How Reproducible Is Feraheme® (Ferumoxytol Injection)? Comparison of Size, Zeta Potential, and Complement Activation of Different Batches over 15 Years
Abstract
1. Introduction
2. Materials and Methods
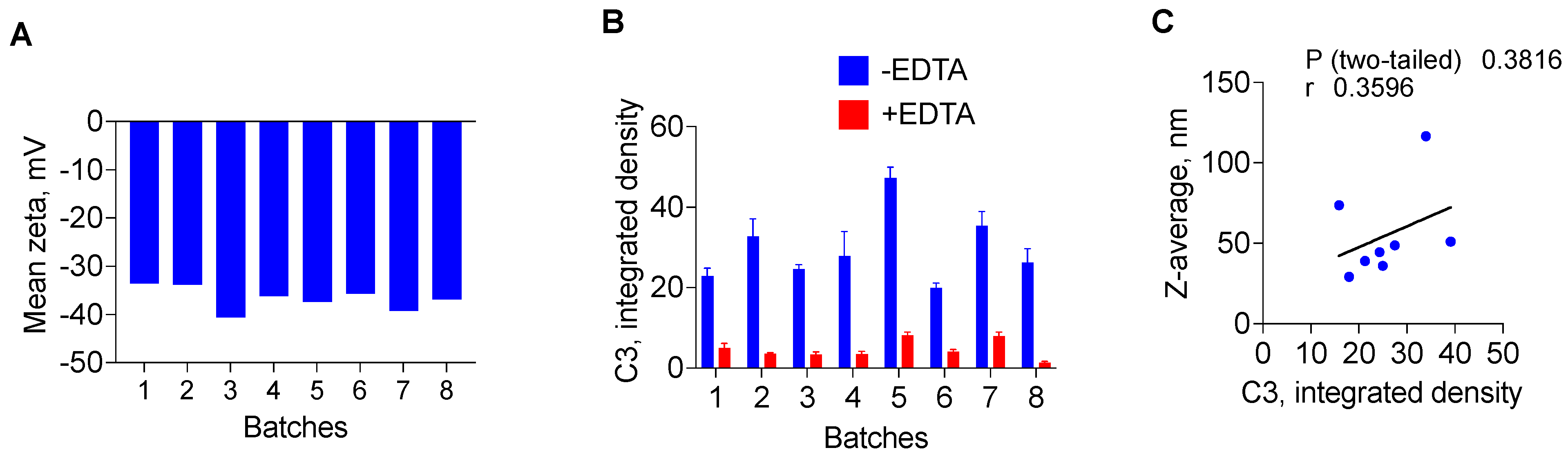
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boström, H.L.B.; Emmerling, S.; Heck, F.; Koschnick, C.; Jones, A.J.; Cliffe, M.J.; Al Natour, R.; Bonneau, M.; Guillerm, V.; Shekhah, O.; et al. How Reproducible is the Synthesis of Zr–Porphyrin Metal–Organic Frameworks? An Interlaboratory Study. Adv. Mater. 2024, 36, e2304832. [Google Scholar] [CrossRef] [PubMed]
- Anchordoquy, T.; Artzi, N.; Balyasnikova, I.V.; Barenholz, Y.; La-Beck, N.M.; Brenner, J.S.; Chan, W.C.W.; Decuzzi, P.; Exner, A.A.; Gabizon, A.; et al. Mechanisms and Barriers in Nanomedicine: Progress in the Field and Future Directions. ACS Nano 2024, 18, 13983–13999. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Chertow, G.M.; Rosner, M. Ferumoxytol for the treatment of iron deficiency anemia. Expert Rev. Hematol. 2018, 11, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A.; Várallyay, C.G.; Manninger, S.; Solymosi, D.; Haluska, M.; Hunt, M.A.; Nesbit, G.; Stevens, A.; Jerosch-Herold, M.; Jacobs, P.M.; et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy. Neurosurgery 2007, 60, 601–612; discussion 611–612. [Google Scholar] [CrossRef] [PubMed]
- Harisinghani, M.; Ross, R.W.; Guimaraes, A.R.; Weissleder, R. Utility of a New Bolus-injectable Nanoparticle for Clinical Cancer Staging. Neoplasia 2007, 9, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Simberg, D.; González-Fernández, A.; Barenholz, Y.; Dobrovolskaia, M.A. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat. Nanotechnol. 2018, 13, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Monte, A.; Dylla, L.; Moghimi, S.M.; Simberg, D. Validation of dot blot immunoassay for measurement of complement opsonization of nanoparticles. J. Immunol. Methods 2024, 528, 113668. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation; U.S. Food and Drug Administration: Rockville, MD, USA, 2018. [Google Scholar]
- Drug Products, Including Biological Products, That Contain Nanomaterials—Guidance for Industry. 2022. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-products-including-biological-products-contain-nanomaterials-guidance-industry (accessed on 30 August 2024).
- Spinowitz, B.S.; Schwenk, M.H.; Jacobs, P.M.; Bolton, W.K.; Kaplan, M.R.; Charytan, C.; Galler, M. The safety and efficacy of ferumoxytol therapy in anemic chronic kidney disease patients. Kidney Int. 2005, 68, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Groman, E.V.; Paul, K.G.; Frigo, T.B.; Bengele, H.; Lewis, J.M. Heat Stable Colloidal Iron Oxides Coated with Reduced Carbohydrates and Uses Thereof. U.S. Patent US7553479B2, 30 June 2009. [Google Scholar]
- Paul, K.G.; Frigo, T.B.; Groman, J.Y.; Groman, E.V. Synthesis of Ultrasmall Superparamagnetic Iron Oxides Using Reduced Polysaccharides. Bioconjug. Chem. 2004, 15, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Spinowitz, B.S.; Kausz, A.T.; Baptista, J.; Noble, S.D.; Sothinathan, R.; Bernardo, M.V.; Brenner, L.; Pereira, B.J.G. Ferumoxytol for Treating Iron Deficiency Anemia in CKD. J. Am. Soc. Nephrol. 2008, 19, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
| Expiration Date | Lot | Z-Average nm | Poly Dispersity Index | Peak 1 Mean Intensity | Peak 2 Mean Intensity | Pk 1 Area Intensity | Pk 2 Area Intensity | |
|---|---|---|---|---|---|---|---|---|
| 1 | 02/2010 | 06021502 | 30 | 0.19 | 32 | 0 | 100 | 0 |
| 1 | 02/2010 | 06021502 | 29 | 0.15 | 33 | 0 | 100 | 0 |
| 2 | 02/2014 | 10021802 | 33 | 0.23 | 38 | 4362 | 98 | 3 |
| 2 | 02/2014 | 10021802 | 31 | 0.20 | 33 | 4320 | 97 | 4 |
| 3 | 09/2023 | LT7285 | 38 | 0.24 | 42 | 4510 | 96 | 4 |
| 3 | 09/2023 | LT7285 | 41 | 0.17 | 38 | 5451 | 99 | 1 |
| 4 | 06/2024 | AL8360D | 43 | 0.28 | 51 | 4136 | 95 | 5 |
| 4 | 06/2024 | AL8360D | 46 | 0.25 | 63 | 3888 | 98 | 2 |
| 5 | 11/2024 | AM4444B | 52 | 0.32 | 60 | 4718 | 95 | 5 |
| 5 | 11/2024 | AM4444B | 51 | 0.31 | 60 | 4716 | 96 | 4 |
| 6 | 04/2025 | AN1173C | 70 | 0.18 | 38 | 0 | 100 | 0 |
| 6 | 04/2025 | AN1173C | 77 | 0.17 | 37 | 0 | 100 | 0 |
| 7 | 08/2025 | AM8228B | 45 | 0.18 | 33 | 0 | 100 | 0 |
| 7 | 08/2025 | AM8228B | 52 | 0.14 | 36 | 0 | 100 | 0 |
| 8 | 11/2025 | AP4322B | 37 | 0.24 | 37 | 5224 | 97 | 3 |
| 8 | 11/2025 | AP4322B | 36 | 0.24 | 42 | 3980 | 96 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ettah, U.; Jacques, S.; Simberg, D. How Reproducible Is Feraheme® (Ferumoxytol Injection)? Comparison of Size, Zeta Potential, and Complement Activation of Different Batches over 15 Years. J. Nanotheranostics 2024, 5, 128-132. https://doi.org/10.3390/jnt5030009
Ettah U, Jacques S, Simberg D. How Reproducible Is Feraheme® (Ferumoxytol Injection)? Comparison of Size, Zeta Potential, and Complement Activation of Different Batches over 15 Years. Journal of Nanotheranostics. 2024; 5(3):128-132. https://doi.org/10.3390/jnt5030009
Chicago/Turabian StyleEttah, Utibeabasi, Sarah Jacques, and Dmitri Simberg. 2024. "How Reproducible Is Feraheme® (Ferumoxytol Injection)? Comparison of Size, Zeta Potential, and Complement Activation of Different Batches over 15 Years" Journal of Nanotheranostics 5, no. 3: 128-132. https://doi.org/10.3390/jnt5030009
APA StyleEttah, U., Jacques, S., & Simberg, D. (2024). How Reproducible Is Feraheme® (Ferumoxytol Injection)? Comparison of Size, Zeta Potential, and Complement Activation of Different Batches over 15 Years. Journal of Nanotheranostics, 5(3), 128-132. https://doi.org/10.3390/jnt5030009






