Copper-Catalyzed Sonogashira-Type Coupling Reaction of Vinylacetylene ortho-Carborane with Boronic Acid in the Synthesis of Luminophores with Phosphorescent Emission
Abstract
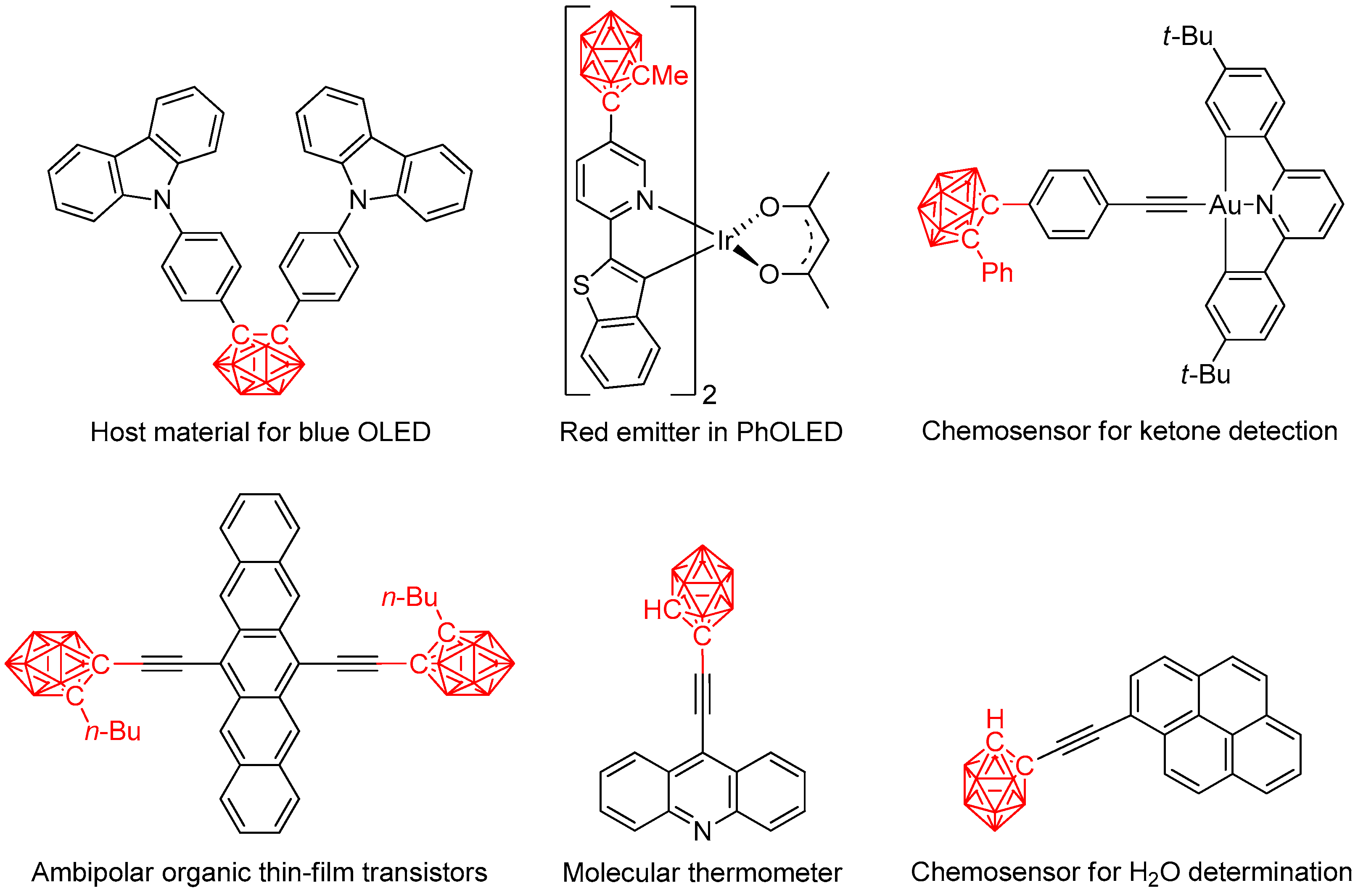
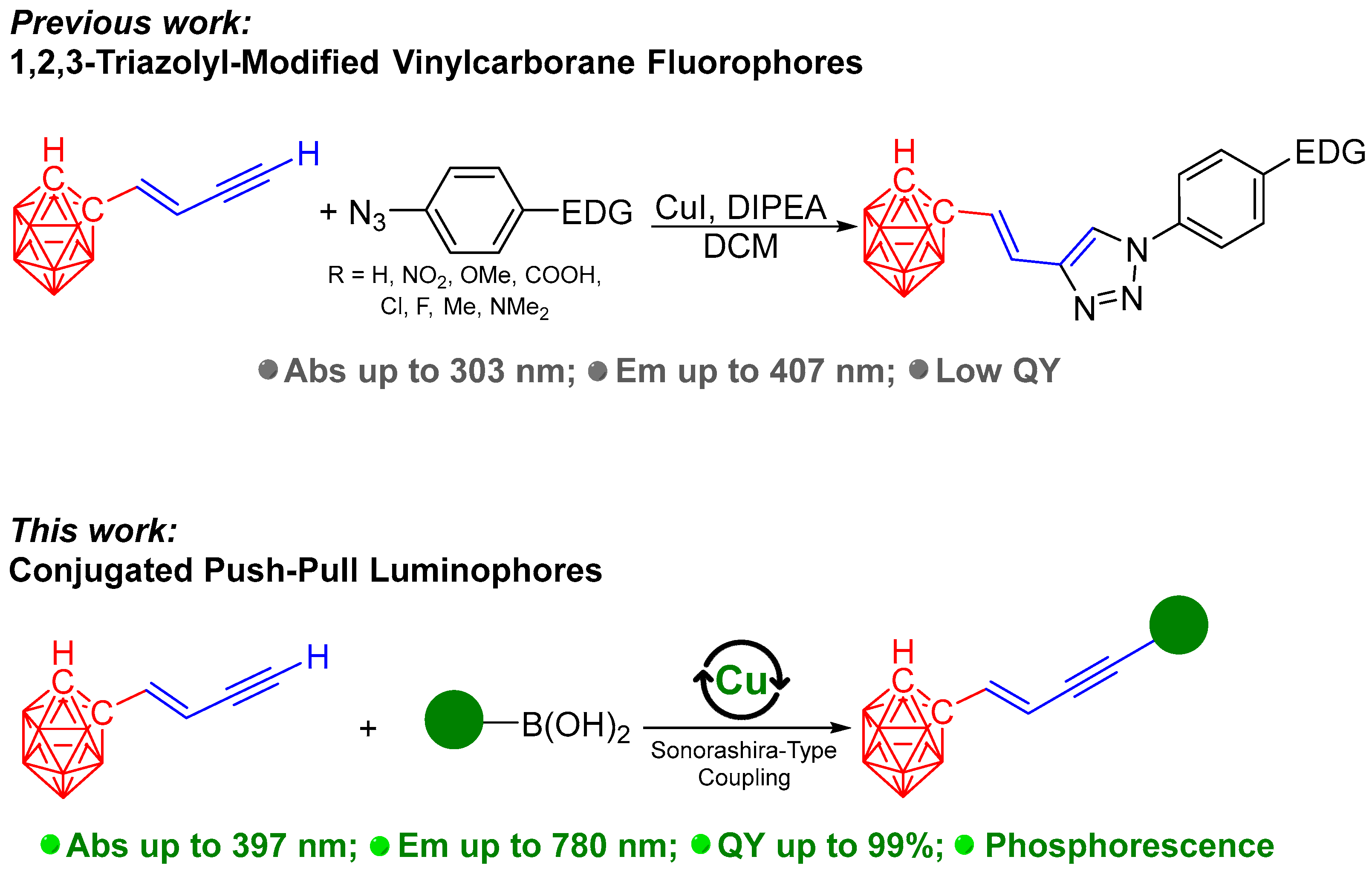
1. Introduction
2. Materials and Methods
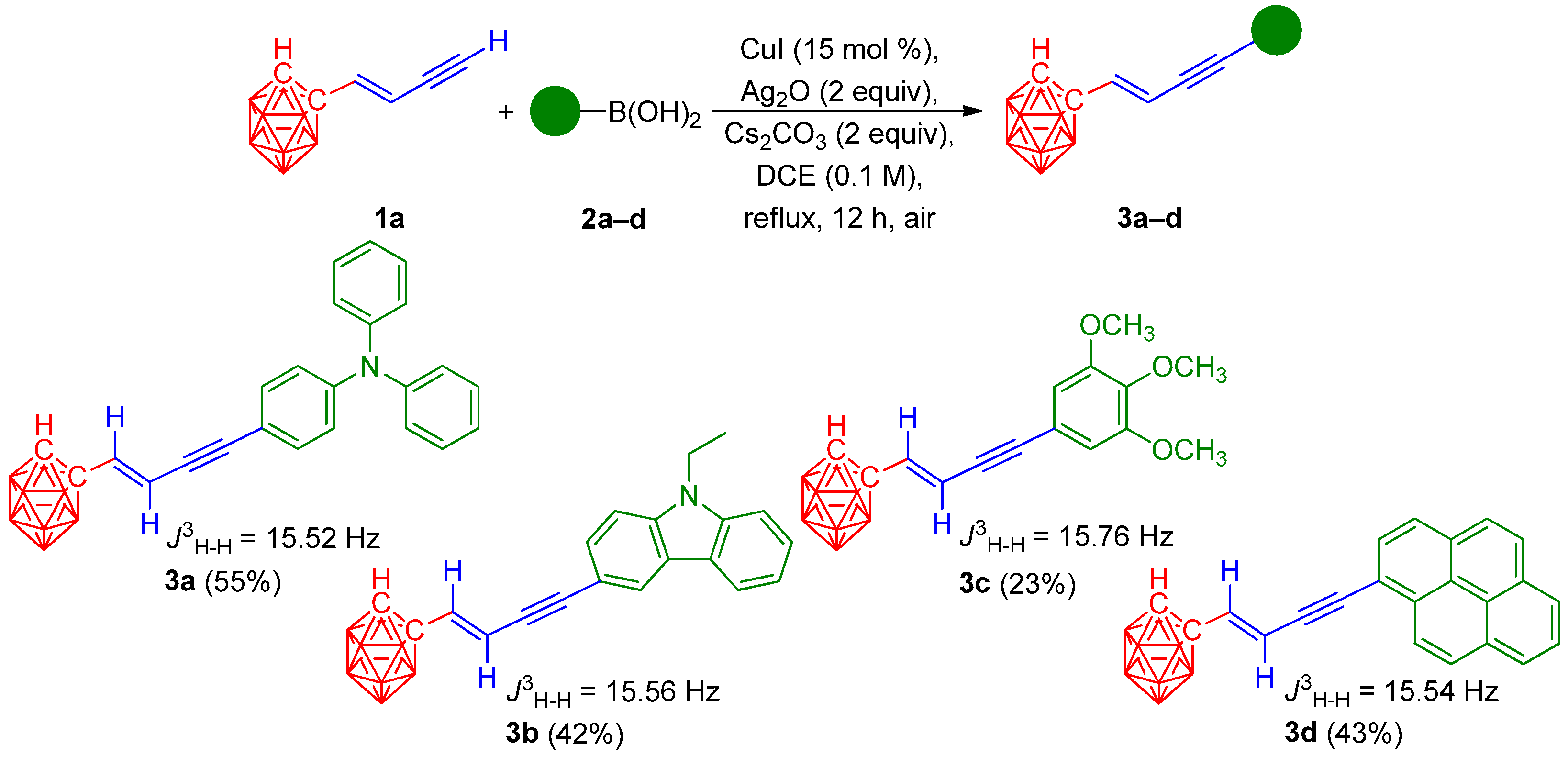
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lipscomb, W.N. Boron Hydrides; Dover, Ed.; Dover Publications: Mineola, NY, USA, 2012; ISBN 978-0-486-48822-6. [Google Scholar]
- Muetterties, E.L. Boron Hydride Chemistry; Academic Press: New York, NY, USA, 1975; ISBN 978-0-12-509650-8. [Google Scholar]
- Electron Deficient Boron and Carbon Clusters; Olah, G.A., Wade, K., Eds.; A Wiley-Interscience Publication; Wiley: Hoboken, NY, USA, 1991; ISBN 978-0-471-52795-4. [Google Scholar]
- Wee, K.-R.; Cho, Y.-J.; Jeong, S.; Kwon, S.; Lee, J.-D.; Suh, I.-H.; Kang, S.O. Carborane-Based Optoelectronically Active Organic Molecules: Wide Band Gap Host Materials for Blue Phosphorescence. J. Am. Chem. Soc. 2012, 134, 17982–17990. [Google Scholar] [CrossRef] [PubMed]
- Furue, R.; Nishimoto, T.; Park, I.S.; Lee, J.; Yasuda, T. Aggregation-Induced Delayed Fluorescence Based on Donor/Acceptor-Tethered Janus Carborane Triads: Unique Photophysical Properties of Nondoped OLEDs. Angew. Chem. Int. Ed. 2016, 55, 7171–7175. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.J.; Chung, J.; Kim, H.; Park, J.; Lee, K.M.; Koh, T.-W.; Lee, Y.S.; Yoo, S.; Do, Y.; Lee, M.H. Deep Red Phosphorescence of Cyclometalated Iridium Complexes by o-Carborane Substitution. Inorg. Chem. 2014, 53, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shi, C.; Huang, M.; Zhang, X.; Sun, F.; Zheng, Y.; Yan, H.; Yang, C.; Yuan, A. Three Types of Charged Ligand-Based Neutral Phosphorescent Iridium(III) Complexes Featuring Nido-Carborane: Synthesis, Structures, and Solution Processed Organic Light-Emitting Diode Applications. Dalton Trans. 2021, 50, 16304–16310. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thilagar, P. Boron Clusters in Luminescent Materials. Chem. Commun. 2016, 52, 1070–1093. [Google Scholar] [CrossRef]
- Kirlikovali, K.O.; Axtell, J.C.; Gonzalez, A.; Phung, A.C.; Khan, S.I.; Spokoyny, A.M. Luminescent Metal Complexes Featuring Photophysically Innocent Boron Cluster Ligands. Chem. Sci. 2016, 7, 5132–5138. [Google Scholar] [CrossRef]
- Shafikov, M.Z.; Suleymanova, A.F.; Czerwieniec, R.; Yersin, H. Design Strategy for Ag(I)-Based Thermally Activated Delayed Fluorescence Reaching an Efficiency Breakthrough. Chem. Mater. 2017, 29, 1708–1715. [Google Scholar] [CrossRef]
- Li, W.; Yan, X.; Zhang, H.; He, R.; Li, M.; Shen, W. Revealing the Unique Properties of Platinum(II) Complexes with Bidentate Bis(o -carborane) Ligands. Eur. J. Inorg. Chem. 2018, 2018, 99–108. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, Q.; Cheng, H.; Sha, Y.; Bregadze, V.I.; Yan, H.; Sun, Z.; Li, X. Boron-Cluster Embedded Necklace-Shaped Nanohoops. Angew. Chem. Int. Ed. 2023, 62, e202213470. [Google Scholar] [CrossRef]
- Guo, J.; Liu, D.; Zhang, J.; Zhang, J.; Miao, Q.; Xie, Z. o-Carborane Functionalized Pentacenes: Synthesis, Molecular Packing and Ambipolar Organic Thin-Film Transistors. Chem. Commun. 2015, 51, 12004–12007. [Google Scholar] [CrossRef]
- Nar, I.; Atsay, A.; Altındal, A.; Hamuryudan, E. o-Carborane, Ferrocene, and Phthalocyanine Triad for High-Mobility Organic Field-Effect Transistors. Inorg. Chem. 2018, 57, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Eo, M.; Bae, H.J.; Hong, M.; Do, Y.; Cho, S.; Lee, M.H. Synthesis and Electron Transporting Properties of Methanofullerene-o-Carborane Dyads in Organic Field-Effect Transistors. Dalton Trans. 2013, 42, 8104. [Google Scholar] [CrossRef] [PubMed]
- Aniés, F.; Qiao, Z.; Nugraha, M.I.; Basu, A.; Anthopoulos, T.D.; Gasparini, N.; Heeney, M. N-Type Polymer Semiconductors Incorporating Para, Meta, and Ortho-Carborane in the Conjugated Backbone. Polymer 2022, 240, 124481. [Google Scholar] [CrossRef]
- Aniés, F.; Furlan, F.; Qiao, Z.; Pirela, V.; Bidwell, M.; Rimmele, M.; Martín, J.; Gasparini, N.; Heeney, M. A Comparison of Para, Meta, and Ortho-Carborane Centred Non-Fullerene Acceptors for Organic Solar Cells. J. Mater. Chem. C 2023, 11, 3989–3996. [Google Scholar] [CrossRef]
- Ye, F.; Zhang, S.; Warby, J.; Wu, J.; Gutierrez-Partida, E.; Lang, F.; Shah, S.; Saglamkaya, E.; Sun, B.; Zu, F.; et al. Overcoming C60-Induced Interfacial Recombination in Inverted Perovskite Solar Cells by Electron-Transporting Carborane. Nat. Commun. 2022, 13, 7454. [Google Scholar] [CrossRef]
- Spokoyny, A.M.; Li, T.C.; Farha, O.K.; Machan, C.W.; She, C.; Stern, C.L.; Marks, T.J.; Hupp, J.T.; Mirkin, C.A. Electronic Tuning of Nickel-Based Bis(Dicarbollide) Redox Shuttles in Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2010, 49, 5339–5343. [Google Scholar] [CrossRef]
- Marshall, J.; Hooton, J.; Han, Y.; Creamer, A.; Ashraf, R.S.; Porte, Y.; Anthopoulos, T.D.; Stavrinou, P.N.; McLachlan, M.A.; Bronstein, H.; et al. Polythiophenes with Vinylene Linked Ortho, Meta and Para-Carborane Sidechains. Polym. Chem. 2014, 5, 6190–6199. [Google Scholar] [CrossRef]
- Şener, S. Ball-Type Dioxy-o-Carborane Bridged Cobaltphthalocyanine: Synthesis, Characterization and DFT Studies For Dye-Sensitized Solar Cells as Photosensitizer. Heterocycl. Commun. 2020, 26, 37–45. [Google Scholar] [CrossRef]
- Kim, B.G.; Jang, W.; Jeon, H.; Lee, S.; Han, W.-S.; Wang, D.H. An Unusual Charge Transfer Accelerator of Monomolecular Cb-OMe (4,4’-(Ortho-Carborane)Bis(N,N-Bis(4-Methoxyphenyl)Aniline) in Perovskite Optoelectronic Devices. Sol. Energy Mater. Sol. Cells 2020, 208, 110414. [Google Scholar] [CrossRef]
- Ochi, J.; Tanaka, K.; Chujo, Y. Dimerization-Induced Solid-State Excimer Emission Showing Consecutive Thermochromic Luminescence Based on Acridine-Modified o-Carboranes. Inorg. Chem. 2021, 60, 8990–8997. [Google Scholar] [CrossRef]
- Peper, S.; Telting-Diaz, M.; Almond, P.; Albrecht-Schmitt, T.; Bakker, E. Perbrominated Closo-Dodecacarborane Anion, 1-HCB11Br11−, as an Ion Exchanger in Cation-Selective Chemical Sensors. Anal. Chem. 2002, 74, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Prokhorov, A.M.; Hofbeck, T.; Czerwieniec, R.; Suleymanova, A.F.; Kozhevnikov, D.N.; Yersin, H. Brightly Luminescent Pt(II) Pincer Complexes with a Sterically Demanding Carboranyl-Phenylpyridine Ligand: A New Material Class for Diverse Optoelectronic Applications. J. Am. Chem. Soc. 2014, 136, 9637–9642. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Yamamoto, H.; Ochi, J.; Tanaka, K.; Chujo, Y. Time-Dependent Emission Enhancement of the Ethynylpyrene-o-Carborane Dyad and Its Application as a Luminescent Color Sensor for Evaluating Water Contents in Organic Solvents. Chem. Asian J. 2019, 14, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, K.; Liu, Z.; Wang, Z.; Hua, C.; Liu, T.; Fang, Y. High-Performance Ketone Sensing in Vapor Phase Enabled by o-Carborane-Modified Cyclometalated Alkynyl-Gold(III) Complex-Based Fluorescent Films. ACS Appl. Mater. Interfaces 2021, 13, 5625–5633. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Du, F.; Tang, L.; Xu, J.; Zhao, Y.; Wu, X.; Li, M.; Shen, J.; Wen, Q.; Cho, C.H.; et al. Carboranes as Unique Pharmacophores in Antitumor Medicinal Chemistry. Mol. Ther. Oncolytics 2022, 24, 400–416. [Google Scholar] [CrossRef]
- Conway-Kenny, R.; Ferrer-Ugalde, A.; Careta, O.; Cui, X.; Zhao, J.; Nogués, C.; Núñez, R.; Cabrera-González, J.; Draper, S.M. Ru(II) and Ir(III) Phenanthroline-Based Photosensitisers Bearing o-Carborane: PDT Agents with Boron Carriers for Potential BNCT. Biomater. Sci. 2021, 9, 5691–5702. [Google Scholar] [CrossRef]
- Zaitsev, A.V.; Alpatova, V.M.; Ol’shevskaya, V.A. Photosensitizers Based on Carborane Conjugates of Meso-Arylporphyrins/Chlorins and Dipyrromethenes for Photodynamic and Boron Neutron Capture Therapies. INEOS OPEN 2021, 3, 188–199. [Google Scholar] [CrossRef]
- Ol’shevskaya, V.A.; Nikitina, R.G.; Savchenko, A.N.; Malshakova, M.V.; Vinogradov, A.M.; Golovina, G.V.; Belykh, D.V.; Kutchin, A.V.; Kaplan, M.A.; Kalinin, V.N.; et al. Novel Boronated Chlorin E6-Based Photosensitizers: Synthesis, Binding to Albumin and Antitumour Efficacy. Bioorg. Med. Chem. 2009, 17, 1297–1306. [Google Scholar] [CrossRef]
- Grimes, R.N. Carboranes in Catalysis. In Carboranes; Elsevier: Amsterdam, The Netherlands, 2016; pp. 929–944. ISBN 978-0-12-801894-1. [Google Scholar]
- Teixidor, F.; Viñas, C.; Planas, J.G.; Romero, I.; Núñez, R. Advances in the Catalytic and Photocatalytic Behavior of Carborane Derived Metal Complexes. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 2022; Volume 71, pp. 1–45. ISBN 978-0-323-98831-5. [Google Scholar]
- Gunther, S.O.; Lai, Q.; Senecal, T.; Huacuja, R.; Bremer, S.; Pearson, D.M.; DeMott, J.C.; Bhuvanesh, N.; Ozerov, O.V.; Klosin, J. Highly Efficient Carborane-Based Activators for Molecular Olefin Polymerization Catalysts. ACS Catal. 2021, 11, 3335–3342. [Google Scholar] [CrossRef]
- Moseev, T.D.; Varaksin, M.V.; Gorlov, D.A.; Charushin, V.N.; Chupakhin, O.N. Transition-Metal-Free C–H/C–Li Coupling of Nonaromatic 2H-Imidazole 1-Oxides with Pentafluorophenyl Lithium in the Design of Novel Fluorophores with Intramolecular Charge Transfer Effect. J. Org. Chem. 2020, 85, 11124–11133. [Google Scholar] [CrossRef]
- Umeno, T.; Seto, R.; Matsumoto, S.; Fujihara, M.; Karasawa, S. Basic Fluorescent Protonation-Type pH Probe Sensitive to Small ΔpKa of Methanol and Ethanol. Anal. Chem. 2022, 94, 10400–10407. [Google Scholar] [CrossRef] [PubMed]
- Durko-Maciag, M.; Popczyk, A.; Rémond, M.; Zheng, Z.; Bretonniere, Y.; Andraud, C.; Mysliwiec, J. Real-Time Tunable Red/Near-Infrared Solid-State Emitters in the First Biological Window: 9,9-Diethyl-2-diphenylaminofluorene-Based Push-Pull Fluorophores for Distributed Feedback and Random Lasing Applications. ChemPhotoChem 2022, 6, e202200008. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Gadirov, R.M.; Samsonova, L.G.; Degtyarenko, K.M.; Kurtcevich, A.E.; Sapozhnikova, E.V.; Medvedeva, M.V.; Svalova, T.S.; Kozitsina, A.N.; Rusinov, G.L.; et al. Impact of an Ortho-Cyano Group on Photophysical Properties and Performance of OLEDs Based on D-A–A Type Pyrazine Push-Pull System. Dye Pigment. 2022, 207, 110716. [Google Scholar] [CrossRef]
- Gomes, L.J.; Carrilho, J.P.; Pereira, P.M.; Moro, A.J. A Near InfraRed Emissive Chemosensor for Zn2+ and Phosphate Derivatives Based on a Di-(2-Picolyl)Amine-Styrylflavylium Push-Pull Fluorophore. Sensors 2023, 23, 471. [Google Scholar] [CrossRef] [PubMed]
- Lavrinchenko, I.A.; Moseev, T.D.; Seleznev, Y.A.; Varaksin, M.V.; Tsmokaluk, A.N.; Charushin, V.N.; Chupakhin, O.N. Blue-Emitting 2-Fluoroaryl-1,2,3-Triazole Fluorophores: Synthesis, Theoretical Calculations, and Optical Properties. Asian J. Org. Chem. 2023, 12, e202300008. [Google Scholar] [CrossRef]
- Głodek, M.; Petrusevich, E.F.; Plażuk, D.; Jacquemin, D.; Ośmiałowski, B. Polyaromatic Hydrocarbon Antennas as Tools for Tuning Properties of Push-Pull Difluoroborates. Dye Pigment. 2023, 212, 111112. [Google Scholar] [CrossRef]
- Pandey, D.; Imran, K.; Kumar Yadav, R.; Kaur, J.; Naqvi, S.; Sharma, A. Push-Pull Intramolecular Charge Transfer Solvatofluorochromic Fluorophore for the Selective and Real-Time Detection of Hydrazine. Microchem. J. 2023, 191, 108912. [Google Scholar] [CrossRef]
- Lavrinchenko, I.A.; Moseev, T.D.; Varaksin, M.V.; Zyryanov, G.V.; Taniya, O.S.; Tsmokalyuk, A.N.; Demidov, O.P.; Borovlev, I.V.; Charushin, V.N.; Chupakhin, O.N. A BF3-Mediated C–H/C–Li Coupling of 1,3,7-Triazapyrene with 2-Thienyllithium in the Design of Push–Pull Fluorophores and Chemosensors for Nitroaromatics. New J. Chem. 2022, 46, 5121–5128. [Google Scholar] [CrossRef]
- Moseev, T.D.; Varaksin, M.V.; Virlova, E.A.; Medvedeva, M.V.; Svalova, T.S.; Melekhin, V.V.; Tsmokaluk, A.N.; Kozitsina, A.N.; Charushin, V.N.; Chupakhin, O.N. Fluoroaromatic 2H-Imidazole-Based Push-Pull Fluorophores: Synthesis, Theoretical Studies, and Application Opportunities as Probes for Sensing the pH in Saliva. Dye Pigment. 2022, 202, 110251. [Google Scholar] [CrossRef]
- Moseev, T.D.; Lavrinchenko, I.A.; Varaksin, M.V.; Pobedinskaya, D.Y.; Demidov, O.P.; Borovlev, I.V.; Charushin, V.N.; Chupakhin, O.N. Meso-Functionalization of Calix[4]Arene with 1,3,7-Triazapyrene in the Design of Novel Fluorophores with the Dual Target Detection of Al3+ and Fe3+ Cations. RSC Adv. 2021, 11, 6407–6414. [Google Scholar] [CrossRef]
- Sakr, A.R.; Georgiev, N.I.; Bojinov, V.B. Design and Synthesis of a Novel ICT Bichromophoric pH Sensing System Based on 1,8-Naphthalimide Fluorophores as a Two-Input Logic Gate and Its Antibacterial Evaluation. Molecules 2023, 28, 3631. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Yu, C.P.; Zhang, G.; Hillier, V.E.; Chan, J.M.W. Pulling with the Pentafluorosulfanyl Acceptor in Push–Pull Dyes. J. Org. Chem. 2017, 82, 11008–11020. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xiao, R.; Feng, B.; Fan, D.; Huang, S.; Bi, A.; Zhong, S.; Feng, X.; Liu, S.; Zeng, W. Elegant Cooperation of AIE, ESIPT and ICT Effects into Tetraarylimidazole-Based Fluorophore and Its Tuning for Ratiometric Detection of Carbon Monoxide. Sens. Actuators B Chem. 2021, 342, 130038. [Google Scholar] [CrossRef]
- Bellomo, C.; Zanetti, D.; Cardano, F.; Sinha, S.; Chaari, M.; Fin, A.; Maranzana, A.; Núñez, R.; Blangetti, M.; Prandi, C. Red Light-Emitting Carborane-BODIPY Dyes: Synthesis and Properties of Visible-Light Tuned Fluorophores with Enhanced Boron Content. Dye Pigment. 2021, 194, 109644. [Google Scholar] [CrossRef]
- Frankær, C.G.; Rosenberg, M.; Santella, M.; Hussain, K.J.; Laursen, B.W.; Sørensen, T.J. Tuning the pKa of a pH Responsive Fluorophore and the Consequences for Calibration of Optical Sensors Based on a Single Fluorophore but Multiple Receptors. ACS Sens. 2019, 4, 764–773. [Google Scholar] [CrossRef]
- Rawat, V.; Vigalok, A. Electronic Tuning of Host-Guest Interactions within the Cavities of Fluorophore-Appended Calix[4]Arenes. Molecules 2022, 27, 5689. [Google Scholar] [CrossRef]
- Grimm, J.B.; Muthusamy, A.K.; Liang, Y.; Brown, T.A.; Lemon, W.C.; Patel, R.; Lu, R.; Macklin, J.J.; Keller, P.J.; Ji, N.; et al. A General Method to Fine-Tune Fluorophores for Live-Cell and in Vivo Imaging. Nat. Methods 2017, 14, 987–994. [Google Scholar] [CrossRef]
- Bureš, F. Fundamental Aspects of Property Tuning in Push–Pull Molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef]
- Malytskyi, V.; Simon, J.-J.; Patrone, L.; Raimundo, J.-M. Thiophene-Based Push–Pull Chromophores for Small Molecule Organic Solar Cells (SMOSCs). RSC Adv. 2015, 5, 354–397. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Brunel, D.; Dumur, F. Recent Advances on Push–Pull Organic Dyes as Visible Light Photoinitiators of Polymerization. Eur. Polym. J. 2020, 133, 109797. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Design of Fluorescent Sensors Based on Azaheterocyclic Push-Pull Systems towards Nitroaromatic Explosives and Related Compounds: A Review. Dye Pigment. 2020, 180, 108414. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Second-order Nonlinear Polarizability of “Push-Pull” Chromophores. A Decade of Progress in Donor-π-acceptor Materials. Chem. Rec. 2022, 22, e202200024. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Butenschön, H.; Misra, R. Tetracyanobutadiene Bridged Push-Pull Chromophores: Development of New Generation Optoelectronic Materials. Chem. Rec. 2023, 23, e202200208. [Google Scholar] [CrossRef] [PubMed]
- Kulhánek, J.; Bureš, F. Imidazole as a Parent π-Conjugated Backbone in Charge-Transfer Chromophores. Beilstein J. Org. Chem. 2012, 8, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Grimes, R.N. Icosahedral Carboranes. In Carboranes; Elsevier: Amsterdam, The Netherlands, 2016; pp. 283–502. ISBN 978-0-12-801894-1. [Google Scholar]
- Smyshliaeva, L.A.; Varaksin, M.V.; Fomina, E.I.; Joy, M.N.; Bakulev, V.A.; Charushin, V.N.; Chupakhin, O.N. Cu(I)-Catalyzed Cycloaddition of Vinylacetylene Ortho-Carborane and Arylazides in the Design of 1,2,3-Triazolyl-Modified Vinylcarborane Fluorophores. Organometallics 2020, 39, 3679–3688. [Google Scholar] [CrossRef]
- Galliamova, L.A.; Varaksin, M.V.; Chupakhin, O.N.; Slepukhin, P.A.; Charushin, V.N. Heterocyclic and Open-Chain Carboranes via Transition-Metal-Free C–H Functionalization of Mono- and Diazine-N-Oxides. Organometallics 2015, 34, 5285–5290. [Google Scholar] [CrossRef]
- Pan, C.; Luo, F.; Wang, W.; Ye, Z.; Cheng, J. Ligand-Free Copper(Ι)-Catalyzed Sonogashira-Type Coupling of Arylboronic Acids with Terminal Alkynes. Tetrahedron Lett. 2009, 50, 5044–5046. [Google Scholar] [CrossRef]
- Chotana, G.A.; Montero Bastidas, J.R.; Miller, S.L.; Smith, M.R.; Maleczka, R.E. One-Pot Iridium Catalyzed C–H Borylation/Sonogashira Cross-Coupling: Access to Borylated Aryl Alkynes. Molecules 2020, 25, 1754. [Google Scholar] [CrossRef]
- Saranya, S.; Saranya, P.V.; Anilkumar, G. Copper-Catalyzed Base-free Protocol for the Sonogashira-type Coupling of Phenylacetylenes with Boronic Acid Derivatives under Air. ChemistrySelect 2022, 7, e202202191. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Q.; Sun, Y.; Zhu, E.; Li, X.; Tan, H.; Chen, G.; Zheng, C. Copper-Catalyzed Umpolung Sonogashira-Type Coupling of Arene Boronic Acids under Visible Light. Chem. Commun. 2023, 59, 5043–5046. [Google Scholar] [CrossRef]
- Mohjer, F.; Mofatehnia, P.; Rangraz, Y.; Heravi, M.M. Pd-Free, Sonogashira Cross-Coupling Reaction. An Update. J. Organomet. Chem. 2021, 936, 121712. [Google Scholar] [CrossRef]
- Liori, A.A.; Stamatopoulos, I.K.; Papastavrou, A.T.; Pinaka, A.; Vougioukalakis, G.C. A Sustainable, User-Friendly Protocol for the Pd-Free Sonogashira Coupling Reaction. Eur. J. Org. Chem. 2018, 2018, 6134–6139. [Google Scholar] [CrossRef]
- Husband, J.T.; Xie, Y.; Wilks, T.R.; Male, L.; Torrent-Sucarrat, M.; Stavros, V.G.; O’Reilly, R.K. Rigidochromism by Imide Functionalisation of an Aminomaleimide Fluorophore. Chem. Sci. 2021, 12, 10550–10557. [Google Scholar] [CrossRef] [PubMed]
- Forster, L.S.; Rund, J.V. Rigidochromism in Metal-Centered Emission of Rh(III) Complexes. Inorg. Chem. Commun. 2003, 6, 78–81. [Google Scholar] [CrossRef]
- Rivera, E.J.; Barbosa, C.; Torres, R.; Rivera, H.; Fachini, E.R.; Green, T.W.; Connick, W.B.; Colón, J.L. Luminescence Rigidochromism and Redox Chemistry of Pyrazolate-Bridged Binuclear Platinum(II) Diimine Complex Intercalated into Zirconium Phosphate Layers. Inorg. Chem. 2012, 51, 2777–2784. [Google Scholar] [CrossRef]
- Norton, A.E.; Zanoni, K.P.S.; Dourges, M.-A.; Ravaro, L.P.; Abdolmaleki, M.K.; De Camargo, A.S.S.; Toupance, T.; Connick, W.B.; Chatterjee, S. Porosity Induced Rigidochromism in Platinum(II) Terpyridyl Luminophores Immobilized at Silica Composites. J. Mater. Chem. C 2021, 9, 6193–6207. [Google Scholar] [CrossRef]
- McCarthy, J.S.; McCormick, M.J.; Zimmerman, J.H.; Hambrick, H.R.; Thomas, W.M.; McMillen, C.D.; Wagenknecht, P.S. Role of the Trifluoropropynyl Ligand in Blue-Shifting Charge-Transfer States in Emissive Pt Diimine Complexes and an Investigation into the PMMA-Imposed Rigidoluminescence and Rigidochromism. Inorg. Chem. 2022, 61, 11366–11376. [Google Scholar] [CrossRef]
- Tu, D.; Leong, P.; Guo, S.; Yan, H.; Lu, C.; Zhao, Q. Highly Emissive Organic Single-Molecule White Emitters by Engineering o-Carborane-Based Luminophores. Angew. Chem. Int. Ed. 2017, 56, 11370–11374. [Google Scholar] [CrossRef]
- Nishino, K.; Uemura, K.; Tanaka, K.; Chujo, Y. Dual emission via remote control of molecular rotation of o-carborane in the excited state by the distant substituents in tolane-modified dyads. New J. Chem. 2018, 42, 4210–4214. [Google Scholar] [CrossRef]
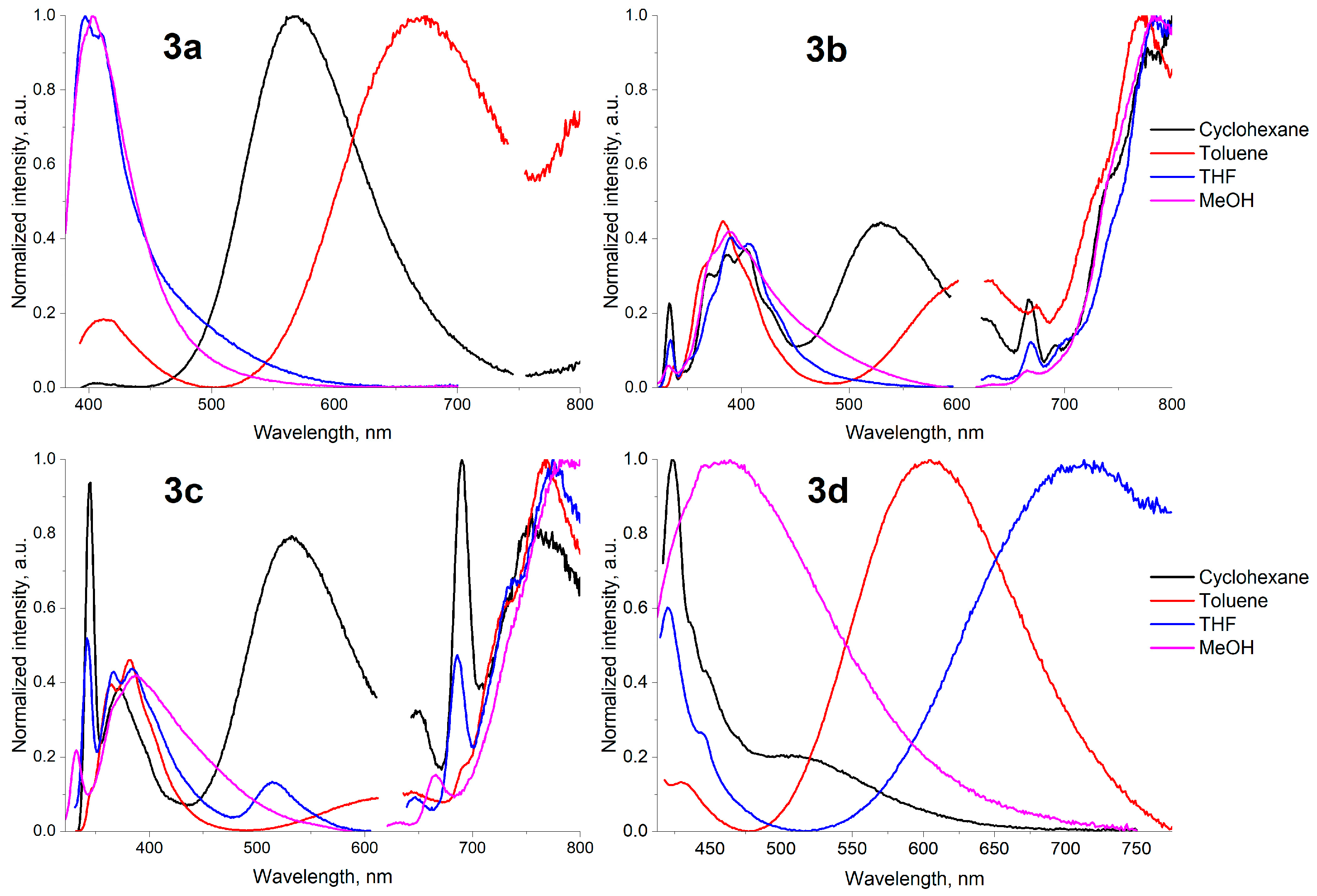
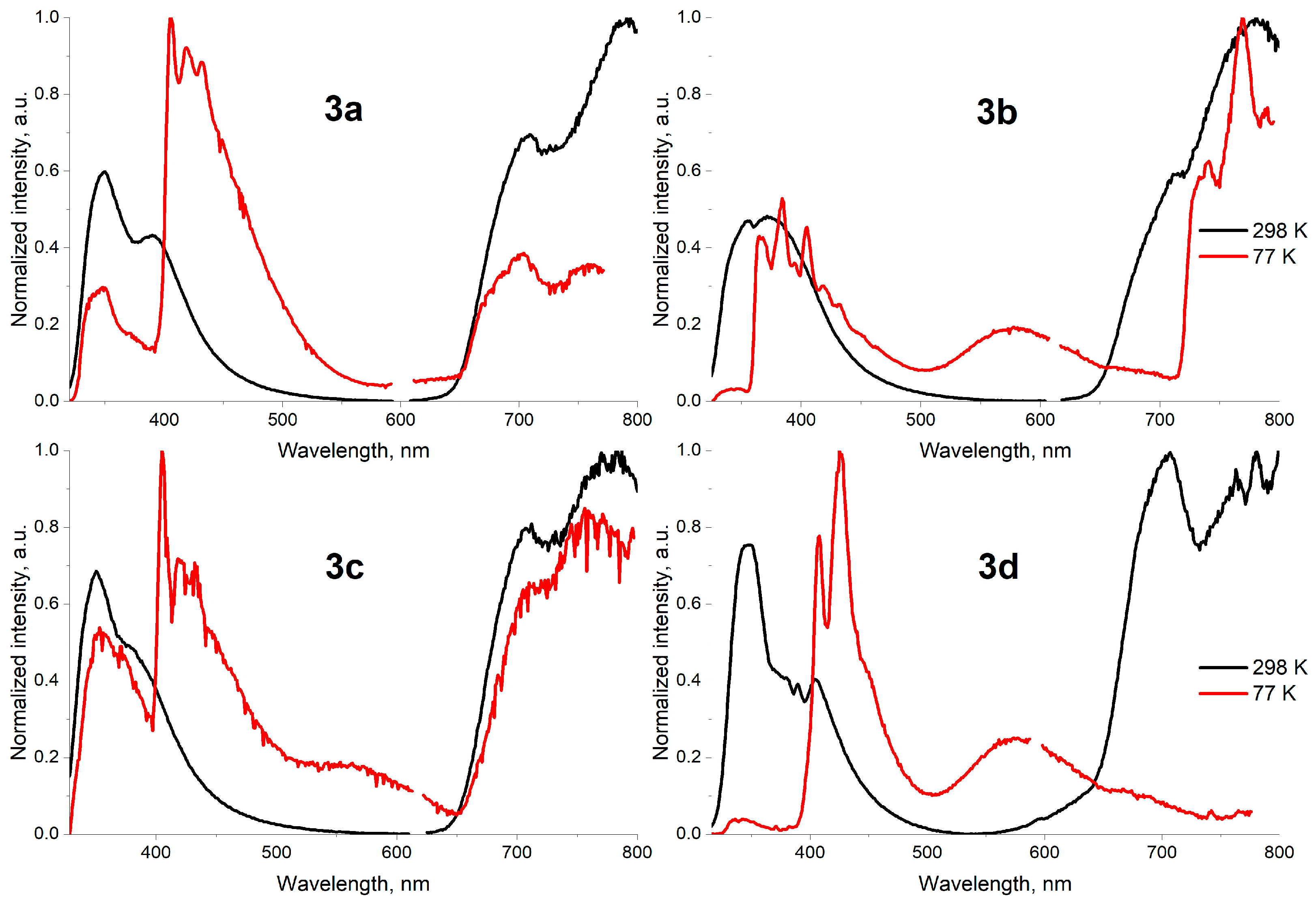
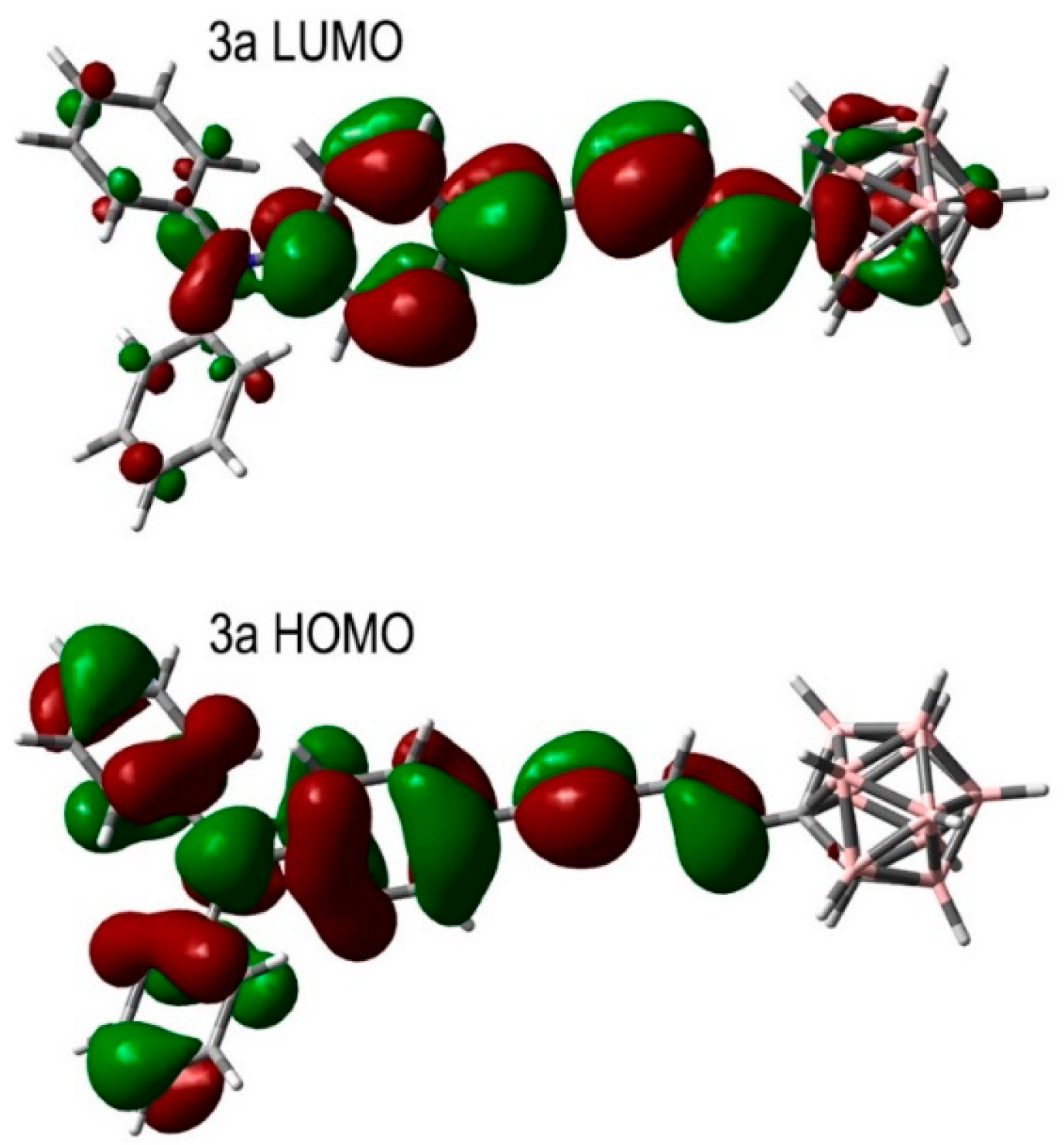
| Entry | Difference from Standard Conditions 1 | Yield 2 (%) |
|---|---|---|
| 1 | None | 60 (55 3) |
| 2 | Reflux 5 h instead of 12 h | 29 |
| 3 | NEt3 instead of Cs2CO3 | <1 |
| 4 | K2CO3 instead of Cs2CO3 | 20 |
| 5 | Ag2CO3 instead of Ag2O | 36 |
| 6 | CuCl instead of CuI | 45 |
| 7 | Toluene instead of DCE | 42 |
| 8 | Addition of Pd(OAc)2 (15 mol%) | 20 |
| 9 | Addition of 1,10-phenanthroline (15 mol%) | 34 |
| 10 | CuI (0.05 mol% instead of 0.15 mol%) | 31 |
| 11 | 2a (2 mmol instead of 1.1 mmol) | 58 |
| 12 | Inert atmosphere (Argon) instead of air | 51 |
| 13 | 4-(Diphenylamino)phenylboronic acid pinacol ester instead of 2a | 57 |
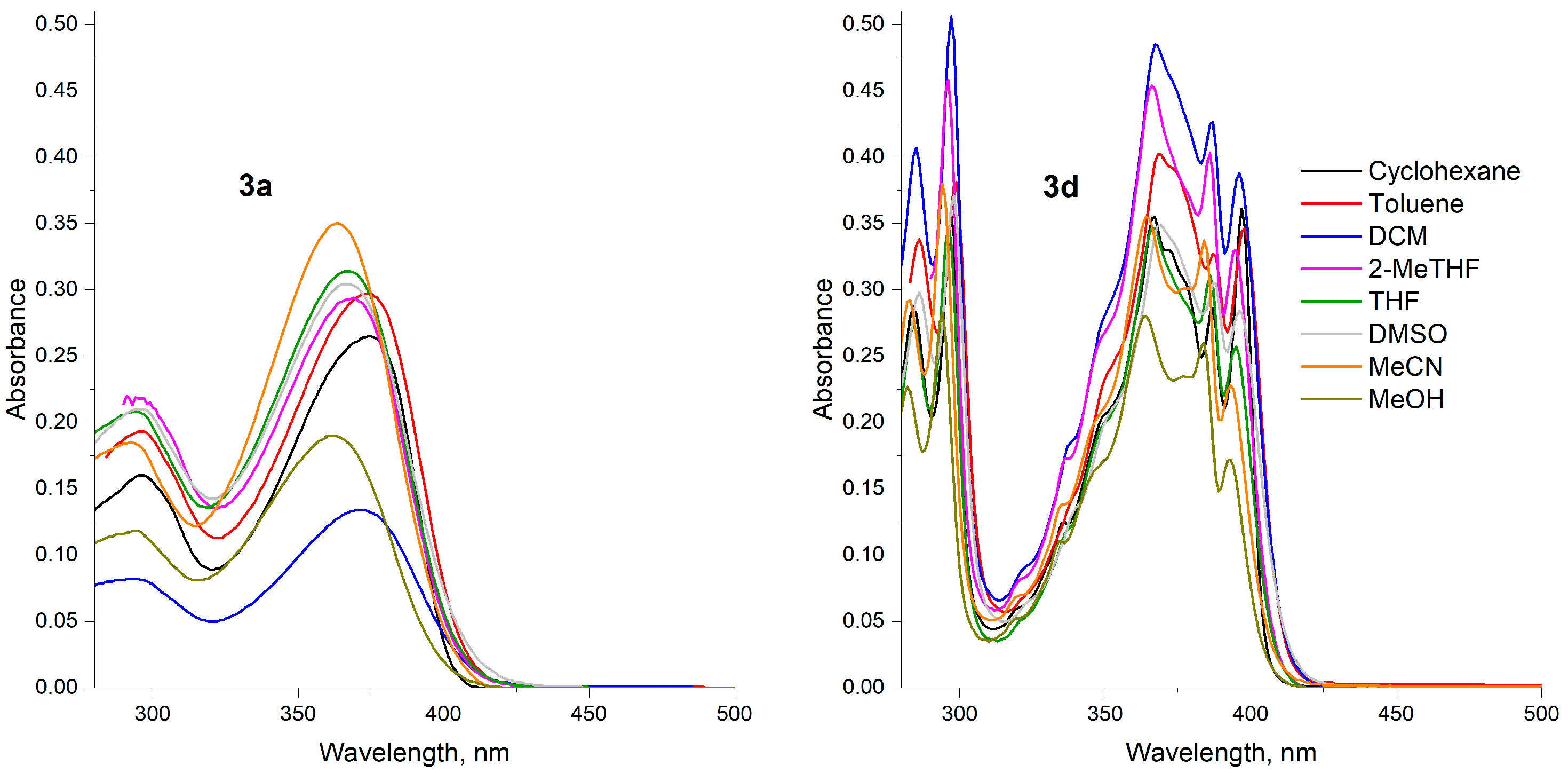
| Entry 1 | CyH | Toluene | DCM | 2-MeTHF | THF | DMSO | MeCN | MeOH |
|---|---|---|---|---|---|---|---|---|
| 3a | 296 (16,799), 374 (26,151) | 296 (16,000), 373 (29,700) | 293 (8200), 372 (13,400) | 294 (21,800), 369 (29,400) | 294 (20,800), 367 (31,400) | 296 (21,000), 367 (30,400) | 293 (18,500), 363 (35,000) | 294 (11,800), 361 (19,000) |
| 3b | 278 (23,845), 304 (29,112), 333 (26,120), 351 (30,267) | 306 (30,700), 336 (26,400), 351 (27,600) | 270 (21,800), 279 (25,500), 305 (32,300), 336 (27,200), 348 (27,800) | 304 (45,800), 335 (36,800), 346 (37,100) | 278 (24,900), 304 (31,900), 334 (26,300), 346 (26,500) | 280 (25,700), 305 (30,900), 337 (24,600), 347 (24,500) | 278 (28,800), 302 (38,200), 334 (30,200), 343 (29,600) | 262 (27,500), 269 (27,000), 277 (25,100), 302 (33,900), 332 (26,600), 343 (25,700) |
| 3c | 313 (18,886), 325 (16,445) | 312 (24,600) | 310 (17,300) | 308 (29,200) | 311 (19,000) | 309 (17,700) | 306 (23,900) | 302 (26,700) |
| 3d | 273 (20,000), 284 (28,700), 297 (35,700), 367 (35,500), 387 (28,600), 397 (36,100) | 286 (33,912), 299 (37,931), 369 (40,058), 387 (32,969), 398 (34,580) | 275 (29,100), 285 (40,700), 297 (50,600), 367 (48,500), 387 (42,600), 396 (38,800) | 296 (45,800), 366 (45,400), 386 (40,300), 395 (33,000) | 284 (27,300), 296 (34,800), 366 (34,700), 386 (31,100), 395 (25,700) | 276 (21,400), 286 (29,800), 298 (37,100), 369 (34,900), 388 (30,500), 396 (28,400) | 283 (29,200), 294 (38,000), 364 (35,500), 384 (33,700), 393 (22,800) | 262 (15,800), 269 (18,300), 282 (22,700), 294 (28,300), 364 (28,000), 384 (26,000), 393 (17,200) |
| № 1 | CyH | Toluene | DCM | THF | DMSO | MeCN | MeOH |
|---|---|---|---|---|---|---|---|
| 3a | 405, 567, 797 | 412, 667, 800 | 403, 810 | 397, 410, 802 | 412, 806 | 408, 795 | 403, 811 |
| ϕ, % | 63 | 10 | <0.1 | <0.1 | 14 | 12 | 13 |
| 3b | 333, 370, 387, 404, 530, 667, 691, 777 | 337, 383, 615, 674, 772 | 336, 373, 390, 783 | 334, 391, 406, 633, 669, 785 | 336, 392, 673, 786 | 332, 373, 391, 408, 633, 651, 665, 787 | 333, 388, 665, 781 |
| ϕ, % | 2 | <0.1 | 1 | 3 | 7 | 20 | 6 |
| 3c | 345, 373, 532, 651, 690, 755 | 365, 382, 643, 767 | 342, 405, 687, 799 | 342, 367, 384, 514, 647, 686, 775 | 341, 405, 633, 682, 798 | 382, 781 | 332, 388, 666, 790 |
| ϕ, % | <0.1 | 1 | 4 | <0.1 | 6 | 23 | <0.1 |
| 3d | 424, 507, 803 | 430, 607 | 416, 449, 728, 817 | 420, 444, 713 | 419, 556 | 417, 428 | 464 |
| ϕ, % | 99 | 9 | <0.1 | 2 | 2 | 1 | <0.1 |
| Entry | Temperature | |
|---|---|---|
| 298 K | 77 K | |
| 3a 1 | 397, 413, 798 | 406, 650, 819 |
| 3b 2 | 355, 372, 715, 780 | 342, 346, 365, 368, 384, 395, 405, 418, 431, 578, 741, 769, 790 |
| 3c 3 | 350, 712, 770, 784 | 353, 371, 405, 418, 432, 756 |
| 3d 4 | 423, 446, 689 | 424, 577 |
| State | Type | Transition | Contribution | |
|---|---|---|---|---|
| S0 → S1 | Abs | HOMO-1 → LUMO | 3% | E = 3.3241 eV λ = 373 nm f = 1.4384 |
| HOMO-1 → LUMO + 4 | 2% | |||
| HOMO → LUMO | 85% | |||
| HOMO → LUMO + 4 | 5% | |||
| S0 → S2 | Abs | HOMO-1 → LUMO + 1 | 3% | E = 4.1238 eV λ = 300 nm f = 0.0170 |
| HOMO → LUMO + 1 | 75% | |||
| HOMO → LUMO + 2 | 4% | |||
| HOMO → LUMO + 5 | 4% | |||
| S0 → S3 | Abs | HOMO-1 → LUMO + 3 | 9% | E = 4.4154 eV λ = 280 nm f = 0.2145 |
| HOMO → LUMO + 3 | 3% | |||
| HOMO → LUMO + 4 | 79% | |||
| S0 → T1 | Em | HOMO-1 → LUMO | 26% | E = 1.9824 eV λ = 625 nm |
| HOMO → LUMO | 69% | |||
| S0 → T2 | Em | HOMO-1 → LUMO | 20% | E = 3.2471 eV λ = 381 nm |
| HOMO → LUMO | 14% | |||
| HOMO → LUMO + 3 | 38% | |||
| S0 → T3 | Em | HOMO-1 → LUMO + 2 | 17% | E = 3.7894 eV λ = 327 nm |
| HOMO → LUMO + 1 | 29% | |||
| HOMO → LUMO + 2 | 22% | |||
| S0 → S1 | Em | HOMO-1 → LUMO | 4% | E = 3.8067 eV λ = 325 nm |
| HOMO → LUMO | 86% | |||
| HOMO → LUMO + 4 | 4% | |||
| S0 → S2 | Em | HOMO → LUMO + 1 | 76% | E = 4.1189 eV λ = 301 nm |
| HOMO → LUMO + 2 | 3% | |||
| S0 → S3 | Em | HOMO → LUMO + 2 | 51% | E = 4.3674 eV λ = 283 nm |
| HOMO → LUMO + 3 | 31% |
| Entry | Compounds 3a–3d | τ, μs | R2 1 |
|---|---|---|---|
| 1 | 3a 2 | 7.08 | 0.99976 |
| 2 | 3b 3 | 7.53 | 0.99959 |
| 3 | 3c 4 | 7.92 | 0.99935 |
| 4 | 3d 5 | 7.15 | 0.99983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moseev, T.D.; Idrisov, T.A.; Varaksin, M.V.; Tsmokaluk, A.N.; Charushin, V.N.; Chupakhin, O.N. Copper-Catalyzed Sonogashira-Type Coupling Reaction of Vinylacetylene ortho-Carborane with Boronic Acid in the Synthesis of Luminophores with Phosphorescent Emission. Reactions 2024, 5, 868-882. https://doi.org/10.3390/reactions5040046
Moseev TD, Idrisov TA, Varaksin MV, Tsmokaluk AN, Charushin VN, Chupakhin ON. Copper-Catalyzed Sonogashira-Type Coupling Reaction of Vinylacetylene ortho-Carborane with Boronic Acid in the Synthesis of Luminophores with Phosphorescent Emission. Reactions. 2024; 5(4):868-882. https://doi.org/10.3390/reactions5040046
Chicago/Turabian StyleMoseev, Timofey D., Tair A. Idrisov, Mikhail V. Varaksin, Anton N. Tsmokaluk, Valery N. Charushin, and Oleg N. Chupakhin. 2024. "Copper-Catalyzed Sonogashira-Type Coupling Reaction of Vinylacetylene ortho-Carborane with Boronic Acid in the Synthesis of Luminophores with Phosphorescent Emission" Reactions 5, no. 4: 868-882. https://doi.org/10.3390/reactions5040046
APA StyleMoseev, T. D., Idrisov, T. A., Varaksin, M. V., Tsmokaluk, A. N., Charushin, V. N., & Chupakhin, O. N. (2024). Copper-Catalyzed Sonogashira-Type Coupling Reaction of Vinylacetylene ortho-Carborane with Boronic Acid in the Synthesis of Luminophores with Phosphorescent Emission. Reactions, 5(4), 868-882. https://doi.org/10.3390/reactions5040046







