A Simple Way to Obtain a Decachloro Derivative of Cobalt Bis(dicarbollide)
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
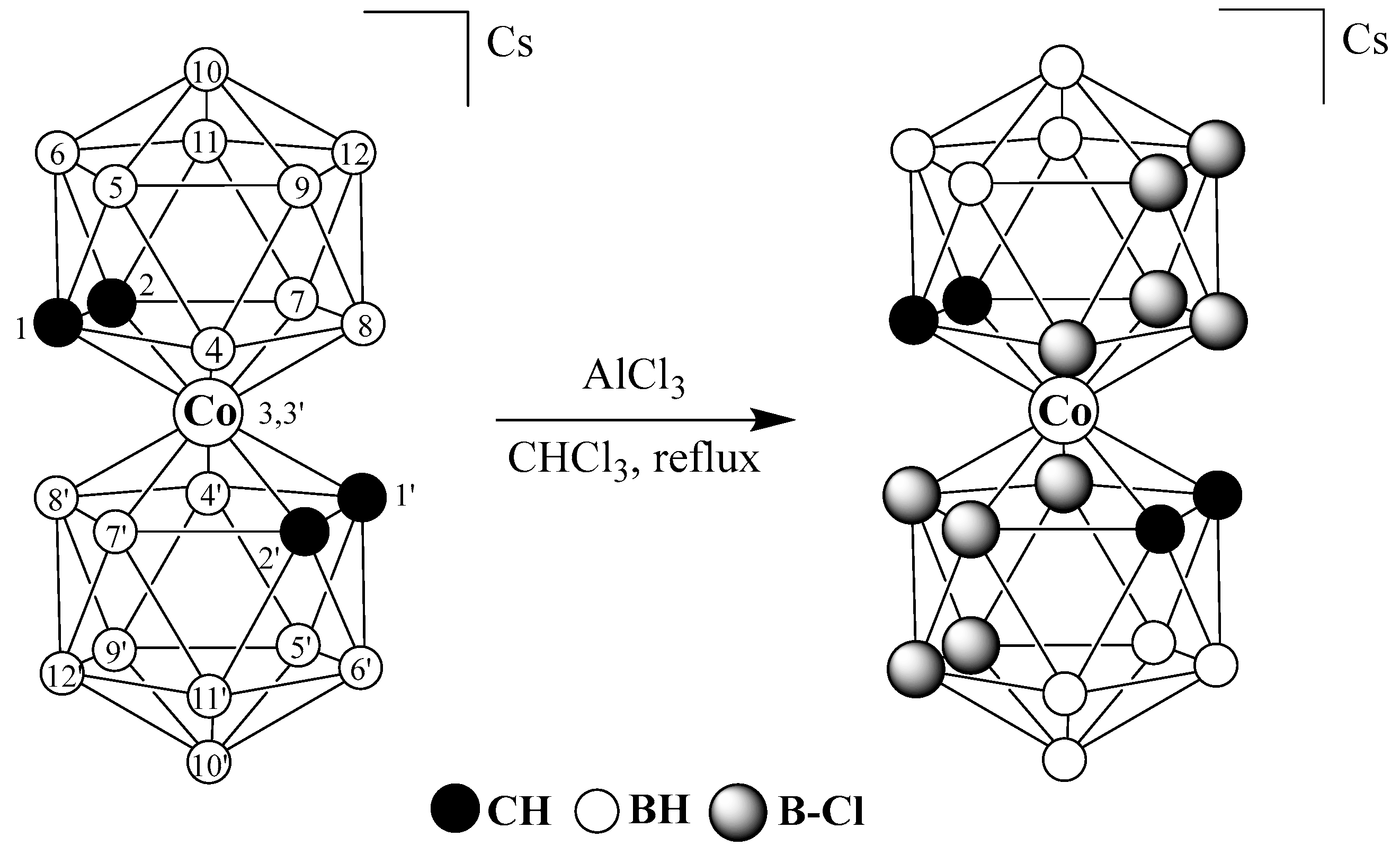
2.2. Synthesis of Cs[3,3′-Co(4,7,8,9,12-Cl5-1,2-C2B9H6)2]
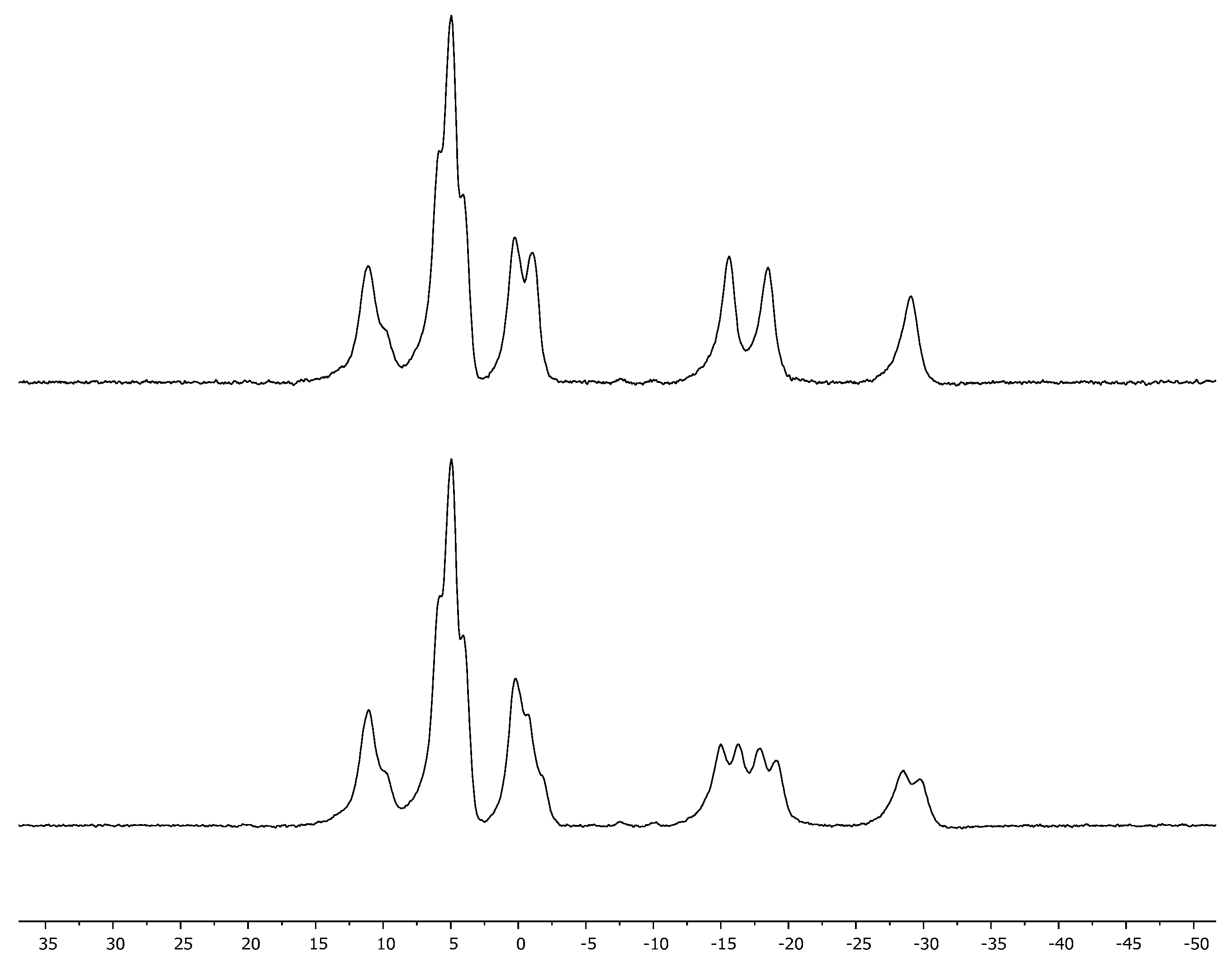
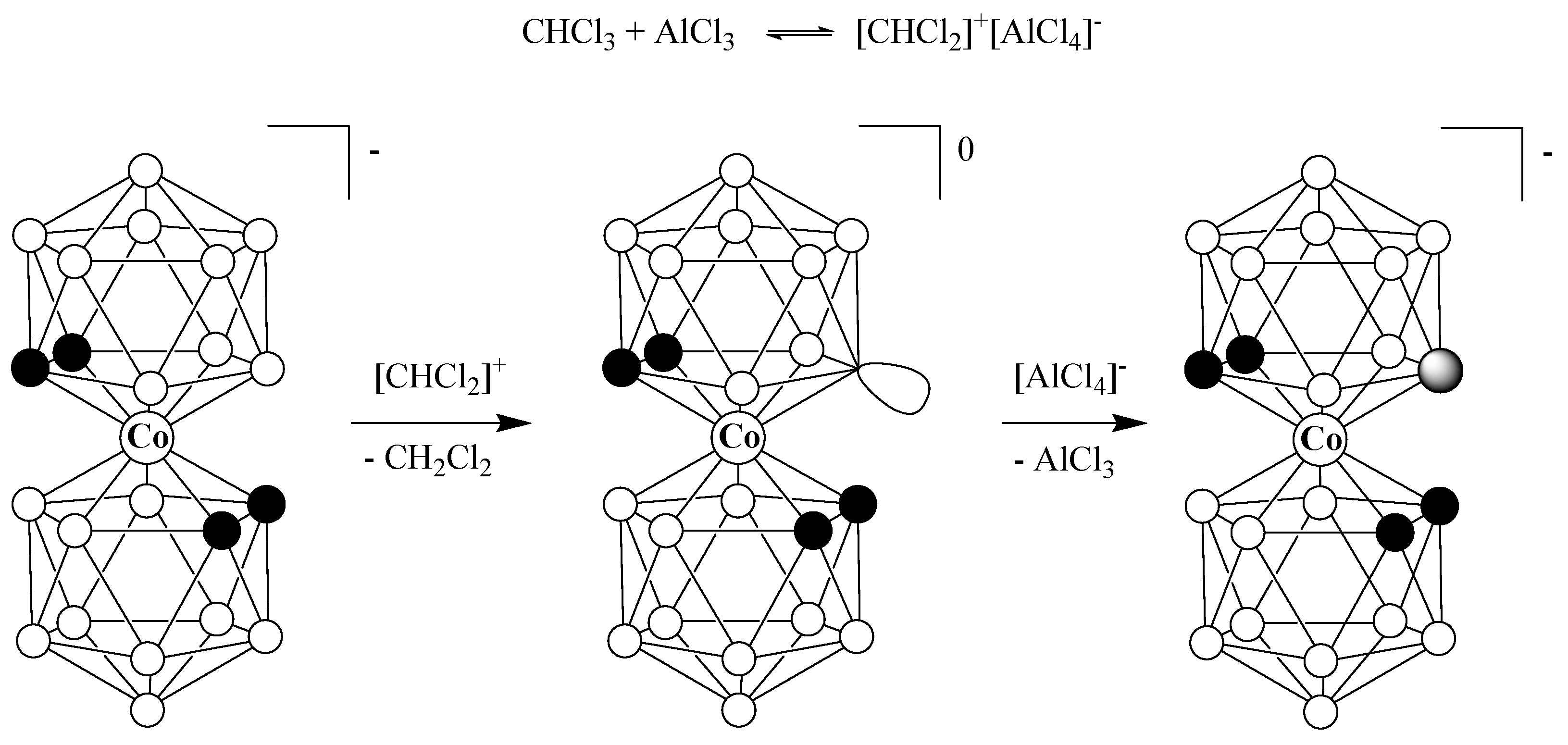
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sivaev, I.B.; Bregadze, V.I. Chemistry of cobalt bis(dicarbollides). A review. Collect. Czech. Chem. Commun. 1999, 64, 783–805. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Swain, B.R.; Mahanta, C.S.; Jena, B.B.; Hosmane, N.S. Cobalt bis(dicarbollide) anion and its derivatives. J. Organomet. Chem. 2017, 849–850, 170–194. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Andrews, T.D. Carborane analogues of cobalticinium ion. J. Chem. Soc. Chem. Commun. 1965, 19, 443–444. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Young, D.; Andrews, T.D.; Hove, D.V.; Pilling, R.L.; Pitts, A.D.; Reintjes, M.; Warren, L.F.; Wegner, P.A. π-Dicarbollyl derivatives of the transition metals. Metallocene analogs. J. Am. Chem. Soc. 1968, 90, 879–896. [Google Scholar] [CrossRef]
- Volovetsky, A.B.; Sukhov, V.S.; Balalaeva, I.V.; Dudenkova, V.V.; Shilyagina, N.Y.; Feofanov, A.V.; Efremenko, A.V.; Grin, M.A.; Mironov, A.F.; Sivaev, I.B.; et al. Pharmacokinetics of chlorin e6-cobalt bis(dicarbollide) conjugate in balb/c mice with engrafted carcinoma. Int. J. Mol. Sci. 2017, 18, 2556. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Sivaev, I.B.; Dubey, R.D.; Semioshkin, A.; Shmal’ko, A.V.; Kosenko, I.D.; Lebedeva, K.V.; Mandal, S.; Sreejyothi, P.; Sarkar, A.; et al. Boron-containing lipids and liposomes: New conjugates of cholesterol with polyhedral boron hydrides. Chem. Eur. J. 2020, 26, 13832–13841. [Google Scholar] [CrossRef]
- Druzina, A.A.; Shmalko, A.V.; Andreichuk, E.P.; Zhidkova, O.B.; Kosenko, I.D.; Semioshkin, A.; Sivaev, I.B.; Mandal, S.; Shen, Z.; Bregadze, V.I. ‘Click’ synthesis of cobalt bis(dicarbollide)-cholesterol conjugates. Mendeleev Commun. 2019, 29, 628–630. [Google Scholar] [CrossRef]
- Couto, M.; Alamón, C.; Nievas, S.; Perona, M.; Dagrosa, M.A.; Teixidor, F.; Cabral, P.; Viñas, C.; Cerecetto, H. Bimodal therapeutic agents against glioblastoma, one of the most lethal forms of cancer. Chem.-Eur. J. 2020, 26, 14335–14340. [Google Scholar] [CrossRef]
- Dubey, R.D.; Sarkar, A.; Shen, Z.; Bregadze, V.I.; Sivaev, I.B.; Druzina, A.A.; Zhidkova, O.B.; Shmal’ko, A.V.; Kosenko, I.D.; Sreejyothi, P.; et al. Effects of linkers on the development of liposomal formulation of cholesterol conjugated cobalt bis(dicarbollides). J. Pharm. Sci. 2021, 110, 1365–1373. [Google Scholar] [CrossRef]
- Couto, M.; Mastandrea, I.; Cabrera, M.; Cabral, P.; Teixidor, F.; Cerecetto, H.; Viñas, C. Small-molecule kinase-inhibitors-loaded boron cluster as hybrid agents for glioma-cell-targeting therapy. Chem.-Eur. J. 2017, 23, 9233–9238. [Google Scholar] [CrossRef]
- Grüner, B.; Brynda, J.; Das, V.; Šicha, V.; Stepankova, J.; Nekvinda, J.; Holub, J.; Pospisilova, K.; Fabry, M.; Pachtl, P.; et al. Metallacarborane sulfamides: Unconventional, specific, and highly selective inhibitors of carbonic anhydrase IX. J. Med. Chem. 2019, 62, 9560–9575. [Google Scholar] [CrossRef] [PubMed]
- Nekvinda, J.; Rozycka, D.; Rykowski, S.; Wyszko, E.; Fedoruk-Wyszomirska, A.; Gurda, D.; Orlicka-Płocka, M.; Giel-Pietraszuk, M.; Kiliszek, A.; Rypniewski, W.; et al. Synthesis of naphthalimide-carborane and metallacarborane conjugates: Anticancer activity, DNA binding ability. Bioorg. Chem. 2020, 94, 103432. [Google Scholar] [CrossRef] [PubMed]
- Kugler, M.; Nekvinda, J.; Holub, J.; El Anwar, S.; Das, V.; Šícha, V.; Pospíšilová, K.; Fábry, M.; Král, V.; Brynda, J.; et al. Inhibitors of CA IX enzyme based on polyhedral boron compounds. ChemBioChem 2021, 22, 2741–2761. [Google Scholar] [CrossRef]
- Murphy, N.; McCarthy, E.; Dwyer, R.; Farras, P. Boron clusters as breast cancer therapeutics. J. Inorg. Biochem. 2021, 218, 111412. [Google Scholar] [CrossRef] [PubMed]
- Anufriev, S.A.; Erokhina, S.A.; Suponitsky, K.Y.; Godovikov, I.A.; Filippov, O.A.; Fabrizi de Biani, F.; Corsini, M.; Chizhov, A.O.; Sivaev, I.B. Methylsulfanyl-stabilized rotamers of cobalt bis(dicarbollide). Eur. J. Inorg. Chem. 2017, 2017, 4444–4451. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Timofeev, S.V.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B. Bis(dicarbollide) complexes of transition metals as a platform for molecular switches. Study of complexation of 8,8′-bis(methylsulfanyl) derivatives of cobalt and iron bis(dicarbollides). Molecules 2020, 25, 5745. [Google Scholar] [CrossRef]
- Saini, A.; Fuentes, I.; Viñas, C.; Zine, N.; Bausells, J.; Errachid, A.; Teixidor, F. A simple membrane with the electroactive [Sulfapyridine-H]+[Co(C2B9H11)2]− for the easy potentiometric detection of sulfonamides. J. Organomet. Chem. 2019, 893, 32–38. [Google Scholar] [CrossRef]
- Tarres, M.; Arderiu, V.S.; Zaulet, A.; Viñas, C.; Fabrizi de Biani, F.; Teixidor, F. How to get the desired reduction voltage in a single framework! Metallacarborane as an optimal probe for sequential voltage tuning. Dalton Trans. 2015, 44, 11690–11695. [Google Scholar] [CrossRef]
- Buades, A.B.; Arderiu, V.S.; Olid-Britos, D.; Viñas, C.; Sillanpää, R.; Haukka, M.; Fontrodona, X.; Paradinas, M.; Ocal, C.; Teixidor, F. Electron accumulative molecules. J. Am. Chem. Soc. 2018, 140, 2957–2970. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Dyachenko, O.A.; Kazheva, O.N.; Kravchenko, A.V.; Sivaev, I.B.; Starodub, V.A. Tetrathiafulvalene-based radical cation salts with transition metal bis(dicarbollide) anions. CrystEngComm 2015, 17, 4754–4767. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Dyachenko, O.A.; Kazheva, O.N.; Kosenko, I.D.; Kravchenko, A.V.; Sivaev, I.B.; Starodub, V.A. Electroconducting radical-cation salts based on tetrathiafulvalene derivatives and transition metal bis(dicarbollides). Russ. J. Gen. Chem. 2019, 89, 971–987. [Google Scholar] [CrossRef]
- Rais, J.; Selucký, P.; Kyrš, M. Extraction of alkali metals into nitrobenzene in the presence of univalent polyhedral borate anions. J. Inorg. Nucl. Chem. 1976, 38, 1376–1378. [Google Scholar] [CrossRef]
- Plešek, J.; Baše, K.; Mareš, F.; Hanousek, F.; Štíbr, B.; Heřmánek, S. Potential uses of metallocarborane sandwich anions for analysis, characterization and isolation of various cations and organic bases. Collect. Czech. Chem. Commun. 1984, 49, 2776–2789. [Google Scholar] [CrossRef]
- Herbst, R.S.; Peterman, D.R.; Tillotson, R.D.; Delmau, L.H. Aspects of the fundamental chemistry of cesium extraction from acidic media by HCCD. Czech. J. Phys. 2006, 56, D477–D482. [Google Scholar] [CrossRef]
- Herbst, R.S.; Peterman, D.R.; Tillotson, R.D.; Delmau, L.H. Fundamental Chemistry of Cesium Extraction from Acidic Media by HCCD in FS-13. Solvent Extr. Ion Exch. 2008, 26, 163–174. [Google Scholar] [CrossRef]
- Romanovskiy, V.N.; Smirnov, I.V.; Babain, V.A.; Todd, T.A.; Herbst, R.S.; Law, J.D.; Brewer, K.N. The Universal solvent extraction (UNEX) process. I. Development of the UNEX process solvent for the separation of cesium, strontium, and the actinides from acidic radioactive waste. Solvent Extr. Ion Exch. 2001, 19, 1–21. [Google Scholar] [CrossRef]
- Buades, A.B.; Viñas, C.; Fontrodona, X.; Teixidor, F. 1.3 V Inorganic sequential redox chain with an all-anionic couple 1−/2− in a single framework. Inorg. Chem. 2021, 60, 16168–16177. [Google Scholar] [CrossRef] [PubMed]
- Mátel, Ľ.; Macášek, F.; Rajec, P.; Heřmánek, S.; Plešek, J. B-Halogen derivatives of the bis(1,2-dicarbollyl)cobalt(III) anion. Polyhedron 1982, 1, 511–519. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Timofeev, S.V.; Zhidkova, O.B.; Suponitsky, K.Y.; Sivaev, I.B. Synthesis, Crystal Structure, and Some Transformations of 9,12-Dichloro-ortho-Carborane. Crystals 2022, 12, 1251. [Google Scholar] [CrossRef]
- Koval, M.; Kaniansky, D.; Mátel, Ľ.; Macáśek, F. Analysis of boron-halogen derivatives of bis(1,2-dicarbollyl)cobalt(III) anions by capillary isotachophoresis. J. Chromatog. A 1982, 243, 144–148. [Google Scholar] [CrossRef]
- Šimuničová, E.; Kanianský, D. Isotachophoretic determination of chloro derivatives of cobaltocarborane without the use of reference analytes. J. Chromatog. A 1987, 390, 121–132. [Google Scholar] [CrossRef]
- Hurlburt, P.K.; Miller, R.L.; Abney, K.D.; Foreman, T.M.; Butcher, R.J.; Kinkhead, S.A. New synthetic routes to B-halogenated derivatives of cobalt dicarbollide. Inorg. Chem. 1995, 34, 5215–5219. [Google Scholar] [CrossRef]
- González-Cardoso, P.; Stoica, A.-I.; Farràs, P.; Pepiol, A.; Viñas, C.; Teixidor, F. Additive tuning of redox potential in metallacarboranes by sequential halogen substitution. Chem. Eur. J. 2010, 16, 6660–6665. [Google Scholar] [CrossRef]
- Wallace, C.H.; Willard, J.E. The exchange reaction between aluminum chloride and carbon tetrachloride. J. Am. Chem. Soc. 1950, 72, 5275–5281. [Google Scholar] [CrossRef]
- Olah, G.A.; Heiliger, L.; Prakash, G.K.S. Stable carbocations. Part 276. Trihalomethyl cations. J. Am. Chem. Soc. 1989, 111, 8020–8021. [Google Scholar] [CrossRef]
- Olah, G.A.; Rasul, G.; Heiliger, L.; Prakash, G.K.S. Preparation, NMR spectroscopic, and ab initio/DFT/GIAO-MP2 studies of halomethyl cations. J. Am. Chem. Soc. 1996, 118, 3580–3583. [Google Scholar] [CrossRef]
- Mercier, H.P.A.; Moran, M.D.; Schrobilgen, G.J.; Steinberg, C.; Suontamo, R.J. The syntheses of carbocations by use of the noble-gas oxidant, [XeOTeF5][Sb(OTeF5)6]: the syntheses and characterization of the CX3+ (X = Cl, Br, OTeF5) and CBr(OTeF5)2+ cations and theoretical studies of CX3+ and BX3 (X = F, Cl, Br, I, OTeF5). J. Am. Chem. Soc. 2004, 126, 5533–5548. [Google Scholar] [CrossRef] [PubMed]
- Lehner, A.J.; Trapp, N.; Scherera, H.; Krossing, I. CCl3+ and CBr3+ salts with the [Al(ORF)4]− and [(FRO)3Al-F-Al(ORF)3]− anions (RF = C(CF3)3). Dalton Trans. 2011, 40, 1448–1452. [Google Scholar] [CrossRef]
- Bach, R.D.; Badger, R.C. A Convenient preparation of 1,3,5,7-tetrachloroadamantane and 1,3,5,7-tetradeuterioadamantane. Synthesis 1979, 7, 529. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Okhlobystin, O.Y.; Semin, G.K.; Babushkina, T.A. Exchange of hydrogen by chlorine in the system barene-CCl4 or CHCl3 under the influence of aluminum chloride. Russ. Chem. Bull. 1965, 14, 1886. [Google Scholar] [CrossRef]
- Akhrem, I.; Orlinkov, A.; Vol’pin, M. A novel family of aprotic organic superacids for low-temperature alkane and cycloalkane transformations. J. Chem. Soc. Chem. Commun. 1993, 8, 671–672. [Google Scholar] [CrossRef]
- Akhrem, I.; Orlinkov, A. Polyhalomethanes combined with lewis acids in alkane chemistry. Chem. Rev. 2007, 107, 2037–2079. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.; Bukala, J. Selective electrophilic activation of alkanes. Acc. Chem. Res. 1993, 26, 370–376. [Google Scholar] [CrossRef]
- Olah, G.A. Superelectrophiles. Angew. Chem. Int. Ed. Engl. 1993, 32, 767–788. [Google Scholar] [CrossRef]
- Olah, G.A.; Rasul, G.; Yudin, A.K.; Burrichter, A.; Chistyakov, A.L.; Stankevich, I.V.; Akhrem, I.S.; Gambaryan, N.P.; Vol’pin, M.E. Trihalomethyl cations and their superelectrophilic activation. J. Am. Chem. Soc. 1996, 118, 1446–1451. [Google Scholar] [CrossRef]
- Akhrem, I.S.; Chistyakov, A.L.; Gambaryan, N.P.; Stankevich, I.V.; Vol’pin, M.E. Polyhalomethanes combined with aluminum halides as generators of superelectrophiles of a novel type. J. Organomet. Chem. 1997, 536-537, 489–495. [Google Scholar] [CrossRef]
- Suri, G.; Liang, F.; Hu, M.; Wang, M.; Bu, R.; Zhang, X.; Wang, H.; Dong, W.; Eerdun, C.; Tsuda, A. direct syntheses of diphenylmethanol derivatives from substituted benzenes and CHCl3 through friedel-crafts alkylation and post-synthetic hydrolysis or alcoholysis catalyzed by alumina. ChemistryOpen 2022, 11, e2022000. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Lewis acidity of boron compounds. Coord. Chem. Rev. 2014, 270–271, 75–78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anufriev, S.A.; Stogniy, M.Y.; Sivaev, I.B. A Simple Way to Obtain a Decachloro Derivative of Cobalt Bis(dicarbollide). Reactions 2023, 4, 148-154. https://doi.org/10.3390/reactions4010008
Anufriev SA, Stogniy MY, Sivaev IB. A Simple Way to Obtain a Decachloro Derivative of Cobalt Bis(dicarbollide). Reactions. 2023; 4(1):148-154. https://doi.org/10.3390/reactions4010008
Chicago/Turabian StyleAnufriev, Sergey A., Marina Yu. Stogniy, and Igor B. Sivaev. 2023. "A Simple Way to Obtain a Decachloro Derivative of Cobalt Bis(dicarbollide)" Reactions 4, no. 1: 148-154. https://doi.org/10.3390/reactions4010008
APA StyleAnufriev, S. A., Stogniy, M. Y., & Sivaev, I. B. (2023). A Simple Way to Obtain a Decachloro Derivative of Cobalt Bis(dicarbollide). Reactions, 4(1), 148-154. https://doi.org/10.3390/reactions4010008







