Evaluating Sodium Hydroxide and Hydrogen Peroxide as Chemical Treatment for Cellulose Extraction from Clitoria fairchildiana Pruning Residues
Abstract
1. Introduction
2. Methodology
2.1. Materials
2.2. Chemical Treatment Steps
2.3. Biomass Yield
- m1: initial mass of the raw biomass in dry weight (g);
- m2: final mass of the treated and dried material (g).
2.4. Biomass Characterization After the Treatments
3. Results and Discussion
3.1. Biomass Characterization and Yield After the Treatments
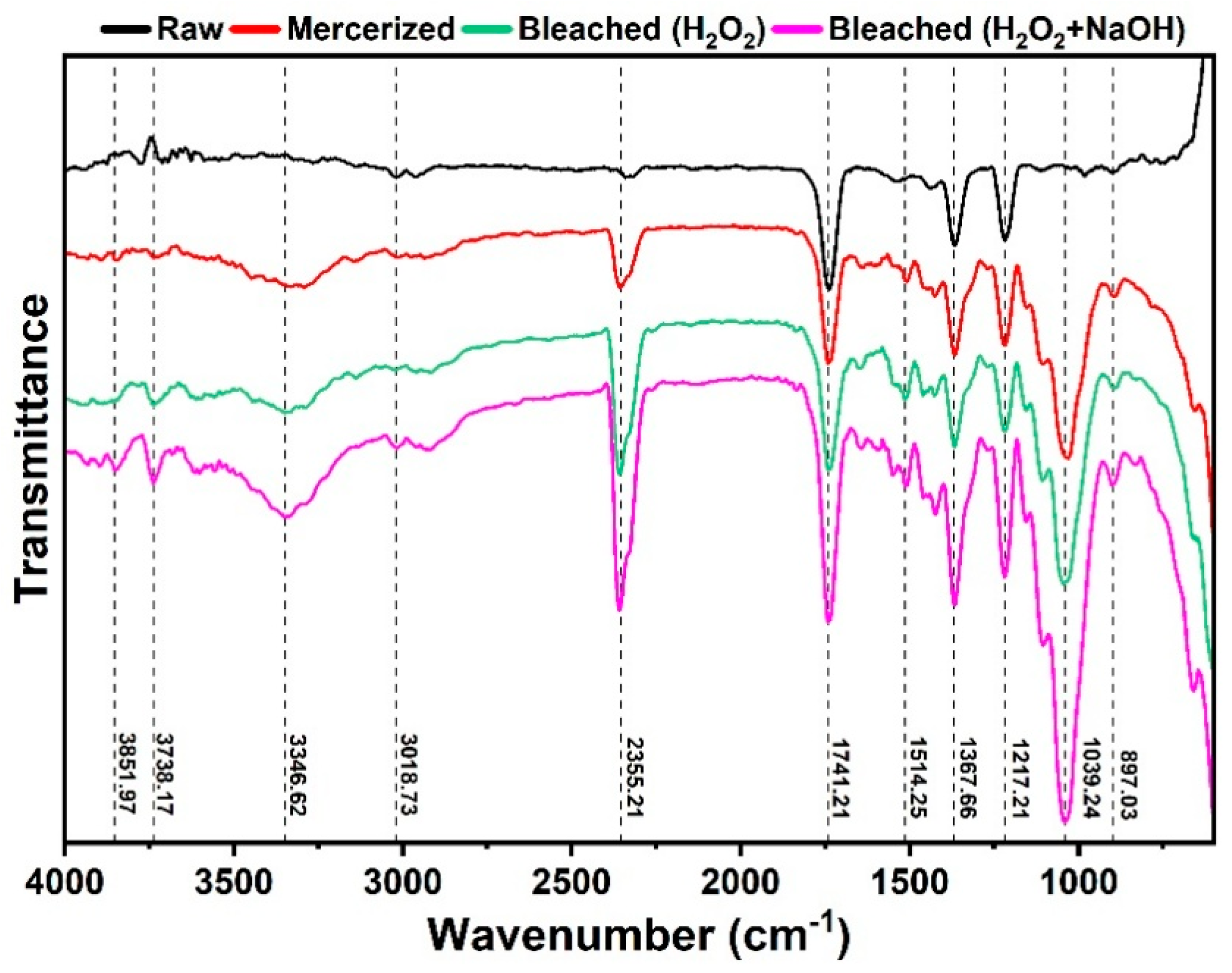
3.2. Fourier Transform Infrared (FTIR) Analysis
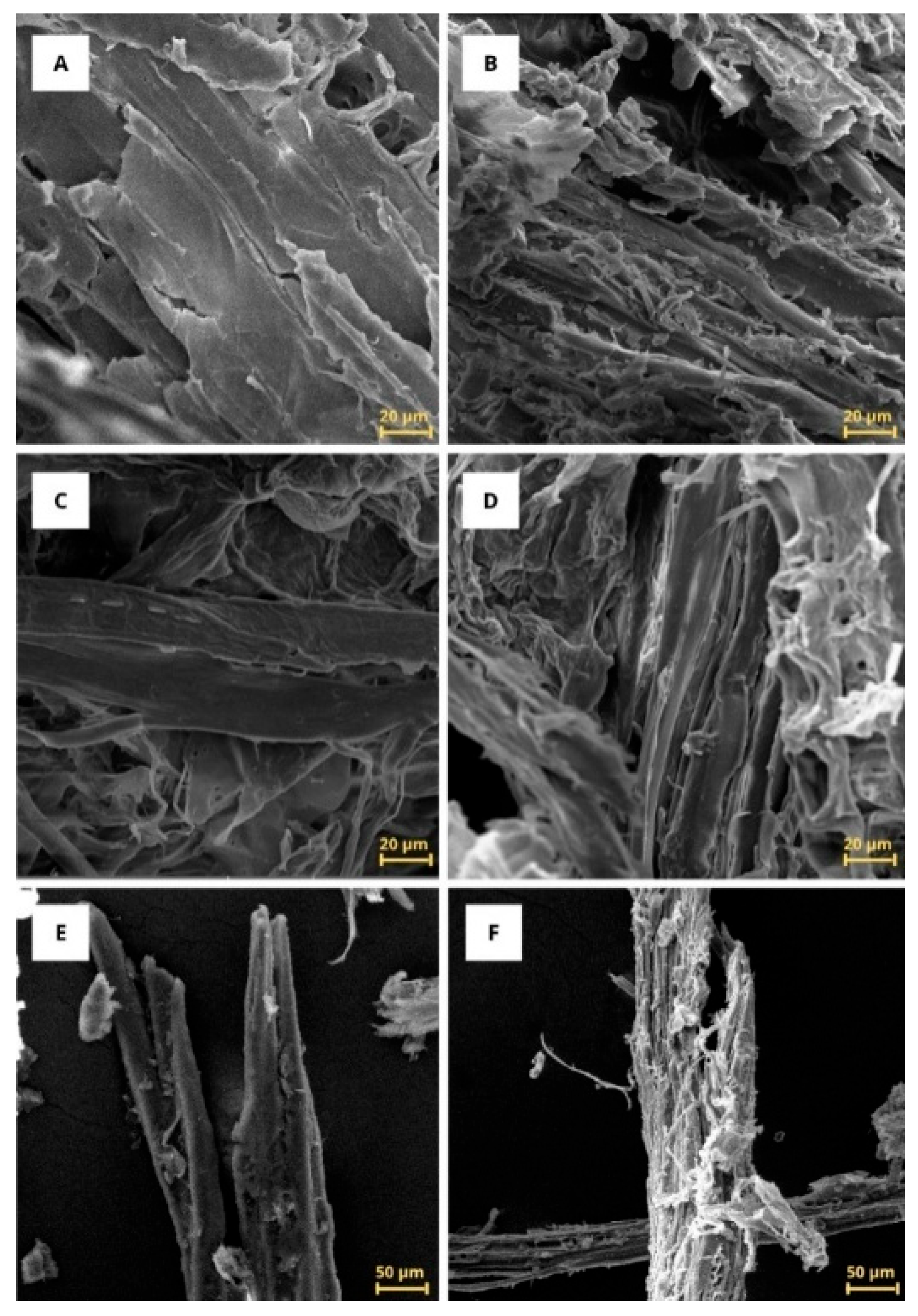
3.3. Scanning Electron Microscopy (SEM)
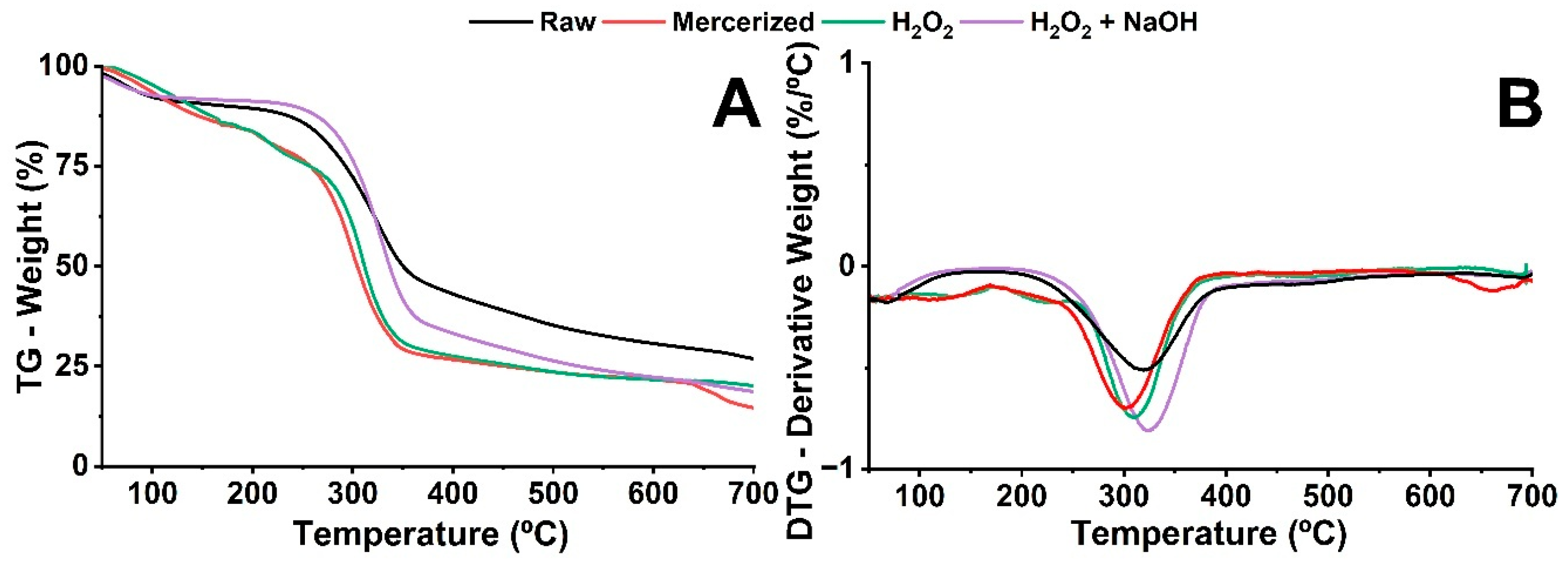
3.4. Thermogravimetric Analysis (TG/DTG)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artilha-Mesquita, C.A.F.; Stafussa, A.P.; Paraiso, C.M.; Rodrigues, L.M.; Silva, L.A.; Santos, S.S.; Marins, A.R.; Madrona, G.S. Evaluation of quality management and its tools: Applicability in the animal food industry. Res. Soc. Dev. 2021, 10, e20210111248. [Google Scholar]
- Zhu, S.; Sun, H.; Um, T.; Li, Q.; Richel, A. Preparation of cellulose nanocrystals from purple sweet potato peels by ultrasound-assisted maleic acid hydrolysis. Food Chem. 2022, 403, 134496. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, F.; Chen, J.; Wang, Y.; Zhou, Z.; Lian, R. Development of seaweed-derived polysaccharide/cellulose nanocrystal-based antiifogging labels loaded with alizarin for monitoring aquatic products freshness. Int. J. Biol. 2023, 235, 126640. [Google Scholar]
- Baraka, F.; Langari, M.M.; Beitia, I.; Dávila, I.; Labidi, J.; Morales, A.; Sillero, L. Enhancing lignocellulosic biomass pretreatment with choline chloride-based deep eutetic solvents. J. Environ. Chem. Eng. 2025, 13, 117087. [Google Scholar] [CrossRef]
- Tiwari, A.; Sanjog, J. Morphological, structural, and thermal properties of cellulose nanocrystals extracted from Indian water chestnut shells (agricultural waste). Next Mater. 2025, 8, 100653. [Google Scholar] [CrossRef]
- Mateo, S.; Peinado, S.; Morillas-Gutiérrez, F.; La Rubia, M.D.; Moya, A.J. Nanocellulose from agricultural wastes: Products and applications—A review. Processes 2021, 9, 1594. [Google Scholar] [CrossRef]
- Zeng, Q.; Ma, J.; Liu, T.; Shi, Y.; Hua, R.; Zhu, Y.; Xu, Y.; Zhu, J. Extraction of xylo-oligosaccharides and carboxylated cellulose nanocrystals from corncob using stepwise treatment with ammonium persulfate. Int. J. Biol. Macromol. 2025, 322, 147142. [Google Scholar] [CrossRef]
- Nyerere, G.; Kyokusiima, S.; Nabaterega, R.; Tumusiime, G.; Kavuma, C. The synergy of maize straw cellulose and sugarcane bagasse fibre on the characteristics of bioplastic packaging film. Bioresour. Technol. Rep. 2024, 28, 102007. [Google Scholar] [CrossRef]
- Techawinyutham, L.; Sundaram, R.S.; Suyambulingam, I.; Mo-on, S.; Srisuk, R.; Divakaran, D.; Rangappa, S.M.; Siengchin, S. Rice husk biowaste derived microcrystalline cellulose reinforced sustainable green composites: A comprehensive characterization for lightweight applications. Int. J. Biol. Macromol. 2025, 299, 140153. [Google Scholar] [CrossRef]
- Bortolatto, R.; Bittencourt, P.R.S.; Yamashita, F. Biodegradable starch/polyvinyl alcohol composites produced by thermoplastic injection containing cellulose extracted from soybean hulls (Glycine max L.). Ind. Crops Prod. 2022, 176, 114383. [Google Scholar] [CrossRef]
- Moriana, R.; Vilaplana, F.; Ek, M. Cellulose Nanocrystals from Forest Residues as Reinforcing Agents for Composites: A Study from Macro- to Nano-Dimensions. Carbohydr. Polym. 2016, 139, 139–149. [Google Scholar] [CrossRef]
- Jaffur, N.; Jeetah, P. Produção de papel de baixo custo a partir de fibras Pandanus utilis em substituição à madeira. Pesqui. Em Meio Ambiente Sustentável 2019, 29, 20. [Google Scholar]
- Alves, M.M.; Alves, E.U.; Bruno, R.L.A.; Silva, K.R.G.; Santos-Moura, S.S.; Barrozo, L.M.; Araújo, L.R. Potencial fisiológico de sementes de Clitoriafairchildiana R.A. Howard.-Fabaceae submetidas a diferentes regimes de luz e temperatura. Ciência Rural 2012, 42, 2199–2205. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Nelo, L.M.A.; Ribeiro, A.P. Arborização urbana: Uma perspectiva sobre o direcionamento dos resíduos em cidades brasileiras. Periódico Técnico e Científico Cidades Verdes 2023, 11, 80–93. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Abrema. Panorama dos Resíduos Sólidos no Brasil; ABREPEL: São Paulo, Brazil, 2023. [Google Scholar]
- Correia, G.R.; Machado, J.C.; Almeida, S.S.; Santos, L.A.C.; Costa, R.L.; Calil, F.N.; Silva-Neto, C.M. Scientific Production on Urban Pruning Waste: A Scientometric Analysis. Braz. J. Phys. Geogr. 2022, 15, 1701–1714. [Google Scholar]
- Li, Y.; Hua, D.; Xu, H.; Zhao, Y.; Jin, F.; Fang, X. Improving biodegradability of corn stover pretreated by different organic acids: Investigation on the hydrolysis/acidification and methanogenic performance. Ind. Crops Prod. 2022, 177, 114395. [Google Scholar] [CrossRef]
- Alizadeh, H.-R.; Kansedo, J.; Tan, I.S.; Tan, Y.H.; Suali, E.; Dini, A. Recent advances on two-step and combined multi-step pretreatment of lignocellulosic biomass for cellulose extraction. Bioresour. Technol. Rep. 2025, 31, 102243. [Google Scholar] [CrossRef]
- Gil-López, D.I.L.; Lois-Correa, J.A.; Sánchez-Pardo, M.E.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Rodríguez-Salazar, A.E.; Orta-Guzmán, V.N. Production of dietary fibers from sugarcane bagasse and sugarcane tops using microwave-assisted alkaline treatments. Ind. Crops Prod. 2019, 135, 159–169. [Google Scholar] [CrossRef]
- Meng, X.; Liu, F.; Xiao, Y.; Cao, J.; Wang, M.; Duan, X. Alterações nas propriedades físico-químicas e funcionais da fibra alimentar insolúvel da palha de trigo sarraceno por tratamento com peróxido de hidrogênio alcalino. Food Chem. X 2019, 3, 100029. [Google Scholar]
- Nair, L.G.; Agrawal, K.; Verma, P. Organosolv pretreatment: An in-depth purview of mechanics of the system. Bioresour. Bioprocess. 2023, 10, 50. [Google Scholar] [CrossRef]
- Jiang, Y.; Ni, P.; Chen, C.; Lu, Y.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Selective Electrochemical H2O2 Production through Two-Electron Oxygen Electrochemistry. Adv. Energy Mater. 2018, 8, 1801909. [Google Scholar] [CrossRef]
- Ranganathan, S.; Sieber, V. Recent Advances in the Direct Synthesis of Hydrogen Peroxide Using Chemical Catalysis—A Review. Catalysts 2018, 8, 379. [Google Scholar] [CrossRef]
- Araújo, A.A.D.S.; Mercuri, L.P.; Seixas, S.R.S.; Storpirtis, S.; Matos, J.D.R. Determinação dos teores de umidade e cinzas de amostras comerciais de guaraná utilizando métodos convencionais e análise térmica. Revista Brasileira de Ciências Farmacêuticas 2006, 42, 269–277. [Google Scholar] [CrossRef]
- Tappi. T 203 CM-99. In Alpha-, Beta- and Gamma-Cellulose in Pulp; TAPPI Press: Atlanta, GA, USA, 2009; 7p. [Google Scholar]
- Gouveia, E.R.; Nascimento, R.T.; Souto-Maior, A.M.; Rocha, G.J.D.M. Validação de metodologia para a caracterização química de bagaço de cana-de-açúcar. Química Nova 2009, 32, 1500–1503. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Juan, J.C.; Phang, S.M. Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans. Carbohydr. Polym. 2016, 151, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Subir, K.B.; Suteera Witayakran, E.H.Y. Water Hyacinth: A Sustainable Lignin-Poor Cellulose Source for the Production of Cellulose Nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 18884–18893. [Google Scholar] [CrossRef]
- Vu, A.N.; Nguyen, L.H.; Tran, H.-C.V.; Yoshimura, K.; Tran, T.D.; Van Le, H.; Nguyen, N.-U.T. Cellulose nanocrystals extracted from rice husk using the formic/peroxyformic acid process: Isolation and structural characterization. RSC Adv. 2024, 14, 2048–2060. [Google Scholar] [CrossRef]
- Tran, M.H.; Phan, D.-P.; Lee, E.Y. Revisão sobre modificações de lignina em relação a ingredientes naturais de proteção UV para protetores solares à base de lignina. Green Chem. 2021, 23, 4633–4646. [Google Scholar] [CrossRef]
- Ji, C.; Wang, Y. Lignin-containing cellulose nanocrystals from maple leaves: A natural Pickering emulsion stabilizer for food preservation. Food Chem. 2025, 463, 141407. [Google Scholar] [CrossRef] [PubMed]
- Tofani, G.; Cornet, I.; Tavernier, S. Multiple Linear Regression to Predict the Brightness of Waste Fibres Mixtures before Bleaching. Chem. Pap. 2022, 76, 4351–4365. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of lignin color via one-step UV irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Zhou, Z.; Li, A.; Zhu, S.; Li, J.; Zhang, W.; Zhang, F.; Chen, K. A feasible approach to chromophores removal and color reduction in industrial lignin via deep eutectic solvent/isopropanol treatment. Int. J. Biol. Macromol. 2025, 290, 138857. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Kantola, A.M.; Ämmälä, A. Nanofibras de celulose de serragem tratada com tioureia ácida, não branqueada e branqueada com peróxido de hidrogênio. J. Clean. Prod. 2023, 423, 138824. [Google Scholar]
- Biswas, S.; Rahaman, T.; Gupta, P.; Mitra, R.; Dutta, S.; Kharlyngdoh, E.; Guha, S.; Ganguly, J.; Pal, A.; Das, M. Cellulose and lignin profiling in seven, economically important bamboo species of India by anatomical, biochemical, FTIR spectroscopy and thermogravimetric analysis. Biomass Bioenergy 2022, 158, 106362. [Google Scholar] [CrossRef]
- Ciftci, D.; Flores, R.A.; Saldaña, M.D. Cellulose Fiber Isolation and Characterization from Sweet Blue Lupin Hull and Canola Straw. J. Polym. Environ. 2018, 26, 2773–2781. [Google Scholar] [CrossRef]
- Sobri, N.S.A.; Harun, S.; Abdul, P.M.; Feng, A.W.; Salleh, M.Z.M. Xylan Solubilisation from Oil Palm Frond and Sago Palm Bark via In-Situ Reductant-Aided Alkaline Peroxide Pretreatment. Ind. Crops Prod. 2025, 232, 121309. [Google Scholar] [CrossRef]
- Díaz, A.B.; Blandino, A.; Belleli, C.; Caro, I. An Effective Process for Pretreating Rice Husk to Enhance Enzyme Hydrolysis. Ind. Eng. Chem. Res. 2014, 53, 10870–10875. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Ornaghi, H.L.; Arantes, V.; Cioffi, M.O.H. Effect of chemical treatment of pineapple crown fiber in the production, chemical composition, crystalline structure, thermal stability and thermal degradation kinetic properties of cellulosic materials. Carbohydr. Res. 2021, 499, 108227. [Google Scholar] [CrossRef]
- Song, Z.; Yang, G.; Liu, X.; Yan, Z.; Yuan, Y.; Liao, Y. Comparison of Seven Chemical Pretreatments of Corn Straw for Improving Methane Yield by Anaerobic Digestion. PLoS ONE 2014, 9, e93801. [Google Scholar] [CrossRef]
- He, Z.-J.; Chen, K.; Liu, Z.-H.; Li, B.-Z.; Yuan, Y.-J. Valorizing renewable cellulose from lignocellulosic biomass toward functional products. J. Clean. Prod. 2023, 414, 137708. [Google Scholar] [CrossRef]
- Jantarat, C.; Kaewpradit, S.; Chingunpitak, J.; Srivaro, S. Characteristics of microcrystalline cellulose derived from oil palm trunk slabs and its potential for use as tablet diluent. Heliyon 2025, 11, e42902. [Google Scholar] [CrossRef]
- Othman, J.A.S.; Ilyas, R.A.; Nordin, A.H.; Ngadi, N.; Alkbir, M.F.M.; Knight, V.F.; Norrrahim, M.N.F. Optimization of delignification and mercerization processes for high-purity cellulose extraction from Semantan bamboo (Gigantochloa scortechinii) using Response Surface Modelling. Carbohydr. Polym. Technol. Appl. 2025, 10, 100784. [Google Scholar] [CrossRef]
- Alam, M.M.; Greco, A.; Rajabimashhadi, Z.; Esposito Corcione, C. Efficient and environmentally friendly techniques for extracting lignin from lignocellulose biomass and subsequent uses: A review. Clean. Mater. 2024, 13, 100253. [Google Scholar] [CrossRef]
- Moura, H.O.M.A.; Campos, L.M.A.; da Silva, V.L.; de Andrade, J.C.F.; de Assumpção, S.M.N.; Pontes, L.A.M.; de Carvalho, L.S. Investigating Acid/Peroxide-Alkali Pretreatment of Sugarcane Bagasse to Isolate High Accessibility Cellulose Applied in Acetylation Reactions. Cellulose 2018, 25, 5669–5685. [Google Scholar] [CrossRef]
- Abderrahim, B.; Abderrahman, E.; Mohamed, A.; Fatima, T.; Abdesselam, T.; Krim, O. Kinetic Thermal Degradation of Cellulose, Polybutylene Succinate and a Green Composite: Comparative Study. World J. Environ. Eng. 2015, 3, 95–110. [Google Scholar]
- Oliveira, L.M.T.M.; Oliveira, L.F.A.M.; Sonsin, A.F.; Duarte, J.L.S.; Soletti, J.I.; Fonseca, E.J.S.; Ribeiro, L.M.O.; Meili, L. Ultrafast diesel oil spill removal by fibers from silk-cotton tree: Characterization and sorption potential evaluation. J. Clean. Prod. 2020, 263, 121448. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, K.; Qian, H.; Ramachandran, B.; Jiang, F. The advances of characterization and evaluation methods for the compatibility and assembly structure stability of food soft matter. Trends Food Sci. Technol. 2021, 112, 753–763. [Google Scholar] [CrossRef]
- Oliveira, L.M.T.M.; Fonseca, E.J.S.; Bernardo, V.B.; Zanta, C.L.P.S.; Oliveira, L.F.A.M.; Oliveira, J.N.S.R.D.; Souza, S.T.D.; Duarte, J.L.D.S. Modified kapok fibers (Ceiba pentandra (L.) Gaerth) for oil spill remediation. Appl. Sci. 2024, 14, 11995. [Google Scholar] [CrossRef]
- Norfarhana, A.S.; Ilyas, R.A.; Ngadi, N.; Othman, M.H.D.; Misenan, M.S.M.; Norrrahim, M.N.F. Revolutionizing lignocellulosic biomass: A review of harnessing the power of ionic liquids for sustainable utilization and extraction. Int. J. Biol. Macromol. 2023, 256, 128256. [Google Scholar] [CrossRef] [PubMed]
- Celina, M.C.; Linde, E.; Martinez, E. Carbonyl Identification and Quantification Uncertainties for Oxidative Polymer Degradation. Polym. Degrad. Stab. 2021, 188, 109550. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef]
- Thi Thuy Van, N.; Gaspillo, P.; Thanh, H.G.T.; Nhi, N.H.T.; Long, H.N.; Tri, N.; Thi Truc Van, N.; Nguyen, T.-T.; Ky Phuong Ha, H. Cellulose from the banana stem: Optimization of extraction by response surface methodology (RSM) and charaterization. Heliyon 2022, 8, e11845. [Google Scholar] [CrossRef]
- Shinde, S.D.; Meng, X.; Kumar, R.; Ragauskas, A.J. Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem. 2018, 20, 2192–2205. [Google Scholar] [CrossRef]
- Ren, M.; Fakayode, O.A.; Kong, F.; Zhou, C.; Chen, L.; Fan, X.; Liang, J.; Li, H. Characterization of cellulose nanocrystals prepared by different delignification methods and application of ultra-light, hydrophobic aerogels as oil absorbent in food systems. Ind. Crops Prod. 2023, 197, 116653. [Google Scholar] [CrossRef]
- Luchese, C.L.; Engel, J.B.; Tessaro, I.C. A Review on the Mercerization of Natural Fibers: Parameters and Effects. Korean J. Chem. Eng. 2024, 41, 571–587. [Google Scholar] [CrossRef]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 2018, 6, 141. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Fathi, M.; Ghoddusi, H.B. Nanoencapsulation of oregano essential oil using cellulose nanocrystals extracted from hazelnut shell to enhance shelf life of fruits: Case study: Pears. Int. J. Biol. Macromol. 2023, 242, 124704. [Google Scholar] [CrossRef]
- Lamaming, J.; Hashim, R.; Sulaiman, O.; Leh, C.P.; Sugimoto, T.; Nordin, N.A. Cellulose nanocrystals isolated from oil palm trunk. Carbohydr. Polym. 2015, 127, 202–208. [Google Scholar] [CrossRef]
- Brindha, R.; Narayana, C.K.; Vijayalakshmi, V.; Nachane, R.P. Effect of different retting processes on yield and quality of banana pseudostem fiber. J. Nat. Fibers 2019, 16, 58–67. [Google Scholar] [CrossRef]
- Pinheiro, M.A.; Ribeiro, M.M.; Rosa, D.L.S.; Nascimento, D.D.C.B.; Da Silva, A.C.R.; Dos Reis, M.A.L.; Monteiro, S.N.; Candido, V.S. Periquiteira (Cochlospermum orinocense): A Promising Amazon Fiber for Application in Composite Materials. Polymers 2023, 15, 2120. [Google Scholar] [CrossRef] [PubMed]
- Apaydın Varol, E.; Mutlu, Ü. TGA-FTIR Analysis of Biomass Samples Based on the Thermal Decomposition Behavior of Hemicellulose, Cellulose, and Lignin. Energies 2023, 16, 3674. [Google Scholar] [CrossRef]
- Raza, M.; Abu-Jdayil, B. Extraction of cellulose nanocrystals from date seeds using transition metal complex-assisted hydrochloric acid hydrolysis. Int. J. Biol. Macromol. 2025, 294, 139477. [Google Scholar] [CrossRef] [PubMed]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of Cellulose and Preparation of Nanocellulose from Sisal Fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Wang, S.; Luo, Z. Interactions of biomass components during pyrolysis: A TG-FTIR study. J. Anal. Appl. Pyrolysis 2011, 90, 213–218. [Google Scholar] [CrossRef]
- Zortea, L.F.; Pinheiro, I.R.; Mulin, L.B.; Mascarenhas, A.R.P.; Nascimento, J.N.; Tonoli, G.H.D.; Moulin, J.C.; Monteiro, S.N.; Oliveira, M.P. Improved non-woven surgical masks with nanostructured cellulosic reinforcement from sugarcane bagasse waste. J. Mater. Res. Technol. 2024, 30, 580–588. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zattera, A.J. Structural Characteristics and thermal Properties of Native Cellulose. In Em cellulose-Fundamental Aspects; van de Ven, T., Godbout, L., Eds.; Intech: London, UK, 2013. [Google Scholar]
- Huamani-Palomino, R.G.; Mayta, S.; Córdova, B.M.; Yáñez-S, M.; Venâncio, T.; Rivera, E.; Quintana, M. Estudo do efeito de agentes branqueadores no índice cristalino de materiais à base de celulose derivados da casca de milho por espectroscopias de RMN de 13C CP/MAS e FT-IR. Carbohydr. Polym. 2024, 346, 122593. [Google Scholar]
- Norfarhana, A.S.; Ilyas, R.A.; Ngadi, N.; Othman, M.H.D.O. Optimization of Ionic Liquid Pretreatment of Sugar Palm Fiber for Cellulose Extraction. J. Mol. Liq. 2024, 398, 124256. [Google Scholar] [CrossRef]
- Burhani, D.; Septevani, A.A. Isolation of nanocellulose from oil palm empty fruit bunches using strong acid hydrolysis. AIP Conf. Proc. 2018, 2024, 020005. [Google Scholar]
- Antony Jose, S.; Cowan, N.; Davidson, M.; Godina, G.; Smith, I.; Xin, J.; Menezes, P.L. A Comprehensive Review on Cellulose Nanofibers, Nanomaterials, and Composites: Manufacturing, Properties, and Applications. Nanomaterials 2025, 15, 356. [Google Scholar] [CrossRef]

| Components (%) | Raw | Mercerized | Bleached (H2O2) | Bleached (H2O2 + NaOH) |
|---|---|---|---|---|
| Klason Lignin | 31.30 ± 0.30 | 19.34 ± 0.87 | 15.62 ± 0.32 | 11.80 ± 1.0 |
| Cellulose | 29.78 ± 3.19 | 33.33 ± 0.38 | 35.43 ± 1.12 | 37.16 ± 0.80 |
| Hemicellulose | 21.84 ± 2.01 | 7.87 ± 0.20 | 4.80 ± 1.18 | 2.96 ± 0.86 |
| Extractives | 4.79 ± 0.10 | - | - | - |
| Ash | 12.37 ± 0.84 | - | - | - |
| Moisture | 6.91 ± 0.11 | - | - | - |
| Biomass yield | 100 * | 61.80 ± 2.32 ** | 57.33 ± 4.40 ** | 55.60 ± 4.10 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.B.; de Lima Araújo, R.R.; Almeida, R.M.R.G.; de Farias Silva, C.E.; Brandão, M.R.P.; de Menezes Bernardino, T.; Lôbo, L.N.; Duarte de Freitas, J.M.; Duarte de Freitas, J. Evaluating Sodium Hydroxide and Hydrogen Peroxide as Chemical Treatment for Cellulose Extraction from Clitoria fairchildiana Pruning Residues. Reactions 2025, 6, 60. https://doi.org/10.3390/reactions6040060
da Silva MB, de Lima Araújo RR, Almeida RMRG, de Farias Silva CE, Brandão MRP, de Menezes Bernardino T, Lôbo LN, Duarte de Freitas JM, Duarte de Freitas J. Evaluating Sodium Hydroxide and Hydrogen Peroxide as Chemical Treatment for Cellulose Extraction from Clitoria fairchildiana Pruning Residues. Reactions. 2025; 6(4):60. https://doi.org/10.3390/reactions6040060
Chicago/Turabian Styleda Silva, Mariana Barboza, Rosana Reis de Lima Araújo, Renata Maria Rosas Garcia Almeida, Carlos Eduardo de Farias Silva, Maria Regina Pereira Brandão, Thiago de Menezes Bernardino, Larissa Nascimento Lôbo, Jeniffer Mclaine Duarte de Freitas, and Johnnatan Duarte de Freitas. 2025. "Evaluating Sodium Hydroxide and Hydrogen Peroxide as Chemical Treatment for Cellulose Extraction from Clitoria fairchildiana Pruning Residues" Reactions 6, no. 4: 60. https://doi.org/10.3390/reactions6040060
APA Styleda Silva, M. B., de Lima Araújo, R. R., Almeida, R. M. R. G., de Farias Silva, C. E., Brandão, M. R. P., de Menezes Bernardino, T., Lôbo, L. N., Duarte de Freitas, J. M., & Duarte de Freitas, J. (2025). Evaluating Sodium Hydroxide and Hydrogen Peroxide as Chemical Treatment for Cellulose Extraction from Clitoria fairchildiana Pruning Residues. Reactions, 6(4), 60. https://doi.org/10.3390/reactions6040060








