Renewable Solvents for Diels–Alder/Cheletropic Reaction Sequences: Preparation of Pentaphenylbenzene and 1,2,4-Triphenyltriphenylene
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Details/Instruments
2.2. Synthesis of Pentaphenylbenzene (5)—General Protocol
2.3. Purification and Analysis of Pentaphenyltriphenylene (5)
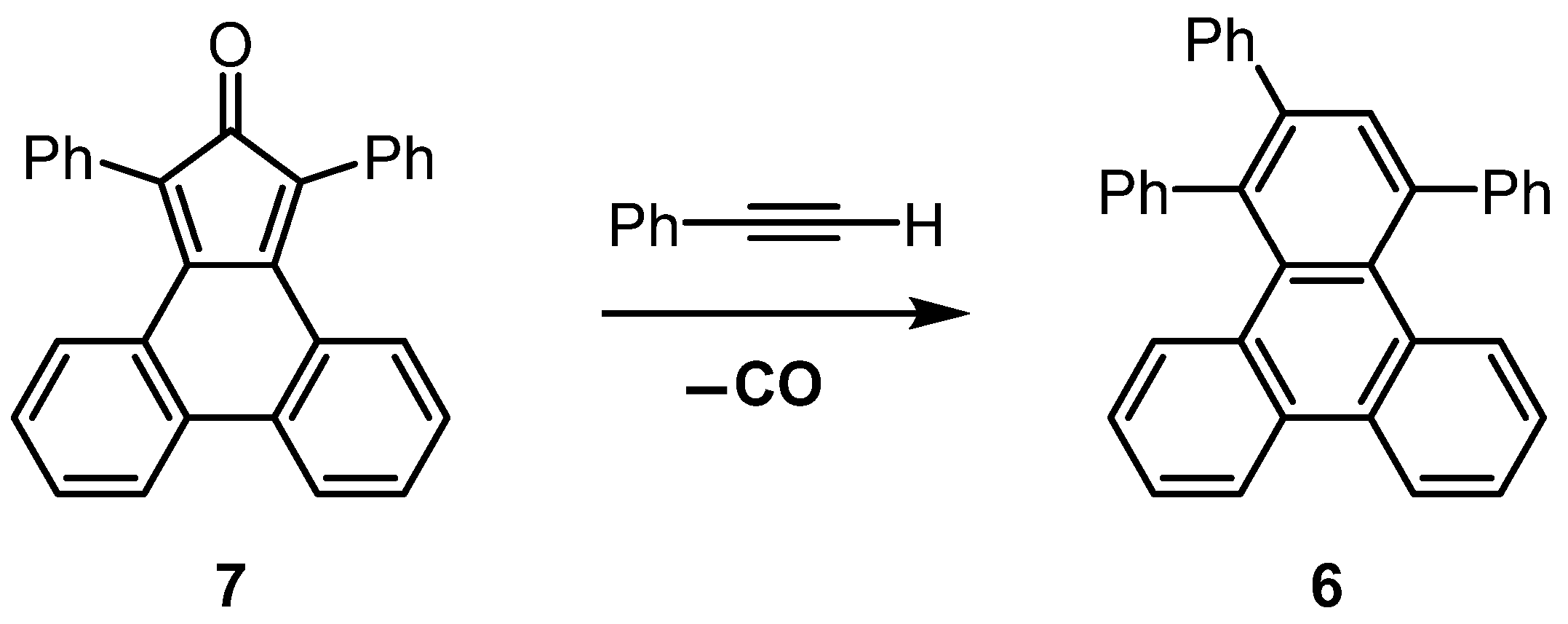
2.4. Synthesis of 1,2,4-Triphenyltriphenylene (6)—General Protocol
2.5. Purification and Analysis of 1,2,4-Triphenyltriphenylene (6)
2.6. Synthesis of 1,2,4-Triphenyltriphenylene (6)—CPME Protocol
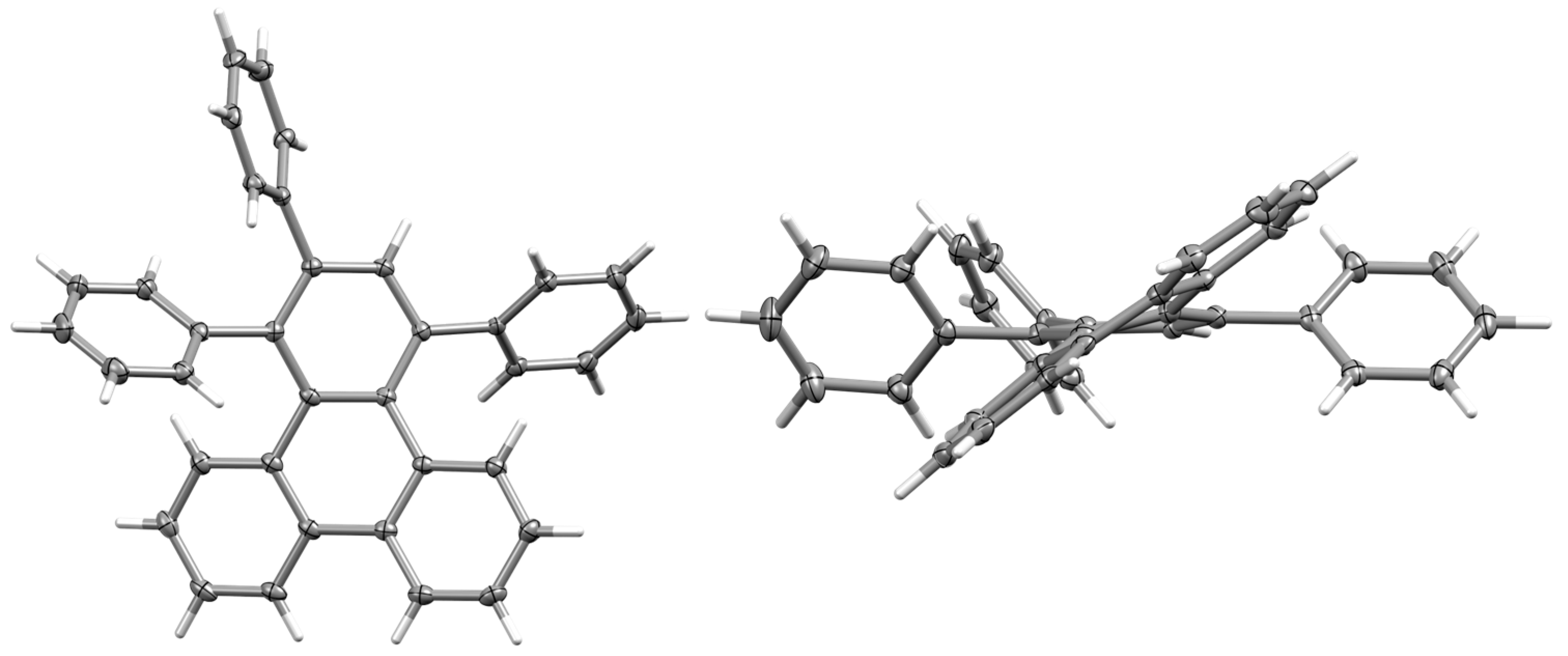
2.7. X-Ray Crystallography
3. Results
3.1. Solvent Selection
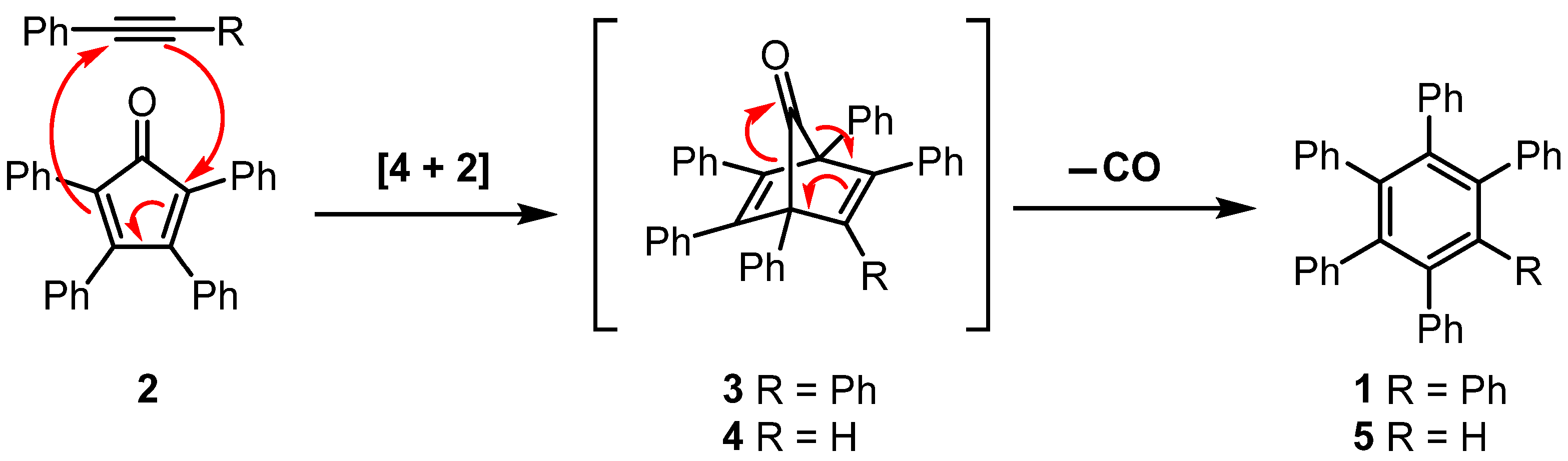
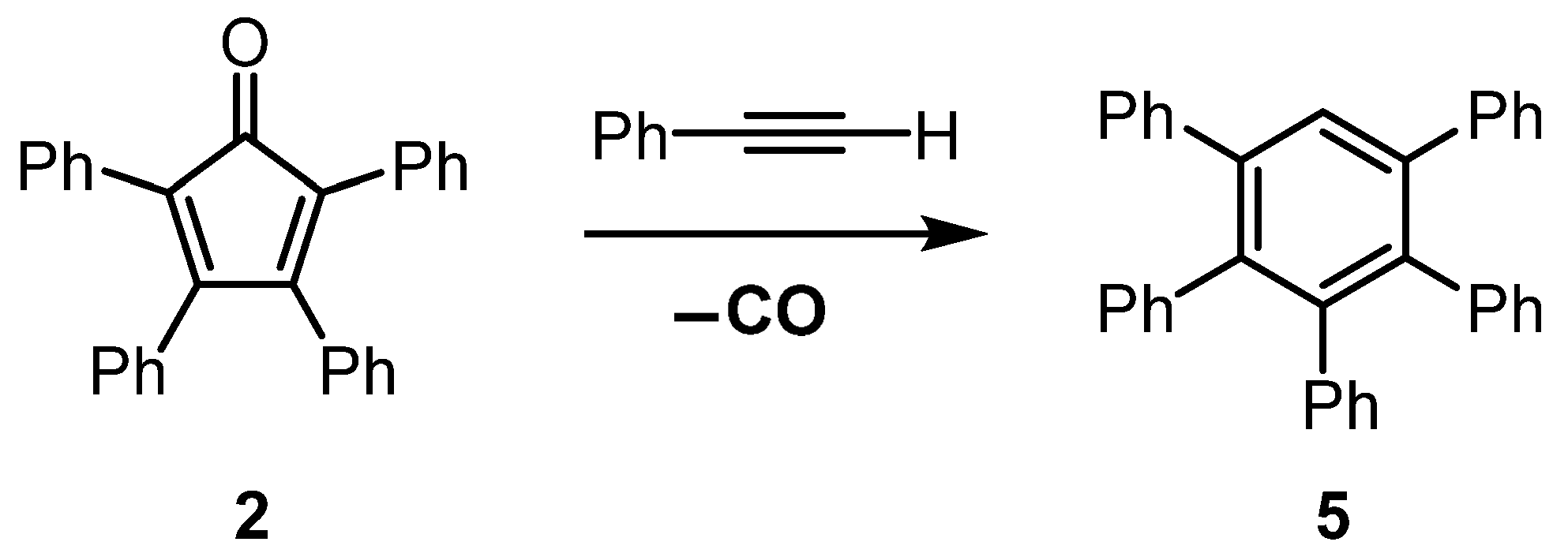
3.2. Synthesis of Pentaphenylbenzene (5)
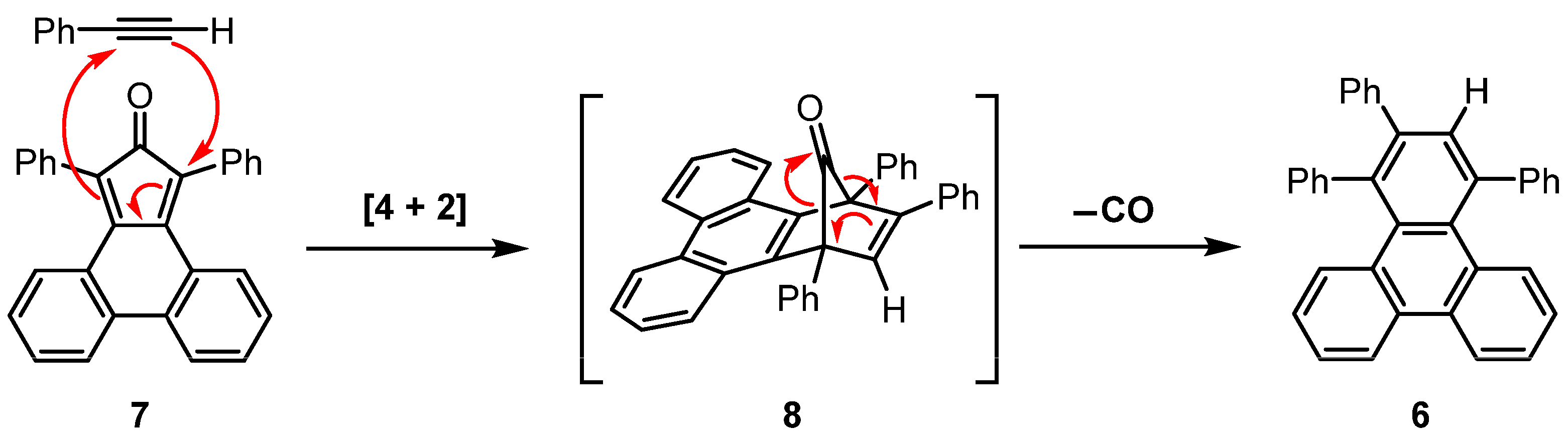
3.3. Synthesis of 1,2,4-Triphenyltriphenylene (6) at Elevated Temperatures
3.4. Synthesis of 1,2,4-Triphenyltriphenylene (6) at Lower Temperatures
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | Attenuated Reflectance Spectroscopy |
| CPME | Cyclopropyl Methyl Ether |
| DCM | Dichloromethane |
| IR | Infrared |
| 2-MeTHF | 2-Methyltetrahydrofuran |
| NMR | Nuclear Magnetic Resonance |
References
- Karunakaran, J.; Qiu, H.; Balaraman, E. Synthesis of diverse heterocyclic frameworks using cyclopentadienones via the Diels-Alder strategy. Org. Chem. Front. 2021, 8, 5608–5650. [Google Scholar] [CrossRef]
- Dyan, O.T.; Borodkin, G.I.; Zaikin, P.A. The Diels–Alder Reaction for the Synthesis of Polycyclic Aromatic Compounds. Eur. J. Org. Chem. 2019, 2019, 7271–7306. [Google Scholar] [CrossRef]
- Krompiec, S.; Kurpanik-Wójcik, A.; Matussek, M.; Gołek, B.; Mieszczanin, A.; Fijołek, A. Diels–Alder Cycloaddition with CO, CO2, SO2, or N2 Extrusion: A Powerful Tool for Material Chemistry. Materials 2022, 15, 172. [Google Scholar] [CrossRef]
- Bergman, H.M.; Beattie, D.D.; Kiel, G.R.; Handford, R.C.; Liu, Y.; Tilley, T.D. A sequential cyclization/π-extension strategy for modular construction of nanographenes enabled by stannole cycloadditions. Chem. Sci. 2022, 13, 5568–5573. [Google Scholar] [CrossRef]
- Vij, V.; Bhalla, V.; Kumar, M. Hexaarylbenzene: Evolution of Properties and Applications of Multitalented Scaffold. Chem. Rev. 2016, 116, 9565–9627. [Google Scholar] [CrossRef]
- Fieser, L.F. Hexaphenylbenzene. Org. Synth. 1966, 46, 44. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Xie, H.; Feng, L.; Lu, H.; Feng, S. Emission enhancement and color adjustment of silicon-cored structure in tetraphenylbenzene with aggregation-enhanced emission. J. Mater. Chem. C 2014, 2, 5601–5606. [Google Scholar] [CrossRef]
- Dusold, C.; Weiss, C.; Hampel, F.; Hirsch, A. Synthesis and crystal packing of perylene-derivatives with extreme sterically demanding pentaphenylbenzene bay-substituents. Chem. Commun. 2021, 57, 583–586. [Google Scholar] [CrossRef]
- Li, L.; Chen, M.; Zhang, H.; Nie, H.; Sun, J.Z.; Qin, A.; Tang, B.Z. Influence of the number and substitution position of phenyl groups on the aggregation-enhanced emission of benzene-cored luminogens. Chem. Commun. 2015, 51, 4830–4833. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jeong, S.J.; Lee, H.W.; Kim, J.; Lee, S.E.; Kim, Y.K.; Yoon, S.S. Efficient blue organic light-emitting diodes using N2,N2,N11,N11,5,6,7,8-octaphenyltriphenylene-2,11-diamine derivatives. Luminescence 2016, 31, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Zhou, G.; Scheiber, H.; Bauer, R.E.; Baumgarten, M.; Anson, C.E.; List, E.J.W.; Müllen, K. Polytriphenylene Dendrimers: A Unique Design for Blue-Light-Emitting Materials. Angew. Chem. Int. Ed. 2008, 47, 8292–8296. [Google Scholar] [CrossRef] [PubMed]
- Wettach, H.; Jester, S.S.; Colsmann, A.; Lemmer, U.; Rehmann, N.; Meerholz, K.; Hoger, S. Deep blue organic light-emitting diodes based on triphenylenes. Synth. Met. 2010, 160, 691–700. [Google Scholar] [CrossRef]
- Batsyts, S.; Hubner, E.G.; Namyslo, J.C.; Gjikaj, M.; Schmidt, A. Synthesis and characterization of propellor-shaped mono to hexacationic quinolinium-substituted benzenes. Org. Biomol. Chem. 2019, 17, 4102–4114. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical-and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Sherwood, J.; De bruyn, M.; Constantinou, A.; Moity, L.; McElroy, C.R.; Farmer, T.J.; Duncan, T.; Raverty, W.; Hunt, A.J.; Clark, J.H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar] [CrossRef]
- Stini, N.A.; Gkizis, P.L.; Kokotos, C.G. Cyrene: A bio-based novel and sustainable solvent for organic synthesis. Green Chem. 2022, 24, 6435–6449. [Google Scholar] [CrossRef]
- Leita, B.A.; Warden, A.C.; Burke, N.; O’Shea, M.S.; Trimm, D. Production of p-cymene and hydrogen from a bio-renewable feedstock-1,8-cineole (eucalyptus oil). Green Chem. 2010, 12, 70–76. [Google Scholar] [CrossRef]
- Ye, L.; Thompson, B.C. p-Cymene: A Sustainable Solvent that is Highly Compatible with Direct Arylation Polymerization (DArP). ACS Macro Lett. 2021, 10, 714–719. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Aparicio, S.; Alcalde, R. The green solvent ethyl lactate: An experimental and theoretical characterization. Green Chem. 2009, 11, 65–78. [Google Scholar] [CrossRef]
- Shukla, K.; Srivastava, V.C. Diethyl carbonate: Critical review of synthesis routes, catalysts used and engineering aspects. RSC Adv. 2016, 6, 32624–32645. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Zabrocka, W.; Bystrzanowska. Diethyl carbonate as a green extraction solvent for chlorophenol determination with dispersive liquid–liquid microextraction. Anal. Methods 2019, 11, 844–850. [Google Scholar] [CrossRef]
- Harrison, E.A. The importance of temperature control in the preparation of phencyclone (1,3-diphenyl-2-H-cyclopenta[I]phenanthrene-2-one). J. Chem. Educ. 1992, 69, 571. [Google Scholar] [CrossRef]
- Allen, C.F.H.; Bell, A. β-Nitrostyrene in the Diene Synthesis. J. Am. Chem. Soc. 1939, 61, 521–522. [Google Scholar] [CrossRef]
- Dilthey, W.; Henkels, S.; Schaefer, A. Hocharylierte aromatische Verbindungen (VI. Mitteil.). Berichte Der Dtsch. Chem. Ges. A/B 1938, 71, 974–979. [Google Scholar] [CrossRef]
- CrysAlisPro, v1.171.38.46. Rigaku Oxford Diffraction. Rigaku Corporation: Oxford, UK, 2015.
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B. Organic Solvents: Physical Properties and Methods of Purification, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1970; pp. 229–230. [Google Scholar]
- Dudkowski, J.J.; Becker, E.I. Electronic Effects and Rates in the Diels-Alder Reaction. J. Org. Chem. 1952, 17, 201–206. [Google Scholar] [CrossRef]
- Thiemann, T.; Iniesta, J.; Walton, D.J. Thermal oxidation of tetracyclones (2,3,4,5-tetraarylcyclopentadienones). J. Chem. Res. 2008, 2008, 173–180. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Castoldi, L.; Domínguez de María, P.; Alcántara, A.R. Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Alcántara, A.R.; Domínguez de María, P. Cyclopentyl Methyl Ether (CPME): A Versatile Eco-Friendly Solvent for Applications in Biotechnology and Biorefineries. ChemSusChem 2019, 12, 2083. [Google Scholar] [CrossRef]
- MacLeod, M.; Mackay, D. An assessment of the environmental fate and exposure of benzene and the chlorobenzenes in Canada. Chemosphere 1999, 38, 1777–1796. [Google Scholar] [CrossRef] [PubMed]
- Wooi, R.G.Y.; White, J.M. CCDC 1020300. CSD Communication 2014. [Google Scholar] [CrossRef]
- Pascal, R.A.; Van Engen, D.; Kahr, B.; McMillan, W.D. Twisted Polycyclic Aromatic Hydrocarbons. Structural Studies of Protic and Deuteriated 1,2,3,4-Tetraphenyltriphenylenes. J. Org. Chem. 1988, 53, 1687–1689. [Google Scholar] [CrossRef]
- Umezawa, Y.; Tsuboyama, S.; Honda, K.; Uzawa, J.; Nishio, M. CH/π Interaction in the Crystal Structure of Organic Compounds. A Database Study. Bull. Chem. Soc. Jpn. 1998, 71, 1207–1213. [Google Scholar] [CrossRef]
- Ogliaruso, M.A.; Romanelli, M.C.; Becker, E.I. Chemistry of Cyclopentadienones. Chem. Rev. 1965, 65, 261–367. [Google Scholar] [CrossRef]
- Yankelevich, S.; Fuchs, B. On Alleged Norbornadien-7-ones. Tetrahedron Lett. 1967, 49, 4945–4949. [Google Scholar] [CrossRef]
- LeBlanc, B.F.; Sheridan, R.S. Photochemical Generation and Direct Observation of 7-Norbornadienone. J. Am. Chem. Soc. 1985, 107, 4554–4556. [Google Scholar] [CrossRef]
- Birney, D.M.; Wiberg, K.B.; Berson, J.A. Geometry of the Transition State of the Decarbonylation of Bicyclo[2.2.1]hepta-2,5-dien-7-one. Experimental and ab Initio Theoretical Studies. J. Am. Chem. Soc. 1988, 110, 6631–6642. [Google Scholar] [CrossRef]
- Lai, C.-H.; Li, E.Y.; Chen, K.-Y.; Chow, T.J.; Chou, P.-T. Theoretical Investigation of Cheletropic Decarbonylation Reactions. J. Chem. Theory Comput. 2006, 2, 1078–1084. [Google Scholar] [CrossRef]

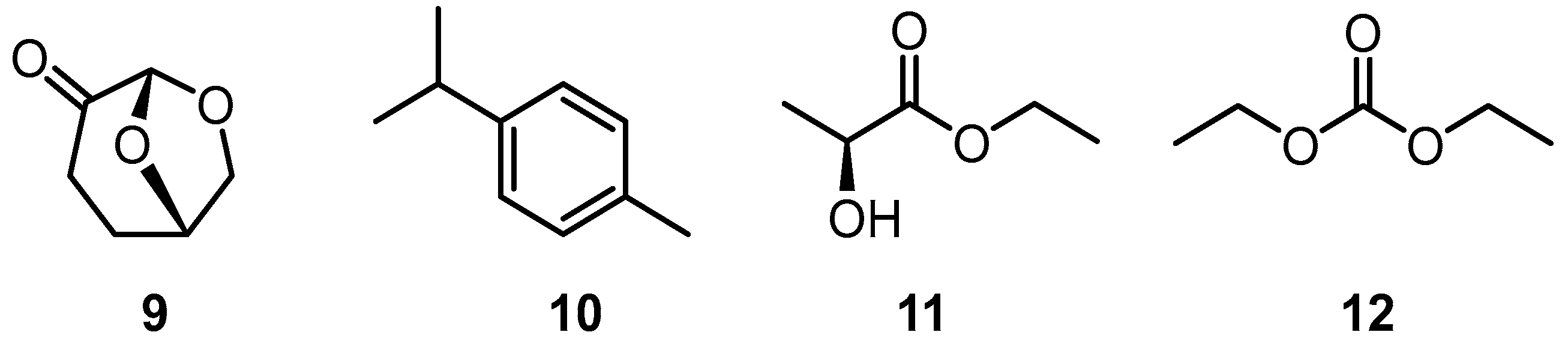

| Entry | Solvent | Boiling Point | References |
|---|---|---|---|
| 1 | Dihydrolevoglucosenone (CyreneTM) | 227 °C | [17,18] |
| 2 | p-Cymene (1-methyl-4-(propan-2-yl)benzene) | 177 °C | [19,20] |
| 3 | Ethyl lactate (ethyl (S)-2-hydroxypropionate) | 151–154 °C | [21,22] |
| 4 | Diethyl carbonate | 126 °C | [23,24] |
| Entry | Solvent | Reaction Time | % Yield | Reference |
|---|---|---|---|---|
| 1 | p-Xylene (1,4-dimethylbenzene) | 180–220 min 1 | 85–90% 2 | This work |
| 2 | Dihydrolevoglucosenone (CyreneTM) | 50–55 min 1 | 75–80% 2 | This work |
| 3 | p-Cymene (1-methyl-4-(propan-2-yl)benzene) | 60–70 min 1 | 80–85% 2 | This work |
| 4 | Ethyl lactate (ethyl (S)-2-hydroxypropionate) | 130–150 min 1 | 87–92% 2 | This work |
| 5 | Diethyl carbonate | 430–450 min 1 | 85–90% 2 | This work |
| 6 | Diphenyl ether | 2 h | 74% 3 | [7] |
| 7 | Diphenyl ether | 16 h | 78% 3 | [9] |
| Entry | Solvent | Reaction Time | % Yield | Reference |
|---|---|---|---|---|
| 1 | p-Xylene (1,4-dimethylbenzene) | 20–25 min 1 | 75–80% 2 | This work |
| 2 | Dihydrolevoglucosenone (CyreneTM) | 5 min 1 | 85–90% 2 | This work |
| 3 | p-Cymene (1-methyl-4-(propan-2-yl)benzene) | 5–10 min 1 | 87–92% 2 | This work |
| 4 | Ethyl lactate (ethyl (S)-2-hydroxypropionate) | 15–20 min 1 | 85–90% 2 | This work |
| 5 | Diethyl carbonate | 25–30 min 1 | 70–75% 2 | This work |
| 6 | o-Xylene (1,2-dimethylbenzene) | 24 h | 95% 3 | [10] |
| 7 | β-Decalol (decahydro-2-naphthol) | Not stated | 59% 4 | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.; Burrows, H.; Chalmers, B.A.; Cordes, D.B.; Davidson, R.M.; Emmens, L.; Fulton, T.V.; Kleinjan, D.; Patterson, I.L.J.; Smellie, I.A. Renewable Solvents for Diels–Alder/Cheletropic Reaction Sequences: Preparation of Pentaphenylbenzene and 1,2,4-Triphenyltriphenylene. Reactions 2025, 6, 41. https://doi.org/10.3390/reactions6030041
Ahmed S, Burrows H, Chalmers BA, Cordes DB, Davidson RM, Emmens L, Fulton TV, Kleinjan D, Patterson ILJ, Smellie IA. Renewable Solvents for Diels–Alder/Cheletropic Reaction Sequences: Preparation of Pentaphenylbenzene and 1,2,4-Triphenyltriphenylene. Reactions. 2025; 6(3):41. https://doi.org/10.3390/reactions6030041
Chicago/Turabian StyleAhmed, Sara, Harry Burrows, Brian A. Chalmers, David B. Cordes, Ruairidh Macleod Davidson, Lauren Emmens, Theodore V. Fulton, Daniel Kleinjan, Iain L. J. Patterson, and Iain A. Smellie. 2025. "Renewable Solvents for Diels–Alder/Cheletropic Reaction Sequences: Preparation of Pentaphenylbenzene and 1,2,4-Triphenyltriphenylene" Reactions 6, no. 3: 41. https://doi.org/10.3390/reactions6030041
APA StyleAhmed, S., Burrows, H., Chalmers, B. A., Cordes, D. B., Davidson, R. M., Emmens, L., Fulton, T. V., Kleinjan, D., Patterson, I. L. J., & Smellie, I. A. (2025). Renewable Solvents for Diels–Alder/Cheletropic Reaction Sequences: Preparation of Pentaphenylbenzene and 1,2,4-Triphenyltriphenylene. Reactions, 6(3), 41. https://doi.org/10.3390/reactions6030041








