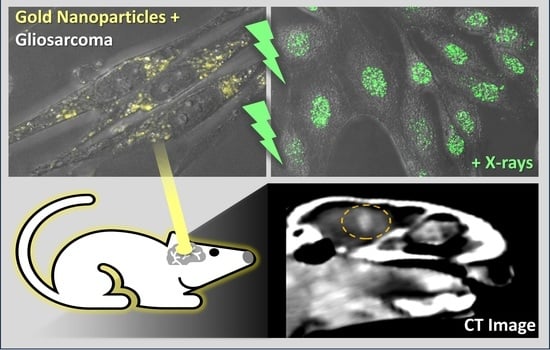
Efficacy of 15 nm Gold Nanoparticles for Image-Guided Gliosarcoma Radiotherapy
Abstract
:1. Introduction
2. Materials and Methods
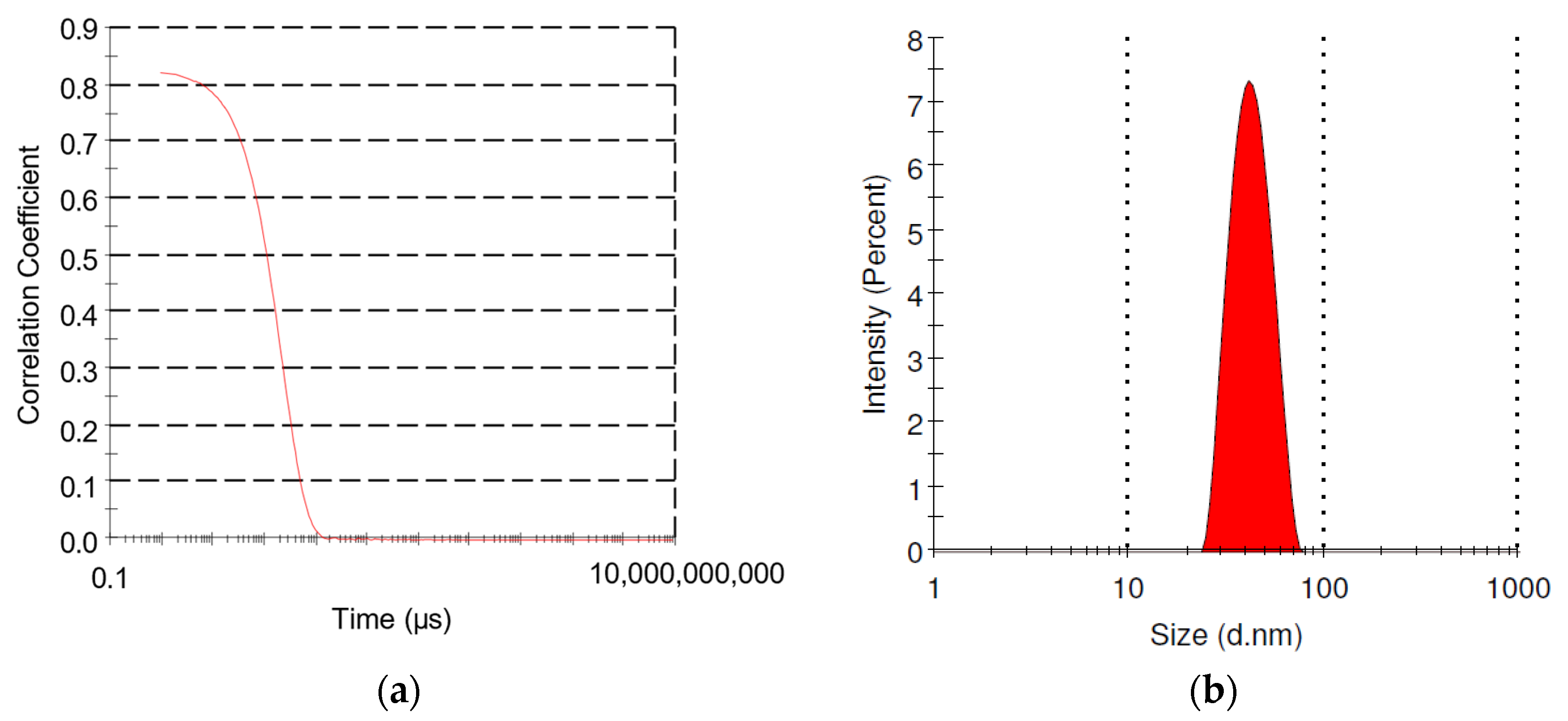
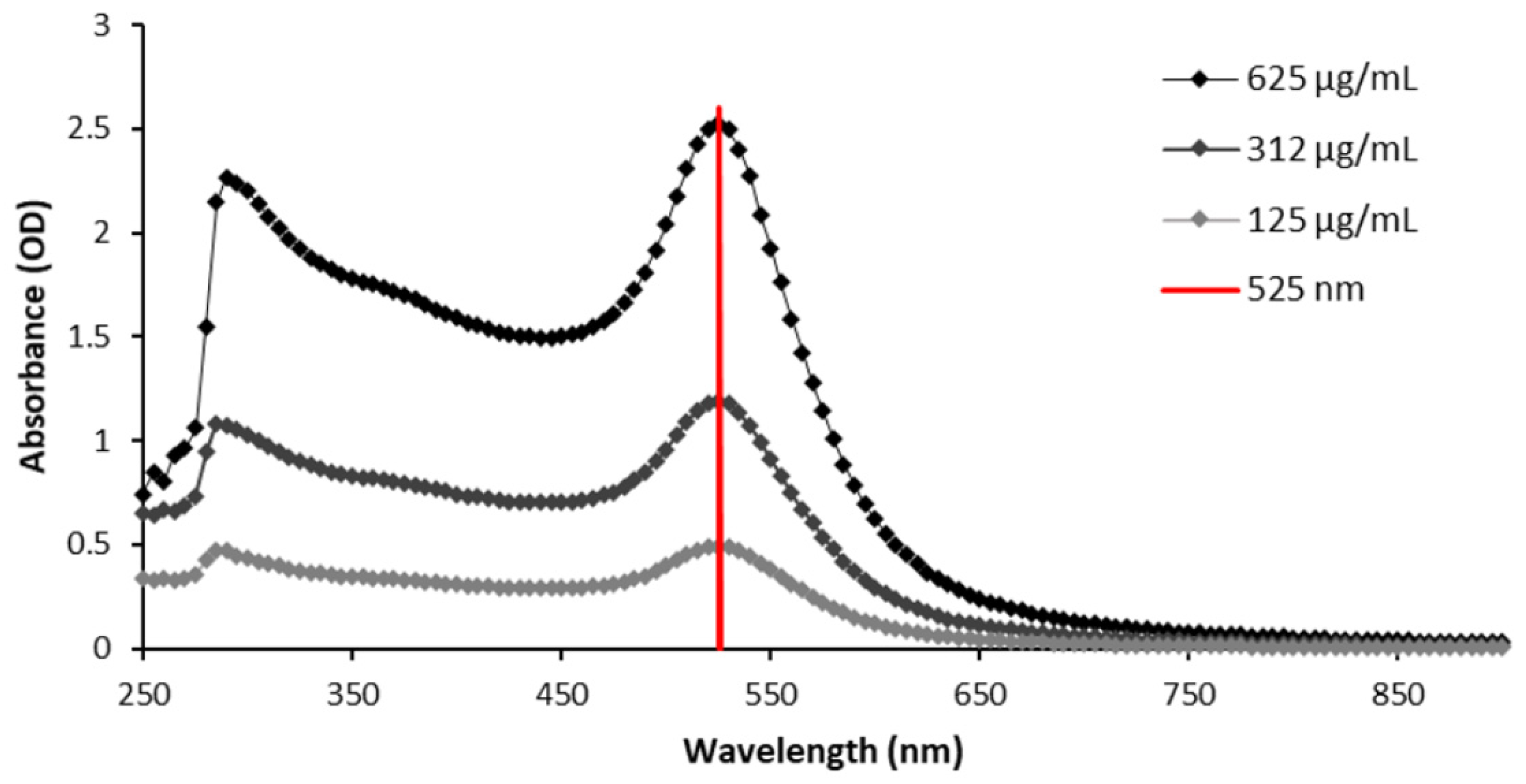
2.1. Size Characterization
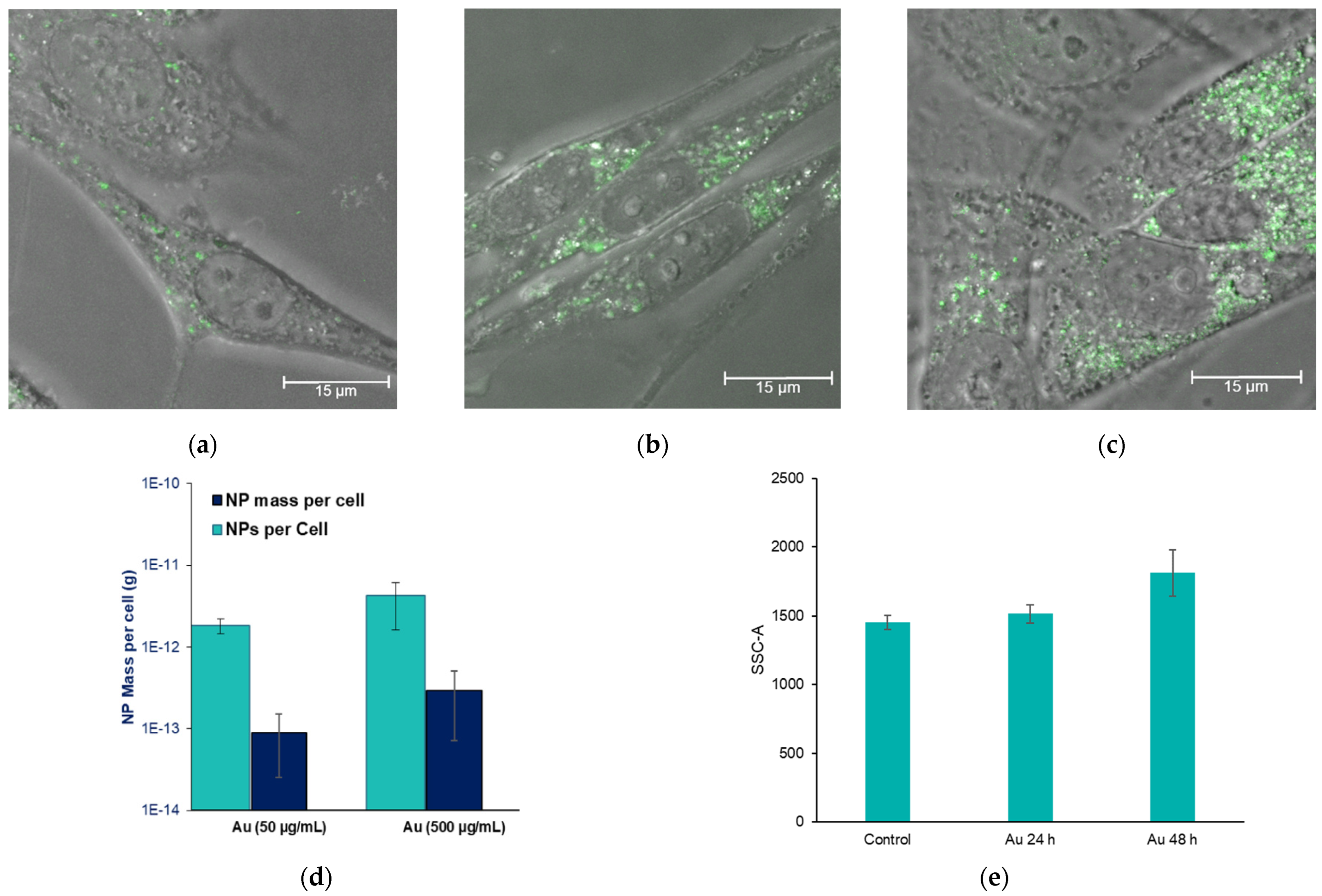
2.2. Nanoparticle Uptake in 9L Gliosarcoma Cells
2.3. Cell Irradiation with Gold Nanopartilces
2.4. Gold Nanoparticle Contrast in Computed Tomography
2.5. Preclinical Brain Cancer Gold Nanoparticle Uptake
3. Results
3.1. GNP Size Characterization
3.2. Gold Nanoparticle Uptake in Brain Cancer Cells
3.3. Gold Nanoparticle Dose Enhancement for Brain Cancer Radiotherapy
3.4. Preclinical Gliosarcoma Imaging with GNPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Neubeck, C.; Seidlitz, A.; Kitzler, H.H.; Beuthien-Baumann, B.; Krause, M. Glioblastoma multiforme: Emerging treatments and stratification markers beyond new drugs. Br. J. Radiol. 2015, 88, 20150354. [Google Scholar] [CrossRef]
- Sizoo, E.M.; Braam, L.; Postma, T.J.; Roeline, H.; Pasman, W.; Heimans, J.J.; Klein, M.; Reijneveld, J.C.; Taphoorn, M.J.B. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro-Oncology 2010, 12, 1162–1166. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, K.; Chen, K.; Xu, C.; Ma, P.; Dang, G.; Yang, Y.; Lei, Q.; Huang, H.; Yu, Y.; et al. Nanoparticle-based medicines in clinical cancer therapy. Nano Today 2022, 45, 101512. [Google Scholar] [CrossRef]
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M.; et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): A multicentre, phase 2-3, randomised, controlled trial. Lancet Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, 309–315. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Konstantinov, K.; McKinnon, S.; Guatelli, S.; Lerch, M.; Rosenfeld, A.; Tehei, M.; Corde, S. First proof of bismuth oxide nanoparticles as efficient radiosensitisers on highly radioresistant cancer cells. Phys. Medica 2016, 32, 1444–1452. [Google Scholar] [CrossRef]
- Engels, E.; Westlake, M.; Li, N.; Vogel, S.; Gobert, Q.; Thorpe, N.; Rosenfeld, A.; Lerch, M.; Corde, S.; Tehei, M. Thulium Oxide Nanoparticles: A new candidate for image-guided radiotherapy. Biomed. Phys. Eng. Express 2018, 4, 044001. [Google Scholar] [CrossRef]
- Mesbahi, A.; Jamali, F.; Garehaghaji, N. Effect of photon beam energy, gold nanoparticle size and concentration on the dose enhancement in radiation therapy. BioImpacts 2013, 3, 29–35. [Google Scholar]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’Sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McMahon, S.J.; Taggart, L.E.; Prise, K.M. Radiosensitization by gold nanoparticles: Effective at megavoltage energies and potential role of oxidative stress. Transl. Cancer Res. 2013, 2, 269–279. [Google Scholar]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef]
- Engels, E.; Bakr, S.; Bolst, D.; Sakata, D.; Li, N.; Lazarakis, P.; McMahon, S.J.; Ivanchenko, V.; Rosenfeld, A.B.; Incerti, S.; et al. Advances in modelling gold nanoparticle radiosensitization using new Geant4-DNA physics models. Phys. Med. Biol. 2020, 65, 225017. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Bobyk, L.; Edouard, M.; Deman, P.; Vautrin, M.; Pernet-Gallay, K.; Delaroche, J.; Adam, J.F.; Estève, F.; Ravanat, J.L.; Elleaume, H. Photoactivation of gold nanoparticles for glioma treatment. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1089–1097. [Google Scholar] [CrossRef]
- Ferrero, V.; Visonà, G.; Dalmasso, F.; Gobbato, A.; Cerello, P.; Strigari, L.; Visentin, S.; Attili, A. Targeted dose enhancement in radiotherapy for breast cancer using gold nanoparticles, part 1: A radiobiological model study. Med. Phys. 2017, 44, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Joh, D.Y.; Sun, L.; Stangl, M.; Al Zaki, A.; Murty, S.; Santoiemma, P.P.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Bhang, D.; et al. Selective targeting of brain tumors with gold nanoparticle induced radiosensitization. PLoS ONE 2013, 8, e62425. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhau, G. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef]
- Domey, J.; Teichgräber, U.; Hilger, I. Gold nanoparticles allow detection of early-stage edema in mice via computed tomography imaging. Int. J. Nanomed. 2015, 10, 3803–3814. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Kim, S.H.; Choi, K.S.; Kim, S.Y.; Byun, S.J.; Kim, K.W.; Park, S.H.; Juhng, S.K.; Yoon, K.H. Colloidal gold nanoparticles as a blood-pool contrast agent for x-ray computed tomography in mice. Investig. Radiol. 2007, 42, 797–806. [Google Scholar] [CrossRef]
- Luo, D.; Johnson, A.; Wang, X.; Li, H.; Erokwu, B.O.; Springer, S.; Lou, J.; Ramamurthy, G.; Flask, C.A.; Burda, C.; et al. Targeted Radiosensitizers for MR-Guided Radiation Therapy of Prostate Cancer. Nano Lett. 2020, 20, 7159–7167. [Google Scholar] [CrossRef]
- Zhao, Y.; Sultan, D.; Detering, L.; Luehmann, H.; Liu, Y. Facile synthesis, pharmacokinetic and systemic clearance evaluation, and positron emission tomography cancer imaging of 64Cu-Au alloy nanoclusters. Nanoscale 2014, 6, 13501–13509. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- He, Y.Q.; Liu, S.P.; Kong, L.; Liu, Z.F. A study on the sizes and concentrations of gold nanoparticles by spectra of absorption, resonance Rayleigh scattering and resonance non-linear scattering. Spectrochim. Acta Part A 2005, 61, 2861–2866. [Google Scholar] [CrossRef]
- Penninckx, S.; Heuskin, A.-C.; Michiels, C.; Lucas, S. Gold Nanoparticles as a Potent Radiosensitizer: A Transdisciplinary Approach from Physics to Patient. Cancers 2020, 12, 2021. [Google Scholar] [CrossRef] [PubMed]
- Verry, C.; Dufort, S.; Villa, J.; Gavard, M.; Iriart, C.; Grand, S.; Charles, J.; Chovelon, B.; Cracowski, J.L.; Quesada, J.L.; et al. Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiother. Oncol. 2021, 160, 159–165. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–442. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Li, X.; Jiang, Y.; Yan, B.; Tonga, G.Y.; Ray, M.M.; Solfiell, D.J.; Rotello, V.M. Cellular imaging of endosome entrapped small gold nanoparticles. MethodsX 2015, 2, 306–315. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Bahamonde, J.; Brenseke, B.; Chan, M.Y.; Kent, R.D.; Vikesland, P.J.; Prater, M.R. Gold Nanoparticle Toxicity in Mice and Rats: Species Differences. Toxicol. Pathol. 2018, 46, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Leopold, L.; Todor, I.; Diaconeasa, Z.; Rugină, D.; Ştefancu, A.; Leopold, N.; Coman, C. Assessment of PEG and BSA-PEG gold nanoparticles cellular interaction. Colloids Surf. A Physicochem. Eng. Asp. 2007, 532, 70–76. [Google Scholar] [CrossRef]
- Corde, S.; Joubert, A.; Adam, J.; Charvet, A.M.; Le Bas, J.F.; Estève, F.; Elleaume, H.; Balosso, J. Synchrotron radiation-based experimental determination of the optimal energy for cell radiotoxicity enhancement following photoelectric effect on stable iodinated compounds. Br. J. Cancer 2004, 91, 544–551. [Google Scholar] [CrossRef]
- McMahon, S.J. The linear quadratic model: Usage, interpretation and challenges. Phys. Med. Biol. 2019, 64, 01TR01. [Google Scholar] [CrossRef] [PubMed]
- Goodhead, D.T. Initial Events in the Cellular Effects of Ionizing Radiations: Clustered Damage in DNA. Int. J. Radiat. Biol. 1994, 65, 7–17. [Google Scholar] [CrossRef]
- Nowotny, R. XMuDat: Photon Attenuation Data on PC, Version 1.0.1. IAEA-NDS-195 International Atomic Energy Agency, Vienna, Austria. 1998. Available online: http://www.mds.iaea.or.at/reports/mds-195.htm (accessed on 24 September 2023).
- Poludniowski, G.; Landry, G.; DeBlois, F.; Evans, P.M.; Verhaegen, F. SpekCalc: A program to calculate photon spectra from tungsten anode X-ray tubes. Phys. Med. Biol. 2009, 54, 433–438. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, S.; Engels, E.; Tehei, M.; Konstantinov, K.; Corde, S.; Oktaria, S.; Incerti, S.; Lerch, M.; Rosenfeld, A.; Guatelli, S. Study of the effect of ceramic Ta2O5 nanoparticle distribution on cellular dose enhancement in a kilovoltage photon field. Phys. Med. 2016, 32, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.; Corde, S.; McKinnon, S.; Incerti, S.; Konstantinov, K.; Rosenfeld, A.; Tehei, M.; Lerch, M.; Guatelli, S. Optimizing dose enhancement with Ta2O5 nanoparticles for synchrotron microbeam activated radiation therapy. Phys. Med. 2016, 32, 1852–1861. [Google Scholar]
- Brown, R.; Tehei, M.; Oktaria, S.; Briggs, A.; Stewart, C.; Konstantinov, K.; Rosenfeld, A.; Corde, S.; Lerch, M. High-Z nanostructured ceramics in radiotherapy: First evidence of Ta2O5-induced dose enhancement on radioresistant cancer cells in an MV photon field. Part. Part. Syst. Charact. 2014, 31, 500–505. [Google Scholar] [CrossRef]
- Berger, M.J.; Coursey, J.S.; Zucker, M.A.; Chang, J. ESTAR, PSTAR, and ASTAR: Computer Programs for Calculating Stopping-Power and Range Tables for Electrons, Protons, and Helium Ions (Version 1.2.3); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2005. Available online: http://physics.nist.gov/Star (accessed on 24 September 2023).
- Dobešová, L.; Gier, T.; Kopečná, O.; Pagáčová, E.; Vičar, T.; Bestvater, F.; Toufar, J.; Bačíková, A.; Kopel, P.; Fedr, R.; et al. Incorporation of Low Concentrations of Gold Nanoparticles: Complex Effects on Radiation Response and Fate of Cancer Cells. Pharmaceutics 2022, 14, 166. [Google Scholar] [CrossRef]
- Pagáčová, E.; Štefančíková, L.; Schmidt-Kaler, F.; Hildenbrand, G.; Vičar, T.; Depeš, D.; Lee, J.H.; Bestvater, F.; Lacombe, S.; Porcel, E.; et al. Challenges and Contradictions of Metal Nano-Particle Applications for Radio-Sensitivity Enhancement in Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 588. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.; Li, N.; Davis, J.A.; Paino, J.; Cameron, M.; Dipuglia, A.; Vogel, S.; Valceski, M.; Khochaiche, A.; O’Keefe, A.; et al. Toward personalized synchrotron microbeam radiation therapy. Sci. Rep. 2020, 10, 8833-1–8833-13. [Google Scholar] [CrossRef] [PubMed]
- Brönnimann, D.; Bouchet, A.; Schneider, C.; Potez, M.; Serduc, R.; Bräuer-Krisch, E.; Graber, W.; von Gunten, S.; Laissue, J.A.; Djonov, V. Synchrotron microbeam irradiation induces neutrophil infiltration, thrombocyte attachment and selective vascular damage in vivo. Sci. Rep. 2016, 6, 33601. [Google Scholar] [CrossRef] [PubMed]
- Le Duc, G.; Miladi, I.; Alric, C.; Mowat, P.; Bräuer-Krisch, E.; Bouchet, A.; Khalil, E.; Billotey, C.; Janier, M.; Lux, F.; et al. Toward an Image-Guided Microbeam Radiation Therapy Using Gadolinium-Based Nanoparticles. ACS Nano 2011, 5, 9566–9574. [Google Scholar] [CrossRef] [PubMed]
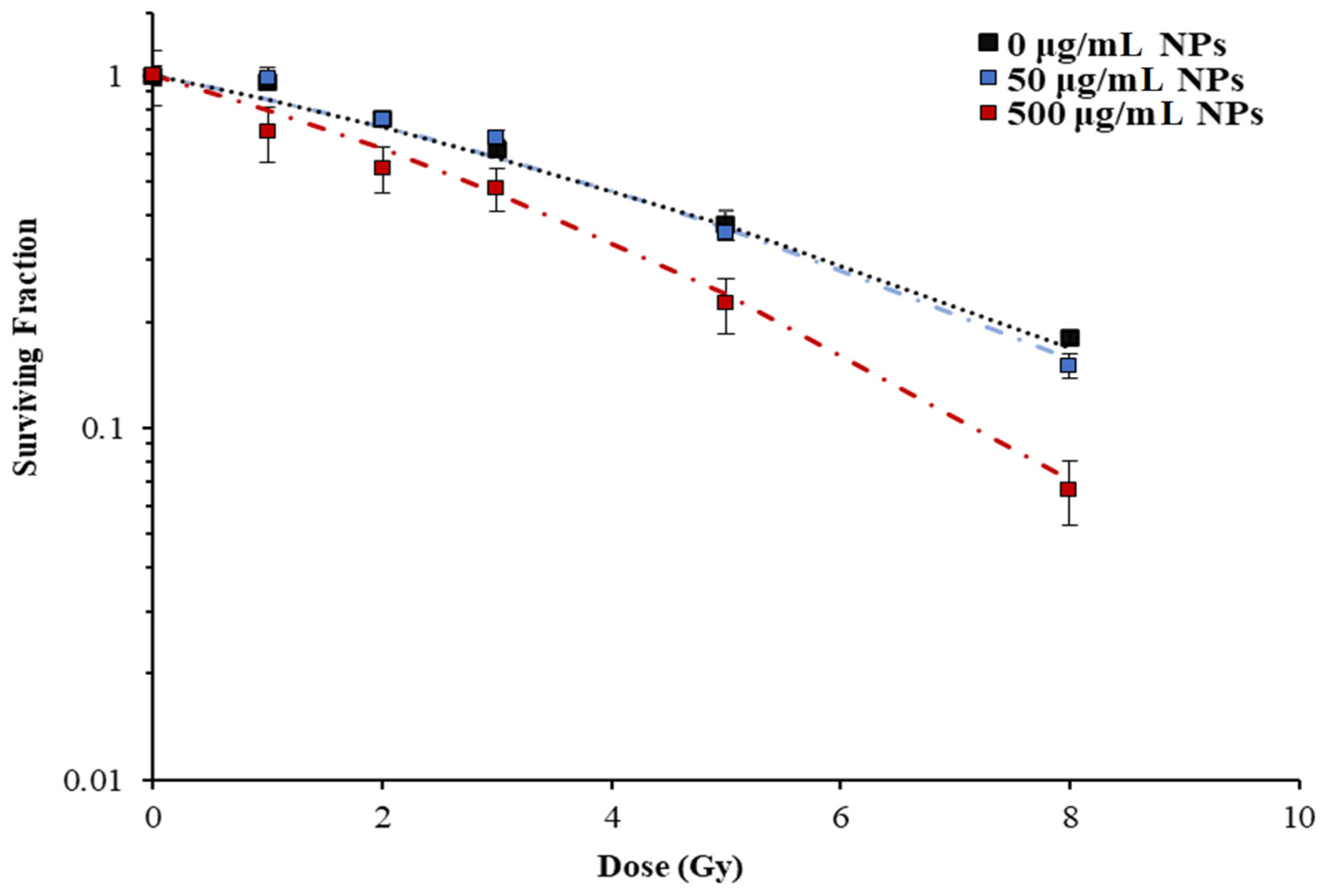
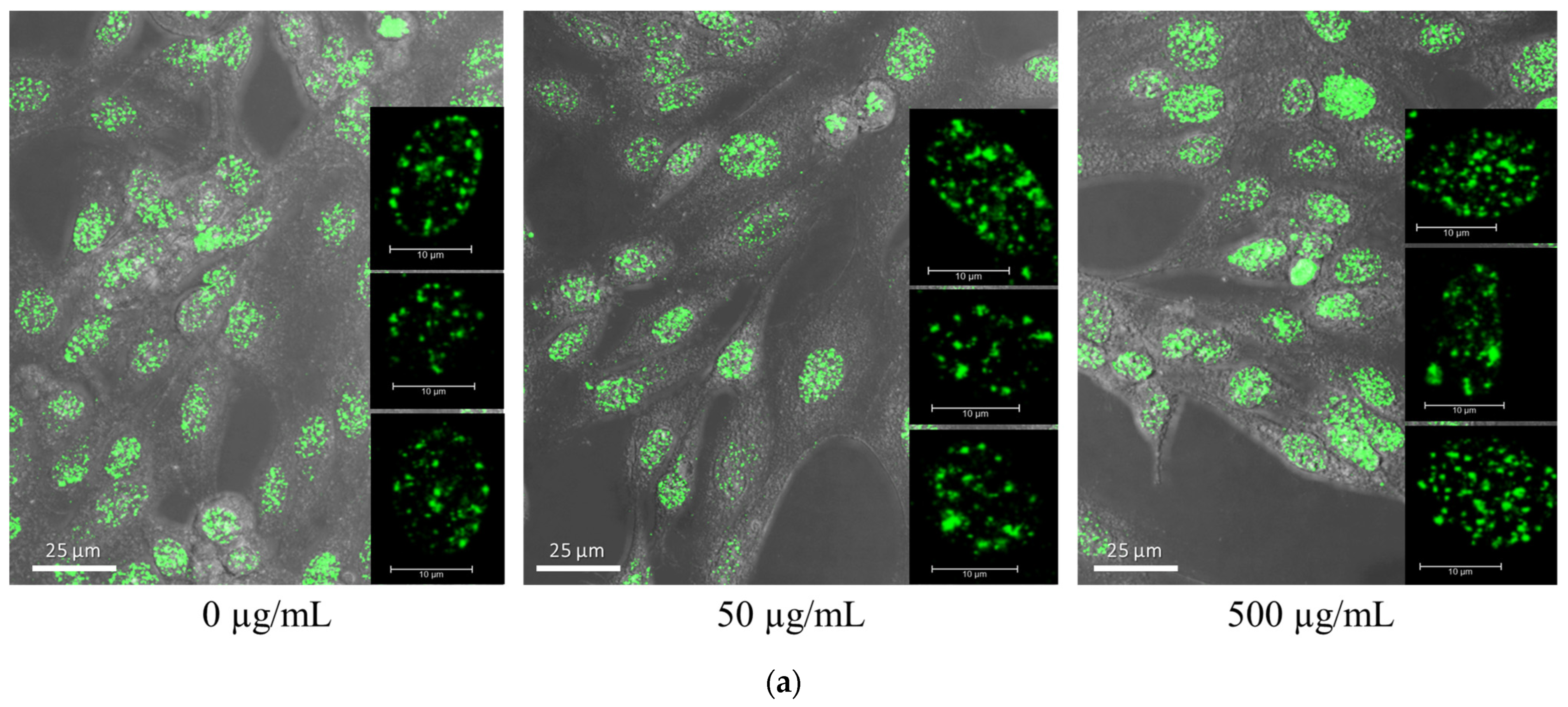

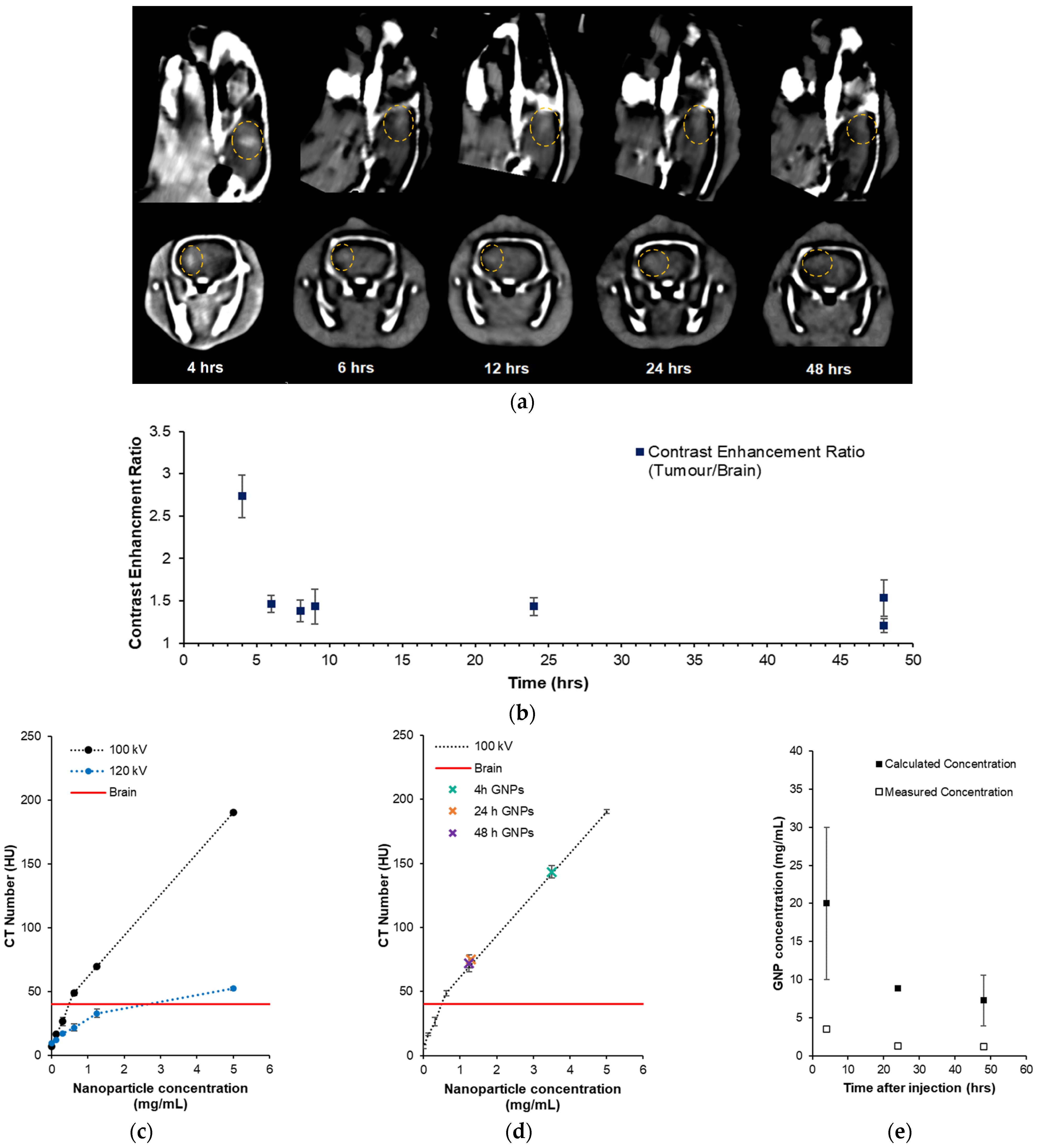
| Treatment | α (Gy−1) | β (Gy−2) | SER10 |
|---|---|---|---|
| 150 kVp, 0 µg/mL GNPs | 0.13 ± 0.03 | 0.0011 ± 0.008 | 1 |
| 150 kVp, 50 µg/mL GNPs | 0.13 ± 0.04 | 0.015 ± 0.005 | 1.13 |
| 150 kVp, 500 µg/mL GNPs | 0.33 ± 0.03 | 0 | 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engels, E.; Lerch, M.; Corde, S.; Tehei, M. Efficacy of 15 nm Gold Nanoparticles for Image-Guided Gliosarcoma Radiotherapy. J. Nanotheranostics 2023, 4, 480-495. https://doi.org/10.3390/jnt4040021
Engels E, Lerch M, Corde S, Tehei M. Efficacy of 15 nm Gold Nanoparticles for Image-Guided Gliosarcoma Radiotherapy. Journal of Nanotheranostics. 2023; 4(4):480-495. https://doi.org/10.3390/jnt4040021
Chicago/Turabian StyleEngels, Elette, Michael Lerch, Stéphanie Corde, and Moeava Tehei. 2023. "Efficacy of 15 nm Gold Nanoparticles for Image-Guided Gliosarcoma Radiotherapy" Journal of Nanotheranostics 4, no. 4: 480-495. https://doi.org/10.3390/jnt4040021
APA StyleEngels, E., Lerch, M., Corde, S., & Tehei, M. (2023). Efficacy of 15 nm Gold Nanoparticles for Image-Guided Gliosarcoma Radiotherapy. Journal of Nanotheranostics, 4(4), 480-495. https://doi.org/10.3390/jnt4040021