Responsive Nanostructure for Targeted Drug Delivery
Abstract
:1. Introduction
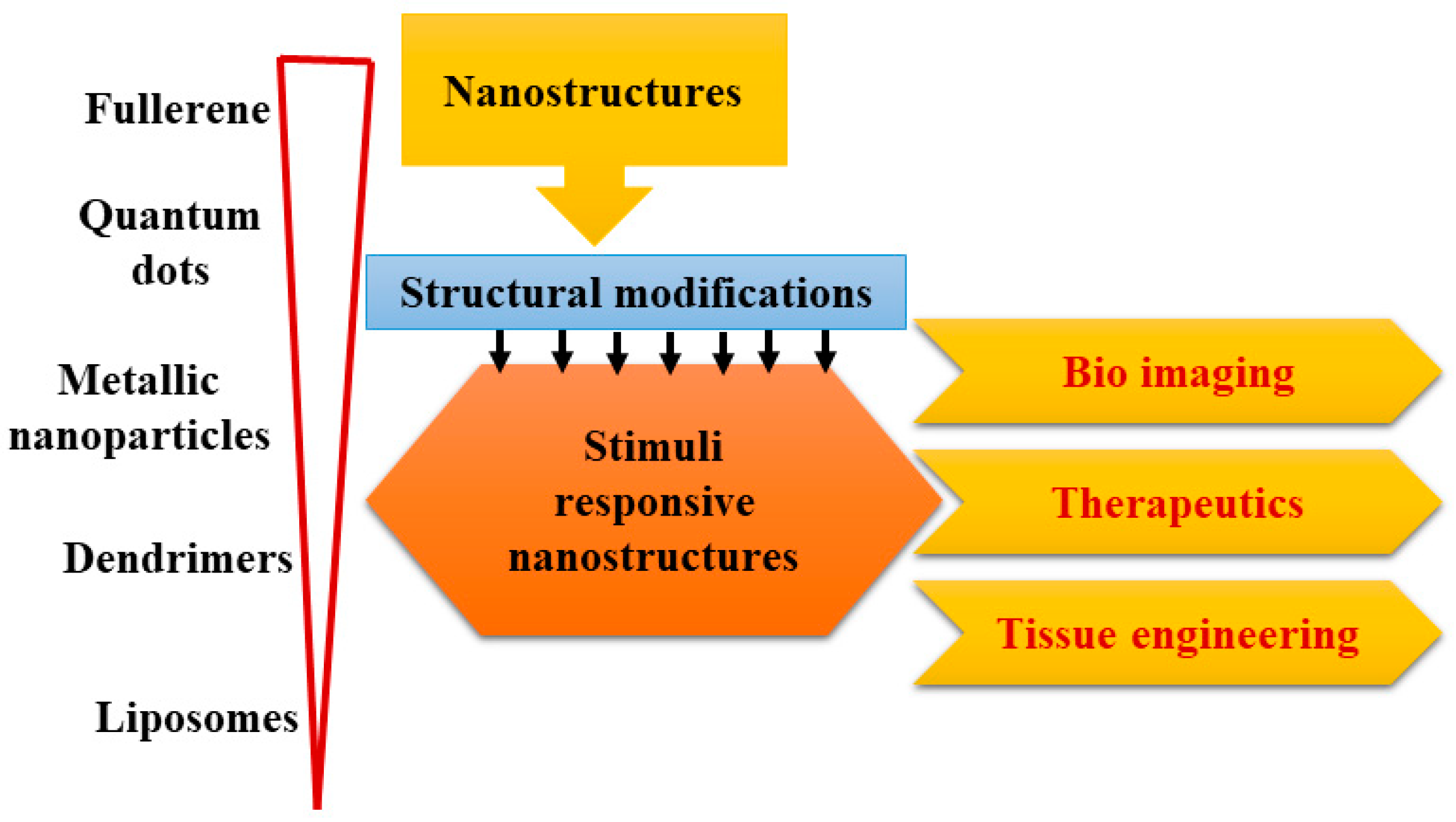
2. Design and Development of Biomaterials Comprising of Responsive Nanostructures
2.1. Liposomes
2.2. Dendrimers
2.3. Quantum Dots
2.4. Fullerene
2.5. Metallic Nanoparticles
3. Applications of Responsive Nanostructures in Various Clinical Applications
3.1. Cancer Therapy
3.1.1. External Stimuli
Photo Responsive
Thermal Responsive
Magnetic Field Responsive
Ultrasound Responsive
3.1.2. Internal Responsive
pH Responsive
Redox Responsive
Enzyme Responsive
Hypoxia Responsive
Combinational Stimuli-Responsive
3.2. Pain Management
| Drug | Photosensitizer | Trigger | Nanometer | Effect | Reference |
|---|---|---|---|---|---|
| Bupivacaine | Copolymer oligo (ethylene glycol) methyl ether methacrylate (OEGMA) and di (ethylene glycol) methyl ether methacrylate coupled to copper sulfide NPs | NIR light | 808 nm | Controlled Release | [121] |
| Lidocaine | PCL MN loading lanthanum hexaboride nanoparticles | NIR light | 808 nm | Lidocaine blood levels (15–20 ng/mL) after each irradiation | [122] |
| Tetrodotoxin | Liposome loading the photosensitizer | NIR light | 730 nm | Sciatic nerve blockade | [123] |
| Tetrodotoxin +Dexmedetomidine | Liposome tethered with gold nanorods | NIR light | 808 nm | Sciatic nerve blockade | [124] |
| Tetrodotoxin | Liposome tethered with gold nanorods and loading the photosensitizer | NIR light | 730 nm | Sciatic nerve blockade | [125] |
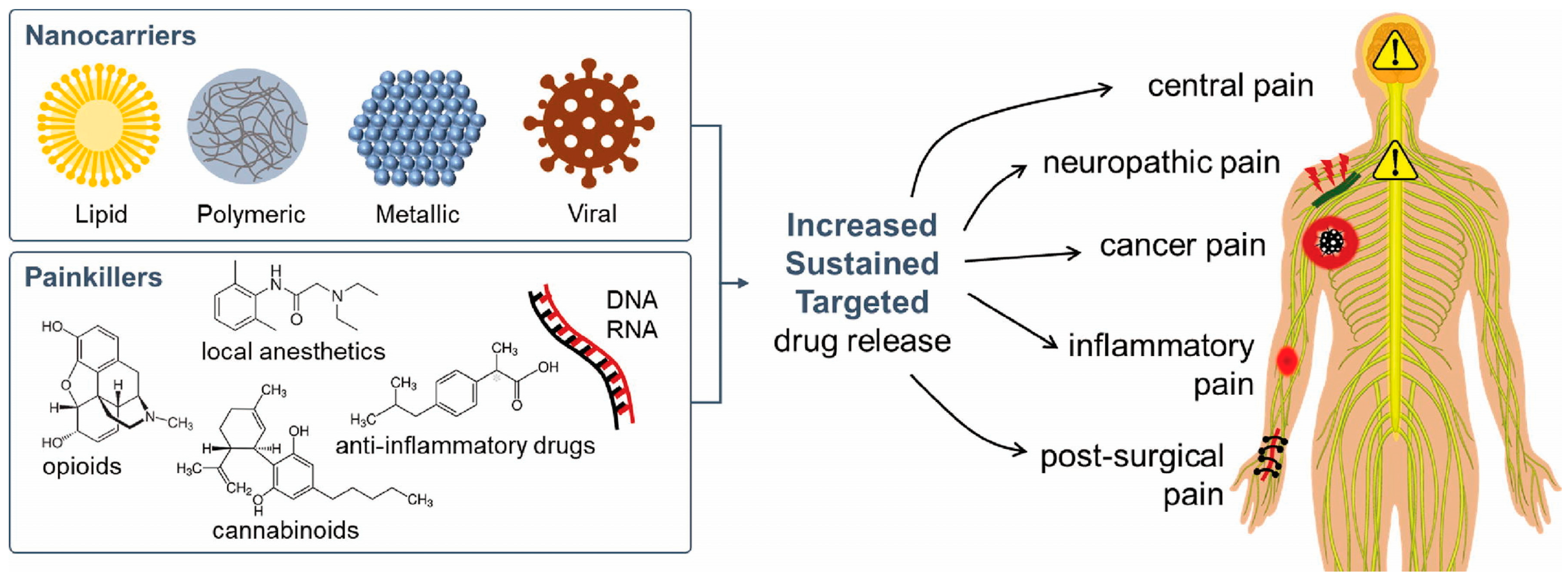
3.3. Inflammatory Disease
3.4. Wound Healing
4. Challenges Faced during Design and Development of Responsive Nanostructures
5. Clinical Perspective and Applicability of Responsive Nanostructures
5.1. Clinical Response in Varied Patient Profiles
5.1.1. Endogenously Activated Particles in Variable Clinical Scenarios
5.1.2. Exogenously Activated Particles in Variable Clinical Scenarios
5.1.3. Personalized Medicine and System Biology
5.1.4. Integrated Nanoplatforms for Personalized Medicine
5.1.5. Critical Issues with Smart Nanocarriers
5.2. Clinical Trial Status of SRNPs
6. Future Outlook
7. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, R.S.; Peppas, N.A. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials 1981, 2, 201–214. [Google Scholar] [CrossRef]
- Petersson, L.; Kvien, I.; Oksman, K. Structure and thermal properties of poly(lactic acid)/cellulose whiskers nanocomposite materials. Compos. Sci. Technol. 2007, 67, 2535–2544. [Google Scholar] [CrossRef]
- Bibi, N.; Ahmed, N.; Khan, G.M. Nanostructures in transdermal drug delivery systems. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 639–668. [Google Scholar] [CrossRef]
- Owens, D.E., III; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Dong, Z.; Kang, Y.; Yuan, Q.; Luo, M.; Gu, Z. H2O2-Responsive Nanoparticle Based on the Supramolecular Self-Assemble of Cyclodextrin. Front. Pharmacol. 2018, 9, 552. [Google Scholar] [CrossRef]
- Torchilin, V.P. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007, 9, E128–E147. [Google Scholar] [CrossRef]
- Ke, C.-J.; Lin, Y.-J.; Hu, Y.-C.; Chiang, W.-L.; Chen, K.-J.; Yang, W.-C.; Liu, H.-L.; Fu, C.-C.; Sung, H.-W. Multidrug release based on microneedle arrays filled with pH-responsive PLGA hollow microspheres. Biomaterials 2012, 33, 5156–5165. [Google Scholar] [CrossRef]
- Onaca, O.; Enea, R.; Hughes, D.W.; Meier, W. Stimuli-Responsive Polymersomes as Nanocarriers for Drug and Gene Delivery. Macromol. Biosci. 2008, 9, 129–139. [Google Scholar] [CrossRef]
- Mendes, P.M. Stimuli-responsive surfaces for bio-applications. Chem. Soc. Rev. 2008, 37, 2512–2529. [Google Scholar] [CrossRef]
- Zahednezhad, F.; Zakeri-Milani, P.; Mojarrad, J.S.; Valizadeh, H. The latest advances of cisplatin liposomal formulations: Essentials for preparation and analysis. Expert Opin. Drug Deliv. 2020, 17, 523–541. [Google Scholar] [CrossRef]
- Lammers, T.; Subr, V.; Ulbrich, K.; Hennink, W.E.; Storm, G.; Kiessling, F. Polymeric nanomedicines for image-guided drug delivery and tumor-targeted combination therapy. Nano Today 2010, 5, 197–212. [Google Scholar] [CrossRef]
- Davidson, P.M.; Sofos, J.N.; Branen, A.L. (Eds.) Antimicrobials in Food; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Rai, M.; dos Santos, C.A. Nanotechnology Applied to Pharmaceutical Technology; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Singh, B.; Shukla, N.; Kim, J.; Kim, K.; Park, M.-H. Stimuli-Responsive Nanofibers Containing Gold Nanorods for On-Demand Drug Delivery Platforms. Pharmaceutics 2021, 13, 1319. [Google Scholar] [CrossRef]
- Li, R.; Cheng, Z.; Wen, R.; Zhao, X.; Yu, X.; Sun, L.; Zhang, Y.; Han, Z.; Yuan, Y.; Kang, L. Novel SA@ Ca2+/RCSPs core–shell structure nanofibers by electrospinning for wound dressings. RSC Adv. 2018, 8, 15558–15566. [Google Scholar]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive hydrogels: Fabrication and biomedical applications. View 2021, 3, 20200112. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Frazar, E.M.; Shah, R.A.; Dziubla, T.D.; Hilt, J.Z. Multifunctional temperature-responsive polymers as advanced biomaterials and beyond. J. Appl. Polym. Sci. 2020, 137, 48770. [Google Scholar] [CrossRef]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef]
- Teixeira, M.; Carbone, C.; Souto, E. Beyond liposomes: Recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog. Lipid Res. 2017, 68, 1–11. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Verma, D.; Gulati, N.; Kaul, S.; Mukherjee, S.; Nagaich, U. Protein Based Nanostructures for Drug Delivery. J. Pharm. 2018, 2018, 9285854. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Xu, Y.; Zhao, Y.; Zhong, H.; Luo, X. Fabrication of BSA@AuNC-Based Nanostructures for Cell Fluoresce Imaging and Target Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 8947–8954. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, N.; Ma, T.; Liu, L.; Zhao, L.; Xie, H. Engineered bovine serum albumin-based nanoparticles with pH-sensitivity for doxorubicin delivery and controlled release. Drug Deliv. 2020, 27, 1156–1164. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Rofstad, E.K. Microenvironment-induced cancer metastasis. Int. J. Radiat. Biol. 2000, 76, 589–605. [Google Scholar] [CrossRef]
- Liu, M.; Du, H.; Zhang, W.; Zhai, G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications. Mater. Sci. Eng. C 2017, 71, 1267–1280. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Gramegna, A.; Amati, F.; Terranova, L.; Sotgiu, G.; Tarsia, P.; Miglietta, D.; Calderazzo, M.A.; Aliberti, S.; Blasi, F. Neutrophil elastase in bronchiectasis. Respir. Res. 2017, 18, 211. [Google Scholar] [CrossRef]
- Ramana, K.V.; Srivastava, S.K. Aldose reductase: A novel therapeutic target for inflammatory pathologies. Int. J. Biochem. Cell Biol. 2010, 42, 17–20. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, D.H. Stimuli-responsive liposomes for drug delivery. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, Y.; Huang, X.; Luby-Phelps, K.K.; Sumer, B.D.; Gao, J. Tunable, Ultrasensitive pH-Responsive Nanoparticles Targeting Specific Endocytic Organelles in Living Cells. Angew. Chem. Int. Ed. 2011, 50, 6109–6114. [Google Scholar] [CrossRef]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control. Release 2008, 132, 164–170. [Google Scholar] [CrossRef]
- Meyer, D.; Shin, B.; Kong, G.; Dewhirst, M.; Chilkoti, A. Drug targeting using thermally responsive polymers and local hyperthermia. J. Control. Release 2001, 74, 213–224. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Saito, G.; Swanson, J.A.; Lee, K.-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli-Responsive Nano-Architecture Drug-Delivery Systems to Solid Tumor Micromilieu: Past, Present, and Future Perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef]
- Suen, W.-L.L.; Chau, Y. Size-dependent internalisation of folate-decorated nanoparticles via the pathways of clathrin and caveolae-mediated endocytosis in ARPE-19 cells. J. Pharm. Pharmacol. 2013, 66, 564–573. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9 (Suppl. S1), 51–63. [Google Scholar] [CrossRef]
- Hao, X.; Wu, J.; Shan, Y.; Cai, M.; Shang, X.; Jiang, J.; Wang, H. Caveolae-mediated endocytosis of biocompatible gold nanoparticles in living Hela cells. J. Phys. Condens. Matter 2012, 24, 164207. [Google Scholar] [CrossRef]
- Buono, C.; Anzinger, J.J.; Amar, M.; Kruth, H.S. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J. Clin. Investig. 2009, 119, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Platel, A.; Carpentier, R.; Becart, E.; Mordacq, G.; Betbeder, D.; Nesslany, F. Influence of the surface charge of PLGA nanoparticles on theirin vitrogenotoxicity, cytotoxicity, ROS production and endocytosis. J. Appl. Toxicol. 2015, 36, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.; Martin, F.; Gabizon, A.; Huang, S.; Papahadjopoulos, D. Sterically stabilized liposomes: A hypothesis on the molecular origin of the extended circulation times. Biochim. Biophys. Acta (BBA)—Biomembr. 1991, 1070, 187–192. [Google Scholar] [CrossRef]
- Chen, Z.; Liao, T.; Wan, L.; Kuang, Y.; Liu, C.; Duan, J.; Xu, X.; Xu, Z.; Jiang, B.; Li, C. Dual-stimuli responsive near-infrared emissive carbon dots/hollow mesoporous silica-based integrated theranostics platform for real-time visualized drug delivery. Nano Res. 2021, 14, 4264–4273. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Labhasetwar, V. Nanotech approaches to drug delivery and imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef]
- Chonn, A.; Cullis, P.R. Recent advances in liposomal drug-delivery systems. Curr. Opin. Biotechnol. 1995, 6, 698–708. [Google Scholar] [CrossRef]
- Thamphiwatana, S.; Fu, V.; Zhu, J.; Lu, D.; Gao, W.; Zhang, L. Nanoparticle-Stabilized Liposomes for pH-Responsive Gastric Drug Delivery. Langmuir 2013, 29, 12228–12233. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Frechet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part A: Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Grayson, S.M.; Fréchet, J.M.J. Convergent Dendrons and Dendrimers: From Synthesis to Applications. Chem. Rev. 2001, 101, 3819–3868. [Google Scholar] [CrossRef] [PubMed]
- Bagley, A.F.; Hill, S.; Rogers, G.S.; Bhatia, S.N. Plasmonic Photothermal Heating of Intraperitoneal Tumors through the Use of an Implanted Near-Infrared Source. ACS Nano 2013, 7, 8089–8097. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, D.; Sk, U.H.; Sakamoto, Y.; Nakase, I.; Kojima, C. Dual stimuli-sensitive dendrimers: Photothermogenic gold nanoparticle-loaded thermo-responsive elastin-mimetic dendrimers. Colloids Surfaces B Biointerfaces 2015, 132, 155–160. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, X.; Zhang, B.; Zhou, Z.; Sun, Q.; Jin, E.; Sui, M.; Tang, J.; Wang, J.; Fan, M. Degradable Dual pH- and Temperature-Responsive Photoluminescent Dendrimers. Chem.—A Eur. J. 2011, 17, 5319–5326. [Google Scholar] [CrossRef]
- Sreenivasan, M. Cytology of a Spontaneous Triploid Coffea Canephora Pierre ex Froehner. Caryologia 1981, 34, 345–349. [Google Scholar] [CrossRef]
- Kastner, M.A. Artificial Atoms. Phys. Today 1993, 46, 24–31. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Xu, Y.-D.; Ma, Y.-Y.; Qiu, L.-L.; Wang, Y.; Kong, J.-L.; Xiong, H.-M. Biodegradable ZnO@polymer Core-Shell Nanocarriers: pH-Triggered Release of Doxorubicin In Vitro. Angew. Chem. Int. Ed. 2013, 52, 4127–4131. [Google Scholar] [CrossRef]
- Youn, Y.S.; Kwag, D.S.; Lee, E.S. Multifunctional nano-sized fullerenes for advanced tumor therapy. J. Pharm. Investig. 2016, 47, 1–10. [Google Scholar] [CrossRef]
- Teoh, S.K.; Ravi, P.; Dai, S.; Tam, K.C. Self-Assembly of Stimuli-Responsive Water-Soluble [60]Fullerene End-Capped Ampholytic Block Copolymer. J. Phys. Chem. B 2005, 109, 4431–4438. [Google Scholar] [CrossRef]
- Yao, Z.L.; Tam, K.C. Synthesis and self-assembly of stmuli responsive poly(2-dimethylamino)ethylmethacrylate)-block-fullerene (PDMAEMA-b-C60) and the demicellization induced by free PDMAEMA chains. Langmuir 2011, 27, 6668–6673. [Google Scholar] [CrossRef]
- Zhou, Z.; Vázquez-González, M.; Willner, I. Stimuli-responsive metal–organic framework nanoparticles for controlled drug delivery and medical applications. Chem. Soc. Rev. 2021, 50, 4541–4563. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Mukherjee, A.; Mukherjee, S. Recent Trends of the Bio-Inspired Nanoparticles in Cancer Theranostics. Front. Pharmacol. 2019, 10, 1264. [Google Scholar] [CrossRef]
- Roome, T.; Aziz, S.; Razzak, A.; Aslam, Z.; Lubna; Jamali, K.S.; Sikandar, B.; Fatima, T.; Abidi, L.; Imran, M.; et al. Opuntioside, opuntiol and its metallic nanoparticles attenuate adjuvant-induced arthritis: Novel suppressors of Toll-like receptors −2 and −4. Biomed. Pharmacother. 2019, 112, 108624. [Google Scholar] [CrossRef]
- Tan, L.-L.; Li, H.; Zhou, Y.; Zhang, Y.; Feng, X.; Wang, B.; Yang, Y.-W. Zn2+-Triggered Drug Release from Biocompatible Zirconium MOFs Equipped with Supramolecular Gates. Small 2015, 11, 3807–3813. [Google Scholar] [CrossRef]
- Mohapatra, A.; Uthaman, S.; Park, I.-K. External and Internal Stimuli-Responsive Metallic Nanotherapeutics for Enhanced Anticancer Therapy. Front. Mol. Biosci. 2021, 7, 597634. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, Y.; Luo, H.; Zhu, A.; Ke, H.; Yang, H.; Chen, H. Light-Responsive Nanoparticles for Highly Efficient Cytoplasmic Delivery of Anticancer Agents. ACS Nano 2017, 11, 12134–12144. [Google Scholar] [CrossRef]
- Li, Y.; Gobin, A.M.; Dryden, G.W.; Kang, X.; Xiao, D.; Li, S.P.; Zhang, G.; Martin, R.C.G. Infrared light-absorbing gold/gold sulfide nanoparticles induce cell death in esophageal adenocarcinoma. Int. J. Nanomedicine 2013, 8, 2153–2161. [Google Scholar] [CrossRef]
- Yao, J.; Zhu, C.; Peng, T.; Ma, Q.; Gao, S. Injectable and Temperature-Sensitive Titanium Carbide-Loaded Hydrogel System for Photothermal Therapy of Breast Cancer. Front. Bioeng. Biotechnol. 2021, 9, 1295. [Google Scholar] [CrossRef]
- Volarić, J.; Szymanski, W.; Simeth, N.A.; Feringa, B.L. Molecular photoswitches in aqueous environments. Chem. Soc. Rev. 2021, 50, 12377–12449. [Google Scholar] [CrossRef]
- Scherbakov, A.M.; Balakhonov, R.Y.; Salnikova, D.I.; Sorokin, D.V.; Yadykov, A.V.; Markosyan, A.I.; Shirinian, V.Z. Light-driven photoswitching of quinazoline analogues of combretastatin A-4 as an effective approach for targeting skin cancer cells. Org. Biomol. Chem. 2021, 19, 7670–7677. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, V.; Fu, H.; Hoorens, M.W.H.; Trinco, G.; Lameijer, L.N.; Marin, E.; Feringa, B.L.; Poelarends, G.J.; Szymanski, W.; Slotboom, D.J.; et al. Structural Aspects of Photopharmacology: Insight into the Binding of Photoswitchable and Photocaged Inhibitors to the Glutamate Transporter Homologue. J. Am. Chem. Soc. 2021, 143, 1513–1520. [Google Scholar] [CrossRef]
- Dunkel, P.; Ilaš, J. Targeted Cancer Therapy Using Compounds Activated by Light. Cancers 2021, 13, 3237. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef]
- Khoee, S.; Karimi, M.R. Dual-drug loaded Janus graphene oxide-based thermoresponsive nanoparticles for targeted therapy. Polymer 2018, 142, 80–98. [Google Scholar] [CrossRef]
- Li, L.; Hagen, T.L.T.; Hossann, M.; Süss, R.; van Rhoon, G.C.; Eggermont, A.M.; Haemmerich, D.; Koning, G.A. Mild hyperthermia triggered doxorubicin release from optimized stealth thermosensitive liposomes improves intratumoral drug delivery and efficacy. J. Control. Release 2013, 168, 142–150. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Z.; Tang, Y. Conjugated Polymers-Based Thermal-Responsive Nanoparticles for Controlled Drug Delivery, Tracking, and Synergistic Photodynamic Therapy/Chemotherapy. ACS Appl. Bio Mater. 2019, 2, 4485–4492. [Google Scholar] [CrossRef]
- Ha, P.T.; Le, T.T.H.; Bui, T.Q.; Pham, H.N.; Ho, A.S.; Nguyen, L.T. Doxorubicin release by magnetic inductive heating and in vivo hyperthermia-chemotherapy combined cancer treatment of multifunctional magnetic nanoparticles. New J. Chem. 2019, 43, 5404–5413. [Google Scholar] [CrossRef]
- Radović, M.; Calatayud, M.P.; Goya, G.F.; Ibarra, M.R.; Antić, B.; Spasojević, V.; Nikolić, N.; Janković, D.; Mirković, M.; Vranješ-Đurić, S. Preparation and in vivo evaluation of multifunctional90Y-labeled magnetic nanoparticles designed for cancer therapy. J. Biomed. Mater. Res. Part A 2014, 103, 126–134. [Google Scholar] [CrossRef]
- Chen, W.; Du, J. Ultrasound and pH Dually Responsive Polymer Vesicles for Anticancer Drug Delivery. Sci. Rep. 2013, 3, srep02162. [Google Scholar] [CrossRef]
- AlSawaftah, N.M.; Awad, N.S.; Paul, V.; Kawak, P.S.; Al-Sayah, M.H.; Husseini, G.A. Transferrin-modified liposomes triggered with ultrasound to treat HeLa cells. Sci. Rep. 2021, 11, 11589. [Google Scholar] [CrossRef]
- Salkho, N.M.; Paul, V.; Kawak, P.; Vitor, R.F.; Martins, A.M.; Al Sayah, M.; Husseini, G.A. Ultrasonically controlled estrone-modified liposomes for estrogen-positive breast cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 462–472. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Puglisi, A.; Bayir, E.; Timur, S.; Yagci, Y. pH-Responsive Polymersome Microparticles as Smart Cyclodextrin-Releasing Agents. Biomacromolecules 2019, 20, 4001–4007. [Google Scholar] [CrossRef]
- Yao, Y.; Saw, P.E.; Nie, Y.; Wong, P.-P.; Jiang, L.; Ye, X.; Chen, J.; Ding, T.; Xu, L.; Yao, H.; et al. Multifunctional sharp pH-responsive nanoparticles for targeted drug delivery and effective breast cancer therapy. J. Mater. Chem. B 2018, 7, 576–585. [Google Scholar] [CrossRef]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef]
- Lin, Z.; Cheng, X. Synthesis and properties of pH sensitive carboxymethylated hydroxypropyl chitosan nanocarriers for delivery of doxorubicin. J. Macromol. Sci. Part A 2021, 58, 600–609. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Ding, X.; Shen, W.; Li, M.; Wagner, E.; Xiao, C.; Chen, X. A Multistage Cooperative Nanoplatform Enables Intracellular Co-Delivery of Proteins and Chemotherapeutics for Cancer Therapy. Adv. Mater. 2020, 32, e2000013. [Google Scholar] [CrossRef]
- Han, S.; Li, Z.-Y.; Zhu, J.-Y.; Han, K.; Zeng, Z.-Y.; Hong, W.; Li, W.-X.; Jia, H.-Z.; Liu, Y.; Zhuo, R.-X.; et al. Dual-pH Sensitive Charge-Reversal Polypeptide Micelles for Tumor-Triggered Targeting Uptake and Nuclear Drug Delivery. Small 2015, 11, 2543–2554. [Google Scholar] [CrossRef]
- Epel, B.; Sundramoorthy, S.V.; Krzykawska-Serda, M.; Maggio, M.C.; Tseytlin, M.; Eaton, G.R.; Eaton, S.S.; Rosen, G.M.; Kao, J.P.; Halpern, H.J. Imaging thiol redox status in murine tumors in vivo with rapid-scan electron paramagnetic resonance. J. Magn. Reson. 2017, 276, 31–36. [Google Scholar] [CrossRef]
- Paris, J.L.; Manzano, M.; Cabañas, M.V.; Vallet-Regí, M. Mesoporous silica nanoparticles engineered for ultrasound-induced uptake by cancer cells. Nanoscale 2018, 10, 6402–6408. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hu, J.; Wang, L.; Li, Y.; Zhu, C.; Chen, C.; Shi, M.; Ju, Z.; Cao, X.; Zhang, Z. Targeted and redox-responsive drug delivery systems based on carbonic anhydrase IX-decorated mesoporous silica nanoparticles for cancer therapy. Sci. Rep. 2020, 10, 14447. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Fukushima, S.; Kanayama, N.; Aoyagi, K.; Nishiyama, N.; Koyama, H.; Kataoka, K. Cyclic RGD Peptide-Conjugated Polyplex Micelles as a Targetable Gene Delivery System Directed to Cells Possessing αvβ3 and αvβ5 Integrins. Bioconjugate Chem. 2007, 18, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Dirisala, A.; Osada, K.; Chen, Q.; Tockary, T.A.; Machitani, K.; Osawa, S.; Liu, X.; Ishii, T.; Miyata, K.; Oba, M.; et al. Optimized rod length of polyplex micelles for maximizing transfection efficiency and their performance in systemic gene therapy against stroma-rich pancreatic tumors. Biomaterials 2014, 35, 5359–5368. [Google Scholar] [CrossRef]
- Lafleur, M.A.; Drew, A.F.; de Sousa, E.L.; Blick, T.; Bills, M.; Walker, E.C.; Williams, E.D.; Waltham, M.; Thompson, E.W. Upregulation of matrix metalloproteinases (MMPs) in breast cancer xenografts: A major induction of stromal MMP-13. Int. J. Cancer 2004, 114, 544–554. [Google Scholar] [CrossRef]
- Naz, S.; Wang, M.; Han, Y.; Hu, B.; Teng, L.; Zhou, J.; Zhang, H.; Chen, J. Enzyme-responsive mesoporous silica nanoparticles for tumor cells and mitochondria multistage-targeted drug delivery. Int. J. Nanomed. 2019, 14, 2533–2542. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, D.; Luo, K.; She, W.; Guo, C.; Yang, Y.; Gu, Z. Peptide Dendrimer-Doxorubicin Conjugate-Based Nanoparticles as an Enzyme-Responsive Drug Delivery System for Cancer Therapy. Adv. Healthc. Mater. 2014, 3, 1299–1308. [Google Scholar] [CrossRef]
- Li, N.; Li, N.; Yi, Q.; Luo, K.; Guo, C.; Pan, D.; Gu, Z. Amphiphilic peptide dendritic copolymer-doxorubicin nanoscale conjugate self-assembled to enzyme-responsive anti-cancer agent. Biomaterials 2014, 35, 9529–9545. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Thambi, T.; Deepagan, V.; Yoon, H.Y.; Han, H.S.; Kim, S.-H.; Son, S.; Jo, D.-G.; Ahn, C.-H.; Suh, Y.D.; Kim, K.; et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 2014, 35, 1735–1743. [Google Scholar] [CrossRef]
- Vaupel, P.; Höckel, M.; Mayer, A. Detection and Characterization of Tumor Hypoxia Using pO2 Histography. Antioxid. Redox Signal 2007, 9, 1221–1236. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Chu, D.; Yang, F.; Li, Z.; Fan, B.; Jin, L.; Li, J. Hypoxia-Responsive Polymeric Micelles for Enhancing Cancer Treatment. Front. Chem. 2020, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, G.; Wang, N.; Guo, F.; Guo, L.; Liu, X. Synthesis of temperature/pH dual-sensitive supramolecular micelles from β-cyclodextrin-poly(N-isopropylacrylamide) star polymer for drug delivery. Colloids Surfaces B Biointerfaces 2018, 172, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, W.; Zhu, Y.; Luo, J.; Liu, R.; Liu, X. Synthesis of Temperature/pH Dual-Stimuli-Response Multicompartmental Microcapsules via Pickering Emulsion for Preprogrammable Payload Release. ACS Appl. Mater. Interfaces 2020, 12, 4821–4832. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, F.; Hou, H.; Hou, J.; Wang, Y.; Zhou, S. Stimuli-responsive nitric oxide generator for light-triggered synergistic cancer photothermal/gas therapy. Nano Res. 2019, 12, 1361–1370. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Liu, X.; Yang, C.; Hu, N.; Dou, L.; Zhao, B.; Zhang, Q.; Suo, Y.; Wang, J. Oxygen-Generating MnO2 Nanodots-Anchored Versatile Nanoplatform for Combined Chemo-Photodynamic Therapy in Hypoxic Cancer. Adv. Funct. Mater. 2018, 28, 1706375. [Google Scholar] [CrossRef]
- Tamarov, K.; Xu, W.; Osminkina, L.; Zinovyev, S.; Soininen, P.; Kudryavtsev, A.; Gongalsky, M.; Gaydarova, A.; Närvänen, A.; Timoshenko, V.; et al. Temperature responsive porous silicon nanoparticles for cancer therapy—Spatiotemporal triggering through infrared and radiofrequency electromagnetic heating. J. Control. Release 2016, 241, 220–228. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Tarner, I.H.; Müller-Ladner, U. Drug delivery systems for the treatment of rheumatoid arthritis. Expert Opin. Drug Deliv. 2008, 5, 1027–1037. [Google Scholar] [CrossRef]
- Indulekha, S.; Arunkumar, P.; Bahadur, D.; Srivastava, R. Thermoresponsive polymeric gel as an on-demand transdermal drug delivery system for pain management. Mater. Sci. Eng. C 2016, 62, 113–122. [Google Scholar] [CrossRef]
- Fateh, S.T.; Moradi, L.; Kohan, E.; Hamblin, M.R.; Dezfuli, A.S. Comprehensive review on ultrasound-responsive theranostic nanomaterials: Mechanisms, structures and medical applications. Beilstein J. Nanotechnol. 2021, 12, 808–862. [Google Scholar] [CrossRef]
- Soto, F.; Jeerapan, I.; Silva-López, C.; Lopez-Ramirez, M.A.; Chai, I.; Xiaolong, L.; Lv, J.; Kurniawan, J.F.; Martin, I.; Chakravarthy, K.; et al. Noninvasive Transdermal Delivery System of Lidocaine Using an Acoustic Droplet-Vaporization Based Wearable Patch. Small 2018, 14, e1803266. [Google Scholar] [CrossRef]
- Ramírez-García, P.D.; Retamal, J.S.; Shenoy, P.; Imlach, W.; Sykes, M.; Truong, N.; Constandil, L.; Pelissier, T.; Nowell, C.J.; Khor, S.Y.; et al. A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain. Nat. Nanotechnol. 2019, 14, 1150–1159. [Google Scholar] [CrossRef]
- Marzoli, F.; Marianecci, C.; Rinaldi, F.; Passeri, D.; Rossi, M.; Minosi, P.; Carafa, M.; Pieretti, S. Long-Lasting, Antinociceptive Effects of pH-Sensitive Niosomes Loaded with Ibuprofen in Acute and Chronic Models of Pain. Pharmaceutics 2019, 11, 62. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Ye, Y.; DiSanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Bian, F.; Cai, L.; Zhao, Y. Encoded Microneedle Arrays for Detection of Skin Interstitial Fluid Biomarkers. Adv. Mater. 2019, 31, e1902825. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Xie, X.; Li, X. Development of photo-magnetic drug delivery system by facile-designed dual stimuli-responsive modified biopolymeric chitosan capped nano-vesicle to improve efficiency in the anesthetic effect and its biological investigations. J. Photochem. Photobiol. B Biol. 2019, 202, 111716. [Google Scholar] [CrossRef]
- de Solorzano, I.O.; Alejo, T.; Abad, M.; Bueno-Alejo, C.; Mendoza, G.; Andreu, V.; Irusta, S.; Sebastian, V.; Arruebo, M. Cleavable and thermo-responsive hybrid nanoparticles for on-demand drug delivery. J. Colloid Interface Sci. 2018, 533, 171–181. [Google Scholar] [CrossRef]
- Chen, M.-C.; Chan, H.-A.; Ling, M.-H.; Su, L.-C. Implantable polymeric microneedles with phototriggerable properties as a patient-controlled transdermal analgesia system. J. Mater. Chem. B 2016, 5, 496–503. [Google Scholar] [CrossRef]
- Rwei, A.Y.; Lee, J.-J.; Zhan, C.; Liu, Q.; Ok, M.T.; Shankarappa, S.A.; Langer, R.; Kohane, D.S. Repeatable and adjustable on-demand sciatic nerve block with phototriggerable liposomes. Proc. Natl. Acad. Sci. USA 2015, 112, 15719–15724. [Google Scholar] [CrossRef]
- Zhan, C.; Wang, W.; McAlvin, J.B.; Guo, S.; Timko, B.P.; Santamaria, C.; Kohane, D.S. Phototriggered Local Anesthesia. Nano Lett. 2015, 16, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Rwei, A.Y.; Wang, B.Y.; Ji, T.; Zhan, C.; Kohane, D.S. Enhanced Triggering of Local Anesthetic Particles by Photosensitization and Photothermal Effect Using a Common Wavelength. Nano Lett. 2017, 17, 7138–7145. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.; Lepetre-Mouelhi, S.; Couvreur, P. Micro- and nanocarriers for pain alleviation. Adv. Drug Deliv. Rev. 2022, 187, 114359. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Minko, T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 2020, 18, 205–227. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, W.; Cai, C.; Wu, Y.; Li, J.; Dong, S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater. Today Bio 2022, 14, 100223. [Google Scholar] [CrossRef]
- Xie, Y.; Tuguntaev, R.G.; Mao, C.; Chen, H.; Tao, Y.; Wang, S.; Yang, B.; Guo, W. Stimuli-responsive polymeric nanomaterials for rheumatoid arthritis therapy. Biophys. Rep. 2020, 6, 193–210. [Google Scholar] [CrossRef]
- Li, Z.; Li, G.; Xu, J.; Li, C.; Han, S.; Zhang, C.; Wu, P.; Lin, Y.; Wang, C.; Zhang, J.; et al. Hydrogel Transformed from Nanoparticles for Prevention of Tissue Injury and Treatment of Inflammatory Diseases. Adv. Mater. 2022, 34, 2109178. [Google Scholar] [CrossRef]
- Sufian, A.; Bhattacherjee, D.; Barman, P.; Srivastava, A.; Thummer, R.P.; Bhabak, K.P. Stimuli-responsive prodrug of non-steroidal anti-inflammatory drug diclofenac: Self-immolative drug release with turn-on near-infrared fluorescence. Chem. Commun. 2022, 58, 7833–7836. [Google Scholar] [CrossRef]
- Fattal, E.; Couvreur, P.; Dubernet, C. “Smart” delivery of antisense oligonucleotides by anionic pH-sensitive liposomes. Adv. Drug Deliv. Rev. 2004, 56, 931–946. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.; Park, S.; Khang, G.; Kang, P.M.; Lee, D. Inflammation-Responsive Antioxidant Nanoparticles Based on a Polymeric Prodrug of Vanillin. Biomacromolecules 2013, 14, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.-L.; Hu, Y.-C.; Liu, H.-Y.; Hsiao, C.-W.; Sureshbabu, R.; Yang, C.-M.; Chung, M.-F.; Chia, W.-T.; Sung, H.-W. Injectable Microbeads with a Thermo-Responsive Shell and a pH-Responsive Core as a Dual-Switch-Controlled Release System. Small 2014, 10, 4100–4105. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, S.; Liu, Z.; Correia, A.; Martins, J.P.; Rahikkala, A.; Fontana, F.; Kemell, M.; Liu, D.; Albertini, B.; Passerini, N.; et al. pH and Reactive Oxygen Species-Sequential Responsive Nano-in-Micro Composite for Targeted Therapy of Inflammatory Bowel Disease. Adv. Funct. Mater. 2018, 28, 1806175. [Google Scholar] [CrossRef]
- Cao, F.; Gui, S.-Y.; Gao, X.; Zhang, W.; Fu, Z.-Y.; Tao, L.-M.; Jiang, Z.-X.; Chen, X.; Qian, H.; Wang, X. Research progress of natural product-based nanomaterials for the treatment of inflammation-related diseases. Mater. Des. 2022, 218, 110686. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Hou, Y.; Meng, X.; Wang, D.; Liu, J.; Sun, F.; Li, Y. Hyaluronic Acid Coated Acid-Sensitive Nanoparticles for Targeted Therapy of Adjuvant-Induced Arthritis in Rats. Molecules 2019, 24, 146. [Google Scholar] [CrossRef]
- Fiore, V.F.; Lofton, M.C.; Roser-Page, S.; Yang, S.C.; Roman, J.; Murthy, N.; Barker, T.H. Polyketal microparticles for therapeutic delivery to the lung. Biomaterials 2010, 31, 810–817. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, M.; Yu, C.; Zhang, X.; Liu, J.; Cheng, X.; Lee, R.J.; Sun, F.; Teng, L.; Li, Y. Multifunctional folate receptor-targeting and pH-responsive nanocarriers loaded with methotrexate for treatment of rheumatoid arthritis. Int. J. Nanomed. 2017, 12, 6735–6746. [Google Scholar] [CrossRef]
- Fang, F.; Ni, Y.; Yu, H.; Yin, H.; Yang, F.; Li, C.; Sun, D.; Pei, T.; Ma, J.; Deng, L.; et al. Inflammatory endothelium-targeted and cathepsin responsive nanoparticles are effective against atherosclerosis. Theranostics 2022, 12, 4200–4220. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, Y.; Zhang, P.; Zhai, S.; Li, W.; Cui, J. Transcutaneous delivery of mung bean-derived nanoparticles for amelioration of psoriasis-like skin inflammation. Nanoscale 2022, 14, 3040–3048. [Google Scholar] [CrossRef]
- Said, S.S.; Campbell, S.; Hoare, T. Externally Addressable Smart Drug Delivery Vehicles: Current Technologies and Future Directions. Chem. Mater. 2019, 31, 4971–4989. [Google Scholar] [CrossRef]
- Banerjee, I.; Mishra, D.; Das, T.; Maiti, T.K. Wound pH-Responsive Sustained Release of Therapeutics from a Poly(NIPAAm-co-AAc) Hydrogel. J. Biomater. Sci. Polym. Ed. 2012, 23, 111–132. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.S.; Herron, C.C.; Hastings, C.L.; Deckers, R.; Noriega, A.L.; Kelly, H.M.; Hennink, W.E.; McDonnell, C.O.; O’Brien, F.J.; Ruiz-Hernández, E.; et al. A stimuli responsive liposome loaded hydrogel provides flexible on-demand release of therapeutic agents. Acta Biomater. 2017, 48, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Pasche, S.; Angeloni, S.; Ischer, R.; Liley, M.; Luprano, J.; Voirin, G. Wearable Biosensors for Monitoring Wound Healing. Adv. Sci. Technol. 2008, 57, 80–87. [Google Scholar] [CrossRef]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-Responsive Poly(N-Isopropylacrylamide)-Cellulose Nanocrystals Hybrid Hydrogels for Wound Dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2018, 203, 423–429. [Google Scholar] [CrossRef]
- Wu, D.; Kang, L.; Tian, J.; Wu, Y.; Liu, J.; Li, Z.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Exosomes Derived from Bone Mesenchymal Stem Cells with the Stimulation of Fe3O4 Nanoparticles and Static Magnetic Field Enhance Wound Healing Through Upregulated miR-21-5p. Int. J. Nanomed. 2020, 15, 7979–7993. [Google Scholar] [CrossRef]
- Wang, X.-F.; Li, M.-L.; Fang, Q.-Q.; Zhao, W.-Y.; Lou, D.; Hu, Y.-Y.; Chen, J.; Tan, W.-Q. Flexible electrical stimulation device with Chitosan-Vaseline® dressing accelerates wound healing in diabetes. Bioact. Mater. 2020, 6, 230–243. [Google Scholar] [CrossRef]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Graphene quantum dot-decorated luminescent porous silicon dressing for theranostics of diabetic wounds. Acta Biomater. 2021, 131, 544–554. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, R.; Momtazi-Borojeni, A.A.; Vakili-Ghartavol, Z.; Aiyelabegan, H.T.; Jaafari, M.R.; Rezayat, S.M.; Bidgoli, S.A. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif. Cells Nanomed. Biotechnol. 2020, 48, 443–451. [Google Scholar] [CrossRef]
- Jha, R.; Singh, A.; Sharma, P.; Fuloria, N.K. Smart carbon nanotubes for drug delivery system: A comprehensive study. J. Drug Deliv. Sci. Technol. 2020, 58, 101811. [Google Scholar] [CrossRef]
- Bratek-Skicki, A. Towards a new class of stimuli-responsive polymer-based materials—Recent advances and challenges. Appl. Surf. Sci. Adv. 2021, 4, 100068. [Google Scholar] [CrossRef]
- Luk, B.T.; Zhang, L. Current Advances in Polymer-Based Nanotheranostics for Cancer Treatment and Diagnosis. ACS Appl. Mater. Interfaces 2014, 6, 21859–21873. [Google Scholar] [CrossRef] [PubMed]
- Dirisala, A.; Uchida, S.; Toh, K.; Li, J.; Osawa, S.; Tockary, T.A.; Liu, X.; Abbasi, S.; Hayashi, K.; Mochida, Y.; et al. Transient stealth coating of liver sinusoidal wall by anchoring two-armed PEG for retargeting nanomedicines. Sci. Adv. 2020, 6, eabb8133. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.M.; MacParland, S.; Ma, X.-Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Caccamo, D.; Pelle, E.; De Luca, C. Innovative Approaches in Environmental Medicine: Redox/Detoxification Biomarkers in Environmental Intolerances. Oxidative Med. Cell. Longev. 2013, 2013, 691624. [Google Scholar] [CrossRef] [PubMed]
- Polenik, P. Curcumin Nanoparticles and Blue Laser Irradiation in Photothermal Inactivation of Selected Oral Pathogens in Vitro. Int. J. Theor. Appl. Nanotechnol. 2020, 8, 8–12. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Chen, R.; Snyder, M. Systems biology: Personalized medicine for the future? Curr. Opin. Pharmacol. 2012, 12, 623–628. [Google Scholar] [CrossRef]
- Zardad, A.-Z.; Choonara, Y.E.; Du Toit, L.C.; Kumar, P.; Mabrouk, M.; Kondiah, P.P.D.; Pillay, V. A Review of Thermo- and Ultrasound-Responsive Polymeric Systems for Delivery of Chemotherapeutic Agents. Polymers 2016, 8, 359. [Google Scholar] [CrossRef]
- Lock, L.L.; LaComb, M.; Schwarz, K.; Cheetham, A.G.; Lin, Y.-A.; Zhang, P.; Cui, H. Self-assembly of natural and synthetic drug amphiphiles into discrete supramolecular nanostructures. Faraday Discuss. 2013, 166, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Calandra, P.; Caschera, D.; Liveri, V.T.; Lombardo, D. How self-assembly of amphiphilic molecules can generate complexity in the nanoscale. Colloids Surfaces A: Physicochem. Eng. Asp. 2015, 484, 164–183. [Google Scholar] [CrossRef]
- Kiselev, M.; Lombardo, D.; Lesieur, P.; Kisselev, A.; Borbely, S.; Simonova, T.; Barsukov, L. Membrane self assembly in mixed DMPC/NaC systems by SANS. Chem. Phys. 2008, 345, 173–180. [Google Scholar] [CrossRef]
- Pirollo, K.F.; Chang, E.H. Does a targeting ligand influence nanoparticle tumor localization or uptake? Trends Biotechnol. 2008, 26, 552–558. [Google Scholar] [CrossRef]
- Li, R.; Peng, F.; Cai, J.; Yang, D.; Zhang, P. Redox dual-stimuli responsive drug delivery systems for improving tumor-targeting ability and reducing adverse side effects. Asian J. Pharm. Sci. 2019, 15, 311–325. [Google Scholar] [CrossRef] [PubMed]

| Biomolecule/Metabolite/Stimuli | Physiological Concentration | Pathological Concentration | Reference |
|---|---|---|---|
| pH | pH of ~7.4 | extracellular pH of tumor tissues is between 6.0 and 7.0 | [28] |
| Temperature | ~37 °C | ~40–42 °C | [29] |
| ROS | Low to moderate levels | Elevated levels in Inflammation and various cancer. | [30] |
| Glutathione | Lower level of expression with similar intracellular concentrations and extracellular concentrations | ~2–10 μM in extracellular environment, ~2–10 mM in intracellular space. | [29] |
| Human Neutrophil Elastase | Lower level of expression | High expression in chronic respiratory disease | [31] |
| Aldose Reductase | Only present in diabetes target tissues | Its catalytic activity is crucial in a variety of inflammatory processes. In human malignancies such as liver, cervical, and ovarian, its gene is overexpressed. | [32] |
| S. No. | Stimuli | Nanocarrier System | Mechanism/Response | Reference |
|---|---|---|---|---|
| 1. | pH, temperature | PNIPAM-Nile red-oil soluble fluorescent green | Independent release of molecules with selectivity, multicompartment microcapsules with programmed release | [107] |
| 2. | Near-infrared, nitric oxide | PpRE@PEG-PpIX nanoparticles | Increase in temperature and intracellular internalization to inhibit cancer cells. | [108] |
| 3. | pH, near-infrared | g-C3N4 and doxorubicin encapsulated MnO2 nanodots | Release of encapsulated drug in acidic environment, elevated oxygen level for effective anticancer activity | [109] |
| 4. | Infrared, radiofrequency | TR-NDA Porous silicon nanoparticles | Trigger the release of encapsulated drug by collapsing the polymeric chain | [110] |
| 5. | pH, enzyme | TLR7/8 agonist conjugated nanovaccine | Receptor signaling activation, dendritic cell maturation, enhance cellular immunity | [67] |
| Materials Used | Therapeutic Agent | Disorder | References |
|---|---|---|---|
| Hyaluronic acid-coated Poly (cyclohexane1,4diylacetone dimethylene ketal) | Dexamethasone | Rheumatoid arthritis | [138] |
| Polyketal | Superoxide dismutase | Lung fibrosis | [139] |
| Folic acid targeted Poly(cyclohexane-1,4-diylacetone dimethylene ketal)/Poly(lactide-co-glycolic acid) blend | Methotrexate | Arthritis | [140] |
| Poly(lactide-co-glycolic acid -Poly ethylene glycol-c(RGDfC) | Rapamycin | Atherosclerosis | [141] |
| mung bean-derived nanoparticles | Mung bean | Psoriasis | [142] |
| Gold nanoparticles | Rosa rugosa | Skin Inflammation | [143] |
| Title of the Study | Drug and Clinical Condition/Use | Status | Stimuli | Clinical Trial No | Phases Covered | Purpose | Reference Citation |
|---|---|---|---|---|---|---|---|
| Phase 2 Study of Thermodox as Adjuvant Therapy with Thermal Ablation (RFA) in Treatment of Metastatic Colorectal Cancer(mCRC) (ABLATE) | ThermoDox in combination with Microwave Hyperthermia (heat). For recurrent regional breast cancer | Completed First Posted: 21 January 2009 Last Update Posted: 30 January 2017 | Thermo-responsive | NCT00826085 | I and II | To evaluate the effects of ThermoDox in combination with therapeutic heating of the chest wall in the treatment of recurrent regional breast cancer. | https://www.clinicaltrials.gov/ct2/show/NCT00826085 |
| ThermoDox™ in Combination with Radiofrequency Ablation (RFA) in Primary and Metastatic Tumors of the Liver | ThermoDox Hepatocellular Carcinoma Liver Neoplasms | Recruitment Status: Completed First Posted: 28 February 2007 Last Update Posted: 7 February 2019 | Thermo-responsive | NCT00441376 | I | To determine the maximum tolerated dose (MTD) of ThermoDox when used in combination with radiofrequency ablation (RFA) in the treatment of primary and metastatic tumors of the liver. | https://clinicaltrials.gov/ct2/show/NCT00441376 |
| Study of Thermodox as Adjuvant Therapy with Thermal Ablation (RFA) in Treatment of Metastatic Colorectal Cancer(mCRC) | Colon Cancer Liver Metastasis Drug: Lyso-Thermosensitive Liposomal Doxorubicin Other: 5% Dextrose Solution Drug: ThermoDox | Recruitment Status: Terminated (trial design contingent on RFA optimization) First Posted: 3 November 2011Results First Posted: 13 October 2022 Last Update Posted: 13 October 2022 | Thermo-responsive | NCT01464593 | II | To determine the safety and efficacy of Thermodox, a thermally sensitive liposomal doxorubicin, in combination with thermal ablation in the treatment of hepatic colorectal liver metastases (CRLM). | https://clinicaltrials.gov/ct2/show/NCT01464593 |
| ThermoDox with Standardized Radiofrequency Ablation (RFA) for Treatment of Hepatocellular Carcinoma (HCC) (OPTIMA) | Drug: ThermoDox Drug: Dummy infusion/Hepatocellular Carcinoma | Recruitment Status: CompletedFirst Posted: 14 April 2014 Last Update Posted: 24 October 2018 | Thermo-responsive | NCT02112656 | III | To determine whether ThermoDox, a thermally sensitive liposomal doxorubicin, is effective in the treatment of non-resectable hepatocellular carcinoma when used in conjunction with standardized radiofrequency ablation (sRFA). | https://clinicaltrials.gov/ct2/show/NCT02112656 |
| MRI Guided High Intensity Focused Ultrasound (HIFU) and ThermoDox for Palliation of Painful Bone Metastases | Painful Bone Metastases Breast Carcinoma Non-small Cell Lung Cancer, Small Cell Lung Cancer Adenocarcinoma Drug: High Intensity Focused Ultrasound (HIFU) in combination with ThermoDox | Recruitment Status: Withdrawn First Posted: 16 July 2012 Last Update Posted: 7 February 2017 | Thermo-responsive | NCT01640847 | II | To evaluate treatment with High Intensity Focused Ultrasound (HIFU)in combination with ThermoDox (liposomal doxorubicin) is safe and effective in reducing pain for patients with painful bone metastases. | MRI Guided High Intensity Focused Ultrasound (HIFU) and ThermoDox for Palliation of Painful Bone Metastases-Full Text View-ClinicalTrials.gov |
| Phase 3 study of ThermoDox with radiofrequency ablation (RFA) in treatment of hepatocellularcarcinoma (HCC) | Hepatocellular Carcinoma Drug: ThermoDoxDrug: 5% Dextrose Solution | Recruitment Status: Completed First Posted: 18 February 2008 Results First Posted: 24 March 2017 Last Update Posted: 25 April 2017 | Thermo-responsive | NCT00617981 | III | To determine whether ThermoDox, a thermally sensitive liposomal doxorubicin, is effective in the treatment of non-resectable hepatocellular carcinoma when used in conjunction with radiofrequency ablation (RFA). | https://clinicaltrials.gov/ct2/show/NCT00617981 |
| A Phase I/II Single Dose Trial to Determine the Safety, Tolerance, Pharmacokinetic Profile, and Preliminary Activity of Intrahepatic Delivery (Via Hepatic Artery Catheterization) of Doxorubicin Hydrochloride Adsorbed to Magnetic Targeted Carriers (MTC-DOX) in Patients with Metastatic Cancer to the Liver. | Metastases, Neoplasm Colorectal Neoplasms Esophageal Neoplasms Stomach Neoplasms Pancreatic Neoplasms Breast Neoplasms Melanoma Sarcoma Gastrointestinal Neoplasms Lung Neoplasms Liver Neoplasms Cholangiocarcinoma Drug: MTC-DOX for Injection Procedure: Chemotherapy/ | Recruitment Status: Completed First Posted: 19 July 2002 Last Update Posted: 24 June 2005 | Iron and carbon magnetic beads (magneto-responsive) | NCT00041808 | I and II | Therapeutic | https://clinicaltrials.gov/ct2/show/NCT00041808 |
| Preoperative staging of pancreatic cancer using superparamagnetic iron oxide magnetic resonance imaging (SPIO MRI) | Pancreatic Cancer | Recruitment Status: Completed First Posted: 12 June 2009 Results First Posted: 25 July 2017 Last Update Posted: 25 July 2017 | Feraheme Iron oxide nanoparticle | NCT00920023 | IV | Diagnostic | https://clinicaltrials.gov/ct2/show/NCT00920023 |
| NanoTherm | Prostate Cancer/ Device: NanoTherm Ablation | Recruitment Status: Recruiting First Posted: 18 August 2021 Last Update Posted: 24 August 2022 | Magnetic field | NCT05010759 | Not Applicable | Stage 2B: NanoTherm ablation of focal prostate cancer in small lesions in Gleason 3 + 4 disease. The outcome of this ablation is validated by a transperineal biopsy at 4 months after ablation. | https://clinicaltrials.gov/ct2/show/NCT05010759 |
| A study of Trastuzumab Emtansine (T-DM1) in combination with Docetaxel, and potentially Pertuzumab, in participants with advanced breast cancer | Breast Cancer/ Drug: Docetaxel Drug: Pertuzumab Drug: Trastuzumab emtansine | Recruitment Status: Completed First Posted: 8 July 2009 Results First Posted: 6 April 2017 Last Update Posted: 6 April 2017 | Targeted antibody drug conjugate | NCT00934856 | I and II | Therapeutic | https://clinicaltrials.gov/ct2/show/NCT00934856 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawar, V.; Maske, P.; Khan, A.; Ghosh, A.; Keshari, R.; Bhatt, M.; Srivastava, R. Responsive Nanostructure for Targeted Drug Delivery. J. Nanotheranostics 2023, 4, 55-85. https://doi.org/10.3390/jnt4010004
Pawar V, Maske P, Khan A, Ghosh A, Keshari R, Bhatt M, Srivastava R. Responsive Nanostructure for Targeted Drug Delivery. Journal of Nanotheranostics. 2023; 4(1):55-85. https://doi.org/10.3390/jnt4010004
Chicago/Turabian StylePawar, Vaishali, Priyanka Maske, Amreen Khan, Arnab Ghosh, Roshan Keshari, Mahek Bhatt, and Rohit Srivastava. 2023. "Responsive Nanostructure for Targeted Drug Delivery" Journal of Nanotheranostics 4, no. 1: 55-85. https://doi.org/10.3390/jnt4010004
APA StylePawar, V., Maske, P., Khan, A., Ghosh, A., Keshari, R., Bhatt, M., & Srivastava, R. (2023). Responsive Nanostructure for Targeted Drug Delivery. Journal of Nanotheranostics, 4(1), 55-85. https://doi.org/10.3390/jnt4010004






