A Brief Review: Advancement in the Synthesis of Amine through the Leuckart Reaction
Abstract
1. Introduction
1.1. Background
1.2. Related Reactions
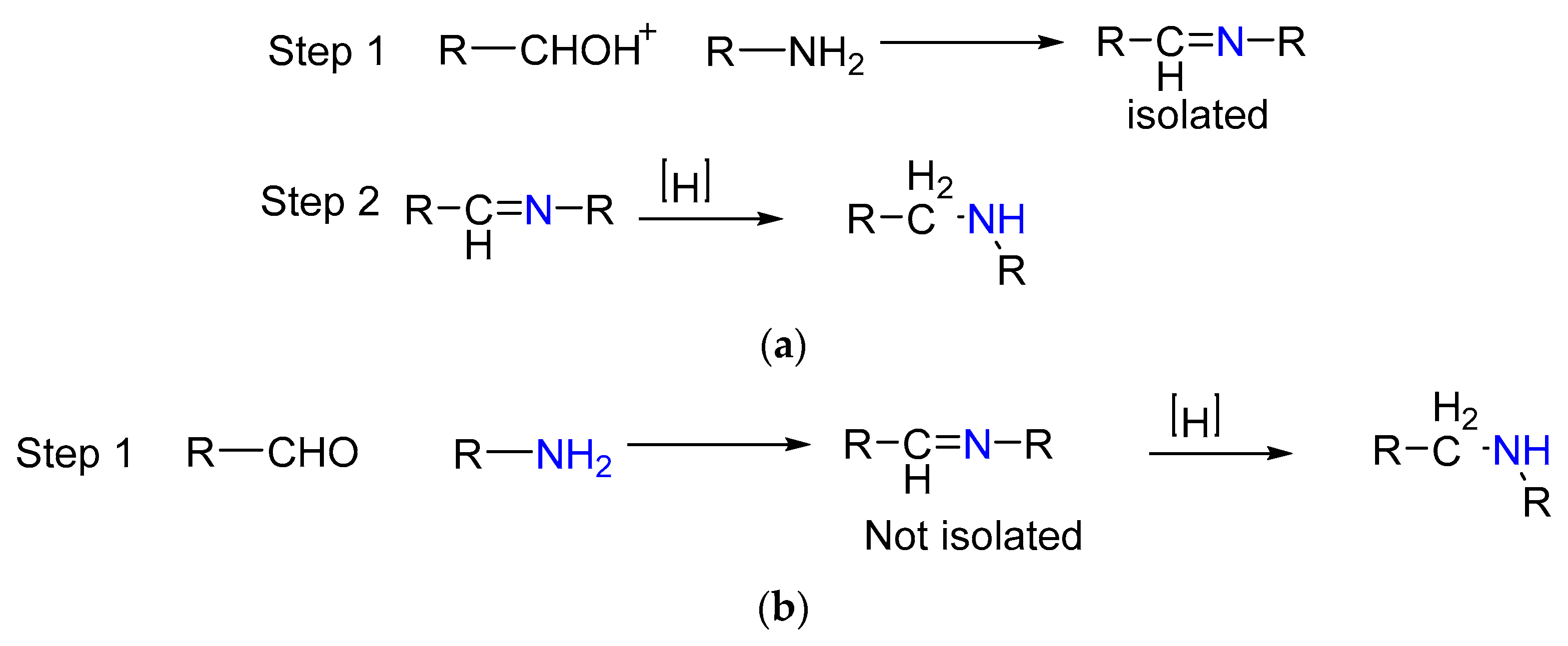
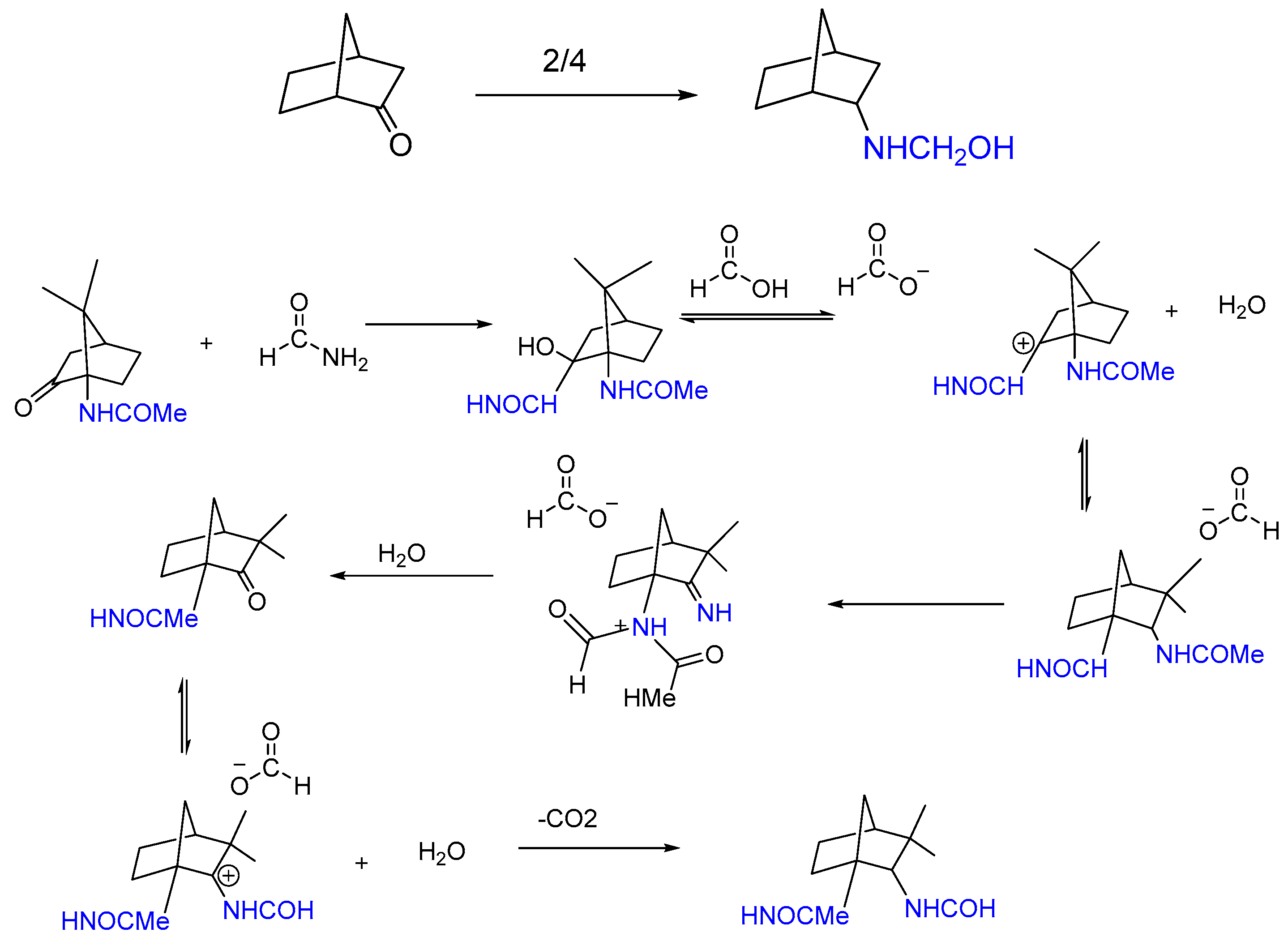
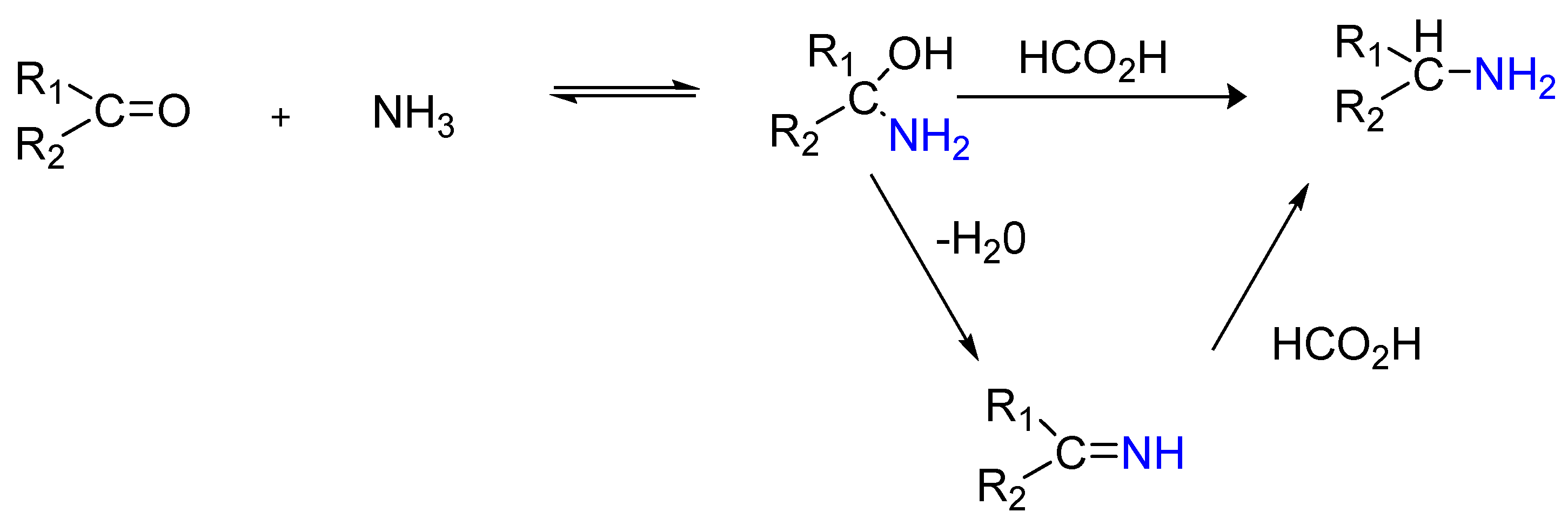
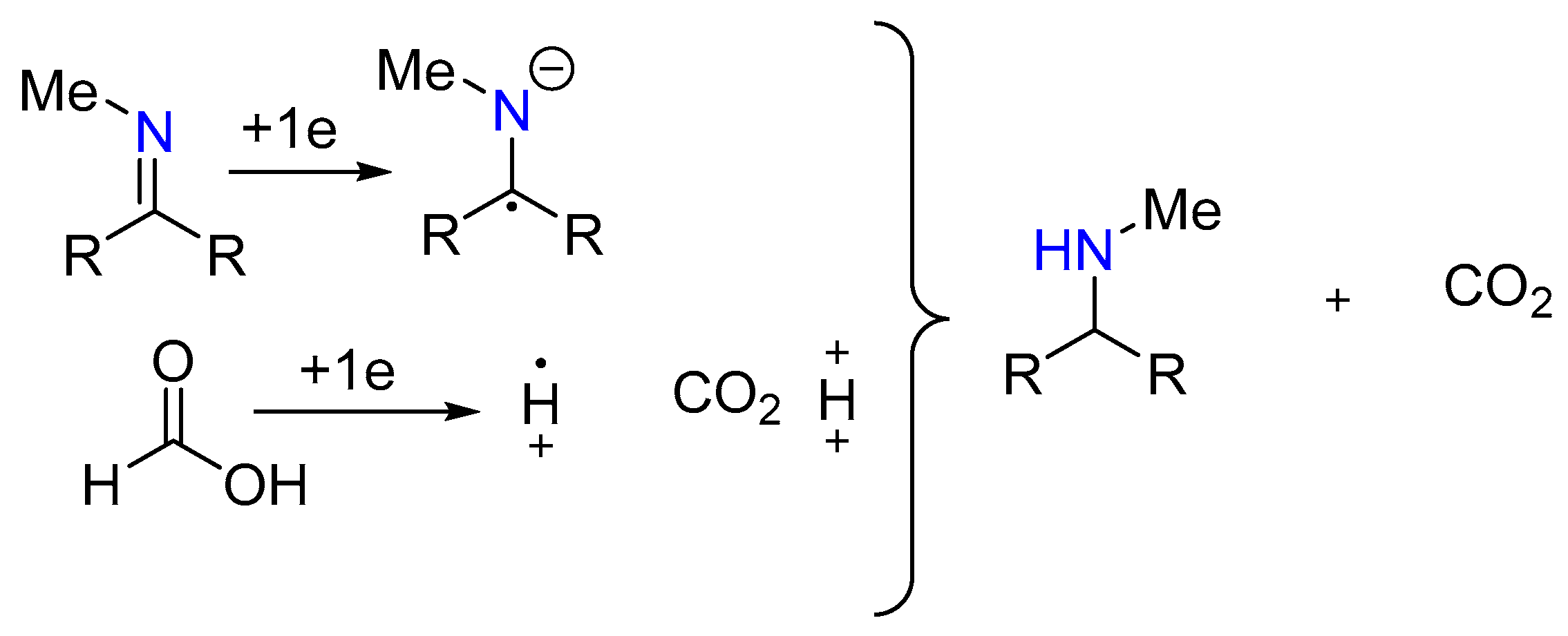
1.3. Mechanism
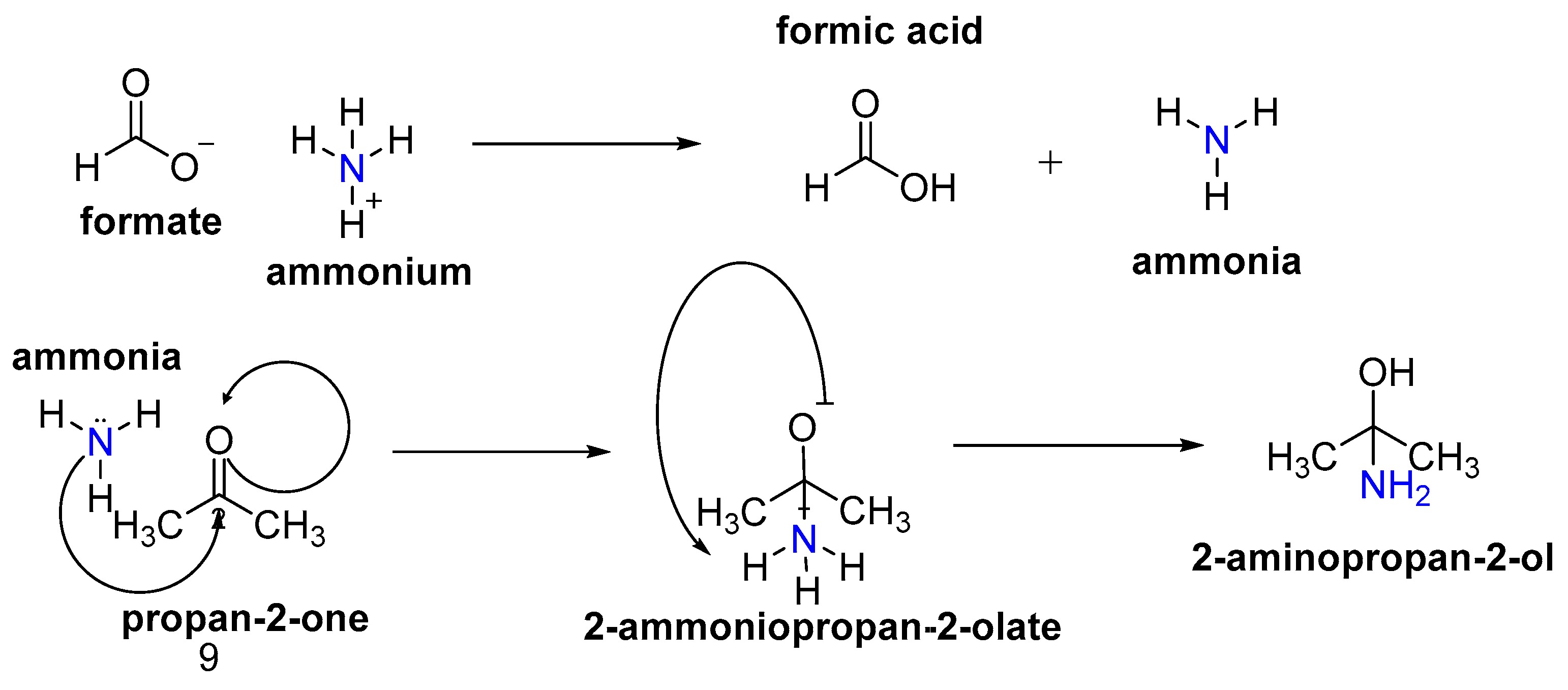
1.4. Kinetic Study of the Leuckart–Wallach Reaction
- (i)
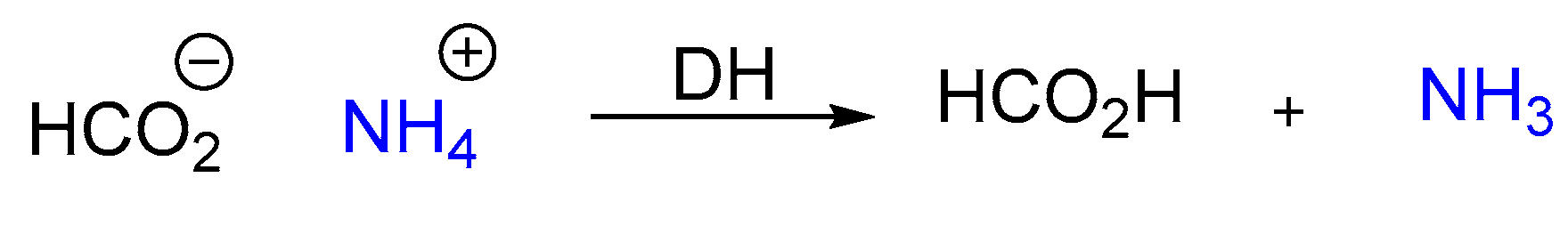
- Dissociation of ammonium formate into HCOOH and NH3;
- (ii)
- Nucleophilic attack of ammonia on the carbonyl carbon;
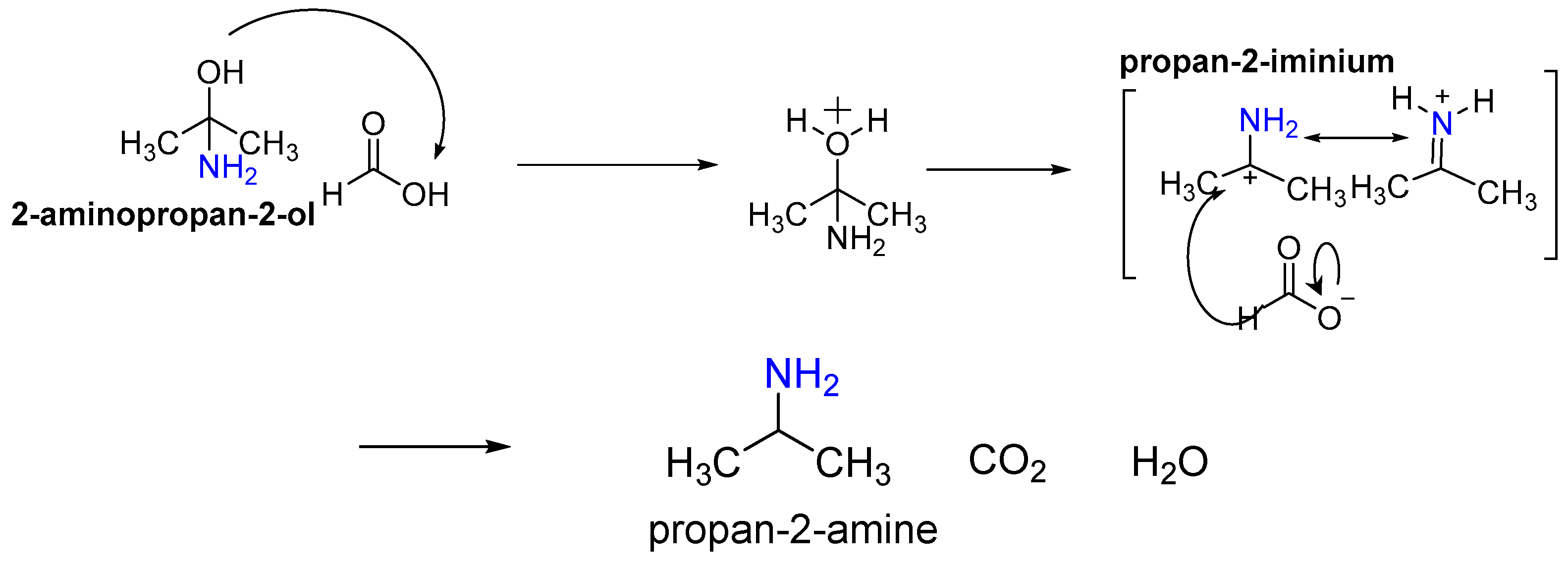
- (iii)
- Dehydration;
- (iv)
- Trans to cis isomerization of the formic acid;
- (v)
- Formation of amphetamine by the reduction of the produced 1-phenyl propane-2-imine. The reaction is spontaneous, and the reaction kinetics are of the first order [26].
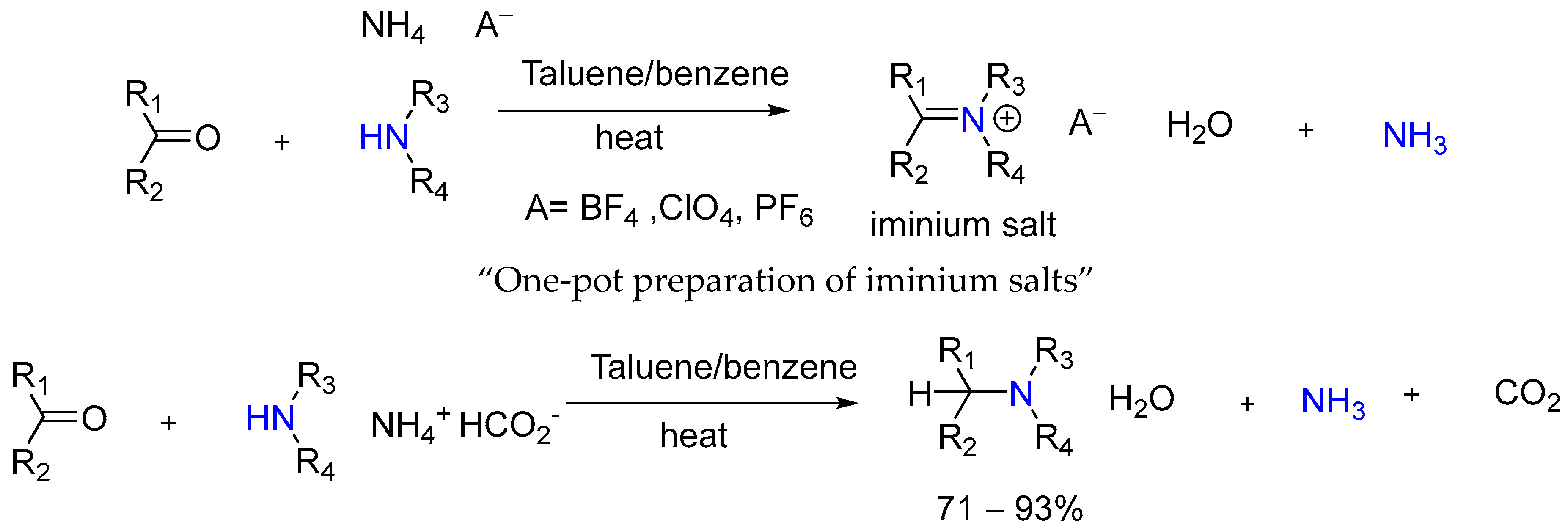
2. Significance of the Leuckart-Type Reaction
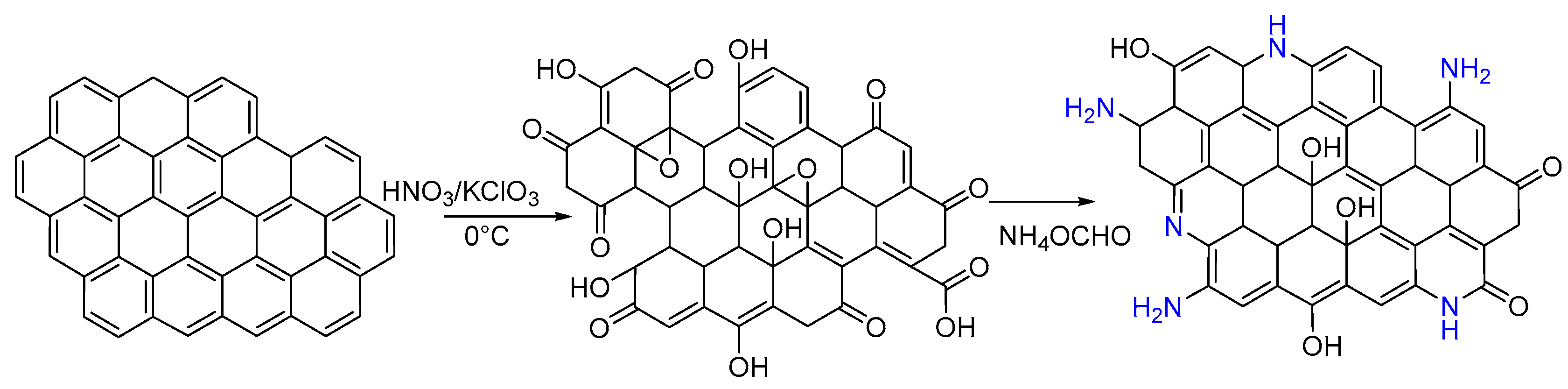
2.1. Synthesis of Animated Graphene and Amphetamine
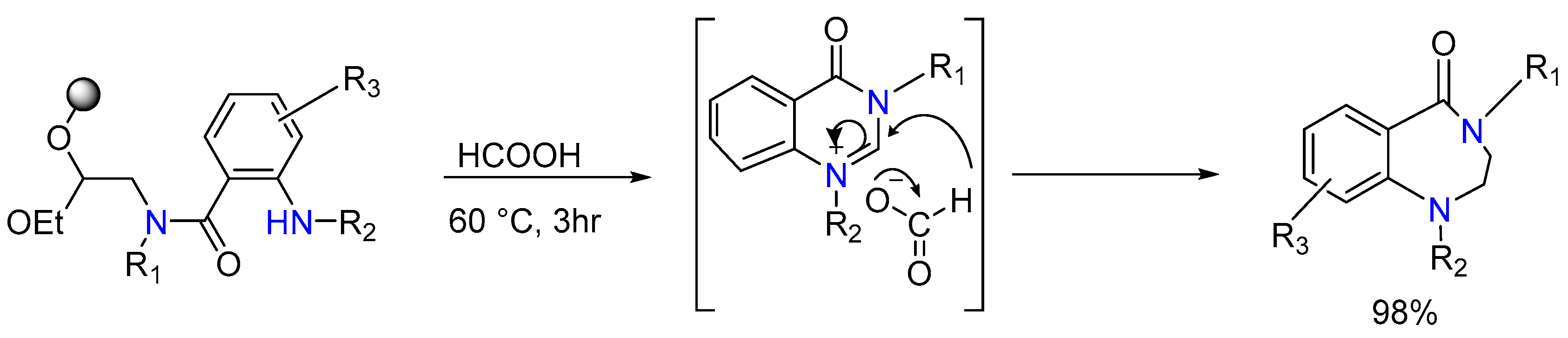
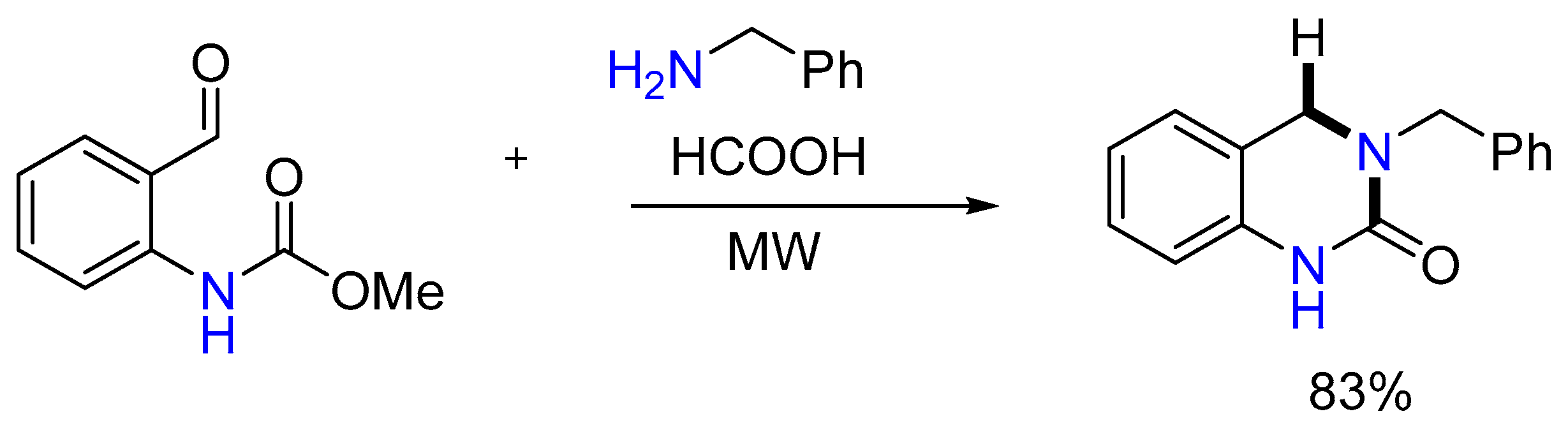
2.2. Synthesis of Tetrahydro-1,4-benzodiazepine-5-one and Arylamine
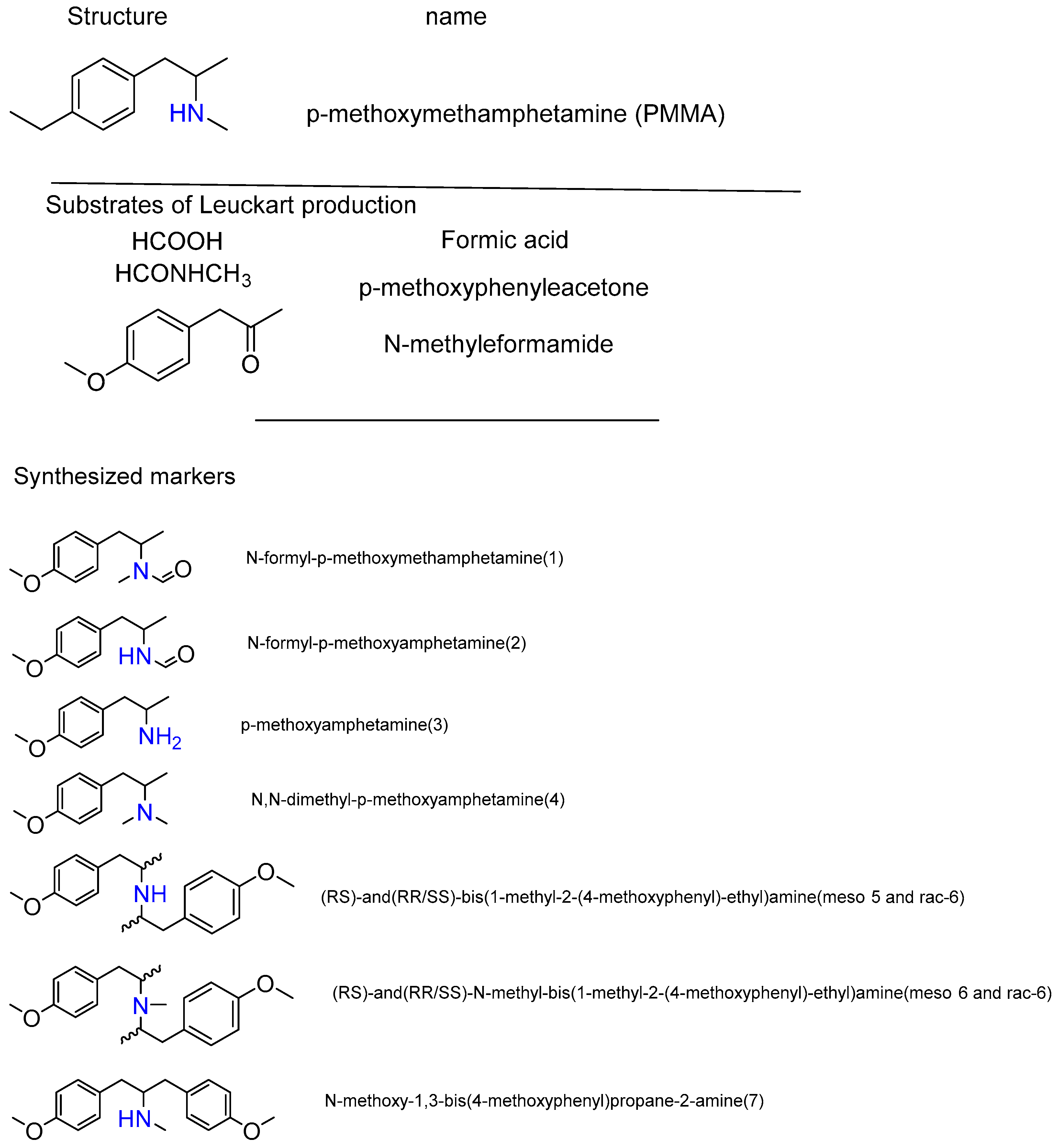
2.3. Synthesis of 4-methylthioamphetamine (4-MTM), (PMMA) and Heterocycles
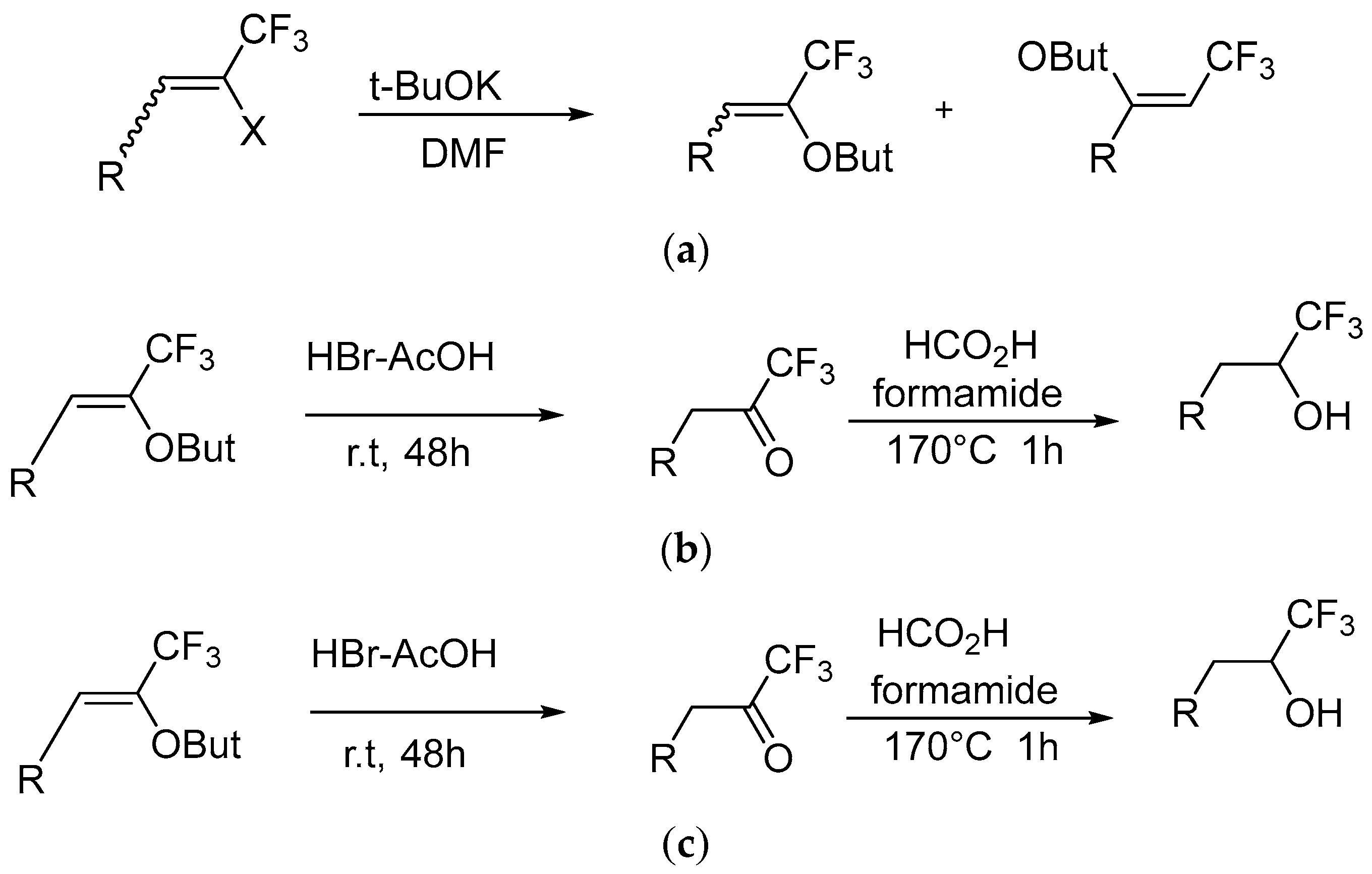
2.4. Synthesis of Trifluoromethyl Alcohol and Isocyanides
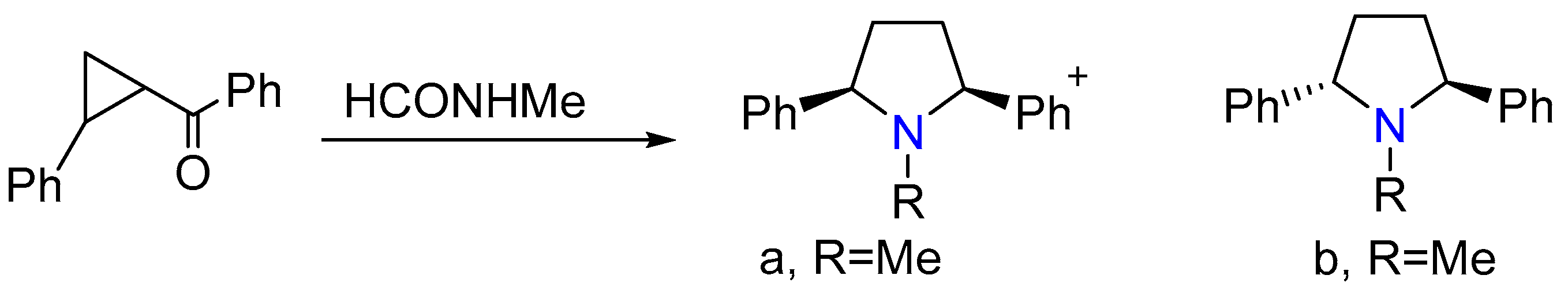
2.5. Synthesis of cis- and trans-l-Methyl-2,5-diphenylpyrrolidines
2.6. Synthesis of Racemic Tert-Leucine and Polyether Amines and PPGs
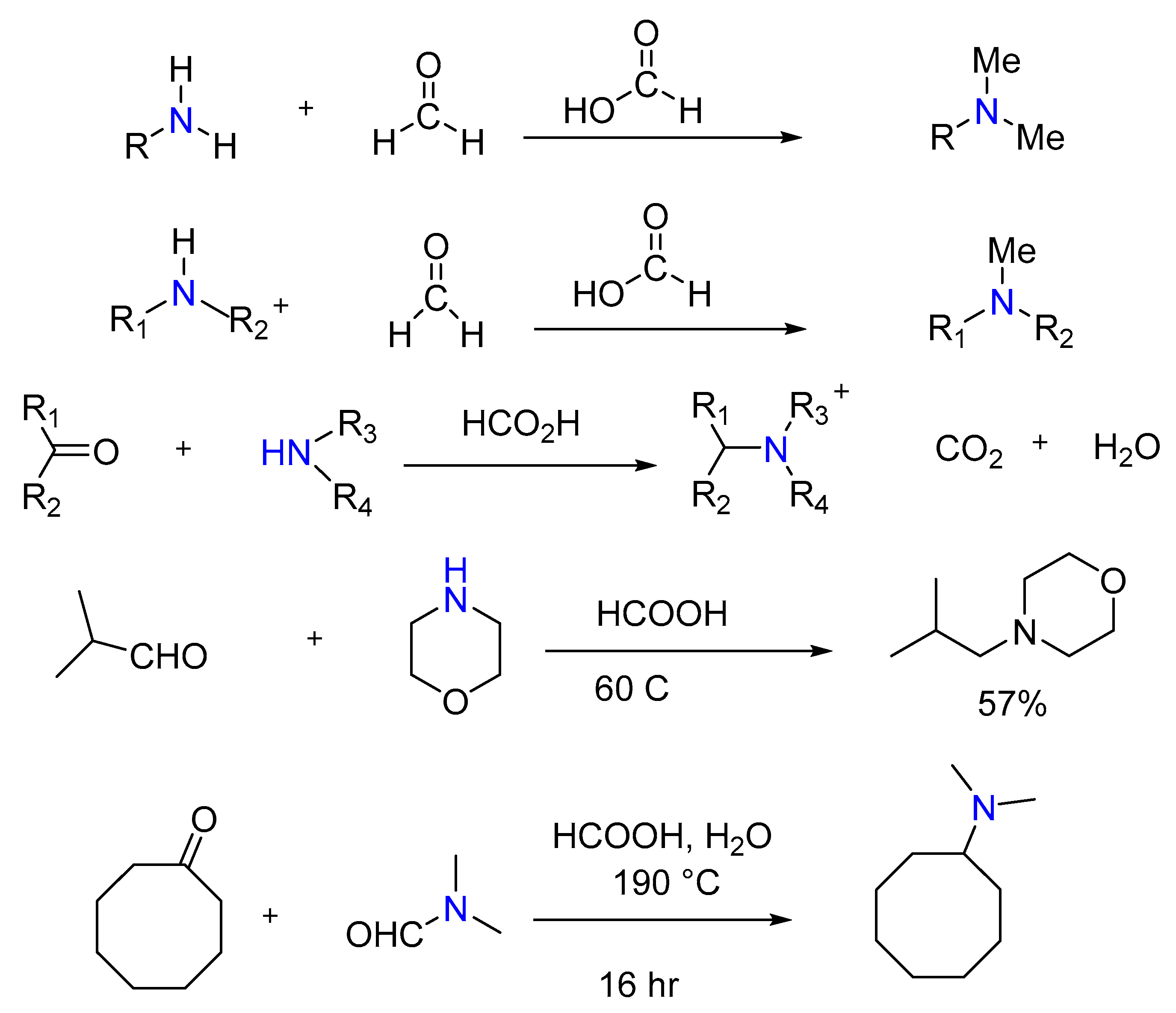
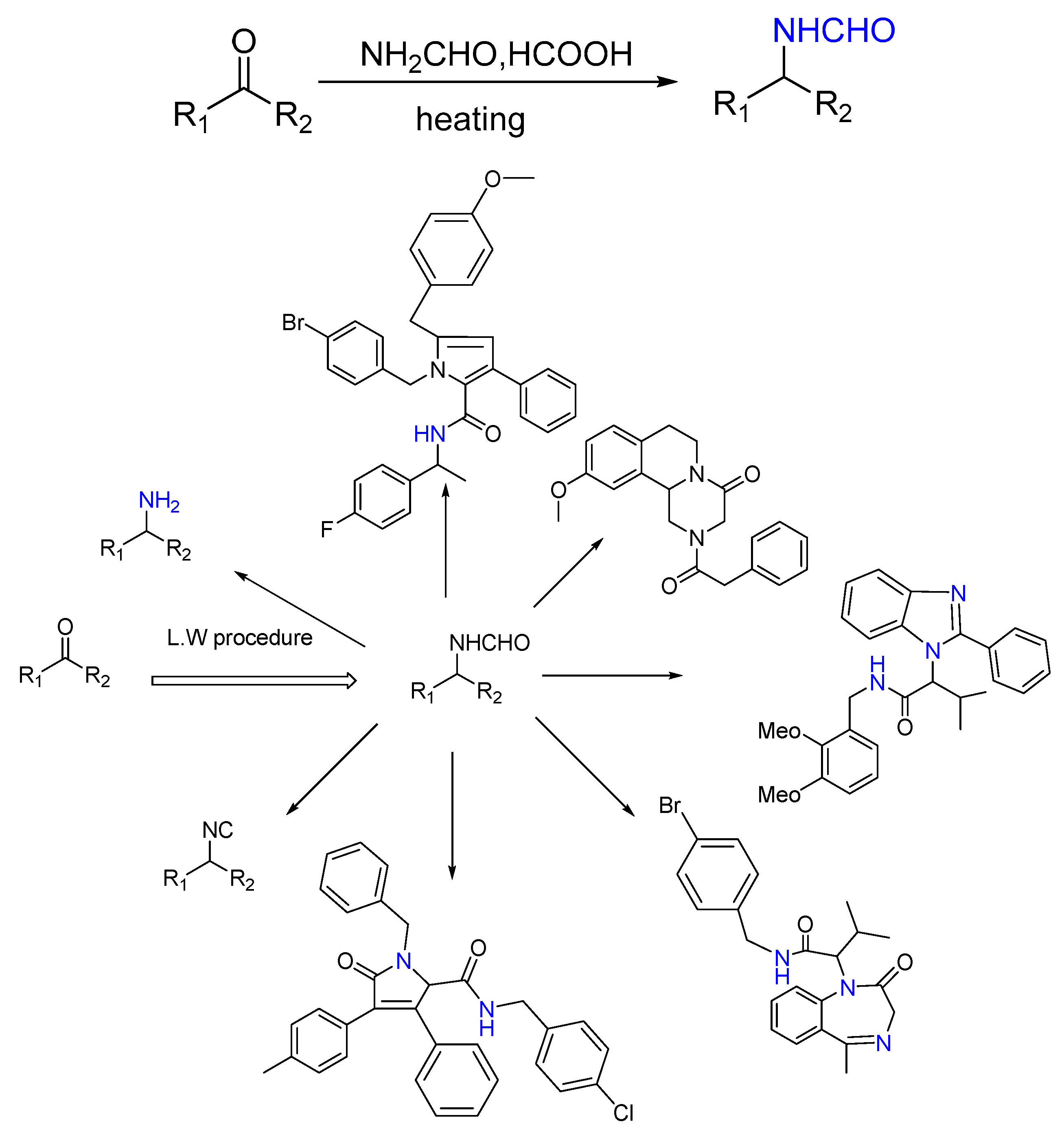
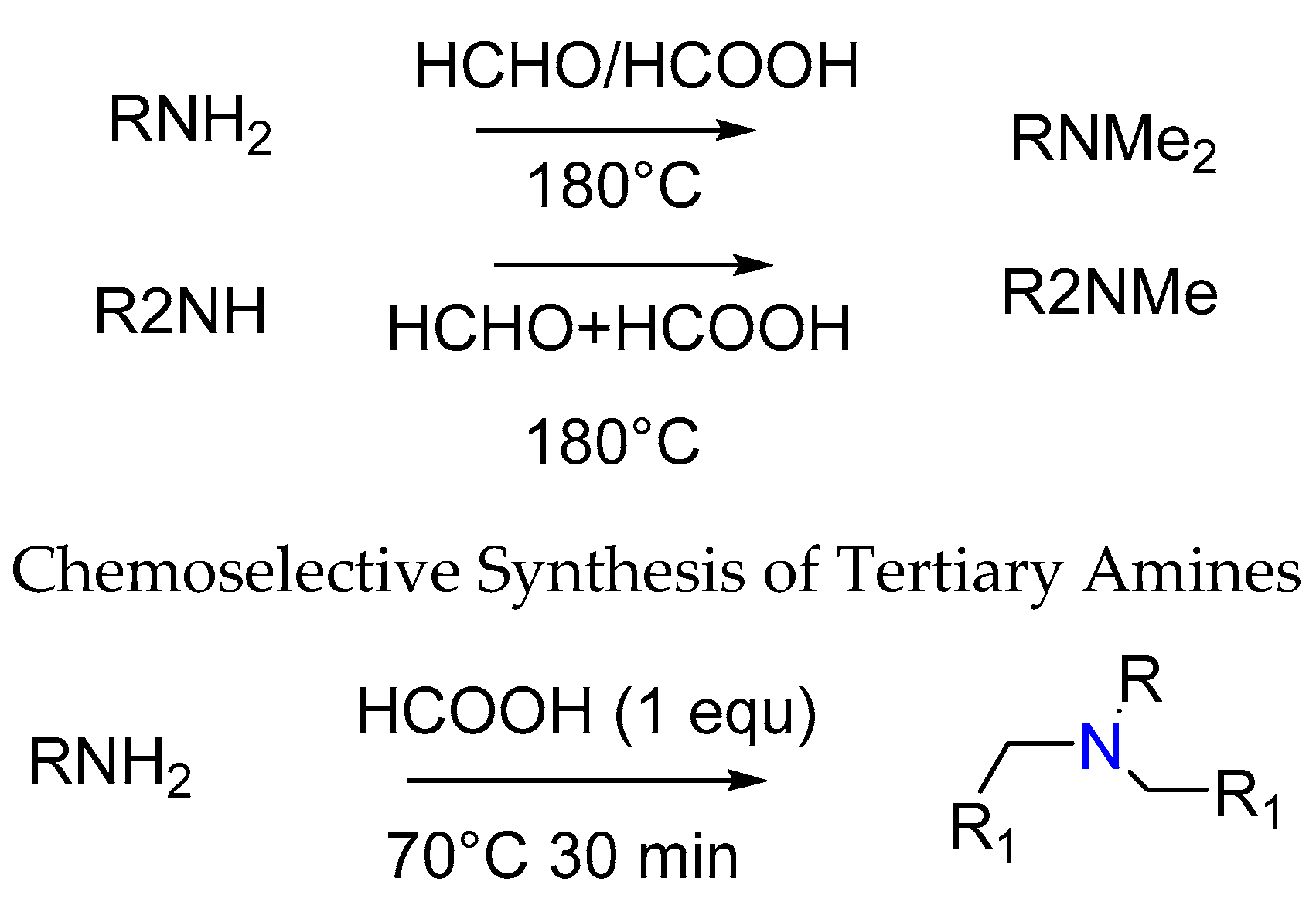
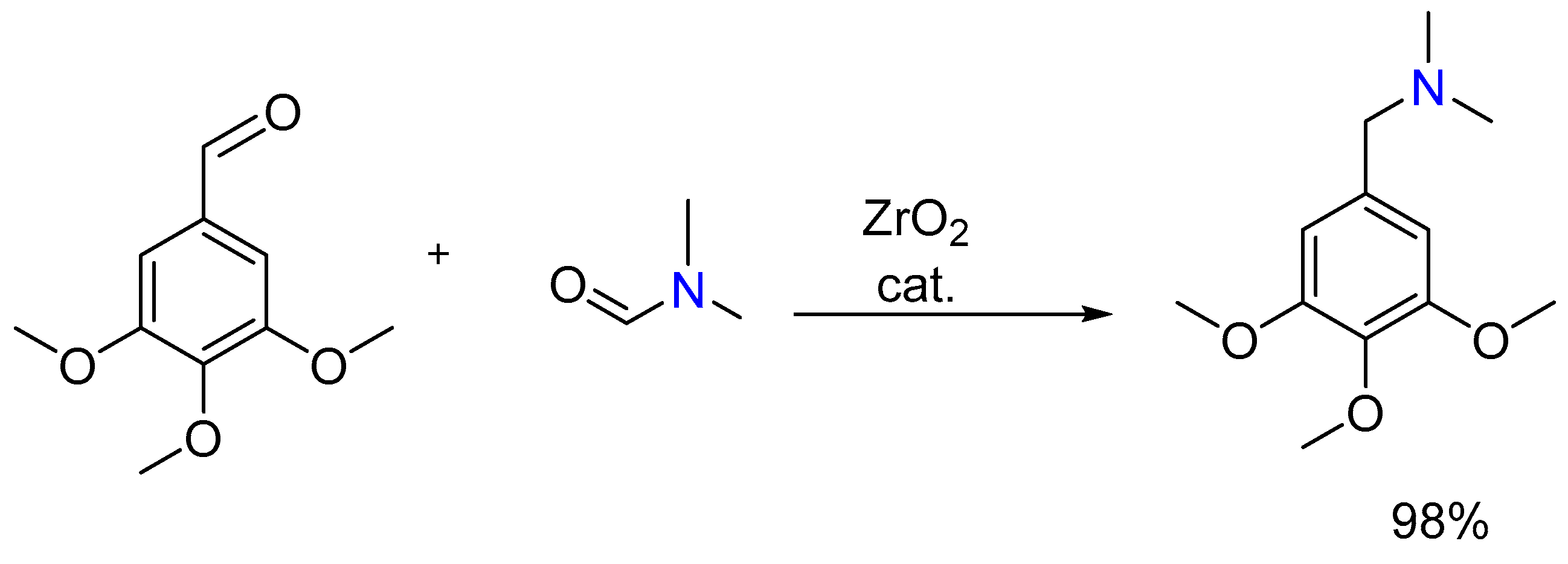
2.7. Synthesis of Tertiary Amines
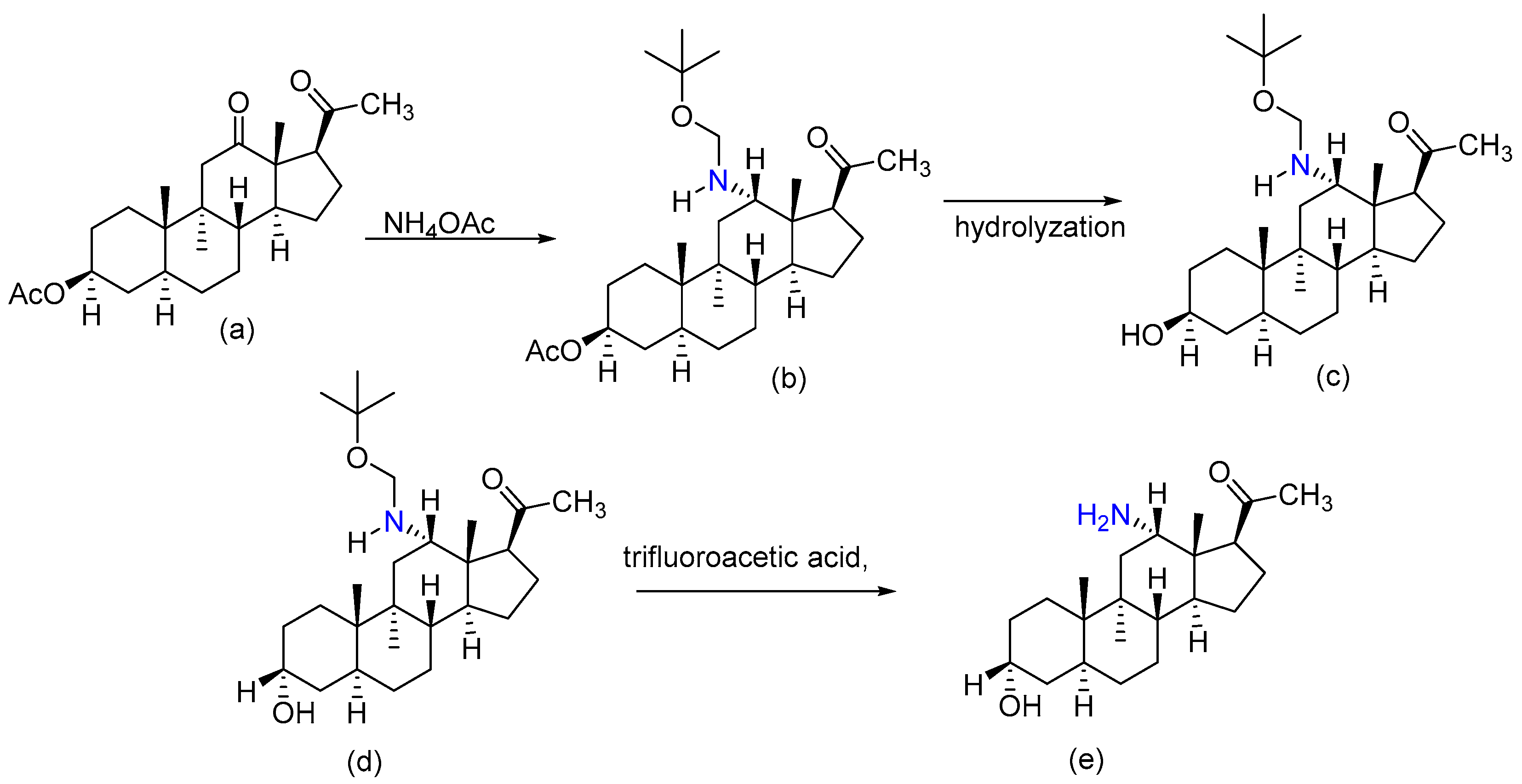
2.8. Synthesizing a 12β-Amino Derivative of Allopregnanolone
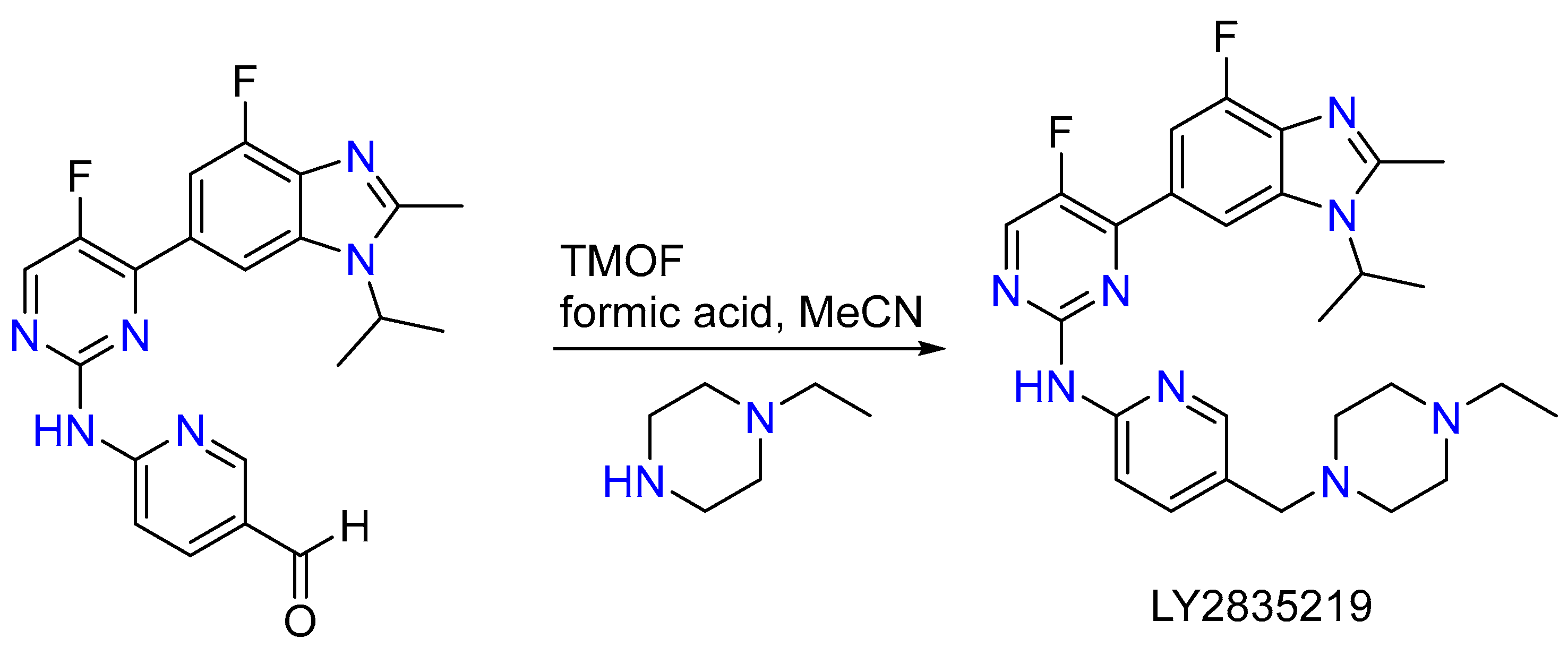
2.9. Synthesis of Abemaciclib, Chiral Bis and Racemic Methamphetamine
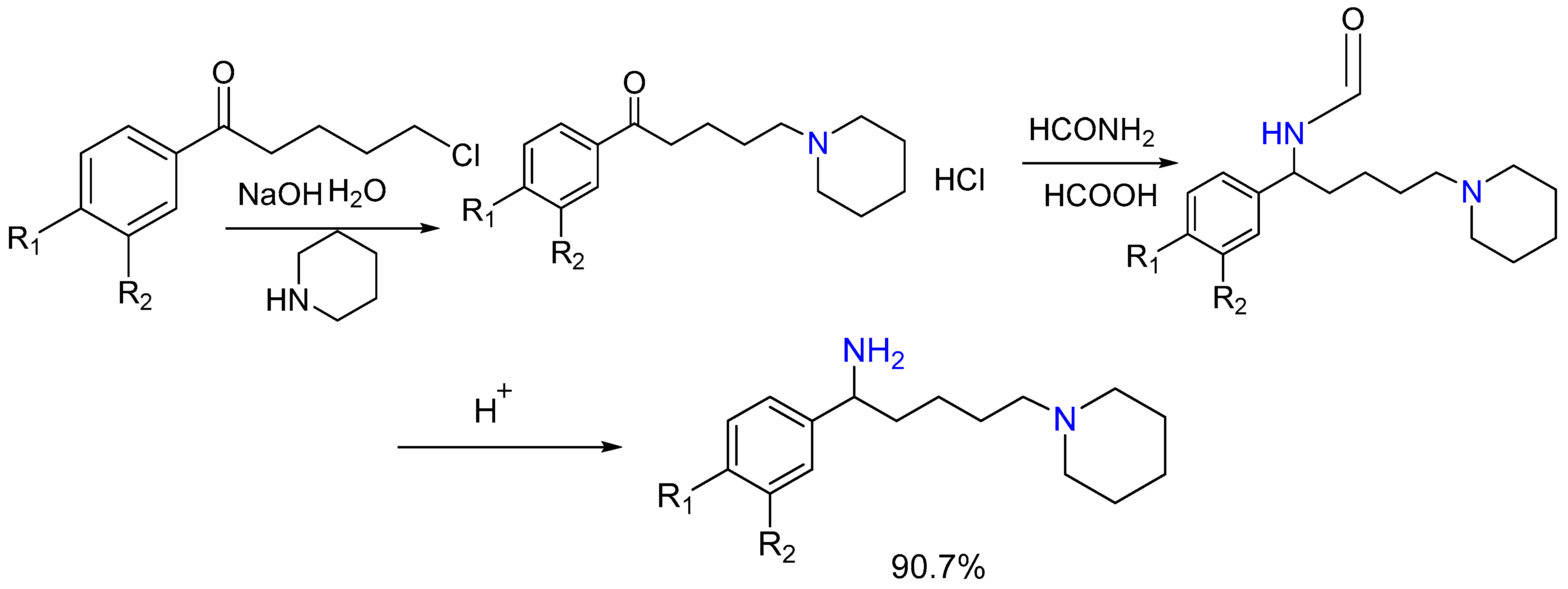
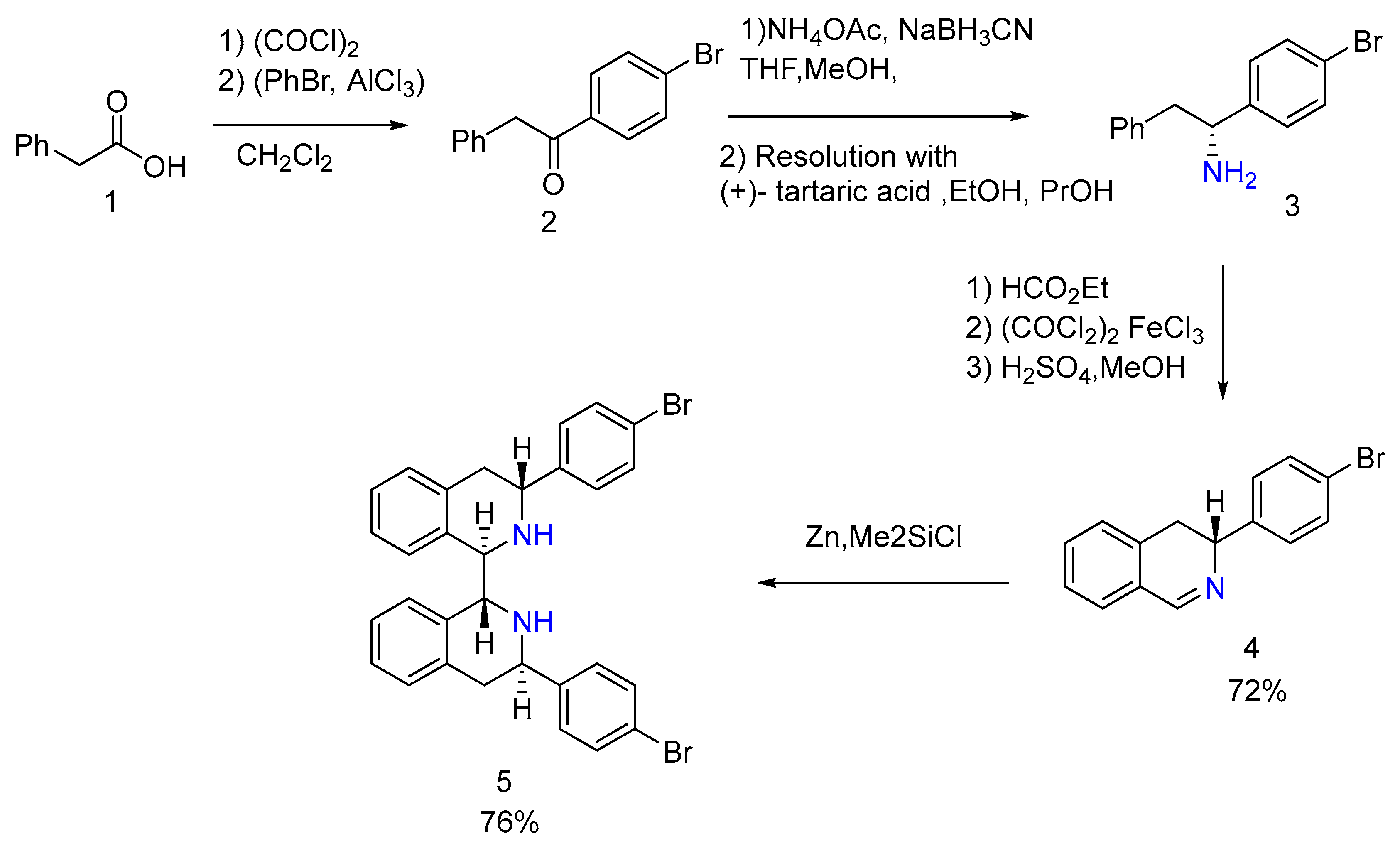
2.10. Synthesis of Hydro naphthylamines
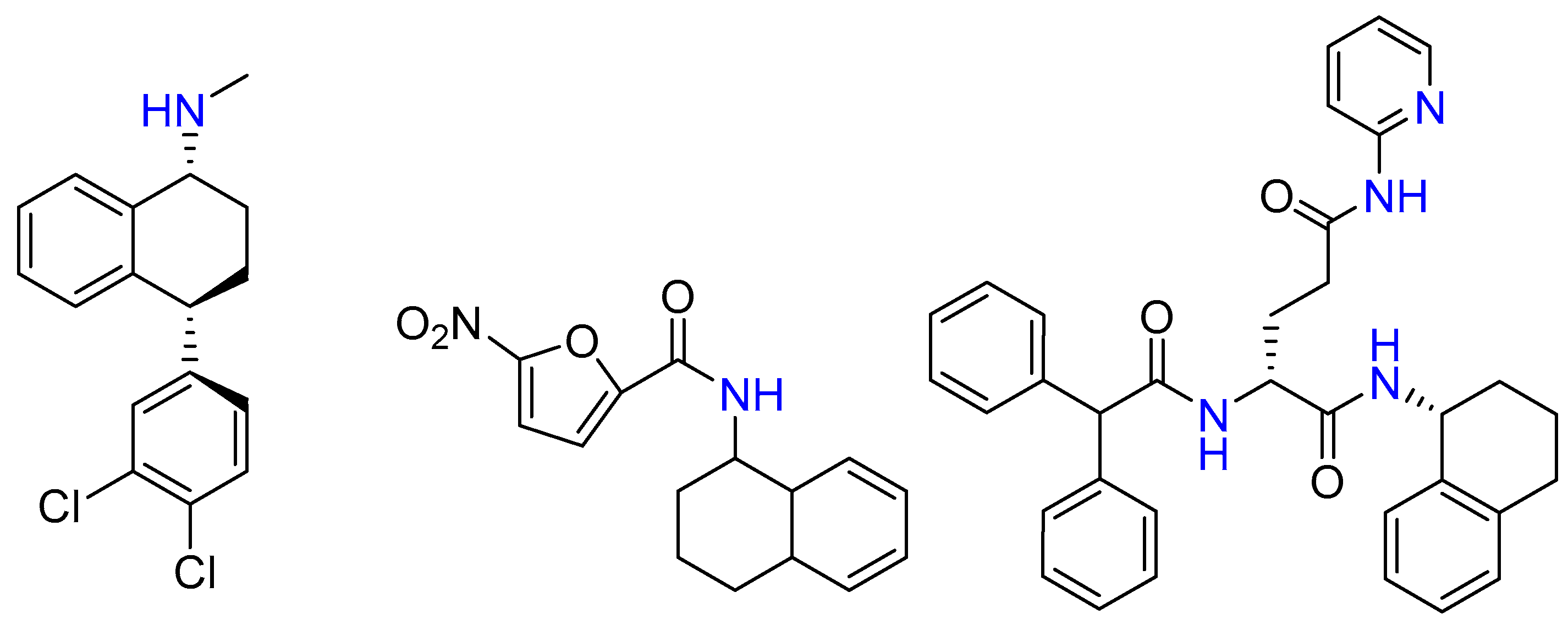
2.11. Synthesis of N-Alkylated-l, 2-Phenylethylamine and Some High-MW Compounds
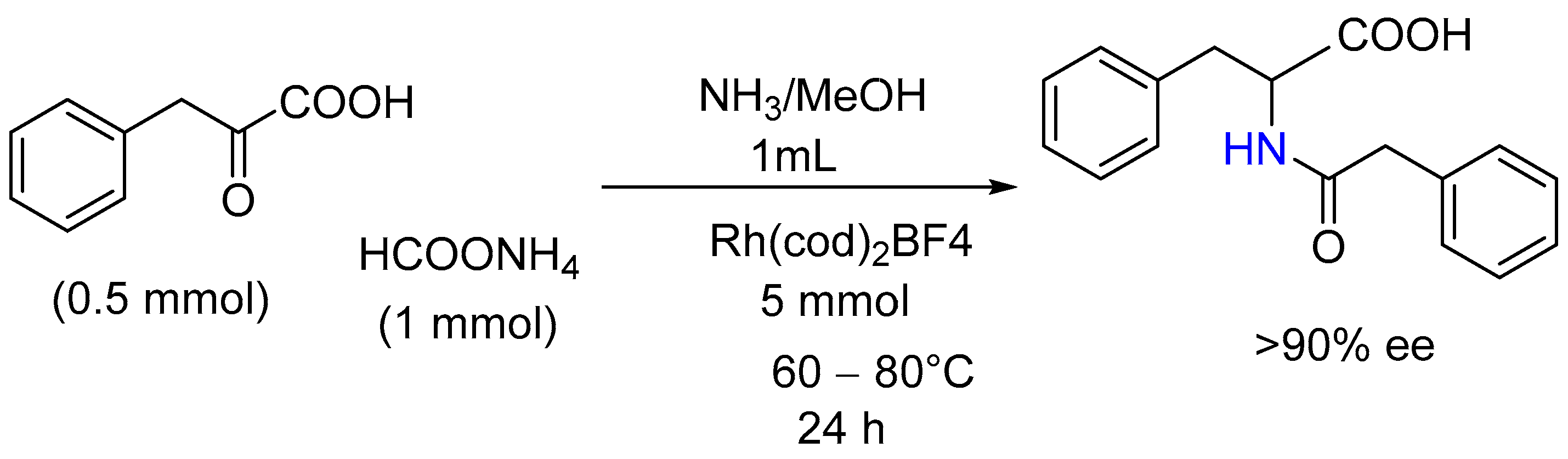
2.12. Enantiospecific Synthesis
3. Recent Advancement
3.1. Catalytic Advancement
3.1.1. Rh(III) Complex Catalysis
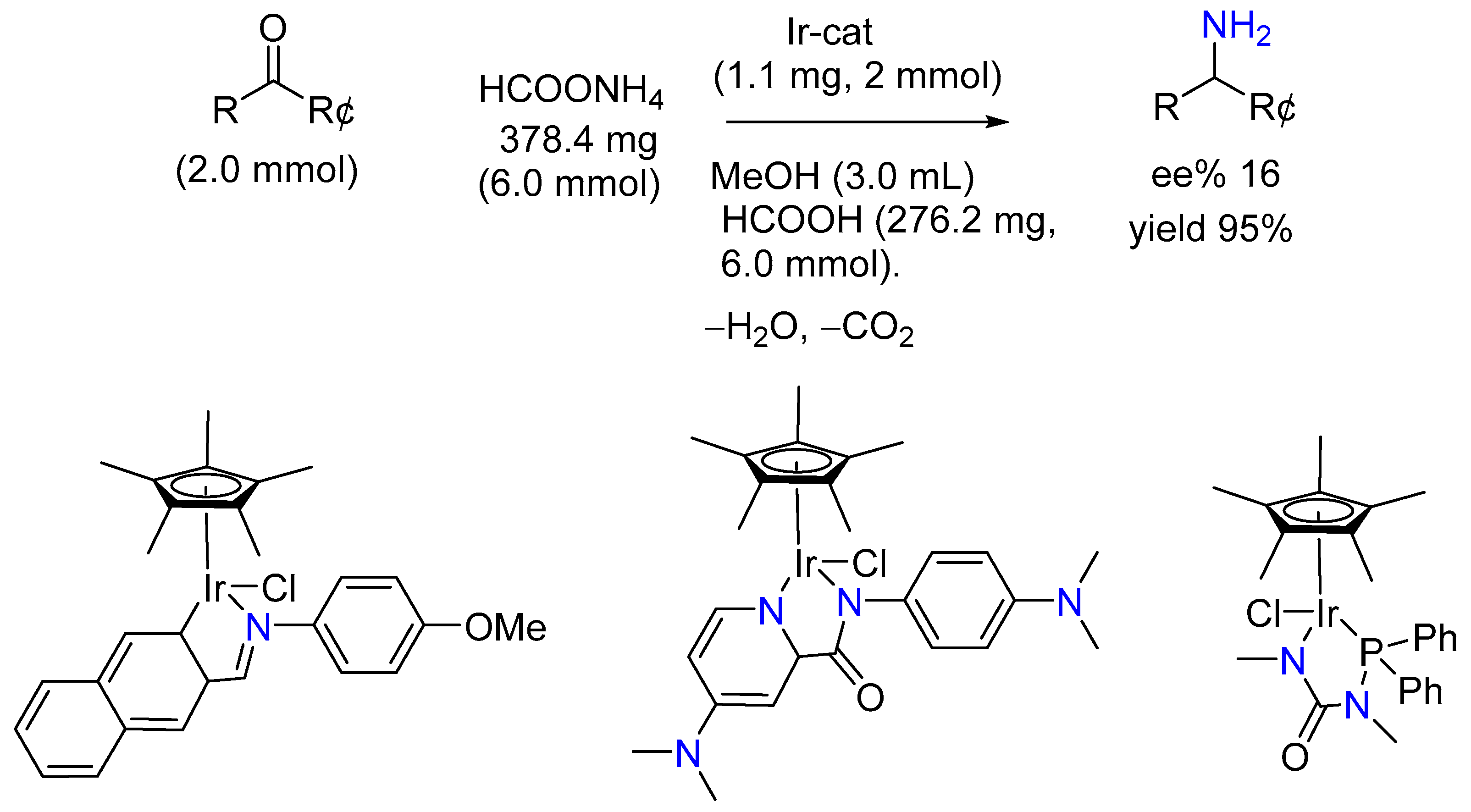
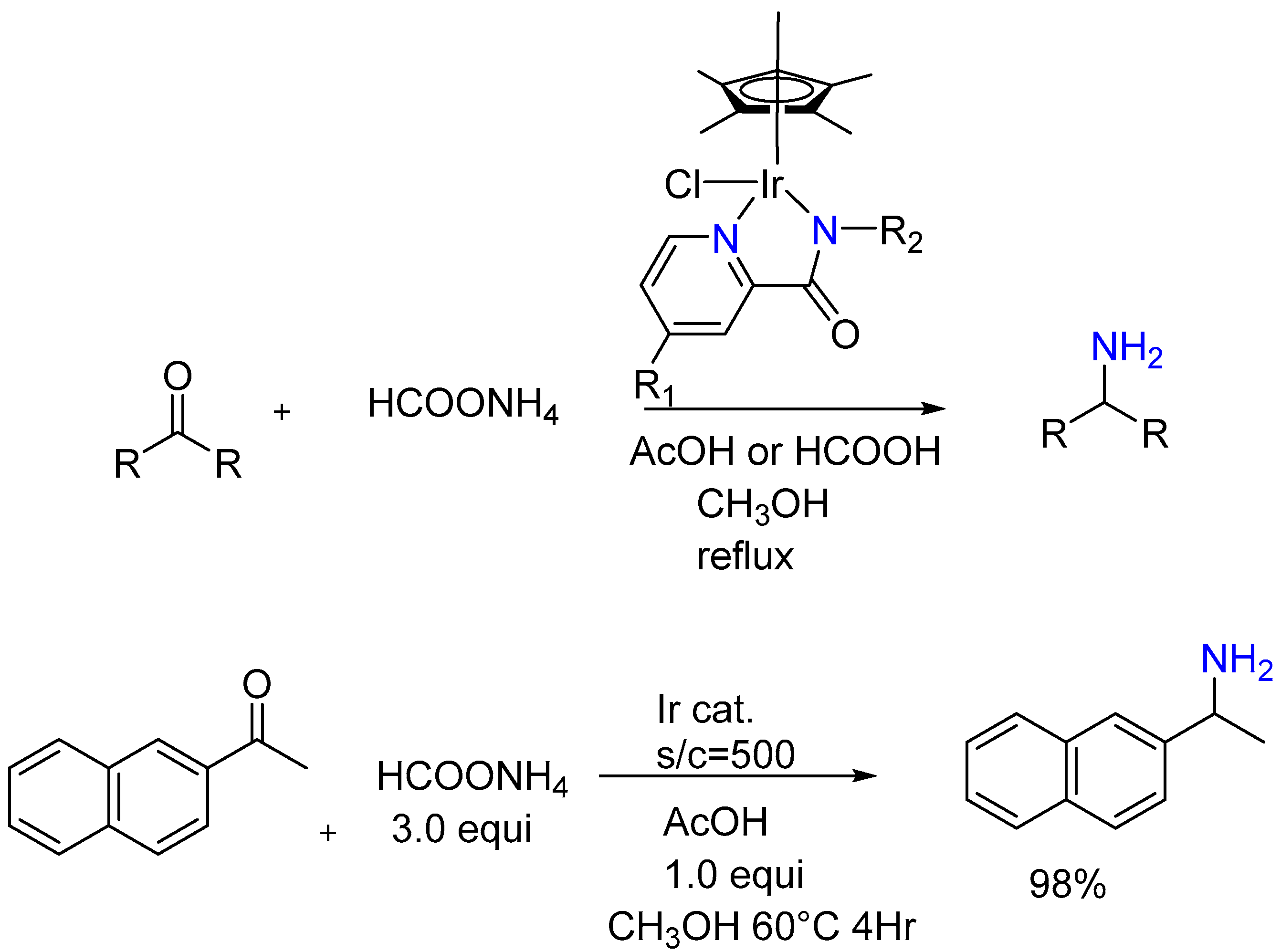
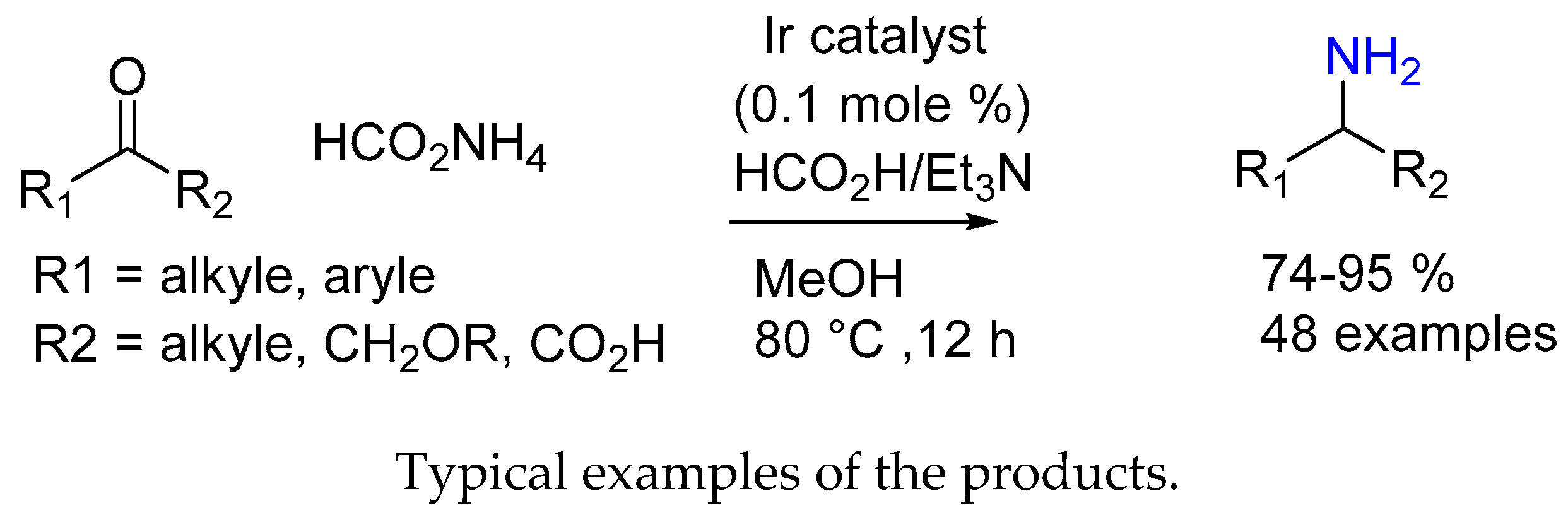
3.1.2. Ir(III) Complex Catalysis (Half-Sandwich Iridium Complexes)
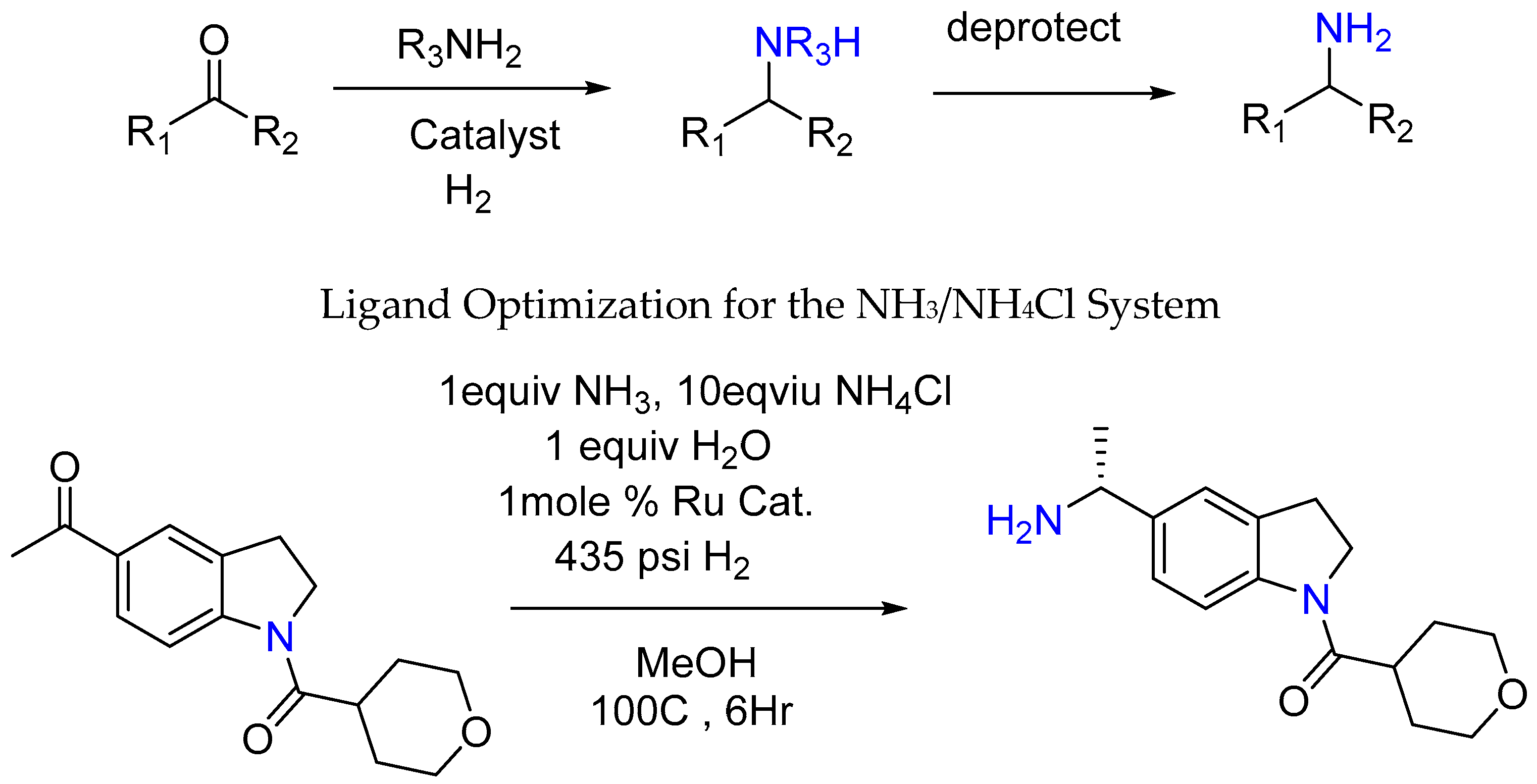
3.1.3. Synthesis of Chiral Amine under Ru and H2 Catalysis
3.1.4. “Leuckart-Type Reaction” under CHT
3.1.5. Synthesis of Tertiary Amines by Bronsted Acid and Lewis Acid Catalysts
3.1.6. Multiple Relay Catalysis for the Asymmetric Synthesis of Amines
3.1.7. Synthesis of Amines by Catalysis with Cp*Ir(III) Complexes
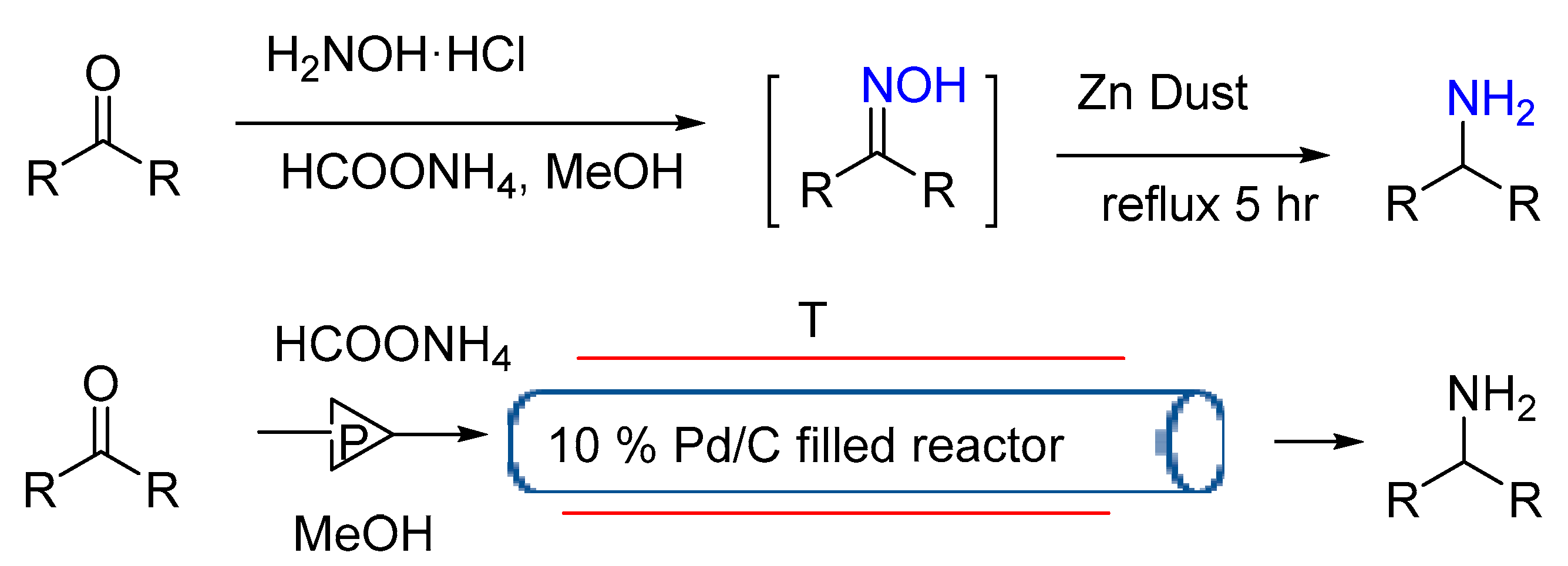
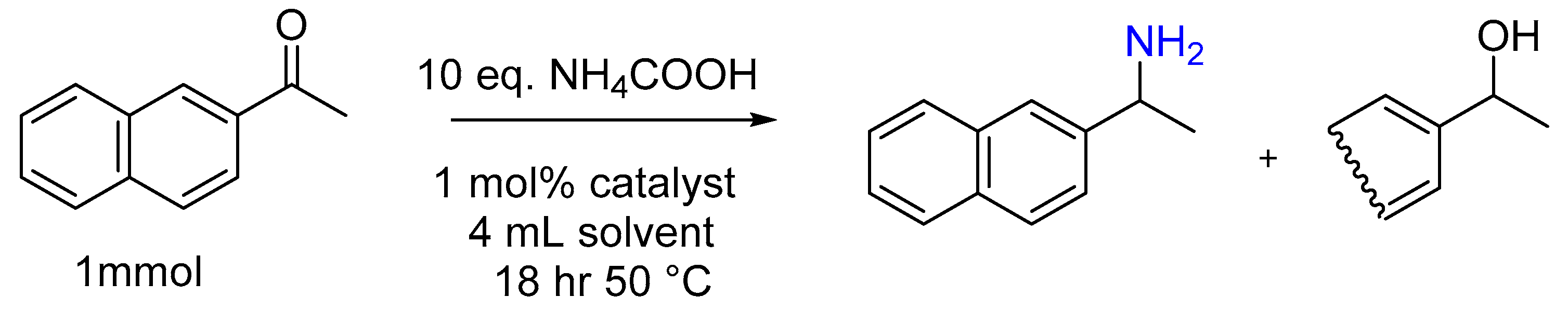
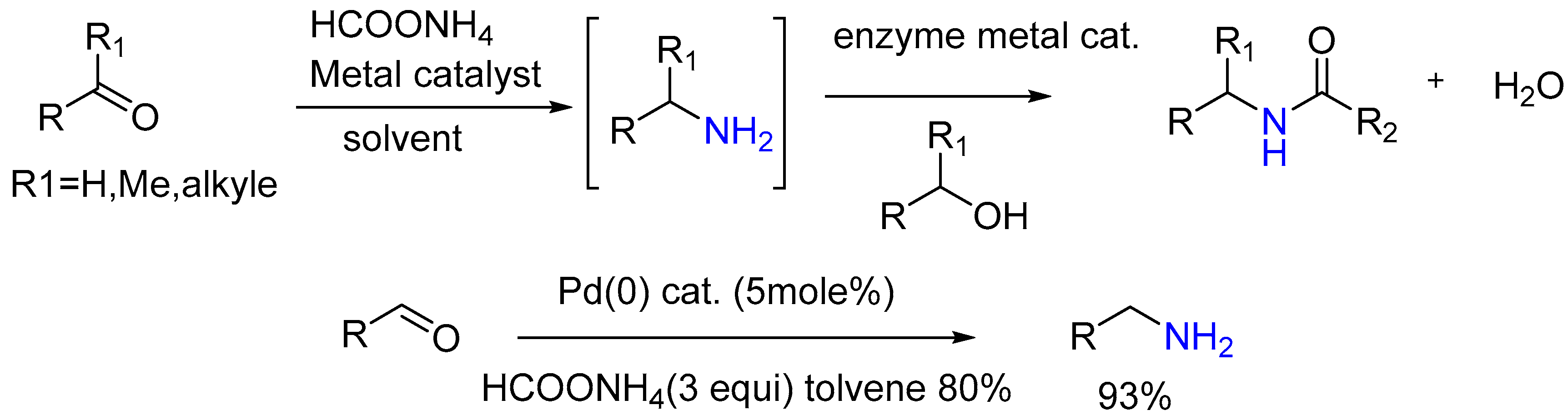
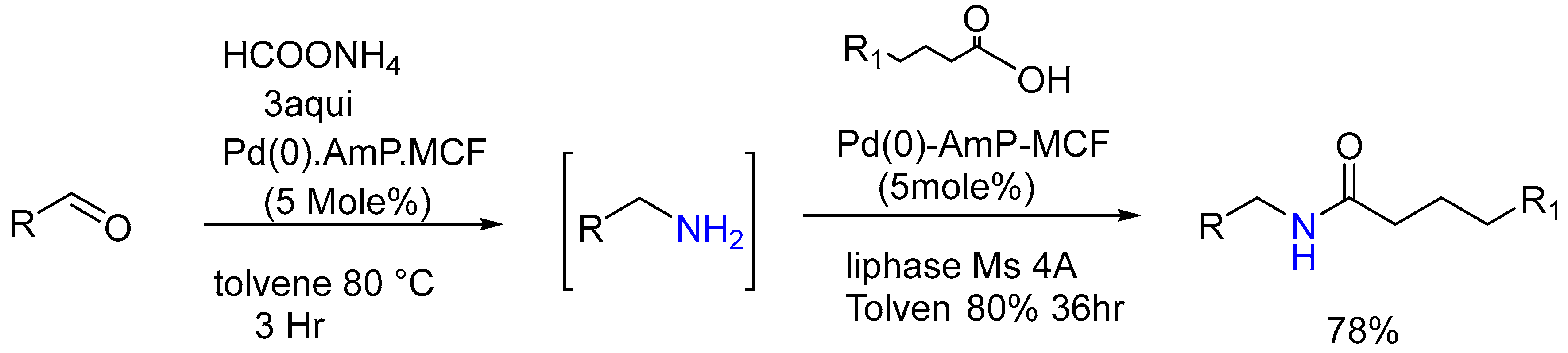
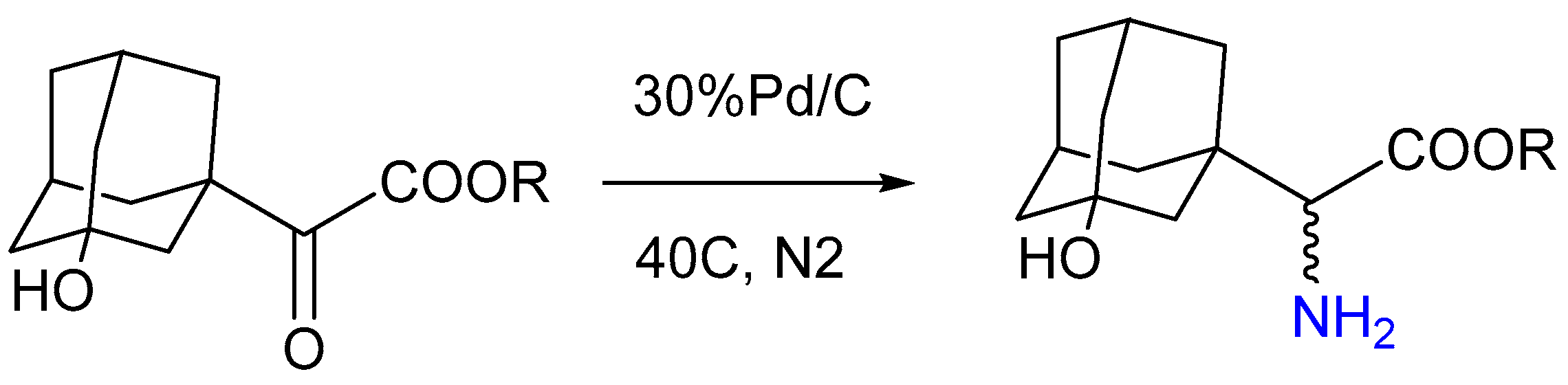
3.1.8. Pd/C Catalysis LW Reaction
3.2. Noncatalytic Advancement
3.2.1. MW-Assisted Synthesis of Formylated Secondary Amines and Isocyanide
3.2.2. LW Reaction under MW in Solvent-Free Conditions
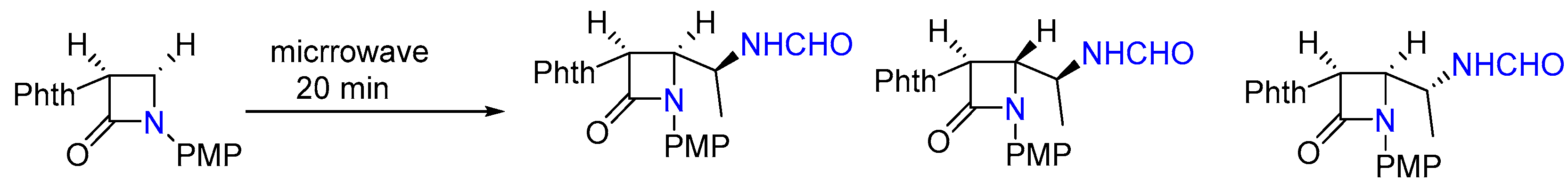
3.2.3. Synthesis of Dimethylated Tertiary Amine and Ethyl Azetidin-2-ones under MW
3.2.4. Metal-Free LW Synthesis of DHQs and Amino-Substituted Pyrrolidines
3.2.5. Modified Leuckart–Wallach Formamide Procedure
4. Outlook and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- August, S.; Einwirkung, D.; Aldehyd, M.; Benglidendiacetimid, T.; Wasser, M.; Interesse, B.R. Leuokert: A new method for the formation of tritylamine. ACS 1885, 469, 72–75. [Google Scholar]
- Ito, K.; Oba, H.; Sekiya, M. Studies on Leuckart–Wallach Reaction Paths. Bull. Chem. Soc. Jpn. 1976, 49, 2485–2490. [Google Scholar] [CrossRef]
- Gilman, H. Organic reactions, volume V. J. Chem. Educ. 1950, 27, 172. [Google Scholar] [CrossRef]
- Carlson, R.; Lejon, T.; Lundstedt, T.; Le Clouerec, E.; Casabó, J.; Coppens, P.; Buchardt, O. An optimized procedure for the reductive amination of acetophenone by the leuckart reaction. Acta Chem. Scand. 1993, 47, 1046–1049. [Google Scholar] [CrossRef]
- Mózo, B.S. Studies on the Leckart Reaction. J. Chem. Inf. Model. 2017, 53, 1689–1699. [Google Scholar] [CrossRef]
- Lee, S.C.; Park, S.B. Novel application of Leuckart–Wallach reaction for synthesis of tetrahydro-1,4-benzodiazepin-5-ones library. Chem. Commun. 2007, 36, 3714–3716. [Google Scholar] [CrossRef]
- Elavarasi, R. Molecular Design, Synthesis, Characterization and Biological Evaluation of 1-Substituted Tetrahydropyrimidine Derivatives by Leuckart Reaction. Ph.D. Dissertation, College of Pharmacy, Madurai Medical College, Madurai, India, 2014. [Google Scholar]
- Pollard, C.B.; Young, D.C. The mechanism of the leuckart reaction. J. Org. Chem. 1951, 16, 661–672. [Google Scholar] [CrossRef]
- Gil, C.; Bra, S. Solid-phase synthesis of biologically active benzoannelated nitrogen heterocycles. J. Com. Chem. 2009, 11, 175–197. [Google Scholar] [CrossRef]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.P.; Boutevin, B. Biobased amines: From synthesis to polymers; present and future. Chem Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef]
- Afanasyev, O.I.; Kuchuk, E.; Usanov, D.L.; Chusov, D. Reductive amination in the synthesis of pharmaceuticals. Chem. Rev. 2019, 119, 11857–11911. [Google Scholar] [CrossRef]
- Sutter, M.; Da Silva, E.; Duguet, N.; Raoul, Y.; Métay, E.; Lemaire, M. Glycerol ether synthesis: A bench test for green chemistry concepts and technologies. Chem. Rev. 2015, 115, 8609–8651. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.; Parikh, H.; Parikh, K. Leuckart–Wallach reaction. In Name Reactions in Organic Synthesis; Cambridge University Press: Cambridge, UK, 2012; pp. 276–280. [Google Scholar] [CrossRef]
- Thakore, R.R.; Takale, B.S.; Casotti, G.; Gao, E.S.; Jin, H.S.; Lipshutz, B.H. Chemoselective reductive aminations in aqueous nanoreactors using parts per million level Pd/C catalysis. Org. Lett. 2020, 22, 6324–6329. [Google Scholar] [CrossRef] [PubMed]
- Chauvier, C.; Cantat, T. A viewpoint on chemical reductions of carbon-oxygen bonds in renewable feedstocks including CO2 and biomass. ACS Catal. 2017, 7, 2107–2115. [Google Scholar] [CrossRef]
- Kitamura, M.; Lee, D.; Hayashi, S.; Tanaka, S.; Yoshimura, M. Catalytic Leuckart–Wallach-type reductive amination of ketones. J. Org. Chem. 2002, 67, 8685–8687. [Google Scholar] [CrossRef]
- De, A.; Ghosal, N.C.; Mahato, S.; Santra, S.; Zyryanov, G.V.; Majee, A. Scope and limitations of Leuckart–Wallach-type reductive amination: Chemoselective synthesis of tertiary amines from aldehydes under neat conditions. ChemistrySelect 2018, 3, 4058–4066. [Google Scholar] [CrossRef]
- García Martínez, A.; Teso Vilar, E.; García Fraile, A.; Martínez-Ruiz, P. Influence of the bridgehead substituent on the stereoselective Leuckart reaction of 2-norbornanones—Skeletal rearrangement versus structural retention. Eur. J. Org. Chem. 2001, 15, 2805–2808. [Google Scholar] [CrossRef]
- Bunnett, J.F.; Marks, J.L. Preparation of tertiary amines by the Leuckart reaction. J. Am. Chem. Soc. 1949, 71, 1587–1589. [Google Scholar] [CrossRef]
- Kang, C.L.; Hnatyk, C.; Heaton, A.R.; Wood, B.; Goyette, C.M.; Gibson, J.M.; Tischler, J.L. A simplified, green synthesis of tertiary amines using the Leuckart–Wallach reaction in subcritical water. Tetrahedron Lett. 2022, 106, 154079. [Google Scholar] [CrossRef]
- De Benneville, P.L.; Macartney, J.H. The behavior of aliphatic aldehydes in the Leuckart–Wallach reaction. J. Am. Chem. Soc. 1950, 72, 3073–3075. [Google Scholar] [CrossRef]
- Skachilova, S.Y.; Zheltukhin, N.K.; Sergeev, V.N.; Davydova, N.K. Reductive amination of sterically hindered arylaminoketones using a modified Leuckart reaction. Pharm. Chem. J. 2018, 52, 545–549. [Google Scholar] [CrossRef]
- Bach, R.D. Preparation of tertiary N,N-dimethylamines by the Leuckart reaction. J. Org. Chem. 1968, 33, 1647–1649. [Google Scholar] [CrossRef]
- Webers, V.J.; Bruce, W.F. The Leuckart reaction: A study of the mechanism. J. Am. Chem. Soc. 1948, 70, 1422–1424. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.; Recio, J.; Batanero, B. Microwave-assisted conversion of carbonyl compounds into formylated secondary amines: New contribution to the Leuckart reaction mechanism in N-methylformamide. Tetrahedron Lett. 2013, 54, 1835–1838. [Google Scholar] [CrossRef]
- Ostovari, H.; Zahedi, E. Kinetic and mechanistic insight into the formation of amphetamine using the Leuckart—Wallach reaction and interaction of the drug with GpC Á CpG base-pair step of DNA: A DFT study. Mon. Für Chem.—Chem. Mon. 2018, 149, 1045–1057. [Google Scholar] [CrossRef]
- Tzitzikas, Z. Innovative Multicomponent Reactions and Their Use in Medicinal Chemistry. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2018; pp. 3–6. [Google Scholar]
- Martínez, A.G.; Teso Vilar, E.; Fraile, A.G.; Martínez-Ruiz, P. Enantioselective synthesis of both enantiomers of vicinal norbornanediamines through the Leuckart reaction of 2-norbornanones. Tetrahedron Asymmetry 2001, 12, 2153–2158. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zhang, J.; Dömling, A. Leuckart–Wallach approach to sugar isocyanides and its IMCRs. Synthesis 2015, 47, 2407–2413. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. The Identification and Analysis of Amphetamine, Methamphetamine and Their Ring-Substituted; United Nations: New York, NY, USA, 2006. [Google Scholar]
- Świst, M.; Wilamowski, J.; Parczewski, A. Basic and neutral route specific impurities in MDMA prepared by different synthesis methods: Comparison of impurity profiles. Forensic Sci. Int. 2005, 155, 100–111. [Google Scholar] [CrossRef]
- Błachut, D.; Wojtasiewicz, K.; Czarnocki, Z. Some pyridine derivatives as “route-specific markers” in 4-methoxyamphetamine (PMA) prepared by the Leuckart method: Studies on the role of the aminating agent in their distribution in the final product. Forensic Sci. Int. 2005, 152, 157–173. [Google Scholar] [CrossRef]
- Świst, M.; Wilamowski, J.; Parczewski, A. Determination of synthesis method of ecstasy based on the basic impurities. Forensic Sci. Int. 2005, 152, 175–184. [Google Scholar] [CrossRef]
- El-Akaad, S.; De Saeger, S.; Beloglazova, N. Chemical molecularly imprinted polymer based capacitive sensing of a specific Leuckart marker 4-methyl-5-phenylpyrimidine in wastewater. Sens. Actuators B Chem. 2021, 343, 130116. [Google Scholar] [CrossRef]
- Abbruscato, T.J.; Trippier, P.C. DARK classics in chemical neuroscience: Methamphetamine. ACS Chem. Neurosci. 2018, 9, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Taglialatela-scafati, O. Recreational drug discovery: Natural products as lead structures for the synthesis of smart drugs. Nat. Prod. Rep. 2014, 31, 880–904. [Google Scholar] [CrossRef] [PubMed]
- Tournier, L.; Zard, S.Z. A practical variation on the Leuckart reaction. Tetrahedron Lett. 2005, 46, 971–973. [Google Scholar] [CrossRef]
- Devassia, T.; Prathapan, S.; Unnikrishnan, P.A. Unusual reactivity of DMAD (dimethyl acetylenedicarboxylate) with N-alkyl-9-anthracenemethanamine. Chem. Data Collect. 2018, 17–18, 9–12. [Google Scholar] [CrossRef]
- Peris, E.; Crabtree, R.H. Key factors in pincer ligand design. Chem. Soc. Rev. 2018, 47, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Vogel, P.; Lam, Y.-H.; Simon, A.; Houk, K.N. Organocatalysis: Fundamentals and comparisons to metal and enzyme catalysis. Catalysts 2016, 6, 128. [Google Scholar] [CrossRef]
- Hussein, M.A.; Dinh, A.H.; Huynh, V.T.; Nguyen, T.V. Synthesis of tertiary amines by direct Brønsted acid catalyzed reductive amination. Chem. Commun. 2020, 56, 8691–8694. [Google Scholar] [CrossRef]
- Gomez, S.; Peters, J.A.; Maschmeyer, T. The reductive animation of aldehydes and ketones and the hydrogenation of nitriles: Mechanistic aspects and selectivity control. Adv. Synth. Catal. 2002, 344, 1037–1057. [Google Scholar] [CrossRef]
- Zhang, F.; Li, T.; Chen, W.; Yan, X.; Wu, X.; Jiang, X.; Zhang, Y.; Wang, X.; He, G. High-performance anion exchange membranes with para-type cations on electron-withdrawing C=O links free backbone. Macromolecules 2020, 53, 10988–10997. [Google Scholar] [CrossRef]
- Frederick, M.O.; Pietz, M.A.; Kjell, D.P.; Richey, R.N.; Tharp, G.A.; Touge, T.; Yokoyama, N.; Kida, M.; Matsuo, T. Development of a Leuckart–Wallach reaction in flow for the synthesis of abemaciclib. Org. Process Res. Dev. 2017, 21, 1447–1451. [Google Scholar] [CrossRef]
- Frederick, M.O.; Kjell, D.P. A synthesis of abemaciclib utilizing a Leuckart–Wallach reaction. Tetrahedron Lett. 2015, 56, 949–951. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Vargas-Astudillo, D.; Yazdani-Pedram, M.; Acosta-Villavicencio, G.; Fuentealba, P.; Contreras-Cid, A.; Verdejo, R.; López-Manchado, M.A. Facile and scalable one-step method for amination of graphene using Leuckart reaction. Chem. Mater. 2017, 29, 6698–6705. [Google Scholar] [CrossRef]
- Hauser, F.M.; Pütz, M.; Rößler, T.; Hulshof, J.W. Identification of specific markers for amphetamines synthesized from glycidic acid pre-precursors and retrospective search in German profiling database. Drug Test. Anal. 2020, 12, 41–52. [Google Scholar] [CrossRef]
- Lee, S.C.; Park, S.B. Solid-phase parallel synthesis of natural product-like diaza-bridged heterocycles through Pictet–Spengler intramolecular cyclization. J. Comb. Chem. 2006, 8, 50–57. [Google Scholar] [CrossRef]
- Yoshida, H.; Shirakawa, E.; Honda, Y.; Hiyama, T. Addition of ureas to arynes: Straightforward synthesis of benzodiazepine and benzodiazepine derivatives. Angew. Chem.—Int. Ed. 2002, 41, 3247–3249. [Google Scholar] [CrossRef]
- Yu, H.; Wu, Z.; Wei, Z.; Zhai, Y.; Ru, S.; Zhao, Q.; Wang, J.; Han, S.; Wei, Y. N-formylation of amines using methanol as a potential formyl carrier by a reusable chromium catalyst. Commun. Chem. 2019, 2, 15. [Google Scholar] [CrossRef]
- Błachut, D.; Wojtasiewicz, K.; Krawczyk, K.; Maurin, J.; Szawkało, J.; Czarnocki, Z. Identification and synthesis of by-products found in 4-methylthioamphetamine (4-MTA) produced by the Leuckart method. Forensic Sci. Int. 2012, 216, 108–120. [Google Scholar] [CrossRef]
- Kochana, J.; Wilamowski, J.; Parczewski, A.; Surma, M. Synthesis of standards of the most important markers of Leuckart p-methoxymethamphetamine (PMMA)—Examination of the influence of experimental conditions and a drug diluent on SPE/TLC profiling. Forensic Sci Int. 2003, 134, 207–213. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M.; Nenajdenko, V.G.; Shastin, A.V.; Balenkova, E.S.; Haufe, G. Synthesis of trifluoromethyl alcohols from tert-butoxy-β-(trifluoromethyl)styrenes and trifluoromethylbenzyl ketones under the conditions of the Leuckart–Wallach reaction. J. Fluor. Chem. 2008, 129, 1052–1055. [Google Scholar] [CrossRef]
- Jaekel, E.E.; Antonietti, M. One-step method for the preparation of cationic nanocellulose in reactive eutectic media. Green Chem. 2021, 23, 2317–2323. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Dömling, A. Towards a facile and convenient synthesis of highly functionalized indole derivatives based on multi-component reactions. Org. Biomol. Chem. 2014, 12, 1649–1651. [Google Scholar] [CrossRef] [PubMed]
- Neochoritis, C.G.; Zarganes-Tzitzikas, T.; Stotani, S.; Domling, A.; Herdtweck, E.; Khoury, K.; Dömling, A. Leuckart–Wallach route toward isocyanides and some applications. ACS Comb. Sci. 2015, 17, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Brkukr, E.; Melumad, D. The synthesis of cis- and trans-1-methyl-2, 5-diphenylpyrrolidines by the Leuckart reaction of 1-benzoyl-2-phenylcyclopropane. J. Org. Chem. 1972, 37, 3949–3950. [Google Scholar] [CrossRef]
- Adger, B.M.; Dyer, U.C.; Lennon, I.C.; Tiffin, P.D.; Ward, S.E. A novel synthesis of tert-leucine via a Leuckart type reaction. Tetrahedron Lett. 1997, 38, 2153–2154. [Google Scholar] [CrossRef]
- Kulyk, O.G.; Biloborodov, D.A.; Cherevatenko, M.A.; Shyriakin, Y.Y.; Lyapunov, A.Y.; Mazepa, A.V.; Vashchenko, V.V.; Orlov, V.D.; Kolosov, M.A. Versatile approaches to a library of building blocks based on 5-acylthiazole skeleton. Synth. Commun. 2020, 50, 3616–3628. [Google Scholar] [CrossRef]
- Smith, P.A.S.; John Macdonald, A. Preparation of tertiary amines by the Leuckart reaction. J. Am. Chem. Soc. 1950, 72, 1037–1038. [Google Scholar] [CrossRef]
- Slavíková, B.; Bujons, J.; Matyáš, L.; Vidal, M.; Babot, Z.; Krištofíková, Z.; Suñol, C.; Kasal, A. Allopregnanolone and pregnanolone analogues modified in the C ring: Synthesis and activity. J. Med. Chem. 2013, 56, 2323–2336. [Google Scholar] [CrossRef]
- Reizman, B.J.; Burt, J.L.; Frank, S.A.; Argentine, M.D.; Garcia-Muñoz, S. Data-driven prediction of risk in drug substance starting materials. Org. Process Res. Dev. 2019, 23, 1429–1441. [Google Scholar] [CrossRef]
- Wilckens, K.; Lentz, D.; Czekelius, C. Synthesis of gold complexes bearing sterically highly encumbered, chiral carbene ligands. Organometallics 2011, 30, 1287–1290. [Google Scholar] [CrossRef]
- Yan, Q.; Xiong, C.; Chu, S.; Liu, Z.; Zhang, Y. Ruthenium-catalyzed ring-opening addition of anilides to 7-azabenzonorbornadienes: A diastereoselective route to hydronaphthylamines. J. Org. Chem. 2018, 83, 5598–5608. [Google Scholar] [CrossRef]
- Kozlov, N.G. Advances in the field of the synthesis of amino derivatives of terpenoids. Chem. Nat. Compd. 1982, 18, 131–143. [Google Scholar] [CrossRef]
- Goodson, L.H.; Wiegand, C.J.W.; Splitter, J.S. Analgesics. I. N-alkylated-1,2-diphenylethylamines prepared by the Leuckart reaction. J. Am. Chem. Soc. 1946, 68, 2174–2175. [Google Scholar] [CrossRef] [PubMed]
- Burckhalter, J.H.; Johnson, S.H. The Leuckart reaction with β-phenyl ketones. J. Am. Chem. Soc. 1951, 73, 4830–4832. [Google Scholar] [CrossRef]
- Chubb, F.; Hay, A.S.; Sandin, R.B. The Leuckart reaction of some 1,5-diketones. J. Am. Chem. Soc. 1953, 75, 6042–6044. [Google Scholar] [CrossRef]
- García Martínez, A.; Teso Vilar, E.; García Fraile, A.; Martínez Ruiz, P.; Macías San Antonio, R.; Martínez Alcázar, M.P. On themechanism of the Leuckart reaction. Enantiospecific preparation of (1R,2R)- and (1S,2S)-N-(3,3-dimethyl-2-formylamino-1-norbornyl)acetamide. Tetrahedron Asymmetry 1999, 10, 1499–1505. [Google Scholar] [CrossRef]
- Internasional, K.S. Studies on the mechanism of the Leuckart reaction. J. Am. Chem. Soc. 1945, 2, 73–80. [Google Scholar]
- Liang, G.; Wang, A.; Li, L.; Xu, G.; Yan, N.; Zhang, T. Production of primary amines by reductive amination of biomass-derived aldehydes/ketones. Angew. Chem. 2017, 129, 3096–3100. [Google Scholar] [CrossRef]
- Irrgang, T.; Kempe, R. Transition-metal-catalyzed reductive amination employing hydrogen. Chem. Rev. 2020, 120, 9583–9674. [Google Scholar] [CrossRef]
- Dong, C.; Wu, Y.; Wang, H.; Peng, J.; Li, Y.; Samart, C.; Ding, M. Facile and efficient synthesis of primary amines via reductive amination over a Ni/Al2O3 catalyst. ACS Sustain. Chem. Eng. 2021, 9, 7318–7327. [Google Scholar] [CrossRef]
- Gokhale, T.A.; Raut, A.B.; Bhanage, B.M. Comparative account of catalytic activity of Ru- and Ni-based nanocomposites towards reductive amination of biomass derived molecules. Mol. Catal. 2021, 510, 111667. [Google Scholar] [CrossRef]
- Coeck, R.; Meeprasert, J.; Li, G.; Altantzis, T.; Bals, S.; Pidko, E.A.; De Vos, D.E. Gold and silver-catalyzed reductive amination of aromatic carboxylic acids to benzylic amines. ACS Catal. 2021, 11, 7672–7684. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Feng, S.; Zhao, Z.; Zhu, H.; Ding, Y. Atomically dispersed Zn-Nx sites in N-doped carbon for reductive N-formylation of nitroarenes with formic acid. ChemCatChem 2020, 12, 1546–1550. [Google Scholar] [CrossRef]
- Palo-Nieto, C.; Afewerki, S.; Anderson, M.; Tai, C.W.; Berglund, P.; Córdova, A. Integrated heterogeneous metal/enzymatic multiple relay catalysis for eco-friendly and asymmetric synthesis. ACS Catal. 2016, 6, 3932–3940. [Google Scholar] [CrossRef]
- Polishchuk, I.; Sklyaruk, J.; Lebedev, Y.; Rueping, M. Air stable iridium catalysts for direct reductive amination of ketones. Chem. Eur. J. 2021, 27, 5919–5922. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, Z.; Jiang, L.; Yu, C.; Lv, K.; Sun, J.; Wang, S. A versatile cobalt catalyst for the reductive amination of carbonyl compounds with nitro compounds by transfer hydrogenation. Appl. Catal. B Environ. 2017, 210, 522–532. [Google Scholar] [CrossRef]
- Chen, S.; Ling, L.L.; Jiang, S.F.; Jiang, H. Selective hydrogenation of nitroarenes under mild conditions by the optimization of active sites in a well defined Co@ NC catalyst. Green Chem. 2020, 22, 5730–5741. [Google Scholar] [CrossRef]
- Gong, W.; Lin, Y.; Chen, C.; Al-Mamun, M.; Lu, H.; Wang, G.; Zhang, H.; Zhao, H. Nitrogen-doped carbon nanotube confined Co–Nx sites for selective hydrogenation of biomass-derived compounds. J. Adv. Mater. 2019, 31, 1808341. [Google Scholar] [CrossRef]
- Senthamarai, T.; Murugesan, K.; Schneidewind, J.; Kalevaru, N.V.; Baumann, W.; Neumann, H.; Kamer, P.C.J.; Beller, M.; Jagadeesh, R.V. Simple ruthenium-catalyzed reductive amination enables the synthesis of a broad range of primary amines. Nat. Commun. 2018, 9, 4123. [Google Scholar] [CrossRef]
- Kadyrov, R.; Riermeier, T.H. Highly enantioselective hydrogen-transfer reductive amination: Catalytic asymmetric synthesis of primary amines. Angew. Chem. 2003, 115, 5630–5632. [Google Scholar] [CrossRef]
- Dos Santos, E.D.A.; Hamel, E.; Bai, R.; Burnett, J.C.; Tozatti, C.S.S.; Bogo, D.; Perdomo, R.T.; Antunes, A.M.M.; Marques, M.M.; Matos, M.D.F.C.; et al. Synthesis and evaluation of diaryl sulfides and diaryl selenide compounds for antitubulin and cytotoxic activity. Bioorg. Med. Chem. Lett. 2013, 23, 4669–4673. [Google Scholar] [CrossRef]
- Tanaka, K.; Miki, T.; Murata, K.; Yamaguchi, A.; Kayaki, Y.; Kuwata, S.; Ikariya, T.; Watanabe, M. Reductive amination of ketonic compounds catalyzed by Cp*Ir(III) complexes bearing a picolinamidato ligand. J. Org. Chem. 2019, 84, 10962–10977. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Pan, Y.M.; Wang, S.G.; Zhang, X.; Yin, Q. Direct reductive amination of ketones with ammonium salt catalysed by Cp* Ir (iii) complexes bearing an amidato ligand. Org. Biomol. Chem. 2021, 19, 8934–8939. [Google Scholar] [CrossRef]
- Falus, P.; Boros, Z.; Hornyánszky, G.; Nagy, J.; Darvas, F.; Ürge, L.; Poppe, L. Reductive amination of ketones: Novel one-step transfer hydrogenations in batch and continuous-flow mode. Tetrahedron Lett. 2011, 52, 1310–1312. [Google Scholar] [CrossRef]
- Ba, M.; Ku, E. A new practical route for the manufacture of (4aR,10aR)-9-methoxy-1-methyl-6-trimethylsilanyl-1,2,3,4,4a,5,10,10a-octahydrobenzo [g] quinoline. Org. Process Res. Dev. 2003, 7, 904–912. [Google Scholar]
- Brewer, A.C.; Ruble, J.; Vandeveer, H.; Frank, S.; Nevill, C.R. Development and scale-up of a direct asymmetric reductive amination with ammonia. Org. Process Res. Dev. 2020, 25, 576–582. [Google Scholar] [CrossRef]
- Hanson, R.W. Catalytic transfer hydrogenation reactions for undergraduate practical programs. J. Chem. Educ. 1997, 74, 430–431. [Google Scholar] [CrossRef]
- Zhang, H.; Tong, X.; Liu, Z.; Wan, J.; Yu, L.; Zhang, Z. The sustainable heterogeneous catalytic reductive amination of lignin models to produce aromatic tertiary amines. Catal. Sci. Technol. 2018, 8, 5396–5400. [Google Scholar] [CrossRef]
- Yang, L.; Lin, J.; Kang, L.; Zhou, W.; Ma, D.Y. Lewis acid-catalyzed reductive amination of aldehydes and ketones with N,N-dimethylformamide as dimethylamino source, reductant and solvent. Adv. Synth. Catal. 2018, 360, 485–490. [Google Scholar] [CrossRef]
- Morisaki, K.; Morimoto, H.; Ohshima, T. Recent progress on catalytic addition reactions to N-unsubstituted imines. ACS Catal. 2020, 10, 6924–6951. [Google Scholar] [CrossRef]
- Li, C.; Meng, Y.; Yang, S.; Li, H. ZIF-67 derived Co/NC nanoparticles enable catalytic Leuckart-type reductive amination of bio-based carbonyls to N-formyl compounds. ChemCatChem 2021, 13, 5166–5177. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, D.; Shi, Y.; Huang, Y.; Chen, S.; Liu, F. Synthesis of S-3-hydroxyadamantylglycine ester by a Pd/C promoted mild Leuckart-Wallach reaction and an L-dibenzoyltartaric acid resolution. J. Chem. Res. 2015, 39, 108–111. [Google Scholar] [CrossRef]
- Rao, H.S.P.; Poonguzhali, E.; Muthukumaran, J. Synthesis and conformational studies on 1-aryl-cis-2, 6-diphenylpiperidines. J. Mol. Struct. 2021, 1232, 130065. [Google Scholar] [CrossRef]
- Hey, D.G.; Meakins, G.D.; Whateley, T.L. The Leuckart reaction with 4-t-butylcyclohexanone. J. Chem. Soc. C: Org. 1967, 1509–1512. [Google Scholar] [CrossRef]
- Parmar, H.; Asada, M.; Kanazawa, Y.; Asakuma, Y.; Phan, C.M.; Pareek, V.; Evans, G.M. Influence of microwaves on the water surface tension. Langmuir 2014, 30(33), 9875–9879. [Google Scholar] [CrossRef] [PubMed]
- Schaarschmidt, D.; Lang, H. Selective syntheses of planar-chiral ferrocenes. Organometallics 2013, 32, 5668–5704. [Google Scholar] [CrossRef]
- Petit, A.; Me, C.; Palomo, C. Leuckart reductive amination of a 4-acetylazetidinone using microwave technology. J. Chem. Res. Synop. 1998, 4, 187. [Google Scholar]
- Bokale-Shivale, S.; Amin, M.A.; Sawant, R.T.; Stevens, M.Y.; Turanli, L.; Hallberg, A.; Waghmode, S.B.; Odell, L.R. Synthesis of substituted 3,4-dihydroquinazolinonesviaa metal free Leuckart–Wallach type reaction. RSC Adv. 2021, 11, 349–353. [Google Scholar] [CrossRef]
- Dohle, W.; Jourdan, F.L.; Menchon, G.; Prota, A.E.; Foster, P.A.; Mannion, P.; Hamel, E.; Thomas, M.P.; Kasprzyk, P.G.; Ferrandis, E.; et al. Quinazolinone-based anticancer agents: Synthesis, antiproliferative SAR, antitubulin activity, and tubulin co-crystal structure. J. Med. Chem. 2018, 61, 1031–1044. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, C.; Jiang, X.; Xue, D.; Liu, Z.-T.; Xiao, J. Catalyst-free transformation of levulinic acid into pyrrolidinones with formic acid. ChemInform 2014, 16, 1093–1096. [Google Scholar] [CrossRef]
- Zhuang, C.; Miao, Z.; Zhu, L.; Dong, G.; Guo, Z.; Wang, S.; Zhang, Y.; Wu, Y.; Yao, J.; Sheng, C.; et al. Discovery, synthesis, and biological evaluation of orally active pyrrolidone derivatives as novel inhibitors of p53-MDM2 protein-protein interaction. J. Med. Chem. 2012, 55, 9630–9642. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Stotani, S.; Mishra, B.; Dömling, A. Efficient isocyanide-less isocyanide-based multicomponent reactions. Org. Lett. 2015, 17, 2002–2005. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Chavez, A.; Banik, B.K. Microwave-induced bismuth salts-mediated synthesis of molecules of medicinal interests. Curr. Med. Chem. 2017, 24, 4677–4713. [Google Scholar] [CrossRef] [PubMed]
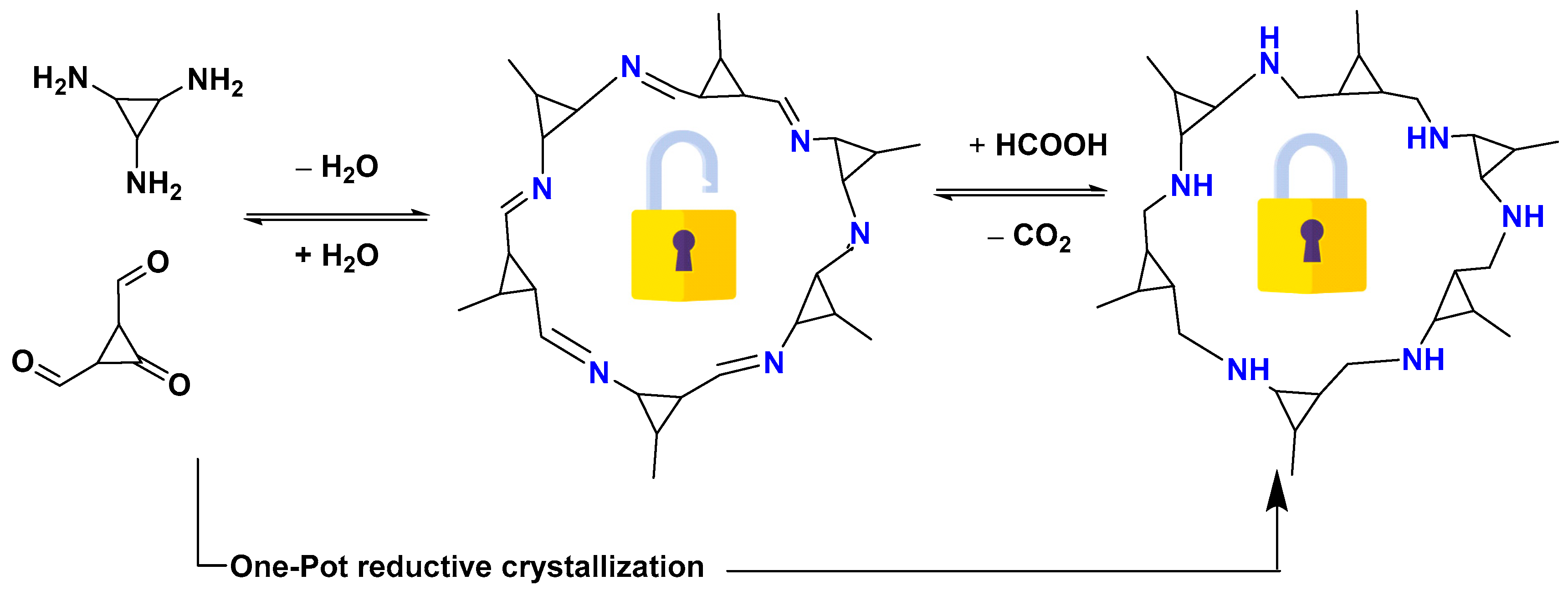
- Grunenberg, L.; Savasci, G.; Terban, M.W.; Duppel, V.; Moudrakovski, I.; Etter, M.; Dinnebier, R.E.; Ochsenfeld, C.; Lotsch, B.V. Amine-linked covalent organic frameworks as a platform for postsynthetic structure interconversion and pore-wall modification. J. Am. Chem. Soc. 2021, 143, 3430–3438. [Google Scholar] [CrossRef] [PubMed]






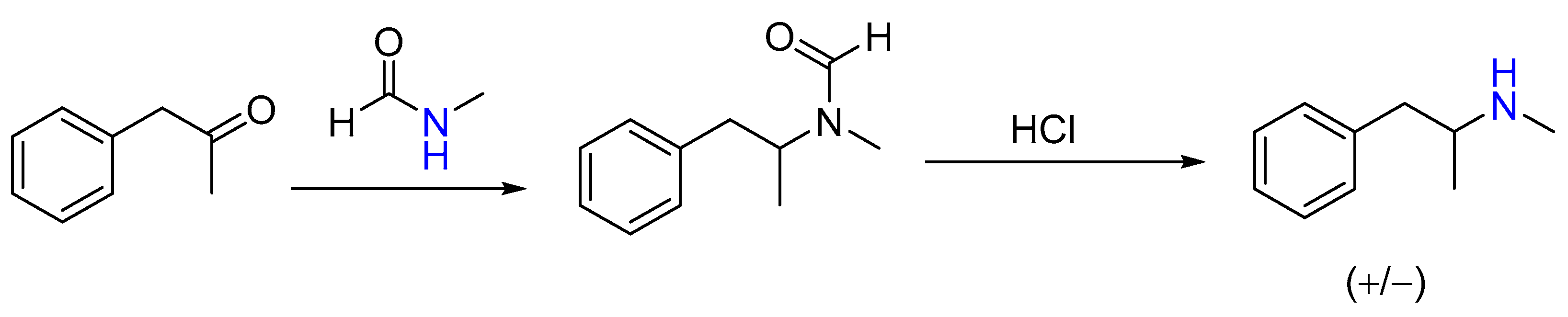

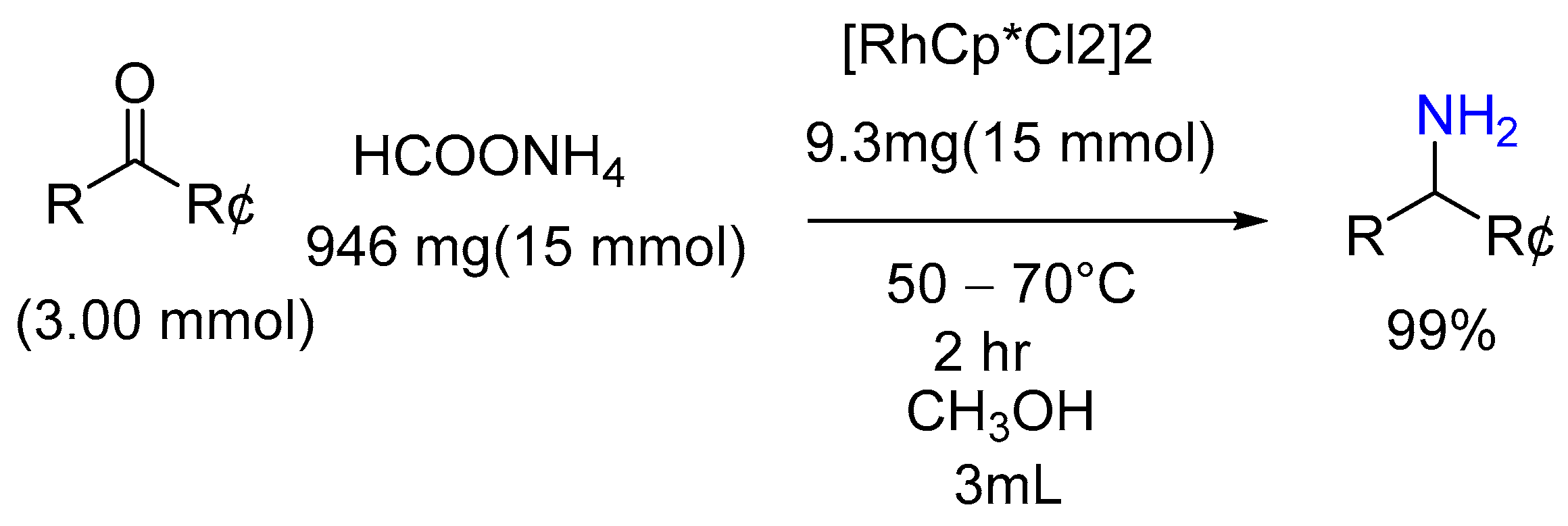
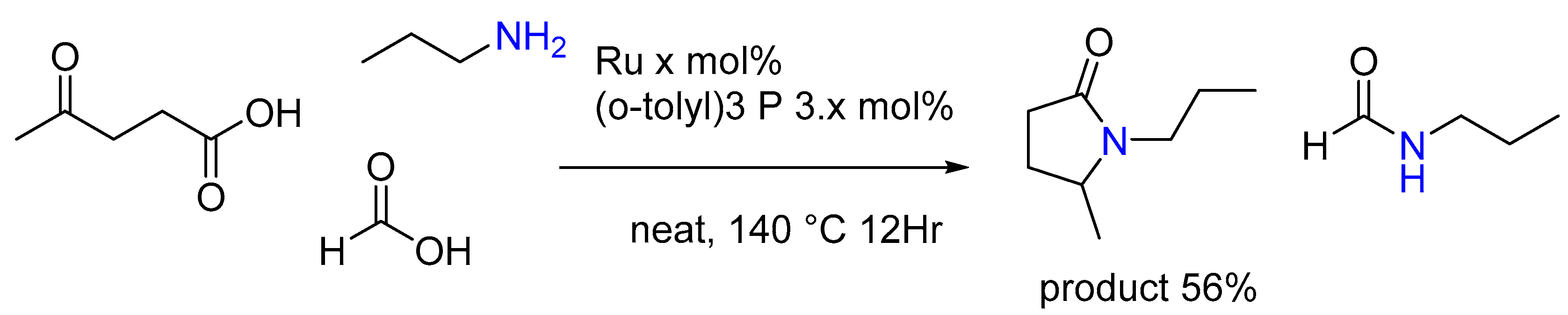
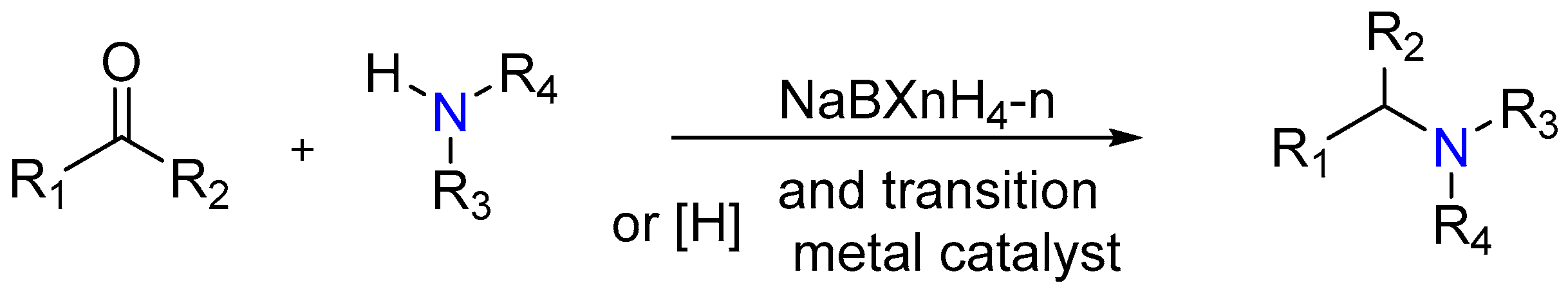

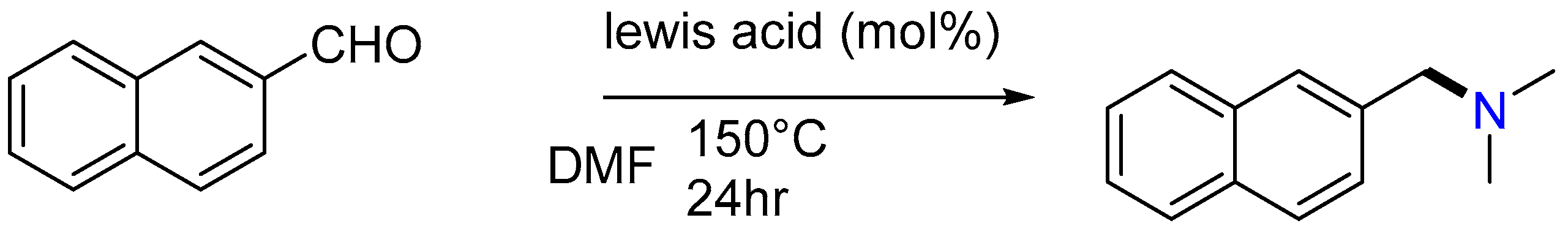


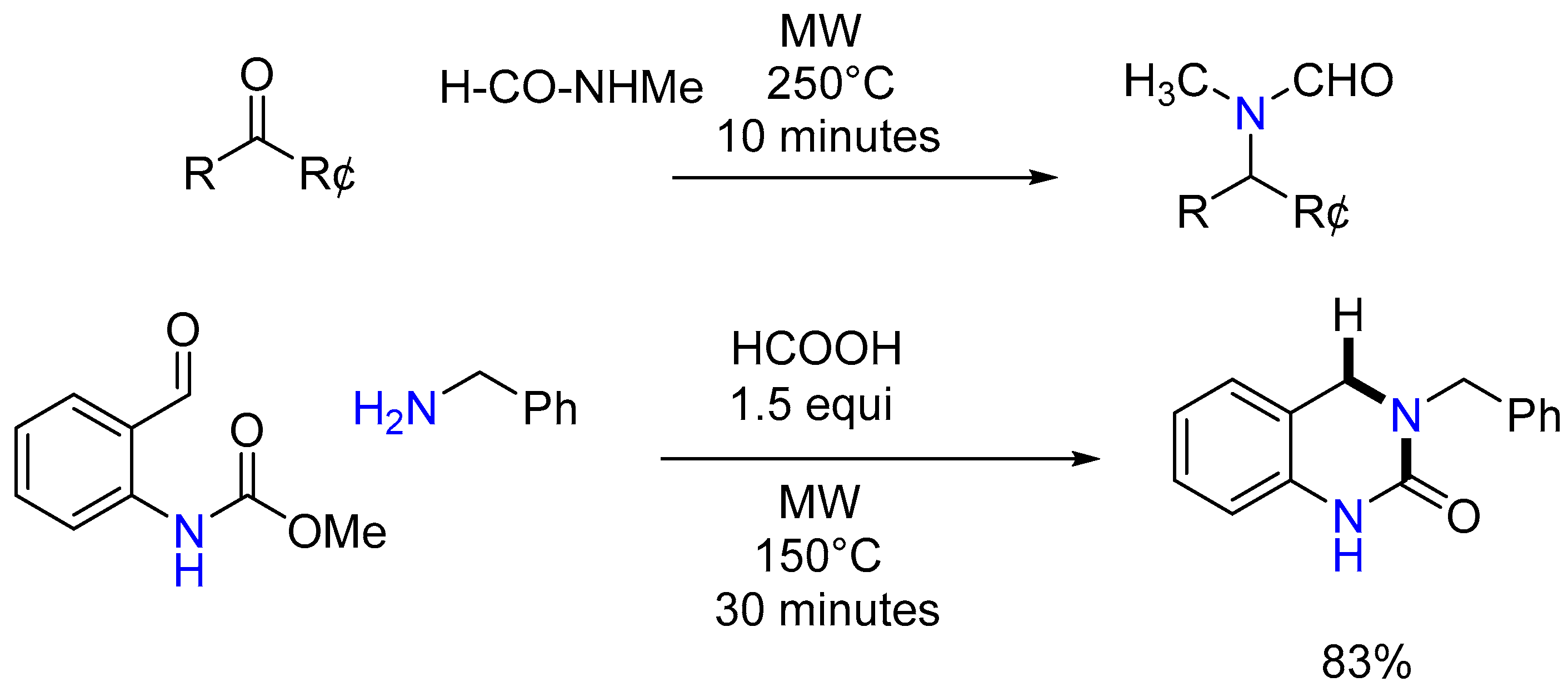
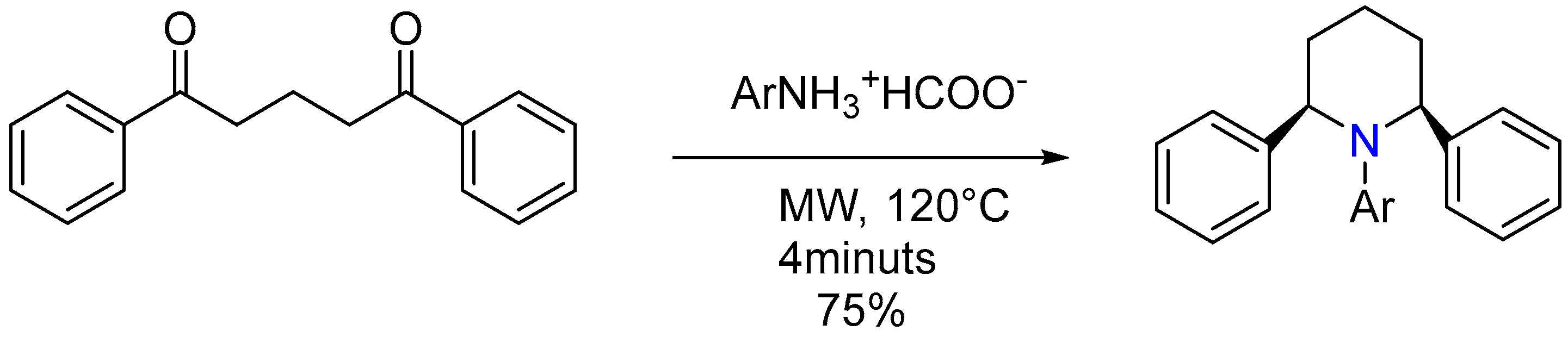
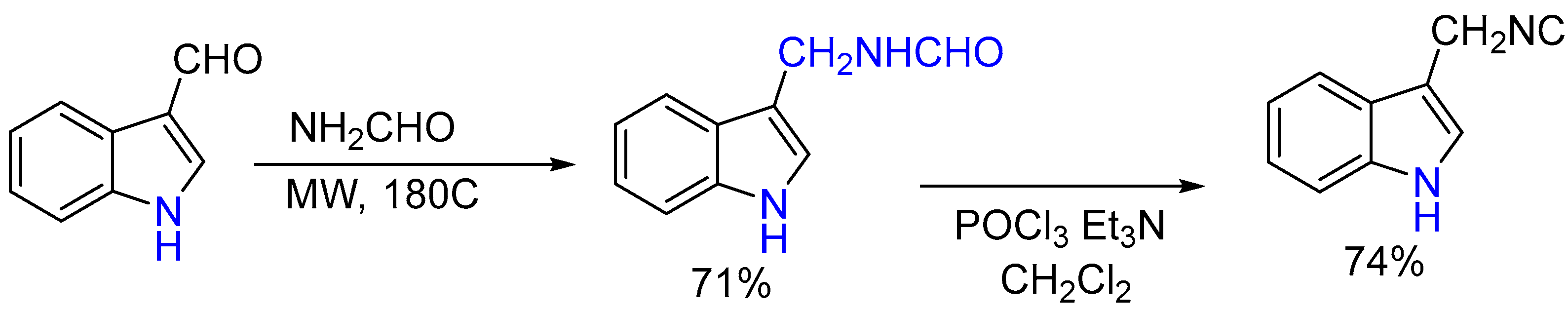
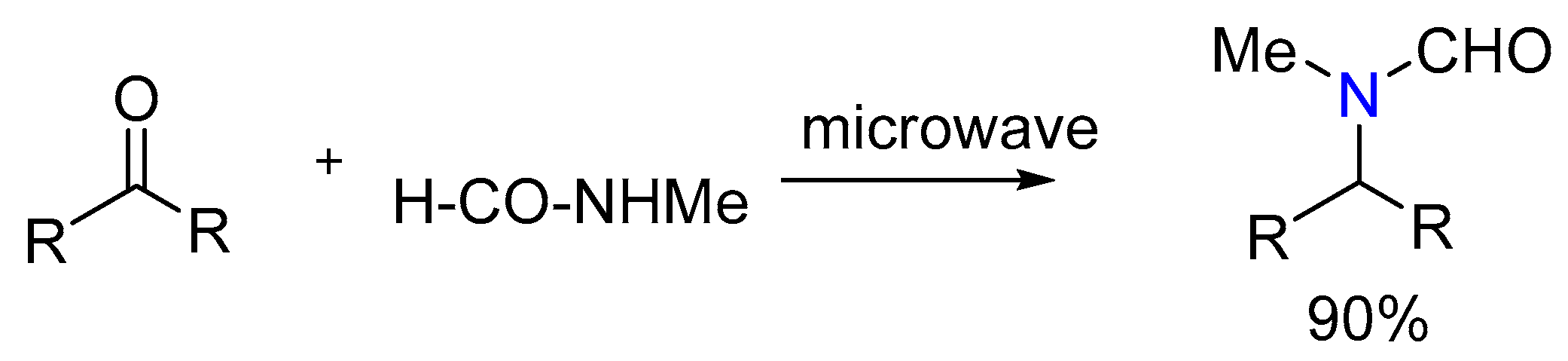
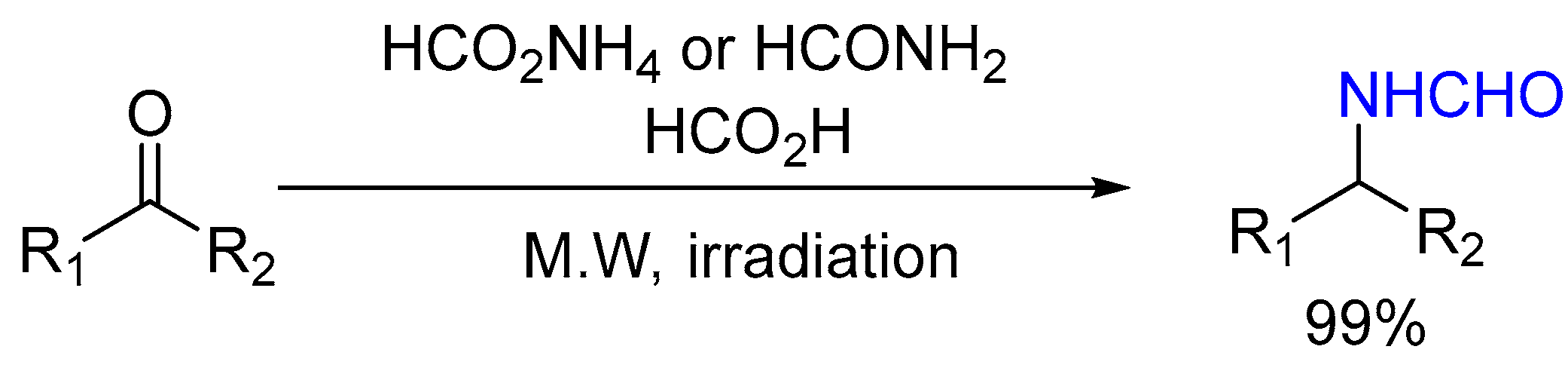

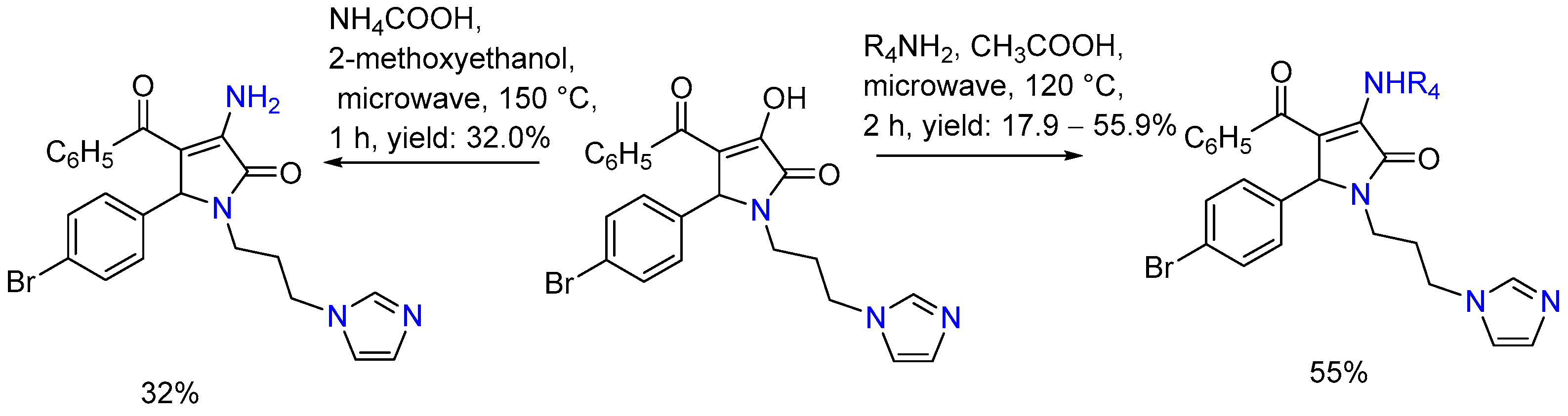
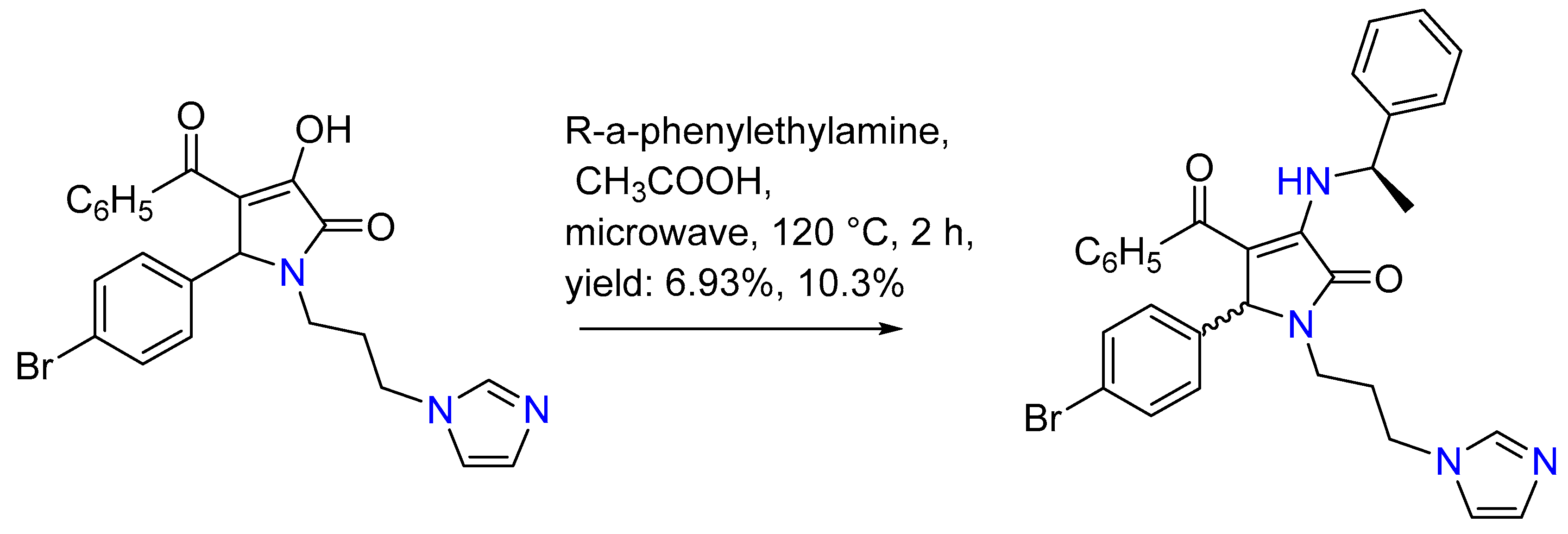
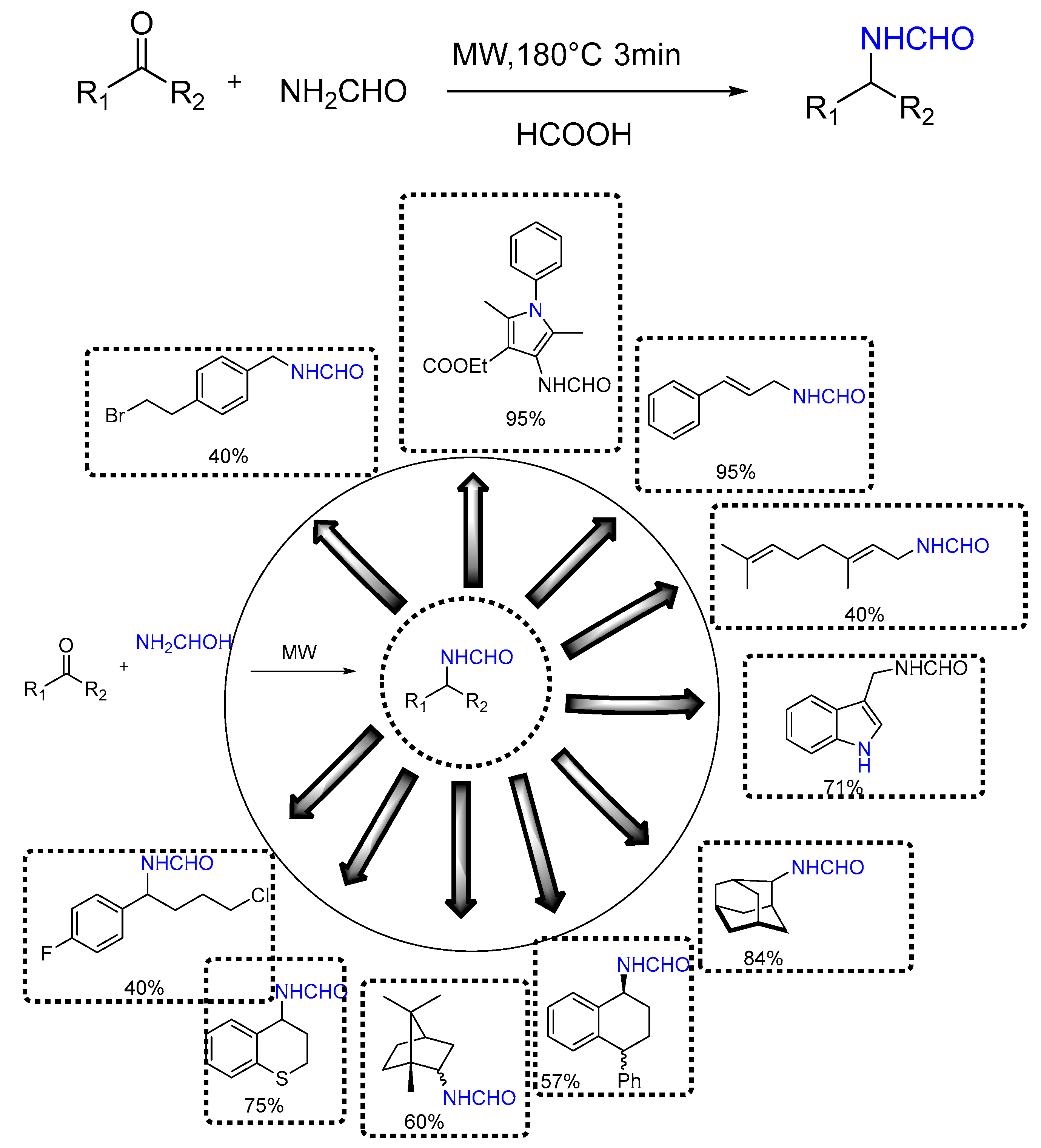
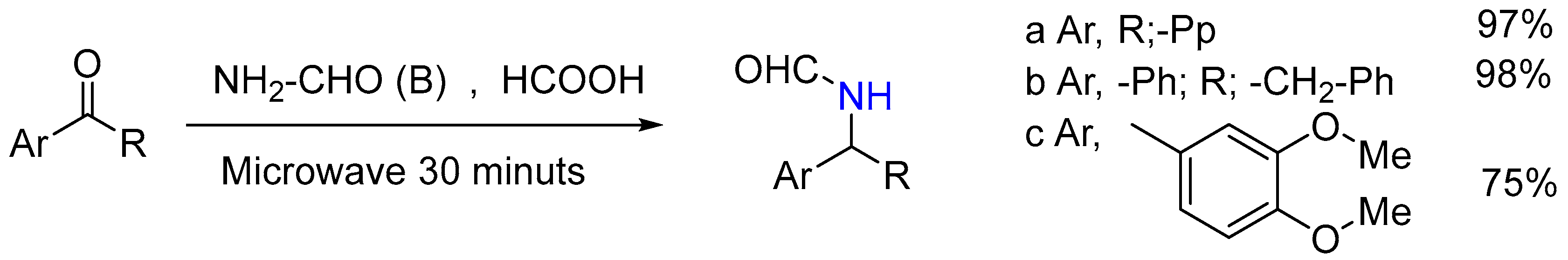
| 1 | Yield of 2% |
|---|---|
| a; cyclohexane | 90% |
| b; acetophenone | 85% |
| c; propophenone | 88% |
| d; 2-Acetylepyradine | 85% |
| e; thiophene—carbaldehyde | 92% |
| f; benzaldehyde | 90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umar, Q.; Luo, M. A Brief Review: Advancement in the Synthesis of Amine through the Leuckart Reaction. Reactions 2023, 4, 117-147. https://doi.org/10.3390/reactions4010007
Umar Q, Luo M. A Brief Review: Advancement in the Synthesis of Amine through the Leuckart Reaction. Reactions. 2023; 4(1):117-147. https://doi.org/10.3390/reactions4010007
Chicago/Turabian StyleUmar, Qasim, and Mei Luo. 2023. "A Brief Review: Advancement in the Synthesis of Amine through the Leuckart Reaction" Reactions 4, no. 1: 117-147. https://doi.org/10.3390/reactions4010007
APA StyleUmar, Q., & Luo, M. (2023). A Brief Review: Advancement in the Synthesis of Amine through the Leuckart Reaction. Reactions, 4(1), 117-147. https://doi.org/10.3390/reactions4010007






