Abstract
Polycyclic aromatic compounds can often be made by a sequence featuring an initial Diels–Alder [4 + 2] cycloaddition reaction, followed by cheletropic extrusion of carbon monoxide. These reactions normally require heating the diene and dieneophile in petrochemical-derived aromatic hydrocarbon solvents, such as xylenes or diphenyl ether. This article summarizes the results of attempts to use renewable solvents in place of those currently in use to prepare pentaphenylbenzene and 1,2,4-triphenyltriphenylene. Dihydrolevoglucosenone, p-cymene, ethyl lactate, diethyl carbonate, and cyclopentyl methyl ether have all been successfully evaluated as renewable solvent alternatives in Diels–Alder/cheletropic reaction sequences. An analysis of the products from the reactions investigated did not show evidence of oxidative degradation of the diene reactants. Furthermore, norbornadien-7-one intermediates were not isolated from any of the reactions tested.
1. Introduction
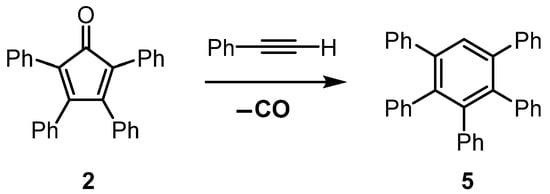
Reaction sequences involving a Diels–Alder [4 + 2] cycloaddition reaction and subsequent thermal cheletropic extrusion of carbon monoxide are well-established methods to prepare polycyclic aromatic compounds [1,2,3,4]. The formation of hexaphenylbenzene (1) from the reaction of tetracyclone (2, 2,3,4,5-tetraphenylcyclopentadienone) and diphenylacetylene serves as a representative example of this type of sequence (Scheme 1) [5,6]. The initial step of the sequence is a Diels–Alder [4 + 2] cycloaddition reaction to form norbornadien-7-one derivative 3. However, intermediate 3 is short-lived and undergoes rapid cheletropic release of carbon monoxide gas to form hexaphenylbenzene. This reaction is conducted at a high temperature, and a mixture of tetracyclone and diphenylacetylene is heated to 300 °C, using benzophenone or benzophenone/diphenyl ether mixtures as the solvent [6].
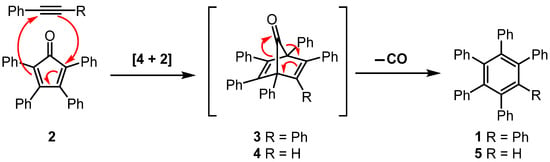
Scheme 1.
Diels–Alder reaction between tetracyclone (2) and diphenylacetylene or phenylacetylene, followed by cheletropic extrusion of carbon monoxide from transient intermediates 3 or 4 to form hexaphenylbenzene (1) or pentaphenylbenzene (5).
The analogous reaction of tetracyclone (2) and phenylacetylene via norbornadien-7-one derivative 4 to form pentaphenylbenzene (5) does not require such forcing conditions and can be achieved when the reaction temperature is <300 °C (Scheme 1) [7].
In recent years, routes to pentaphenylbenzene analogues have become more prominent due to these compounds having applications in optoelectronics and sensing [8,9]. 1,2,4-Triphenyltriphenylene (6) has also attracted attention since it is fluorescent; it has been shown that blue light is emitted when samples of this material in solution are irradiated with a UV or visible light source (Figure 1) [10]. Moreover, larger and more complex structures that incorporate triphenylene motifs have been investigated for potential use as blue light emitters in Organic Light-Emitting Diodes (OLEDs) [10,11,12].

Figure 1.
A sample of 1,2,4-triphenyltriphenylene (6) dissolved in dichloromethane and illuminated with a 405 nm laser.
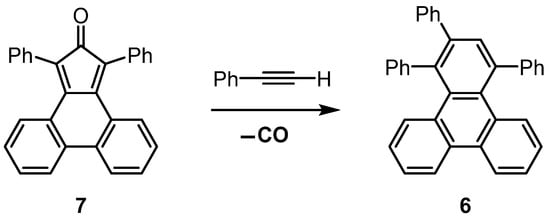
1,2,4-Triphenyltriphenylene (6) is usually prepared by a Diels–Alder [4 + 2] cycloaddition reaction of phencyclone (7, 1,3-diphenyl-2H-cyclopenta[l]phenanthren-2-one) with phenylacetylene. The initial cycloadduct formed is norbornadien-7-one derivative 8 (Scheme 2); however, this transient intermediate then undergoes facile cheletropic release of carbon monoxide gas to obtain the desired product (6).
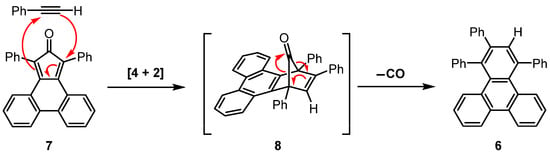
Scheme 2.
Diels–Alder reaction between phencyclone (7) and phenylacetylene, followed by cheletropic extrusion of carbon monoxide from transient intermediate 8 to form 1,2,4-triphenyltriphenylene (6).
Recent interest in pentaphenylbenzene and 1,2,4-triphenyltriphenylene derivatives led us to investigate possible alternative conditions to prepare compounds of this type. In this paper, we explore modifications to the preparative routes to pentaphenylbenzene (5) and 1,2,4-triphenyltriphenylene (6), the key objective being to try and identify suitable sustainable alternative solvents to those used in older protocols. The synthetic procedures currently available often employ aromatic hydrocarbons such as toluene or xylene isomers as the reaction solvent [10,11]. When higher reaction temperatures are required, high boiling point solvents such as diphenyl ether, benzophenone, or decahydro-2-naphthol (often referred to as β-decalol) are often necessary [7,12,13]. The recent literature attention has been directed toward addressing the need to become less reliant on solvents derived from petrochemical sources and replace these with renewable alternatives. For the purposes of this article, the term “renewable” refers to solvents that can be obtained from a feedstock that is not petrochemical; often the feedstocks come from readily available biomass. Several sources have recently been identified, and key examples include lignocellulosic biomass, citrus waste, and plant-derived oils [14,15,16]. Furthermore, these renewable solvents are often less hazardous to manipulate and have a reduced environmental impact. The short study described in this paper investigates whether selected renewable solvents are compatible with the reaction conditions required to prepare and isolate pentaphenylbenzene (5) and 1,2,4-triphenyltriphenylene (6). The work presented herein is focused on the use of dihydrolevoglucosenone (9, CyreneTM), p-cymene (10, 1-methyl-4-(propan-2-yl)benzene), ethyl lactate (11, ethyl (S)-2-hydroxypropionate), and diethyl carbonate (12) as replacements for the solvents employed in previous studies (Figure 2) [17,18,19,20,21,22,23,24].
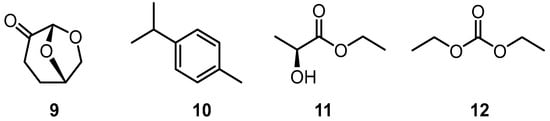
Figure 2.
Structures of the solvents to be evaluated: dihydrolevoglucosenone (9, CyreneTM), p-cymene (10, 1-methyl-4-(propan-2-yl)benzene), ethyl lactate (11, ethyl (S)-2-hydroxypropionate), and diethyl carbonate (12).
2. Materials and Methods
2.1. Experimental Details/Instruments
Tetracyclone was used as supplied (Sigma-Aldrich Company Ltd., Gillingham, UK). Phencyclone was prepared according to the method initially reported by E. A. Harrison [25]. Melting points were recorded on a Stuart SMP3 melting point apparatus (tolerance ±1.5 °C at 300 °C). The melting point apparatus was calibrated using samples of phenolphthalein (mp 261–263 °C). IR spectra were recorded on a Nicolet Summit FTIR instrument with an Everest diamond ATR accessory. NMR spectra were obtained for 1H at 500.13 MHz and for 13C at 125.77 MHz using a Bruker AVIII_HD 500 instrument. Spectra were run at 25 °C in CDCl3. Chemical shifts are reported in ppm to the high frequency of the reference, and coupling constants J are reported in Hz.
2.2. Synthesis of Pentaphenylbenzene (5)—General Protocol
Tetracyclone (200 mg, 0.5 mmol, 1 equivalent) and phenylacetylene (61 mg, 0.6 mmol, 1.2 equivalents) were mixed in the selected solvent (2 mL). The mixture was heated to reflux until the red colour of tetracyclone was no longer visible. On completion of the reaction, the mixture was cooled to room temperature and methanol (10 mL) was added. After 5 min of mixing time, the mixture was cooled in an ice bath to ensure the crude product precipitated.
2.3. Purification and Analysis of Pentaphenyltriphenylene (5)
The crude product was crystallized from toluene/cyclohexane. A colourless crystalline solid was formed, and this was filtered off under suction and washed with ice-chilled cyclohexane (2 × 1 mL) to afford product 5. mp 250 °C (lit. [26] 246 °C). IR (ATR) 3077.5, 3054.4, 3021.7 (ArCH), 1598.9, 1488.3, 1441.0, 1073.9, 759.8, 695.6 cm−1; 1H NMR (500 MHz, CDCl3); 7.62 (1H, s, ArH), 7.18–7.22 (10H, m, ArH), 6.96–6.98 (6H, m, ArH), 6.89–6.92 (7H, m, ArH), 6.82–6.84 (2H, m, ArH). 13C NMR (126 MHz, CDCl3); 141.8 (ArCq), 141.7 (ArCq), 140.8 (ArCq), 140.4 (ArCq), 140.0 (ArCq), 139.3 (ArCq), 131.6 (ArCH), 131.5 (ArCH), 131.4 (ArCH), 130.0 (ArCH), 127.6 (ArCH), 126.9 (ArCH), 126.7 (ArCH), 126.3 (ArCH), 125.6 (ArCH), 125.4 (ArCH).
2.4. Synthesis of 1,2,4-Triphenyltriphenylene (6)—General Protocol
Phencyclone (200 mg, 0.5 mmol, 1 equivalent) and phenylacetylene (61 mg, 0.6 mmol, 1.2 equivalents) were mixed in the selected solvent (2 mL). The mixture was heated to reflux until the green colour of phencyclone was no longer visible. On completion of the reaction, the mixture was cooled to room temperature and methanol (10 mL) was added. After 5 min mixing time, the mixture was cooled in an ice bath to ensure the crude product precipitated.
2.5. Purification and Analysis of 1,2,4-Triphenyltriphenylene (6)
The crude product was crystallized from DCM/methanol, CPME/methanol or 2-Me-THF/methanol mixtures. A colourless crystalline solid was formed, this was filtered off under suction and washed with ice-cold methanol (2 × 1 mL) to afford product 6. mp 259 °C (lit. [27] 250 °C). IR (ATR) 3051.7, 3025.4 (ArCH), 1595.6, 1493.0, 1441.2, 757.1, 697.4 cm−1; 1H NMR (500 MHz, CDCl3); 8.47 (2H, d, J = 8.2 Hz, ArH), 7.76 (1H, d, J = 8.4 Hz, ArH), 7.70 (1H, s, ArH), 7.54–7.58 (3H, m, ArH), 7.40–7.50 (5H, m, ArH), 7.18–7.24 (6H, m, ArH), 7.10–7.16 (6H, m, ArH). 13C NMR (126 MHz, CDCl3); 144.4 (ArCq), 142.1 (ArCq), 141.7 (ArCq), 140.2 (ArCq), 138.2 (ArCq), 136.6 (ArCq), 132.4 (ArCH), 132.1 (ArCH), 131.9 (ArCq), 131.7 (ArCq), 131.4 (ArCq), 130.8 (ArCq), 130.2 (ArCH), 130.1 (ArCH), 129.9 (ArCH), 129.8 (ArCH), 129.7 (ArCH), 129.1 (ArCH), 128.4 (ArCH), 127.7 (ArCH), 127.2 (ArCH), 126.7 (ArCH), 126.6 (ArCH), 126.4 (ArCH), 126.2 (ArCH), 125.6 (ArCH), 125.3 (ArCH), 123.3 (ArCH).
2.6. Synthesis of 1,2,4-Triphenyltriphenylene (6)—CPME Protocol
Phencyclone (200 mg, 0.5 mmol, 1 equivalent) and phenylacetylene (61 mg, 0.6 mmol, 1.2 equivalents), were mixed in CPME (2 mL). The mixture was heated to reflux until the green colour of phencyclone was no longer visible. On completion of the reaction, a further portion of CPME (2–4 mL) was added to the reaction flask. Methanol was then added in small portions until the solution became turbid, and the mixture was cooled to allow the product to crystallize. The crystalline material formed was filtered off under suction and washed with ice-cold methanol (2 × 1 mL) to obtain product 6 (191 mg, 0.42 mmol, 80% yield).
2.7. X-Ray Crystallography
X-ray diffraction data for compound 6 were collected at 100 K using a Rigaku FR-X Ultrahigh Brilliance Microfocus RA generator/confocal optics [Mo Kα radiation (λ = 0.71073 Å)] with an XtaLAB P200 diffractometer. Intensity data were collected (using a calculated strategy) and processed (including correction for Lorentz, polarization, and absorption) using CrysAlisPro [28]. The structure was solved by dual-space methods (SHELXT) [29] and refined by full-matrix least-squares against F2 (SHELXL-2019/3) [30]. Non-hydrogen atoms were refined anisotropically, and hydrogen atoms were refined using a riding model. In the early stages of modelling, the structure showed multiple apparent dichloromethane solvates, all of which were disordered over multiple orientations. Attempts to model these resulted in unusually elongated thermal ellipsoids for the chlorine sites, poor agreement with expected values for the C-Cl bonds, and significant residual electron density among the disordered solvates. Attempts to treat the residual electron density using the SQUEEZE [31] routine implemented in PLATON [32] were made, retaining some of the modelled disordered dichloromethane, but this did not result in the expected behaviour for the residual electron density. Following this, all the modelled solvates were removed, and SQUEEZE was used to remove the contribution to the diffraction pattern of the poorly ordered electron density in the void spaces (856 Å3, 18.5% of unit cell volume). All calculations except SQUEEZE were performed using the Olex2 interface [33]. CCDC 2465931 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures (accessed on 25 June 2025).
Selected crystallographic data: C36H24 M = 456.55, monoclinic, a = 10.05173(18), b = 24.8852(5), c = 22.3496(4) Å, β = 91.4305(17)°, Vol. = 5588.76(18) Å3, T = 100 K, space group P21/c (no. 14), Z = 8, 62,090 reflections measured, 13,006 unique (Rint = 0.0242), which were used in all calculations. The final R1 [I > 2σ (I)] was 0.0493 and wR2 (all data) was 0.1359.
3. Results
3.1. Solvent Selection
The key objective of this study was to identify replacement solvents for use in the synthesis of pentaphenylbenzene (5) and 1,2,4-triphenyltriphenylene (6) from tetracyclone (2) and phencyclone (7), respectively. It was desirable for the alternative solvents to be available from renewable sources and to pose fewer toxicity and disposal problems than those currently in use. Existing methods for the preparation of pentaphenylbenzene (5) often use diphenyl ether as the reaction solvent, and these procedures employ conventional or microwave heating [7,8,9]. Diphenyl ether has a boiling point of 258 °C; however, its melting point range is 25–26 °C and is therefore solid at room temperature [34]. The key feature of this substance is the high boiling point to enable short reaction times; we therefore initially selected solvents that had boiling points as close to 200 °C as possible and were liquid at room temperature. Dihydrolevoglucosenone (9, CyreneTM) emerged as the closest candidate (Table 1, entry 1); however, 1-methyl-4-(propan-2-yl)benzene (10, “p-cymene”) has a slightly lower boiling point (Table 1, entry 2), which was considered close enough for consideration. Both of these solvents are considered renewable since they can be made from biomass feedstocks. Dihydrolevoglucosenone is made from lignocellulosic materials, and 1-methyl-4-(propan-2-yl)benzene can be prepared from eucalyptus oil or citrus fruit waste [17,18,19,20].

Table 1.
Boiling points of the renewable solvents selected for study.
In early studies, 1,2,4-triphenyltriphenylene (6) was successfully prepared by heating a mixture of phencyclone (7) and phenylacetylene without solvent [27]. This initial work reported that the reaction was initiated at approximately 100 °C and that the detection of carbon monoxide gas was used to follow the progress of the reaction. While these conditions were effective, a 2.5:1 molar ratio of phenylacetylene to phencyclone was required, and the product was purified by recrystallization from chlorobenzene. Later work has focused on routes that do not rely on a large excess of the phenylacetylene dienophile, and these also demonstrate simpler purification methods. Typical examples involve heating phencyclone (7) in xylenes, diphenyl ether, or β-decalol (decahydro-2-naphthol) for several hours [10,11,12,13]. Once the reaction is complete, the product is often purified by the addition of an alcohol solvent to the reaction mixture and then allowing the product (6) to crystallize. In this study, it was anticipated that ethyl lactate or diethyl carbonate could serve as renewable alternatives to high-boiling-point hydrocarbon solvents. Ethyl lactate is derived from the fermentation of carbohydrate biomass and has a boiling point of ~150 °C (Table 1, entry 3). Diethyl carbonate has a slightly lower boiling point (Table 1, entry 4), and modern processes allow this solvent to be prepared from sustainable feedstocks, such as urea. Furthermore, the alternative solvents (10 and 12) initially selected for studies on the preparation of pentaphenylbenzene (5) would also be evaluated for suitability for producing samples of 1,2,4-triphenyltriphenylene (6). p-Xylene (1,4-dimethylbenzene) was used as a representative aromatic hydrocarbon solvent since xylene isomers have been used in previous experiments [10]. The reaction times and ease of isolation of products when sustainable solvents were used would then be compared with the results obtained from the use of p-xylene.
3.2. Synthesis of Pentaphenylbenzene (5)
The reaction of tetracylone (2) with phenylacetylene (Scheme 3) was attempted in refluxing dihydrolevoglucosenone (9, CyreneTM), p-cymene (10, 1-methyl-4-(propan-2-yl)benzene), ethyl lactate (11, ethyl (S)-2-hydroxypropionate), and diethyl carbonate (12) (see Table 2). Trials found that the reactions conducted at higher temperature proceeded faster; this observation aligns with the results from an earlier study where reaction times in refluxing toluene and p-cymene were compared [35]. Dihydrolevoglucosenone has the highest boiling point; it was found that the reaction proceeded to completion within an hour (Table 2, entry 2). The reaction in refluxing p-cymene was also found to proceed within a similar period of time (Table 2, entry 3). The reaction times in refluxing ethyl lactate and diethyl carbonate were much slower (Table 2, entries 4 and 5); this outcome is not unexpected since these solvents have lower boiling points.
Scheme 3.
Diels–Alder/cheletropic extrusion reaction sequence to form pentaphenylbenzene (5).

Table 2.
Reaction times and yields for the synthesis of pentaphenylbenzene (5) in a selection of solvents.
The ease of recovery of the pentaphenylbenzene product was also examined in each case. After each reaction was complete, the reaction flask was allowed to cool to room temperature, a portion of methanol was added, and then the resulting mixture was cooled in ice. In all cases, the product was found to precipitate or crystallize from the solution. This allowed the product to be isolated by vacuum filtration. The material isolated at this stage was found to be the desired product, but often with some residual solvent present. The pentaphenylbenzene samples obtained from the reactions were readily crystallized from toluene/cyclohexane mixtures. The material obtained in all cases had physical properties consistent with those expected from the previous literature reports (IR and NMR data are provided in the Supporting Information document) [7,9]. For comparison purposes, Table 2 (entries 6 and 7) also provides solvents, reaction times, and % yields for previous syntheses of pentaphenylbenzene from tetracyclone. In both cases (Table 2 entries 6 and 7), the product was obtained in good yield; however, petrochemical-derived diphenyl ether was used as the reaction solvent. The products were also purified and isolated by use of flash column chromatography on silica; this process consumes a comparatively large volume of solvent relative to the solvents required for the crystallization processes employed herein.
It was noteworthy that the reactions that required heating for longer than 60 min did not lead to a low % yield of the product. There were some initial concerns that prolonged heating could reduce the reaction yield; this was because previous studies had reported by-product formation after extensive heating of tetracylone in the presence of atmospheric oxygen [36]. Scheme 4 illustrates a key outcome from a previous study on the formation of oxidative degradation products obtained by heating tetracyclone to 135 °C in diphenyl ether for 36 h. The reactions presented in this article did not lead to the formation of diketone 13 or α-pyrone 14 by-products, nor were they isolated from any of the sequences outlined in Table 2.
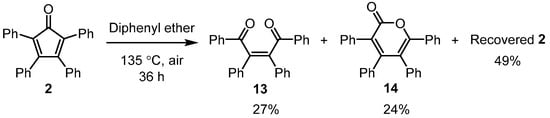
Scheme 4.
Air oxidation of tetracyclone (2) to form ketone 13 and α-pyrone 14 [27].
3.3. Synthesis of 1,2,4-Triphenyltriphenylene (6) at Elevated Temperatures
The reaction of phencyclone (7) with phenylacetylene (Scheme 5) was attempted using the same selection of solvents as highlighted in Section 3.2 (See Table 3). These experiments again demonstrated that the reactions conducted at higher temperatures proceeded faster; however, the reaction times overall were significantly shorter. For example, the reaction in refluxing p-cymene was found to proceed within a 5–10 min period (Table 3, entry 3). The reaction in dihydrolevoglucosenone was found to proceed within 5 min; often the reaction was complete before the solvent reached reflux temperature (Table 3, entry 2). The reaction times in refluxing ethyl lactate and diethyl carbonate were notably faster when phencylone was used as the diene (Table 3, entries 4 and 5). The reaction times associated with these solvents compared well with the conditions often used in contemporary work (Table 3, entries 6 and 7).
Scheme 5.
Diels–Alder/cheletropic extrusion reaction sequence to form 1,2,4,triphenyltriphenylene (6).

Table 3.
Reaction times and yields for the synthesis of 1,2,4-triphenyltriphenylene (6) in a selection of solvents.
In each case, the ease of recovery of the 1,2,4-triphenyltriphenylene product was investigated. In all the experiments, the product was found to readily precipitate when the reaction mixtures were treated with methanol and cooled in ice. As was the case with samples of pentaphenylbenzene, the material isolated was found to be the desired product, but often with some residual solvent present. The 1,2,4-triphenyltriphenylene samples obtained from the reactions were readily crystallized using three different solvent systems. It was observed that 1,2,4-triphenyltriphenylene had limited solubility in methanol between 0 °C and 25 °C; however, it was found to be very soluble in dichloromethane (DCM) over this temperature range. Good-quality crystalline material was routinely obtained by the following method: A sample of material precipitated from a reaction mixture was dissolved in a small volume of DCM (just enough for the solid to dissolve). Methanol was then added dropwise until the mixture became slightly turbid. Crystalline 1,2,4-triphenyltriphenylene was observed to form within a few minutes, though the mixtures were allowed to crystallize for a few hours before being isolated by filtration. Although DCM was effective, we were concerned about the use of a chlorinated organic solvent and sought to identify some renewable alternatives. 2-Methyltetrahydrofuan (2-MeTHF) and cyclopropyl methyl ether (CPME) have both found use as DCM substitutes in recent years, and both solvents were evaluated in this study [37,38]. 1,2,4-Triphenyltriphenylene was found to be less soluble in CPME and 2-MeTHF than in DCM. However, samples of crude product could be purified by heating 200–300 mg of material in CPME or 2-MeTHF until the solid dissolved. On cooling, methanol was added in small portions until the mixture became turbid; the product was then allowed to crystallize over a period of 2–3 h. The material obtained from all the crystallization methods was found to have physical properties consistent with those expected from the previous literature reports (IR and NMR data are provided in the Supporting Information document). Table 3 (entries 6 and 7) also provides solvents, reaction times, and % yields for previous syntheses of 1,2,4-triphenyltriphenylene from phencyclone. In both cases (Table 3 entries 6 and 7), the reactions used petrochemical-derived hydrocarbons as the reaction solvent. The method described in entry 7 states that prior to purification by vacuum sublimation, the crude product was treated with chlorobenzene. The original method described by Dilthey in 1938 also uses chlorobenzene in the purification process [27]. As is the case with many chlorinated organic solvents, chlorobenzene is recognized as a persistent aromatic pollutant in aquatic environments and soil. Although methods of remediation are currently being identified, alternatives to this solvent are now preferred [39].
Crystals of 1,2,4-triphenyltriphenylene (6) suitable for single-crystal X-ray diffraction were obtained by dissolving the compound in dichloromethane, followed by enough methanol to initiate slow crystallization. The structure presented (Figure 3) is a solvate of the structure of 6 previously reported by Wooi and White [40]. The two structures show near-identical molecular geometries, despite the solvation differences. In addition, the X-ray crystal structure of the 1,2,3,4-tetraphenyltriphenylene was previously acquired and provides a further useful comparison [41]. A key feature of all three structures is the pronounced twisting distortion of the triphenylene ring due to non-bonded interactions between the benzo-fused triphenylene ring protons and the phenyl ring substituents. The triphenyl substituted structures show very similar triphenylene distortion, minimizing the steric clash between the phenyl rings and the proximal triphenylene ring protons and leading to intramolecular CH···π interactions at H···centroid distances of 2.87–2.95 Å (corresponding C···centroid separations of 3.5526(16)–3.6229(15)). These distances are at or beyond the conventional van der Waals radii, but CH···π interactions have been suggested to be effective at distances beyond this limit [42].
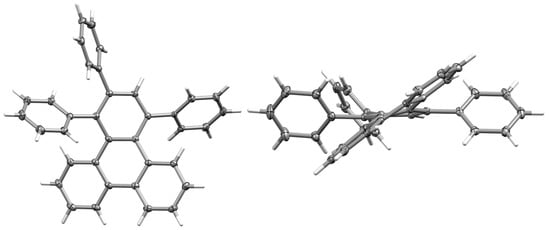
Figure 3.
Two views of the molecular structure of 6 (anisotropic displacement ellipsoids are set at the 50% probability level. (left) View of one independent molecule in the structure; (right) view showing the twist across the triphenylene.
3.4. Synthesis of 1,2,4-Triphenyltriphenylene (6) at Lower Temperatures
The purification methods for 1,2,4-triphenyltriphenylene described in Section 3.3 demonstrated that the product could be crystallized from a mixture of cyclopentylmethyl ether (CPME) and methanol. To simplify the procedure, attempts were made to prepare 1,2,4-triphenyltriphenylene from phencyclone and phenylacetylene in refluxing CPME. It was expected under these conditions that the product could then be crystallized directly from the reaction mixture and avoid the need for an additional crystallization step. Furthermore, it was also anticipated that the boiling point of CPME (106 °C) would be sufficiently high to allow the reaction to take place within a reasonable period of time. Work by Dilthey in 1938 noted that the reaction of phenylacetylene and phencyclone showed “strong” evolution of CO gas when the reaction temperature reached 100 °C, and refluxing CPME would exceed this temperature threshold [27]. Previous experiments have demonstrated that the reaction can occur at temperatures < 100 °C; however, the reaction times were significantly longer [43]. In trial reactions, phencyclone and phenylacetylene were refluxed together in CPME until the green colour of phencyclone was no longer visible. The reactions were typically complete within 50–60 min, and the crude product often precipitated during cooling. The best method for purification was found to increase the volume of CPME in the flask (twice or three times the volume) then heat the mixture to reflux. Methanol was then added dropwise down the reflux condenser until the solution became slightly turbid. On cooling, a crystalline product formed, and this was readily isolated by filtration.
4. Discussion
All of the reactions of phenylacetylene with tetracyclone and phencyclone in the renewable solvents highlighted in Table 1 resulted in pentaphenylbenzene (5) and 1,2,4-triphenyltriphenylene (6) as products. The spectroscopic and physical properties of the products from the reactions reported herein are consistent with pentaphenylbenzene (5) and 1,2,4-triphenyltriphenylene (6) rather than norbornadien-7-ones 4 and 8 (Scheme 1 and Scheme 2, Figure 4). If norbornadien-7-ones were isolated, they would be expected to have 13C NMR spectra containing signals at ~200 ppm due to having a bridgehead carbonyl carbon. The IR spectra of norbornadiene-7-ones and related compounds are also very distinctive; the stretching frequency of the C=O bond usually occurs in the 1780–1800 cm−1 range due to angle compression [44]. The products isolated during our study did not show any spectroscopic evidence for a C=O group being present in the NMR or IR spectra.
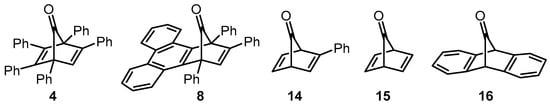
Figure 4.
Example norbornadien-7-one compounds.
The results of this study are not unexpected, since norbornadien-7-one derivatives such as 4 and 8 are known to be unstable. The cheletropic release of carbon monoxide is likely to follow rapidly after the initial [4 + 2] cycloaddition reaction has taken place [44,45]. Some early studies have claimed the successful isolation of norbornadien-7-one derivatives (14 for example), although subsequent investigations have revealed that norbornadien-7-one 15 is particularly unstable [44,45,46,47]. However, it has been reported that dibenzo analogues such as 16 are stable enough to be isolated at room temperature [46,47]. Although intermediates 4 and 8 are partially benzo-fused, it is apparent from the results that we have obtained that they are not stabilized enough to be isolated. Pentaphenylbenzene (5) and 1,2,4-triphenyltriphenylene (6) were the sole isolated products; therefore, these observations align with the results of Dilthey’s original study of the reaction of phencyclone with phenylacetylene [27]. The results reported herein indicate that the renewable solvents examined in this study can be used in place of petrochemical-derived solvents for Diels–Alder/cheletropic reaction sequences to prepare compounds 5 and 6. In addition to the use of solvents that can be obtained from sustainable feedstocks, the methods developed in this study also reduce the amount of solvent used in the purification steps compared to previous protocols [7,9,10,12]. Previous routes to pentaphenylbenzene (5) that involve the reaction of tetracyclone with phenylacetylene have employed flash column chromatography on silica to purify the product [7,9]. This method of purification uses larger volumes of solvent in comparison to the crystallization process described in this work. In contrast, previous methods for the preparation of 1,2,4-triphenyltriphenylene (6) from the reaction of phencyclone with phenylacetylene do not rely on chromatography techniques for purification of the product [10,12,27]. However, crude samples of 6 are reported to be precipitated or recrystallised from chlorinated solvents (such as chlorobenzene). As part of the work reported herein, modified purification methods for compound 6 have been described that avoid the need to use undesirable chlorinated solvents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/reactions6030041/s1, Figure S1: 500 MHz (CDCl3) 1H NMR spectrum of compound 5 (with expansion); Figure S2: 126 MHz (CDCl3) 13C DEPTQ NMR spectrum of compound 5 (with expansion); Figure S3: 500 MHz (CDCl3) Stacked plot of 1H NMR spectra obtained from crude samples of compound 5; Figure S4: 500 MHz (CDCl3) 1H NMR spectrum of compound 6 (with expansion); Figure S5: 126 MHz (CDCl3) 13C DEPTQ NMR spectrum of compound 6 (with expansion); Figure S6: 500 MHz (CDCl3) Stacked plot of 1H NMR spectra obtained from crude samples of compound 6; Figure S7: ATR-IR spectrum of compound 5; Figure S8: ATR-IR spectrum of compound 6. File S1: diels-alder_2025_checkcif; Video S1: Recording 2025-06-20 134727 trimmed.
Author Contributions
Synthetic steps, crystallization trials, and preliminary analysis have been conducted by S.A., H.B., B.A.C., R.M.D., L.E., T.V.F., D.K., I.A.S., and I.L.J.P.; D.B.C. collected the x-ray data and solved the structure; D.B.C., I.L.J.P., and I.A.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All supplementary data is provided as “Supplementary Materials”. The X-ray structural data can be obtained from The Cambridge Crystallographic Data Centre (www.ccdc.cam.ac.uk/structures, accessed on 25 June 2025) as deposition number 2465931.
Acknowledgments
The authors express gratitude to the University of St Andrews School of Chemistry for use of laboratory facilities and provision of materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ATR | Attenuated Reflectance Spectroscopy |
| CPME | Cyclopropyl Methyl Ether |
| DCM | Dichloromethane |
| IR | Infrared |
| 2-MeTHF | 2-Methyltetrahydrofuran |
| NMR | Nuclear Magnetic Resonance |
References
- Karunakaran, J.; Qiu, H.; Balaraman, E. Synthesis of diverse heterocyclic frameworks using cyclopentadienones via the Diels-Alder strategy. Org. Chem. Front. 2021, 8, 5608–5650. [Google Scholar] [CrossRef]
- Dyan, O.T.; Borodkin, G.I.; Zaikin, P.A. The Diels–Alder Reaction for the Synthesis of Polycyclic Aromatic Compounds. Eur. J. Org. Chem. 2019, 2019, 7271–7306. [Google Scholar] [CrossRef]
- Krompiec, S.; Kurpanik-Wójcik, A.; Matussek, M.; Gołek, B.; Mieszczanin, A.; Fijołek, A. Diels–Alder Cycloaddition with CO, CO2, SO2, or N2 Extrusion: A Powerful Tool for Material Chemistry. Materials 2022, 15, 172. [Google Scholar] [CrossRef]
- Bergman, H.M.; Beattie, D.D.; Kiel, G.R.; Handford, R.C.; Liu, Y.; Tilley, T.D. A sequential cyclization/π-extension strategy for modular construction of nanographenes enabled by stannole cycloadditions. Chem. Sci. 2022, 13, 5568–5573. [Google Scholar] [CrossRef]
- Vij, V.; Bhalla, V.; Kumar, M. Hexaarylbenzene: Evolution of Properties and Applications of Multitalented Scaffold. Chem. Rev. 2016, 116, 9565–9627. [Google Scholar] [CrossRef]
- Fieser, L.F. Hexaphenylbenzene. Org. Synth. 1966, 46, 44. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Xie, H.; Feng, L.; Lu, H.; Feng, S. Emission enhancement and color adjustment of silicon-cored structure in tetraphenylbenzene with aggregation-enhanced emission. J. Mater. Chem. C 2014, 2, 5601–5606. [Google Scholar] [CrossRef]
- Dusold, C.; Weiss, C.; Hampel, F.; Hirsch, A. Synthesis and crystal packing of perylene-derivatives with extreme sterically demanding pentaphenylbenzene bay-substituents. Chem. Commun. 2021, 57, 583–586. [Google Scholar] [CrossRef]
- Li, L.; Chen, M.; Zhang, H.; Nie, H.; Sun, J.Z.; Qin, A.; Tang, B.Z. Influence of the number and substitution position of phenyl groups on the aggregation-enhanced emission of benzene-cored luminogens. Chem. Commun. 2015, 51, 4830–4833. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jeong, S.J.; Lee, H.W.; Kim, J.; Lee, S.E.; Kim, Y.K.; Yoon, S.S. Efficient blue organic light-emitting diodes using N2,N2,N11,N11,5,6,7,8-octaphenyltriphenylene-2,11-diamine derivatives. Luminescence 2016, 31, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Zhou, G.; Scheiber, H.; Bauer, R.E.; Baumgarten, M.; Anson, C.E.; List, E.J.W.; Müllen, K. Polytriphenylene Dendrimers: A Unique Design for Blue-Light-Emitting Materials. Angew. Chem. Int. Ed. 2008, 47, 8292–8296. [Google Scholar] [CrossRef] [PubMed]
- Wettach, H.; Jester, S.S.; Colsmann, A.; Lemmer, U.; Rehmann, N.; Meerholz, K.; Hoger, S. Deep blue organic light-emitting diodes based on triphenylenes. Synth. Met. 2010, 160, 691–700. [Google Scholar] [CrossRef]
- Batsyts, S.; Hubner, E.G.; Namyslo, J.C.; Gjikaj, M.; Schmidt, A. Synthesis and characterization of propellor-shaped mono to hexacationic quinolinium-substituted benzenes. Org. Biomol. Chem. 2019, 17, 4102–4114. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical-and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Sherwood, J.; De bruyn, M.; Constantinou, A.; Moity, L.; McElroy, C.R.; Farmer, T.J.; Duncan, T.; Raverty, W.; Hunt, A.J.; Clark, J.H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar] [CrossRef]
- Stini, N.A.; Gkizis, P.L.; Kokotos, C.G. Cyrene: A bio-based novel and sustainable solvent for organic synthesis. Green Chem. 2022, 24, 6435–6449. [Google Scholar] [CrossRef]
- Leita, B.A.; Warden, A.C.; Burke, N.; O’Shea, M.S.; Trimm, D. Production of p-cymene and hydrogen from a bio-renewable feedstock-1,8-cineole (eucalyptus oil). Green Chem. 2010, 12, 70–76. [Google Scholar] [CrossRef]
- Ye, L.; Thompson, B.C. p-Cymene: A Sustainable Solvent that is Highly Compatible with Direct Arylation Polymerization (DArP). ACS Macro Lett. 2021, 10, 714–719. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Aparicio, S.; Alcalde, R. The green solvent ethyl lactate: An experimental and theoretical characterization. Green Chem. 2009, 11, 65–78. [Google Scholar] [CrossRef]
- Shukla, K.; Srivastava, V.C. Diethyl carbonate: Critical review of synthesis routes, catalysts used and engineering aspects. RSC Adv. 2016, 6, 32624–32645. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Zabrocka, W.; Bystrzanowska. Diethyl carbonate as a green extraction solvent for chlorophenol determination with dispersive liquid–liquid microextraction. Anal. Methods 2019, 11, 844–850. [Google Scholar] [CrossRef]
- Harrison, E.A. The importance of temperature control in the preparation of phencyclone (1,3-diphenyl-2-H-cyclopenta[I]phenanthrene-2-one). J. Chem. Educ. 1992, 69, 571. [Google Scholar] [CrossRef]
- Allen, C.F.H.; Bell, A. β-Nitrostyrene in the Diene Synthesis. J. Am. Chem. Soc. 1939, 61, 521–522. [Google Scholar] [CrossRef]
- Dilthey, W.; Henkels, S.; Schaefer, A. Hocharylierte aromatische Verbindungen (VI. Mitteil.). Berichte Der Dtsch. Chem. Ges. A/B 1938, 71, 974–979. [Google Scholar] [CrossRef]
- CrysAlisPro, v1.171.38.46. Rigaku Oxford Diffraction. Rigaku Corporation: Oxford, UK, 2015.
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B. Organic Solvents: Physical Properties and Methods of Purification, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1970; pp. 229–230. [Google Scholar]
- Dudkowski, J.J.; Becker, E.I. Electronic Effects and Rates in the Diels-Alder Reaction. J. Org. Chem. 1952, 17, 201–206. [Google Scholar] [CrossRef]
- Thiemann, T.; Iniesta, J.; Walton, D.J. Thermal oxidation of tetracyclones (2,3,4,5-tetraarylcyclopentadienones). J. Chem. Res. 2008, 2008, 173–180. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Castoldi, L.; Domínguez de María, P.; Alcántara, A.R. Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Alcántara, A.R.; Domínguez de María, P. Cyclopentyl Methyl Ether (CPME): A Versatile Eco-Friendly Solvent for Applications in Biotechnology and Biorefineries. ChemSusChem 2019, 12, 2083. [Google Scholar] [CrossRef]
- MacLeod, M.; Mackay, D. An assessment of the environmental fate and exposure of benzene and the chlorobenzenes in Canada. Chemosphere 1999, 38, 1777–1796. [Google Scholar] [CrossRef] [PubMed]
- Wooi, R.G.Y.; White, J.M. CCDC 1020300. CSD Communication 2014. [Google Scholar] [CrossRef]
- Pascal, R.A.; Van Engen, D.; Kahr, B.; McMillan, W.D. Twisted Polycyclic Aromatic Hydrocarbons. Structural Studies of Protic and Deuteriated 1,2,3,4-Tetraphenyltriphenylenes. J. Org. Chem. 1988, 53, 1687–1689. [Google Scholar] [CrossRef]
- Umezawa, Y.; Tsuboyama, S.; Honda, K.; Uzawa, J.; Nishio, M. CH/π Interaction in the Crystal Structure of Organic Compounds. A Database Study. Bull. Chem. Soc. Jpn. 1998, 71, 1207–1213. [Google Scholar] [CrossRef]
- Ogliaruso, M.A.; Romanelli, M.C.; Becker, E.I. Chemistry of Cyclopentadienones. Chem. Rev. 1965, 65, 261–367. [Google Scholar] [CrossRef]
- Yankelevich, S.; Fuchs, B. On Alleged Norbornadien-7-ones. Tetrahedron Lett. 1967, 49, 4945–4949. [Google Scholar] [CrossRef]
- LeBlanc, B.F.; Sheridan, R.S. Photochemical Generation and Direct Observation of 7-Norbornadienone. J. Am. Chem. Soc. 1985, 107, 4554–4556. [Google Scholar] [CrossRef]
- Birney, D.M.; Wiberg, K.B.; Berson, J.A. Geometry of the Transition State of the Decarbonylation of Bicyclo[2.2.1]hepta-2,5-dien-7-one. Experimental and ab Initio Theoretical Studies. J. Am. Chem. Soc. 1988, 110, 6631–6642. [Google Scholar] [CrossRef]
- Lai, C.-H.; Li, E.Y.; Chen, K.-Y.; Chow, T.J.; Chou, P.-T. Theoretical Investigation of Cheletropic Decarbonylation Reactions. J. Chem. Theory Comput. 2006, 2, 1078–1084. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).