Transient Behavior of CO and CO2 Hydrogenation on Fe@SiO2 Core–Shell Model Catalysts—A Stoichiometric Analysis of Experimental Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation and Characterization
2.2. Catalytic Experiments
2.3. Evaluation of Rates of the Stoichiometric Reactions
3. Results and Discussion
3.1. Catalyst Characterization
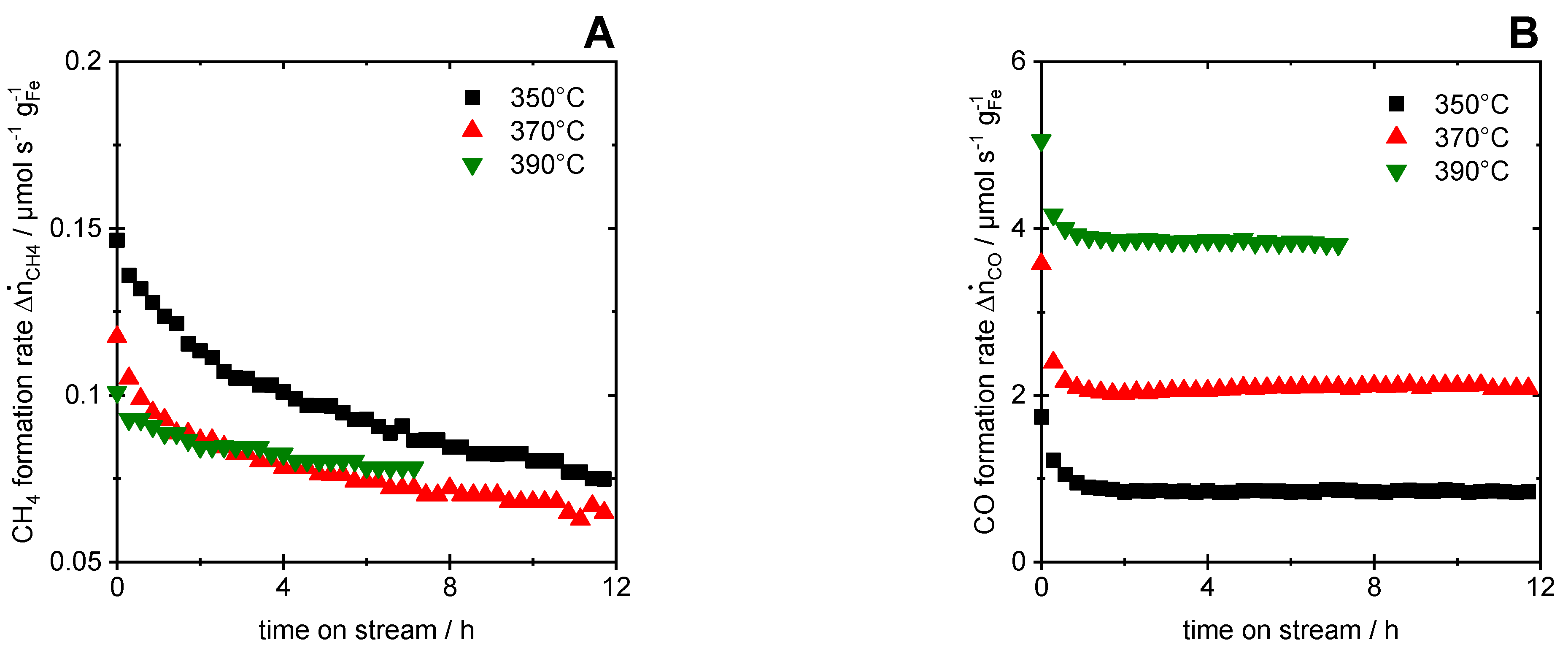
3.2. Hydrogenation of CO
3.3. Hydrogenation of CO2
4. Conclusions
- The evaluation method via stoichiometric analysis was successfully applied to the experimental data. The method allows the contributions of the individual key reactions to be determined and individually evaluated, especially for CO hydrogenation. By comparing the extent of the key reactions with the formation rates of the carbonaceous products CO, CO2 and CH4, it was thus possible to draw conclusions about the state of the catalytically active phase from the analysis of the gaseous effluent. This procedure forms a good basis for further kinetic investigations.
- The results for CO hydrogenation showed a slowly and monotonically increasing formation rate for the products CH4 and C2+ with TOS, while the extent of the Boudouard reaction remained rather constant for all temperatures. The resulting increasing carburization of the catalyst favored the observed increase in the CH4 and C2+ formation rate. The comparison between the CH4 and CO2 formation rates and the associated extents of reaction revealed that CO methanation was the dominant reaction.
- CO hydrogenation after CO2 treatment showed a significant change in product distribution, with lower CH4 as well as higher C2+ and CO2 formation rates over the entire 12 h period. Furthermore, the CO2 treatment led to the formation of Fe oxide species, which affected the product spectrum in the first two hours until the catalyst reached the state before CO2 treatment. Hence, intermittent CO2 treatment affected the active phase of the catalyst significantly.
- During CO2 hydrogenation, only the formation of CH4 and CO was detected. CO was the dominant product over the entire 12 h measurement period, presumably formed via the RWGS under the given conditions. The decreasing course of the CH4 formation rate with TOS indicated a continuous change in the catalytically active iron phase, even though the interpretation of this trend is limited due to the small values.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Köne, A.Ç.; Büke, T. Forecasting of CO2 emissions from fuel combustion using trend analysis. Renew. Sustain. Energy Rev. 2010, 14, 2906–2915. [Google Scholar] [CrossRef]
- bp Statistical Review of World Energy 2021, 70th ed.; BP p.l.c.: London, UK, 2021.
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Li, L.; Zhao, N.; Wei, W.; Sun, Y. A review of research progress on CO2 capture, storage, and utilization in Chinese Academy of Sciences. Fuel 2013, 108, 112–130. [Google Scholar] [CrossRef]
- Nam, S.-S.; Kim, H.; Kishan, G.; Choi, M.-J.; Lee, K.-W. Catalytic conversion of carbon dioxide into hydrocarbons over iron supported on alkali ion-exchanged Y-zeolite catalysts. Appl. Catal. A Gen. 1999, 179, 155–163. [Google Scholar] [CrossRef]
- Kuei, C.-K.; Lee, M.-D. Hydrogenation of carbon dioxide by hybrid catalysts, direct synthesis of aromatics from carbon dioxide and hydrogen. Can. J. Chem. Eng. 1991, 69, 347–354. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M.A. Production of synthetic natural gas (SNG) from coal and dry biomass—A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- Gahleitner, G. Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrogen Energy 2013, 38, 2039–2061. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Riedel, T.; Claeys, M.; Schulz, H.; Schaub, G.; Nam, S.-S.; Jun, K.-W.; Choi, M.-J.; Kishan, G.; Lee, K.-W. Comparative study of Fischer–Tropsch synthesis with H2/CO and H2/CO2 syngas using Fe- and Co-based catalysts. Appl. Catal. A Gen. 1999, 186, 201–213. [Google Scholar] [CrossRef]
- Geng, S.; Jiang, F.; Xu, Y.; Liu, X. Iron-Based Fischer-Tropsch Synthesis for the Efficient Conversion of Carbon Dioxide into Isoparaffins. ChemCatChem 2016, 8, 1303–1307. [Google Scholar] [CrossRef]
- Albrecht, M.; Rodemerck, U.; Schneider, M.; Bröring, M.; Baabe, D.; Kondratenko, E.V. Unexpectedly efficient CO2 hydrogenation to higher hydrocarbons over non-doped Fe2O3. Appl. Catal. B Environ. 2017, 204, 119–126. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.-L.; Zboril, R.; Varma, R.S. Core-shell nanoparticles: Synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.A.; Pragt, J.J.; Vos, H.J.; Bargeman, G.; de Groot, M.T. Novel efficient process for methanol synthesis by CO2 hydrogenation. Chem. Eng. J. 2016, 284, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Bukhtiyarova, M.; Lunkenbein, T.; Kähler, K.; Schlögl, R. Methanol Synthesis from Industrial CO2 Sources: A Contribution to Chemical Energy Conversion. Catal. Lett. 2017, 147, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Sterner, M.; Specht, M. Power-to-Gas and Power-to-X—The History and Results of Developing a New Storage Concept. Energies 2021, 14, 6594. [Google Scholar] [CrossRef]
- Sabatier, P.; Senderens, J.B. Direct hydrogenation of oxides of carbon in presence of various finely divided metals. C. R. Acad. Sci. 1902, 134, 689–691. [Google Scholar]
- Wang, W.; Gong, J. Methanation of carbon dioxide: An overview. Front. Chem. Sci. Eng. 2011, 5, 2–10. [Google Scholar] [CrossRef]
- Alig, L.; Fritz, M.; Schneider, S. First-Row Transition Metal (De)Hydrogenation Catalysis Based on Functional Pincer Ligands. Chem. Rev. 2019, 119, 2681–2751. [Google Scholar] [CrossRef]
- Bonzel, H.P.; Krebs, H.J. Surface science approach to heterogeneous catalysis: CO hydrogenation on transition metals. Surf. Sci. 1982, 117, 639–658. [Google Scholar] [CrossRef]
- Oza, R.; Shah, N.; Patel, S. Recovery of nickel from spent catalysts using ultrasonication-assisted leaching. J. Chem. Technol. Biotechnol. 2011, 86, 1276–1281. [Google Scholar] [CrossRef]
- Vuyyuru, K.R.; Pant, K.K.; Krishnan, V.V.; Nigam, K.D.P. Recovery of Nickel from Spent Industrial Catalysts Using Chelating Agents. Ind. Eng. Chem. Res. 2010, 49, 2014–2024. [Google Scholar] [CrossRef]
- Barrientos, J.; González, N.; Lualdi, M.; Boutonnet, M.; Järås, S. The effect of catalyst pellet size on nickel carbonyl-induced particle sintering under low temperature CO methanation. Appl. Catal. A Gen. 2016, 514, 91–102. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Wissing, L.; Skjøth-Rasmussen, M.S. High temperature methanation: Catalyst considerations. Catal. Today 2013, 215, 233–238. [Google Scholar] [CrossRef]
- Kirchner, J.; Baysal, Z.; Kureti, S. Activity and Structural Changes of Fe-based Catalysts during CO2 Hydrogenation towards CH4—A Mini Review. ChemCatChem 2020, 12, 981–988. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Du, J. Superparamagnetic nanoparticles for biomedical applications. J. Mater. Chem. B 2020, 8, 354–367. [Google Scholar] [CrossRef]
- Du, H.; Deng, F.; Kommalapati, R.R.; Amarasekara, A.S. Iron based catalysts in biomass processing. Renew. Sustain. Energy Rev. 2020, 134, 110292. [Google Scholar] [CrossRef]
- Lee, M.-D.; Lee, J.-F.; Chang, C.-S. Catalytic behavior and phase composition change of iron catalyst in hydrogenation of carbon dioxide. Catal. Rev. 1990, 23, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Zambrzycki, C.; Shao, R.; Misra, A.; Streb, C.; Herr, U.; Güttel, R. Iron Based Core-Shell Structures as Versatile Materials: Magnetic Support and Solid Catalyst. Catalysts 2021, 11, 72. [Google Scholar] [CrossRef]
- Byron Smith, R.J.; Loganathan, M.; Shantha, M.S. A Review of the Water Gas Shift Reaction Kinetics. Int. J. Chem. React. Eng. 2010, 8. [Google Scholar] [CrossRef]
- Newsome, D.S. The Water-Gas Shift Reaction. Catal. Rev. 1980, 21, 275–318. [Google Scholar] [CrossRef]
- Kirchner, J.; Anolleck, J.K.; Lösch, H.; Kureti, S. Methanation of CO2 on iron based catalysts. Appl. Catal. B Environ. 2018, 223, 47–59. [Google Scholar] [CrossRef]
- Visconti, C.G.; Martinelli, M.; Falbo, L.; Fratalocchi, L.; Lietti, L. CO2 hydrogenation to hydrocarbons over Co and Fe-based Fischer-Tropsch catalysts. Catal. Today 2016, 277, 161–170. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, P.; Zhang, X.; Zhang, G.; Li, R.; Li, W.; Senftle, T.; Liu, W.; Wang, J.; Wang, Y.; et al. Dynamic structural evolution of iron catalysts involving competitive oxidation and carburization during CO2 hydrogenation. Sci. Adv. 2022, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Li, W.; Wu, X.; Wang, S.; Deng, Q.; Huang, M. Recent Advances in the Synthesis, Surface Modifications and Applications of Core-Shell Magnetic Mesoporous Silica Nanospheres. Chem. Asian J. 2020, 15, 1248–1265. [Google Scholar] [CrossRef] [PubMed]
- de Smit, E.; Weckhuysen, B.M. The renaissance of iron-based Fischer-Tropsch synthesis: On the multifaceted catalyst deactivation behaviour. Chem. Soc. Rev. 2008, 37, 2758–2781. [Google Scholar] [CrossRef]
- Pérez-Alonso, F.J.; Herranz, T.; Rojas, S.; Ojeda, M.; López Granados, M.; Terreros, P.; Fierro, J.L.G.; Gracia, M.; Gancedo, J.R. Evolution of the bulk structure and surface species on Fe–Ce catalysts during the Fischer–Tropsch synthesis. Green Chem. 2007, 9, 663–670. [Google Scholar] [CrossRef]
- Herranz, T.; Rojas, S.; Pérez-Alonso, F.J.; Ojeda, M.; Terreros, P.; Fierro, J.L.G. Genesis of iron carbides and their role in the synthesis of hydrocarbons from synthesis gas. J. Catal. 2006, 243, 199–211. [Google Scholar] [CrossRef]
- Schulz, H.; vein Steen, E.; Claeys, M. Selectivity and mechanism of Fischer-Tropsch synthesis with iron and cobalt catalysts. In Natural Gas Conversion II—Proceedings of the Third Natural Gas Conversion Symposium; Elsevier: Amsterdam, Netherlands, 1994; pp. 455–460. ISBN 9780444895356. [Google Scholar]
- Su, X.; Xu, J.; Liang, B.; Duan, H.; Hou, B.; Huang, Y. Catalytic carbon dioxide hydrogenation to methane: A review of recent studies. J. Energy Chem. 2016, 25, 553–565. [Google Scholar] [CrossRef]
- Claeys, M.; van Steen, E.; Botha, T.; Crous, R.; Ferreira, A.; Harilal, A.; Moodley, D.J.; Moodley, P.; Du Plessis, E.; Visagie, J.L. Oxidation of Hägg Carbide during High-Temperature Fischer–Tropsch Synthesis: Size-Dependent Thermodynamics and In Situ Observations. ACS Catal. 2021, 11, 13866–13879. [Google Scholar] [CrossRef]
- Zhu, M.; Wachs, I.E. Iron-Based Catalysts for the High-Temperature Water–Gas Shift (HT-WGS) Reaction: A Review. ACS Catal. 2016, 6, 722–732. [Google Scholar] [CrossRef]
- Owen, R.E.; Mattia, D.; Plucinski, P.; Jones, M.D. Kinetics of CO2 Hydrogenation to Hydrocarbons over Iron-Silica Catalysts. Chemphyschem 2017, 18, 3211–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loiland, J.A.; Wulfers, M.J.; Marinkovic, N.S.; Lobo, R.F. Fe/γ-Al2O3 and Fe–K/γ-Al2O3 as reverse water-gas shift catalysts. Catal. Sci. Technol. 2016, 6, 5267–5279. [Google Scholar] [CrossRef] [Green Version]
- Riedel, T.; Schulz, H.; Schaub, G.; Jun, K.-W.; Hwang, J.-S.; Lee, K.-W. Fischer–Tropsch on iron with H2/CO and H2/CO2 as synthesis gases: The episodes of formation of the Fischer–Tropsch regime and construction of the catalyst. Top. Catal. 2003, 26, 41–54. [Google Scholar] [CrossRef]
- Kirchner, J.; Zambrzycki, C.; Baysal, Z.; Güttel, R.; Kureti, S. Fe based core–shell model catalysts for the reaction of CO2 with H2. Reac. Kinet. Mech. Cat. 2020, 131, 119–128. [Google Scholar] [CrossRef]
- Kirchner, J.; Zambrzycki, C.; Kureti, S.; Güttel, R. CO2 Methanation on Fe Catalysts Using Different Structural Concepts. Chem. Ing. Tech. 2020, 92, 603–607. [Google Scholar] [CrossRef] [Green Version]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Zeng, B.; Hou, B.; Jia, L.; Wang, J.; Chen, C.; Sun, Y.; Li, D. Studies of Cobalt Particle Size Effects on Fischer-Tropsch Synthesis over Core-Shell-Structured Catalysts. ChemCatChem 2013, 5, 3794–3801. [Google Scholar] [CrossRef]
- Straß-Eifert, A.; Sheppard, T.L.; Damsgaard, C.D.; Grunwaldt, J.-D.; Güttel, R. Stability of Cobalt Particles IN and Outside HZSM-5 under CO Hydrogenation Conditions Studied by ex situ and in situ Electron Microscopy. ChemCatChem 2021, 13, 718–729. [Google Scholar] [CrossRef]
- Feyen, M.; Weidenthaler, C.; Güttel, R.; Schlichte, K.; Holle, U.; Lu, A.-H.; Schüth, F. High-temperature stable, iron-based core-shell catalysts for ammonia decomposition. Chemistry 2011, 17, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Tai, Z.; Isaacs, M.A.; Durndell, L.J.; Parlett, C.M.A.; Lee, A.F.; Wilson, K. Magnetically-separable Fe3O4@SiO2@SO4-ZrO2 core-shell nanoparticle catalysts for propanoic acid esterification. Mol. Catal. 2018, 449, 137–141. [Google Scholar] [CrossRef]
- Yao, L.H.; Li, Y.X.; Zhao, J.; Ji, W.J.; Au, C.T. Core–shell structured nanoparticles (M@SiO2, Al2O3, MgO. M=Fe, Co, Ni, Ru) and their application in COx-free H2 production via NH3 decomposition. Catal. Today 2010, 158, 401–408. [Google Scholar] [CrossRef]
- Hudson, R.; Rivière, A.; Cirtiu, C.M.; Luska, K.L.; Moores, A. Iron-iron oxide core-shell nanoparticles are active and magnetically recyclable olefin and alkyne hydrogenation catalysts in protic and aqueous media. ChemComm 2012, 48, 3360–3362. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.; Qin, H.; Kang, S.; Bai, J.; Wang, Z.; Li, Y.; Zheng, Z.; Li, X. Effect of graphitic carbon modification on the catalytic performance of Fe@SiO2-GC catalysts for forming lower olefins via Fischer-Tropsch synthesis. J. Colloid Interface Sci. 2018, 516, 16–22. [Google Scholar] [CrossRef]
- Aris, R. Elementary Chemical Reactor Analysis; Butterworths: Boston, MA, USA, 1989; ISBN 0-409-90221-7. [Google Scholar]
- Shao, R.; Zambrzycki, C.; Guttel, R.; Herr, U. Model-Independent Size Distribution Determination of Superparamagnetic Nanoparticles. IEEE Trans. Magn. 2022, 58, 1–5. [Google Scholar] [CrossRef]
- Zieliński, J.; Zglinicka, I.; Znak, L.; Kaszkur, Z. Reduction of Fe2O3 with hydrogen. Appl. Catal. A Gen. 2010, 381, 191–196. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses, 2nd ed.; Completely rev. and Extended ed.; Wiley-VCH: Weinheim, Germany, 2003; ISBN 3527302743. [Google Scholar]
- Eliason, S.A.; Bartholomew, C.H. Reaction and deactivation kinetics for Fischer–Tropsch synthesis on unpromoted and potassium-promoted iron catalysts. Appl. Catal. A Gen. 1999, 186, 229–243. [Google Scholar] [CrossRef]
- Anderson, J.R. Catalysis: Science and Technology; Springer: Berlin/Heidelberg, Germany, 1982; ISBN 9783642932236. [Google Scholar]
- Xu, M.-W.P.; Brown, J.J. Mechanism of Iron Catalysis of Carbon Monoxide Decomposition in Refractories. J. Am. Ceram. Soc. 1989, 72, 110–115. [Google Scholar] [CrossRef]
- Mirzaei, A.A.; Kiai, R.M.; Atashi, H.; Arsalanfar, M.; Shahriari, S. Kinetic study of CO hydrogenation over co-precipitated iron–nickel catalyst. J. Ind. Eng. Chem. 2012, 18, 1242–1251. [Google Scholar] [CrossRef]
- Spencer, M.S. On the activation energies of the forward and reverse water-gas shift reaction. Catal. Lett. 1995, 32, 9–13. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Rivera-Utrilla, J.; Ferro-Garcia, M.A. Gasification of active carbons of different texture impregnated with nickel, cobalt and iron. Carbon 1987, 25, 703–708. [Google Scholar] [CrossRef]
- Wenzel, M.; Aditya Dharanipragada, N.V.R.; Galvita, V.V.; Poelman, H.; Marin, G.B.; Rihko-Struckmann, L.; Sundmacher, K. CO production from CO2 via reverse water–gas shift reaction performed in a chemical looping mode: Kinetics on modified iron oxide. J. CO2 Util. 2017, 17, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Landau, M.V.; Meiri, N.; Utsis, N.; Vidruk Nehemya, R.; Herskowitz, M. Conversion of CO2, CO, and H2 in CO2 Hydrogenation to Fungible Liquid Fuels on Fe-Based Catalysts. Ind. Eng. Chem. Res. 2017, 56, 13334–13355. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, C.; Zhang, C.; Zhang, Z.; Liu, X.; Yang, Z.; Zhu, M.; Meng, B.; Xu, J.; Han, Y.-F. The study of structure-performance relationship of iron catalyst during a full life cycle for CO2 hydrogenation. J. Catal. 2019, 378, 51–62. [Google Scholar] [CrossRef]
- Ilsemann, J.; Straß-Eifert, A.; Friedland, J.; Kiewidt, L.; Thöming, J.; Bäumer, M.; Güttel, R. Cobalt@Silica Core-Shell Catalysts for Hydrogenation of CO/CO2 Mixtures to Methane. ChemCatChem 2019, 11, 4884–4893. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z. Catalytic Conversion of Carbon Oxides in Confined Spaces: Status and Prospects. ChemCatChem 2020, 12, 3960–3981. [Google Scholar] [CrossRef]
- Sengupta, S.; Jha, A.; Shende, P.; Maskara, R.; Das, A.K. Catalytic performance of Co and Ni doped Fe-based catalysts for the hydrogenation of CO2 to CO via reverse water-gas shift reaction. J. Environ. Chem. Eng. 2019, 7, 102911. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Hamdeh, H.H.; Shafer, W.D.; Liu, F.; Hopps, S.D.; Thomas, G.A.; Davis, B.H. Hydrogenation of Carbon Dioxide over Co–Fe Bimetallic Catalysts. ACS Catal. 2016, 6, 913–927. [Google Scholar] [CrossRef]
- Lopez Luna, M.; Timoshenko, J.; Kordus, D.; Rettenmaier, C.; Chee, S.W.; Hoffman, A.S.; Bare, S.R.; Shaikhutdinov, S.; Roldan Cuenya, B. Role of the Oxide Support on the Structural and Chemical Evolution of Fe Catalysts during the Hydrogenation of CO2. ACS Catal. 2021, 11, 6175–6185. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrzycki, C.; Güttel, R. Transient Behavior of CO and CO2 Hydrogenation on Fe@SiO2 Core–Shell Model Catalysts—A Stoichiometric Analysis of Experimental Data. Reactions 2022, 3, 374-391. https://doi.org/10.3390/reactions3030027
Zambrzycki C, Güttel R. Transient Behavior of CO and CO2 Hydrogenation on Fe@SiO2 Core–Shell Model Catalysts—A Stoichiometric Analysis of Experimental Data. Reactions. 2022; 3(3):374-391. https://doi.org/10.3390/reactions3030027
Chicago/Turabian StyleZambrzycki, Christian, and Robert Güttel. 2022. "Transient Behavior of CO and CO2 Hydrogenation on Fe@SiO2 Core–Shell Model Catalysts—A Stoichiometric Analysis of Experimental Data" Reactions 3, no. 3: 374-391. https://doi.org/10.3390/reactions3030027
APA StyleZambrzycki, C., & Güttel, R. (2022). Transient Behavior of CO and CO2 Hydrogenation on Fe@SiO2 Core–Shell Model Catalysts—A Stoichiometric Analysis of Experimental Data. Reactions, 3(3), 374-391. https://doi.org/10.3390/reactions3030027





