Overview of the Catalytic Liquefaction of Waste Plastics Process Development, Operation and Product Quality †
Abstract
1. Introduction
2. Basic Concept of Chemistry and Reaction Procedure FCC Catalyst
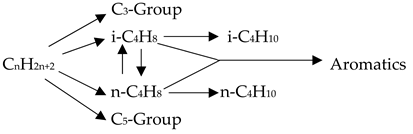
3. Experimental Procedure
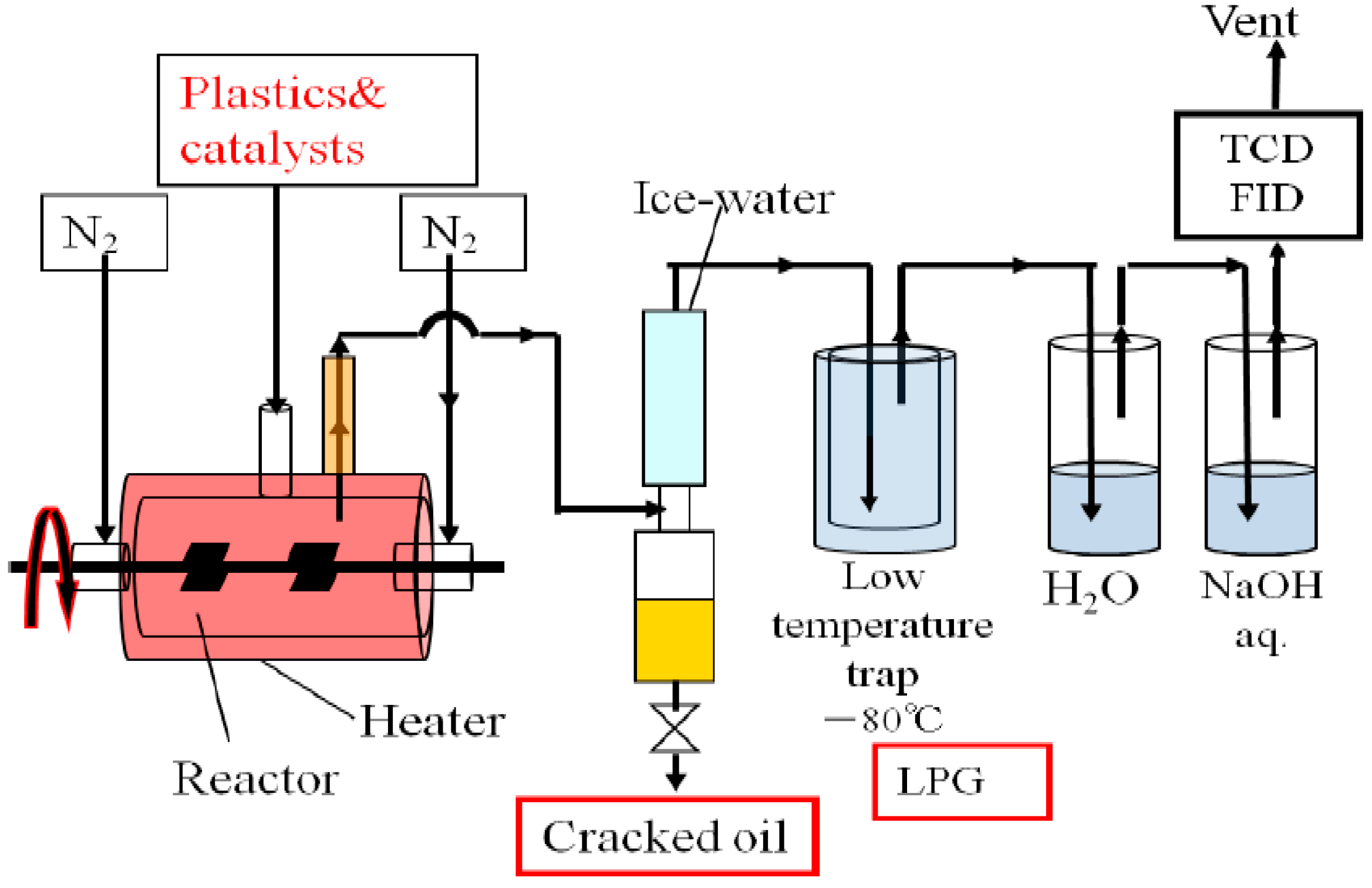
3.1. Reaction of Catalytic Cracking Procedure
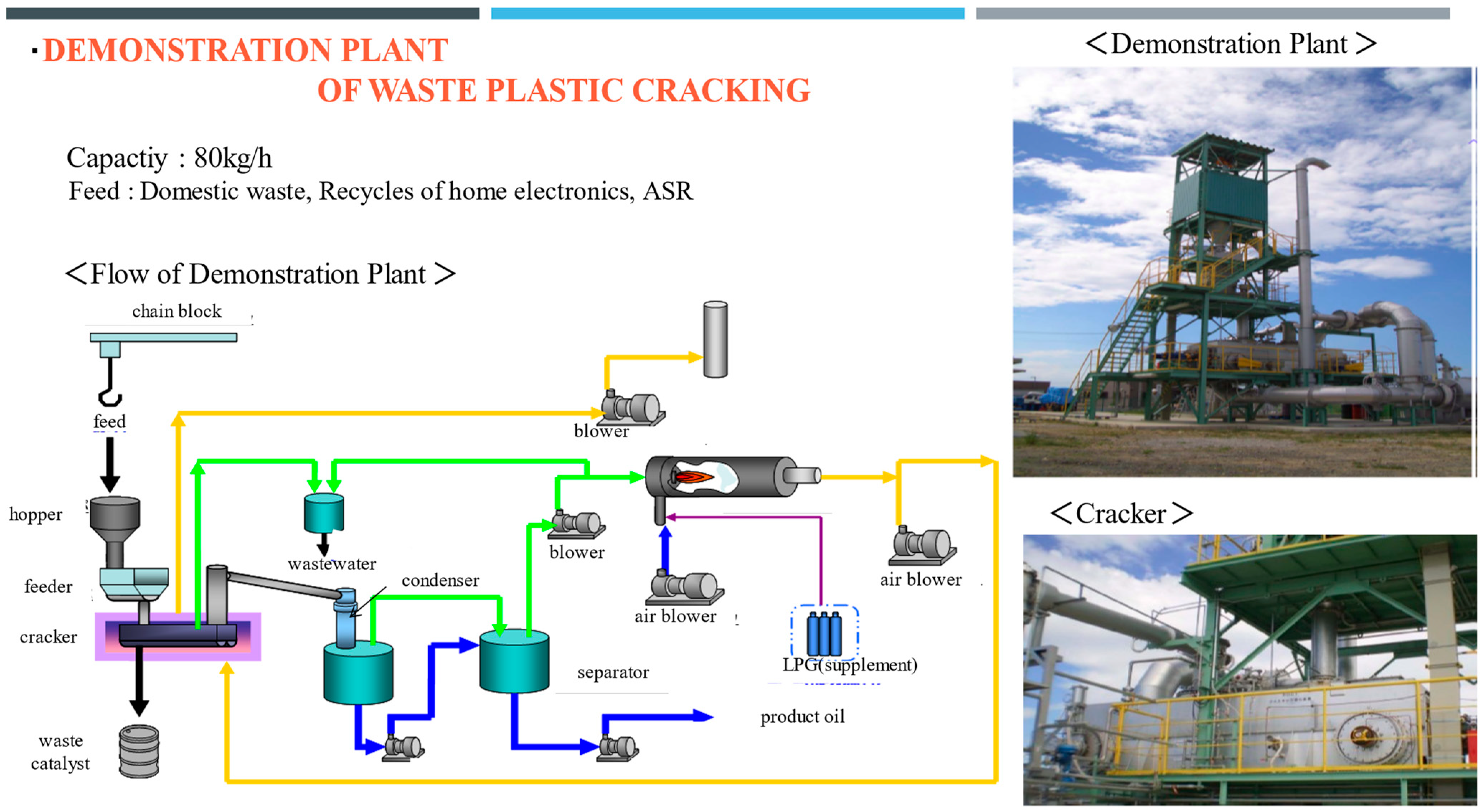
3.2. Procedure of the Demonstration Plant
3.3. Characterization of Products
4. Results and Discussion
4.1. Catalytic and Non-Catalytic Cracking of Polyethylene (High Density PE)
4.2. Catalytic Cracking of a Variety of Recycled Plastics
- The main product is Oil (naphtha, kerosene and gas oil) ~60 wt%, LPG: 5~20 wt%
- The oil yield and residue yield are highest for RDF, whose values are nearly 70 wt% and 11 wt%, respectively. On the other hand, PE (LLDPE) gives lower oil yield (60–65 wt%) and residue yield (5 wt%).
- The yield of light hydrocarbons such as LPG and naphtha is much higher for LLDPE
- The yield of residue, in another word coke, is highest for RDF (11 wt%) and lowest for LLDPE (4 wt%).
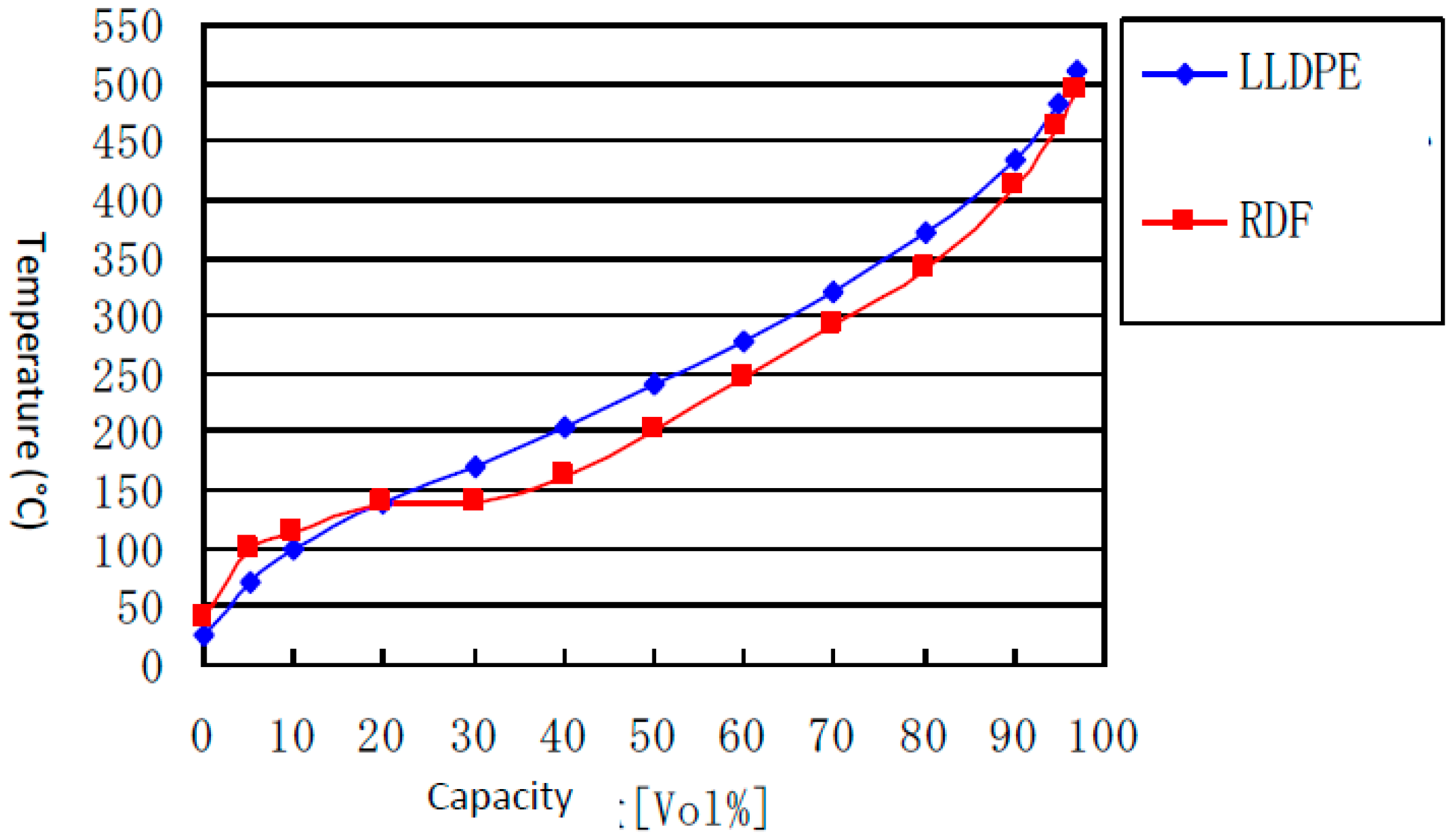
- The distillation curve of oil as shown in Figure 5. shows similar trend for oil from LLDPE, and RDF with slight difference at around 150 °C.
- PET, which commonly exists with low content in the waste plastic exhibits bad effect after being converted to terephthalic acid (TPA). In the present catalytic system PET is converted to methyl benzenes.
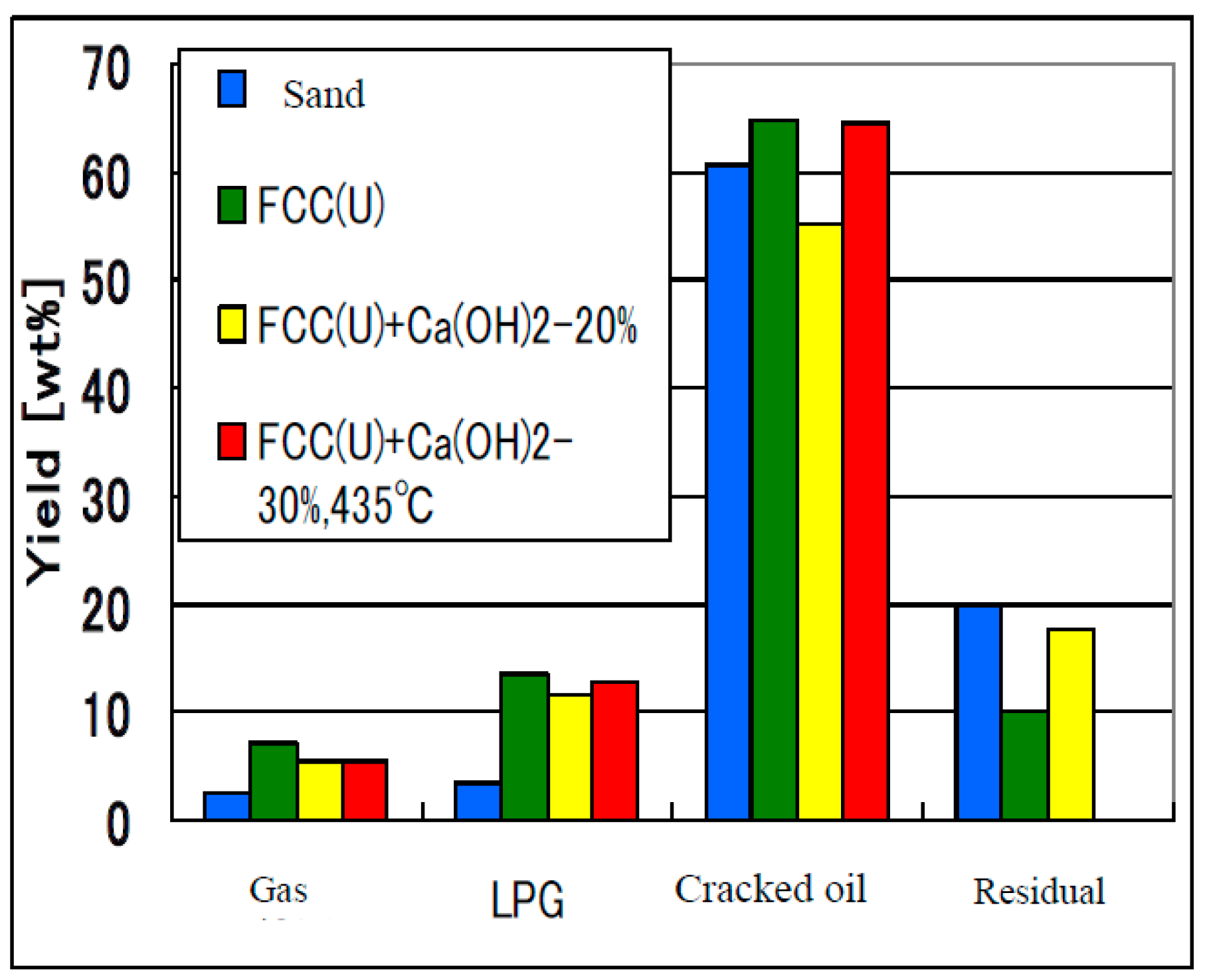
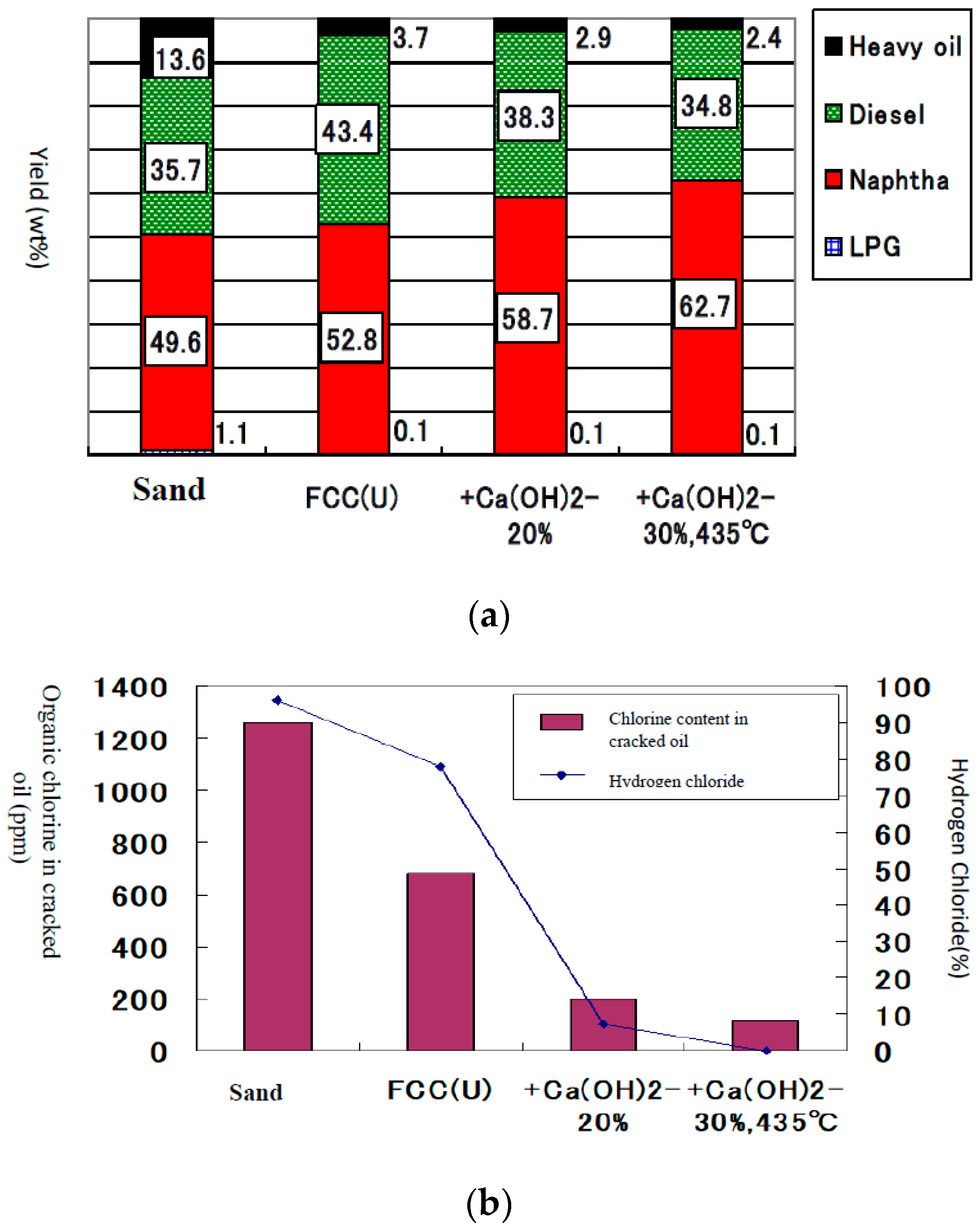
4.3. Cracking Performance of RDF Containing PVC and PET
4.4. Deposition of Coke in the Catalyst, Catalytic Activity and Its Recovery
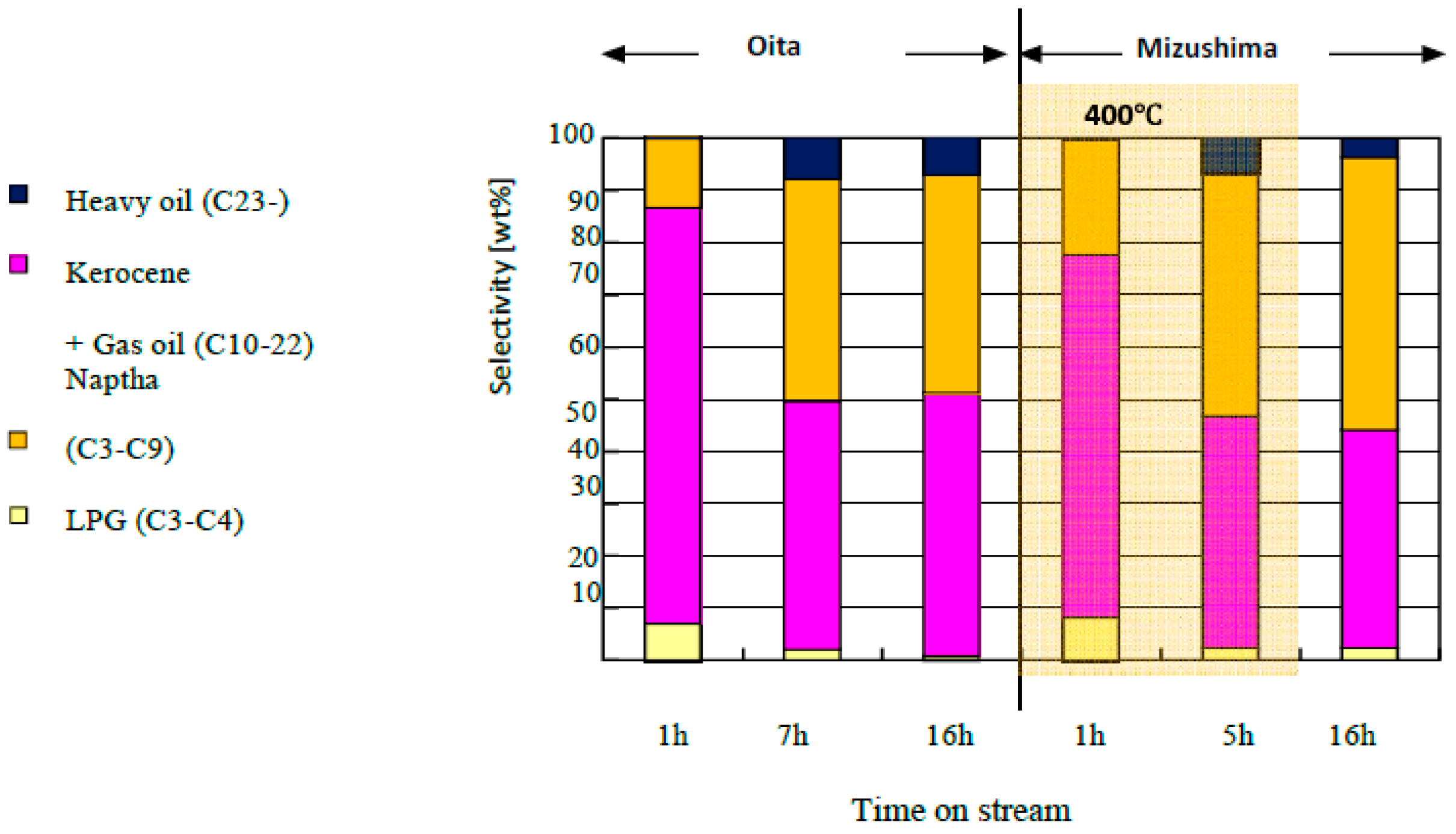
4.5. Results of Continuous Operation of the Demonstration Plant
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plastic Management Institute. An Introduction to Plastic Recycling Plastic Waste in Japan. 2022. Available online: http://www.pwmi.or.jp/ei/index.htm (accessed on 22 April 2022).
- Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH. Waste to Energy Management Options in Municipal Solid Waste Management; Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH: Eschborn, Germany, 2017. [Google Scholar]
- Buekens, A.G.; Huang, H. Catalytic Plastics Cracking for recovery of Gasoline-Range Hydrocarbons from Municipal Plastic Wastes. Resour. Conserv. Recycl. 1998, 23, 163–181. [Google Scholar] [CrossRef]
- Fluid Catalytic Cracking Handbook, 4th ed.; Sadeghbeig, R., Ed.; Butterworth-Heinemann: Oxford, UK, 2020. [Google Scholar]
- Tani, H.; Fujimoto, K. Catalytic Cracking of Polyolefins in the Molten Phase-Basic study for the process development of waste plastics liquefaction. J. Environ. Sci. Eng. B 2017, 6, 352–361. [Google Scholar]
- Fujimoto, K.; Tani, H.; Yamawaki, T. Producing Naphtha from Mixed Waste Plastics; Identiplast: London, UK, 2010. [Google Scholar]
- Nakamura, I.; Fujimoto, K. Development of new disposable catalyst for waste plastics treatment for high quality transportation fuel. Catal. Today 1996, 27, 175–179. [Google Scholar] [CrossRef]
- Nakamura, I.; Fujimoto, K. Development of new disposable catalyst for waste plastics. J. Pet. Inst. Jpn. 1996, 39, 245–249. [Google Scholar] [CrossRef][Green Version]
- Venuto, P.; Habib, T. Fluid Catalytic Cracking with Zeolite Catalysts; Marcel Dekker: New York, NY, USA, 1979. [Google Scholar]
- Kunugi, T.; Fujimoto, K.; Sakai, T. Formation of Middle Olefins by Catalytic Cracking of Petroleum Hydrocarbon. Sekiy Gakka Shi 1968, 11, 103. (In Japanese) [Google Scholar] [CrossRef]
- Fujimoto, K.; Kunugi, T. The Kinetics of Secondary Reactions in Catalytic Cracking of Paraffins. J. Jpn. Pet. Inst. 1969, 12, 33–37. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342. [Google Scholar] [CrossRef] [PubMed]
- JIS K 2249-1; Crude Petroleum Products Determination of Density. Japanese Standards Association: Tokyo, Japan, 2011.
- JIS K 2283; Testing Method of Kinematic Viscosity and Calculation Method for Viscosity Index of Crude Oil and Petroleum Products. Japanese Standards Association: Tokyo, Japan, 1983.
- Speight, J.G. The Chemistry and Technology of Petroleum, 3rd ed.; Mercel Dekker, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Yang, M.G.; Nakamura, I.; Fujimoto, K. Hydrothermal Cracking of Heavy Oils and Its Model Compound. Catal. Today 1998, 43, 237–280. [Google Scholar] [CrossRef]
- Purification Plant for Waste Plastics (HICOP System). Available online: https://kankyo-energy.jp/products/ (accessed on 21 August 2024).
- Started Japan’s First Demonstration Study of Waste Plastic Recycling Including Mixed Waste Plastics at Chiba Complex. Construct a Waste Plastics Recycling System by New Liquefaction Technology and Our Petroleum Refining & Petrochemical Plants. Available online: https://www.idemitsu.com/en/news/2021/210507_2.html/ (accessed on 21 August 2024).
- Commencement of Basic Design for Commercialization of Used Plastic Recycling—Commencement of Consideration of Business Alliance for Raw Material Procurement and Construction of Chemical Recycling System. Available online: https://www.idemitsu.com/jp/news/2022/220913.html/ (accessed on 21 August 2024).



| No Catalyst [wt%] | Catalytic [wt%] | |
|---|---|---|
| Gas (C1~C2) | 1.5 | 2.9 |
| LPG (C3~C4) | 2.3 | 5.2 |
| Naphtha (C5~C8) | 2.7 | 21.5 |
| Kerosene (C9~C12) | 13.1 | 20.8 |
| Light oil (C13~C24) | 37.8 | 36.6 |
| Heavy oi (C25~) | 29.9 | 12.1 |
| Residual | 12.6 | 0.9 |
| No Catalyst a [wt%] | Catalytic b [wt%] | |
|---|---|---|
| n-paraffin | 55.4 | 11.0 |
| Olefins | 32.0 | 22.5 |
| i-paraffin | 2.1 | 11.5 |
| Aromatic | 4.5 | 45.0 |
| Unknown | 6.0 | 10.0 |
| Component | Composition (wt%) |
|---|---|
| Polyethylene (PE) | 49 |
| Polypropylene (PP) | 28 |
| Polystyrene (PS) | 11 |
| Polyethylene Terephthalate (PET) | 2 |
| Polyvinyl Chloride | 1 |
| Others | 9 |
| Product Yield (%) | CLPE | LLDPE | RDF |
|---|---|---|---|
| Oil | 59.8 | 64.0 | 69.4 |
| LPG | 24.1 | 18.3 | 5.1 |
| Dry Gas | 5.0 | 1.4 | 9.3 |
| Residue | 8.1 | 4.2 | 11.2 |
| Polymer/Catalyst (w/w) | 2.5 | 6.3 | 14.0 |
| Composition (wt%) | CLPE | LLDPE | RDF |
|---|---|---|---|
| Naphtha | 53.6 | 42.0 | 55.8 |
| Kerosene | 40.1 | 48.2 | 39.9 |
| Heavy Oil | 3.8 | 9.3 | 3.8 |
| Component (vol%) | CLPE | RDF |
|---|---|---|
| Saturate | 36.6 | 19.3 |
| Olefin | 32.7 | 24.7 |
| 1 ring aromatics | 22.4 | 41.8 |
| 2 ring aromatics | 6.0 | 6.6 |
| 3 ring aromatics | 2.3 | 7.6 |
| Raw Materials | MR Raw Materials |
|---|---|
| Catalysts: FCC waste catalysts [g] | 1000 |
| Ca(OH)2 [g] | 250 |
| Reaction temperature [°C] | 430 |
| Reaction Pressure [MPa] | 0.1 |
| Carrier gas flow [mL/min] | 100 |
| Stirring rate [rpm] | 50 |
| Input [g/h] | 100 |
| MR (1) * (PP/PE) | MR (2) ** | ASR *** | ||
|---|---|---|---|---|
| Plant | Demo Plant | Demo Plant | Lab Plant | Demo Plant |
| Oil Yield (%) | 49.6 | 65.5 | 65.3 | 60.5 |
| LPG Yield (%) | 27.0 | ~16.0 | ~16.0 | 9.0 |
| Residual (%) | 2.7 | 0.0 | 12.0 | 29.7 |
| Density@15°C (g/cm3) | 0.789 | 0.822 | 0.811 | 0.837 |
| Water (wtppm) | 87.0 | 140.0 | 851.0 | 756.0 |
| Diene (g/100 g) | 2.4 | 2.4 | 1.1 | 6.9 |
| Br (mg-Br2/100 g) | 43.6 | 34.6 | 27.2 | 42.9 |
| S (wtppm) | 6.0 | 6.0 | 3.0 | 339.0 |
| Cl (wtppm) | 48.0 | 30.0 | 4.0 | 86.0 |
| N (wtppm) | 542.0 | 576.0 | 254.0 | 6866.0 |
| Basic N (wtppm) | 326.0 | 280.0 | 146.0 | 2300.0 |
| Total Acid (mg-KOH/g) | 0.02 | <0.01 | 0.05 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinakruang, J.; Tani, H.; Fujimoto, K. Overview of the Catalytic Liquefaction of Waste Plastics Process Development, Operation and Product Quality. Reactions 2024, 5, 740-752. https://doi.org/10.3390/reactions5040036
Srinakruang J, Tani H, Fujimoto K. Overview of the Catalytic Liquefaction of Waste Plastics Process Development, Operation and Product Quality. Reactions. 2024; 5(4):740-752. https://doi.org/10.3390/reactions5040036
Chicago/Turabian StyleSrinakruang, Jumluck, Haruki Tani, and Kaoru Fujimoto. 2024. "Overview of the Catalytic Liquefaction of Waste Plastics Process Development, Operation and Product Quality" Reactions 5, no. 4: 740-752. https://doi.org/10.3390/reactions5040036
APA StyleSrinakruang, J., Tani, H., & Fujimoto, K. (2024). Overview of the Catalytic Liquefaction of Waste Plastics Process Development, Operation and Product Quality. Reactions, 5(4), 740-752. https://doi.org/10.3390/reactions5040036





