Abstract
In oncology, tremendous research has been conducted on the use of alternative minimally invasive techniques for cancer treatment and diagnosis. The use of biophotonic techniques as a standalone treatment or together with conventional imaging techniques has gained interest among researchers in recent years, while biophotonic therapies such as photothermal and photodynamic therapies tend to bring the use of non-ionizing radiation in therapy back into the spotlight due to the progressive development of optical instrumentation, enhancement agents, molecular probes, light sources and nanocarriers. Thus, the coupling of non-ionizing with ionizing radiation (IR) and the combination of nanomedicine with nuclear medicine procedures are considered to be revolutionary strategies to optimize the therapeutic efficacy of biophotonic modalities and to develop theranostic applications for the better diagnosis and treatment of cancer. Recently, the low-intensity Cerenkov light emitted by tissues as a byproduct of the IR–biostructure interaction has been suggested as an effective internal light source that can trigger phototherapy and guide radiotherapy dosimetry using Cerenkov imaging. This review also provides an overview of in vitro and in vivo studies regarding the use of Cerenkov radiation produced by X-rays or radionucleotides and combined with nanoparticles as a hybrid method to induce enhanced photothermal and photodynamic therapies.
1. Introductory Remarks
Cancer is still one of the main causes of morbidity and mortality worldwide, despite the development of several imaging and treatment modalities since the very beginning of the 20th century. According to a 2020 World Health Organization (WHO) report, in the year 2018, 18.1 million people around the globe had cancer and 9.6 million died from the disease, while, by 2040, those figures will nearly double [1]. In the same report, the WHO summarizes the spectrum of cancer control interventions: primary prevention, screening and early diagnosis, multimodal treatment and survivorship, and palliative care. However, the survival and the quality of life with certain types of cancer are still poor. In fact, the complexity, diversity and heterogeneity of tumors strongly affect the therapeutic outcome of any treatment effort.
Moreover, according to Augustine et al. [2], as the response to conventional treatments is still variable among patients, particularly for poorly characterized cancers, a mostly personalized treatment approach is proposed, as the “one-size-fits-all” model can have limited effectiveness. Each cancer varies among different types and patients, making even widespread and universally applied therapies that have been used for years, such as chemotherapy, produce different results and vary in effectiveness among patients. In fact, chemotherapeutic agents may vary in their effectiveness even for a certain tumor due to heterogeneity and cell plasticity [2]. So, in order for a treatment to be more effective, a new type of medicine, “personalized medicine”, was introduced, which, according to the American National Human Genome Research Institute [3], uses each patient’s individual genetic and molecular profiles in order to administer a more appropriate approach tailored to each patient’s diagnosis and therapy, leading to the highest therapeutic efficiency along with the best safety profile [4]. The drawbacks of personalized medicine are mainly economically driven, since genome and molecular profiles would require more lab tests and processes, making them more time-consuming and potentially causing national and provincial inequities due to the lack of some patients’ access to personalized testing and, overall, unequal opportunities to better health care [5].
This approach ascertains a clinical need for treatment regimens specific to an individual or a certain group of patients [2]. The development of new biophysical methods and techniques for the early (in terms of the time of appearance and extent) diagnosis and treatment of pathological biostructures is of great interest. Moreover, it requires a combination of knowledge and experience in both classical biomedical sciences, such as biology, biochemistry, and biomedical diagnosis and treatment, and interdisciplinary applications of biophysics, medical physics, informatics and biomedical engineering. Certainly, cancer treatment is one of the most interdisciplinary fields of basic and clinical research. New techniques, which act in addition to existing methods or as alternative diagnostic methods, have been developed in recent years. Among these, bio-optical techniques based on the use of laser and laser-like radiation seem to be promising.
At the same time, combined noninvasive targeted therapies, covering the entire spectrum of electromagnetic radiation, promise better outcomes in the fight against cancer and other diseases [6]. An interplay between ionizing and non-ionizing radiation is considered to optimize both the diagnostic and therapeutic efficacy of biophotonic modalities. Recently, there has been great interest in new biomedical modalities that combine optical and ionizing radiation, known as radioluminescence, for radiation monitoring therapy, phototherapy and nanoparticle-based molecular imaging [7].
The use of photons in the visible spectrum emitted through Cerenkov radiation is considered by several research groups to be a factor for the deeply penetrating energy stimulation of photosensitizers for photodiagnosis and/or photodynamic therapy (PDT) and photothermal therapy (PTT). After nearly a century since its discovery, in the last decades, the Cerenkov effect has received particular interest in the biomedical fields of diagnosis and therapy, resulting in new ideas for applications. To overcome the limitations imposed by Cerenkov’s low light intensity, several efforts have been made, mainly regarding the local enhancement of the light intensity using nanoparticles. Nanoparticles are biophotonic systems that serve either as diagnostic fluorescence agents for noninvasive tumor detection or as noninvasive tumor destruction systems with photodynamic and photothermal anticancer properties. The advantages of combining nanotechnology and Cerenkov luminescence have already been demonstrated in preclinical models [8], and it has been suggested as an effective internal light source for cancer phototherapy.
As the combination of nano-biophotonic techniques with up-to-date nuclear medicine procedures could provide a valuable benefit for theranostic applications in oncology, in this work, we briefly present an overview of the ionizing and non-ionizing radiation interplay in cancer management. Furthermore, preclinical and theoretical examples of the combined ionizing and non-ionizing Cerenkov luminescence of nanomaterials are given.
2. Novelties in Cancer Diagnosis
Once a symptom or a routine screening test result indicates a malignancy, several conventional procedures are usually suggested to the patient for cancer diagnosis. These may involve biochemical laboratory and imaging tests and scans, or even the surgical excision for biopsy and histopathologic confirmation, which, for a long time, has been considered the “gold standard” of malignancy diagnosis. Conventional medical imaging uses various physical factors (e.g., X-rays, ultrasounds, magnetic fields and radio waves) to discriminate biophysical parameters, such as the tissue density (X-rays), wave reflectance (ultrasound—US) or hydration state and properties (magnetic resonance imaging—MRI). In the last decades, the introduction of multimodal imaging systems has opened up a new avenue in the preclinical and clinical confrontation of cancer, aiming to improve the interpretation accuracy by correlating anatomical, functional and morphological information extracted from different imaging units. Multimodality imaging has been the subject of much research, particularly in the preclinical setting [9].
The widespread use of ionizing-radiation-based medical imaging for cancer diagnosis, particularly computed tomography (CT), mammography, fluoroscopic procedures and nuclear medicine examinations, has led to increased exposure to ionizing radiation. Even though the A.L.A.R.A. (As Low As Reasonably Achievable) principle minimizes patients’ exposure, the risk of radiation-induced secondary effects, or even malignancies, still remains. Hence, non-ionizing biomedical imaging has been proposed as an alternative for cancer diagnosis because, unlike X-ray imaging, it does not expose patients to ionizing radiation. Importantly, novelties in biomedical health research aim to increase image resolution versus reduced radiation exposure. Moreover, another drawback of conventional medical imaging technologies is attributed to the need for big installations (from a machine and shielding point of view), with high operating expenses.
Non-ionizing imaging includes several modalities for noninvasive or semi-invasive imaging based on different physical factors, other than tissue-penetrating ionizing radiation. Some representative diagnostic procedures are endoscopy (based on conventional light illumination of internal organs and tissues via physical body cavities), spectroscopy (based on non-ionizing electromagnetic waves), ultrasound imaging (based on high-frequency sound waves) and magnetic resonance imaging (based on strong magnetic fields, magnetic field gradients and radio waves). It is generally accepted that visible and near-infrared light, conventional ultrasound mechanical waves and magnetic fields are, in principle, harmless to the human body. However, for the improvement of the contrast resolution (e.g., the sensitivity and specificity of diagnostic images), it is recommended that specific contrast pharmaceuticals (such as nanoparticles of gadolinium or iron compounds for MRI and microbubbles for ultrasound) be injected [10].
Optical imaging uses monochromatic photon beams of laser light from ultraviolet to infrared wavelengths to obtain detailed images of organs, tissues, cells and even molecules, from point-of-care to laboratory testing and from screening and diagnostic imaging to treatment monitoring [11]. Although non-ionizing radiation does not penetrate the human body, the so-called optical imaging or biophotonic techniques offer the advantages of minimally or noninvasive methods for looking inside the body via optical fibers and wave-guides [12] in a less harmful manner than X-rays. Consequently, optical imaging can be used for repeated procedures to monitor the progression of a disease or the results of a treatment. Among different optical imaging techniques, we list the following: Laser-Induced Fluorescence spectroscopy (LIFs), endoscopy, Optical Coherence Tomography (OCT) [13], Photoacoustic Imaging (PI) [14], Diffuse Optical Tomography (DOT) [6], Diffuse Optical Imaging (DOI) [15], Raman Spectroscopy [16], Super-resolution Microscopy (e.g., photoactivated localization microscopy—PALM) [17], etc. [18] (Figure 1). According to Chen et al. [19], imaging technologies can be categorized into macroscopic, mesoscopic and microscopic scales, depending on their spatial resolution and penetration depth. In addition, biomedical imaging technologies can also be categorized as anatomical, physiological, cellular or molecular, depending on the imaging information obtained [19]. Emphasizing the contribution of optical imaging to pathological diagnosis, we have to point out that for some medical disciplines, e.g., dermatology, no other imaging technology was available before that could readily reveal details of sub-surface skin tissue at a clinically useful resolution, except surgical extraction and biopsy.
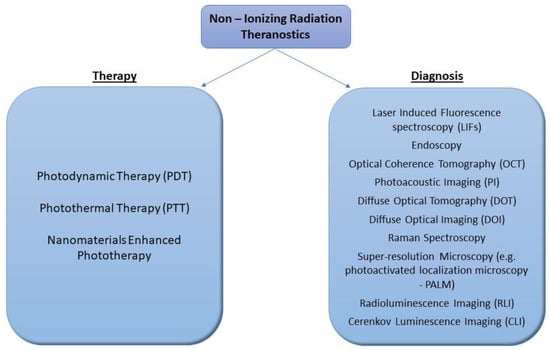
Figure 1.
Schematic illustration of different uses of non-ionizing radiation theranostics in cancer treatment and diagnosis.
Certainly, during the multiple years of optical imaging applications, it was anticipated by the scientific community that optical imaging modalities would not be able to replace conventional X-ray imaging. Furthermore, optical imaging covers several emerging diagnostic methods, and its acceptance in clinical practice required the validation of images obtained with other well-established techniques to discriminate not only neoplastic/non-tumor tissue but also malignant events at the molecular and cellular levels. It is noteworthy to mention that optical imaging applications demand safe optical powers lower than the maximum permissible exposure (MPE) values [11]. According to a current statement by the International Commission on Non-Ionizing Radiation Protection (ICNIRP), a literature search of existing regulations and potential health risks did not reveal any studies indicating adverse effects associated with the use of optical radiation in diagnostic procedures [20].
Recently, Pogue and Wilson [21] summarized several aspects of X-ray and optical technologies, “the two central pillars for human imaging and therapy”, as they said, giving various examples of the ways in which optical and X-ray sciences and technologies intersect. At this point, it is worth emphasizing that optical technologies tend to be highly specific depending on the organ to be examined and the suspected disease and diagnosis. This leads to the appropriate ergonomic adaptation of respective biomedical technologies, resulting in different devices for different specialties (e.g., the OCT system in ophthalmology is very different from the analog in dermatology). This is in contrast to X-ray imaging and therapy systems, which are much more general-purpose, with a given device covering a range of clinical procedures, as Pogue and Wilson mentioned in their work [21].
With the progressive development in both “noninvasive” imaging instrumentation and imaging materials (e.g., enhancement agents, molecular probes, tracers and nanocarriers), the field of imaging science is growing exponentially. Furthermore, the convergence of nuclear clinical imaging systems with advanced radiological imaging technology has resulted in the development of combined imaging modalities, such as PET/CT, SPECT/CT and PET/MRI, with the latest addition being total-body PET-CT [22,23]. Last year, Noltes et al. [23] in their editorial urged, “let us all embrace optical imaging as a growing branch on the clinical molecular imaging tree and a global opportunity to enrich our molecular armamentarium for the benefit of the patient”. According to the same team, optical imaging, apart from its use in preclinical studies, has already been translated into routine clinical care by both surgery- and endoscopy-focused groups in phase I–III clinical trials [23]. The inter-crossing of in vivo imaging techniques with bioluminescence, fluorescence and MRI enables the recording of events at the cellular and even molecular levels, which is expected to accelerate diagnosis [24]. Moreover, in an effort to set “a big picture to Biophotonics imaging” agenda, Laura Marcu and her colleagues discussed the relevant works presented by experts in the field during the 5th International Conference on Biophotonics (ICOB), held in 2017 in Fremantle, Western Australia [25]. In this position paper, they present the three main themes from the conference that capture the current status and future directions of biophotonics imaging, namely, (1) biophotonics at the nano- to microscale level; (2) biophotonics at the meso- to macroscale level; and (3) biophotonics and the clinical translation conundrum [25].
3. New Trends in Cancer Therapy: A SHIFT to Non-Ionizing Radiation
The armamentarium of cancer treatments includes invasive and noninvasive therapeutic modalities. Treatment options depend on the type, location and stage of the disease. The most conventional therapies are surgery (an invasive treatment modality), radiotherapy (a noninvasive, loco-regional treatment modality) and chemotherapy (a noninvasive, systemic treatment modality). Nowadays, other therapeutic methods are also considered (e.g., immunotherapy, hormone therapy, biological therapy, photodynamic therapy) as well as combinations of these (e.g., radiosurgery) [6,26].
In oncology, treating cancer with a beam of photons is currently an established therapeutic technique, one familiar to patients undergoing X-ray radiotherapy. The history of radiotherapy began in 1895, when Röntgen discovered X-rays, and in the following more than 125 years of being on the scene, radiotherapy has been significantly improved. More specifically, it expanded from its initial empirical application with the aid of the newly developed fields of radiation biology, medical physics and nanomedicine to a fairly complex and efficient therapeutic approach [6,26,27].
Radiotherapy can be sorted into two major types of ionizing radiation: (a) photon-based radiative energy (X-rays and gamma rays) and (b) particle-based radiative energy (such as electrons, protons, neutrons, carbon ions, alpha particles and beta particles). In radiotherapy, radiation is used in different forms, such as external beam radiation therapy, brachytherapy or internal radiation and radiopharmaceuticals, aiming to destroy cancer cells mainly by causing irreversible damage to their DNA. External beam radiation therapy uses linear accelerators in order to produce ionizing radiation and direct it locally to patients’ region of interest, while in brachytherapy (from Greek words “brachy”, which means short—in terms of time—and “therapy”), a radioactive source is placed inside of the patient’s body for a short period of time in order for the area of interest to be irradiated internally. Radioisotope therapy, classified under nuclear medicine, uses radiopharmaceuticals that are injected, in a specific amount, into a patient and then accumulate near the cancerous area and irradiate it [28]. Nevertheless, high-energy photons are not the only choice, as the ionizing radiation releasing energy into the cancerous tumor can lead to significant damage to healthy tissues surrounding the tumor as a side effect [29]. Usually, radiotherapy is performed by directing ionizing radiation to a specified volume, and in order to maximize the radiation dose in the tumor area and to minimize the dose in the surrounding healthy tissue, multiple beams are used at different angles of incidence, in different shapes and with modulated intensity.
The laser discovery in 1960 gave the opportunity to introduce new therapeutic modalities in the cancer battle, in both invasive and noninvasive modes. Laser beams are monochromatic, coherent and collimated, resulting in laser-based procedures that have high spectral, spatial and temporal resolutions. Depending on the photobiological laser–matter interaction mechanisms, laser light, in place of a scalpel, can be used to surgically remove cancerous or precancerous growths or to relieve symptoms. Laser surgery causes less bleeding and damage to normal tissue than standard surgical tools do, and there is a lower risk of infection. Furthermore, laser light can be used non-surgically for photodynamic therapy (PDT) and plasmonic photothermal therapy (PPTT) for cancer and other diseases [30]. In the next two sub-sections, we briefly mention some aspects of these phototherapies, PDT and PTT.
3.1. Photodynamic Therapy (PDT)
The agents are the photosensitizers (PSs), photosensitive drugs that are administered intravenously or topically to the patient and accumulate selectively in the cancer mass after a time interval or a couple of days. Subsequently, the pathological area is irradiated with monochromatic light of an appropriate wavelength matching the PS absorption peak, more precisely light from a laser source or a laser-like (i.e., light-emitting diode—LED) source. Depending on the mechanism that takes place, the PS will absorb the incident photon energy and produce highly cytotoxic molecules, such as singlet oxygen (1O2) [30,31]. The photo-generated singlet oxygen (1O2) attacks cellular organelles, causing their destruction through direct cellular damage, vascular shutdown and the activation of an immune response against targeted cells [32].
During the new era of PDT, several improvements have been developed in all three necessary PDT constituents. Many different PDT photosensitizers have been introduced and approved for use in PDT and fluorescence diagnosis since the first porphyrin and hematoporphyrin derivative compounds, forming the so-called photosensitive drug or photosensitizer generations for cancer and other disease applications (there are at least three PS generations, the first being hematoporphyrin derivatives, developed in the early 1970s). In parallel, several biophotonic technologies required to generate and transmit the appropriate light for PDT in patients use various light sources (e.g., lasers emitting in various wavelengths, LED arrays, lamps) and light-guiding modalities (e.g., optical fibers for endoscopic or interstitial applications). At present, diode lasers are the most common light sources for clinical PDT [33]. Certainly, PDT is considered a promising strategy with a noninvasive nature and spatiotemporal selectivity. In regard to theoretical and experimental efforts for accurate light, PS and ROS dosimetry in PDT (i.e., the biophysical features of PDT), we have to admit that this is a complex and still-open research field, regardless of whether many papers have been published [32,33,34,35,36,37,38,39].
Following vast basic biomedical research and preclinical exploration, PDT has been applied to treat a wide range of medical diseases, including wet age-related macular degeneration, acne and certainly malignant tumors [40]. As very aptly noted by Juzeniene, Peng and Moan in 2007, the field of PDT is large, embracing several subspecialties, from laser technology and optical penetration through diffusing media to a number of medical fields, including dermatology, gastroenterology, ophthalmology, blood sterilization and treatment of microbial/viral diseases [41]. However, there are many problems that hinder the widespread clinical use of PDT, such as the selective approach of the photosensitizer to the malignant tumor and the appropriate value of the laser light radiation dose throughout the tumor. Although several PDT drugs have been approved for clinical use, the method has not gained acceptance as a first-line treatment option. More than a decade ago, Agostinis et al. [42] provided a comprehensive review of PDT, referring, among others, to the paradox of one of the good properties of PDT, namely, the highly localized nature of its action, also being one of its current limitations, as “the treatment is ineffective against metastatic lesions which are the most frequent cause of death in cancer patients” [42].
Another major drawback is the limited penetration of visible light deep in the tissue [18], which influences the photodynamic therapy’s efficacy. Non-ionizing light delivered to deeper tissues, up to 1 cm, has been limited to the wavelength’s emission in the so-called optical window, spanning approximately from the orange/red region of the visible spectrum into the near-infrared range (from 600 to 1300 nm). Obviously, it is expected that the illumination of specific photosensitizers revealing reduced penetration in the treated tissue lesion will result in problematic treatment and—potentially—in a future recurrence of the tumor.
Consequently, the translation of PDT from preclinical to clinical applications is mostly performed on human diseases that are either easily accessible or limited to superficial or relatively transparent layers of tissues (such as skin pathologies and chorioretinal conditions) [43,44]. Research to improve the therapeutic possibilities of PDT is oriented toward (i) the design of new photosensitizers with minimum cytotoxicity and maximum tumor selectivity: newer-generation substances are intended to absorb light radiation toward the near-infrared part of the spectrum for deeper light penetration into the tissues; (ii) the combined application of photodynamic therapy together with other techniques, such as in combination with radiotherapy, hyperthermia and radiosensitive substances.
3.2. Photothermal Therapy (PTT)
Photothermal therapy (PTT) is a minimally invasive therapeutic scheme. PTT is a newly developed and encouraging type of hyperthermia that employs photon energy (provided by monochromatic electromagnetic wave beams) to generate heat sufficient to cause cell destruction [30,32,45]. Certainly, the use of laser radiation in PTT offers an alternative path to using radio-frequencies (RFs), microwaves or high-intensity focused ultrasound (HIFU) [32]. In photothermal therapy, local and controlled temperature effects are sought for the cancerous tissue only. This leads to the destruction of cancer cells by either apoptosis, necroptosis or necrosis, or it can be used in combination with radiotherapy.
Recent advances in nanotechnology gave rise to a generation of various metallic nanoparticles that can act as photosensitizers for PTT. Functionalized metallic nanoparticles can be photothermal sensitizing agents due to their unique optical and electrical properties. Metallic nanoparticles can resonate plasmonically upon their exposure to light. Surface plasmon resonance is a nanoscale electronic effect that causes metallic nanoparticles to absorb and scatter electromagnetic radiation of wavelengths considerably larger than the particles themselves [46].
According to this phenomenon, the electromagnetic wave that is incident on small dielectric particles causes collective transitions of the particles’ electrons between occupied and unoccupied electronic energy bands, thus creating a form of resonance with the incident electromagnetic field. Hence, the free electrons present in the material oscillate collectively, producing so-called Localized Surface Plasmons (LSPs) [47]. When the frequency of the incident electromagnetic wave is tuned to the oscillation frequency of the electron cloud, the absorption and scattering of light are enhanced [48]. Τhe interaction of the electromagnetic wave with a homogeneous particle depends on the particle’s size, the refractive index of the particle and the medium in which it is located, as well as its construction material [49]. Plasmonic oscillations can be tuned in a wide spectral range, from visible to NIR, when the NP shape deviates from the highly symmetric nanospherical shape to nanoshells (NSs), nanocages (NCgs) and nanorods (NRs) [45]. The resonated nanoparticles convert the electromagnetic energy into heat via a non-irradiated process, causing hyperthermic damage to cancer cells.
Plasmonic photothermal therapy (PPTT) using gold-based nanospheres was reported for the first time in 2003 by Lin and co-workers [50]. Thirty-nanometer gold nanoparticles functionalized with anti-mouse IgG were incubated with T lymphocytes labeled with anti-human CD8 mouse IgG. Irradiation with a nanosecond pulsed laser at a 565 nm wavelength led to over 90% cell death. In vivo, the use of near-infrared light (NIR) for PTT is the optimal choice, as it penetrates deeper into tissues with higher spatial precision. PEG-AuNSs injected interstitially into tumors in mice and then irradiated with NIR light at 4W/cm2 for 4–6 nm induced a temperature increase and irreversible tissue damage. Recently, silica-encapsulated gold nanochains (AuNCs@SiO2) with strong surface plasmon resonance at NIR-II (λ = 1–1.7 μm) demonstrated high photothermal conversion efficiency at 1.064 μm, inducing severe cell death and remarkable tumor growth inhibition in mice [51].
However, the main drawback of PTT, as in PDT as well, is the limited penetration depth of light, leading to the incomplete elimination of tumors even if the therapeutic effect is high. This can in turn lead to tumor recurrence [52]. Recently, Upconverting Nanoparticles (UCNPs) have been demonstrated in biophotonics as hopeful theranostic probes for tissues [53,54]. UNCNPs have the unique ability to absorb near-infrared light and emit light at shorter wavelengths, closer to the visible or ultraviolet light region. Sharma et al. [55] provided a general review of the application of different kinds of theranostic nanoparticles for in vivo image-guided light-activated therapy in cancer. They demonstrated that nanoparticle-mediated phototherapies, such as photodynamic therapy and photothermal therapy, are earnestly considered efficient interventional strategies against several cancer types [55].
4. Radioluminescence Combined with Nanoparticles in Imaging and Phototherapy
The link between thera(peutics) and diag(nostics) led to the development of a new interdisciplinary field, entitled “theranostics”, which became very exciting for scientists after the first reports during the last decade [32,56,57,58] as a key hallmark of cancer nanomedicine. A very interesting approach is to combine existing optical imaging technology with sophisticated nanoparticle-based optical contrast agents for high-resolution in vivo cancer imaging. Recently, Kemp and Kwon published a comprehensive review on cancer theranostic improvements through nanotechnology, pointing out that “the strategy against cancer needs to shift from finding new therapies to improving existing therapies and diagnostics in innovative, effective, and plausible ways” [59]. They provide insights into the current clinical and preclinical nanotechnological applications for cancer drug therapy, diagnostics, imaging and radiation therapy. Moreover, several gold nanomaterials have recently been translated into clinical evaluations for pathologies other than cancer, such as atherosclerotic lesions and other diseases [60].
In recent decades, several papers have presented the existing and potential interfaces between biophotonic and X-ray technologies that enable biomedical diagnostic and therapeutic applications [21]. As we mentioned, the main disadvantage of optical imaging is that the penetration depth is limited because the energy of photons is low, much lower than that of ionizing radiation photons, thereby making it nearly impossible to image deep tissues in large biological targets. Due to the small size of experimental animals, the depth of penetration is not a problem for them. Therefore, optical imaging and therapy are widely used, especially for preclinical research [61].
Radioluminescence, which is normally produced by the interaction of ionizing radiation with a scintillator material, can be used as a light source in PDT. The use of X-ray sources in combination with scintillating nanoparticles can overcome the barrier of light tissue penetration [62]. NaGdF4:Eu3+ NPs were used to convert X-rays into optical photons within the 600–700 nm range to perform X-ray luminescence optical tomography [62]. Bilirubin-photodynamic nanoparticles (PEGylated bilirubin-encapsulated CaWO4 nanoparticles or “PEG-BR/CWO NPs”) can mediate combined radiotherapy/PDT in solid tumors due to the radioluminescence properties of their CaWO4 nanoparticle cores. PEG-BR/CWO NPs emit UV-A and visible light from their CaWO4 cores, which excites bilirubin and leads to the production of singlet oxygen (Figure 2). The clonogenic survival array demonstrates the phototherapy enhancement due to PEG-BR/CWO NPs.
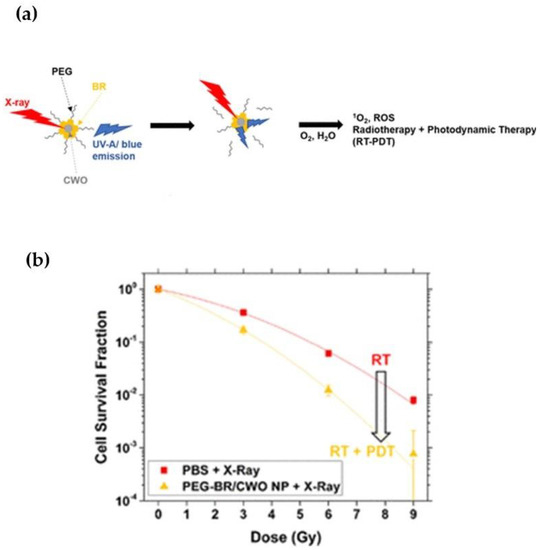
Figure 2.
(a) Schematic image of the PEG-BR/CWO NP mechanism of action. (b) PEG-BR/CWO NP initial clonogenic survival assay. HN31 cells were plated in 6-well plates at 0.2 × 103 (0 Gy), 0.8 × 103 (3 Gy), 1.6 × 103 (6 Gy) and 5.0 × 103 (9 Gy) in triplicate for each treatment group. Cells were incubated with PBS, PEG-BR micelles (0.2 mg/mL PEG-BR) and PEG-BR/CWO NPs (0.2 mg/mL CWO nanoparticle) for 4 h prior to radiotherapy. Irradiation was performed with a 320 kV X-ray irradiator (2 Gy/min). Reprinted with permission from Ref. [62]. Copyright 2020, American Chemical Society.
Cerenkov Radiation Coupled with Nanoparticles Mediates Imaging and Phototherapy Enhancement
Cerenkov radiation (CR) is a type of electromagnetic radiation that is emitted when a charged particle moves through a dielectric medium, with a velocity greater than the phase velocity of light in that medium. The emission is a result of the polarization of the medium’s molecules along the charged particle’s trajectory. During the depolarization of these molecules, after the particle’s passage, radiation, mainly blue in color, is released.
More specifically, the light is emitted at the angle θ, forming a cone around the particle’s direction, which depends on the particle’s relative velocity, β = (v is the velocity of the particle and c is the speed of light in a vacuum), and the refractive index, n, of the medium in which the particle moves, with the relation cos θ = . The CR spectrum is continuous, peaks in the ultraviolet–blue region and then decreases inversely to the square of the wavelength (λ) through the visible region [63] according to the Frank–Tamm equation:
where n is the number of Cerenkov photons that are produced per unit of length (l) and wavelength (λ), and a ≈ is the fine-structure constant [64].
The use of Cerenkov luminescence has been suggested by some research groups as an effective alternative solution to overcome the limitation of the penetration depth and become the bridge between ionizing and non-ionizing radiation (Figure 3). Cerenkov light is spontaneously generated within tissues subjected to ionizing radiation in a continuous spectrum from the near-ultraviolet through the visible spectrum (highly weighted to the ultraviolet/blue spectral region) [65]. The characteristic blue glow of Cerenkov luminescence (CL) is produced when a charged particle traveling faster than the phase velocity of light interacts with a dielectric medium, such as water or tissue. Astronauts in space (crews of Apollos 11, 12 and 13 [66]), as well as patients with cancer undergoing radiation therapy, have reported seeing blue flashes of light when encountering high-energy radiation, even if their eyes are closed [67]. This phenomenon has been attributed to the excitation of retinal pigments by the generation of Cerenkov light inside the eye and can be very helpful in the setting of stereotactic radiosurgery and in brain radiation therapy [67].
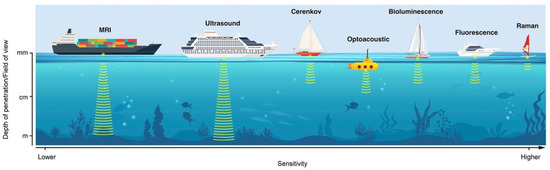
Figure 3.
Schematic representation of various optical imaging modalities discussed in this review. Different modalities are represented as different boats, each with its individual pros and cons and its specific utility. The x-axis represents sensitivity (lower to high, left to right). The y-axis represents the depth of penetration/field of view, as indicated below each boat. Reprinted with permission from Ref. [68]. Copyright 2020, Society of Nuclear Medicine and Molecular Imaging.
In the last decade, the use of Cerenkov radiation emission has been considered by several research groups to be a determining factor for in situ dosimetry in radiotherapy [69,70]. A big international effort by several collaborating research groups, led by Dr. Brian Pogue, published both experimental and theoretical results from their investigation into fluorescence, phosphorescence and absorption spectroscopy methods using Cerenkov light release [71,72,73,74,75]. To cancer patients receiving radiotherapy schemes at Dartmouth-Hitchcock Medical Center, they applied a real-time monitoring protocol for radiation dose verification and for patient positioning throughout each treatment fraction via a Cerenkov imaging system [76]. They realized that, during the radiotherapy session, the Cerenkov effect occurs when photons or electron beams interact with the irradiated tissue volume and that this internally emitted light reflects the shape of the treatment beam on the patient’s skin surface. So, several research groups, especially the Dartmouth team led by Lesley Jarvis and Brian Pogue, developed proper imaging devices (e.g., cameras) to capture the intensity of this light, which is relative to the delivered dose, in an effort to realize real-time monitoring [76]. A major problem that Pogue’s team emphasized was the difficulty in making clinical measurements in a conventional radiation treatment environment where ambient room light is always present, raising significant concern for patient and physician compliance [77]. Several efforts were oriented to develop, test and implement the appropriate imaging devices for Cerenkov emission registration. A very illustrative image of Cerenkov blue light from a human head phantom for radiation therapy was published by Pogue’s group in 2012, enabled by a Cerenkov imaging system, which provides the real-time visualization of each radiation beam (Figure 4).
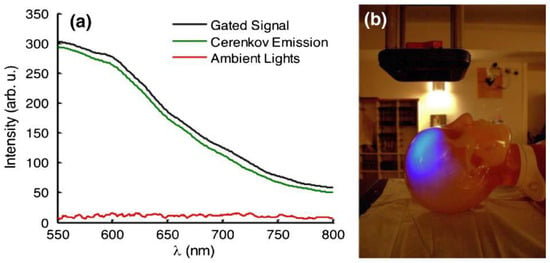
Figure 4.
(a) The gated detection of Cerenkov emission with room lights on is shown with spectra acquired from a scattering phantom. Cerenkov emission is obtained by calculating the difference between the gated signal and ambient lighting signal. (b) A photograph of the room with a corresponding image of Cerenkov emission from a human head phantom for radiation therapy overlaid to illustrate the amount of ambient lighting present for all experiments. Reprinted with permission from Ref. [78]. Copyright 2012, Optica Publishing Group.
For applications other than radiation dosimetry, Briam Pogue and collaborating groups reported their efforts on tumor pO2 measurements using Cerenkov-excited phosphorescence imaging with a systemically delivered phosphorescent oxygen-sensitive probe, PtG4 [71,72], or Cerenkov-excited luminescence imaging (CELI) [73]. Furthermore, they recognized that moving from phantom experiments to the systemic administration of any drug to human beings requires extensive toxicological and other tests before clinical translation. Thus, they developed and evaluated the possibility of oxygen measurements with CELI using a model implantable sensor [75]. This technique makes use of the Cerenkov light generated within tissues subjected to radiation beams to excite the phosphorescence of PtG4, and the phosphorescence decay time of the probe is used to quantify tissue pO2. CELI offers several advantages, including the real-time reporting of oxygenation in tumors at the time of delivering the radiation, either as a monodose or during each fraction in tumors undergoing multiple fractions of radiotherapy.
Cerenkov luminescence imaging (CLI) can be an emerging hybrid method that uses light emission from many commonly used medical radioisotopes [63,79]. The term CLI was coined by Robertson et al. in 2009 when they studied Cerenkov emission from the PET probe 18F-FDG in well plates and a xenograft tumor in a mouse [80]. Boschi and Spinelli summarized several studies referring to CLI in the biomedical field. They organized them in a table to help interested viewers for a fast consultation, subdividing CLI into different areas of applications (cancer imaging, tomography, Cerenkov-induced fluorescence, intraoperative modality, medical applications in humans, models and others) [81]. Liu et al. used CL produced by β-tracer iodine-131 as an internal light source to illuminate three different CdSe–ZnS core–shell Quantum Dots (QDs) (QD655, QD705 and QD800) both in vitro and in vivo (Figure 5). The excited QDs allowed for multiplexing optical imaging, producing fluorescence in much higher optical intensities [82], whereas the number of luminescent photons produced from radionuclide radiation is small.
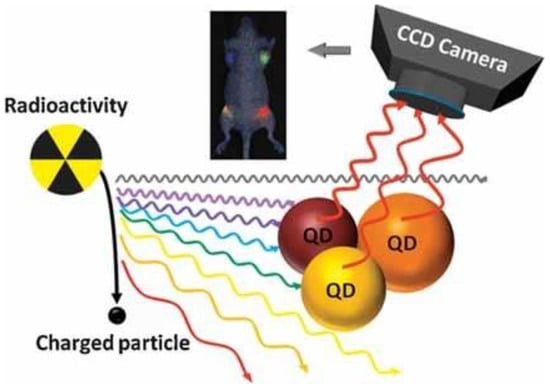
Figure 5.
Radiation-luminescence-excited QDs for optical imaging. Reprinted with permission from Ref. [82]. Copyright 2010, John Wiley and Sons.
Cerenkov radiation emission can also be used for the internal stimulation of photosensitizers resulting in photodiagnosis and/or phototherapy. Fan et al. (2016) reported some potential challenges to overcome the so-called Achilles’ heel of photodynamic therapy, i.e., the tissue penetration limitation of excitation light for deep-seated diseases [40]. They present a series of deep PDT techniques based on different excitation sources, including NIR light, X-ray radiation and internal self-luminescence. They anticipate that “deep PDT will be developed as a versatile, depth/oxygen-independent and minimally invasive strategy for treating a variety of malignant tumors at deep locations”.
According to published ex vivo and in situ studies, the combination of PTT with RT can have a synergistic therapeutic effect that can overcome the limitations of each single treatment [83]. PTT can enhance the effects of RT by attenuating the repair of double-strand breaks (DSBs) caused by RT [84,85]. Novel G. Lan et al. synthesized a novel nanoscale metal–organic framework (nMOF) by hierarchically incorporating three high-Z components, Hf12, DBB-Ir and W18 (W18@Hf12-DBB-Ir), which, upon X-ray irradiation, significantly enhance hydroxyl radical generation. These distinct reactive oxygen species, W18@Hf12-DBB-Ir, demonstrate synergetic cell killing with high anticancer efficacy [86].
Clinical results have shown that the combination of hyperthermia and radiotherapy (ionizing radiation) can increase response rates from 16% to 26% [87]. Cells in hypoxic, low-pH areas and cells in the S-phase are both relatively radioresistant. The increased blood flow caused by hyperthermia can improve tissue oxygenation, which temporarily increases radiosensitivity [88]. Studies have shown that thermal treatment combined with traditional or accelerated radiation fractionation for 7 days may increase the tumor growth delay by up to 17 days [89].
Apart from Cerenkov luminescence optical imaging, the exploitation of Cerenkov radiation can provide a very important option for the in vivo photoactivation of pharmaceuticals, particularly photosensitizers, for photodynamic therapy [90].
However, the majority of the energy from CR is dissipated through molecular excitation, ionization, Bremsstrahlung radiation and heat [91]. For example, CR represents less than 0.006% of the total energy released during the radioactive decay of 18F [91]. To give a numerical example from the literature [63], assuming an average refractive index of 1.33 for water and 1.4 for soft tissue and using the Frank–Tamm equation, the number of Cerenkov photons produced by a beta particle moving in the two media, for a wavelength range of 400–1000 nm, per length unit, is calculated to be 20 photons/mm and 23 photons/mm, respectively. Cerenkov radiation production in biological tissue is a very complicated process and can be generated by primary and secondary particles produced during different interactions with the medium’s atoms. However, most of the photons originate from the primary beta particles of the radioisotope, which have enough energy to overcome the Cerenkov threshold in that medium and cover an area of only a few millimeters around the radioisotope molecule. Secondary particles can produce Cerenkov photons further from the source, but their contribution is important only in high-energy isotopes used in PET, such as 89Zr [63]. In biological tissues, ultraviolet light (λ < 380 nm) is greatly absorbed by molecules such as hemoglobin, water and melanin, while in the early near-infrared region (600–1600 nm), scattering is more dominant, leading to Cerenkov radiation’s spectrum having a more complex shape and the conical spatial distribution of photons in the tissue being disturbed [63], adding more difficulty to the detection or use of this radiation for therapy.
Moreover, the effect of CR-PDT can be limited when the radionuclide and a photosensitizer are administered separately [92]. The strategy of coupling radionuclides with a photosensitizer can lead to the continuous generation of ROS throughout the blood circulation period, making the modality effectively a normal chemotherapeutic agent [93]. Thus, the reduction in the adverse effects of CR-PDT is of major significance and still remains challenging. The combination of the commonly used clinical diagnostic radiotracers in PET imaging with nanoparticles loaded with photosensitizers can have an effective therapeutic photodynamic action [94]. Radionuclides can be transformed from chemotherapeutics into phototherapeutics by utilizing CL to trigger photoactivated nanoparticles. Kotagiri et al. used transferrin-coated TiO2 nanoparticles doped with the photosensitizer titanocene to target human fibrosarcoma (HT1080) cells injected in mice [95]. They demonstrated that CR emitted by 18F-fluorodeoxyglucose (18F-FDG), the commonly used radiotracer in PET, mediates the excitation of TiO2 NPs, which leads to the production of cytotoxic hydroxyl and superoxide radicals, achieving depth-independent PDT. Duan et al. compared the activation of Dextran-modified TiO2 nanoparticles (D-TiO2 NPs) between the radiotracers Gallium-68 (Ga-68) and 18F. They proved that Ga-68 is a higher-yield photon emitter than 18F for Cerenkov-induced PDT and displayed more DNA damage to 4T1 cells [96]. TiO2 NPs present a high absorption of UV light (λ = 275–390 nm) and can serve as efficient photocatalysts producing Radical Oxygen Species (ROS) (Figure 6).
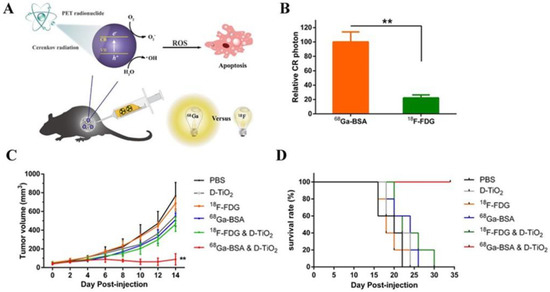
Figure 6.
(A) Schematic of the CR-mediated excitation of TiO2 NPs to generate ROS that induced cell apoptosis of cancer cells. (B) Relative amounts of CR photons generated by intracellular 68Ga-BSA and 18F-FDG. (C) Tumor growth curves of different groups of mice after treatments indicated. (D) Survival curve indicates that co-injection of Ga-BSA and D-TiO2 NPs was the optimal choice among methods. ** p < 0.01. Reprinted with permission from Ref. [96]. Copyright 2018, American Chemical Society.
Recently, Ruijie Qian et al. engineered a nanocarrier co-loaded with the radionuclide 131I and a photosensitizer precursor for combined radiotherapy and PDT. They designed tumor-targeting nanoparticles (131I-EM@ALA) by loading the photosensitizer 5-aminolevulinic acid (ALA) into an 131I-labeled exosome mimetic (EM) to achieve combined antitumor therapy. 131I also served as an internal light source for CR, and the drug-loaded nanoparticles effectively targeted tumors [97].
Hybrid nanotheranostic formulations have been developed for PET to provide synergetic PDT/PTT in breast-tumor-bearing mice. Radiolabeled core–satellite nanoconstructs of copper sulfide (CuS) nanoparticles were prepared by [89Zr]-labeled hollow mesoporous silica nanoparticles (HMSNs) and citrate-capped CuS nanosatellites. CuS mediates hyperthermia, which enhances the oxygenation of blood vessels and decreases tumor hypoxia, the major barrier to the effectiveness of PDT [98]. Many computational simulations have been conducted to calculate the CR yield arising from radiotracers and radiotherapy beams. According to Monte Carlo simulations, the fluence rate of CR was found to be in the order of 0.01–1 nW/cm2 per MBq/g for radionuclides and 1–100 µW/cm2 per Gy/s [99]. The CR fluence depends on the radiation source, the optical properties of the tissue and the given wavelength. The energy fluence of CR-mediated phototherapy reaches the order of nJ/cm2 for radionuclides and mJ/cm2 for radiotherapy beams. Ngwa et al. performed Monte Carlo simulations to compare the CR produced during external beam radiotherapy (EBRT) using titanium dioxide (titania) nanoparticles (NPs) as inclusions in radiotherapy biomaterials to that produced by radionuclides. The simulations showed that 6 MV radiation produced about 10 times higher CR per unit dose deposition than 18F. They confirm the CR enhancement experimentally by irradiating human lung cancer cells with or without 0.5 μg/g 2nm titania NPs at 2 Gy with a 6MV photon beam. According to clonogenic assays, the survival fraction of the cancer cells was reduced by 20% with NPs, confirming the synergistic effect of ionizing radiation and titania NPs. Table 1 provides a list summarizing the recent in vivo and in vitro studies with NPs and different Cerenkov sources. Figure 7 illustrates the interconnection between the advanced radiation biophysics, biophotonic and nanotechnology techniques for cancer management and the interplay of Cerenkov radiation for applications showing promise for significantly improving patient prognoses and quality of life.

Table 1.
Combined nanoparticle–radiation treatments for various types of NPs and Cerenkov sources.
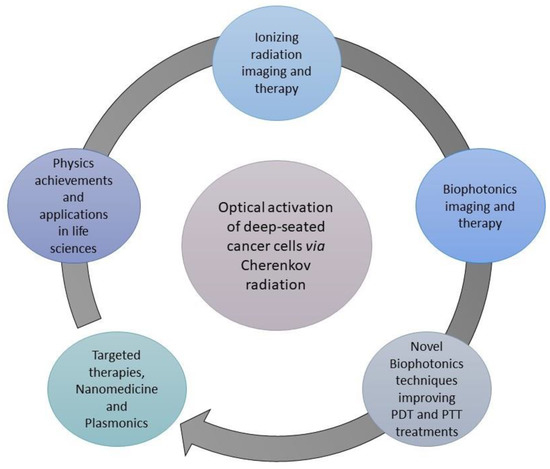
Figure 7.
Schematic illustration of the interconnection of advanced radiation biophysics, biophotonic and nanotechnology techniques with the Cerenkov radiation for cancer management.
5. Conclusions
The novelty of using nano-biophotonic techniques, combined with up-to-date nuclear medicine imaging procedures and radiotherapy, could provide an effective strategy in preclinical and clinical environments for theranostic applications. Many studies have been conducted with various protocols based on different types of nanoparticles, Cerenkov radiation sources and irradiation conditions. Each of them demonstrates that Cerenkov luminescence can be the bridge between ionizing and non-ionizing radiation to overcome the limitation of the penetration depth for more effective and targeted photothermal therapies.
Author Contributions
Conceptualization, E.S. and M.M.; writing—original draft preparation, E.S., M.M. and K.K., writing—review and editing, E.S., M.M., K.K., G.K., G.T., K.P. and E.P.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/330745 (accessed on 1 December 2022).
- Augustine, R.; Kalva, S.N.; Ahmad, R.; Zahid, A.A.; Hasan, S.; Nayeem, A.; McClements, L.; Hasan, A. 3D Bioprinted cancer models: Revolutionizing personalized cancer therapy. Transl. Oncol. 2021, 14, 101015. [Google Scholar] [CrossRef] [PubMed]
- Personalized Medicine. Available online: https://www.genome.gov/genetics-glossary/Personalized-Medicine (accessed on 30 November 2022).
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.; Kamel-Reid, S.; Batist, G.; Chia, S.; Blanke, C.; Moore, M.; Sawyer, M.B.; Desjardins, C.; Dubois, A.; Pun, J.; et al. Benefits, issues, and recommendations for personalized medicine in oncology in Canada. Curr. Oncol. 2013, 20, e475–e483. [Google Scholar] [CrossRef] [PubMed]
- Kareliotis, G.; Tremi, I.; Kaitatzi, M.; Drakaki, E.; Serafetinides, A.A.; Makropoulou, M.; Georgakilas, A.G. Combined radiation strategies for novel and enhanced cancer treatment. Int. J. Radiat. Biol. 2020, 96, 1087–1103. [Google Scholar] [CrossRef]
- Klein, J.S.; Sun, C.; Pratx, G. Radioluminescence in biomedicine: Physics, applications, and models. Phys. Med. Biol. 2019, 64, 04tr01. [Google Scholar] [CrossRef]
- Shaffer, T.M.; Pratt, E.C.; Grimm, J. Utilizing the power of Cerenkov light with nanotechnology. Nat. Nanotechnol. 2017, 12, 106–117. [Google Scholar] [CrossRef]
- Amirrashedi, M.; Zaidi, H.; Ay, M.R. Towards quantitative small-animal imaging on hybrid PET/CT and PET/MRI systems. Clin. Transl. Imaging 2020, 8, 243–263. [Google Scholar] [CrossRef]
- van Zandwijk, J.K.; Simonis, F.F.J.; Heslinga, F.G.; Hofmeijer, E.I.S.; Geelkerken, R.H.; Ten Haken, B. Comparing the signal enhancement of a gadolinium based and an iron-oxide based contrast agent in low-field MRI. PLoS ONE 2021, 16, e0256252. [Google Scholar] [CrossRef]
- Yun, S.H.; Kwok, S.J.J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 0008. [Google Scholar] [CrossRef]
- Optical Imaging Fact Sheet; National Institute of Health: Bethesda, MA, USA, 2019. Available online: www.nibib.nih.gov (accessed on 2 December 2020).
- Shu, X.; Beckmann, L.; Zhang, H. Visible-Light optical coherence tomography: A review. J. Biomed. Opt. 2017, 22, 121707. [Google Scholar] [CrossRef]
- Wang, L.V.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Akbari Sari, A.; Mobinizadeh, M.; Azadbakht, M. A systematic review of the effects of diffuse optical imaging in breast diseases. Iran. J. Cancer Prev. 2013, 6, 44–51. [Google Scholar] [PubMed]
- Kouri, M.A.; Spyratou, E.; Karnachoriti, M.; Kalatzis, D.; Danias, N.; Arkadopoulos, N.; Seimenis, I.; Raptis, Y.S.; Kontos, A.G.; Efstathopoulos, E.P. Raman Spectroscopy: A Personalized Decision-Making Tool on Clinicians & rsquo; Hands for In Situ Cancer Diagnosis and Surgery Guidance. Cancers 2022, 14, 1144. [Google Scholar] [PubMed]
- Zhong, H. Photoactivated localization microscopy (PALM): An optical technique for achieving ~10-nm resolution. Cold Spring Harb. Protoc. 2010, 2010, pdb.top91. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, S.; Wierwille, J.; Naphas, R.; Li, Q.; Blackwell, T.R.; Winnard, P.T.; Raman, V.; Glunde, K. Integrated Optical Coherence Tomography (OCT) and Fluorescence Laminar Optical Tomography (FLOT). IEEE J. Sel. Top. Quantum Electron. 2010, 16, 755–766. [Google Scholar] [CrossRef]
- ICNIRP Statement on Diagnostic Devices Using Non-ionizing Radiation: Existing Regulations and Potential Health Risks. Health Phys. 2017, 112, 305–321. [CrossRef]
- Pogue, B.W.; Wilson, B.C. Optical and x-ray technology synergies enabling diagnostic and therapeutic applications in medicine. J. Biomed. Opt. 2018, 23, 121610. [Google Scholar] [CrossRef]
- Cherry, S.R.; Jones, T.; Karp, J.S.; Qi, J.; Moses, W.W.; Badawi, R.D. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J. Nucl. Med. 2018, 59, 3–12. [Google Scholar] [CrossRef]
- Noltes, M.E.; van Dam, G.M.; Nagengast, W.B.; van der Zaag, P.J.; Slart, R.; Szymanski, W.; Kruijff, S.; Dierckx, R. Let’s embrace optical imaging: A growing branch on the clinical molecular imaging tree. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4120–4128. [Google Scholar] [CrossRef]
- Youn, H.; Hong, K.-J. In Vivo Noninvasive Small Animal Molecular Imaging. Osong Public Health Res. Perspect. 2012, 3, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Laura, M.; Stephen, A.B.; Mark, R.H.; Jürgen, P.; Brian, C.W. Biophotonics: The big picture. J. Biomed. Opt. 2017, 23, 021103. [Google Scholar] [CrossRef]
- Spyratou, E.; Makropoulou, M.; Efstathopoulos, E.P.; Georgakilas, A.G.; Sihver, L. Recent Advances in Cancer Therapy Based on Dual Mode Gold Nanoparticles. Cancers 2017, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Tremi, I.; Spyratou, E.; Souli, M.; Efstathopoulos, E.P.; Makropoulou, M.; Georgakilas, A.G.; Sihver, L. Requirements for Designing an Effective Metallic Nanoparticle (NP)-Boosted Radiation Therapy (RT). Cancers 2021, 13, 3185. [Google Scholar] [CrossRef] [PubMed]
- Radiotherapy Overview. Available online: https://www.nhs.uk/conditions/radiotherapy/ (accessed on 1 December 2022).
- Makropoulou, M. Cancer and electromagnetic radiation therapy: Quo Vadis? arXiv 2016. [Google Scholar] [CrossRef]
- Kareliotis, G.; Chronopoulou, E.; Makropoulou, M. In Silico, Combined Plasmonic Photothermal and Photodynamic Therapy in Mice. J. Nanotheranostics 2022, 3, 4. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Makropoulou, M.; Kareliotis, G.; Spyratou, E.; Drakaki, E.; Serafetinides, A.A.; Efstathopoulos, E. Non-ionizing, laser radiation in Theranostics: The need for dosimetry and the role of Medical Physics. Phys. Med. 2019, 63, 7–18. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Kareliotis, G.; Xanthopoulos, S.; Drakaki, E.; Papachristou, M.; Datseris, I.; Bouziotis, P.; Makropoulou, M. Photodynamic Therapy of 4T1 tumors in NOD-SCID mice. arXiv 2020. [Google Scholar] [CrossRef]
- Kareliotis, G.; Liossi, S.; Makropoulou, M. Assessment of singlet oxygen dosimetry concepts in photodynamic therapy through computational modeling. Photodiagnosis Photodyn. Ther. 2018, 21, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Baochang, L.; Thomas, J.F.; Michael, S.P. Comparison of photodynamic therapy with different excitation wavelengths using a dynamic model of aminolevulinic acid-photodynamic therapy of human skin. J. Biomed. Opt. 2012, 17, 088001. [Google Scholar] [CrossRef]
- Wang, K.K.-H.; Finlay, J.C.; Busch, T.M.; Hahn, S.M.; Zhu, T.C. Explicit dosimetry for photodynamic therapy: Macroscopic singlet oxygen modeling. J. Biophotonics 2010, 3, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.C.; Kim, M.M.; Liang, X.; Finlay, J.C.; Busch, T.M. In-Vivo singlet oxygen threshold doses for PDT. Photonics Lasers Med. 2015, 4, 59–71. [Google Scholar] [CrossRef]
- de Bruijn, H.S.; Brooks, S.; van der Ploeg-van den Heuvel, A.; ten Hagen, T.L.M.; de Haas, E.R.M.; Robinson, D.J. Light Fractionation Significantly Increases the Efficacy of Photodynamic Therapy Using BF-200 ALA in Normal Mouse Skin. PLoS ONE 2016, 11, e0148850. [Google Scholar] [CrossRef]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Juzeniene, A.; Peng, Q.; Moan, J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem. Photobiol. Sci. 2007, 6, 1234–1245. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Mpourazanis, G.; Konschake, W.; Vogiatzis, R.; Papalexis, P.; Georgakopoulou, V.E.; Ntritsos, G.; Sklapani, P.; Trakas, N. The Role and Effectiveness of Photodynamic Therapy on Patients With Actinic Keratosis: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e26390. [Google Scholar] [CrossRef]
- Newman, D.K. Photodynamic therapy: Current role in the treatment of chorioretinal conditions. Eye 2016, 30, 202–210. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Pissuwan, D.; Valenzuela, S.M.; Cortie, M.B. Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotechnol. 2006, 24, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Quinten, M. Optical Properties of Nanoparticle Systems: Mie and Beyond; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Wrigglesworth, E.; Johnston, J. Mie theory and the dichroic effect for spherical gold nanoparticles: An experimental approach. Nanoscale Adv. 2021, 3, 3530–3536. [Google Scholar] [CrossRef] [PubMed]
- Hergert, W.; Wriedt, T. The Mie Theory; Springer: Berlin/Heidelberg, Germany, 2012; Volume 169. [Google Scholar]
- Pitsillides, C.M.; Joe, E.K.; Wei, X.; Anderson, R.R.; Lin, C.P. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys. J. 2003, 84, 4023–4032. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, L.; Sun, T.; Zhang, Y.; Liu, Y.; Gong, M.; Xu, Z.; Du, M.; Liu, Y.; Liu, G.; et al. Activatable NIR-II Plasmonic Nanotheranostics for Efficient Photoacoustic Imaging and Photothermal Cancer Therapy. Adv. Mater. 2021, 33, e2006532. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shao, Z.; Zhao, Y. Solutions to the Drawbacks of Photothermal and Photodynamic Cancer Therapy. Adv. Sci. 2021, 8, 2002504. [Google Scholar] [CrossRef]
- Ether, D.S.; Pires, L.B.; Umrath, S.; Martinez, D.; Ayala, Y.; Pontes, B.; Araujo, G.D.S.; Frases, S.; Ingold, G.-L.; Rosa, F.S.S.; et al. Probing the Casimir force with optical tweezers. Europhys. Lett. 2015, 112, 44001. [Google Scholar] [CrossRef]
- Machta, B.B.; Veatch, S.L.; Sethna, J.P. Critical Casimir Forces in Cellular Membranes. Phys. Rev. Lett. 2012, 109, 138101. [Google Scholar] [CrossRef]
- Sharma, S.; Zvyagin, A.V.; Roy, I. Theranostic Applications of Nanoparticle-Mediated Photoactivated Therapies. J. Nanotheranostics 2021, 2, 9. [Google Scholar] [CrossRef]
- Fan, Z.; Fu, P.P.; Yu, H.; Ray, P.C. Theranostic nanomedicine for cancer detection and treatment. J. Food Drug Anal. 2014, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Soenen, S.; Braeckmans, K.; Skirtach, A. Towards Theranostic Multicompartment Microcapsules: In-Situ Diagnostics and Laser-induced Treatment. Theranostics 2013, 3, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Kwon, Y.J. Cancer nanotechnology: Current status and perspectives. Nano Converg. 2021, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Lee, H.-L.; Chiou, J.-F.; Lo, L.-W. Recent Advances in Gold Nanomaterials for Photothermal Therapy. J. Nanotheranostics 2022, 3, 8. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Wang, Y.X.; Lin, Y.; Zhang, J.S.; Yang, F.; Zhou, Q.L.; Liao, Y.Y. Advance of molecular imaging technology and targeted imaging agent in imaging and therapy. Biomed. Res. Int. 2014, 2014, 819324. [Google Scholar] [CrossRef]
- Pizzuti, V.J.; Viswanath, D.; Torregrosa-Allen, S.E.; Currie, M.P.; Elzey, B.D.; Won, Y.Y. Bilirubin-Coated Radioluminescent Particles for Radiation-Induced Photodynamic Therapy. ACS Appl. Bio. Mater. 2020, 3, 4858–4872. [Google Scholar] [CrossRef]
- Ciarrocchi, E.; Belcari, N. Cerenkov luminescence imaging: Physics principles and potential applications in biomedical sciences. EJNMMI Phys. 2017, 4, 14. [Google Scholar] [CrossRef]
- Spinelli, A.E.; Boschi, F. Photodynamic Therapy Using Cerenkov and Radioluminescence Light. Front. Phys. 2021, 9, 637120. [Google Scholar] [CrossRef]
- Jelley, J.V. SPECIAL ARTICLE: Cerenkov radiation and its applications. Br. J. Appl. Phys. 1955, 6, 227–232. [Google Scholar] [CrossRef]
- Fazio, G.G.; Jelley, J.V.; Charman, W.N. Generation of Cherenkov Light Flashes by Cosmic Radiation within the Eyes of the Apollo Astronauts. Nature 1970, 228, 260–264. [Google Scholar] [CrossRef]
- Tendler, I.I.; Hartford, A.; Jermyn, M.; LaRochelle, E.; Cao, X.; Borza, V.; Alexander, D.; Bruza, P.; Hoopes, J.; Moodie, K.; et al. Experimentally Observed Cherenkov Light Generation in the Eye During Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, G.; Roberts, S.; Kossatz, S.; Reiner, T. Optical Imaging Modalities: Principles and Applications in Preclinical Research and Clinical Settings. J. Nucl. Med. 2020, 61, 1419–1427. [Google Scholar] [CrossRef]
- Jarvis, L.A.; Hachadorian, R.L.; Jermyn, M.; Bruza, P.; Alexander, D.A.; Tendler, I.I.; Williams, B.B.; Gladstone, D.J.; Schaner, P.E.; Zaki, B.I.; et al. Initial Clinical Experience of Cherenkov Imaging in External Beam Radiation Therapy Identifies Opportunities to Improve Treatment Delivery. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.R.; Rahman, M.; Zhang, R.; Williams, B.B.; Gladstone, D.J.; Pogue, B.W.; Bruza, P. Dosimetry for FLASH Radiotherapy: A Review of Tools and the Role of Radioluminescence and Cherenkov Emission. Front. Phys. 2020, 8, 328. [Google Scholar] [CrossRef]
- Holt, R.W.; Zhang, R.; Esipova, T.V.; Vinogradov, S.A.; Glaser, A.K.; Gladstone, D.J.; Pogue, B.W. Cherenkov excited phosphorescence-based pO2 estimation during multi-beam radiation therapy: Phantom and simulation studies. Phys. Med. Biol. 2014, 59, 5317–5328. [Google Scholar] [CrossRef]
- Cao, X.; Rao Allu, S.; Jiang, S.; Jia, M.; Gunn, J.R.; Yao, C.; LaRochelle, E.P.; Shell, J.R.; Bruza, P.; Gladstone, D.J.; et al. Tissue pO2 distributions in xenograft tumors dynamically imaged by Cherenkov-excited phosphorescence during fractionated radiation therapy. Nat. Commun. 2020, 11, 573. [Google Scholar] [CrossRef]
- Pogue, B.W.; Feng, J.; LaRochelle, E.P.; Bruža, P.; Lin, H.; Zhang, R.; Shell, J.R.; Dehghani, H.; Davis, S.C.; Vinogradov, S.A.; et al. Maps of in vivo oxygen pressure with submillimetre resolution and nanomolar sensitivity enabled by Cherenkov-excited luminescence scanned imaging. Nat. Biomed. Eng. 2018, 2, 254–264. [Google Scholar] [CrossRef]
- Zhang, R.; Glaser, A.; Esipova, T.V.; Kanick, S.C.; Davis, S.C.; Vinogradov, S.; Gladstone, D.; Pogue, B.W. Čerenkov radiation emission and excited luminescence (CREL) sensitivity during external beam radiation therapy: Monte Carlo and tissue oxygenation phantom studies. Biomed. Opt. Express 2012, 3, 2381–2394. [Google Scholar] [CrossRef]
- Cao, X.; Gunn, J.R.; Allu, S.R.; Bruza, P.; Jiang, S.; Vinogradov, S.A.; Pogue, B.W. Implantable sensor for local Cherenkov-excited luminescence imaging of tumor pO2 during radiotherapy. J. Biomed. Opt. 2020, 25, 112704. [Google Scholar] [CrossRef]
- Keen, C.E. Visualizing the Treatment Beam Improves Radiation Therapy Delivery; Physics World: Bristol, UK, 2021. [Google Scholar]
- Cherenkov Emission Imaging and Spectroscopy Using a Pulsed Linear Accelerator and the Subsequent Deep-Tissue Imaging of Molecular Oxygen Sensors in a Human Body Phantom; © 2018 Princeton Instruments, Inc. Available online: https://www.princetoninstruments.com/wp-content/uploads/2020/04/AppNote_CherenkovDeepTissueMolecularOxygenHumanPhantom.pdf (accessed on 1 December 2022).
- Glaser, A.K.; Zhang, R.; Davis, S.C.; Gladstone, D.J.; Pogue, B.W. Time-gated Cherenkov emission spectroscopy from linear accelerator irradiation of tissue phantoms. Opt. Lett. 2012, 37, 1193–1195. [Google Scholar] [CrossRef]
- Pratt, E.C. Cerenkov Imaging. Available online: https://www.mskcc.org/research/ski/labs/jan-grimm/cerenkov-imaging (accessed on 11 November 2022).
- Robertson, R.; Germanos, M.S.; Li, C.; Mitchell, G.S.; Cherry, S.R.; Silva, M.D. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys. Med. Biol. 2009, 54, N355–N365. [Google Scholar] [CrossRef] [PubMed]
- Boschi, F.; Spinelli, A. Cerenkov Luminescence Imaging at a Glance. Curr. Mol. Imaging 2014, 3, 106–117. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Xing, B.; Han, P.; Gambhir, S.S.; Cheng, Z. Radiation-Luminescence-Excited Quantum Dots for in vivo Multiplexed Optical Imaging. Small 2010, 6, 1087–1091. [Google Scholar] [CrossRef]
- Zhang, A.-W.; Guo, W.-H.; Qi, Y.-F.; Wang, J.-Z.; Ma, X.-X.; Yu, D.-X. Synergistic Effects of Gold Nanocages in Hyperthermia and Radiotherapy Treatment. Nanoscale Res. Lett. 2016, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chatterjee, D.K.; Lee, M.H.; Krishnan, S. Gold nanoparticles in breast cancer treatment: Promise and potential pitfalls. Cancer Lett. 2014, 347, 46–53. [Google Scholar] [CrossRef]
- Atkinson, R.L.; Zhang, M.; Diagaradjane, P.; Peddibhotla, S.; Contreras, A.; Hilsenbeck, S.G.; Woodward, W.A.; Krishnan, S.; Chang, J.C.; Rosen, J.M. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci. Transl. Med. 2010, 2, 55ra79. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Veroneau, S.S.; Luo, T.; You, E.; Lin, W. Nanoscale Metal-Organic Framework Hierarchically Combines High-Z Components for Multifarious Radio-Enhancement. J. Am. Chem. Soc. 2019, 141, 6859–6863. [Google Scholar] [CrossRef]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef]
- van der Zee, J. Heating the patient: A promising approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef]
- Griffin, R.J.; Dings, R.P.; Jamshidi-Parsian, A.; Song, C.W. Mild temperature hyperthermia and radiation therapy: Role of tumour vascular thermotolerance and relevant physiological factors. Int. J. Hyperth. 2010, 26, 256–263. [Google Scholar] [CrossRef]
- Ran, C.; Zhang, Z.; Hooker, J.; Moore, A. In Vivo Photoactivation Without “Light”: Use of Cherenkov Radiation to Overcome the Penetration Limit of Light. Mol. Imaging Biol. 2012, 14, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Pratx, G.; Kapp, D.S. Is Cherenkov luminescence bright enough for photodynamic therapy? Nat. Nanotechnol. 2018, 13, 354. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Yang, W.; He, Z.; Jia, H.; Wang, H.; Zhao, W.; Gao, L.; Zhang, Z.; Gao, F.; Gao, X. A chlorin-lipid nanovesicle nucleus drug for amplified therapeutic effects of lung cancer by internal radiotherapy combined with the Cerenkov radiation-induced photodynamic therapy. Biomater. Sci. 2020, 8, 4841–4851. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ni, D.; Rosenkrans, Z.T.; Barnhart, T.E.; Wei, H.; Ferreira, C.A.; Lan, X.; Engle, J.W.; He, Q.; Yu, F.; et al. A “Missile-Detonation” Strategy to Precisely Supply and Efficiently Amplify Cerenkov Radiation Energy for Cancer Theranostics. Adv. Mater. 2019, 31, e1904894. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Li, J.; Wang, P.; Lang, J.; Yang, Y. Cherenkov Luminescence in Tumor Diagnosis and Treatment: A Review. Photonics 2022, 9, 390. [Google Scholar] [CrossRef]
- Kotagiri, N.; Sudlow, G.P.; Akers, W.J.; Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015, 10, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Liu, H.; Xu, Y.; Han, Y.; Xu, M.; Zhang, Z.; Liu, Z. Activating TiO(2) Nanoparticles: Gallium-68 Serves as a High-Yield Photon Emitter for Cerenkov-Induced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 5278–5286. [Google Scholar] [CrossRef]
- Qian, R.; Wang, K.; Guo, Y.; Li, H.; Zhu, Z.; Huang, X.; Gong, C.; Gao, Y.; Guo, R.; Yang, B.; et al. Minimizing adverse effects of Cerenkov radiation induced photodynamic therapy with transformable photosensitizer-loaded nanovesicles. J. Nanobiotechnol. 2022, 20, 203. [Google Scholar] [CrossRef]
- Goel, S.; Ferreira, C.A.; Chen, F.; Ellison, P.A.; Siamof, C.M.; Barnhart, T.E.; Cai, W. Activatable Hybrid Nanotheranostics for Tetramodal Imaging and Synergistic Photothermal/Photodynamic Therapy. Adv. Mater. 2018, 30, 1704367. [Google Scholar] [CrossRef]
- Glaser, A.K.; Zhang, R.; Andreozzi, J.M.; Gladstone, D.J.; Pogue, B.W. Cherenkov radiation fluence estimates in tissue for molecular imaging and therapy applications. Phys. Med. Biol. 2015, 60, 6701–6718. [Google Scholar] [CrossRef]
- Kamkaew, A.; Cheng, L.; Goel, S.; Valdovinos, H.F.; Barnhart, T.E.; Liu, Z.; Cai, W. Cerenkov Radiation Induced Photodynamic Therapy Using Chlorin e6-Loaded Hollow Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 26630–26637. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Ferreira, C.A.; Barnhart, T.E.; Quach, V.; Yu, B.; Jiang, D.; Wei, W.; Liu, H.; Engle, J.W.; Hu, P.; et al. Magnetic Targeting of Nanotheranostics Enhances Cerenkov Radiation-Induced Photodynamic Therapy. J. Am. Chem. Soc. 2018, 140, 14971–14979. [Google Scholar] [CrossRef] [PubMed]
- Reed, N.A.; Raliya, R.; Tang, R.; Xu, B.; Mixdorf, M.; Achilefu, S.; Biswas, P. Electrospray Functionalization of Titanium Dioxide Nanoparticles with Transferrin for Cerenkov Radiation Induced Cancer Therapy. ACS Appl. Bio Mater. 2019, 2, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Zheleznyak, A.; Mixdorf, M.; Ghai, A.; Prior, J.; Black, K.C.L.; Shokeen, M.; Reed, N.; Biswas, P.; Achilefu, S. Osteotropic Radiolabeled Nanophotosensitizer for Imaging and Treating Multiple Myeloma. ACS Nano 2020, 14, 4255–4264. [Google Scholar] [CrossRef]
- Ouyang, Z.; Liu, B.; Yasmin-Karim, S.; Sajo, E.; Ngwa, W. Nanoparticle-aided external beam radiotherapy leveraging the Cerenkov effect. Phys. Med. 2016, 32, 944–947. [Google Scholar] [CrossRef]
- Gu, X.; Shen, C.; Li, H.; Goldys, E.M.; Deng, W. X-ray induced photodynamic therapy (PDT) with a mitochondria-targeted liposome delivery system. J. Nanobiotechnol. 2020, 18, 87. [Google Scholar] [CrossRef]
- Huang, H.; He, L.; Zhou, W.; Qu, G.; Wang, J.; Yang, N.; Gao, J.; Chen, T.; Chu, P.K.; Yu, X.F. Stable black phosphorus/Bi2O3 heterostructures for synergistic cancer radiotherapy. Biomaterials 2018, 171, 12–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).