Confinement of LiAlH4 in a Mesoporous Carbon Black for Improved Near-Ambient Release of H2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. N2 Adsorption
2.3. X-ray Diffraction
2.4. Temperature-Programmed Desorption
3. Results
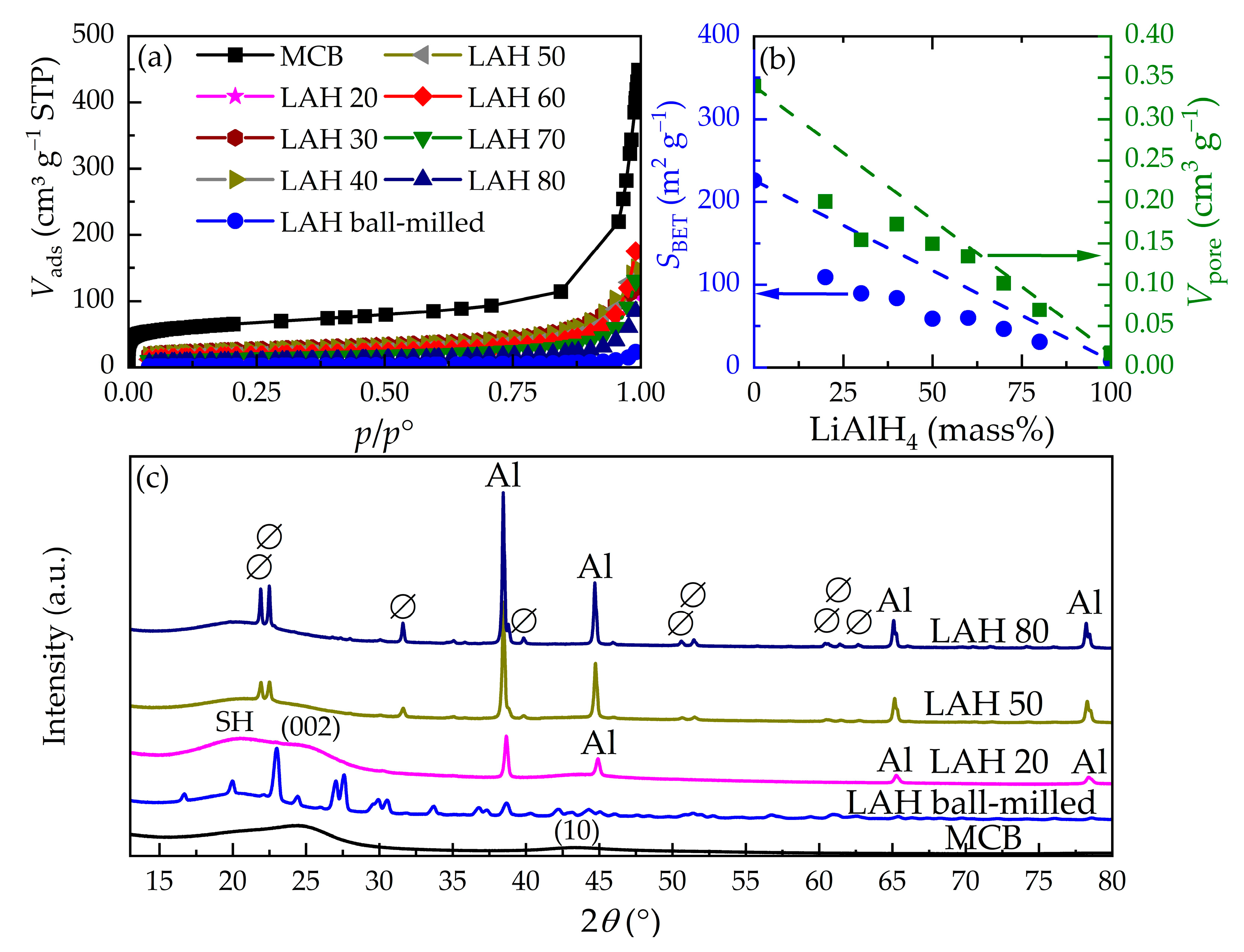
3.1. Physical Characterisation
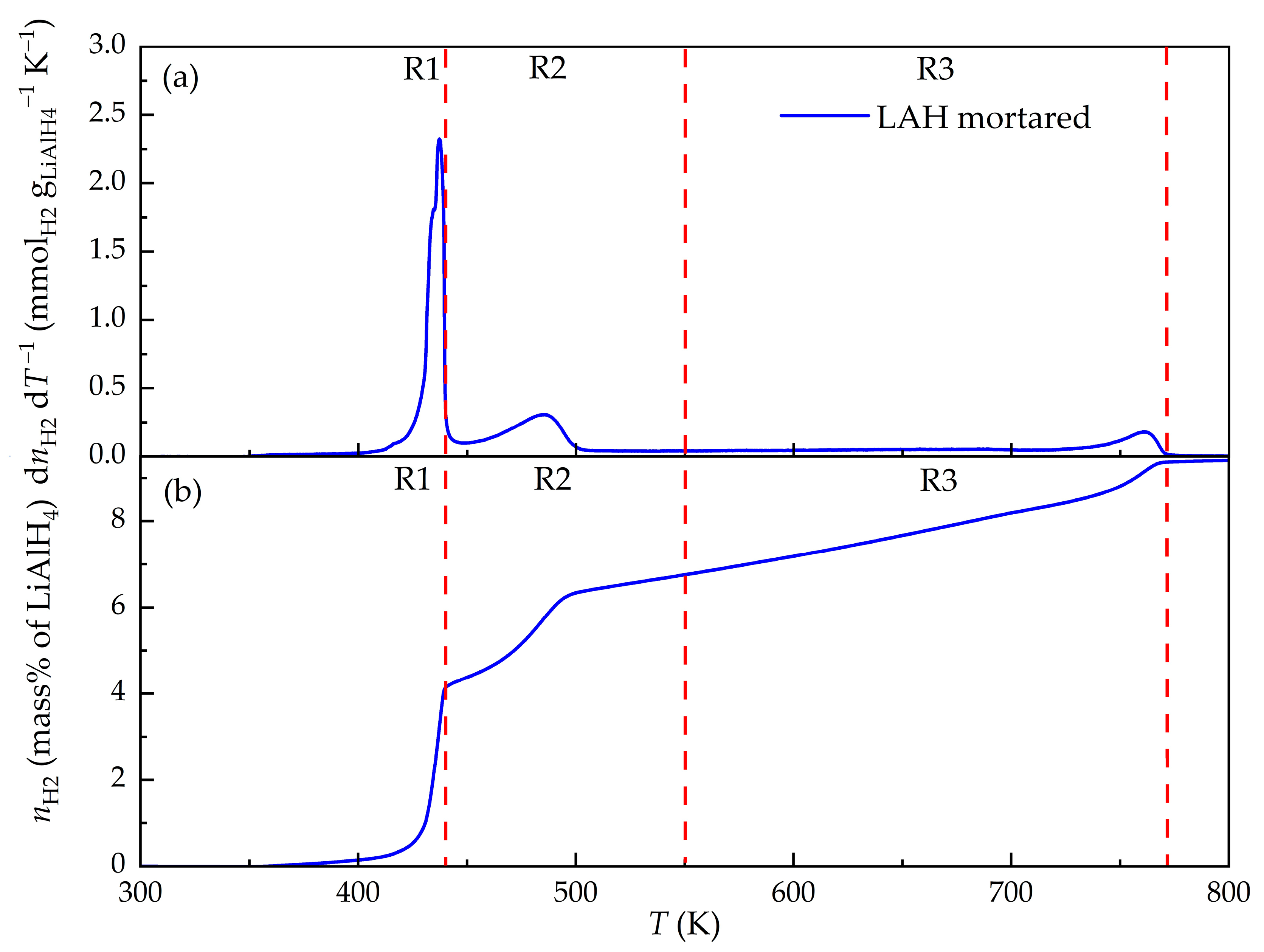
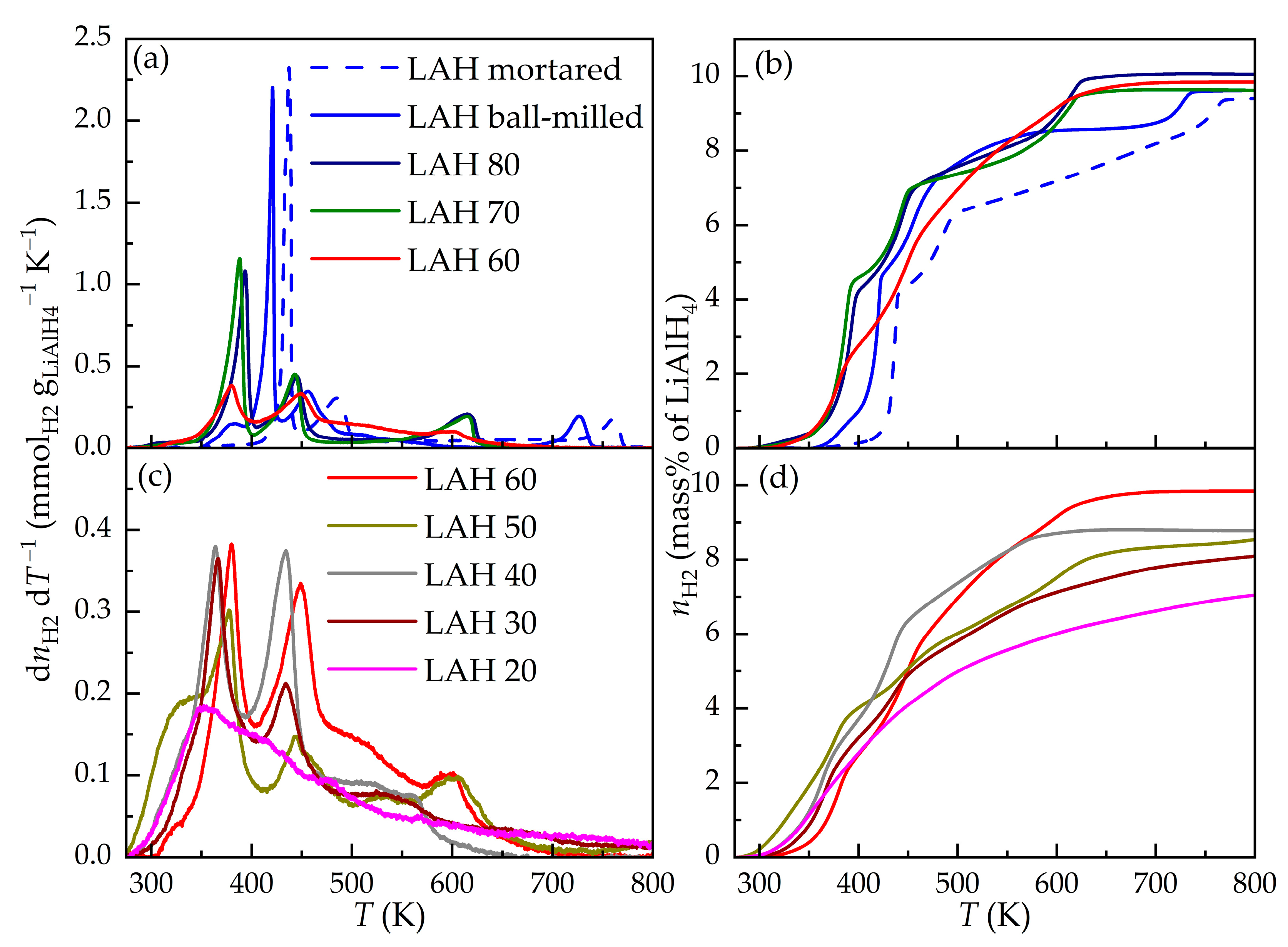
3.2. Temperature-Programmed Desorption
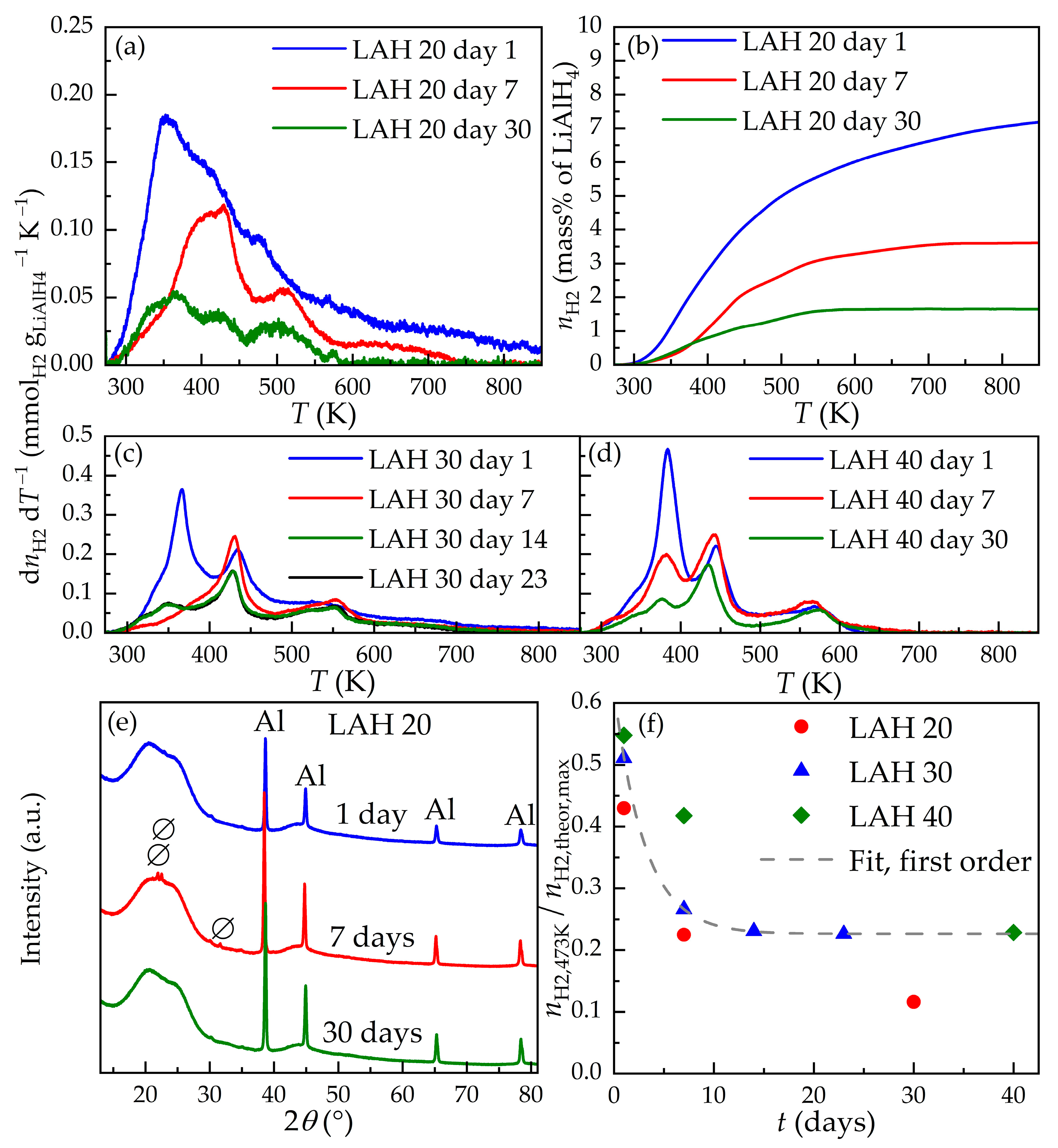
3.3. Decomposition during Storage
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ku, A.Y.; Kocs, E.A.; Afzal, S.; Ewan, M.; Glenn, J.R.; Toma, F.; Vickers, J.; Weeks, B.; White, A.A. Opportunities for the Materials Research Community to Support the Development of the H2 Economy. MRS Energy Sustain. 2023. [Google Scholar] [CrossRef]
- Nazir, H.; Muthuswamy, N.; Louis, C.; Jose, S.; Prakash, J.; Buan, M.E.M.; Flox, C.; Chavan, S.; Shi, X.; Kauranen, P.; et al. Is the H2 Economy Realizable in the Foreseeable Future? Part III: H2 Usage Technologies, Applications, and Challenges and Opportunities. Int. J. Hydrogen Energy 2020, 45, 28217–28239. [Google Scholar] [CrossRef] [PubMed]
- Nazir, G.; Rehman, A.; Hussain, S.; Aftab, S.; Heo, K.; Ikram, M.; Patil, S.A.; Aizaz Ud Din, M. Recent Advances and Reliable Assessment of Solid-State Materials for Hydrogen Storage: A Step Forward toward a Sustainable H2 Economy. Adv. Sustain. Syst. 2022, 6, 2200276. [Google Scholar] [CrossRef]
- Nazir, H.; Muthuswamy, N.; Louis, C.; Jose, S.; Prakash, J.; Buan, M.E.; Flox, C.; Chavan, S.; Shi, X.; Kauranen, P.; et al. Is the H2 Economy Realizable in the Foreseeable Future? Part II: H2 Storage, Transportation, and Distribution. Int. J. Hydrogen Energy 2020, 45, 20693–20708. [Google Scholar] [CrossRef]
- So, S.H.; Sung, S.J.; Yang, S.J.; Park, C.R. Where to Go for the Development of High-Performance H2 Storage Materials at Ambient Conditions? Electron. Mater. Lett. 2023, 19, 1–18. [Google Scholar] [CrossRef]
- Løvvik, O.M.; Opalka, S.M.; Brinks, H.W.; Hauback, B.C. Crystal Structure and Thermodynamic Stability of the Lithium Alanates LiAlH4 and Li3AlH6. Phys. Rev. B 2004, 69, 134117. [Google Scholar] [CrossRef]
- Dymova, T.N.; Aleksandrov, D.P.; Konoplev, V.N.; Silina, T.A.; Sizareva, A.S. Spontaneous and Thermal-Decomposition of Lithium Tetrahydroaluminate LiAlH4-the Promoting Effect of Mechanochemical Action on the Process. Koord. Khimiya 1994, 20, 279–285. [Google Scholar]
- Ares, J.R.; Aguey-Zinsou, K.-F.; Porcu, M.; Sykes, J.M.; Dornheim, M.; Klassen, T.; Bormann, R. Thermal and Mechanically Activated Decomposition of LiAlH4. Mater. Res. Bull. 2008, 43, 1263–1275. [Google Scholar] [CrossRef]
- Jang, J.-W.; Shim, J.-H.; Cho, Y.W.; Lee, B.-J. Thermodynamic Calculation of LiH↔Li3AlH6↔LiAlH4 Reactions. J. Alloys Compd. 2006, 420, 286–290. [Google Scholar] [CrossRef]
- Sazelee, N.A.; Ismail, M. Recent Advances in Catalyst-Enhanced LiAlH4 for Solid-State Hydrogen Storage: A Review. Int. J. Hydrogen Energy 2021, 46, 9123–9141. [Google Scholar] [CrossRef]
- Varin, R.A.; Zbroniec, L. Decomposition Behavior of Unmilled and Ball Milled Lithium Alanate (LiAlH4) Including Long-Term Storage and Moisture Effects. J. Alloys Compd. 2010, 504, 89–101. [Google Scholar] [CrossRef]
- Cho, Y.; Li, S.; Snider, J.L.; Marple, M.A.T.; Strange, N.A.; Sugar, J.D.; El Gabaly, F.; Schneemann, A.; Kang, S.; Kang, M.; et al. Reversing the Irreversible: Thermodynamic Stabilization of LiAlH4 Nanoconfined Within a Nitrogen-Doped Carbon Host. ACS Nano 2021, 15, 10163–10174. [Google Scholar] [CrossRef] [PubMed]
- Che Mazlan, N.S.; Asyraf Abdul Halim Yap, M.F.; Ismail, M.; Yahya, M.S.; Ali, N.A.; Sazelee, N.; Seok, Y.B. Reinforce the Dehydrogenation Process of LiAlH4 by Accumulating Porous Activated Carbon. Int. J. Hydrogen Energy 2023, 48, 16381–16391. [Google Scholar] [CrossRef]
- Pratthana, C.; Yang, Y.; Rawal, A.; Aguey-Zinsou, K.-F. Nanoconfinement of Lithium Alanate for Hydrogen Storage. J. Alloys Compd. 2022, 926, 166834. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Nanoconfined Lithium Aluminium Hydride (LiAlH4) and Hydrogen Reversibility. Int. J. Hydrogen Energy 2017, 42, 14144–14153. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, L.; Zhao, S.; Wu, Z.; Wang, S.; Yin, D.; Wang, Q.; Wang, L.; Wang, C.; Cheng, Y. Dehydrogenation Behavior and Mechanism of LiAlH4 Adding Nano-CeO2 with Different Morphologies. Nano Res. 2023, 16, 9426–9434. [Google Scholar] [CrossRef]
- Liu, S.-S.; Li, Z.-B.; Jiao, C.-L.; Si, X.-L.; Yang, L.-N.; Zhang, J.; Zhou, H.-Y.; Huang, F.-L.; Gabelica, Z.; Schick, C.; et al. Improved Reversible Hydrogen Storage of LiAlH4 by Nano-Sized TiH2. Int. J. Hydrogen Energy 2013, 38, 2770–2777. [Google Scholar] [CrossRef]
- Rafi-ud-din; Zhang, L.; Ping, L.; Xuanhui, Q. Catalytic Effects of Nano-Sized TiC Additions on the Hydrogen Storage Properties of LiAlH4. J. Alloys Compd. 2010, 508, 119–128. [Google Scholar] [CrossRef]
- Cheng, H.; Zheng, J.; Xiao, X.; Liu, Z.; Ren, X.; Wang, X.; Li, S.; Chen, L. Ultra-Fast Dehydrogenation Behavior at Low Temperature of LiAlH4 Modified by Fluorographite. Int. J. Hydrogen Energy 2020, 45, 28123–28133. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Xu, Q.; Takeshita, H.T.; Sakai, T. Reversible Hydrogen Storage via Titanium-Catalyzed LiAlH4 and Li3AlH6. J. Phys. Chem. B 2001, 105, 11214–11220. [Google Scholar] [CrossRef]
- Brinks, H.W.; Fossdal, A.; Fonneløp, J.E.; Hauback, B.C. Crystal Structure and Stability of LiAlD4 with TiF3 Additive. J. Alloys Compd. 2005, 397, 291–295. [Google Scholar] [CrossRef]
- Gao, J.; Adelhelm, P.; Verkuijlen, M.H.W.; Rongeat, C.; Herrich, M.; van Bentum, P.J.M.; Gutfleisch, O.; Kentgens, A.P.M.; de Jong, K.P.; de Jongh, P.E. Confinement of NaAlH4 in Nanoporous Carbon: Impact on H2 Release, Reversibility, and Thermodynamics. J. Phys. Chem. C 2010, 114, 4675–4682. [Google Scholar] [CrossRef]
- Palm, R.; Kurig, H.; Aruväli, J.; Lust, E. NaAlH4/Microporous Carbon Composite Materials for Reversible Hydrogen Storage. Microporous Mesoporous Mater. 2018, 264, 8–12. [Google Scholar] [CrossRef]
- Tuul, K.; Palm, R. Influence of Nanoconfinement on the Hydrogen Release Processes from Sodium Alanate. Reactions 2021, 2, 1. [Google Scholar] [CrossRef]
- Tuul, K.; Palm, R.; Aruväli, J.; Lust, E. Dehydrogenation and Low-Pressure Hydrogenation Properties of NaAlH4 Confined in Mesoporous Carbon Black for Hydrogen Storage. Int. J. Hydrogen Energy 2023, 48, 19646–19656. [Google Scholar] [CrossRef]
- Palm, R.; Tuul, K.; Elson, F.; Nocerino, E.; Forslund, O.K.; Hansen, T.C.; Aruväli, J.; Månsson, M. In Situ Neutron Diffraction of NaAlD4/Carbon Black Composites during Decomposition/Deuteration Cycles and the Effect of Carbon on Phase Segregation. Int. J. Hydrogen Energy 2022, 47, 34195–34204. [Google Scholar] [CrossRef]
- Santiago, D.; Rodríguez-Calero, G.G.; Rivera, H.; Tryk, D.A.; Scibioh, M.A.; Cabrera, C.R. Platinum Electrodeposition at High Surface Area Carbon Vulcan-XC-72R Material Using a Rotating Disk-Slurry Electrode Technique. J. Electrochem. Soc. 2010, 157, F189. [Google Scholar] [CrossRef]
- Lázaro, M.J.; Calvillo, L.; Celorrio, V.; Pardo, J.; Perathoner, S.; Moliner, R. Study and Application of Vulcan XC-72 in Low Temperature Fuel Cells. In Carbon Black: Production, Properties and Uses; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 41–68. ISBN 978-1-61209-535-6. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kabekkodu, S.N.; Faber, J.; Fawcett, T. New Powder Diffraction File (PDF-4) in Relational Database Format: Advantages and Data-Mining Capabilities. Acta Crystallogr. B 2002, 58, 333–337. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High Temperature PEM Fuel Cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
| Compound | SBET/ m2 g−1 | Vpore/ cm3 g−1 | TR1,peak 1/ K | nH2,800K/nH2,theoretical 2 |
|---|---|---|---|---|
| LAH mortared | - | - | 437 | 0.89 |
| LAH ball-milled | 8 | 0.017 | 420 | 0.91 |
| LAH 80 | 31 | 0.069 | 393 | 0.95 |
| LAH 70 | 46 | 0.10 | 388 | 0.91 |
| LAH 60 | 60 | 0.13 | 380 | 0.93 |
| LAH 50 | 59 | 0.15 | 376 | 0.81 |
| LAH 40 | 84 | 0.17 | 364 | 0.83 |
| LAH 30 | 89 | 0.15 | 365 | 0.77 |
| LAH 20 | 109 | 0.20 | - | 0.67 |
| MCB ball-milled | 226 | 0.34 |
| t/days | SBET/ m2 g−1 | Vpore/ cm3 g−1 | nH2,800K/nH2,theoretical 1 |
|---|---|---|---|
| 1 | 52 | 0.12 | 0.69 |
| 7 | 89 | 0.161 | 0.34 |
| 30 | 109 | 0.20 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramah, P.; Palm, R.; Tuul, K.; Aruväli, J.; Månsson, M.; Lust, E. Confinement of LiAlH4 in a Mesoporous Carbon Black for Improved Near-Ambient Release of H2. Reactions 2023, 4, 635-646. https://doi.org/10.3390/reactions4040035
Ramah P, Palm R, Tuul K, Aruväli J, Månsson M, Lust E. Confinement of LiAlH4 in a Mesoporous Carbon Black for Improved Near-Ambient Release of H2. Reactions. 2023; 4(4):635-646. https://doi.org/10.3390/reactions4040035
Chicago/Turabian StyleRamah, Pavle, Rasmus Palm, Kenneth Tuul, Jaan Aruväli, Martin Månsson, and Enn Lust. 2023. "Confinement of LiAlH4 in a Mesoporous Carbon Black for Improved Near-Ambient Release of H2" Reactions 4, no. 4: 635-646. https://doi.org/10.3390/reactions4040035
APA StyleRamah, P., Palm, R., Tuul, K., Aruväli, J., Månsson, M., & Lust, E. (2023). Confinement of LiAlH4 in a Mesoporous Carbon Black for Improved Near-Ambient Release of H2. Reactions, 4(4), 635-646. https://doi.org/10.3390/reactions4040035










