Enhancing Antibody Exposure in the Central Nervous System: Mechanisms of Uptake, Clearance, and Strategies for Improved Brain Delivery
Abstract
:1. Introduction
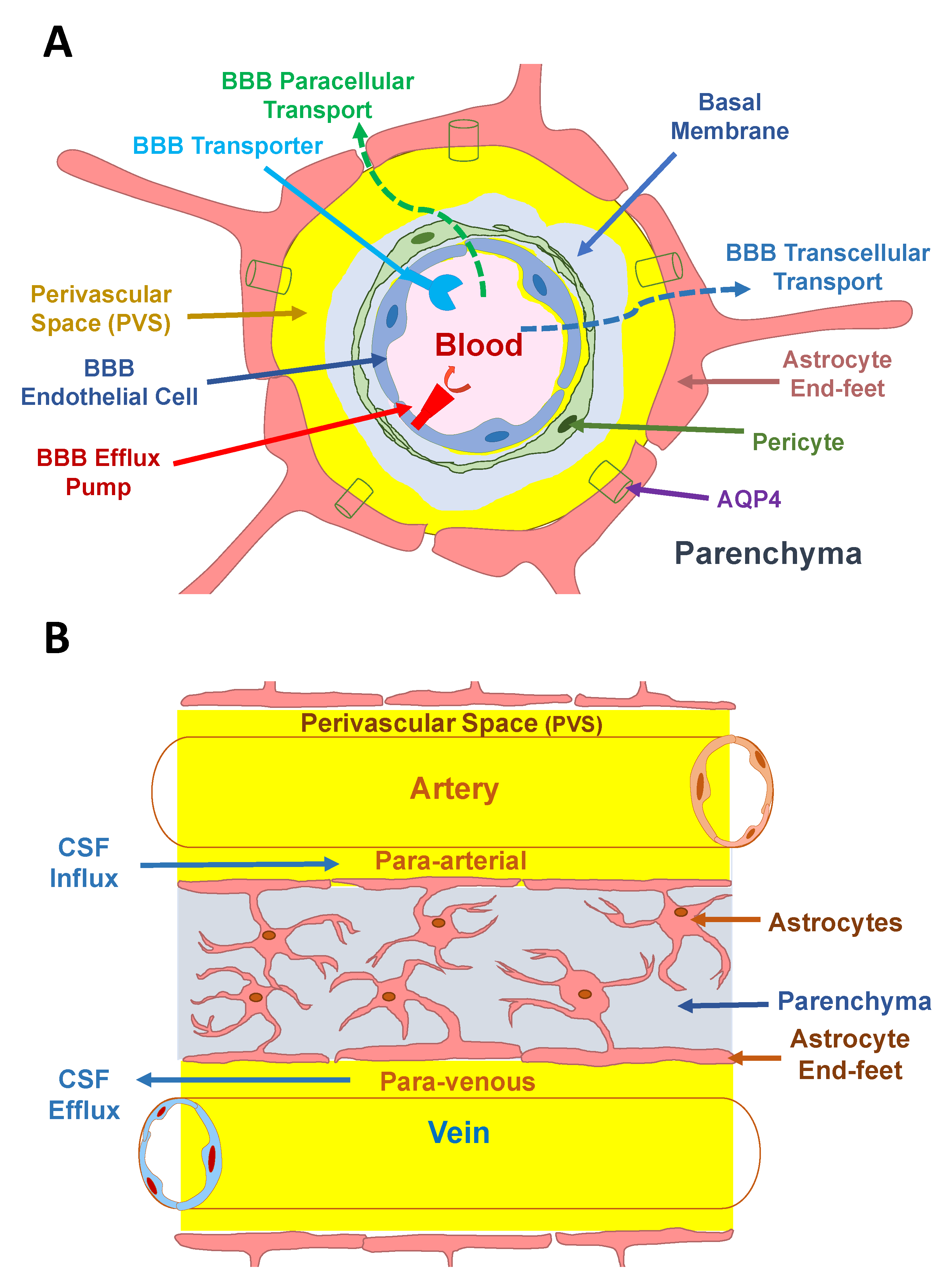
1.1. The Blood–Brain Barrier
1.2. The Blood–Cerebrospinal Fluid (CSF) Barrier (BCSFB)
2. Mechanisms of Antibody Uptake into the CNS
2.1. Crossing the BCSFB
2.2. Non-Specific Endocytosis
2.3. Antibody Clearance from the CNS
2.4. Neonatal Fc Receptor
3. The Glymphatic System and Bulk Convective Flow
4. Diffusion
5. Antibody Delivery Strategies into the CNS
5.1. Receptor-Mediated Transcytosis (Trojan Horse Method)
5.2. Osmotic BBB Opening
5.3. Focused Ultrasound Microbubbles
5.4. BBB-Modulating Peptides
5.5. Enhancing Antibody Retention
5.6. Brain Delivery Using Nanoparticles
5.7. Intranasal Brain Delivery of Proteins and Peptides
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergman, I.; Burckart, G.J.; Pohl, C.R.; Venkataramanan, R.; Barmada, M.A.; Griffin, J.A.; Cheung, N.K. Pharmacokinetics of IgG and IgM anti-ganglioside antibodies in rats and monkeys after intrathecal administration. J. Pharmacol. Exp. Ther. 1998, 284, 111–115. [Google Scholar] [PubMed]
- Noguchi, Y.; Kato, M.; Ozeki, K.; Ishigai, M. Pharmacokinetics of an intracerebroventricularly administered antibody in rats. mAbs 2017, 9, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Kemshead, J.T.; Hopkins, K.; Pizer, B.; Papanastassiou, V.; Coakham, H.; Bullimore, J.; Chandler, C. Dose escalation with repeated intrathecal injections of 131I-labelled MAbs for the treatment of central nervous system malignancies. Br. J. Cancer 1998, 77, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J. Anti-Amyloid Monoclonal Antibodies are Transformative Treatments that Redefine Alzheimer's Disease Therapeutics. Drugs 2023, 83, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Gandy, S.; Ehrlich, M.E. Moving the Needle on Alzheimer's Disease with an Anti-Oligomer Antibody. N. Engl. J. Med. 2023, 388, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, S.; Khan, K.; Barbour, R.; Doan, M.; Chen, M.; Guido, T.; Gill, D.; Basi, G.; Schenk, D.; Seubert, P.; et al. Immunotherapy reduces vascular amyloid-beta in PDAPP mice. J. Neurosci. 2008, 28, 6787–6793. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier delivery. Drug Discov Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2018, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef]
- Spector, R.; Keep, R.F.; Robert Snodgrass, S.; Smith, Q.R.; Johanson, C.E. A balanced view of choroid plexus structure and function: Focus on adult humans. Exp. Neurol. 2015, 267, 78–86. [Google Scholar] [CrossRef]
- Chang, H.Y.; Morrow, K.; Bonacquisti, E.; Zhang, W.; Shah, D.K. Antibody pharmacokinetics in rat brain determined using microdialysis. mAbs 2018, 10, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Abuqayyas, L.; Balthasar, J.P. Investigation of the role of FcgammaR and FcRn in mAb distribution to the brain. Mol. Pharm. 2013, 10, 1505–1513. [Google Scholar] [CrossRef]
- Garg, A.; Balthasar, J.P. Investigation of the influence of FcRn on the distribution of IgG to the brain. AAPS J. 2009, 11, 553–557. [Google Scholar] [CrossRef]
- Bard, F.; Fox, M.; Friedrich, S.; Seubert, P.; Schenk, D.; Kinney, G.G.; Yednock, T. Sustained levels of antibodies against Abeta in amyloid-rich regions of the CNS following intravenous dosing in human APP transgenic mice. Exp. Neurol. 2012, 238, 38–43. [Google Scholar] [CrossRef]
- Bohrmann, B.; Baumann, K.; Benz, J.; Gerber, F.; Huber, W.; Knoflach, F.; Messer, J.; Oroszlan, K.; Rauchenberger, R.; Richter, W.F.; et al. Gantenerumab: A novel human anti-Abeta antibody demonstrates sustained cerebral amyloid-beta binding and elicits cell-mediated removal of human amyloid-beta. J. Alzheimers Dis. 2012, 28, 49–69. [Google Scholar] [CrossRef]
- Antibodies in late-stage clinical studies. Available online: https://www.antibodysociety.org/antibodies-in-late-stage-clinical-studies/ (accessed on 12 July 2023).
- Antibody therapeutics approved or in regulatory review in the EU or US. Available online: https://www.antibodysociety.org/resources/approved-antibodies/ (accessed on 12 July 2023).
- Cavaco, M.; Gaspar, D.; Arb Castanho, M.; Neves, V. Antibodies for the Treatment of Brain Metastases, a Dream or a Reality? Pharmaceutics 2020, 12, 62. [Google Scholar] [CrossRef]
- Selewski, D.T.; Shah, G.V.; Segal, B.M.; Rajdev, P.A.; Mukherji, S.K. Natalizumab (Tysabri). AJNR Am. J. Neuroradiol. 2010, 31, 1588–1590. [Google Scholar] [CrossRef] [PubMed]
- Withington, C.G.; Turner, R.S. Amyloid-Related Imaging Abnormalities With Anti-amyloid Antibodies for the Treatment of Dementia Due to Alzheimer’s Disease. Front. Neurol. 2022, 13, 862369. [Google Scholar] [CrossRef]
- Kouhi, A.; Pachipulusu, V.; Kapenstein, T.; Hu, P.; Epstein, A.L.; Khawli, L.A. Brain Disposition of Antibody-Based Therapeutics: Dogma, Approaches and Perspectives. Int. J. Mol. Sci. 2021, 22, 6442. [Google Scholar] [CrossRef] [PubMed]
- Ineichen, B.V.; Plattner, P.S.; Good, N.; Martin, R.; Linnebank, M.; Schwab, M.E. Nogo-A Antibodies for Progressive Multiple Sclerosis. CNS Drugs 2017, 31, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, S.; Tortorella, C.; Gasperini, C. Anti lingo 1 (opicinumab) a new monoclonal antibody tested in relapsing remitting multiple sclerosis. Expert. Rev. Neurother. 2017, 17, 1081–1089. [Google Scholar] [CrossRef]
- Ciric, B.; Howe, C.L.; Paz Soldan, M.; Warrington, A.E.; Bieber, A.J.; Van Keulen, V.; Rodriguez, M.; Pease, L.R. Human monoclonal IgM antibody promotes CNS myelin repair independent of Fc function. Brain Pathol. 2003, 13, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Fisher, T.L.; Reilly, C.A.; Winter, L.A.; Pandina, T.; Jonason, A.; Scrivens, M.; Balch, L.; Bussler, H.; Torno, S.; Seils, J.; et al. Generation and preclinical characterization of an antibody specific for SEMA4D. mAbs 2016, 8, 150–162. [Google Scholar] [CrossRef]
- Florian, H.; Arnold, S.E.; Bateman, R.; Braunstein, J.B.; Budur, K.; Kerwin, D.R.; Soares, H.; Wang, D.; Holtzman, D.M. BBV-8E12, A humanized anti-Tau monoclonal antibody for treating early Alzheimer’s disease: Updated design and baseline characteristics of a Phase 2 study. Alzheimer’s Dementia 2019, 15, 251–252. [Google Scholar] [CrossRef]
- Rubenstein, J.L.; Combs, D.; Rosenberg, J.; Levy, A.; McDermott, M.; Damon, L.; Ignoffo, R.; Aldape, K.; Shen, A.; Lee, D.; et al. Rituximab therapy for CNS lymphomas: Targeting the leptomeningeal compartment. Blood 2003, 101, 466–468. [Google Scholar] [CrossRef]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert. Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef]
- Reiber, H. Proteins in cerebrospinal fluid and blood: Barriers, CSF flow rate and source-related dynamics. Restor. Neurol. Neurosci. 2003, 21, 79–96. [Google Scholar]
- Van De Vyver, A.J.; Walz, A.C.; Heins, M.S.; Abdolzade-Bavil, A.; Kraft, T.E.; Waldhauer, I.; Otteneder, M.B. Investigating brain uptake of a non-targeting monoclonal antibody after intravenous and intracerebroventricular administration. Front. Pharmacol. 2022, 13, 958543. [Google Scholar] [CrossRef]
- Ruano-Salguero, J.S.; Lee, K.H. Antibody transcytosis across brain endothelial-like cells occurs nonspecifically and independent of FcRn. Sci. Rep. 2020, 10, 3685. [Google Scholar] [CrossRef]
- Yu, Y.J.; Watts, R.J. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics 2013, 10, 459–472. [Google Scholar] [CrossRef]
- Mantle, J.L.; Lee, K.H. Immunoglobulin G transport increases in an in vitro blood-brain barrier model with amyloid-beta and with neuroinflammatory cytokines. Biotechnol. Bioeng. 2019, 116, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Atwal, J.K.; Chen, Y.; Chiu, C.; Mortensen, D.L.; Meilandt, W.J.; Liu, Y.; Heise, C.E.; Hoyte, K.; Luk, W.; Lu, Y.; et al. A therapeutic antibody targeting BACE1 inhibits amyloid-beta production in vivo. Sci. Transl. Med. 2011, 3, 84ra43. [Google Scholar] [CrossRef] [PubMed]
- Triguero, D.; Buciak, J.B.; Yang, J.; Pardridge, W.M. Blood-brain barrier transport of cationized immunoglobulin G: Enhanced delivery compared to native protein. Proc. Natl. Acad. Sci. USA 1989, 86, 4761–4765. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, A.K.; Eisenberg, J.B.; Pardridge, W.M. Absorptive-mediated endocytosis of cationized albumin and a beta-endorphin-cationized albumin chimeric peptide by isolated brain capillaries. Model system of blood-brain barrier transport. J. Biol. Chem. 1987, 262, 15214–15219. [Google Scholar] [CrossRef]
- Zhang, Y.; Pardridge, W.M. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J. Neuroimmunol. 2001, 114, 168–172. [Google Scholar] [CrossRef]
- Wolak, D.J.; Pizzo, M.E.; Thorne, R.G. Probing the extracellular diffusion of antibodies in brain using in vivo integrative optical imaging and ex vivo fluorescence imaging. J. Control Release 2015, 197, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Schlachetzki, F.; Zhu, C.; Pardridge, W.M. Expression of the neonatal Fc receptor (FcRn) at the blood-brain barrier. J. Neurochem. 2002, 81, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Yip, V.; Palma, E.; Tesar, D.B.; Mundo, E.E.; Bumbaca, D.; Torres, E.K.; Reyes, N.A.; Shen, B.Q.; Fielder, P.J.; Prabhu, S.; et al. Quantitative cumulative biodistribution of antibodies in mice: Effect of modulating binding affinity to the neonatal Fc receptor. MAbs 2014, 6, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J.; Zhang, Y.; Zhang, Y.; Xia, C.F.; Pardridge, W.M. Fusion antibody for Alzheimer’s disease with bidirectional transport across the blood-brain barrier and abeta fibril disaggregation. Bioconjug Chem. 2007, 18, 447–455. [Google Scholar] [CrossRef]
- Cooper, P.R.; Ciambrone, G.J.; Kliwinski, C.M.; Maze, E.; Johnson, L.; Li, Q.; Feng, Y.; Hornby, P.J. Efflux of monoclonal antibodies from rat brain by neonatal Fc receptor, FcRn. Brain Res. 2013, 1534, 13–21. [Google Scholar] [CrossRef]
- Deane, R.; Sagare, A.; Hamm, K.; Parisi, M.; LaRue, B.; Guo, H.; Wu, Z.; Holtzman, D.M.; Zlokovic, B.V. IgG-assisted age-dependent clearance of Alzheimer's amyloid beta peptide by the blood-brain barrier neonatal Fc receptor. J. Neurosci. 2005, 25, 11495–11503. [Google Scholar] [CrossRef] [PubMed]
- Aukland, K.; Reed, R.K. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol. Rev. 1993, 73, 1–78. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Ding, Z.; Fan, X.; Zhang, Y.; Yao, M.; Wang, G.; Dong, Y.; Liu, J.; Song, W. The glymphatic system: A new perspective on brain diseases. Front. Aging Neurosci. 2023, 15, 1179988. [Google Scholar] [CrossRef]
- Yang, L.; Kress, B.T.; Weber, H.J.; Thiyagarajan, M.; Wang, B.; Deane, R.; Benveniste, H.; Iliff, J.J.; Nedergaard, M. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J. Transl. Med. 2013, 11, 107. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef]
- Takano, K.; Yamada, M. Contrast-enhanced magnetic resonance imaging evidence for the role of astrocytic aquaporin-4 water channels in glymphatic influx and interstitial solute transport. Magn. Reson. Imaging 2020, 71, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Cserr, H.F.; Cooper, D.N.; Suri, P.K.; Patlak, C.S. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol. 1981, 240, F319–F328. [Google Scholar] [CrossRef] [PubMed]
- Gomolka, R.S.; Hablitz, L.M.; Mestre, H.; Giannetto, M.; Du, T.; Hauglund, N.L.; Xie, L.; Peng, W.; Martinez, P.M.; Nedergaard, M.; et al. Loss of aquaporin-4 results in glymphatic system dysfunction via brain-wide interstitial fluid stagnation. Elife 2023, 12, e82232. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, C.; Sykova, E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998, 21, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Begley, D.J. Brain superhighways. Sci. Transl. Med. 2012, 4, 147fs129. [Google Scholar] [CrossRef]
- Verkman, A.S.; Tradtrantip, L.; Smith, A.J.; Yao, X. Aquaporin Water Channels and Hydrocephalus. Pediatr. Neurosurg. 2017, 52, 409–416. [Google Scholar] [CrossRef]
- Pizzo, M.E.; Wolak, D.J.; Kumar, N.N.; Brunette, E.; Brunnquell, C.L.; Hannocks, M.J.; Abbott, N.J.; Meyerand, M.E.; Sorokin, L.; Stanimirovic, D.B.; et al. Intrathecal antibody distribution in the rat brain: Surface diffusion, perivascular transport and osmotic enhancement of delivery. J. Physiol. 2018, 596, 445–475. [Google Scholar] [CrossRef]
- Pardridge, W.M. Molecular Trojan horses for blood-brain barrier drug delivery. Curr. Opin. Pharmacol. 2006, 6, 494–500. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Buciak, J.L.; Friden, P.M. Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J. Pharmacol. Exp. Ther. 1991, 259, 66–70. [Google Scholar] [PubMed]
- Friden, P.M.; Walus, L.R.; Musso, G.F.; Taylor, M.A.; Malfroy, B.; Starzyk, R.M. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc. Natl. Acad. Sci. USA 1991, 88, 4771–4775. [Google Scholar] [CrossRef]
- Roth, R.A.; Cassell, D.J.; Wong, K.Y.; Maddux, B.A.; Goldfine, I.D. Monoclonal antibodies to the human insulin receptor block insulin binding and inhibit insulin action. Proc. Natl. Acad. Sci. USA 1982, 79, 7312–7316. [Google Scholar] [CrossRef]
- Bickel, U.; Yoshikawa, T.; Landaw, E.M.; Faull, K.F.; Pardridge, W.M. Pharmacologic effects in vivo in brain by vector-mediated peptide drug delivery. Proc. Natl. Acad. Sci. USA 1993, 90, 2618–2622. [Google Scholar] [CrossRef]
- Friden, P.M.; Walus, L.R.; Watson, P.; Doctrow, S.R.; Kozarich, J.W.; Backman, C.; Bergman, H.; Hoffer, B.; Bloom, F.; Granholm, A.C. Blood-brain barrier penetration and in vivo activity of an NGF conjugate. Science 1993, 259, 373–377. [Google Scholar] [CrossRef]
- Wu, D.; Yang, J.; Pardridge, W.M. Drug targeting of a peptide radiopharmaceutical through the primate blood-brain barrier in vivo with a monoclonal antibody to the human insulin receptor. J. Clin. Invest. 1997, 100, 1804–1812. [Google Scholar] [CrossRef]
- Gosk, S.; Vermehren, C.; Storm, G.; Moos, T. Targeting anti-transferrin receptor antibody (OX26) and OX26-conjugated liposomes to brain capillary endothelial cells using in situ perfusion. J. Cereb. Blood Flow. Metab. 2004, 24, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Moos, T.; Morgan, E.H. Restricted transport of anti-transferrin receptor antibody (OX26) through the blood-brain barrier in the rat. J. Neurochem. 2001, 79, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Wu, S.; Li, Y.; Zhang, W.; Burrell, M.; Webster, C.I.; Shah, D.K. Brain pharmacokinetics of anti-transferrin receptor antibody affinity variants in rats determined using microdialysis. mAbs 2021, 13, 1874121. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J.; et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011, 3, 84ra44. [Google Scholar] [CrossRef]
- Niewoehner, J.; Bohrmann, B.; Collin, L.; Urich, E.; Sade, H.; Maier, P.; Rueger, P.; Stracke, J.O.; Lau, W.; Tissot, A.C.; et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 2014, 81, 49–60. [Google Scholar] [CrossRef]
- Haqqani, A.S.; Thom, G.; Burrell, M.; Delaney, C.E.; Brunette, E.; Baumann, E.; Sodja, C.; Jezierski, A.; Webster, C.; Stanimirovic, D.B. 2018. Intracellular sorting and transcytosis of the rat transferrin receptor antibody OX26 across the blood-brain barrier in vitro is dependent on its binding affinity. J. Neurochem. 2018, 146, 735–752. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J.; Hui, E.K.; Lu, J.Z.; Pardridge, W.M. IgG-enzyme fusion protein: Pharmacokinetics and anti-drug antibody response in rhesus monkeys. Bioconjug Chem. 2013, 24, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Giugliani, R.; Nestrasil, I.; Chen, S.; Pardridge, W.M.; Rioux, P. Intravenous Infusion of Iduronidase-IgG and Its Impact on the Central Nervous System in Children with Hurler Syndrome. Mol. Genet. Metab. 2017, 120, S55–S56. [Google Scholar] [CrossRef]
- Leoh, L.S.; Daniels-Wells, T.R.; Martinez-Maza, O.; Penichet, M.L. Insights into the effector functions of human IgG3 in the context of an antibody targeting transferrin receptor 1. Mol. Immunol. 2015, 67 Pt B, 407–415. [Google Scholar] [CrossRef]
- Okuyama, T.; Eto, Y.; Sakai, N.; Minami, K.; Yamamoto, T.; Sonoda, H.; Yamaoka, M.; Tachibana, K.; Hirato, T.; Sato, Y. Iduronate-2-Sulfatase with Anti-human Transferrin Receptor Antibody for Neuropathic Mucopolysaccharidosis II: A Phase 1/2 Trial. Mol. Ther. 2019, 27, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, T.; Eto, Y.; Sakai, N.; Nakamura, K.; Yamamoto, T.; Yamaoka, M.; Ikeda, T.; So, S.; Tanizawa, K.; Sonoda, H.; et al. A Phase 2/3 Trial of Pabinafusp Alfa, IDS Fused with Anti-Human Transferrin Receptor Antibody, Targeting Neurodegeneration in MPS-II. Mol. Ther. 2021, 29, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Giugliani, R.; Martins, A.M.; So, S.; Yamamoto, T.; Yamaoka, M.; Ikeda, T.; Tanizawa, K.; Sonoda, H.; Schmidt, M.; Sato, Y. Iduronate-2-sulfatase fused with anti-hTfR antibody, pabinafusp alfa, for MPS-II: A phase 2 trial in Brazil. Mol. Ther. 2021, 29, 2378–2386. [Google Scholar] [CrossRef]
- Kullic, L.; Vogt, A.; Alcarez, F.; Barrington, P.; Marchesi, M.; Svoboda, H. Brain Shuttle AD: Investigationg Safety, Tolerability, and PK/PD of RG6102 in Prodromal/Mild-to-Moderate AD. J. Neurol. Neurosurg. Psychiatry 2020, 27, 2019–2020. [Google Scholar]
- Weber, F.; Bohrmann, B.; Niewoehner, J.; Fischer, J.A.A.; Rueger, P.; Tiefenthaler, G.; Moelleken, J.; Bujotzek, A.; Brady, K.; Singer, T.; et al. Brain Shuttle Antibody for Alzheimer’s Disease with Attenuated Peripheral Effector Function due to an Inverted Binding Mode. Cell Rep. 2018, 22, 149–162. [Google Scholar] [CrossRef]
- Rapoport, S.I. Effect of concentrated solutions on blood-brain barrier. Am. J. Physiol. 1970, 219, 270–274. [Google Scholar] [CrossRef]
- Rapoport, S.I.; Hori, M.; Klatzo, I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am. J. Physiol. 1972, 223, 323–331. [Google Scholar] [CrossRef]
- Mayhan, W.G.; Heistad, D.D. Permeability of blood-brain barrier to various sized molecules. Am. J. Physiol. 1985, 248 Pt 2, H712–H718. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A.; Barnett, P.A.; Hellstrom, I.; Hellstrom, K.E.; Beaumier, P.; McCormick, C.I.; Weigel, R.M. Delivery of melanoma-associated immunoglobulin monoclonal antibody and Fab fragments to normal brain utilizing osmotic blood-brain barrier disruption. Cancer Res. 1988, 48, 4725–4729. [Google Scholar] [PubMed]
- Neuwelt, E.A.; Minna, J.; Frenkel, E.; Barnett, P.A.; McCormick, C.I. Osmotic blood-brain barrier opening to IgM monoclonal antibody in the rat. Am. J. Physiol. 1986, 250 Pt 2, R875–R883. [Google Scholar] [CrossRef] [PubMed]
- Bullard, D.E.; Bourdon, M.; Bigner, D.D. Comparison of various methods for delivering radiolabeled monoclonal antibody to normal rat brain. J. Neurosurg. 1984, 61, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.T.; Albrecht, P.; Rapoport, S.I. Entry of neutralizing antibody to measles into brain and cerebrospinal fluid of immunized monkeys after osmotic opening of the blood-brain barrier. Exp. Neurol. 1976, 53, 768–779. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Barnett, P.A.; McCormick, C.I.; Frenkel, E.P.; Minna, J.D. Osmotic blood-brain barrier modification: Monoclonal antibody, albumin, and methotrexate delivery to cerebrospinal fluid and brain. Neurosurgery 1985, 17, 419–423. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Specht, H.D.; Barnett, P.A.; Dahlborg, S.A.; Miley, A.; Larson, S.M.; Brown, P.; Eckerman, K.F.; Hellstrom, K.E.; Hellstrom, I. Increased delivery of tumor-specific monoclonal antibodies to brain after osmotic blood-brain barrier modification in patients with melanoma metastatic to the central nervous system. Neurosurgery 1987, 20, 885–895. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Specht, H.D.; Hill, S.A. Permeability of human brain tumor to 99mTc-gluco-heptonate and 99mTc-albumin. Implications for monoclonal antibody therapy. J. Neurosurg. 1986, 65, 194–198. [Google Scholar] [CrossRef]
- Remsen, L.G.; Trail, P.A.; Hellstrom, I.; Hellstrom, K.E.; Neuwelt, E.A. Enhanced delivery improves the efficacy of a tumor-specific doxorubicin immunoconjugate in a human brain tumor xenograft model. Neurosurgery 2000, 46, 704–709. [Google Scholar] [CrossRef]
- Muldoon, L.L.; Neuwelt, E.A. BR96-DOX immunoconjugate targeting of chemotherapy in brain tumor models. J. Neurooncol 2003, 65, 49–62. [Google Scholar] [CrossRef]
- Nadal, A.; Fuentes, E.; Pastor, J.; McNaughton, P.A. Plasma albumin is a potent trigger of calcium signals and DNA synthesis in astrocytes. Proc. Natl. Acad. Sci. USA 1995, 92, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.A.; Ryu, J.K.; Chang, K.J.; Etxeberria, A.; Bardehle, S.; Mendiola, A.S.; Kamau-Devers, W.; Fancy, S.P.J.; Thor, A.; Bushong, E.A.; et al. Fibrinogen Activates BMP Signaling in Oligodendrocyte Progenitor Cells and Inhibits Remyelination after Vascular Damage. Neuron 2017, 96, 1003–1012.e1007. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, T.S.; Johansson, B.B.; Kalimo, H.; Olsson, Y. Structural changes in the rat brain after carotid infusions of hyperosmolar solutions. An electron microscopic study. Acta Neuropathol. 1988, 77, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Burks, S.R.; Kersch, C.N.; Witko, J.A.; Pagel, M.A.; Sundby, M.; Muldoon, L.L.; Neuwelt, E.A.; Frank, J.A. Blood-brain barrier opening by intracarotid artery hyperosmolar mannitol induces sterile inflammatory and innate immune responses. Proc. Natl. Acad. Sci. USA 2021, 118, e2021915118. [Google Scholar] [CrossRef]
- Lossinsky, A.S.; Vorbrodt, A.W.; Wisniewski, H.M. Scanning and transmission electron microscopic studies of microvascular pathology in the osmotically impaired blood-brain barrier. J. Neurocytol. 1995, 24, 795–806. [Google Scholar] [CrossRef]
- Warwick, R.; Pond, J. Trackless Lesions in Nervous Tissues Produced by High Intensity Focused Ultrasound (High-Frequency Mechanical Waves). J. Anat. 1968, 102, 387–405. [Google Scholar]
- Vykhodtseva, N.I.; Hynynen, K.; Damianou, C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med. Biol. 1995, 21, 969–979. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.; Wang, S.; Tung, Y.S.; Morrison, B., 3rd; Konofagou, E.E. Molecules of various pharmacologically-relevant sizes can cross the ultrasound-induced blood-brain barrier opening in vivo. Ultrasound Med. Biol. 2010, 36, 58–67. [Google Scholar] [CrossRef]
- Chen, H.; Konofagou, E.E. The size of blood-brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J. Cereb. Blood Flow. Metab. 2014, 34, 1197–1204. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc. Natl. Acad. Sci. USA 2006, 103, 11719–11723. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem. Biophys. Res. Commun. 2006, 340, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Shi, Q.; Zhang, Y.; Power, C.; Hoesch, C.; Antonelli, S.; Schroeder, M.K.; Caldarone, B.J.; Taudte, N.; Schenk, M.; et al. Focused ultrasound with anti-pGlu3 Abeta enhances efficacy in Alzheimer's disease-like mice via recruitment of peripheral immune cells. J. Control Release 2021, 336, 443–456. [Google Scholar] [CrossRef]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef] [PubMed]
- Lutz, K.L.; Siahaan, T.J. Modulation of the cellular junction protein E-cadherin in bovine brain microvessel endothelial cells by cadherin peptides. Drug Deliv. 1997, 4, 187–193. [Google Scholar] [CrossRef]
- Makagiansar, I.T.; Avery, M.; Hu, Y.; Audus, K.L.; Siahaan, T.J. Improving the selectivity of HAV-peptides in modulating E-cadherin-E-cadherin interactions in the intercellular junction of MDCK cell monolayers. Pharm. Res. 2001, 18, 446–453. [Google Scholar] [CrossRef]
- Sinaga, E.; Jois, S.D.; Avery, M.; Makagiansar, I.T.; Tambunan, U.S.; Audus, K.L.; Siahaan, T.J. Increasing paracellular porosity by E-cadherin peptides: Discovery of bulge and groove regions in the EC1-domain of E-cadherin. Pharm. Res. 2002, 19, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Kiptoo, P.; Sinaga, E.; Calcagno, A.M.; Zhao, H.; Kobayashi, N.; Tambunan, U.S.; Siahaan, T.J. Enhancement of drug absorption through the blood-brain barrier and inhibition of intercellular tight junction resealing by E-cadherin peptides. Mol. Pharm. 2011, 8, 239–249. [Google Scholar] [CrossRef]
- Tabanor, K.; Lee, P.; Kiptoo, P.; Choi, I.Y.; Sherry, E.B.; Eagle, C.S.; Williams, T.D.; Siahaan, T.J. Brain Delivery of Drug and MRI Contrast Agent: Detection and Quantitative Determination of Brain Deposition of CPT-Glu Using LC-MS/MS and Gd-DTPA Using Magnetic Resonance Imaging. Mol. Pharm. 2016, 13, 379–390. [Google Scholar] [CrossRef]
- Laksitorini, M.D.; Kiptoo, P.K.; On, N.H.; Thliveris, J.A.; Miller, D.W.; Siahaan, T.J. Modulation of intercellular junctions by cyclic-ADT peptides as a method to reversibly increase blood-brain barrier permeability. J. Pharm. Sci. 2015, 104, 1065–1075. [Google Scholar] [CrossRef]
- On, N.H.; Kiptoo, P.; Siahaan, T.J.; Miller, D.W. Modulation of blood-brain barrier permeability in mice using synthetic E-cadherin peptide. Mol. Pharm. 2014, 11, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Alaofi, A.; On, N.; Kiptoo, P.; Williams, T.D.; Miller, D.W.; Siahaan, T.J. Comparison of Linear and Cyclic His-Ala-Val Peptides in Modulating the Blood-Brain Barrier Permeability: Impact on Delivery of Molecules to the Brain. J. Pharm. Sci. 2016, 105, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Ulapane, K.R.; On, N.; Kiptoo, P.; Williams, T.D.; Miller, D.W.; Siahaan, T.J. Improving Brain Delivery of Biomolecules via BBB Modulation in Mouse and Rat: Detection using MRI, NIRF, and Mass Spectrometry. Nanotheranostics 2017, 1, 217–231. [Google Scholar] [CrossRef]
- Kopec, B.M.; Kiptoo, P.; Zhao, L.; Rosa-Molinar, E.; Siahaan, T.J. Noninvasive Brain Delivery and Efficacy of BDNF to Stimulate Neuroregeneration and Suppression of Disease Relapse in EAE Mice. Mol. Pharm. 2020, 17, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Kopec, B.M.; Zhao, L.; Rosa-Molinar, E.; Siahaan, T.J. Non-invasive Brain Delivery and Efficacy of BDNF in APP/PS1 Transgenic Mice as a Model of Alzheimer's Disease. Med. Res. Arch. 2020, 8, 2043. [Google Scholar] [CrossRef]
- Ulapane, K.R.; Kopec, B.M.; Siahaan, T.J. In Vivo Brain Delivery and Brain Deposition of Proteins with Various Sizes. Mol. Pharm. 2019, 16, 4878–4889. [Google Scholar] [CrossRef]
- Ulapane, K.R.; Kopec, B.M.; Siahaan, T.J. Improving In Vivo Brain Delivery of Monoclonal Antibody Using Novel Cyclic Peptides. Pharmaceutics 2019, 11, 568. [Google Scholar] [CrossRef]
- Sajesh, B.V.; On, N.H.; Omar, R.; Alrushaid, S.; Kopec, B.M.; Wang, W.G.; Sun, H.D.; Lillico, R.; Lakowski, T.M.; Siahaan, T.J.; et al. Validation of Cadherin HAV6 Peptide in the Transient Modulation of the Blood-Brain Barrier for the Treatment of Brain Tumors. Pharmaceutics 2019, 11, 481. [Google Scholar] [CrossRef]
- Nakano, R.; Takagi-Maeda, S.; Ito, Y.; Kishimoto, S.; Osato, T.; Noguchi, K.; Kurihara-Suda, K.; Takahashi, N. A new technology for increasing therapeutic protein levels in the brain over extended periods. PLoS ONE 2019, 14, e0214404. [Google Scholar] [CrossRef]
- Bukhari, S.N.A. Nanotherapeutics for Alzheimer's Disease with Preclinical Evaluation and Clinical Trials: Challenges, Promises and Limitations. Curr. Drug Deliv. 2021, 19, 17–31. [Google Scholar] [CrossRef]
- Zinger, A.; Soriano, S.; Baudo, G.; De Rosa, E.; Taraballi, F.; Villapol, S. Biomimetic Nanoparticles as a Theranostic Tool for Traumatic Brain Injury. Adv. Funct. Mater. 2021, 31, 2100722. [Google Scholar] [CrossRef]
- Han, L.; Jiang, C. Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm. Sin. B 2021, 11, 2306–2325. [Google Scholar] [CrossRef]
- Busatto, S.; Morad, G.; Guo, P.; Moses, M.A. The role of extracellular vesicles in the physiological and pathological regulation of the blood-brain barrier. FASEB Bioadv. 2021, 3, 665–675. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through, B.B.B. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Nanobiotechnology-based strategies for crossing the blood-brain barrier. Nanomedicine 2012, 7, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, M.; Ma, X.; Chen, H.; Gao, X. Harnessing Exosomes for the Development of Brain Drug Delivery Systems. Bioconjug. Chem. 2019, 30, 994–1005. [Google Scholar] [CrossRef]
- Lea-Banks, H.; Hynynen, K. Sub-millimetre precision of drug delivery in the brain from ultrasound-triggered nanodroplets. J. Control Release 2021, 338, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yang, Y.; Lin, W.; Liu, H.; Liu, H.; Yang, Y.; Chen, Y.; Fu, X.; Deng, J. Cell-penetrating peptide-siRNA conjugate loaded YSA-modified nanobubbles for ultrasound triggered siRNA delivery. Colloids Surf. B Biointerfaces 2015, 136, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Wang, Y.; Zhang, L.; Liang, M.; Fu, S.; Cui, L.; Yang, M.; Gong, W.; Li, Z.; Yu, L.; et al. Glioma targeting peptide modified apoferritin nanocage. Drug Deliv. 2018, 25, 1013–1024. [Google Scholar] [CrossRef]
- Albright, B.H.; Storey, C.M.; Murlidharan, G.; Castellanos Rivera, R.M.; Berry, G.E.; Madigan, V.J.; Asokan, A. Mapping the Structural Determinants Required for AAVrh.10 Transport across the Blood-Brain Barrier. Mol. Ther. 2018, 26, 510–523. [Google Scholar] [CrossRef]
- Agbandje-McKenna, M.; Kleinschmidt, J. AAV capsid structure and cell interactions. Methods Mol. Biol. 2011, 807, 47–92. [Google Scholar] [CrossRef] [PubMed]
- Madigan, V.J.; Asokan, A. Engineering AAV receptor footprints for gene therapy. Curr. Opin. Virol. 2016, 18, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Furubayashi, T.; Arai, M.; Inoue, D.; Kimura, S.; Kiriyama, A.; Kusamori, K.; Katsumi, H.; Yutani, R.; Sakane, T.; et al. Delivery of Oxytocin to the Brain for the Treatment of Autism Spectrum Disorder by Nasal Application. Mol. Pharm. 2018, 15, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Alonso, M.J. Nose-to-brain peptide delivery—The potential of nanotechnology. Bioorg Med. Chem. 2018, 26, 2888–2905. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.E.; Salameh, T.S.; Banks, W.A. Intranasal Delivery of Proteins and Peptides in the Treatment of Neurodegenerative Diseases. AAPS J. 2015, 17, 780–787. [Google Scholar] [CrossRef]
- Dufes, C.; Olivier, J.C.; Gaillard, F.; Gaillard, A.; Couet, W.; Muller, J.M. Brain delivery of vasoactive intestinal peptide (VIP) following nasal administration to rats. Int. J. Pharm. 2003, 255, 87–97. [Google Scholar] [CrossRef]
| Alzheimer’s Disease | |||
|---|---|---|---|
| Name (Brand) | Target | mAb Type | US Approval (Status) |
| Aducanumab (Aduhelm) | Amyloid beta | Human IgG1 | 2021 |
| Lecanemab (Leqembi) | Amyloid beta | Humanized IgG1 | 2023 |
| Donanemab | Amyloid beta | Humanized IgG1 | 2nd Review |
| LY3372993; Remternetug | Amyloid beta | Human IgG1 | Phase 3 |
| Crenezumab | Amyloid beta | Humanized IgG4 | Phase 3 |
| Gantenerumab | Amyloid beta | Human IgG1 | Phase 3 |
| Solanezumab | Monomers | Humanized IgG1 | Phase 3 |
| E2814 | Tau protein | Humanized IgG1 | Phase 2/3 |
| Semorinemab | Tau protein | Humanized IgG4 | Phase 2 |
| BIB092 | Tau protein | Human mAb | Phase 2 |
| ABBV-8E12 | Tau protein | Human mAb | Phase 2 |
| Zagotenemab | Tau protein | Human mAb | Phase 2 |
| JNJ-63733657 | Tau protein | Human mAb | Phase 1 |
| AL002 | TREM-2 Receptor | Human mAb | Phase 1 |
| AL003 | SIGLEC-3 | Human mAb | Phase 1 |
| Frontotemporal Dementia | |||
| AL001; Latozinemab | Sortilin | Human IgG1 | Phase 3 |
| Glioblastoma | |||
| 125I-mAb 425 | EGFR | Human mAb | Phase 2 |
| Depatuxizumab mafodotin | EGFR | IgG1 ADC | Phase 2b/3 |
| [188Re]-labeled Nimotuzumab | EGFR | Humanized mAb | Phase 1 |
| 131I-chTNT-1/B MAb | DNA-histone H1 complex | Human mAb | Phase 1/2 |
| 131I-BC-2 mAb | Tenascin | Human mAb | Phase 2 |
| 211At-labeled 81C6 mAb | Tenascin | Human mAb | Phase 1/2 |
| biotin-coupled BC-4 + Avidin + [90Y]-Biotin | Tenascin | Human mAb | Phase 1/2 |
| Neuroblastoma | |||
| Dinutuximab (Unituxin) | GD2 | Chimeric IgG1 | 2015 |
| 131I-omburtamab | BT-H3 | Murine mAb | Phase 2/3 |
| Multiple Sclerosis (MS) | |||
| Daclizumab (Zinbryta) | CD25 | Humanized IgG1 | 2016 |
| Divozilimab (Ivlizi) | CD20 | Humanized IgG1 | 2023 |
| Ocrelizumab (OCREVU) | CD20 | Humanized IgG1 | 2017 |
| Ublituximab (BRIUMVI) | CD20 | Humanized IgG1 | 2022 |
| Alemtuzumab (Lemtrada) | CD52 | Humanized IgG1 | 2014 |
| Natalizumab (Tysabri) | α4 integrin | Humanized IgG4 | 2014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwinghamer, K.; Siahaan, T.J. Enhancing Antibody Exposure in the Central Nervous System: Mechanisms of Uptake, Clearance, and Strategies for Improved Brain Delivery. J. Nanotheranostics 2023, 4, 463-479. https://doi.org/10.3390/jnt4040020
Schwinghamer K, Siahaan TJ. Enhancing Antibody Exposure in the Central Nervous System: Mechanisms of Uptake, Clearance, and Strategies for Improved Brain Delivery. Journal of Nanotheranostics. 2023; 4(4):463-479. https://doi.org/10.3390/jnt4040020
Chicago/Turabian StyleSchwinghamer, Kelly, and Teruna J. Siahaan. 2023. "Enhancing Antibody Exposure in the Central Nervous System: Mechanisms of Uptake, Clearance, and Strategies for Improved Brain Delivery" Journal of Nanotheranostics 4, no. 4: 463-479. https://doi.org/10.3390/jnt4040020
APA StyleSchwinghamer, K., & Siahaan, T. J. (2023). Enhancing Antibody Exposure in the Central Nervous System: Mechanisms of Uptake, Clearance, and Strategies for Improved Brain Delivery. Journal of Nanotheranostics, 4(4), 463-479. https://doi.org/10.3390/jnt4040020







