósito, L.; de Camargo, B.A.F.; da Silva, P.B.; Chorilli, M. Exploiting Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery against Cutaneous Fungal Infections. Crit. Rev. Microbiol. 2021, 47, 79–90. [Google Scholar] [CrossRef]
- Gao, W.; Bigham, A.; Ghomi, M.; Zarrabi, A.; Rabiee, N.; Saeb, M.R.; Nuri Ertas, Y.; Goel, A.; Sharifi, E.; Ashrafizadeh, M.; et al. Micelle-Engineered Nanoplatforms for Precision Oncology. Chem. Eng. J. 2024, 495, 153438. [Google Scholar] [CrossRef]
- Quezada, V.; Guzmán-Satoque, P.; Rincón-Garcia, M.C.; Reyes, L.H.; Cruz, J.C. Physicochemical and Biochemical Characterization of Antimicrobial Peptides. In Antimicrobial Peptides; Elsevier: Amsterdam, The Netherlands, 2025; pp. 259–299. [Google Scholar]
- Zheng, Y.; Oz, Y.; Gu, Y.; Ahamad, N.; Shariati, K.; Chevalier, J.; Kapur, D.; Annabi, N. Rational Design of Polymeric Micelles for Targeted Therapeutic Delivery. Nano Today 2024, 55, 102147. [Google Scholar] [CrossRef]
- Yousefpour Marzbali, M.; Yari Khosroushahi, A. Polymeric Micelles as Mighty Nanocarriers for Cancer Gene Therapy: A Review. Cancer Chemother. Pharmacol. 2017, 79, 637–649. [Google Scholar] [CrossRef]
- Bolanos-Barbosa, A.D.; Rodríguez, C.F.; Acuña, O.L.; Cruz, J.C.; Reyes, L.H. The Impact of Yeast Encapsulation in Wort Fermentation and Beer Flavor Profile. Polymers 2023, 15, 1742. [Google Scholar] [CrossRef]
- Rodríguez-Soto, M.A.; Riveros-Cortés, A.; Orjuela-Garzón, I.C.; Fernández-Calderón, I.M.; Rodríguez, C.F.; Vargas, N.S.; Ostos, C.; Camargo, C.M.; Cruz, J.C.; Kim, S.; et al. Redefining Vascular Repair: Revealing Cellular Responses on PEUU—Gelatin Electrospun Vascular Grafts for Endothelialization and Immune Responses on in Vitro Models. Front. Bioeng. Biotechnol. 2024, 12, 1410863. [Google Scholar] [CrossRef] [PubMed]
- Cajot, S.; Schol, D.; Danhier, F.; Préat, V.; Gillet De Pauw, M.; Jérôme, C. In vitro investigations of smart drug delivery systems based on redox-sensitive cross-linked micelles. Macromol. Biosci. 2013, 13, 1661–1670. [Google Scholar] [CrossRef]
- Christie, R.J.; Nishiyama, N.; Kataoka, K. Minireview: Delivering the Code: Polyplex Carriers for Deoxyribonucleic Acid and Ribonucleic Acid Interference Therapies. Endocrinology 2010, 151, 466–473. [Google Scholar] [CrossRef]
- Jain, M.; Yu, X.; Schneck, J.P.; Green, J.J. Nanoparticle Targeting Strategies for Lipid and Polymer-Based Gene Delivery to Immune Cells In Vivo. Small Sci. 2024, 4, 2400248. [Google Scholar] [CrossRef]
- Zheng, Y.; Luo, S.; Xu, M.; He, Q.; Xie, J.; Wu, J.; Huang, Y. Transepithelial Transport of Nanoparticles in Oral Drug Delivery: From the Perspective of Surface and Holistic Property Modulation. Acta Pharm. Sin. B 2024, 14, 3876–3900. [Google Scholar] [CrossRef]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- van den Berg, A.I.S.; Yun, C.-O.; Schiffelers, R.M.; Hennink, W.E. Polymeric Delivery Systems for Nucleic Acid Therapeutics: Approaching the Clinic. J. Control. Release 2021, 331, 121–141. [Google Scholar] [CrossRef]
- Lächelt, U.; Wagner, E. Nucleic Acid Therapeutics Using Polyplexes: A Journey of 50 Years (and Beyond). Chem. Rev. 2015, 115, 11043–11078. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, M.; Fu, Y.; Li, Y.; Gong, T.; Zhang, Z.; Sun, X. Enhanced Intranasal Delivery of MRNA Vaccine by Overcoming the Nasal Epithelial Barrier via Intra- and Paracellular Pathways. J. Control. Release 2016, 228, 9–19. [Google Scholar] [CrossRef]
- Cheng, J.; Zeidan, R.; Mishra, S.; Liu, A.; Pun, S.H.; Kulkarni, R.P.; Jensen, G.S.; Bellocq, N.C.; Davis, M.E. Structure−Function Correlation of Chloroquine and Analogues as Transgene Expression Enhancers in Nonviral Gene Delivery. J. Med. Chem. 2006, 49, 6522–6531. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, T.; Li, Y.; Huang, G.; White, M.A.; Gao, J. Investigation of Endosome and Lysosome Biology by Ultra PH-Sensitive Nanoprobes. Adv. Drug Deliv. Rev. 2017, 113, 87–96. [Google Scholar] [CrossRef]
- Hu, Y.-B.; Dammer, E.B.; Ren, R.-J.; Wang, G. The Endosomal-Lysosomal System: From Acidification and Cargo Sorting to Neurodegeneration. Transl. Neurodegener. 2015, 4, 18. [Google Scholar] [CrossRef]
- Gallops, C.; Ziebarth, J.; Wang, Y. A Polymer Physics Perspective on Why PEI Is an Effective Nonviral Gene Delivery Vector. In Polymers in Therapeutic Delivery; ACS Publications: Washington, DC, USA, 2020; pp. 1–12. [Google Scholar]
- Degors, I.M.S.; Wang, C.; Rehman, Z.U.; Zuhorn, I.S. Carriers Break Barriers in Drug Delivery: Endocytosis and Endosomal Escape of Gene Delivery Vectors. Acc. Chem. Res. 2019, 52, 1750–1760. [Google Scholar] [CrossRef]
- Kumarasamy, R.V.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Mironescu, M.; Palanisamy, C.P. Clinical Applications and Therapeutic Potentials of Advanced Nanoparticles: A Comprehensive Review on Completed Human Clinical Trials. Front. Nanotechnol. 2024, 6, 1479993. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, W.; Xin, H.; Liu, R.; Wang, Q.; Cai, W.; Peng, X.; Yang, F.; Xin, H. Nanoparticles Advanced from Preclinical Studies to Clinical Trials for Lung Cancer Therapy. Cancer Nanotechnol. 2023, 14, 28. [Google Scholar] [CrossRef]
- Perez, E.A.; Awada, A.; O’Shaughnessy, J.; Rugo, H.S.; Twelves, C.; Im, S.-A.; Gómez-Pardo, P.; Schwartzberg, L.S.; Diéras, V.; Yardley, D.A.; et al. Etirinotecan Pegol (NKTR-102) versus Treatment of Physician’s Choice in Women with Advanced Breast Cancer Previously Treated with an Anthracycline, a Taxane, and Capecitabine (BEACON): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Oncol. 2015, 16, 1556–1568. [Google Scholar] [CrossRef]
- Subbiah, V.; Grilley-Olson, J.E.; Combest, A.J.; Sharma, N.; Tran, R.H.; Bobe, I.; Osada, A.; Takahashi, K.; Balkissoon, J.; Camp, A.; et al. Phase Ib/II Trial of NC-6004 (Nanoparticle Cisplatin) Plus Gemcitabine in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, R.; Xu, J.; Zou, Y.; Wang, T.; Liu, J. Recent Developments of Polymeric Delivery Systems in Gene Therapeutics. Polym. Chem. 2024, 15, 1908–1931. [Google Scholar] [CrossRef]
- Luo, R.; Le, H.; Wu, Q.; Gong, C. Nanoplatform-Based In Vivo Gene Delivery Systems for Cancer Therapy. Small 2024, 20, 2312153. [Google Scholar] [CrossRef]
- Feun, L.; Savaraj, N. Pegylated Arginine Deiminase: A Novel Anticancer Enzyme Agent. Expert. Opin. Investig. Drugs 2006, 15, 815–822. [Google Scholar] [CrossRef]
- Langer, C.J.; O’Byrne, K.J.; Socinski, M.A.; Mikhailov, S.M.; Leśniewski-Kmak, K.; Smakal, M.; Ciuleanu, T.E.; Orlov, S.V.; Dediu, M.; Heigener, D.; et al. Phase III Trial Comparing Paclitaxel Poliglumex (CT-2103, PPX) in Combination with Carboplatin Versus Standard Paclitaxel and Carboplatin in the Treatment of PS 2 Patients with Chemotherapy-Naïve Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2008, 3, 623–630. [Google Scholar] [CrossRef]
- Erathodiyil, N.; Ying, J.Y. Functionalization of Inorganic Nanoparticles for Bioimaging Applications. Acc. Chem. Res. 2011, 44, 925–935. [Google Scholar] [CrossRef]
- Rodríguez, C.F.; Castilla-Bolanos, M.A.; Ortiz, L.; Rodriguez, K.A.G.; Osma, J.F.; Camargo, C.M.; Cruz, J.C. Study of Spheroids Fusion via Multiphysics Simulations: Feasibility of Applying Permanent Magnetic Field Gradients. Biol. Life Sci. Forum 2022, 20, 30. [Google Scholar] [CrossRef]
- Gupta, A.; Moyano, D.F.; Parnsubsakul, A.; Papadopoulos, A.; Wang, L.-S.; Landis, R.F.; Das, R.; Rotello, V.M. Ultrastable and Biofunctionalizable Gold Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 14096–14101. [Google Scholar] [CrossRef]
- Son, S.; Nam, J.; Kim, J.; Kim, S.; Kim, W.J. I-Motif-Driven Au Nanomachines in Programmed SiRNA Delivery for Gene-Silencing and Photothermal Ablation. ACS Nano 2014, 8, 5574–5584. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, H.; Tang, C.; Cheng, Y.; Cheng, L. Exploration of Ultrasound-Sensitive Biomaterials in Cancer Theranostics. Adv. Funct. Mater. 2024, 34, 2313454. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Liu, Z.; Ma, Y.; Huang, Y.; Chen, Y. High-Efficiency Loading and Controlled Release of Doxorubicin Hydrochloride on Graphene Oxide. J. Phys. Chem. C 2008, 112, 17554–17558. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.J. Photothermally Controlled Gene Delivery by Reduced Graphene Oxide–Polyethylenimine Nanocomposite. Small 2014, 10, 117–126. [Google Scholar] [CrossRef]
- Zhi, F.; Dong, H.; Jia, X.; Guo, W.; Lu, H.; Yang, Y.; Ju, H.; Zhang, X.; Hu, Y. Functionalized Graphene Oxide Mediated Adriamycin Delivery and MiR-21 Gene Silencing to Overcome Tumor Multidrug Resistance In Vitro. PLoS ONE 2013, 8, e60034. [Google Scholar] [CrossRef]
- Zhang, L.; Ouyang, S.; Zhang, H.; Qiu, M.; Dai, Y.; Wang, S.; Wang, Y.; Ou, J. Graphene Oxide Induces Dose-dependent Lung Injury in Rats by Regulating Autophagy. Exp. Ther. Med. 2021, 21, 462. [Google Scholar] [CrossRef]
- Kong, C.; Chen, J.; Li, P.; Wu, Y.; Zhang, G.; Sang, B.; Li, R.; Shi, Y.; Cui, X.; Zhou, T. Respiratory Toxicology of Graphene-Based Nanomaterials: A Review. Toxics 2024, 12, 82. [Google Scholar] [CrossRef]
- Drasler, B.; Kucki, M.; Delhaes, F.; Buerki-Thurnherr, T.; Vanhecke, D.; Korejwo, D.; Chortarea, S.; Barosova, H.; Hirsch, C.; Petri-Fink, A.; et al. Single Exposure to Aerosolized Graphene Oxide and Graphene Nanoplatelets Did Not Initiate an Acute Biological Response in a 3D Human Lung Model. Carbon 2018, 137, 125–135. [Google Scholar] [CrossRef]
- Padín-González, E.; Lancaster, P.; Bottini, M.; Gasco, P.; Tran, L.; Fadeel, B.; Wilkins, T.; Monopoli, M.P. Understanding the Role and Impact of Poly (Ethylene Glycol) (PEG) on Nanoparticle Formulation: Implications for COVID-19 Vaccines. Front. Bioeng. Biotechnol. 2022, 10, 882363. [Google Scholar] [CrossRef] [PubMed]
- Biswal, M.R.; Bhatia, S. Carbon Dot Nanoparticles: Exploring the Potential Use for Gene Delivery in Ophthalmic Diseases. Nanomaterials 2021, 11, 935. [Google Scholar] [CrossRef]
- Das, R.P.; Gandhi, V.V.; Singh, B.G.; Kunwar, A. Passive and Active Drug Targeting: Role of Nanocarriers in Rational Design of Anticancer Formulations. Curr. Pharm. Des. 2019, 25, 3034–3056. [Google Scholar] [CrossRef]
- A Comprehensive Review of Passive and Active Nanoparticle Targeting Technics. Available online: https://insidetx.com/review/a-comprehensive-review-of-passive-and-active-nanoparticle-targeting-technics/ (accessed on 5 February 2025).
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Kami, D.; Kitani, T.; Kishida, T.; Mazda, O.; Toyoda, M.; Tomitaka, A.; Ota, S.; Ishii, R.; Takemura, Y.; Watanabe, M.; et al. Pleiotropic functions of magnetic nanoparticles for ex vivo gene transfer. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1165–1174. [Google Scholar] [CrossRef]
- Lv, Z.; Li, D.; Tang, X.; Pulli, B.; Cheng, J.; Zhao, P.; Yuan, X.; Luo, Q.; Cai, H.; Ye, M.; et al. Theranostic nanoparticles based on bioreducible polyethylenimine-coated iron oxide for reduction-responsive gene delivery and magnetic resonance imaging. Int. J. Nanomed. 2014, 9, 3347–3361. [Google Scholar] [CrossRef]
- Seaberg, J.; Montazerian, H.; Hossen, M.N.; Bhattacharya, R.; Khademhosseini, A.; Mukherjee, P. Hybrid Nanosystems for Biomedical Applications. ACS Nano 2021, 15, 2099–2142. [Google Scholar] [CrossRef]
- Ding, X.; Yin, C.; Zhang, W.; Sun, Y.; Zhang, Z.; Yang, E.; Sun, D.; Wang, W. Designing Aptamer-Gold Nanoparticle-Loaded pH-Sensitive Liposomes Encapsulate Morin for Treating Cancer. Nanoscale Res. Lett. 2020, 15, 1–17. [Google Scholar] [CrossRef]
- Yu, B.; Xue, X.; Yin, Z.; Cao, L.; Li, M.; Huang, J. Engineered Cell Membrane-Derived Nanocarriers: The Enhanced Delivery System for Therapeutic Applications. Front. Cell Dev. Biol. 2022, 10, 844050. [Google Scholar] [CrossRef]
- Knights-Mitchell, S.S.; Romanowski, M. Near-Infrared Activated Release of Doxorubicin from Plasmon Resonant Liposomes. Nanotheranostics 2018, 2, 295–305. [Google Scholar] [CrossRef]
- Mahalunkar, S.; Yadav, A.S.; Gorain, M.; Pawar, V.; Braathen, R.; Weiss, S.; Bogen, B.; Gosavi, S.W.; Kundu, G.C. Functional design of pH-responsive folate-targeted polymer-coated gold nanoparticles for drug delivery and in vivo therapy in breast cancer. Int. J. Nanomed. 2019, 14, 8285–8302. [Google Scholar] [CrossRef]
- Bishop, C.J.; Liu, A.L.; Lee, D.S.; Murdock, R.J.; Green, J.J. Layer-by-layer inorganic/polymeric nanoparticles for kinetically controlled multigene delivery. J. Biomed. Mater. Res. Part A 2015, 104, 707–713. [Google Scholar] [CrossRef]
- Rodrigues, B.d.S.; Banerjee, A.; Kanekiyo, T.; Singh, J. Functionalized liposomal nanoparticles for efficient gene delivery system to neuronal cell transfection. Int. J. Pharm. 2019, 566, 717–730. [Google Scholar] [CrossRef]
- Hossen, N.; Wang, L.; Chinthalapally, H.R.; Robertson, J.D.; Fung, K.-M.; Wilhelm, S.; Bieniasz, M.; Bhattacharya, R.; Mukherjee, P. Switching the intracellular pathway and enhancing the therapeutic efficacy of small interfering RNA by auroliposome. Sci. Adv. 2020, 6, eaba5379. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Zheng, W.; Cong, L.; Guo, Z.; Xie, Y.; Wang, L.; Tang, R.; Feng, Q.; Hamada, Y.; et al. Thermo-triggered Release of CRISPR-Cas9 System by Lipid-Encapsulated Gold Nanoparticles for Tumor Therapy. Angew. Chem. Int. Ed. Engl. 2017, 57, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Daniels, A.N.; Singh, M. Sterically Stabilized siRNA:Gold Nanocomplexes Enhance c-MYC Silencing in a Breast Cancer Cell Model. Nanomedicine 2019, 14, 1387–1401. [Google Scholar] [CrossRef]
- Ben Djemaa, S.; David, S.; Hervé-Aubert, K.; Falanga, A.; Galdiero, S.; Allard-Vannier, E.; Chourpa, I.; Munnier, E. Formulation and in vitro evaluation of a siRNA delivery nanosystem decorated with gH625 peptide for triple negative breast cancer theranosis. Eur. J. Pharm. Biopharm. 2018, 131, 99–108. [Google Scholar] [CrossRef]
- Kim, J.-M.; Shin, E.; Ryou, S.-M.; Yeom, J.-H.; Lee, K. Gene delivery platforms. Biotechnol. Bioprocess Eng. 2013, 18, 637–647. [Google Scholar] [CrossRef]
- Lekkala, V.V.V.; Reddy, M.C.; Reddy, V.C.; Kanthirigala, S.K.; Chitta, S.; Reddy, K.R.; Lomada, D. Advancements in nanoparticles-based therapies for biomedical applications. Nano-Struct. Nano-Objects 2024, 40, 101365. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Perrigue, P.M.; Murray, R.A.; Mielcarek, A.; Henschke, A.; Moya, S.E. Degradation of Drug Delivery Nanocarriers and Payload Release: A Review of Physical Methods for Tracing Nanocarrier Biological Fate. Pharmaceutics 2021, 13, 770. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Li, T.; Xu, M.; Chen, Y.; Wu, C.; Dang, X.; Liu, Y. Multifunctional Core/Shell Nanoparticles Cross-linked Polyetherimide-folic Acid as Efficient Notch-1 siRNA Carrier for Targeted Killing of Breast Cancer. Sci. Rep. 2014, 4, 7072. [Google Scholar] [CrossRef]
- Bai, X.; Smith, Z.L.; Wang, Y.; Butterworth, S.; Tirella, A. Sustained Drug Release from Smart Nanoparticles in Cancer Therapy: A Comprehensive Review. Micromachines 2022, 13, 1623. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Hussein, W.; Refaat, T. Passive Targeting of Nanoparticles to Cancer: A Comprehensive Review of the Literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer Active Targeting by Nanoparticles: A Comprehensive Review of Literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef]
- Kumari, M.; Acharya, A.; Krishnamurthy, P.T. Antibody-Conjugated Nanoparticles for Target-Specific Drug Delivery of Chemotherapeutics. Beilstein J. Nanotechnol. 2023, 14, 912–926. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhao, J.; Yi, D.; Di, Z.; Li, L. Peptide Nucleic Acid (PNA)-Guided Peptide Engineering of an Aptamer Sensor for Protease-Triggered Molecular Imaging. Angew. Chem. Int. Ed. 2021, 60, 22659–22663. [Google Scholar] [CrossRef]
- Zheng, L.; Bandara, S.R.; Tan, Z.; Leal, C. Lipid Nanoparticle Topology Regulates Endosomal Escape and Delivery of RNA to the Cytoplasm. Proc. Natl. Acad. Sci. USA 2023, 120, e2301067120. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, L. Lipid Nanoparticles for Gene Delivery. Adv. Genet. 2014, 88, 13–36. [Google Scholar]
- Khan, M. Polymers as Efficient Non-Viral Gene Delivery Vectors: The Role of the Chemical and Physical Architecture of Macromolecules. Polymers 2024, 16, 2629. [Google Scholar] [CrossRef]
- Bolhassani, A.; Javanzad, S.; Saleh, T.; Hashemi, M.; Aghasadeghi, M.R.; Sadat, S.M. Polymeric Nanoparticles. Hum. Vaccin. Immunother. 2014, 10, 321–332. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, N. Cationic Polymer Optimization for Efficient Gene Delivery. Mini-Rev. Med. Chem. 2010, 10, 108–125. [Google Scholar] [CrossRef]
- Jiao, L.; Sun, Z.; Sun, Z.; Liu, J.; Deng, G.; Wang, X. Nanotechnology-Based Non-Viral Vectors for Gene Delivery in Cardiovascular Diseases. Front. Bioeng. Biotechnol. 2024, 12, 1349077. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene Therapy in Cancer Treatment: Why Go Nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef]
- Lin, G.; Revia, R.A.; Zhang, M. Inorganic Nanomaterial-Mediated Gene Therapy in Combination with Other Antitumor Treatment Modalities. Adv. Funct. Mater. 2021, 31, 2007096. [Google Scholar] [CrossRef]
- Li, M.; Tang, Q.; Wan, H.; Zhu, G.; Yin, D.; Lei, L.; Li, S. Functional Inorganic Nanoparticles in Cancer: Biomarker Detection, Imaging, and Therapy. APL Mater. 2024, 12, 100601. [Google Scholar] [CrossRef]
- He, W.-T.; Xue, Y.-N.; Peng, N.; Liu, W.-M.; Zhuo, R.-X.; Huang, S.-W. One-Pot Preparation of Polyethylenimine-Silica Nanoparticles as Serum-Resistant Gene Delivery Vectors: Intracellular Trafficking and Transfection. J. Mater. Chem. 2011, 21, 10496. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; Li, L.; Song, M.; Dong, M. Biomembrane-Wrapped Gene Delivery Nanoparticles for Cancer Therapy. Front. Bioeng. Biotechnol. 2023, 11, 1211753. [Google Scholar] [CrossRef]
- Xu, X.; Xia, T. Recent Advances in Site-Specific Lipid Nanoparticles for MRNA Delivery. ACS Nanosci. Au 2023, 3, 192–203. [Google Scholar] [CrossRef]
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric Lipid Hybrid Nanoparticles (PLNs) as Emerging Drug Delivery Platform—A Comprehensive Review of Their Properties, Preparation Methods, and Therapeutic Applications. Pharmaceutics 2021, 13, 1291. [Google Scholar] [CrossRef]
- Kazemian, P.; Yu, S.-Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J.D. Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components. Mol. Pharm. 2022, 19, 1669–1686. [Google Scholar] [CrossRef]
- Li, C.; Yang, T.; Weng, Y.; Zhang, M.; Zhao, D.; Guo, S.; Hu, B.; Shao, W.; Wang, X.; Hussain, A.; et al. Ionizable Lipid-Assisted Efficient Hepatic Delivery of Gene Editing Elements for Oncotherapy. Bioact. Mater. 2022, 9, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Narain, R. The Effect of Polymer Architecture, Composition, and Molecular Weight on the Properties of Glycopolymer-Based Non-Viral Gene Delivery Systems. Biomaterials 2011, 32, 5279–5290. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Parveen, R.; Chatterji, B.P. Toxicology of Nanoparticles in Drug Delivery. Curr. Pathobiol. Rep. 2021, 9, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rajala, A.; Rajala, R. Lipid Nanoparticles for Ocular Gene Delivery. J. Funct. Biomater. 2015, 6, 379–394. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Jahan, I. Innovative Hybrid Nanostructures: Pioneering Advances in Modern Therapy. Front. Nanotechnol. 2024, 6, 1458894. [Google Scholar] [CrossRef]
- Li, W.; Liu, R.; Wang, Y.; Zhao, Y.; Gao, X. Temporal Techniques: Dynamic Tracking of Nanomaterials in Live Cells. Small 2013, 9, 1585–1594. [Google Scholar] [CrossRef]
- Parhamifar, L.; Moghimi, S.M. Total Internal Reflection Fluorescence (TIRF) Microscopy for Real-Time Imaging of Nanoparticle-Cell Plasma Membrane Interaction. In Nanoparticles in Biology and Medicine; Humana Press: Totowa, NJ, USA, 2012; pp. 473–482. [Google Scholar]
- Pu, R.; Zhan, Q.; Peng, X.; Liu, S.; Guo, X.; Liang, L.; Qin, X.; Zhao, Z.W.; Liu, X. Super-Resolution Microscopy Enabled by High-Efficiency Surface-Migration Emission Depletion. Nat. Commun. 2022, 13, 6636. [Google Scholar] [CrossRef]
- Archontakis, E.; Woythe, L.; van Hoof, B.; Albertazzi, L. Mapping the Relationship between Total and Functional Antibodies Conjugated to Nanoparticles with Spectrally-Resolved Direct Stochastic Optical Reconstruction Microscopy (SR-DSTORM). Nanoscale Adv. 2022, 4, 4402–4409. [Google Scholar] [CrossRef]
- Mehta, K.J. Iron Oxide Nanoparticles in Mesenchymal Stem Cell Detection and Therapy. Stem Cell Rev. Rep. 2022, 18, 2234–2261. [Google Scholar] [CrossRef]
- Stambuk, H. Targeted Silica Nanoparticles for Real-Time Image-Guided Intraoperative Mapping of Nodal Metastases. Available online: https://clinicaltrials.gov/study/NCT02106598 (accessed on 30 January 2025).
- Mastrogiacomo, S.; Dou, W.; Jansen, J.A.; Walboomers, X.F. Magnetic Resonance Imaging of Hard Tissues and Hard Tissue Engineered Bio-Substitutes. Mol. Imaging Biol. 2019, 21, 1003–1019. [Google Scholar] [CrossRef]
- Farinha, P.; Coelho, J.M.P.; Reis, C.P.; Gaspar, M.M. A Comprehensive Updated Review on Magnetic Nanoparticles in Diagnostics. Nanomaterials 2021, 11, 3432. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Yari Kalashgrani, M.; Omidifar, N.; Lai, C.W.; Vijayakameswara Rao, N.; Gholami, A.; Chiang, W.-H. The Pivotal Role of Quantum Dots-Based Biomarkers Integrated with Ultra-Sensitive Probes for Multiplex Detection of Human Viral Infections. Pharmaceuticals 2022, 15, 880. [Google Scholar] [CrossRef]
- Forte, E.; Fiorenza, D.; Torino, E.; Costagliola di Polidoro, A.; Cavaliere, C.; Netti, P.A.; Salvatore, M.; Aiello, M. Radiolabeled PET/MRI Nanoparticles for Tumor Imaging. J. Clin. Med. 2019, 9, 89. [Google Scholar] [CrossRef]
- Piperno, A.; Sciortino, M.T.; Giusto, E.; Montesi, M.; Panseri, S.; Scala, A. Recent Advances and Challenges in Gene Delivery Mediated by Polyester-Based Nanoparticles. Int. J. Nanomed. 2021, 16, 5981–6002. [Google Scholar] [CrossRef]
- Wei, H.; Bruns, O.T.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wiśniowska, A.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly Small Iron Oxide Nanoparticles as Positive MRI Contrast Agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef]
- Ahn, S.; Jung, S.; Lee, S. Gold Nanoparticle Contrast Agents in Advanced X-Ray Imaging Technologies. Molecules 2013, 18, 5858–5890. [Google Scholar] [CrossRef]
- Du, M.; Chen, Y.; Tu, J.; Liufu, C.; Yu, J.; Yuan, Z.; Gong, X.; Chen, Z. Ultrasound Responsive Magnetic Mesoporous Silica Nanoparticle-Loaded Microbubbles for Efficient Gene Delivery. ACS Biomater. Sci. Eng. 2020, 6, 2904–2912. [Google Scholar] [CrossRef]
- Albukhaty, S.; Sulaiman, G.M.; Al-Karagoly, H.; Mohammed, H.A.; Hassan, A.S.; Alshammari, A.A.A.; Ahmad, A.M.; Madhi, R.; Almalki, F.A.; Khashan, K.S.; et al. Iron Oxide Nanoparticles: The Versatility of the Magnetic and Functionalized Nanomaterials in Targeting Drugs, and Gene Deliveries with Effectual Magnetofection. J. Drug Deliv. Sci. Technol. 2024, 99, 105838. [Google Scholar] [CrossRef]
- Bauherr, S.; Larsberg, F.; Petrich, A.; Sperber, H.S.; Klose-Grzelka, V.; Luckner, M.; Azab, W.; Schade, M.; Höfer, C.T.; Lehmann, M.J.; et al. Macropinocytosis and Clathrin-Dependent Endocytosis Play Pivotal Roles for the Infectious Entry of Puumala Virus. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef]
- Rueda-Gensini, L.; Cifuentes, J.; Castellanos, M.C.; Puentes, P.R.; Serna, J.A.; Muñoz-Camargo, C.; Cruz, J.C. Tailoring Iron Oxide Nanoparticles for Efficient Cellular Internalization and Endosomal Escape. Nanomaterials 2020, 10, 1816. [Google Scholar] [CrossRef] [PubMed]
- Innes, E.; Yiu, H.H.P.; McLean, P.; Brown, W.; Boyles, M. Simulated Biological Fluids–A Systematic Review of Their Biological Relevance and Use in Relation to Inhalation Toxicology of Particles and Fibres. Crit. Rev. Toxicol. 2021, 51, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.J.; Kim, H.J.; Lim, S.B.; Naito, M.; Miyata, K. Recent Progress in the Endosomal Escape Mechanism and Chemical Structures of Polycations for Nucleic Acid Delivery. Macromol. Biosci. 2024, 24, 202300366. [Google Scholar] [CrossRef]
- Al-Deen, F.N.; Ho, J.; Selomulya, C.; Ma, C.; Coppel, R. Superparamagnetic Nanoparticles for Effective Delivery of Malaria DNA Vaccine. Langmuir 2011, 27, 3703–3712. [Google Scholar] [CrossRef]
- Lee, H.; Kim, I.-K.; Park, T.G. Intracellular Trafficking and Unpacking of SiRNA/Quantum Dot-PEI Complexes Modified with and without Cell Penetrating Peptide: Confocal and Flow Cytometric FRET Analysis. Bioconjug. Chem. 2010, 21, 289–295. [Google Scholar] [CrossRef]
- Song, W.; Du, J.; Sun, T.; Zhang, P.; Wang, J. Gold Nanoparticles Capped with Polyethyleneimine for Enhanced SiRNA Delivery. Small 2010, 6, 239–246. [Google Scholar] [CrossRef]
- Shirsat, S.D.; Londhe, P.V.; Gaikwad, A.P.; Rizwan, M.; Laha, S.S.; Khot, V.M.; Achal, V.; Tabish, T.A.; Thorat, N.D. Endosomal Escape in Magnetic Nanostructures: Recent Advances and Future Perspectives. Mater. Today Adv. 2024, 22, 100484. [Google Scholar] [CrossRef]
- Xu, X.; Koivisto, O.; Liu, C.; Zhou, J.; Miihkinen, M.; Jacquemet, G.; Wang, D.; Rosenholm, J.M.; Shu, Y.; Zhang, H. Effective Delivery of the CRISPR/Cas9 System Enabled by Functionalized Mesoporous Silica Nanoparticles for GFP-Tagged Paxillin Knock-In. Adv. Ther. 2021, 4, 2000072. [Google Scholar] [CrossRef]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in Gene Therapy: Current Status and Clinical Applications. J. Control. Release 2023, 362, 667–691. [Google Scholar] [CrossRef]
- Gao, J.-Q.; Zhao, Q.-Q.; Lv, T.-F.; Shuai, W.-P.; Zhou, J.; Tang, G.-P.; Liang, W.-Q.; Tabata, Y.; Hu, Y.-L. Gene-Carried Chitosan-Linked-PEI Induced High Gene Transfection Efficiency with Low Toxicity and Significant Tumor-Suppressive Activity. Int. J. Pharm. 2010, 387, 286–294. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, B. Polyethyleneimine-Based Drug Delivery Systems for Cancer Theranostics. J. Funct. Biomater. 2022, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Jerjes, W.; Theodossiou, T.A.; Hirschberg, H.; Høgset, A.; Weyergang, A.; Selbo, P.K.; Hamdoon, Z.; Hopper, C.; Berg, K. Photochemical Internalization for Intracellular Drug Delivery. From Basic Mechanisms to Clinical Research. J. Clin. Med. 2020, 9, 528. [Google Scholar] [CrossRef]
- Petrášek, Z.; Phillips, D. A Time-Resolved Study of Concentration Quenching of Disulfonated Aluminium Phthalocyanine Fluorescence. Photochem. Photobiol. Sci. 2003, 2, 236–244. [Google Scholar] [CrossRef]
- Shieh, M.-J.; Peng, C.-L.; Lou, P.-J.; Chiu, C.-H.; Tsai, T.-Y.; Hsu, C.-Y.; Yeh, C.-Y.; Lai, P.-S. Non-Toxic Phototriggered Gene Transfection by PAMAM-Porphyrin Conjugates. J. Control. Release 2008, 129, 200–206. [Google Scholar] [CrossRef]
- Tsolekile, N.; Ncapayi, V.; Obiyenwa, G.K.; Matoetoe, M.; Songca, S.; Oluwafemi, O.S. Synthesis of Meso-Tetra-(4-Sulfonatophenyl) Porphyrin (TPPS4)–CuInS/ZnS Quantum Dots Conjugate as an Improved Photosensitizer. Int. J. Nanomed. 2019, 14, 7065–7078. [Google Scholar] [CrossRef]
- Balukova, A.; Bokea, K.; Barber, P.R.; Ameer-Beg, S.M.; MacRobert, A.J.; Yaghini, E. Cellular Imaging and Time-Domain FLIM Studies of Meso-Tetraphenylporphine Disulfonate as a Photosensitising Agent in 2D and 3D Models. Int. J. Mol. Sci. 2024, 25, 4222. [Google Scholar] [CrossRef]
- Xie, X.; Yue, T.; Gu, W.; Cheng, W.; He, L.; Ren, W.; Li, F.; Piao, J.-G. Recent Advances in Mesoporous Silica Nanoparticles Delivering SiRNA for Cancer Treatment. Pharmaceutics 2023, 15, 2483. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, K.; Zhang, X.; Chao, Z.; Yang, Y.; Ye, D.; Dai, Z.; Liu, Y.; Ju, H. Photo-Tearable Tape Close-Wrapped Upconversion Nanocapsules for near-Infrared Modulated Efficient SiRNA Delivery and Therapy. Biomaterials 2018, 163, 55–66. [Google Scholar] [CrossRef]
- Jeong, E.H.; Ryu, J.H.; Jeong, H.; Jang, B.; Lee, H.Y.; Hong, S.; Lee, H.; Lee, H. Efficient Delivery of SiRNAs by a Photothermal Approach Using Plant Flavonoid-Inspired Gold Nanoshells. Chem. Commun. 2014, 50, 13388–13390. [Google Scholar] [CrossRef]
- Wunderbaldinger, P.; Josephson, L.; Weissleder, R. Tat Peptide Directs Enhanced Clearance and Hepatic Permeability of Magnetic Nanoparticles. Bioconjug. Chem. 2002, 13, 264–268. [Google Scholar] [CrossRef]
- Paez-Perez, M.; Russell, I.A.; Cicuta, P.; Di Michele, L. Modulating Membrane Fusion through the Design of Fusogenic DNA Circuits and Bilayer Composition. Soft Matter 2022, 18, 7035–7044. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, R.V.; Akimov, S.A.; Dashinimaev, E.B.; Bashkirov, P.V. Boosting Lipofection Efficiency Through Enhanced Membrane Fusion Mechanisms. Int. J. Mol. Sci. 2024, 25, 13540. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhang, Y.; Liu, N.; Wei, Q.; Yang, F.; Li, J. Stimulus-Responsive Nanodelivery and Release Systems for Cancer Gene Therapy: Efficacy Improvement Strategies. Int. J. Nanomed. 2024, 19, 7099–7121. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Hsu, H.-L.; Lan, Y.-H.; Chen, J.-P. Thermosensitive Cationic Magnetic Liposomes for Thermoresponsive Delivery of CPT-11 and SLP2 ShRNA in Glioblastoma Treatment. Pharmaceutics 2023, 15, 1169. [Google Scholar] [CrossRef]
- Lee, S.; Min, S.; Kim, G.; Lee, S. Recent Advances in the Design of Organic Photothermal Agents for Cancer Treatment: A Review. Coord. Chem. Rev. 2024, 506, 215719. [Google Scholar] [CrossRef]
- Strambio-De-Castillia, C.; Niepel, M.; Rout, M.P. The Nuclear Pore Complex: Bridging Nuclear Transport and Gene Regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 490–501. [Google Scholar] [CrossRef]
- Nie, Y.; Fu, G.; Leng, Y. Nuclear Delivery of Nanoparticle-Based Drug Delivery Systems by Nuclear Localization Signals. Cells 2023, 12, 1637. [Google Scholar] [CrossRef]
- Oka, M.; Yoneda, Y. Importin α: Functions as a Nuclear Transport Factor and Beyond. Proc. Jpn. Acad. Ser. B 2018, 94, 259–274. [Google Scholar] [CrossRef]
- Palmeri, D.; Malim, M.H. Importin β Can Mediate the Nuclear Import of an Arginine-Rich Nuclear Localization Signal in the Absence of Importin α. Mol. Cell Biol. 1999, 19, 1218–1225. [Google Scholar] [CrossRef]
- Lu, J.; Wu, T.; Zhang, B.; Liu, S.; Song, W.; Qiao, J.; Ruan, H. Types of Nuclear Localization Signals and Mechanisms of Protein Import into the Nucleus. Cell Commun. Signal. 2021, 19, 60. [Google Scholar] [CrossRef]
- Peng, L.-H.; Niu, J.; Zhang, C.-Z.; Yu, W.; Wu, J.-H.; Shan, Y.-H.; Wang, X.-R.; Shen, Y.-Q.; Mao, Z.-W.; Liang, W.-Q.; et al. TAT Conjugated Cationic Noble Metal Nanoparticles for Gene Delivery to Epidermal Stem Cells. Biomaterials 2014, 35, 5605–5618. [Google Scholar] [CrossRef]
- Vankayala, R.; Kuo, C.; Nuthalapati, K.; Chiang, C.; Hwang, K.C. Nucleus-Targeting Gold Nanoclusters for Simultaneous In Vivo Fluorescence Imaging, Gene Delivery, and NIR-Light Activated Photodynamic Therapy. Adv. Funct. Mater. 2015, 25, 5934–5945. [Google Scholar] [CrossRef]
- Raposo, C.D.; Canelas, A.B.; Barros, M.T. Human Lectins, Their Carbohydrate Affinities and Where to Find Them. Biomolecules 2021, 11, 188. [Google Scholar] [CrossRef]
- Chettri, D.; Boro, M.; Sarkar, L.; Verma, A.K. Lectins: Biological Significance to Biotechnological Application. Carbohydr. Res. 2021, 506, 108367. [Google Scholar] [CrossRef]
- Tammam, S.N.; Azzazy, H.M.E.; Lamprecht, A. How Successful Is Nuclear Targeting by Nanocarriers? J. Control. Release 2016, 229, 140–153. [Google Scholar] [CrossRef]
- Ahmed, M.; Deng, Z.; Liu, S.; Lafrenie, R.; Kumar, A.; Narain, R. Cationic Glyconanoparticles: Their Complexation with DNA, Cellular Uptake, and Transfection Efficiencies. Bioconjug. Chem. 2009, 20, 2169–2176. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; He, Y.; Li, Y. Sandwich-Type Au-PEI/DNA/PEI-Dexa Nanocomplex for Nucleus-Targeted Gene Delivery in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2014, 6, 14196–14206. [Google Scholar] [CrossRef]
- Shahbazi, R.; Ozcicek, I.; Ozturk, G.; Ulubayram, K. Functionalized Gold Nanoparticles Manifested as Potent Carriers for Nucleolar Targeting. Nanotechnology 2017, 28, 025103. [Google Scholar] [CrossRef]
- Knipe, J.M.; Peters, J.T.; Peppas, N.A. Theranostic Agents for Intracellular Gene Delivery with Spatiotemporal Imaging. Nano Today 2013, 8, 21–38. [Google Scholar] [CrossRef]
- Muhammad, K.; Zhao, J.; Ullah, I.; Guo, J.; Ren, X.; Feng, Y. Ligand Targeting and Peptide Functionalized Polymers as Non-Viral Carriers for Gene Therapy. Biomater. Sci. 2020, 8, 64–83. [Google Scholar] [CrossRef]
- Yao, J.; Fan, Y.; Li, Y.; Huang, L. Strategies on the Nuclear-Targeted Delivery of Genes. J. Drug Target. 2013, 21, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Shi, P.; Cheng, Z.; Zhao, K.; Chen, Y.; Zhang, A.; Gan, W.; Zhang, Y. Active Targeting Schemes for Nano-Drug Delivery Systems in Osteosarcoma Therapeutics. J. Nanobiotechnol. 2023, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Sega, E.I.; Low, P.S. Tumor Detection Using Folate Receptor-Targeted Imaging Agents. Cancer Metastasis Rev. 2008, 27, 655–664. [Google Scholar] [CrossRef]
- Du, B.; Gu, X.; Han, X.; Ding, G.; Wang, Y.; Li, D.; Wang, E.; Wang, J. Lipid-Coated Gold Nanoparticles Functionalized by Folic Acid as Gene Vectors for Targeted Gene Delivery in Vitro and in Vivo. ChemMedChem 2017, 12, 1768–1775. [Google Scholar] [CrossRef]
- Pan, X.; Guan, J.; Yoo, J.-W.; Epstein, A.J.; Lee, L.J.; Lee, R.J. Cationic Lipid-Coated Magnetic Nanoparticles Associated with Transferrin for Gene Delivery. Int. J. Pharm. 2008, 358, 263–270. [Google Scholar] [CrossRef]
- Kotcherlakota, R.; Vydiam, K.; Jeyalakshmi Srinivasan, D.; Mukherjee, S.; Roy, A.; Kuncha, M.; Rao, T.N.; Sistla, R.; Gopal, V.; Patra, C.R. Restoration of P53 Function in Ovarian Cancer Mediated by Gold Nanoparticle-Based EGFR Targeted Gene Delivery System. ACS Biomater. Sci. Eng. 2019, 5, 3631–3644. [Google Scholar] [CrossRef]
- Liao, S.; Wu, G.; Xie, Z.; Lei, X.; Yang, X.; Huang, S.; Deng, X.; Wang, Z.; Tang, G. PH Regulators and Their Inhibitors in Tumor Microenvironment. Eur. J. Med. Chem. 2024, 267, 116170. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Chen, Z.; Geng, Y.; Xie, X.; Shen, X.; Li, T.; Li, S.; Wu, C.; Liu, Y. Chemo-Photodynamic Combined Gene Therapy and Dual-Modal Cancer Imaging Achieved by PH-Responsive Alginate/Chitosan Multilayer-Modified Magnetic Mesoporous Silica Nanocomposites. Biomater. Sci. 2017, 5, 1001–1013. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic Efficacy of Nanoparticles and Routes of Administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Wijagkanalan, W. Glycosylated Carriers for Cell-Selective and Nuclear Delivery of Nucleic Acids. Front. Biosci. 2011, 16, 2970. [Google Scholar] [CrossRef] [PubMed]
- Sadauskas, E.; Wallin, H.; Stoltenberg, M.; Vogel, U.; Doering, P.; Larsen, A.; Danscher, G. Kupffer Cells Are Central in the Removal of Nanoparticles from the Organism. Part. Fibre Toxicol. 2007, 4, 10. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, L. Passive Lung-Targeted Drug Delivery Systems via Intravenous Administration. Pharm. Dev. Technol. 2014, 19, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H. Selective Organ Targeting Nanoparticles: From Design to Clinical Translation. Nanoscale Horiz. 2023, 8, 1155–1173. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on In Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing Factors and Strategies of Enhancing Nanoparticles into Tumors in Vivo. Acta Pharm. Sin. B 2021, 11, 2265–2285. [Google Scholar] [CrossRef]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-Targeted Nanomedicine for the Diagnosis and Treatment of Atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef]
- Kasuya, T.; Kuroda, S. Nanoparticles for Human Liver-Specific Drug and Gene Delivery Systems: In Vitro and in Vivo Advances. Expert. Opin. Drug Deliv. 2009, 6, 39–52. [Google Scholar] [CrossRef]
- Varshosaz, J. Nanoparticles for Targeted Delivery of Therapeutics and Small Interfering RNAs in Hepatocellular Carcinoma. World J. Gastroenterol. 2015, 21, 12022. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, S.; Tong, L.; Li, J.; Chen, F.; Han, Y.; Zhao, M.; Xiong, W. Superparamagnetic Iron Oxide Nanoparticles Mediated 131I-HVEGF SiRNA Inhibits Hepatocellular Carcinoma Tumor Growth in Nude Mice. BMC Cancer 2014, 14, 114. [Google Scholar] [CrossRef]
- Wu, C.; Gong, F.; Pang, P.; Shen, M.; Zhu, K.; Cheng, D.; Liu, Z.; Shan, H. An RGD-Modified MRI-Visible Polymeric Vector for Targeted SiRNA Delivery to Hepatocellular Carcinoma in Nude Mice. PLoS ONE 2013, 8, e66416. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Padilla, D.; Durfee, P.N.; Brown, P.A.; Hanna, T.N.; Liu, J.; Phillips, B.; Carter, M.B.; et al. The Targeted Delivery of Multicomponent Cargos to Cancer Cells by Nanoporous Particle-Supported Lipid Bilayers. Nat. Mater. 2011, 10, 389–397. [Google Scholar] [CrossRef]
- Karlsson, J.; Luly, K.M.; Tzeng, S.Y.; Green, J.J. Nanoparticle Designs for Delivery of Nucleic Acid Therapeutics as Brain Cancer Therapies. Adv. Drug Deliv. Rev. 2021, 179, 113999. [Google Scholar] [CrossRef]
- Grafals-Ruiz, N.; Rios-Vicil, C.I.; Lozada-Delgado, E.L.; Quiñones-Díaz, B.I.; Noriega-Rivera, R.A.; Martínez-Zayas, G.; Santana-Rivera, Y.; Santiago-Sánchez, G.S.; Valiyeva, F.; Vivas-Mejía, P.E. Brain targeted gold liposomes improve RNAi delivery for glioblastoma. Int. J. Nanomed. 2020, 15, 2809–2828. [Google Scholar] [CrossRef]
- Jung, J.; Solanki, A.; Memoli, K.A.; Kamei, K.; Kim, H.; Drahl, M.A.; Williams, L.J.; Tseng, H.; Lee, K. Selective Inhibition of Human Brain Tumor Cells through Multifunctional Quantum-Dot-Based SiRNA Delivery. Angew. Chem. Int. Ed. 2010, 49, 103–107. [Google Scholar] [CrossRef]
- Kubczak, M.; Michlewska, S.; Bryszewska, M.; Aigner, A.; Ionov, M. Nanoparticles for Local Delivery of SiRNA in Lung Therapy. Adv. Drug Deliv. Rev. 2021, 179, 114038. [Google Scholar] [CrossRef]
- Taratula, O.; Garbuzenko, O.B.; Chen, A.M.; Minko, T. Innovative Strategy for Treatment of Lung Cancer: Targeted Nanotechnology-Based Inhalation Co-Delivery of Anticancer Drugs and SiRNA. J. Drug Target. 2011, 19, 900–914. [Google Scholar] [CrossRef]
- Li, D.; Son, Y.; Jang, M.; Wang, S.; Zhu, W. Nanoparticle Based Cardiac Specific Drug Delivery. Biology 2023, 12, 82. [Google Scholar] [CrossRef]
- Bondue, T.; van den Heuvel, L.; Levtchenko, E.; Brock, R. The Potential of RNA-Based Therapy for Kidney Diseases. Pediatr. Nephrol. 2023, 38, 327–344. [Google Scholar] [CrossRef]
- Chen, L.; Hong, W.; Ren, W.; Xu, T.; Qian, Z.; He, Z. Recent Progress in Targeted Delivery Vectors Based on Biomimetic Nanoparticles. Signal Transduct. Target. Ther. 2021, 6, 225. [Google Scholar] [CrossRef]
- Zhuo, C.; Zhang, J.; Lee, J.-H.; Jiao, J.; Cheng, D.; Liu, L.; Kim, H.-W.; Tao, Y.; Li, M. Spatiotemporal Control of CRISPR/Cas9 Gene Editing. Signal Transduct. Target. Ther. 2021, 6, 238. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, P.K.; Behera, S.; Lockey, R.F.; Mohapatra, S.; Mohapatra, S. Multifunctional Magnetic Nanoparticles for Targeted Delivery. Nanomedicine 2010, 6, 64–69. [Google Scholar] [CrossRef]
- Yang, W.; Xia, B.; Wang, L.; Ma, S.; Liang, H.; Wang, D.; Huang, J. Shape Effects of Gold Nanoparticles in Photothermal Cancer Therapy. Mater. Today Sustain. 2021, 13, 100078. [Google Scholar] [CrossRef]
- Jia, X.; Lv, M.; Fei, Y.; Dong, Q.; Wang, H.; Liu, Q.; Li, D.; Wang, J.; Wang, E. Facile One-Step Synthesis of NIR-Responsive SiRNA-Inorganic Hybrid Nanoplatform for Imaging-Guided Photothermal and Gene Synergistic Therapy. Biomaterials 2022, 282, 121404. [Google Scholar] [CrossRef]
- Chen, G.; Ma, B.; Xie, R.; Wang, Y.; Dou, K.; Gong, S. NIR-Induced Spatiotemporally Controlled Gene Silencing by Upconversion Nanoparticle-Based SiRNA Nanocarrier. J. Control. Release 2018, 282, 148–155. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, F.; Liu, X.; Xing, B. NIR Light Controlled Photorelease of SiRNA and Its Targeted Intracellular Delivery Based on Upconversion Nanoparticles. Nanoscale 2013, 5, 231–238. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, J.; Luan, X.; Liu, X.; Li, X.; Yang, J.; Huang, T.; Sun, L.; Wang, Y.; Lin, Y.; et al. Near-Infrared Upconversion–Activated CRISPR-Cas9 System: A Remote-Controlled Gene Editing Platform. Sci. Adv. 2019, 5, eaav7199. [Google Scholar] [CrossRef]
- Liao, H.; Wang, S.; Wang, X.; Dai, D.Z.; Zhang, Y.; Zhu, C.; Li, J. Imaging-Assisted Antisense Oligonucleotide Delivery for Tumor-Targeted Gene Therapy. Chem. Biomed. Imaging 2024, 2, 313–330. [Google Scholar] [CrossRef]
- Chen, L.; Li, G.; Wang, X.; Li, J.; Zhang, Y. Spherical Nucleic Acids for Near-Infrared Light-Responsive Self-Delivery of Small-Interfering RNA and Antisense Oligonucleotide. ACS Nano 2021, 15, 11929–11939. [Google Scholar] [CrossRef]
- Kaushik, A.; Yndart, A.; Atluri, V.; Tiwari, S.; Tomitaka, A.; Gupta, P.; Jayant, R.D.; Alvarez-Carbonell, D.; Khalili, K.; Nair, M. Magnetically Guided Non-Invasive CRISPR-Cas9/GRNA Delivery across Blood-Brain Barrier to Eradicate Latent HIV-1 Infection. Sci. Rep. 2019, 9, 3928. [Google Scholar] [CrossRef]
- Kofoed, R.H.; Aubert, I. Focused Ultrasound Gene Delivery for the Treatment of Neurological Disorders. Trends Mol. Med. 2024, 30, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Bruhn, M.; de Ávila, B.E.; Beltrán-Gastélum, M.; Zhao, J.; Ramírez-Herrera, D.E.; Angsantikul, P.; Vesterager Gothelf, K.; Zhang, L.; Wang, J. Active Intracellular Delivery of a Cas9/SgRNA Complex Using Ultrasound-Propelled Nanomotors. Angew. Chem. Int. Ed. 2018, 57, 2657–2661. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.L.; Zieba, J.K.; Li, X.; Campbell, D.B.; Williams, M.R.; Vogt, D.L.; Bupp, C.P.; Edgerly, Y.M.; Rajasekaran, S.; Hartog, N.L.; et al. Gene Therapy for Genetic Syndromes: Understanding the Current State to Guide Future Care. BioTech 2024, 13, 1. [Google Scholar] [CrossRef]
- Bowling, K.M.; Thompson, M.L.; Finnila, C.R.; Hiatt, S.M.; Latner, D.R.; Amaral, M.D.; Lawlor, J.M.J.; East, K.M.; Cochran, M.E.; Greve, V.; et al. Genome Sequencing as a First-Line Diagnostic Test for Hospitalized Infants. Genet. Med. 2022, 24, 851–861. [Google Scholar] [CrossRef]
- Burkett, B.J.; Bartlett, D.J.; McGarrah, P.W.; Lewis, A.R.; Johnson, D.R.; Berberoğlu, K.; Pandey, M.K.; Packard, A.T.; Halfdanarson, T.R.; Hruska, C.B.; et al. A Review of Theranostics: Perspectives on Emerging Approaches and Clinical Advancements. Radiol. Imaging Cancer 2023, 5, e220157. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Sivakumar, A.S.; Lee, C.-H.; Kim, S.J. Diabetes Mellitus and Diabetic Foot Ulcer: Etiology, Biochemical and Molecular Based Treatment Strategies via Gene and Nanotherapy. Biomed. Pharmacother. 2022, 151, 113134. [Google Scholar] [CrossRef]
- Cesur-Ergün, B.; Demir-Dora, D. Gene Therapy in Cancer. J. Gene Med. 2023, 25, e3550. [Google Scholar] [CrossRef]
- Raghuraman, A.; Lawrence, R.; Shetty, R.; Avanthika, C.; Jhaveri, S.; Pichardo, B.V.; Mujakari, A. Role of Gene Therapy in Sickle Cell Disease. Dis. A Mon. 2024, 70, 101689. [Google Scholar] [CrossRef]
- Cring, M.R.; Sheffield, V.C. Gene Therapy and Gene Correction: Targets, Progress, and Challenges for Treating Human Diseases. Gene Ther. 2022, 29, 3–12. [Google Scholar] [CrossRef]
- Kumar, K.R.; Cowley, M.J.; Davis, R.L. Next-Generation Sequencing and Emerging Technologies. Semin. Thromb. Hemost. 2019, 45, 661–673. [Google Scholar] [CrossRef]
- Markert, M.L.; Gupton, S.E.; McCarthy, E.A. Experience with Cultured Thymus Tissue in 105 Children. J. Allergy Clin. Immunol. 2022, 149, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wu, D.M.; Xue, Y.; Wang, S.K.; Chung, M.J.; Ji, X.; Rana, P.; Zhao, S.R.; Mai, S.; Cepko, C.L. AAV Cis-Regulatory Sequences Are Correlated with Ocular Toxicity. Proc. Natl. Acad. Sci. USA 2019, 116, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Flotte, T.R. Moving Forward After Two Deaths in a Gene Therapy Trial of Myotubular Myopathy. Hum. Gene Ther. 2020, 31, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Hulot, J.-S.; Ishikawa, K.; Hajjar, R.J. Gene Therapy for the Treatment of Heart Failure: Promise Postponed. Eur. Heart J. 2016, 37, 1651–1658. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Reimbursement Team. Idecabtagene Vicleucel (Abecma). Can. J. Health Technol. 2021, 1. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T-Cell Therapy in Patients with Relapsed or Refractory Multiple Myeloma (CARTITUDE-1): A Phase 1b/2 Open-Label Study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Chesney, J.; Lewis, K.D.; Kluger, H.; Hamid, O.; Whitman, E.; Thomas, S.; Wermke, M.; Cusnir, M.; Domingo-Musibay, E.; Phan, G.Q.; et al. Efficacy and Safety of Lifileucel, a One-Time Autologous Tumor-Infiltrating Lymphocyte (TIL) Cell Therapy, in Patients with Advanced Melanoma after Progression on Immune Checkpoint Inhibitors and Targeted Therapies: Pooled Analysis of Consecutive Cohorts of the C-144-01 Study. J. Immunother. Cancer 2022, 10, e005755. [Google Scholar] [CrossRef]
- de Groot, P.M.; Arevalo, O.; Shah, K.; Strange, C.D.; Shroff, G.S.; Ahuja, J.; Truong, M.T.; de Groot, J.F.; Vlahos, I. Imaging Primer on Chimeric Antigen Receptor T-Cell Therapy for Radiologists. RadioGraphics 2022, 42, 176–194. [Google Scholar] [CrossRef]
- Viardot, A. Perspektiven Der CAR-T-Zell-Therapie. Pathologe 2020, 41, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Lee, S.M.; Doger de Spéville, B.; Gettinger, S.N.; Häfliger, S.; Sukari, A.; Papa, S.; Rodríguez-Moreno, J.F.; Graf Finckenstein, F.; Fiaz, R.; et al. Lifileucel, an Autologous Tumor-Infiltrating Lymphocyte Monotherapy, in Patients with Advanced Non–Small Cell Lung Cancer Resistant to Immune Checkpoint Inhibitors. Cancer Discov. 2024, 14, 1389–1402. [Google Scholar] [CrossRef]
- Dodero, A.; Bramanti, S.; Di Trani, M.; Pennisi, M.; Ljevar, S.; Chiappella, A.; Massimo, M.; Guidetti, A.; Corrado, F.; Nierychlewska, P.M.; et al. Outcome after Chimeric Antigen Receptor (CAR) T-cell Therapy Failure in Large B-cell Lymphomas. Br. J. Haematol. 2024, 204, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.; Wang, M.; Arnason, J.; Purev, E.; Maloney, D.G.; Andreadis, C.; Sehgal, A.; et al. Two-Year Follow-up of Lisocabtagene Maraleucel in Relapsed or Refractory Large B-Cell Lymphoma in TRANSCEND NHL 001. Blood 2024, 143, 404–416. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Shah, N.N.; Fry, T.J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019, 16, 372–385. [Google Scholar] [CrossRef]
- Sehgal, A.; Hoda, D.; Riedell, P.A.; Ghosh, N.; Hamadani, M.; Hildebrandt, G.C.; Godwin, J.E.; Reagan, P.M.; Wagner-Johnston, N.; Essell, J.; et al. Lisocabtagene Maraleucel as Second-Line Therapy in Adults with Relapsed or Refractory Large B-Cell Lymphoma Who Were Not Intended for Haematopoietic Stem Cell Transplantation (PILOT): An Open-Label, Phase 2 Study. Lancet Oncol. 2022, 23, 1066–1077. [Google Scholar] [CrossRef]
- Wagner, M.; Lunt, C.; Araujo, D.; Blay, J.-Y.; Druta, M.; Attia, S.; Morales, C.M.V.; Ganjoo, K.; Schuetze, S.M.; Van Winkle, E.; et al. 54O Health State Impacts of Afamitresgene Autoleucel (Afami-Cel) in Advanced Synovial Sarcoma and Myxoid/Round Cell Liposarcoma: SPEARHEAD-1 Cohort 1. ESMO Open 2024, 9, 102444. [Google Scholar] [CrossRef]
- Singh, N. Approval of the First TCR-Based Cell Therapy. Mol. Ther. 2024, 32, 3195. [Google Scholar] [CrossRef]
- Hong, D.S.; Van Tine, B.A.; Biswas, S.; McAlpine, C.; Johnson, M.L.; Olszanski, A.J.; Clarke, J.M.; Araujo, D.; Blumenschein, G.R.; Kebriaei, P.; et al. Autologous T Cell Therapy for MAGE-A4+ Solid Cancers in HLA-A*02+ Patients: A Phase 1 Trial. Nat. Med. 2023, 29, 104–114. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Araujo, D.M.; Abdul Razak, A.R.; Agulnik, M.; Attia, S.; Blay, J.-Y.; Carrasco Garcia, I.; Charlson, J.A.; Choy, E.; Demetri, G.D.; et al. Afamitresgene Autoleucel for Advanced Synovial Sarcoma and Myxoid Round Cell Liposarcoma (SPEARHEAD-1): An International, Open-Label, Phase 2 Trial. Lancet 2024, 403, 1460–1471. [Google Scholar] [CrossRef]
- Yoon, J.G.; Smith, D.A.; Tirumani, S.H.; Caimi, P.F.; Ramaiya, N.H. CAR T-Cell Therapy: An Update for Radiologists. Am. J. Roentgenol. 2021, 217, 1461–1474. [Google Scholar] [CrossRef] [PubMed]
- Engeland, K. Cell Cycle Regulation: P53-P21-RB Signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the Complexity of P53 in a New Era of Tumor Suppression. Cancer Cell 2024, 42, 946–967. [Google Scholar] [CrossRef]
- Qi, L.; Li, G.; Li, P.; Wang, H.; Fang, X.; He, T.; Li, J. Twenty Years of Gendicine® RAd-P53 Cancer Gene Therapy: The First-in-Class Human Cancer Gene Therapy in the Era of Personalized Oncology. Genes Dis. 2024, 11, 101155. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ullah, N.; Zhang, Z.; Zhen, Y.; Din, A.-U.; Cui, H.; Wang, M. Recent Insights into the Functions and Mechanisms of Antisense RNA: Emerging Applications in Cancer Therapy and Precision Medicine. Front. Chem. 2024, 11, 1335330. [Google Scholar] [CrossRef]
- Steger, C.M.; Bonaros, N.; Rieker, R.J.; Bonatti, J.; Schachner, T. Gene Therapy with Antisense Oligonucleotides Silencing C-Myc Reduces Neointima Formation and Vessel Wall Thickness in a Mouse Model of Vein Graft Disease. Exp. Mol. Pathol. 2018, 105, 1–9. [Google Scholar] [CrossRef]
- Zhao, B.S.; Roundtree, I.A.; He, C. Post-Transcriptional Gene Regulation by MRNA Modifications. Nat. Rev. Mol. Cell Biol. 2017, 18, 31–42. [Google Scholar] [CrossRef]
- Zahran, S.S.; Ragab, F.A.; Soliman, A.M.; El-Gazzar, M.G.; Mahmoud, W.R.; Ghorab, M.M. Utility of Sulfachloropyridazine in the Synthesis of Novel Anticancer Agents as Antiangiogenic and Apoptotic Inducers. Bioorg. Chem. 2024, 148, 107411. [Google Scholar] [CrossRef]
- Wei, T.; Cheng, J.; Ji, Y.; Cao, X.; Ding, S.; Liu, Q.; Wang, Z. Baculovirus-Mediated Endostatin and Angiostatin Activation of Autophagy through the AMPK/AKT/MTOR Pathway Inhibits Angiogenesis in Hepatocellular Carcinoma. Open Life Sci. 2024, 19, 20220914. [Google Scholar] [CrossRef]
- Taghizadeh-Tabarsi, R.; Akbari-Birgani, S.; Amjadi, M.; Mohammadi, S.; Nikfarjam, N.; Kusamori, K. Aptamer-Guided Graphene Oxide Quantum Dots for Targeted Suicide Gene Therapy in an Organoid Model of Luminal Breast Cancer. Sci. Rep. 2024, 14, 24104. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Su, W.; Liu, Z.; Zhou, M.; Chen, S.; Chen, Y.; Lu, D.; Liu, Y.; Fan, Y.; Zheng, Y.; et al. CD44 Antibody-Targeted Liposomal Nanoparticles for Molecular Imaging and Therapy of Hepatocellular Carcinoma. Biomaterials 2012, 33, 5107–5114. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Sun, Z.; Wang, B.; Liu, X.; Hu, B.; Chen, N.; Zhang, S.; Yu, Z. Suicide Gene Delivery by Morphology-Adaptable Enantiomeric Peptide Assemblies for Combined Ovarian Cancer Therapy. Acta Biomater. 2024, 175, 250–261. [Google Scholar] [CrossRef]
- Kang, S.J.; Jeong, H.Y.; Kim, M.W.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Park, Y.S. Anti-EGFR Lipid Micellar Nanoparticles Co-Encapsulating Quantum Dots and Paclitaxel for Tumor-Targeted Theranosis. Nanoscale 2018, 10, 19338–19350. [Google Scholar] [CrossRef]
- Szymaszek, P.; Tyszka-Czochara, M.; Ortyl, J. Application of Photoactive Compounds in Cancer Theranostics: Review on Recent Trends from Photoactive Chemistry to Artificial Intelligence. Molecules 2024, 29, 3164. [Google Scholar] [CrossRef]
- Kadkhoda, J.; Tarighatnia, A.; Barar, J.; Aghanejad, A.; Davaran, S. Recent Advances and Trends in Nanoparticles Based Photothermal and Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2022, 37, 102697. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Ayan, S.; Gunaydin, G.; Yesilgul-Mehmetcik, N.; Gedik, M.E.; Seven, O.; Akkaya, E.U. Proof-of-Principle for Two-Stage Photodynamic Therapy: Hypoxia Triggered Release of Singlet Oxygen. Chem. Commun. 2020, 56, 14793–14796. [Google Scholar] [CrossRef]
- Gündüz, E.Ö.; Gedik, M.E.; Günaydın, G.; Okutan, E. Amphiphilic Fullerene-BODIPY Photosensitizers for Targeted Photodynamic Therapy. ChemMedChem 2022, 17, e202100693. [Google Scholar] [CrossRef]
- Wang, X.; Maswikiti, E.P.; Zhu, J.-Y.; Ma, Y.; Zheng, P.; Yu, Y.; Wang, B.; Gao, L.; Chen, H. Photodynamic Therapy Combined with Immunotherapy for an Advanced Esophageal Cancer with an Obstruction Post Metal Stent Implantation: A Case Report and Literature Review. Photodiagn. Photodyn. Ther. 2022, 37, 102671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yee, D.; Wang, C. Quantum Dots for Cancer Diagnosis and Therapy: Biological and Clinical Perspectives. Nanomedicine 2008, 3, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Arkin, G.; Zeng, W.; Huang, Y.; Su, L.; Guo, F.; Ye, J.; Wen, G.; Xu, J.; Liu, Y. Ultrasound Image-Guided Cancer Gene Therapy Using IRGD Dual-Targeted Magnetic Cationic Microbubbles. Biomed. Pharmacother. 2024, 172, 116221. [Google Scholar] [CrossRef]
- Neufeld, M.J.; Lutzke, A.; Pratx, G.; Sun, C. High-Z Metal–Organic Frameworks for X-ray Radiation-Based Cancer Theranostics. Chem. A Eur. J. 2021, 27, 3229–3237. [Google Scholar] [CrossRef]
- Gong, T.; Li, Y.; Lv, B.; Wang, H.; Liu, Y.; Yang, W.; Wu, Y.; Jiang, X.; Gao, H.; Zheng, X.; et al. Full-Process Radiosensitization Based on Nanoscale Metal–Organic Frameworks. ACS Nano 2020, 14, 3032–3040. [Google Scholar] [CrossRef]
- Richardson, J.; Bowtell, R.; Mader, K.; Melia, C. Pharmaceutical Applications of Magnetic Resonance Imaging (MRI). Adv. Drug Deliv. Rev. 2005, 57, 1191–1209. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, F.; Davies, G.; Williams, G.R. Theranostics for MRI-guided Therapy: Recent Developments. VIEW 2022, 3, 20200134. [Google Scholar] [CrossRef]
- Chen, W.-H.; Luo, G.-F.; Lei, Q.; Cao, F.-Y.; Fan, J.-X.; Qiu, W.-X.; Jia, H.-Z.; Hong, S.; Fang, F.; Zeng, X.; et al. Rational Design of Multifunctional Magnetic Mesoporous Silica Nanoparticle for Tumor-Targeted Magnetic Resonance Imaging and Precise Therapy. Biomaterials 2016, 76, 87–101. [Google Scholar] [CrossRef]
- Li, L.; Yang, W.-W.; Xu, D.-G. Stimuli-Responsive Nanoscale Drug Delivery Systems for Cancer Therapy. J. Drug Target. 2019, 27, 423–433. [Google Scholar] [CrossRef]
- Harilal, S.; Jose, J.; Parambi, D.G.T.; Kumar, R.; Unnikrishnan, M.K.; Uddin, M.S.; Mathew, G.E.; Pratap, R.; Marathakam, A.; Mathew, B. Revisiting the Blood-Brain Barrier: A Hard Nut to Crack in the Transportation of Drug Molecules. Brain Res. Bull. 2020, 160, 121–140. [Google Scholar] [CrossRef]
- Awuah, W.A.; Ahluwalia, A.; Tan, J.K.; Sanker, V.; Roy, S.; Ben-Jaafar, A.; Shah, D.M.; Tenkorang, P.O.; Aderinto, N.; Abdul-Rahman, T.; et al. Theranostics Advances in the Treatment and Diagnosis of Neurological and Neurosurgical Diseases. Arch. Med. Res. 2025, 56, 103085. [Google Scholar] [CrossRef]
- Artusi, S.; Miyagawa, Y.; Goins, W.F.; Cohen, J.B.; Glorioso, J.C. Herpes Simplex Virus Vectors for Gene Transfer to the Central Nervous System. Diseases 2018, 6, 74. [Google Scholar] [CrossRef]
- Teschemacher, A.G.; Wang, S.; Lonergan, T.; Duale, H.; Waki, H.; Paton, J.F.R.; Kasparov, S. Targeting Specific Neuronal Populations Using Adeno- and Lentiviral Vectors: Applications for Imaging and Studies of Cell Function. Exp. Physiol. 2005, 90, 61–69. [Google Scholar] [CrossRef]
- Bitetti, I.; Lanzara, V.; Margiotta, G.; Varone, A. Onasemnogene Abeparvovec Gene Replacement Therapy for the Treatment of Spinal Muscular Atrophy: A Real-World Observational Study. Gene Ther. 2023, 30, 592–597. [Google Scholar] [CrossRef]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L.; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, I.J.; Park, S.W.; Kim, Y.B.; Ko, Y.; Kim, S.U. Human Neural Stem Cells Genetically Modified to Express Human Nerve Growth Factor (NGF) Gene Restore Cognition in the Mouse with Ibotenic Acid-Induced Cognitive Dysfunction. Cell Transpl. 2012, 21, 2487–2496. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.-A. Viral Vector-Mediated Transgenic Cell Therapy in Regenerative Medicine: Safety of the Process. Expert. Opin. Biol. Ther. 2015, 15, 559–567. [Google Scholar] [CrossRef]
- Guzmán-Sastoque, P.; Sotelo, S.; Esmeral, N.P.; Albarracín, S.L.; Sutachan, J.-J.; Reyes, L.H.; Muñoz-Camargo, C.; Cruz, J.C.; Bloch, N.I. Assessment of CRISPRa-Mediated Gdnf Overexpression in an In Vitro Parkinson’s Disease Model. Front. Bioeng. Biotechnol. 2024, 12, 1420183. [Google Scholar] [CrossRef]
- Kordower, J.H.; Herzog, C.D.; Dass, B.; Bakay, R.A.E.; Stansell, J.; Gasmi, M.; Bartus, R.T. Delivery of Neurturin by AAV2 (CERE-120)-mediated Gene Transfer Provides Structural and Functional Neuroprotection and Neurorestoration in MPTP-treated Monkeys. Ann. Neurol. 2006, 60, 706–715. [Google Scholar] [CrossRef]
- Zuo, F.-X.; Bao, X.-J.; Sun, X.-C.; Wu, J.; Bai, Q.-R.; Chen, G.; Li, X.-Y.; Zhou, Q.-Y.; Yang, Y.-F.; Shen, Q.; et al. Transplantation of Human Neural Stem Cells in a Parkinsonian Model Exerts Neuroprotection via Regulation of the Host Microenvironment. Int. J. Mol. Sci. 2015, 16, 26473–26492. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; McHugh, J.; Tork, C.; Shelley, B.; Hayes, A.; Bellantuono, I.; Aebischer, P.; Svendsen, C.N. Direct Muscle Delivery of GDNF With Human Mesenchymal Stem Cells Improves Motor Neuron Survival and Function in a Rat Model of Familial ALS. Mol. Ther. 2008, 16, 2002–2010. [Google Scholar] [CrossRef]
- Lieb, A.; Qiu, Y.; Dixon, C.L.; Heller, J.P.; Walker, M.C.; Schorge, S.; Kullmann, D.M. Biochemical Autoregulatory Gene Therapy for Focal Epilepsy. Nat. Med. 2018, 24, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.D.; Deng, P.; Torrest, A.; Stewart, H.; Pollock, K.; Gruenloh, W.; Annett, G.; Tempkin, T.; Wheelock, V.; Nolta, J.A. Developing Stem Cell Therapies for Juvenile and Adult-Onset Huntington’s Disease. Regen. Med. 2015, 10, 623–646. [Google Scholar] [CrossRef]
- Azzouz, M.; Martin-Rendon, E.; Barber, R.D.; Mitrophanous, K.A.; Carter, E.E.; Rohll, J.B.; Kingsman, S.M.; Kingsman, A.J.; Mazarakis, N.D. Multicistronic Lentiviral Vector-Mediated Striatal Gene Transfer of Aromatic l-Amino Acid Decarboxylase, Tyrosine Hydroxylase, and GTP Cyclohydrolase I Induces Sustained Transgene Expression, Dopamine Production, and Functional Improvement in a Rat Model of Parkinson’s Disease. J. Neurosci. 2002, 22, 10302–10312. [Google Scholar] [CrossRef]
- Palfi, S.; Gurruchaga, J.M.; Lepetit, H.; Howard, K.; Ralph, G.S.; Mason, S.; Gouello, G.; Domenech, P.; Buttery, P.C.; Hantraye, P.; et al. Long-Term Follow-Up of a Phase I/II Study of ProSavin, a Lentiviral Vector Gene Therapy for Parkinson’s Disease. Hum. Gene Ther. Clin. Dev. 2018, 29, 148–155. [Google Scholar] [CrossRef]
- Gowing, G.; Svendsen, S.; Svendsen, C.N. Ex Vivo Gene Therapy for the Treatment of Neurological Disorders. Prog. Brain Res. 2017, 230, 99–132. [Google Scholar]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics-Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems; Springer International Publishing: Cham, Switzerland, 2016; pp. 33–93. [Google Scholar]
- Foster, A.; Nigam, S.; Tatum, D.S.; Raphael, I.; Xu, J.; Kumar, R.; Plakseychuk, E.; Latoche, J.D.; Vincze, S.; Li, B.; et al. Novel Theranostic Agent for PET Imaging and Targeted Radiopharmaceutical Therapy of Tumour-Infiltrating Immune Cells in Glioma. EBioMedicine 2021, 71, 103571. [Google Scholar] [CrossRef]
- Mansur, A.A.P.; Caires, A.J.; Carvalho, S.M.; Capanema, N.S.V.; Carvalho, I.C.; Mansur, H.S. Dual-Functional Supramolecular Nanohybrids of Quantum Dot/Biopolymer/Chemotherapeutic Drug for Bioimaging and Killing Brain Cancer Cells in Vitro. Colloids Surf. B Biointerfaces 2019, 184, 110507. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, L.; Liang, Z.; Kou, Z.; Chen, Y.; Shi, G.; Li, X.; Liang, Y.; Wang, F.; Shi, Y. Effects of Aptamer to U87-EGFRvIII Cells on the Proliferation, Radiosensitivity, and Radiotherapy of Glioblastoma Cells. Mol. Ther. Nucleic Acids 2018, 10, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Zong, T.; Mei, L.; Gao, H.; Shi, K.; Chen, J.; Wang, Y.; Zhang, Q.; Yang, Y.; He, Q. Enhanced Glioma Targeting and Penetration by Dual-Targeting Liposome Co-Modified with T7 and TAT. J. Pharm. Sci. 2014, 103, 3891–3901. [Google Scholar] [CrossRef] [PubMed]
- Sanati, M.; Afshari, A.R.; Amini, J.; Mollazadeh, H.; Jamialahmadi, T.; Sahebkar, A. Targeting Angiogenesis in Gliomas: Potential Role of Phytochemicals. J. Funct. Foods 2022, 96, 105192. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Chen, X.; Zhang, Y.; Zhang, K.; Zheng, H.; Wei, Y.; Zheng, H.; Zhu, J.; Wu, F.; et al. Angiopep-2 Modified Lipid-Coated Mesoporous Silica Nanoparticles for Glioma Targeting Therapy Overcoming BBB. Biochem. Biophys. Res. Commun. 2021, 534, 902–907. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Su, C.-Y.; Cheng, H.-W.; Chu, C.-Y.; Jeng, L.-B.; Chiang, C.-S.; Shyu, W.-C.; Chen, S.-Y. Stem Cell–Nanomedicine System as a Theranostic Bio-Gadolinium Agent for Targeted Neutron Capture Cancer Therapy. Nat. Commun. 2023, 14, 285. [Google Scholar] [CrossRef]
- El-Gamal, F.R.; Akl, M.A.; Mowafy, H.A.; Mukai, H.; Kawakami, S.; Afouna, M.I. Synthesis and Evaluation of High Functionality and Quality Cell-Penetrating Peptide Conjugated Lipid for Octaarginine Modified PEGylated Liposomes In U251 and U87 Glioma Cells. J. Pharm. Sci. 2022, 111, 1719–1727. [Google Scholar] [CrossRef]
- Agyare, E.K.; Jaruszewski, K.M.; Curran, G.L.; Rosenberg, J.T.; Grant, S.C.; Lowe, V.J.; Ramakrishnan, S.; Paravastu, A.K.; Poduslo, J.F.; Kandimalla, K.K. Engineering Theranostic Nanovehicles Capable of Targeting Cerebrovascular Amyloid Deposits. J. Control. Release 2014, 185, 121–129. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Chong, S.Y.; Wang, X.; Chen, H.; Chen, C.; Ng, L.G.; Wang, J.; Liu, B. In Vivo Three-Photon Imaging of Lipids Using Ultrabright Fluorogens with Aggregation-Induced Emission. Adv. Mater. 2021, 33, 2007490. [Google Scholar] [CrossRef]
- Feng, G.; Li, J.L.Y.; Claser, C.; Balachander, A.; Tan, Y.; Goh, C.C.; Kwok, I.W.H.; Rénia, L.; Tang, B.Z.; Ng, L.G.; et al. Dual Modal Ultra-Bright Nanodots with Aggregation-Induced Emission and Gadolinium-Chelation for Vascular Integrity and Leakage Detection. Biomaterials 2018, 152, 77–85. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface Functionalized Exosomes as Targeted Drug Delivery Vehicles for Cerebral Ischemia Therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, Y.; Lan, Q.; Wu, W.; Li, Z.; Ma, X.; Yu, L. Exosomes Derived from Mesenchymal Stem Cells Ameliorate Hypoxia/Reoxygenation-Injured ECs via Transferring MicroRNA-126. Stem Cells Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hu, X.; Li, L.; Fang, Y.; Yang, Y.; Gu, J.; Xu, J.; Chu, L. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in Ischemic Stroke Mice via Upregulation of MiR-21-5p. Biomolecules 2022, 12, 883. [Google Scholar] [CrossRef] [PubMed]
- Akhter, A.S.; Bankiewicz, K.S.; Lonser, R.R. Real-Time Magnetic Resonance Imaging During Convective Gene Therapy Perfusion of the Brain. JAMA Surg. 2024, 159, 457. [Google Scholar] [CrossRef]
- Feng, L.; Long, H.-Y.; Liu, R.-K.; Sun, D.-N.; Liu, C.; Long, L.-L.; Li, Y.; Chen, S.; Xiao, B. A Quantum Dot Probe Conjugated with Aβ Antibody for Molecular Imaging of Alzheimer’s Disease in a Mouse Model. Cell Mol. Neurobiol. 2013, 33, 759–765. [Google Scholar] [CrossRef]
- Kwon, H.J.; Cha, M.-Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef]
- Zhang, N.; Yan, F.; Liang, X.; Wu, M.; Shen, Y.; Chen, M.; Xu, Y.; Zou, G.; Jiang, P.; Tang, C.; et al. Localized Delivery of Curcumin into Brain with Polysorbate 80-Modified Cerasomes by Ultrasound-Targeted Microbubble Destruction for Improved Parkinson’s Disease Therapy. Theranostics 2018, 8, 2264–2277. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, L.-K.; Zhang, L.; Zhuang, S.; Zhan, X.; Chen, W.-Y.; Du, S.; Yin, L.; You, R.; Li, C.-H.; et al. Inhibition by Multifunctional Magnetic Nanoparticles Loaded with Alpha-Synuclein RNAi Plasmid in a Parkinson’s Disease Model. Theranostics 2017, 7, 344–356. [Google Scholar] [CrossRef]
- Trivedi, R.; Shende, P. A Stimuli-Responsive Nanocarrier for Diagnosis of Seizures and Inhibition of Glutaminase in Epilepsy. Int. J. Pharm. 2023, 642, 123203. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S. Endgame: Glybera Finally Recommended for Approval as the First Gene Therapy Drug in the European Union. Mol. Ther. 2012, 20, 1831–1832. [Google Scholar] [CrossRef]
- Gaudet, D.; Méthot, J.; Kastelein, J. Gene Therapy for Lipoprotein Lipase Deficiency. Curr. Opin. Lipidol. 2012, 23, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.; Miller, L.E.; Miller, A.L.; Bhattacharyya, R. The FDA Approval of Delandistrogene Moxeparvovec-Rokl for Duchenne Muscular Dystrophy: A Critical Examination of the Evidence and Regulatory Process. Expert. Opin. Biol. Ther. 2024, 24, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.A.; Moeller, I.H.; Khan, S.; Haegel, H.; Hollenstein, A.; Steiner, G.; Wandel, C.; Murphy, A.P.; Asher, D.R.; Palatinsky, E.; et al. Immunologic Investigations into Transgene Directed Immune-Mediated Myositis Following Delandistrogene Moxeparvovec Gene Therapy. Sci. Rep. 2025, 15, 4. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic Cardiomyopathy. Circ. Res. 2017, 121, 784–802. [Google Scholar] [CrossRef]
- Mundisugih, J.; Kizana, E. Crossing the Threshold of Therapeutic Hope for Patients With PKP2 Arrhythmogenic Cardiomyopathy. Circ. Genom. Precis. Med. 2024, 17, e004572. [Google Scholar] [CrossRef]
- Rybalka, E.; Timpani, C.; Debruin, D.; Bagaric, R.; Campelj, D.; Hayes, A. The Failed Clinical Story of Myostatin Inhibitors against Duchenne Muscular Dystrophy: Exploring the Biology behind the Battle. Cells 2020, 9, 2657. [Google Scholar] [CrossRef]
- Kim, Y.; Landstrom, A.P.; Shah, S.H.; Wu, J.C.; Seidman, C.E. Gene Therapy in Cardiovascular Disease: Recent Advances and Future Directions in Science: A Science Advisory From the American Heart Association. Circulation 2024, 150, e471–e480. [Google Scholar] [CrossRef]
- Shariati, L.; Esmaeili, Y.; Rahimmanesh, I.; Babolmorad, S.; Ziaei, G.; Hasan, A.; Boshtam, M.; Makvandi, P. Advances in Nanobased Platforms for Cardiovascular Diseases: Early Diagnosis, Imaging, Treatment, and Tissue Engineering. Environ. Res. 2023, 238, 116933. [Google Scholar] [CrossRef]
- Huang, W.; Huo, M.; Cheng, N.; Wang, R. New Forms of Electrospun Nanofibers Applied in Cardiovascular Field. Front. Cardiovasc. Med. 2022, 8, 801077. [Google Scholar] [CrossRef]
- Sivaraman, B.; Ramamurthi, A. Multifunctional Nanoparticles for Doxycycline Delivery towards Localized Elastic Matrix Stabilization and Regenerative Repair. Acta Biomater. 2013, 9, 6511–6525. [Google Scholar] [CrossRef]
- Kobuszewska, A.; Kolodziejek, D.; Wojasinski, M.; Jastrzebska, E.; Ciach, T.; Brzozka, Z. Lab-on-a-Chip System Integrated with Nanofiber Mats Used as a Potential Tool to Study Cardiovascular Diseases (CVDs). Sens. Actuators B Chem. 2021, 330, 129291. [Google Scholar] [CrossRef]
- Sen, I.; Clouse, W.D.; Lauria, A.L.; Calderon, D.R.; Anderson, P.B.; DeMartino, R.R.; Rasmussen, T.E. Outcomes of Arterial Bypass With the Human Acellular Vessel for Chronic Limb-Threatening Ischemia Performed Under the FDA Expanded Access Program. Mayo Clin. Proc. 2024, 99, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Ud Din, S.R.; Khan, S.U.; Gul, R.; Kiani, F.A.; Wahab, A.; Zhong, M. Nanoparticle: A Promising Player in Nanomedicine and Its Theranostic Applications for the Treatment of Cardiovascular Diseases. Curr. Probl. Cardiol. 2023, 48, 101599. [Google Scholar] [CrossRef] [PubMed]
- Schwartze, J.T.; Havenga, M.; Bakker, W.A.M.; Bradshaw, A.C.; Nicklin, S.A. Adenoviral Vectors for Cardiovascular Gene Therapy Applications: A Clinical and Industry Perspective. J. Mol. Med. 2022, 100, 875–901. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, R.; Li, C.; Tao, H.; Dou, Y.; Wang, Y.; Hu, H.; Zhang, J. A Targeting Nanotherapy for Abdominal Aortic Aneurysms. J. Am. Coll. Cardiol. 2018, 72, 2591–2605. [Google Scholar] [CrossRef]
- Elizondo-Benedetto, S.; Sastriques-Dunlop, S.; Detering, L.; Arif, B.; Heo, G.S.; Sultan, D.; Luehmann, H.; Zhang, X.; Gao, X.; Harrison, K.; et al. Chemokine Receptor 2 Is A Theranostic Biomarker for Abdominal Aortic Aneurysms. medRxiv 2023. [Google Scholar] [CrossRef]
- Hou, L.; Kim, J.J.; Woo, Y.J.; Huang, N.F. Stem Cell-Based Therapies to Promote Angiogenesis in Ischemic Cardiovascular Disease. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H455–H465. [Google Scholar] [CrossRef]
- Wang, F.; Wen, L.; Liu, J.; Peng, W.; Meng, Z.; Chen, Q.; Wang, Y.; Ke, B.; Guo, Y.; Mi, P. Albumin Nanocomposites with MnO2/Gd2O3 Motifs for Precise MR Imaging of Acute Myocardial Infarction in Rabbit Models. Biomaterials 2020, 230, 119614. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, Y.; Zhao, S.; Zhang, P.; Zhang, Y.; Bi, J.; Liu, G.; Yue, Z. Carbon Dots Based Photoelectrochemical Sensors for Ultrasensitive Detection of Glutathione and Its Applications in Probing of Myocardial Infarction. Biosens. Bioelectron. 2018, 99, 251–258. [Google Scholar] [CrossRef]
- Vinodhini, A.; Govindaraju, K.; Singaravelu, G.; Mohamed Sadiq, A.; Kumar, V.G. Cardioprotective Potential of Biobased Gold Nanoparticles. Colloids Surf. B Biointerfaces 2014, 117, 480–486. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Chen, Z.-Y.; Wang, Y.-X.; Lin, Y.; Yang, F.; Zhou, Q.-L. New Progress in Angiogenesis Therapy of Cardiovascular Disease by Ultrasound Targeted Microbubble Destruction. Biomed. Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tsumaru, S.; Masumoto, H.; Minakata, K.; Izuhara, M.; Yamazaki, K.; Ikeda, T.; Ono, K.; Sakata, R.; Minatoya, K. Therapeutic Angiogenesis by Local Sustained Release of MicroRNA-126 Using Poly Lactic-Co-Glycolic Acid Nanoparticles in Murine Hindlimb Ischemia. J. Vasc. Surg. 2018, 68, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Cho, H.-J.; Choi, M.; Lee, W.S.; Chung, B.H.; Lee, J.-S. In Vivo Toxicity Assessment of Angiogenesis and the Live Distribution of Nano-Graphene Oxide and Its PEGylated Derivatives Using the Developing Zebrafish Embryo. Carbon 2015, 93, 431–440. [Google Scholar] [CrossRef]
- Mannucci, P.M. Hemophilia Treatment Innovation: 50 Years of Progress and More to Come. J. Thromb. Haemost. 2023, 21, 403–412. [Google Scholar] [CrossRef]
- Ozelo, M.C.; Mahlangu, J.; Pasi, K.J.; Giermasz, A.; Leavitt, A.D.; Laffan, M.; Symington, E.; Quon, D.V.; Wang, J.-D.; Peerlinck, K.; et al. Valoctocogene Roxaparvovec Gene Therapy for Hemophilia A. N. Engl. J. Med. 2022, 386, 1013–1025. [Google Scholar] [CrossRef]
- Heo, Y.-A. Etranacogene Dezaparvovec: First Approval. Drugs 2023, 83, 347–352. [Google Scholar] [CrossRef]
- Cuker, A.; Kavakli, K.; Frenzel, L.; Wang, J.-D.; Astermark, J.; Cerqueira, M.H.; Iorio, A.; Katsarou-Fasouli, O.; Klamroth, R.; Shapiro, A.D.; et al. Gene Therapy with Fidanacogene Elaparvovec in Adults with Hemophilia B. N. Engl. J. Med. 2024, 391, 1108–1118. [Google Scholar] [CrossRef]
- Affan, M.; Dar, M.S. Donislecel-the First Approved Pancreatic Islet Cell Therapy Medication for Type 1 Diabetes: A Letter to the Editor. Ir. J. Med. Sci. (1971) 2024, 193, 231–232. [Google Scholar] [CrossRef]
- Sy, S.L.; Munshi, M.M.; Toschi, E. Can Smart Pens Help Improve Diabetes Management? J. Diabetes Sci. Technol. 2022, 16, 628–634. [Google Scholar] [CrossRef]
- Percival, M.W.; Zisser, H.; Jovanovic, L.; Doyle, F.J. Closed-Loop Control and Advisory Mode Evaluation of an Artificial Pancreatic β Cell: Use of Proportional-Integral-Derivative Equivalent Model-Based Controllers. J. Diabetes Sci. Technol. 2008, 2, 636–644. [Google Scholar] [CrossRef]
- Amer, M.G.; Embaby, A.S.; Karam, R.A.; Amer, M.G. Role of Adipose Tissue Derived Stem Cells Differentiated into Insulin Producing Cells in the Treatment of Type I Diabetes Mellitus. Gene 2018, 654, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Thakur, G.; Lee, H.-J.; Jeon, R.-H.; Lee, S.-L.; Rho, G.-J. Small Molecule-Induced Pancreatic β-Like Cell Development: Mechanistic Approaches and Available Strategies. Int. J. Mol. Sci. 2020, 21, 2388. [Google Scholar] [CrossRef] [PubMed]
- Guide, S.V.; Gonzalez, M.E.; Bağcı, I.S.; Agostini, B.; Chen, H.; Feeney, G.; Steimer, M.; Kapadia, B.; Sridhar, K.; Quesada Sanchez, L.; et al. Trial of Beremagene Geperpavec (B-VEC) for Dystrophic Epidermolysis Bullosa. N. Engl. J. Med. 2022, 387, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Guide, S.V.; Ayala, D.; Gonzalez, M.E.; Lucky, A.W.; Bagci, I.S.; Marinkovich, M.P. Practical Considerations Relevant to Treatment with the Gene Therapy Beremagene Geperpavec-Svdt for Dystrophic Epidermolysis Bullosa. J. Dermatol. Treat. 2024, 35, 2350232. [Google Scholar] [CrossRef]
- Locatelli, F.; Thompson, A.A.; Kwiatkowski, J.L.; Porter, J.B.; Thrasher, A.J.; Hongeng, S.; Sauer, M.G.; Thuret, I.; Lal, A.; Algeri, M.; et al. Betibeglogene Autotemcel Gene Therapy for Non–β0/β0 Genotype β-Thalassemia. N. Engl. J. Med. 2022, 386, 415–427. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Hussain, R.M.; Berrocal, A.M.; Nagiel, A. Voretigene Neparvovec-Rzyl for Treatment of RPE65-Mediated Inherited Retinal Diseases: A Model for Ocular Gene Therapy Development. Expert. Opin. Biol. Ther. 2020, 20, 565–578. [Google Scholar] [CrossRef]
- Bommakanti, N.; Young, B.K.; Sisk, R.A.; Berrocal, A.M.; Duncan, J.L.; Bakall, B.; Mathias, M.T.; Ahmed, I.; Chorfi, S.; Comander, J.; et al. Classification and Growth Rate of Chorioretinal Atrophy after Voretigene Neparvovec-Rzyl for RPE65-Mediated Retinal Degeneration. Ophthalmol. Retin. 2024, 8, 42–48. [Google Scholar] [CrossRef]
- Torres-Vanegas, J.D.; Rincon-Tellez, N.; Guzmán-Sastoque, P.; Valderrama-Rincon, J.D.; Cruz, J.C.; Reyes, L.H. Production and Purification of Outer Membrane Vesicles Encapsulating Green Fluorescent Protein from Escherichia Coli: A Step towards Scalable OMV Technologies. Front. Bioeng. Biotechnol. 2024, 12, 1436352. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current Hurdles to the Translation of Nanomedicines from Bench to the Clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing Nanotechnology to Improve Targeted Cancer Treatment: Overcoming Hurdles in Its Clinical Implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Rauscher, H.; Silva, P.; Slikker, W.; Sokull-Kluettgen, B.; et al. Regulatory Landscape of Nanotechnology and Nanoplastics from a Global Perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Hiremath, P. Challenges Associated and Approaches for Successful Translation of Nanomedicines Into Commercial Products. Nanomedicine 2017, 12, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Paradise, J. Regulating Nanomedicine at the Food and Drug Administration. AMA J. Ethics 2019, 21, E347–E355. [Google Scholar] [CrossRef]
- Gaur, N.; Sharma, N.; Dahiya, A.; Yadav, P.; Ojha, H.; Goyal, R.K.; Sharma, R.K. Toxicity and Regulatory Concerns for Nanoformulations in Medicine. In The ELSI Handbook of Nanotechnology; Wiley & Sons: New York, NY, USA, 2020; pp. 333–357. [Google Scholar]
- Pepic, I.; Hafner, A.; Lovric, J.; Perina Lakos, G. Nanotherapeutics in the EU: An Overview on Current State and Future Directions. Int. J. Nanomed. 2014, 9, 1005–1023. [Google Scholar] [CrossRef]
- Rathore, A.S.; Winkle, H. Quality by Design for Biopharmaceuticals. Nat. Biotechnol. 2009, 27, 26–34. [Google Scholar] [CrossRef]
- Gnoth, S.; Jenzsch, M.; Simutis, R.; Lübbert, A. Process Analytical Technology (PAT): Batch-to-Batch Reproducibility of Fermentation Processes by Robust Process Operational Design and Control. J. Biotechnol. 2007, 132, 180–186. [Google Scholar] [CrossRef]
- Rodríguez, C.F.; Guzmán-Sastoque, P.; Gantiva-Diaz, M.; Gómez, S.C.; Quezada, V.; Muñoz-Camargo, C.; Osma, J.F.; Reyes, L.H.; Cruz, J.C. Low-Cost Inertial Microfluidic Device for Microparticle Separation: A Laser-Ablated PMMA Lab-on-a-Chip Approach without a Cleanroom. HardwareX 2023, 16, e00493. [Google Scholar] [CrossRef]
- Rodríguez, C.F.; Báez-Suárez, M.; Muñoz-Camargo, C.; Reyes, L.H.; Osma, J.F.; Cruz, J.C. Zweifach–Fung Microfluidic Device for Efficient Microparticle Separation: Cost-Effective Fabrication Using CO2 Laser-Ablated PMMA. Micromachines 2024, 15, 932. [Google Scholar] [CrossRef]
- Rodríguez, C.F.; Andrade-Pérez, V.; Vargas, M.C.; Mantilla-Orozco, A.; Osma, J.F.; Reyes, L.H.; Cruz, J.C. Breaking the Clean Room Barrier: Exploring Low-Cost Alternatives for Microfluidic Devices. Front. Bioeng. Biotechnol. 2023, 11, 1176557. [Google Scholar] [CrossRef]
- Colombo, S.; Beck-Broichsitter, M.; Bøtker, J.P.; Malmsten, M.; Rantanen, J.; Bohr, A. Transforming Nanomedicine Manufacturing toward Quality by Design and Microfluidics. Adv. Drug Deliv. Rev. 2018, 128, 115–131. [Google Scholar] [CrossRef]
- Kumah, E.A.; Fopa, R.D.; Harati, S.; Boadu, P.; Zohoori, F.V.; Pak, T. Human and Environmental Impacts of Nanoparticles: A Scoping Review of the Current Literature. BMC Public Health 2023, 23, 1059. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Hull, M.S.; Steevens, J.A.; Dontsova, K.M.; Chappell, M.A.; Gunter, J.C.; Weiss, C.A., Jr. Factors Influencing the Partitioning and Toxicity of Nanotubes in the Aquatic Environment. Environ. Toxicol. Chem. 2008, 27, 1932–1941. [Google Scholar] [CrossRef]
- Tervonen, T.; Linkov, I.; Figueira, J.R.; Steevens, J.; Chappell, M.; Merad, M. Risk-Based Classification System of Nanomaterials. J. Nanoparticle Res. 2009, 11, 757–766. [Google Scholar] [CrossRef]
- Bortoleto, G.G.; de Oliveira Borges, S.S.; Bueno, M.I.M.S. X-Ray Scattering and Multivariate Analysis for Classification of Organic Samples: A Comparative Study Using Rh Tube and Synchrotron Radiation. Anal. Chim. Acta 2007, 595, 38–42. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.; Huang, R. Nanoparticles-induced Potential Toxicity on Human Health: Applications, Toxicity Mechanisms, and Evaluation Models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Interface science and technology. Green Nanotechnol. 2019, 28, 145–198. [Google Scholar]
- Erstling, J.A.; Hinckley, J.A.; Bag, N.; Hersh, J.; Feuer, G.B.; Lee, R.; Malarkey, H.F.; Yu, F.; Ma, K.; Baird, B.A.; et al. Ultrasmall, Bright, and Photostable Fluorescent Core–Shell Aluminosilicate Nanoparticles for Live-Cell Optical Super-Resolution Microscopy. Adv. Mater. 2021, 33, 2006829. [Google Scholar] [CrossRef]
- Jin, D.; Xi, P.; Wang, B.; Zhang, L.; Enderlein, J.; van Oijen, A.M. Nanoparticles for Super-Resolution Microscopy and Single-Molecule Tracking. Nat. Methods 2018, 15, 415–423. [Google Scholar] [CrossRef]
- Gargas, D.J.; Chan, E.M.; Ostrowski, A.D.; Aloni, S.; Altoe, M.V.P.; Barnard, E.S.; Sanii, B.; Urban, J.J.; Milliron, D.J.; Cohen, B.E.; et al. Engineering Bright Sub-10-Nm Upconverting Nanocrystals for Single-Molecule Imaging. Nat. Nanotechnol. 2014, 9, 300–305. [Google Scholar] [CrossRef]
- Wu, C.; Schneider, T.; Zeigler, M.; Yu, J.; Schiro, P.G.; Burnham, D.R.; McNeill, J.D.; Chiu, D.T. Bioconjugation of Ultrabright Semiconducting Polymer Dots for Specific Cellular Targeting. J. Am. Chem. Soc. 2010, 132, 15410–15417. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhanghao, K.; Wang, H.; Liu, Y.; Wang, F.; Zhang, X.; Shi, K.; Gao, J.; Jin, D.; Xi, P. Versatile Application of Fluorescent Quantum Dot Labels in Super-Resolution Fluorescence Microscopy. ACS Photonics 2016, 3, 1611–1618. [Google Scholar] [CrossRef]
- Fan, W.; Bu, W.; Shi, J. On The Latest Three-Stage Development of Nanomedicines Based on Upconversion Nanoparticles. Adv. Mater. 2016, 28, 3987–4011. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, M.L.; Huang, C.Z. Recent Advances of Carbon Dots in Imaging-Guided Theranostics. TrAC Trends Anal. Chem. 2021, 134, 116116. [Google Scholar] [CrossRef]
- Rahman, M. Magnetic Resonance Imaging and Iron-Oxide Nanoparticles in the Era of Personalized Medicine. Nanotheranostics 2023, 7, 424–449. [Google Scholar] [CrossRef]
- Bilgin, G.B.; Bilgin, C.; Burkett, B.J.; Orme, J.J.; Childs, D.S.; Thorpe, M.P.; Halfdanarson, T.R.; Johnson, G.B.; Kendi, A.T.; Sartor, O. Theranostics and Artificial Intelligence: New Frontiers in Personalized Medicine. Theranostics 2024, 14, 2367–2378. [Google Scholar] [CrossRef]
- Tutar, Y. (Ed.) Advances in Cancer Nanotheranostics for Experimental and Personalized Medicine; Bentham Science Publishers: Sharjah, United Arab Emirates, 2020; ISBN 9789811456916. [Google Scholar]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Krauss, A.C.; Gao, X.; Li, L.; Manning, M.L.; Patel, P.; Fu, W.; Janoria, K.G.; Gieser, G.; Bateman, D.A.; Przepiorka, D.; et al. FDA Approval Summary: (Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 2685–2690. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).