Expanding the Equilibrium Solubility and Dissolution Thermodynamics of Benzoic Acid in Aqueous Alcoholic Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Determinations
2.3. Solid Phase Characterization
2.3.1. Differential Scanning Calorimetry Analysis
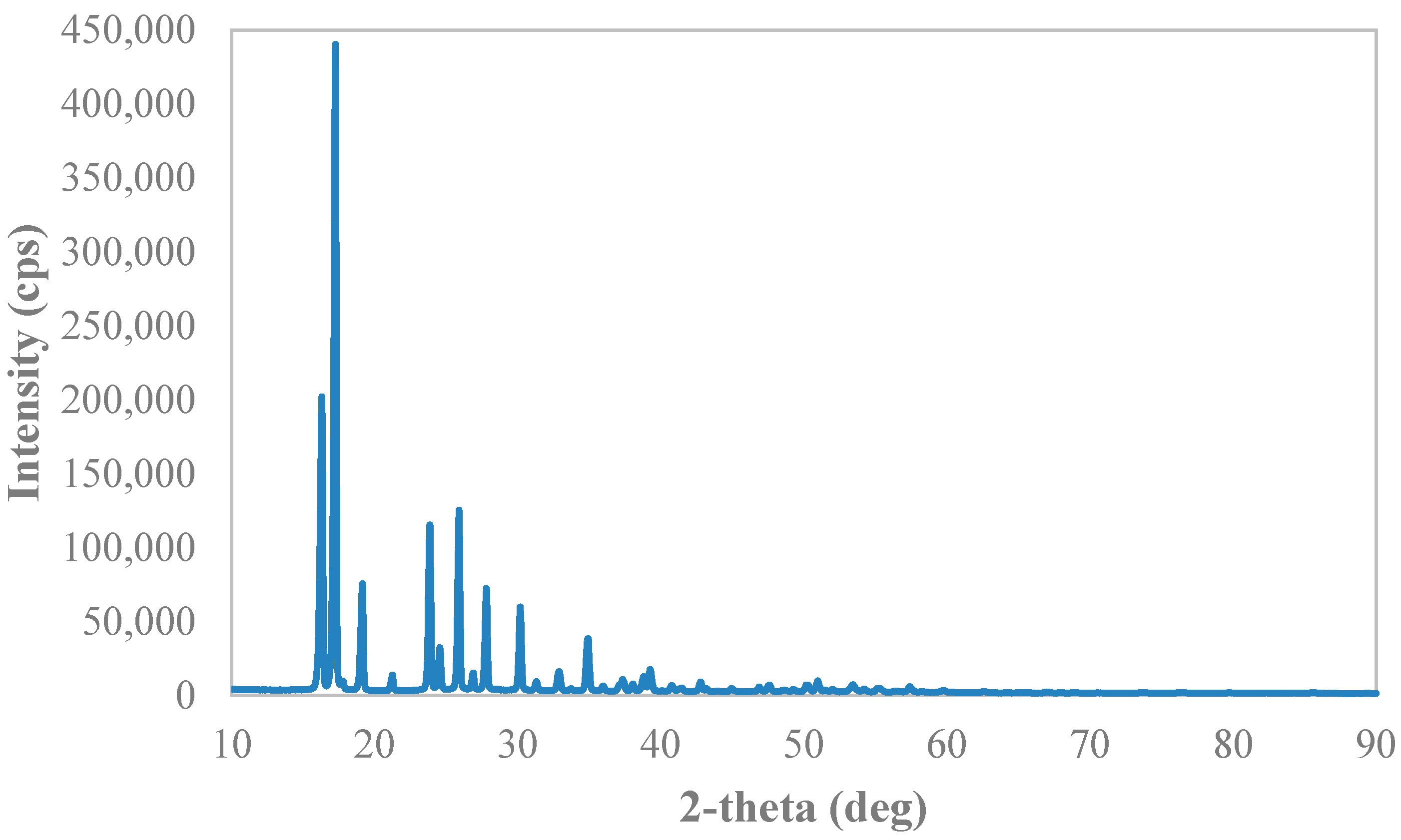
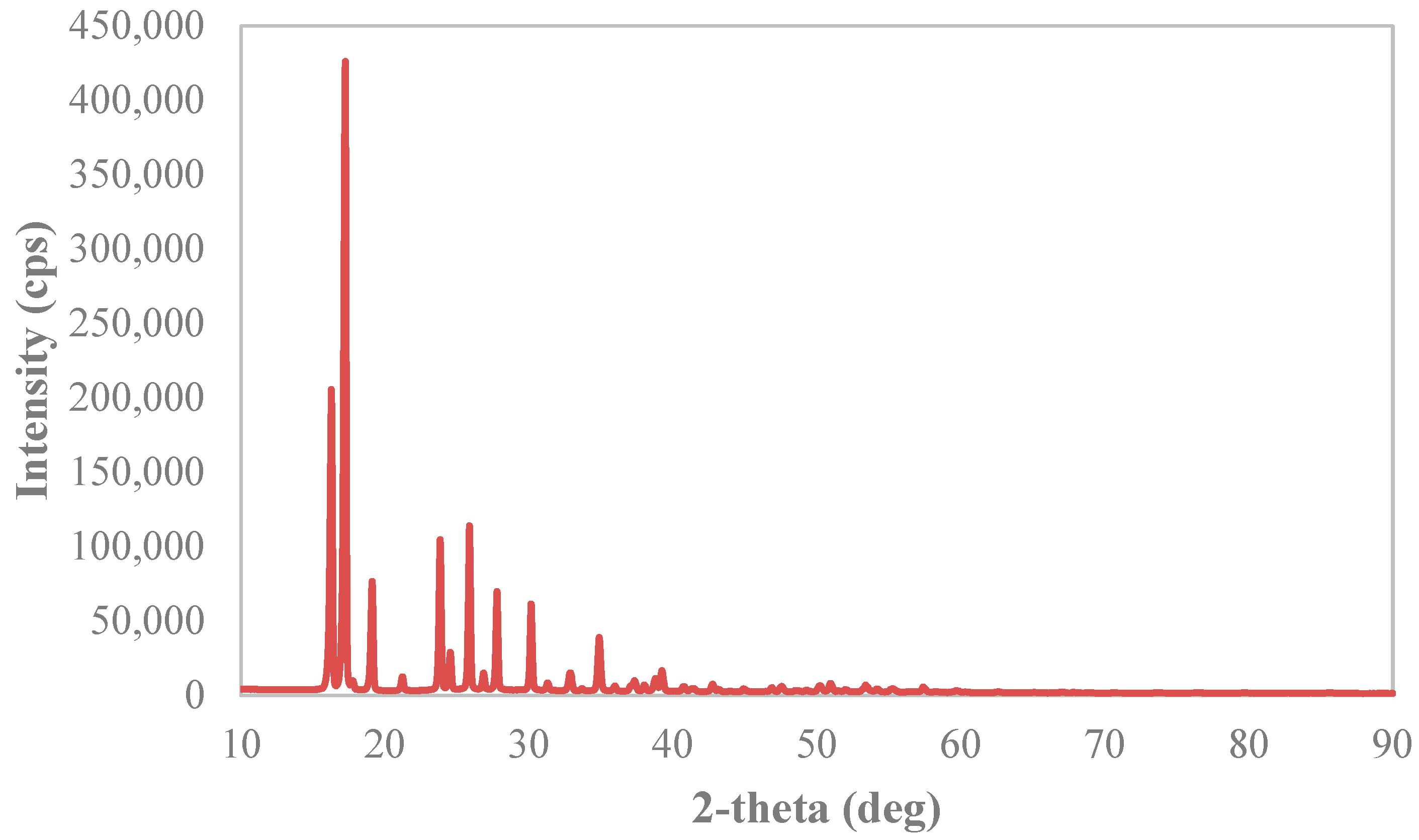
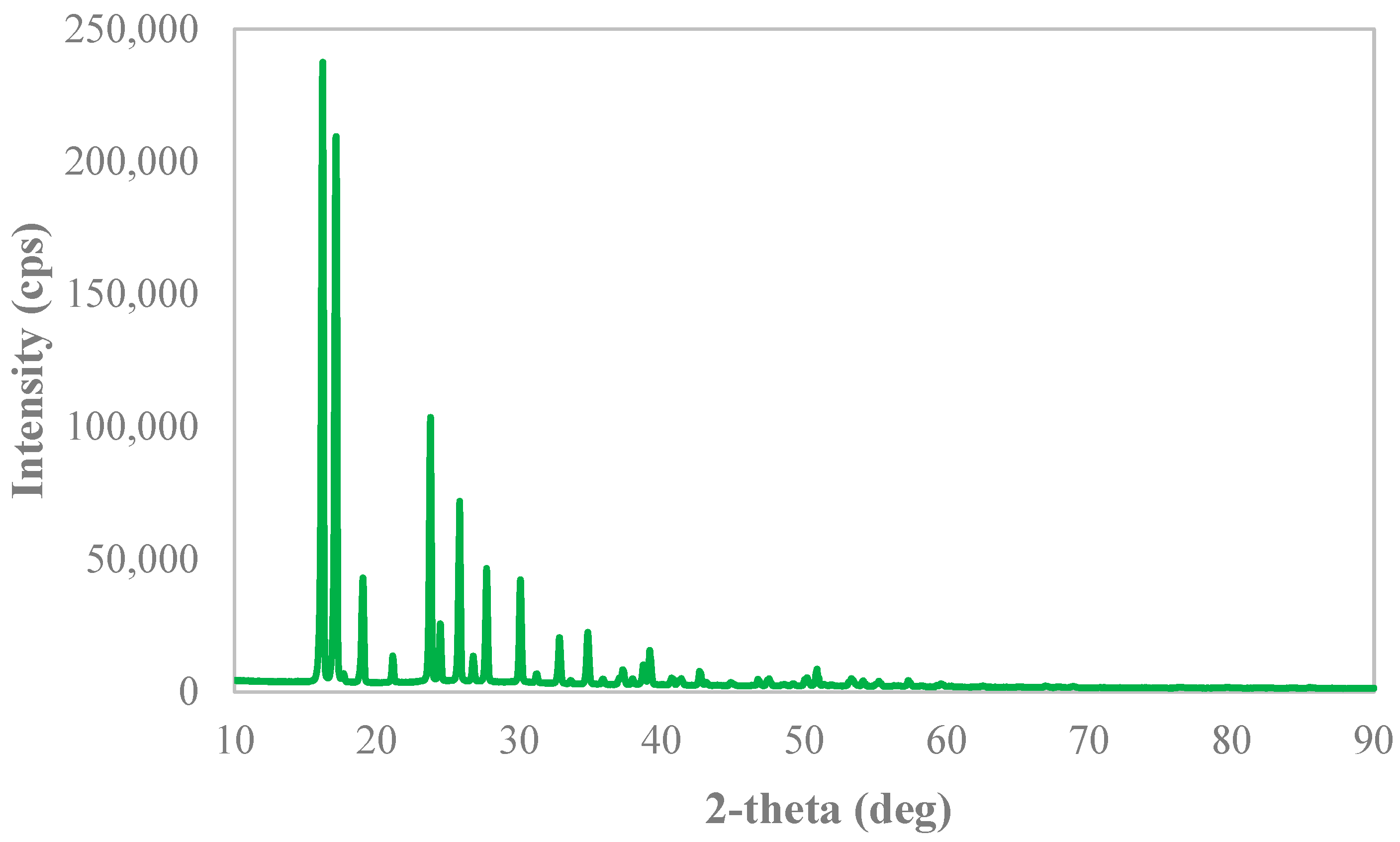
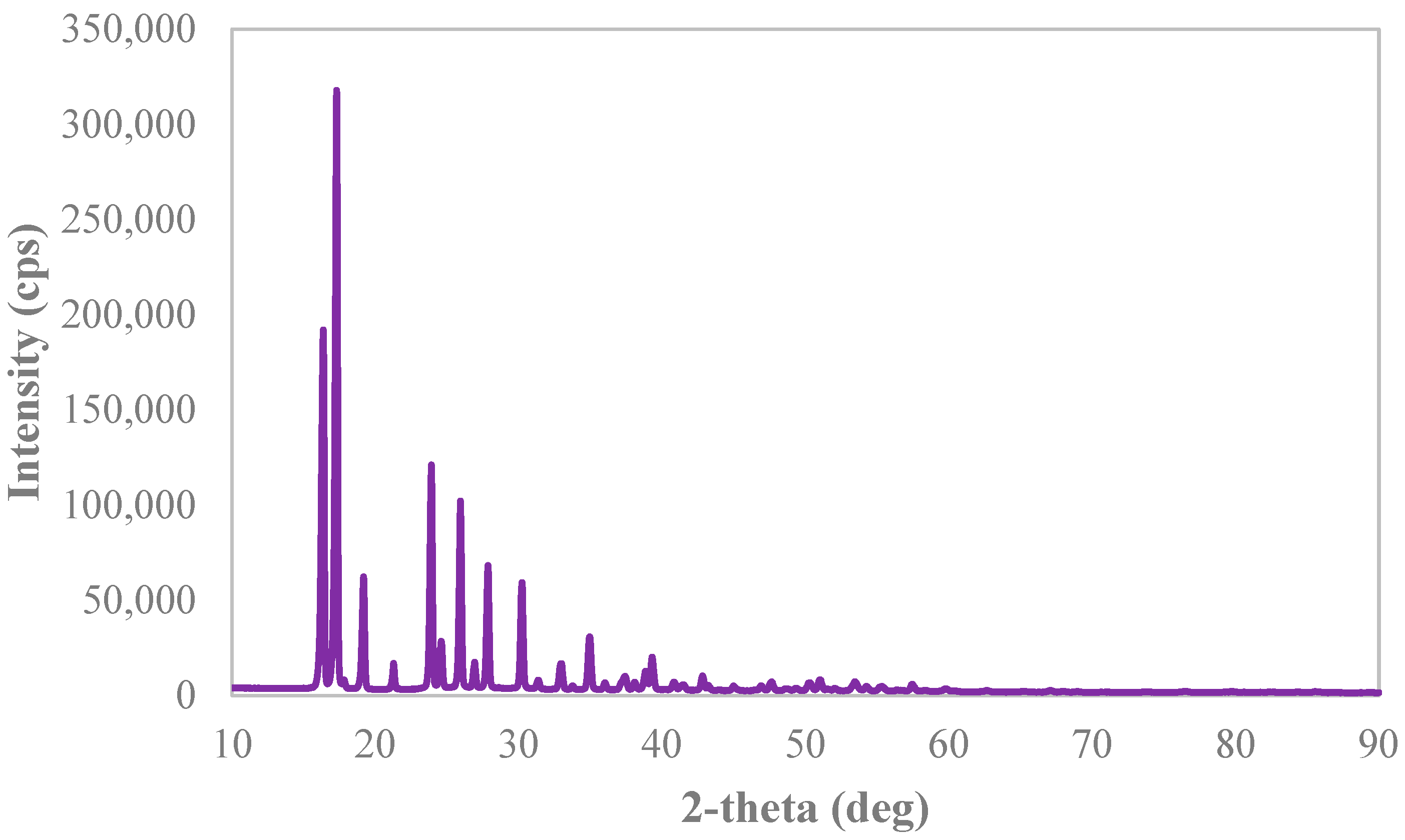
2.3.2. X-ray Diffraction Analysis
3. Results and Discussion
3.1. Mole Fraction Solubility of Benzoic Acid
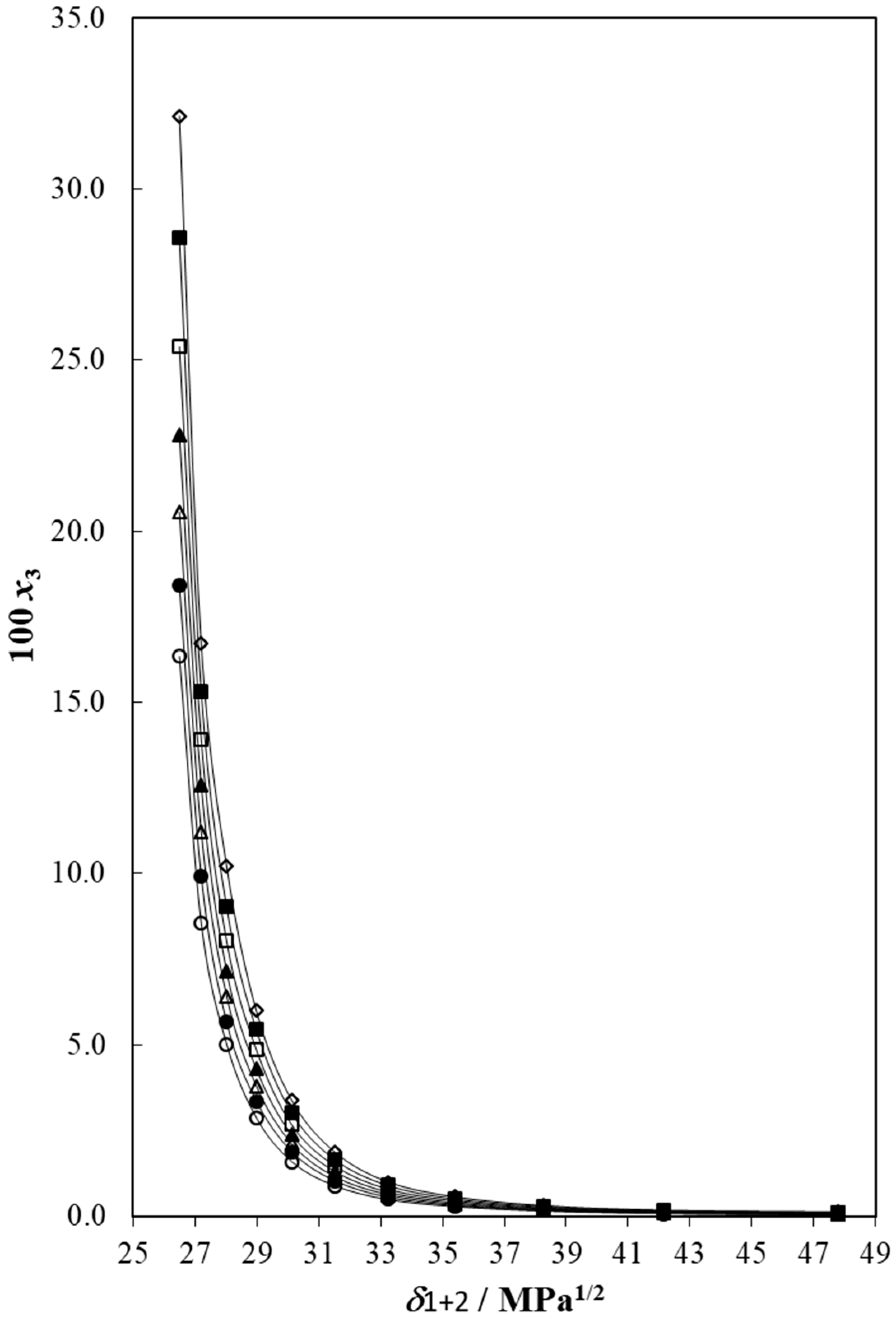
3.2. The Cosolvency Models
3.3. Solid Phases’ Analyses
3.4. Ideal Solubility and Activity Coefficients of Benzoic Acid in Mixed Solvents
3.5. Apparent Thermodynamic Functions of Dissolution
3.6. Apparent Thermodynamic Quantities of Mixing
3.7. Thermodynamic Quantities of Solvation
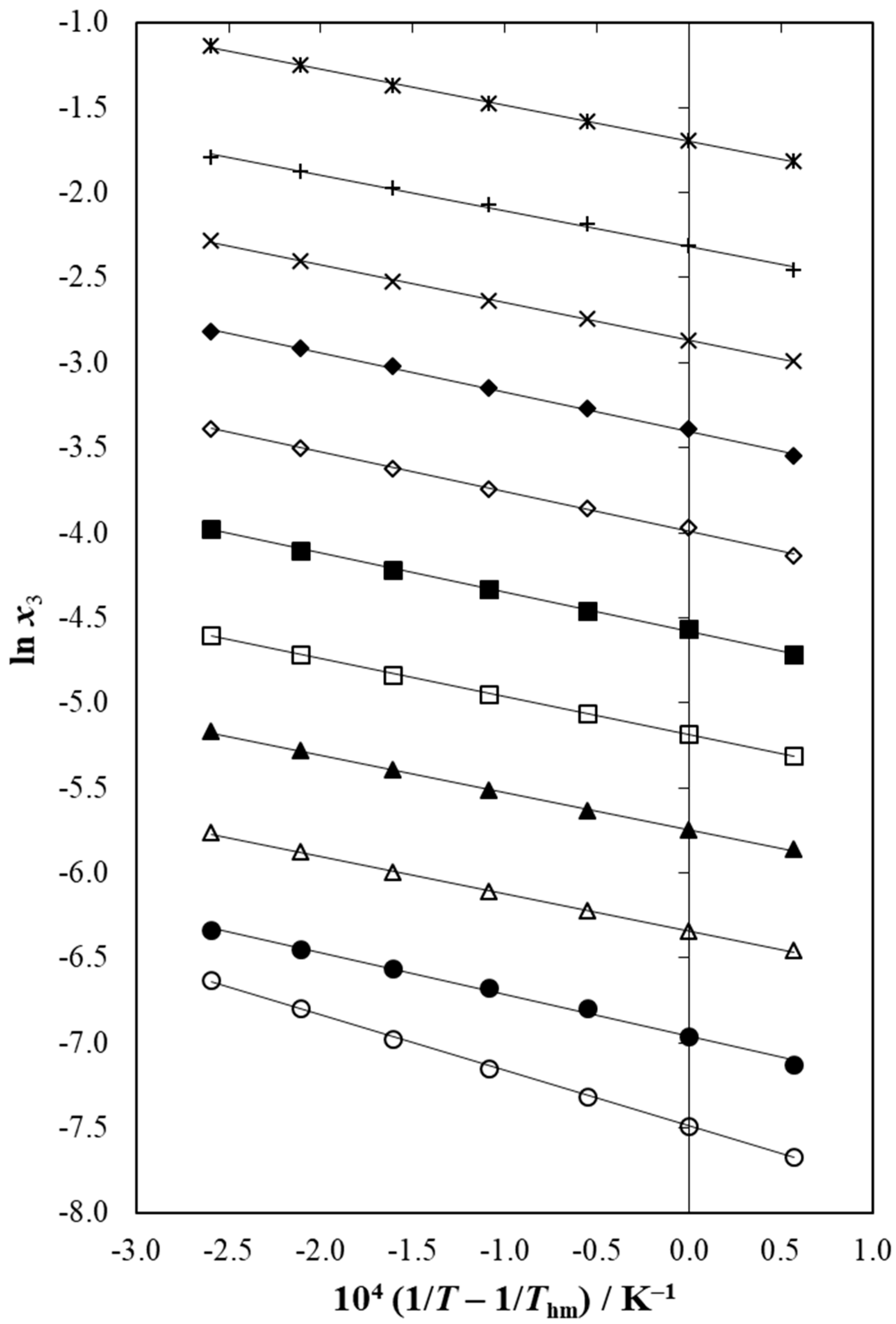
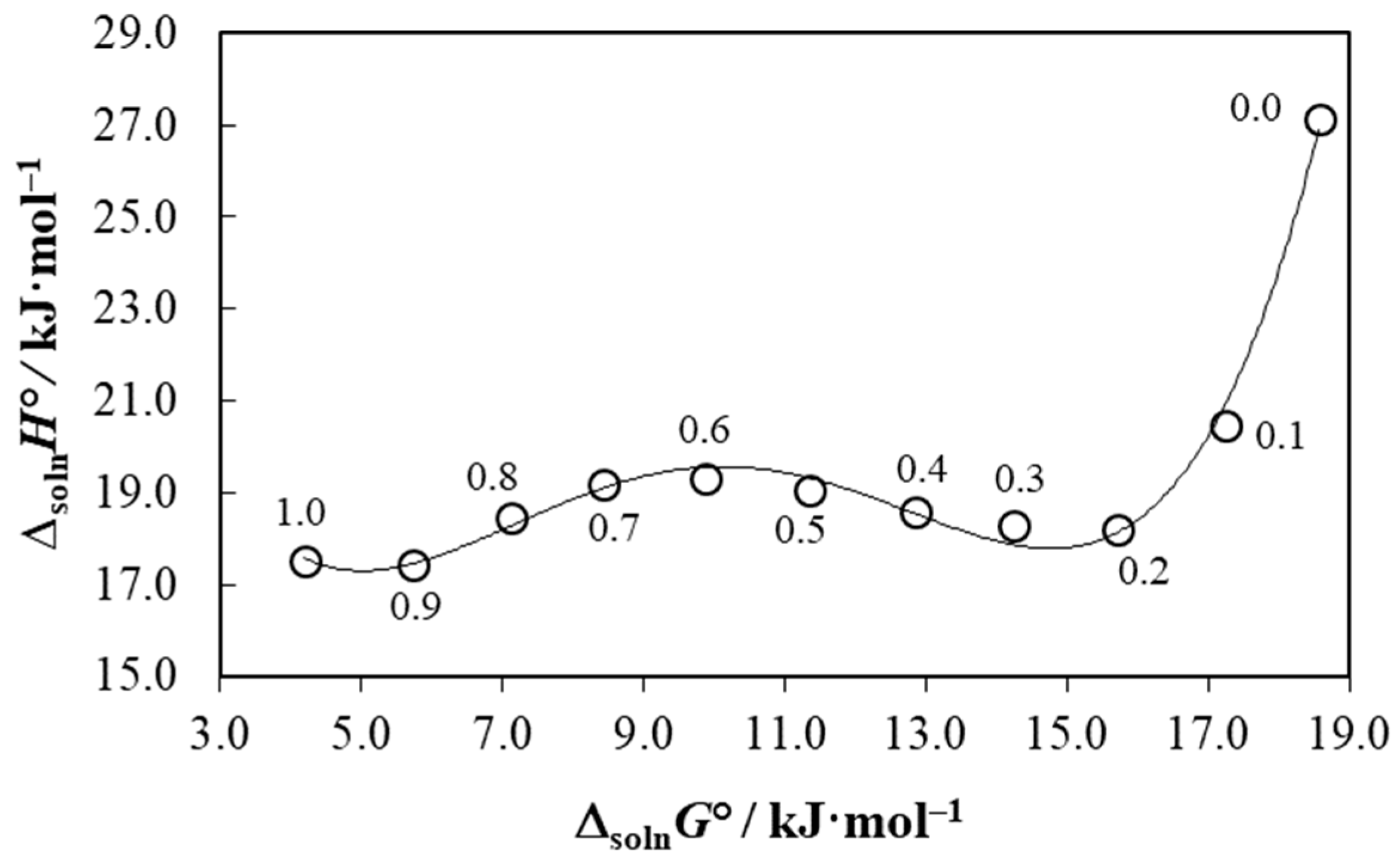
3.8. Enthalpy-Entropy Compensation Analysis
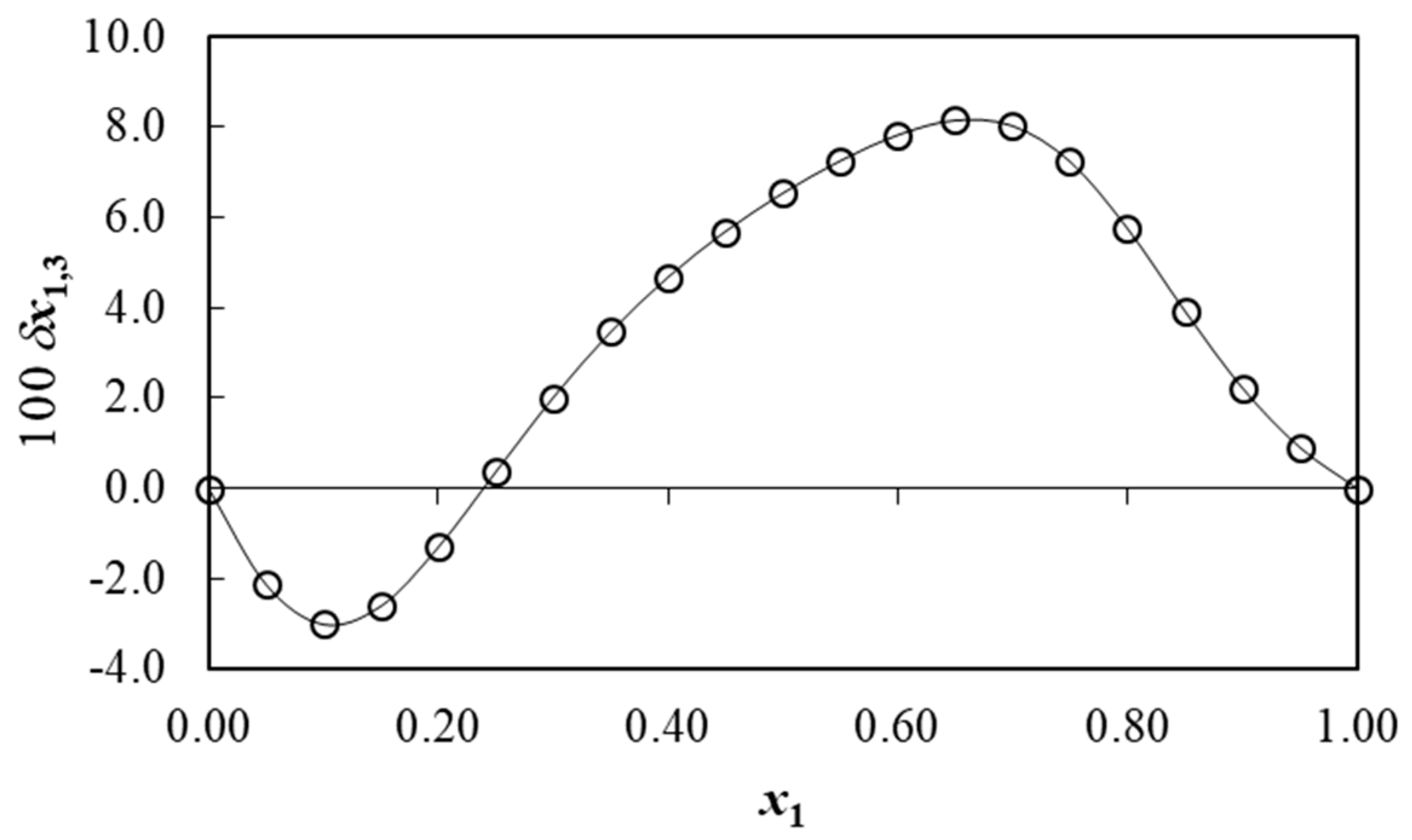
3.9. Preferential Solvation of Benzoic Acid
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. DSC Thermograms




Appendix B. X-ray Diffraction Spectra
References
- Jouyban, A. Handbook of Solubility Data for Pharmaceutical; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Marcus, Y. Solvent Mixtures: Properties and Selective Solvation; Marcel Dekker, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Marcus, Y. On the preferential solvation of drugs and PAHs in binary solvent mixtures. J. Mol. Liq. 2008, 140, 61–67. [Google Scholar] [CrossRef]
- Marcus, Y. Preferential solvation of drugs in binary solvent mixtures. Pharm. Anal. Acta 2017, 8, 1000537. [Google Scholar] [CrossRef]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E.; Obenchain, J.R., Jr.; Gallipeau, J.A.R.; D’Arecea, M.A. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; Merck & Co., Inc.: Whitehouse Station, NJ, USA, 2001. [Google Scholar]
- Long, B.; Li, J.; Zhang, R.; Wan, L. Solubility of benzoic acid in acetone, 2-propanol, acetic acid and cyclohexane: Experimental measurement and thermodynamic modeling. Fluid Phase Equilib. 2010, 297, 113–120. [Google Scholar] [CrossRef]
- Xue, J.; Yu, C.; Zeng, Z.; Xue, W.; Chen, Y. Solubility of benzoic acid in six alcohols within (288.15 to 336.15 K) and thermodynamic properties in the dissolution process. Asian J. Chem. Sci. 2018, 3, 1–12. [Google Scholar] [CrossRef]
- Sandeepa, K.; Kumar, K.R.; Neeharika, T.S.V.R.; Satyavathi, B.; Thella, P.K. Solubility measurement and thermodynamic modeling of benzoic acid in monosolvents and binary mixtures. J. Chem. Eng. Data 2018, 63, 2028–2037. [Google Scholar] [CrossRef]
- AbouEllef, E.M.; Gomaa, E.A.; Mashaly, M.S. Thermodynamic solvation parameters for saturated benzoic acid and some of its derivatives in binary mixtures of ethanol and water. J. Biochem. Tech. 2018, 9, 42–47. [Google Scholar]
- Muhammad, S.; Sanam, S.; Khan, H.; Muhammad, A.; Sultana, S. Temperature dependent solubility of benzoic acid in aqueous phase and aqueous mixtures of aliphatic alcohols. Z. Phys. Chem. 2020, 234, 1771–1787. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; American Pharmacists Association and Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Wang, H.; Wang, Q.; Xiong, Z.; Chen, C.; Shen, B. Solubilities of benzoic acid in binary (benzyl alcohol + benzaldehyde) solvent mixtures. J. Chem. Thermodyn. 2015, 83, 61–66. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Xu, X.; Yang, A.; Luo, W.; Luo, Y. Solubility of benzoic acid in twelve organic solvents: Experimental measurement and thermodynamic modeling. J. Chem. Thermodyn. 2020, 150, 106234. [Google Scholar] [CrossRef]
- Li, D.Q.; Liu, J.C.; Liu, D.Z.; Wang, F.A. Solubilities of terephthalaldehydic, p-toluic, benzoic, terephthalic and isophthalic acids in N,N-dimethylformamide from 294.75 to 370.45 K. Fluid Phase Equilib. 2002, 200, 69–74. [Google Scholar] [CrossRef]
- Cheng, W.T.; Feng, S.; Cui, X.; Cheng, F. Solubility of benzoic acid in ethanol, benzene, acetic acid and ethyl acetate from 291.69 to 356.27 K. Adv. Mater. Res. 2012, 518–523, 3975–3979. [Google Scholar] [CrossRef]
- Thati, J.; Nordstrom, F.L.; Rasmuson, A.C. Solubility of benzoic acid in mono-solvents and binary mixtures. J. Chem. Eng. Data 2010, 55, 5124–5127. [Google Scholar] [CrossRef]
- Kumari, A.; Sandeepa, K.; Kumar, T.P.; Satyavathi, B. Solubility, thermodynamic properties, and derived excess properties of benzoic acid in (acetic acid + water) and (acetic acid + toluene) binary mixtures. J. Chem. Eng. Data 2016, 61, 67–77. [Google Scholar] [CrossRef]
- Jorge, K. Soft Drinks/Chemical Composition. In Encyclopaedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Finglas, P., Toldra, F., Eds.; Academic Press: New York, NY, USA, 2003; p. 5346. [Google Scholar]
- Oliveira, A.C.; Coelho, M.G.; Pires, R.F.; Franco, M.R. Solubility of benzoic acid in mixed solvents. J. Chem. Eng. Data 2007, 52, 298–300. [Google Scholar] [CrossRef]
- Pires, R.F.; Franco, M.R., Jr. Solubility of benzoic acid in aqueous solutions containing ethanol or n-propanol. J. Chem. Eng. Data 2008, 53, 2704–2706. [Google Scholar] [CrossRef]
- Chertkoff, M.J.; Martin, A.N. The solubility of benzoic acid in mixed solvents. J. Am. Pharm. Assoc. 1960, 49, 444–447. [Google Scholar] [CrossRef]
- Sahay, H.; Kumar, S.; Upadhyay, S.N.; Upadhya, Y.D. Solubility of benzoic acid in aqueous polymeric solutions. J. Chem. Eng. Data 1981, 26, 181–183. [Google Scholar] [CrossRef]
- Mendez-Santiago, J.; Teja, A.S. Solubility of benzoic acid in mixtures of CO2 + hexane. J. Chem. Eng. Data 2012, 57, 3438–3442. [Google Scholar] [CrossRef]
- Sandeepa, K.; Neeharika, T.S.V.R.; Kumar, K.R.; Satyavathi, B.; Thella, P.K. Determination of solid−liquid phase equilibrium of benzoic acid in mono, binary, and ternary systems and their correlation. J. Chem. Eng. Data 2021, 66, 793–804. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Hu, J.; Liu, M.; Cai, Z.; Xu, Y.; Sun, B. The solubilities of benzoic acid and its nitro-derivatives, 3-nitro and 3,5-dinitrobenzoic acids. J. Chem. Res. 2021, 45, 1100–1106. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Belhachemi, B.; Makhlouf, H.; Belhachemi, M.H. Determination and correlation of solubilities of benzoic acid, salicylic acid, resorcinol and hydroquinone in water and in 1-octanol at temperatures from 297.25 K to 334.45 K. Int. J. Thermophys. 2021, 42, 1. [Google Scholar] [CrossRef]
- Pal, A.; Lahiri, S.C. Solubility and the thermodynamics of transfer of benzoic acid in mixed solvents. Indian J. Chem. 1989, 28A, 276–279. [Google Scholar]
- Barton, A.F.M. Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Marcus, Y. The Properties of Solvents; John Wiley & Sons: Chichester, UK, 1998. [Google Scholar]
- Martin, A.; Bustamante, P.; Chun, A.H.C. Physical Pharmacy: Physical Chemical Principles in the Pharmaceutical Sciences, 4th ed.; Lea & Febiger: Philadelphia, PA, USA, 1993. [Google Scholar]
- Connors, K.A. Thermodynamics of Pharmaceutical Systems: An Introduction for Students of Pharmacy; Wiley–Interscience: Hoboken, NJ, USA, 2002. [Google Scholar]
- Rubino, J.T. Cosolvents and cosolvency. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Boylan, J.C., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1988; Volume 3, pp. 375–398. [Google Scholar]
- Yalkowsky, S.H. Solubility and Solubilization in Aqueous Media; American Chemical Society and Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Fedors, R.F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Jouyban, A.; Acree, W.E., Jr. Mathematical derivation of the Jouyban-Acree model to represent solute solubility data in mixed solvents at various temperatures. J. Mol. Liq. 2018, 256, 541–547. [Google Scholar] [CrossRef]
- Jouyban-Gharamaleki, A.; Valaee, L.; Barzegar-Jalali, M.; Clark, B.J.; Acree, W.E., Jr. Comparison of various cosolvency models for calculating solute solubility in water-cosolvent mixtures. Int. J. Pharm. 1999, 177, 93–101. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; Roseman, T.J. Solubilization of drugs by cosolvents. In Techniques of Solubilization of Drugs; Yalkowsky, S.H., Ed.; Marcel Dekker: New York, NY, USA, 1981; pp. 91–134. [Google Scholar]
- Jouyban, A.; Romero, S.; Chan, H.K.; Clark, B.J.; Bustamante, P. A cosolvency model to predict solubility of drugs at several temperatures from a limited number of solubility measurements. Chem. Pharm. Bull. 2002, 50, 594–599. [Google Scholar] [CrossRef] [Green Version]
- Dadmand, S.; Kamari, F.; Acree, W.E., Jr.; Jouyban, A. Solubility prediction of drugs in binary solvent mixtures at various temperatures using a minimum number of experimental data points. AAPS PharmSciTech 2019, 20, 10. [Google Scholar] [CrossRef]
- NIST Web Book of Chemistry. SRD 69, Benzoic Acid. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C65850&Mask=4 (accessed on 2 July 2022).
- Feld, R.; Lehmann, M.S.; Muir, K.W.; Speakman, J.C. The crystal structure of Benzoic Acid: A redetermination with X-rays at room temperature; a summary of neutron-diffraction work at temperatures down to 5 K. Z. Fiir. Krist. Cryst. Mater. 1981, 157, 215–231. [Google Scholar] [CrossRef]
- Cárdenas, Z.J.; Jiménez, D.M.; Delgado, D.R.; Almanza, O.A.; Jouyban, A.; Martínez, F.; Acree, W.E., Jr. Solubility and preferential solvation of some n-alkyl parabens in methanol + water mixtures at 298.15 K. J. Chem. Thermodyn. 2017, 108, 26–37. [Google Scholar] [CrossRef]
- Kristl, A.; Vesnaver, G. Thermodynamic investigation of the effect of octanol–water mutual miscibility on the partitioning and solubility of some guanine derivatives. J. Chem. Soc. Faraday Trans. 1995, 91, 995–998. [Google Scholar] [CrossRef]
- Jouyban, A.; Acree, W.E., Jr.; Martínez, F. Dissolution thermodynamics and preferential solvation of ketoconazole in some {ethanol (1) + water (2)} mixtures. J. Mol. Liq. 2020, 313, 113579. [Google Scholar] [CrossRef]
- Akay, S.; Kayan, B.; Jouyban, A.; Martínez, F.; Acree, W.E. Solubility of coumarin in (ethanol + water) mixtures: Determination, correlation, thermodynamics and preferential solvation. J. Mol. Liq. 2021, 339, 116761. [Google Scholar] [CrossRef]
- Bevington, P.R. Data Reduction and Error Analysis for the Physical Sciences; McGraw-Hill Book, Co.: New York, NY, USA, 1969; pp. 56–65. [Google Scholar]
- Carstensen, J.T. Modeling and Data Treatment in the Pharmaceutical Sciences; Technomic Publishing Co., Inc.: Lancaster, PA, USA, 1996; pp. 127–159. [Google Scholar]
- Barrante, J.R. Applied Mathematics for Physical Chemistry, 2nd ed.; Prentice Hall, Inc.: Upper Saddle River, NJ, USA, 1998; 227p. [Google Scholar]
- Ruidiaz, M.A.; Delgado, D.R.; Martínez, F.; Marcus, Y. Solubility and preferential solvation of indomethacin in 1,4-dioxane + water solvent mixtures. Fluid Phase Equilib. 2010, 299, 259–265. [Google Scholar] [CrossRef]
- Perlovich, G.L.; Kurkov, S.V.; Kinchin, A.N.; Bauer-Brandl, A. Thermodynamics of solutions III: Comparison of the solvation of (+)-naproxen with other NSAIDs. Eur. J. Pharm. Biopharm. 2004, 57, 411–420. [Google Scholar] [CrossRef]
- Delgado, D.R.; Almanza, O.A.; Martínez, F.; Peña, M.A.; Jouyban, A.; Acree, W.E., Jr. Solution thermodynamics and preferential solvation of sulfamethazine in (methanol + water) mixtures. J. Chem. Thermodyn. 2016, 97, 264–276. [Google Scholar] [CrossRef]
- Jouyban, K.; Agha, E.M.H.; Hemmati, S.; Martinez, F.; Kuentz, M.; Jouyban, A. Solubility of 5-aminosalicylic acid in N-methyl-2-pyrrolidone + water mixtures at various temperatures. J. Mol. Liq. 2020, 310, 113143. [Google Scholar] [CrossRef]
- Romdhani, A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Solubility of acetaminophen in (ethanol + propylene glycol + water) mixtures: Measurement, correlation, thermodynamics, and volumetric contribution at saturation. J. Mol. Liq. 2020, 318, 114065. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Blok, J.G. The vapour pressure of benzoic acid. J. Chem. Thermodyn. 1982, 14, 201–206. [Google Scholar] [CrossRef]
- Tomlinson, E. Enthalpy-entropy compensation analysis of pharmaceutical, biochemical and biological systems. Int. J. Pharm. 1983, 13, 115–144. [Google Scholar] [CrossRef]
- Leffler, J.E.; Grunwald, E. Rates and Equilibria of Organic Reactions: As Treated by Statistical, Thermodynamic and Extrathermodynamic Methods; Dover Publications Inc.: New York, NY, USA, 1989. [Google Scholar]
- Akay, S.; Kayan, B.; Martínez, F. Solubility of fluconazole in (ethanol + water) mixtures: Determination, correlation, dissolution thermodynamics and preferential solvation. J. Mol. Liq. 2021, 333, 115987. [Google Scholar] [CrossRef]
- Bustamante, P.; Romero, S.; Reillo, A. Thermodynamics of paracetamol in amphiprotic and amphiprotic-aprotic solvent mixtures. Pharm. Pharmacol. Commun. 1995, 1, 505–507. [Google Scholar] [CrossRef]
- Bustamante, P.; Romero, S.; Peña, A.; Escalera, B.; Reillo, A. Nonlinear enthalpy-entropy compensation for the solubility of drugs in solvent mixtures: Paracetamol, acetanilide and nalidixic acid in dioxane-water. J. Pharm. Sci. 1998, 87, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.; Peña, M.A.; Bustamante, P. Thermodynamic analysis and enthalpy-entropy compensation for the solubility of indomethacin in aqueous and non-aqueous mixtures. Fluid Phase Equilib. 2011, 308, 98–106. [Google Scholar] [CrossRef]
- Delgado, D.R.; Peña, M.A.; Martínez, F. Preferential solvation of acetaminophen in ethanol + water solvent mixtures according to the inverse Kirkwood-Buff integrals method. Rev. Colomb. Cienc. Quím. Farm. 2013, 42, 298–314.
- Jiménez, D.M.; Cárdenas, Z.J.; Delgado, D.R.; Martínez, F.; Jouyban, A. Preferential solvation of methocarbamol in aqueous binary cosolvent mixtures at 298.15 K. Phys. Chem. Liq. 2014, 52, 726–737. [Google Scholar] [CrossRef]
- Ben–Naim, A. Preferential solvation in two- and in three-component systems. Pure Appl. Chem. 1990, 62, 25–34. [Google Scholar] [CrossRef]
- Marcus, Y. Solubility and solvation in mixed solvent systems. Pure Appl. Chem. 1990, 62, 2069–2076. [Google Scholar] [CrossRef] [Green Version]


| Compound | CAS | Formula | Molar Mass/g·mol−1 | Source | Purity in Mass Fraction | Analytic Technique a |
|---|---|---|---|---|---|---|
| Benzoic acid | 65-85-0 | C7H6O2 | 122.12 | Sigma-Aldrich | ≥0.995 b | HPLC |
| Ethanol | 64-17-5 | C2H6O | 46.07 | Merck | >0.995 b | GC |
| Acetonitrile | 75-05-8 | C2H3N | 41.05 | Merck | ≥0.999 b | GC |
| Formic acid | 64-18-6 | CH2O2 | 46.03 | Merck | ≥0.999 b | GC |
| Water | 7732-18-5 | H2O | 18.02 | Obtained by Millipore Milli-Q-Gradient water purification system | >0.999 | - |
| w1 b,c | x1 b,c | T/K c | ||||||
|---|---|---|---|---|---|---|---|---|
| 293.15 | 298.15 | 303.15 | 308.15 | 313.15 | 318.15 | 323.15 | ||
| 0.00 | 0.0000 | 4.69 × 10−4 | 5.61 × 10−4 | 6.64 × 10−4 | 7.87 × 10−4 | 9.34 × 10−4 | 1.12 × 10−3 | 1.32 × 10−3 |
| 0.10 | 0.0417 | 8.05 × 10−4 | 9.51 × 10−4 | 1.12 × 10−3 | 1.26 × 10−3 | 1.42 × 10−3 | 1.58 × 10−3 | 1.77 × 10−3 |
| 0.20 | 0.0891 | 1.57 × 10−3 | 1.75 × 10−3 | 1.98 × 10−3 | 2.22 × 10−3 | 2.48 × 10−3 | 2.81 × 10−3 | 3.14 × 10−3 |
| 0.30 | 0.1436 | 2.84 × 10−3 | 3.19 × 10−3 | 3.56 × 10−3 | 4.02 × 10−3 | 4.54 × 10−3 | 5.07 × 10−3 | 5.69 × 10−3 |
| 0.40 | 0.2068 | 4.92 × 10−3 | 5.61 × 10−3 | 6.34 × 10−3 | 7.09 × 10−3 | 7.94 × 10−3 | 8.98 × 10−3 | 1.00 × 10−2 |
| 0.50 | 0.2812 | 8.93 × 10−3 | 1.04 × 10−2 | 1.16 × 10−2 | 1.31 × 10−2 | 1.48 × 10−2 | 1.65 × 10−2 | 1.87 × 10−2 |
| 0.60 | 0.3698 | 1.60 × 10−2 | 1.88 × 10−2 | 2.11 × 10−2 | 2.38 × 10−2 | 2.67 × 10−2 | 3.01 × 10−2 | 3.38 × 10−2 |
| 0.70 | 0.4772 | 2.88 × 10−2 | 3.37 × 10−2 | 3.80 × 10−2 | 4.30 × 10−2 | 4.86 × 10−2 | 5.44 × 10−2 | 6.00 × 10−2 |
| 0.80 | 0.6101 | 5.03 × 10−2 | 5.67 × 10−2 | 6.43 × 10−2 | 7.16 × 10−2 | 8.04 × 10−2 | 9.03 × 10−2 | 0.102 |
| 0.90 | 0.7788 | 8.56 × 10−2 | 9.93 × 10−2 | 0.112 | 0.126 | 0.139 | 0.153 | 0.167 |
| 1.00 | 1.0000 | 0.163 | 0.184 | 0.206 | 0.228 | 0.254 | 0.286 | 0.321 |
| Ideal | 0.194 | 0.213 | 0.233 | 0.255 | 0.278 | 0.304 | 0.331 | |
| Group | Number | ΔU°/kJ·mol−1 | V°/cm3·mol−1 |
|---|---|---|---|
| Phenyl ring | 1 | 31.9 | 71.4 |
| –COOH | 1 | 27.6 | 28.5 |
| Σ | 59.5 | 99.9 | |
| δ3 = (59,500/99.9)1/2 = 24.4 MPa1/2 | |||
| w1 b,c | x1 b,c | T/K c | ||||||
|---|---|---|---|---|---|---|---|---|
| 293.15 | 298.15 | 303.15 | 308.15 | 313.15 | 318.15 | 323.15 | ||
| 0.00 | 0.0000 | 413 | 379 | 351 | 324 | 298 | 272 | 251 |
| 0.10 | 0.0417 | 241 | 223 | 209 | 202 | 197 | 192 | 186 |
| 0.20 | 0.0891 | 123 | 121 | 117 | 115 | 112 | 108 | 106 |
| 0.30 | 0.1436 | 68.2 | 66.5 | 65.4 | 63.3 | 61.2 | 59.9 | 58.2 |
| 0.40 | 0.2068 | 39.4 | 37.9 | 36.7 | 36.0 | 35.1 | 33.8 | 32.9 |
| 0.50 | 0.2812 | 21.7 | 20.4 | 20.1 | 19.4 | 18.9 | 18.4 | 17.6 |
| 0.60 | 0.3698 | 12.1 | 11.3 | 11.0 | 10.7 | 10.4 | 10.1 | 9.78 |
| 0.70 | 0.4772 | 6.73 | 6.31 | 6.12 | 5.92 | 5.72 | 5.58 | 5.52 |
| 0.80 | 0.6101 | 3.85 | 3.75 | 3.62 | 3.56 | 3.46 | 3.36 | 3.24 |
| 0.90 | 0.7788 | 2.26 | 2.14 | 2.07 | 2.02 | 2.00 | 1.98 | 1.98 |
| 1.00 | 1.0000 | 1.18 | 1.15 | 1.13 | 1.12 | 1.10 | 1.06 | 1.03 |
| w1 b,c | x1 b,c | ΔsolnG°/kJ·mol−1 c | ΔsolnH°/kJ·mol−1 c | ΔsolnS°/J·mol−1·K−1 c | TΔsolnS°/kJ·mol−1 c | ζH d | ζTS d |
|---|---|---|---|---|---|---|---|
| 0.00 | 0.0000 | 18.57 | 27.15 | 28.79 | 8.58 | 0.760 | 0.240 |
| 0.10 | 0.0417 | 17.25 | 20.45 | 10.72 | 3.20 | 0.865 | 0.135 |
| 0.20 | 0.0891 | 15.72 | 18.19 | 8.30 | 2.48 | 0.880 | 0.120 |
| 0.30 | 0.1436 | 14.25 | 18.29 | 13.57 | 4.04 | 0.819 | 0.181 |
| 0.40 | 0.2068 | 12.86 | 18.60 | 19.26 | 5.74 | 0.764 | 0.236 |
| 0.50 | 0.2812 | 11.35 | 19.07 | 25.89 | 7.72 | 0.712 | 0.288 |
| 0.60 | 0.3698 | 9.89 | 19.30 | 31.55 | 9.41 | 0.672 | 0.328 |
| 0.70 | 0.4772 | 8.43 | 19.19 | 36.09 | 10.76 | 0.641 | 0.359 |
| 0.80 | 0.6101 | 7.11 | 18.46 | 38.06 | 11.35 | 0.619 | 0.381 |
| 0.90 | 0.7788 | 5.74 | 17.41 | 39.15 | 11.67 | 0.599 | 0.401 |
| 1.00 | 1.0000 | 4.21 | 17.53 | 44.70 | 13.33 | 0.568 | 0.432 |
| Ideal | 3.84 | 14.06 | 34.28 | 10.22 | 0.579 | 0.421 |
| w1 b,c | x1 b,c | ΔmixG°/kJ·mol−1 c | ΔmixH°/kJ·mol−1 c | ΔmixS°/J·mol−1·K−1 c | TΔmixS°/kJ·mol−1 c | ζH d | ζTS d |
|---|---|---|---|---|---|---|---|
| 0.00 | 0.0000 | 14.73 | 13.09 | −5.49 | −1.64 | 0.889 | 0.111 |
| 0.10 | 0.0417 | 13.41 | 6.39 | −23.56 | −7.02 | 0.476 | 0.524 |
| 0.20 | 0.0891 | 11.88 | 4.14 | −25.98 | −7.74 | 0.348 | 0.652 |
| 0.30 | 0.1436 | 10.41 | 4.23 | −20.71 | −6.18 | 0.407 | 0.593 |
| 0.40 | 0.2068 | 9.02 | 4.54 | −15.01 | −4.48 | 0.504 | 0.496 |
| 0.50 | 0.2812 | 7.51 | 5.01 | −8.39 | −2.50 | 0.667 | 0.333 |
| 0.60 | 0.3698 | 6.05 | 5.24 | −2.73 | −0.81 | 0.866 | 0.134 |
| 0.70 | 0.4772 | 4.59 | 5.13 | 1.82 | 0.54 | 0.905 | 0.095 |
| 0.80 | 0.6101 | 3.27 | 4.40 | 3.78 | 1.13 | 0.796 | 0.204 |
| 0.90 | 0.7788 | 1.90 | 3.35 | 4.87 | 1.45 | 0.698 | 0.302 |
| 1.00 | 1.0000 | 0.37 | 3.47 | 10.42 | 3.11 | 0.528 | 0.472 |
| w1 b,c | x1 b,c | ΔsolvG°/kJ·mol−1 c | ΔsolvH°/kJ·mol−1 c | ΔsolvS°/J·mol−1·K−1 c | TΔsolvS°/kJ·mol−1 c | ζH d | ζTS d | εH e | εS e |
|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.0000 | 12.99 | −64.96 | −261.43 | −77.94 | 0.455 | 0.545 | 0.666 | 0.795 |
| 0.10 | 0.0417 | 11.67 | −71.66 | −279.49 | −83.33 | 0.462 | 0.538 | 0.838 | 0.924 |
| 0.20 | 0.0891 | 10.14 | −73.91 | −281.91 | −84.05 | 0.468 | 0.532 | 0.896 | 0.941 |
| 0.30 | 0.1436 | 8.67 | −73.81 | −276.65 | −82.48 | 0.472 | 0.528 | 0.893 | 0.903 |
| 0.40 | 0.2068 | 7.28 | −73.50 | −270.95 | −80.78 | 0.476 | 0.524 | 0.885 | 0.863 |
| 0.50 | 0.2812 | 5.77 | −73.03 | −264.32 | −78.81 | 0.481 | 0.519 | 0.873 | 0.816 |
| 0.60 | 0.3698 | 4.31 | −72.81 | −258.66 | −77.12 | 0.486 | 0.514 | 0.868 | 0.775 |
| 0.70 | 0.4772 | 2.85 | −72.91 | −254.12 | −75.77 | 0.490 | 0.510 | 0.870 | 0.743 |
| 0.80 | 0.6101 | 1.53 | −73.65 | −252.15 | −75.18 | 0.495 | 0.505 | 0.889 | 0.729 |
| 0.90 | 0.7788 | 0.16 | −74.69 | −251.06 | −74.85 | 0.499 | 0.501 | 0.916 | 0.721 |
| 1.00 | 1.0000 | −1.37 | −74.57 | −245.51 | −73.20 | 0.505 | 0.495 | 0.913 | 0.682 |
| x1 a | D/kJ·mol−1 | G1,3/cm3·mol−1 | G2,3/cm3·mol−1 | Vcor/cm3·mol−1 | 100 δx1,3 |
|---|---|---|---|---|---|
| 0.00 | −34.92 | −353.4 | −98.8 | 583 | 0.00 |
| 0.05 | −31.28 | −340.2 | −136.4 | 601 | −2.13 |
| 0.10 | −27.94 | −320.5 | −173.1 | 628 | −3.01 |
| 0.15 | −24.91 | −297.0 | −206.3 | 663 | −2.61 |
| 0.20 | −22.17 | −272.3 | −234.6 | 704 | −1.31 |
| 0.25 | −19.71 | −248.6 | −258.1 | 748 | 0.36 |
| 0.30 | −17.51 | −227.2 | −277.8 | 791 | 2.01 |
| 0.35 | −15.56 | −208.7 | −295.1 | 832 | 3.46 |
| 0.40 | −13.85 | −193.3 | −311.9 | 873 | 4.68 |
| 0.45 | −12.36 | −180.6 | −329.7 | 911 | 5.69 |
| 0.50 | −11.09 | −170.3 | −350.3 | 949 | 6.54 |
| 0.55 | −10.01 | −161.8 | −375.1 | 985 | 7.26 |
| 0.60 | −9.12 | −154.4 | −404.3 | 1021 | 7.83 |
| 0.65 | −8.40 | −147.5 | −436.3 | 1055 | 8.15 |
| 0.70 | −7.83 | −140.0 | −464.3 | 1085 | 8.03 |
| 0.75 | −7.42 | −131.2 | −476.7 | 1112 | 7.24 |
| 0.80 | −7.13 | −121.4 | −461.8 | 1134 | 5.76 |
| 0.85 | −6.97 | −112.2 | −419.6 | 1154 | 3.94 |
| 0.90 | −6.91 | −104.9 | −363.8 | 1174 | 2.23 |
| 0.95 | −6.94 | −100.0 | −309.4 | 1197 | 0.92 |
| 1.00 | −7.05 | −97.0 | −264.0 | 1222 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akay, S.; Kayan, B.; Peña, M.Á.; Jouyban, A.; Martínez, F.; Acree, W.E., Jr. Expanding the Equilibrium Solubility and Dissolution Thermodynamics of Benzoic Acid in Aqueous Alcoholic Mixtures. Reactions 2022, 3, 392-414. https://doi.org/10.3390/reactions3030028
Akay S, Kayan B, Peña MÁ, Jouyban A, Martínez F, Acree WE Jr. Expanding the Equilibrium Solubility and Dissolution Thermodynamics of Benzoic Acid in Aqueous Alcoholic Mixtures. Reactions. 2022; 3(3):392-414. https://doi.org/10.3390/reactions3030028
Chicago/Turabian StyleAkay, Sema, Berkant Kayan, M. Ángeles Peña, Abolghasem Jouyban, Fleming Martínez, and William E. Acree, Jr. 2022. "Expanding the Equilibrium Solubility and Dissolution Thermodynamics of Benzoic Acid in Aqueous Alcoholic Mixtures" Reactions 3, no. 3: 392-414. https://doi.org/10.3390/reactions3030028
APA StyleAkay, S., Kayan, B., Peña, M. Á., Jouyban, A., Martínez, F., & Acree, W. E., Jr. (2022). Expanding the Equilibrium Solubility and Dissolution Thermodynamics of Benzoic Acid in Aqueous Alcoholic Mixtures. Reactions, 3(3), 392-414. https://doi.org/10.3390/reactions3030028












