1. Introduction
In recent years, post-lithium-ion technologies, such as sodium-ion batteries (SIBs), have been gaining popularity. Sodium-containing raw materials are significantly cheaper than lithium-containing ones and are widely distributed [
1,
2,
3]. In addition, in SIBs, aluminum can be applied as an anode current collector instead of copper in lithium-ion batteries, making the battery cheaper. Since SIBs operate on the same concept as lithium-ion batteries, already existing facilities can be used for their production; that is important in commercialization. It is anticipated that these batteries would eventually take the place of lithium ones, at least in a number of applications, including stationary energy storage, urban public transport, and industrial machinery [
4,
5].
Currently, SIB technology is in its emergence and many aspects require further optimization to make the technology truly widespread in practice. Selection of anode-active material for SIBs is one of the key problems. At present, carbon materials (especially hard carbon) are considered the most promising SIB anode. However, the sodiation potential for these materials is only slightly higher than 0 V vs. Na/Na
+, which can lead to the formation of dendrites and cause safety issues (especially at high current densities) [
6,
7].
Titanium dioxide, TiO
2, is regarded as an alternative anode material for alkali metal-ion batteries, including SIBs, owing to its high theoretical capacity of 335 mAh g
–1, appropriate operating voltage, non-toxicity, natural abundance, and low cost [
6,
8]. TiO
2 is a polymorphic substance. In nature, it exists in four crystalline forms: brookite (orthorhombic, space group:
Pbca), rutile (tetragonal,
P4
2/
mnm), anatase (tetragonal,
I4
1/
amd), and bronze TiO
2(B) (monoclinic,
C2/
m). Although all of them are being studied to use as an SIB anode material, TiO
2(B) has attracted the greatest interest due to open channel crystal structure providing faster ion diffusion and better cycling stability [
9]. Even though TiO
2(B) exists in nature, it is suggested to synthesize it for battery application. Since Marchand et al. proposed it in 1980 [
10], hydrothermal synthesis remains one of the most convenient and widespread methods for preparing bronze-phase TiO
2. Adjusting the conditions of this method allows it to synthesize nanocrystalline TiO
2(B) of various forms such as nanospheres, nanotubes, nanowires, nanoribbons, nanofibers, etc. [
11]. After 2013, when the use of bronze-phase TiO
2 as an anode for SIBs has been for the first time suggested [
12], promising performance characteristics have been evidenced [
13,
14,
15]. Nevertheless, due to a lack of thorough research, the mechanism underlying the electrochemical de-/sodiation of TiO
2(B) remains unclear. Huang et al. [
12], who were the first to explore TiO
2(B) nanotubes as a SIB anode within the potential range of 1.0–3.0 V, proposed that the solid solution mechanism is dominant in the electrochemical Na
+ insertion and extraction processes. Next, Dawson and Robertson [
16], applying DFT calculations, proved that sodium intercalation is thermodynamically favorable for TiO
2(B) and takes place at an estimated potential of 1.39 V. But what happens during TiO
2(B) de-/sodiation at lower potentials? Using
in situ X-ray diffraction, Wu et al. [
17] found that the initial sodiation of nanospherical TiO
2(B) is accompanied by a decrease in the intensity of (110) reflection; at low potentials, no reflection can be detected anymore. Upon the subsequent desodiation process, this peak is not restored. The authors suggest that, like anatase [
18], TiO
2(B) becomes fully amorphous during the initial sodiation caused by the reorganization of its crystal structure due to the uptake of such a large ion as Na
+. Lin and colleagues [
19], utilizing
operando Raman spectroscopy, showed that the first sodiation of TiO
2(B)/anatase dual-phase nanowires at temperatures near 0 °C occurs with partial retention of TiO
2(B) crystallinity and amorphization of the anatase up to a potential of 0.01 V. Applying
in situ X-ray diffraction, Lee et al. [
20] demonstrated that as sodium is stored into TiO
2(B) nanowires during the initial cycle, a decrease in the intensity of (110) and (020) reflections occurs and a new peak at 31.5° from an unknown phase appears. Upon the Na
+ release process, the intensity of (110) and (020) reflections increase, but the peak from the unidentified phase remains. The authors presume that during deep sodiation (at potentials of around 0.01 V) of TiO
2(B), a local phase transition with a formation of electrochemically inactive sodium titanate of unknown composition occurs. Similarly, Zhang et al. [
21], using
in situ X-ray diffraction, observed that (110) reflection recovers during the first desodiation of TiO
2(B) nanobelts. In this regard, it is also interesting to mention a recent work in which the DFT method was applied by Zou et al. to determine the storage sites for Na
+ ions within the TiO
2(B) lattice [
22].
The goal of this research is to unravel the reaction mechanism driving the electrochemical de-/sodiation process of bronze-phase TiO2. For this purpose, the systematic studies of electrodes based on TiO2(B) nanobelts cycled in sodium half-cells by using a number of in situ and ex situ methods (X-ray photoelectron spectroscopy, X-ray diffraction, Raman spectroscopy, and gas chromatography–mass spectrometry) were performed.
2. Experiment Materials and Methods
Bronze-phase titanium dioxide nanobelts were synthesized by a hydrothermal method described in [
23] and illustrated in
Figure S1. In a typical procedure, 0.1 g commercial nanoparticulate anatase TiO
2 was dispersed in 15 mL NaOH aqueous solution (14 M) under vigorous stirring. Then, the dispersion was transferred to a 20 mL autoclave (stainless-steel reactor with a Teflon liner) and heated at 170 °C for 72 h. Such harsh synthesis conditions were chosen in order to obtain a single-phase product. After cooling the autoclave naturally to room temperature, the reaction product was separated by centrifugation. By rinsing the centrifuged material in an aqueous solution of HCl (0.05 M), Na
+ was exchanged by H
+. The resulting protonated form was washed with deionized water to a neutral pH, dried at 80 °C, and vacuum annealed at 450 °C for 3 h. The yield of the TiO
2 in the synthesis was about 90%.
The morphology and elemental composition of as-prepared TiO2(B) were monitored by scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) using a Hitachi S5500 microscope (Tokyo, Japan).
The working electrodes were prepared by a tape casting method (Doctor Blade technique) from a slurry prepared by mixing of TiO
2(B), Super P carbon black, and polyvinylidene fluoride with a weight ratio of 8:1:1 in 1-Methyl-2-pyrrolidone using a planetary ball mill at 100 rpm rotational speed. An aluminum and copper foil were used as a current collector. After coating the slurry onto the current collector with a film thickness of 60 μm, drying in air at 60 °C to a constant weight, pressing on a roller press, the cutting-out of the electrodes were performed. The vacuum thermal treatment of working electrodes was carried out at a temperature of 110 °C for 12 h. The active material loading for a typical electrode was 2–4 mg cm
–2. The half-cells were assembled in a VBOX PRO glovebox (Vilitek, Moscow, Russia) in an atmosphere of dried and purified argon (H
2O, O
2 < 1 ppm) using CR2025 cases or split-test electrochemical cell. Metallic Na was applied as a counter/reference electrode. The separator was a Whattman glass fiber membrane. The electrolyte was 1.0 M solution of NaClO
4 in propylene carbonate containing 2 wt.% fluoroethylene carbonate (FEC). It should be noted that the introduction of FEC additive into the SIB carbonate-based electrolyte helps to adjust the solid electrolyte interphase (SEI) layer, mitigating irreversible capacity losses in the first-cycle [
24]. There are other strategies for reducing initial capacity losses such as formation protocols [
25], pre-sodiation [
26], but their discussion and evaluation of their effectiveness are beyond the scope of this paper. Electrochemical studies were carried out at room temperature using Elins (Chernogolovka, Moscow Oblast, Russia) and Solartron (Leicester, UK) workstations. The half-cells were charged and discharged in galvanostatic mode at different current densities ranging from 10 to 650 mA g
–1. Hereinbelow, the terms “charge” and “discharge” correspond to TiO
2(B) sodiation and desodiation, respectively. The results presented in all respective graphs were obtained by averaging over three measurements. Cyclic voltammetry (CV) tests were performed at various scan rates of 0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 mV s
–1. Electrochemical impedance spectroscopy experiments were carried out in the frequency range from 1 MHz to 0.1 Hz with AC amplitude of 10 mV. The collected EIS data were fitted using Zview software from Scribner Associates (Southern Pines, NC, USA).
For
ex situ experiments, half-cells were transferred to the glovebox and disassembled carefully to extract the TiO
2(B) electrodes, which were non-sodiated, fully sodiated or fully desodiated. Then, the electrodes were washed by propylene carbonate solvent and dried. At least three half-cells were used for all experiments. X-ray diffraction (XRD) data were collected on a Rigaku SmartLab diffractometer (Tokyo, Japan) with a HyPix-3000 detector (1D measurement mode, Ni filter) in parafocusing Bragg–Brentano geometry over 2θ range of 5 to 90° with a step of 0.01° using Cu K
α-radiation [
27]. The ICDD’s PDF-2 (2015) database was used for phase identification. Raman spectra were recorded on an Alpha 500 spectrometer (WiTec, Germany): the excitation wavelength of the Nd:YAG laser was 532 nm; the accumulation time was 100 s; the spectral resolution was 4 cm
–1. The WITec Project software (version 1.6) was applied for Raman data processing. XPS spectra were acquired on a SPECS spectrometer (Hünstetten, Hessen, Germany) equipped with a hemispherical Phoibos-150 analyzer employing a non-monochromatized Al K
α X-ray source. The measurements were performed under a vacuum of 6·10
–8 Torr at an analyzer transmission energy of 15 eV. Calibration of binding energy scale was conducted using the peak of C 1s-electrons at 285.0 eV. The XPSPEAK program (version 4.1) was used to analyze the experimental XPS spectra. Argon ion sputtering treatment of the cycled electrode surface was applied prior to XPS studies in order to reduce the influence of the species of the passivating SEI layer. The binding energy and element concentration errors were within ± 0.1 eV and 10 rel%, respectively.
In situ gas chromatography–mass spectrometry (GC-MS) analysis of gas evolution during initial electrochemical sodiation of TiO2(B) was performed using a specially designed handmade split-test cell on a Shimadzu GCMS-QP2010 spectrometer (Kyoto, Japan) upgraded to determine oxygen and carbon dioxide. The experiments were conducted using a deactivated metal capillary of 15 m in length and 0.25 mm in inner diameter as a GC column. The cell was purged with helium (Grade A) at a rate of 1.32 mL s–1. The MS interface temperature was 200 °C; the ion source temperature was 200 °C; the detector voltage was 1.4 kV. The SIM parameters were the following: m/z 32 (O2); m/z 44 (CO2). The signal-to-noise ratio was 1:60, and stability (deviation) of the mass scale for the 4 h operation did not exceed 1 amu.
3. Results and Discussion
Figure 1a,b show SEM micrographs of as-synthesized TiO
2(B) sample at different magnification. The material consists of nanobelts, which are several nanometers in thickness, a few tens of nanometers in width, and up to several microns in length. EDX spectrum of the sample (
Figure 1c) demonstrates the presence of characteristic lines of O (72.7 at%) and Ti (27.3 at%). There is also a carbon peak in the spectrum, which is explained by using a carbon tape as a substrate as well as surface contamination of nanoparticles.
Figure S2 presents SEM micrographs of TiO
2(B)-based electrodes before and after initial de-/sodiation. As can be seen from these data, the electrode surface becomes smooth after the first sodiation and remains so during the subsequent desodiation process. This is caused by the formation of passivating SEI layer at the electrode. EDX analysis of the electrodes (
Figure S3 and
Table S1), besides O, Ti, C, and F elements, reveals the presence of Na at the surface. However, since sodium is also a component of the SEI, it is impossible to determine how much of it has entered the TiO
2(B) based on these data.
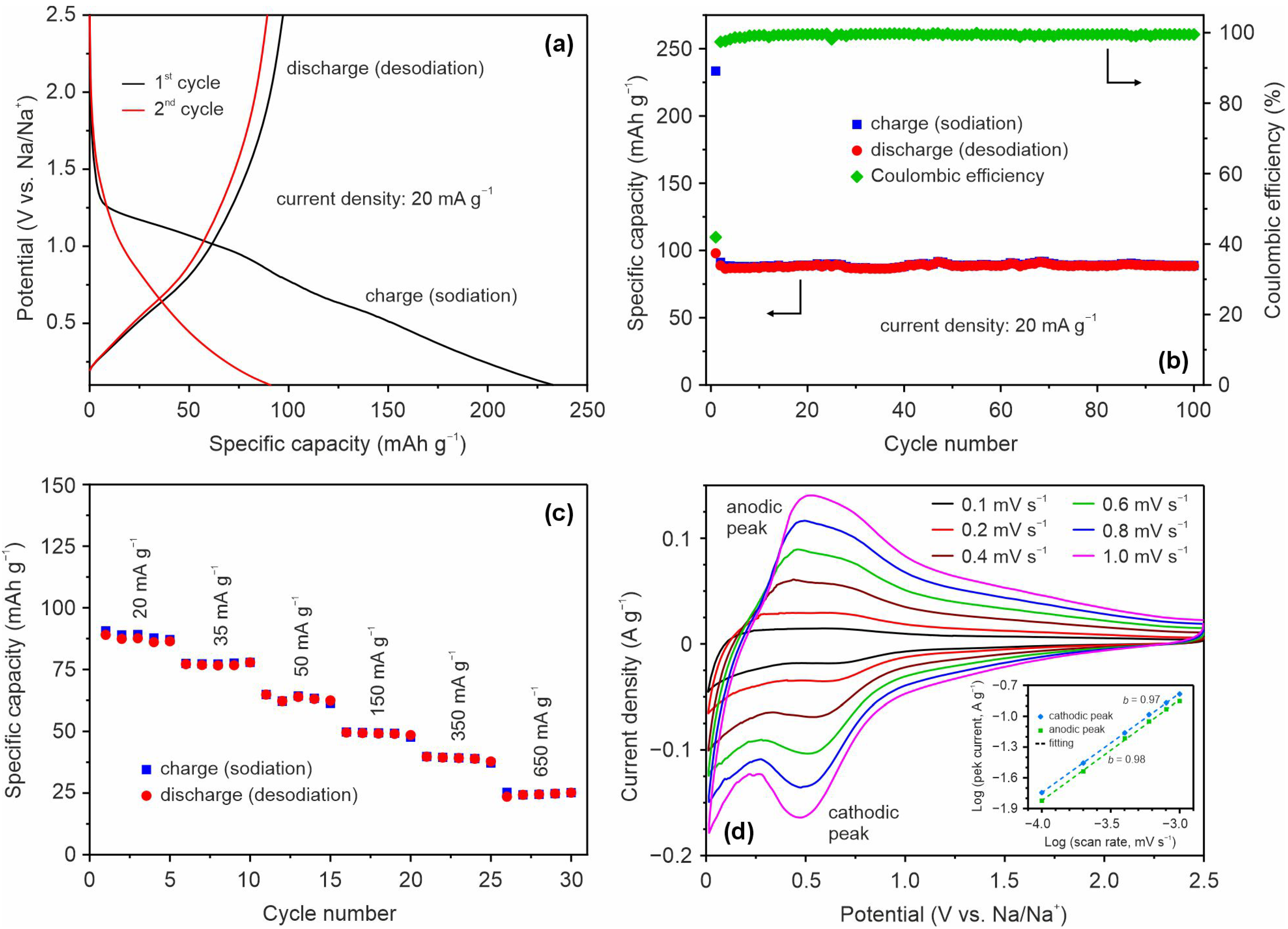
Figure 2a displays galvanostatic charge–discharge curves of the first and second cycles for an electrode based on TiO
2(B) nanobelts recorded in a sodium half-cell at a current density of 20 mA g
–1 in the potential range of 0.1–2.5 V. In general, the shape of these profiles corresponds to that of electrochemical sodiation and desodiation of TiO
2(B), as shown in the literature [
17]. As can be seen from the graph, there is a difference between the charge (sodiation process) curves of the first and second cycles. Unlike the initial, the subsequent profile is sloping. Such a discrepancy may indicate amorphization of TiO
2(B) or structural rearrangement (phase transition) during the first sodiation. This, along with the SEI formation through the electrolyte decomposition process occurring when a battery’s voltage exceeds the electrochemical window, can be responsible for the irreversible capacity loss in the first cycle. The initial charge (sodiation) capacity of TiO
2(B) nanobelts reaches about 232 mAh g
–1. The capacity corresponding to the reversible process in the first cycle was 97 mAh g
–1. The second cycle charge (sodiation) and discharge (desodiation) capacities were nearly 91 and 89 mAh g
–1, respectively.
The specific capacity in the further 100 charge/discharge cycles (
Figure 2b) remains almost unchanged, indicating stable cycling performance of TiO
2 (B) nanobelts.
Figure 2c shows the results of rate capability measurements for the TiO
2(B) electrode. As seen from this plot, the de-/sodiation capacity of 78, 62, 49, 38, and 28 mAh g
–1 was achieved at 35, 50, 150, 350, and 650 mA g
–1, respectively.
The sodium-ion storage of TiO
2(B) anodes was considered to have a pseudocapacitive type. To gain insight into the kinetic of Na
+ uptake and release, CV studies at different scan rates were performed, as shown in
Figure 2d. Although the registered CV curves obviously have similar features in shape, the intensities of the peaks and the difference between anodic and cathodic peak potentials become larger when the scan rate increases. In general, there are two mechanisms, diffusion- and/or surface-controlled processes, which provide the charge storage in battery materials. By plotting the logarithm of the current density versus the logarithm of the scan rate,
b-values determining the nature of the charge storage mechanism can be found from the slopes. The
b value of 0.5 indicates that the charge storage process exhibits diffusion-dominant behavior. The
b value of 1.0 means that fast surface capacitive reactions (either pseudocapacitive or EDLC) dominate. The inset in
Figure 2d shows log (
i)–log (
v) plots for the TiO
2 (B) electrode. The fitted
b-values based on cathodic and anodic peaks are found to be 0.97 and 0.98, respectively, indicating that de-/sodiation of TiO
2(B) is governed by a pseudocapacitive faradaic process. This means that it is possible to develop fast-charging SIB based on such anode material. The limitations of the capacity, which was observed in
Figure 2a–c, are obviously due to the poor particle morphology and insufficient electronic conductivity of the nanobelts hindering Na
+ ions from reaching and occupying all available sites within the TiO
2(B) structure. This is also evidenced by the relatively high series resistance (
Rs) of 16 Ω, determined using the EIS data (
Figure S4). Techniques like morphology engineering, conductive coating, and doping can be used to overcome this drawback [
28,
29]. However, this issue is not the subject of our study. Incidentally, the SEI layer resistance (
Rf) is estimated from the EIS data is 19.3 Ω (
Figure S4).
Table 1 shows the surface chemical composition, determined by XPS analysis, of the TiO
2(B)-based electrodes before (i.e., non-polarized electrode taken out of the non-cycled half-cell) and after the first sodiation. According to these data, the analyzed samples contain Na, F, O, Ti, C, and Al. Chlorine was not detected in the samples, which indicates that they were washed thoroughly in propylene carbonate. The electrode composition includes the polyvinylidene fluoride binder, while the electrolyte contains FEC, which is decomposed with a formation of NaF. This results in the presence of fluorine in the samples. Obviously, a current collector is responsible for the aluminum. A small amount of sodium found in the non-sodiated TiO
2(B) electrode is associated with SEI components derived from sodium perchlorate decomposition. The data clearly displays that the Na concentration in the electrode increases during sodiation. This is caused by both: (i) the electrochemical reaction between TiO
2(B) and sodium; (ii) the formation of a SEI layer (the rise in fluorine content confirms the SEI’s growing contribution to these data).
The oxygen detected by XPS analysis is attributed to the TiO2(B) and, possibly, Na2O (a decomposition product of NaClO4). According to the XPS data, carbon existed in several chemical states in the samples. Among them, the component with a binding energy of 285.0 eV, corresponding to the electroconductive additive in the electrode composition, predominates.
The XPS spectrum of Ti 2p
3/2 (
Figure 3a) for the non-sodiated electrode indicates that titanium is in the oxidation state of +4, i.e., ascribed to TiO
2(B). After sodiation, the Ti 2p
3/2 peak (
Figure 3b) shows two components. The first one, having a binding energy of 459.2 eV, corresponds to tetravalent titanium, and the second one, located at 456.5 eV, is attributed to titanium in the +3 oxidation state. According to the XPS data, metallic titanium was not detected in the electrode. This means that sodiation of TiO
2(B) occurs without involving a conversion reaction (without reduction of Ti
4+ to metallic titanium). Hence, the XPS results obtained here for TiO
2(B) differ from those reported for anatase in [
18].
Figure 4a shows X-ray diffraction patterns of the as-prepared TiO
2(B) sample as well as electrodes subjected to a de-/sodiation during the first and 30th cycles. As seen, all the reflections in the XRD pattern of the as-synthesized sample could be well indexed to the bronze-phase titanium dioxide having a monoclinic structure with a
C2/
m space group (JCPDS card No. 74-1940). The observed diffraction peak broadening is associated with the small sizes of the TiO
2(B) particles. Beyond bronze-phase TiO
2, diffraction peaks of metallic copper (used as a current collector) are detected at approximately 43.3, 50.5, 74.1, and 90.0° (marked by *) in the XRD patterns of electrodes. No other reflections, characteristic of other phases, e.g., titanates, metallic titanium, were detected in XRD patterns of the studied electrodes. Comparison of XRD patterns for the electrodes with that for the as-prepared sample reveals the disappearance of (110), (310), and (020) reflections of TiO
2(B) after initial sodiation. During subsequent desodiation, these peaks do not reappear. Other diffraction peaks of TiO
2(B) remained during cycling. This means that in the processes of electrochemical uptake and release of sodium, the TiO
2(B) structure is preserved. The observed disappearance of some peaks in patterns of the electrodes is explained by the partial amorphization of TiO
2(B), which is probably caused by the large size of the sodium ion; the absence of corresponding reflections (halo) indicates a small contribution from this process.
Figure 4b illustrates Raman spectra recorded for the as-synthesized TiO
2(B) sample and electrodes at the sodiated and desodiated states during the initial cycle. As seen, the spectrum of the as-prepared material contains modes in the range up to 900 cm
–1. It is known that the Raman spectrum of bronze-phase TiO
2 exhibits 18 active modes: 12
Ag and 6
Bg [
30,
31]. In Raman spectrum of the as-synthesized sample, the bands belonging to TiO
2(B) are observed at around 114 cm
–1 (
Ag(1) +
Bg(1)), 146 cm
–1 (
Ag(2)), 188 cm
–1 (
Ag(3) +
Bg(2)), 239 cm
–1 (
Bg(3)), 261 cm
–1 (
Bg(4) +
Ag(4)), 373 cm
–1 (
Ag(5) +
Ag(6)), 413 cm
–1 (
Ag(7) +
Ag(8)), 475 cm
–1 (
Ag(9) +
Bg(5)), 531 cm
–1 (
Ag(10)), 640 cm
–1 (
Ag(11) +
Bg(6)), and 855 cm
–1 (
Ag(12)). The electrodes, alongside the features of bronze-phase TiO
2, shows a Raman band near 1580 cm
–1 (inset in
Figure 4b), which corresponds to the Super P electroconductive additive. During the first sodiation process, the lines of TiO
2(B) reveal notable changes: in the range up to 750 cm
–1, they shift and decrease in intensity. Some of them become more intense upon subsequent desodiation. The obtained data indicate that structure of TiO
2(B) undergoes changes (becomes partially disordered based on XRD data) during cycling as a SIB anode-active material.
A previously published work on understanding the electrochemical sodium storage mechanism of anatase [
18] defines that the process occurs with the involvement of a conversion-type reaction accompanied by the reduction in a part of Ti
4+ to metal and the release of oxygen (based on GC–MS data). Somewhat later, in [
32], it was reported that the formation of metallic titanium during electrochemical de-/sodiation of anatase was refuted. Regarding oxygen, the authors of this study suggested that its release was caused by the structural rearrangement of anatase during the first Na
+ uptake. In order to clarify this circumstance with respect to bronze-phase titanium dioxide,
in situ GC–MS studies of the gas medium in a sodium half-cell with a TiO
2(B)-based electrode during initial sodiation were carried out.
Figure 5 displays the experimental data acquired during these experiments. As seen, the charge profile (sodiation process) shown in this graph has some differences in shape from that in
Figure 2. This could be because at a lower current density, the electrolyte decomposition at the electrode happens more actively. Carbon dioxide is the gaseous product of reduction in cyclic carbonate-based organic electrolytes [
33,
34]. In our case, CO
2 content, as expected, increases somewhat during the first charge (sodiation), i.e., before the formation of stable SEI layer at the electrode surface. The acquired data indicate that the oxygen concentration in the half-cell does not increase upon the initial sodiation of TiO
2(B). Together with the findings of
ex situ XPS, XRD, and Raman studies, this suggests that the changes that occurred with the bronze-phase titanium dioxide during Na
+ uptake are not accompanied by the release of oxygen.
Thus, the obtained results show that electrochemical interaction of TiO
2(B) with sodium involves a reversible intercalation process. The conversion reaction, as it was previously proposed for anatase [
18], does not happen for bronze TiO
2. However, during the initial sodiation, although the crystal structure of TiO
2(B) is, as a whole, preserved, it is subject to partial amorphization. The obtained data is a good complement to existing assumptions of other researchers [
12,
14,
16,
17,
18,
19,
20,
21,
22,
35] and unravel the electrochemical reaction mechanism of bronze-phase titanium dioxide in sodium-ion batteries.