Catalytic Performances of Sn-Beta Catalysts Prepared from Different Heteroatom-Containing Beta Zeolites for the Retro-Aldol Fragmentation of Glucose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Synthesis
2.3. Post-Synthetic Modification of M-Beta Zeolites by Solid-State Incorporation
2.4. Catalyst Characterisation
2.5. Kinetic Studies
2.6. Activity Definitions
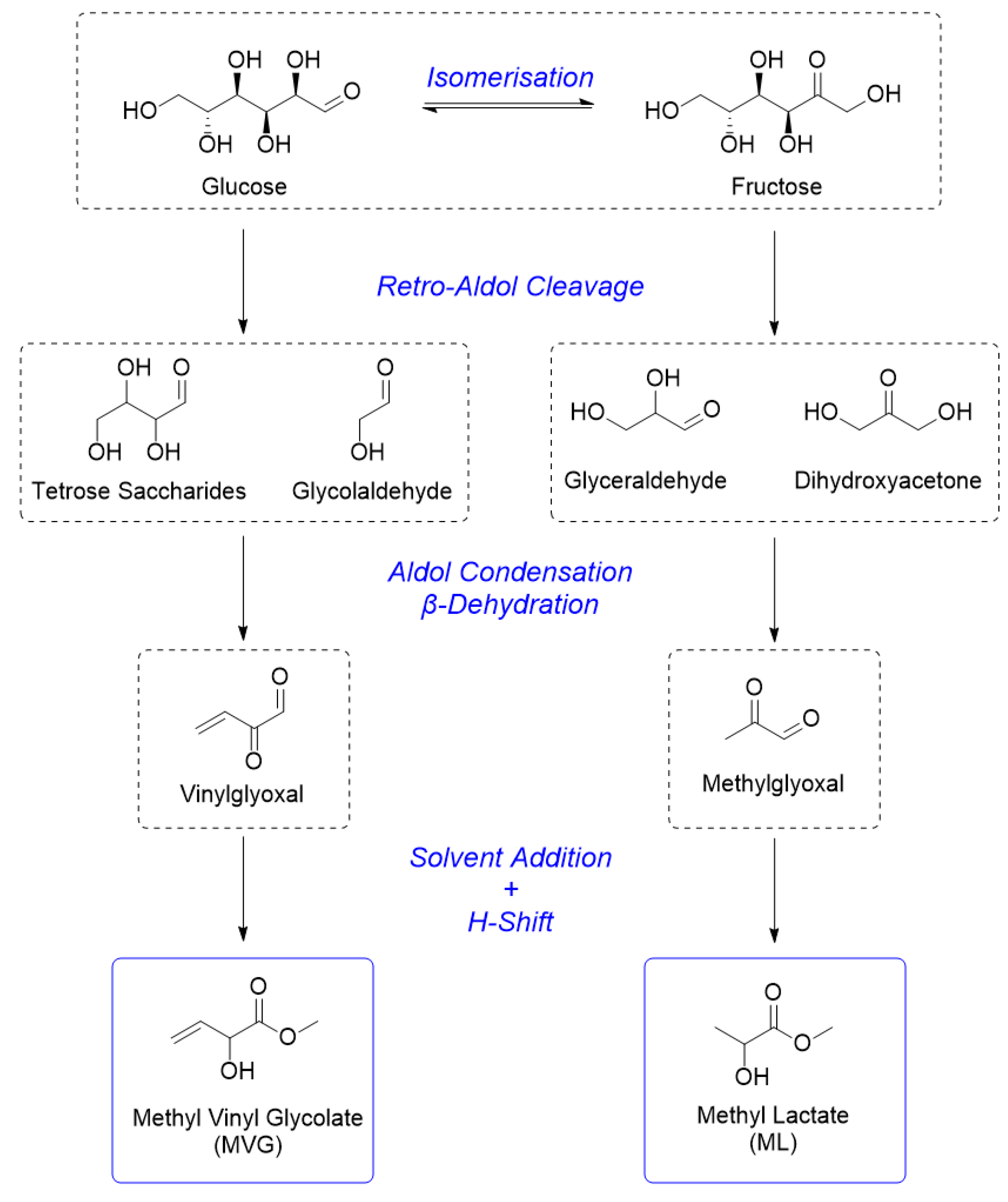
3. Results and Discussion
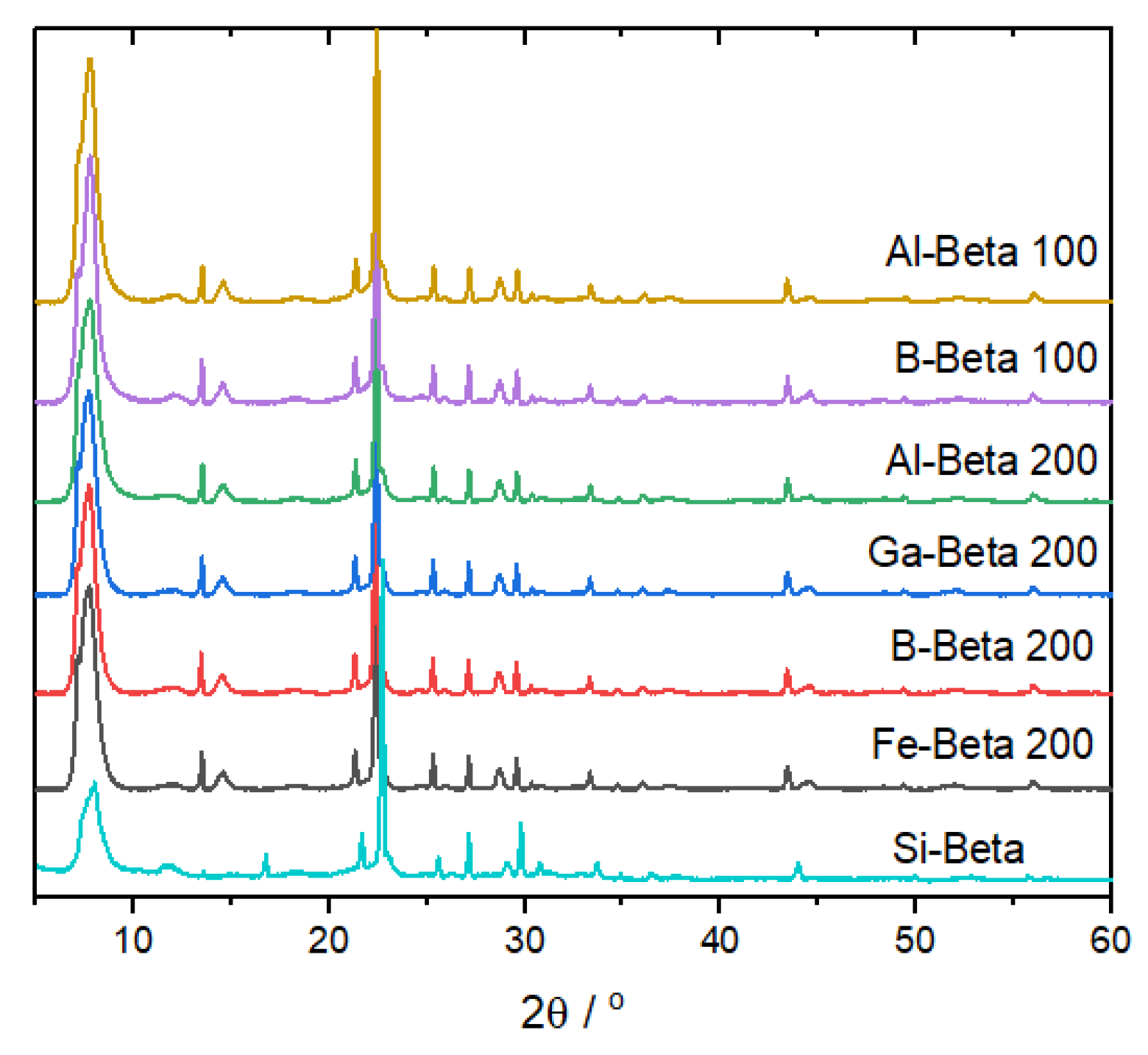
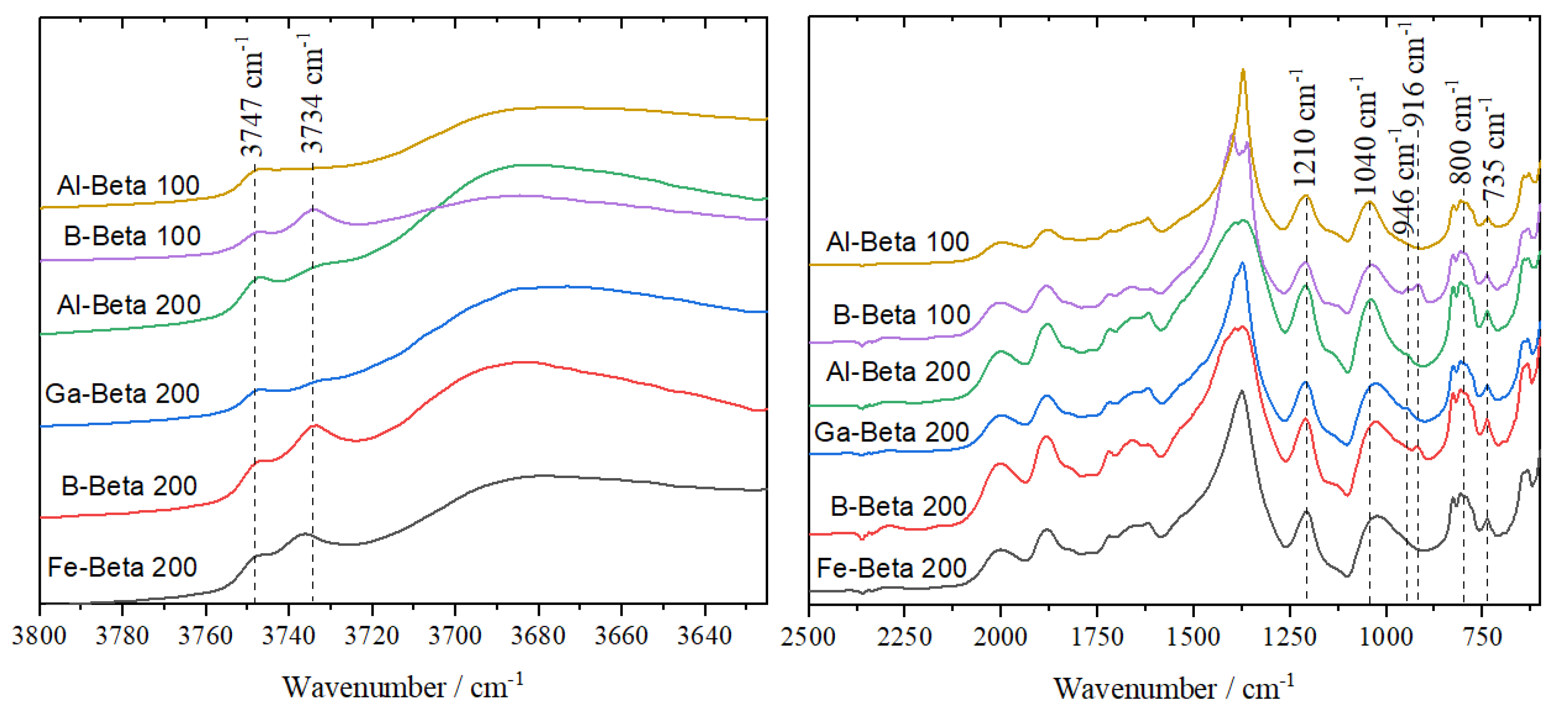
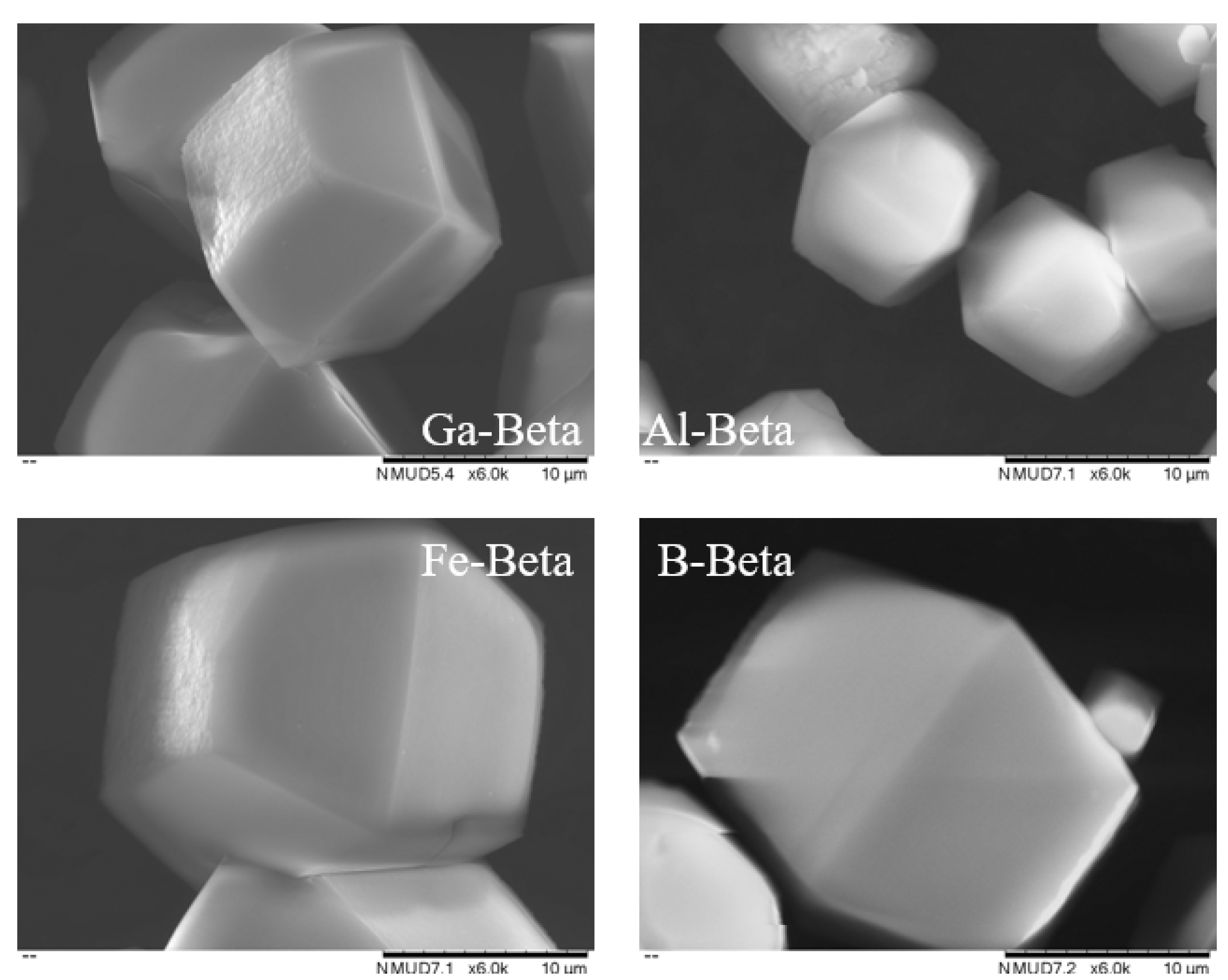
3.1. Catalyst Synthesis and Characterisation
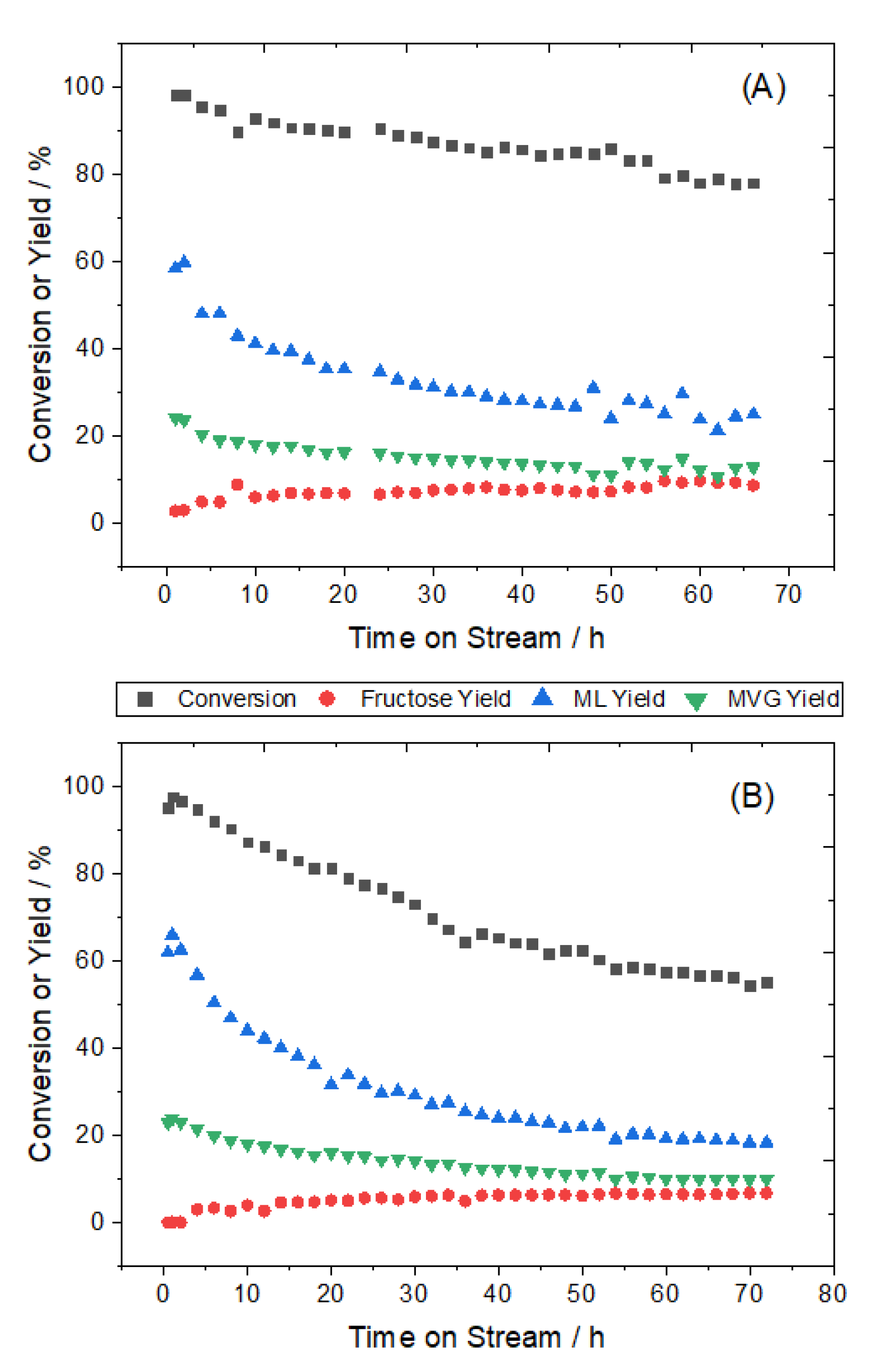
3.2. Kinetic Evaluation of the Synthesised Catalyst Materials
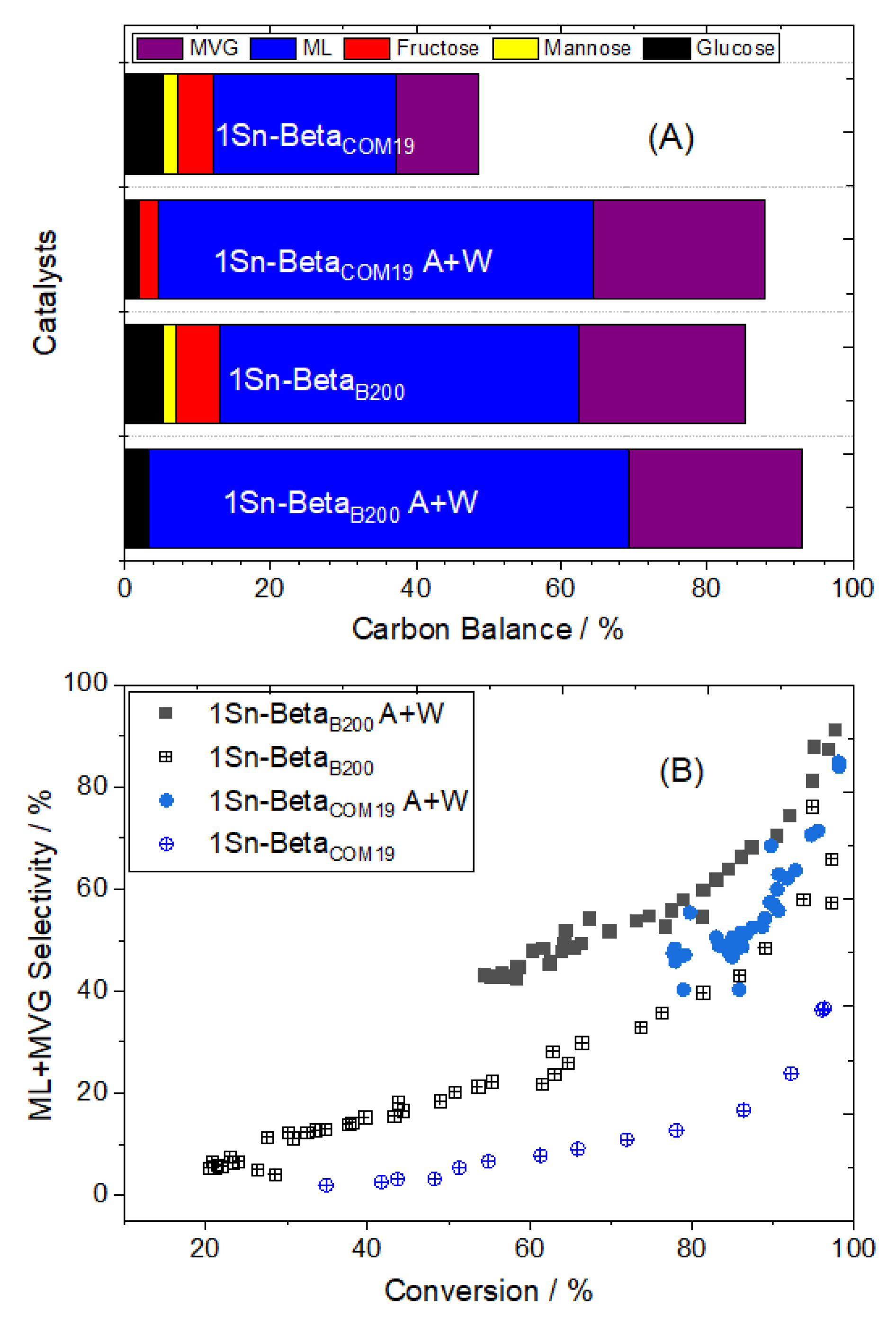
3.3. Effect of Water and Alkali Salts on the Kinetic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pachamuthu, M.P.; Shanthi, K.; Luque, R.; Ramanathan, A. SnTUD-1: A solid acid catalyst for three component coupling reactions at room temperature. Green Chem. 2013, 15, 2158–2166. [Google Scholar] [CrossRef]
- Corma, A.; Domine, M.E.; Nemeth, L.; Valencia, S. Al-Free Sn-Beta Zeolite as a Catalyst for the Selective Reduction of Carbonyl Compounds (Meerwein-Ponndorf-Verley Reaction). J. Am. Chem. Soc. 2002, 124, 3194–3195. [Google Scholar] [CrossRef] [PubMed]
- Takahara, I.; Saito, M.; Inaba, M.; Murata, K. Dehydration of Ethanol into Ethylene over Solid Acid Catalysts. Catal. Lett. 2005, 105, 249–252. [Google Scholar] [CrossRef]
- Esteban, J.; Yustos, P.; Ladero, M. Catalytic Processes from Biomass-Derived Hexoses and Pentoses: A Recent Literature Overview. Catalysts 2018, 8, 637. [Google Scholar] [CrossRef]
- Li, Y.-P.; Head-Gordon, M.; Bell, A.T. Analysis of the Reaction Mechanism and Catalytic Activity of Metal-Substituted Beta Zeolite for the Isomerization of Glucose to Fructose. ACS Catal. 2014, 4, 1537–1545. [Google Scholar] [CrossRef]
- Moliner, M.; Roman-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Natl. Acad. Sci. USA 2010, 107, 6164–6168. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Wang, G.; Chen, F.; Zhu, J.; Wang, C.; Bian, C.; Pan, S.; Xiao, F.-S. Hierarchical Sn-Beta Zeolite Catalyst for the Conversion of Sugars to Alkyl Lactates. ACS Sustain. Chem. Eng. 2017, 5, 3123–3131. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Li, X.; Ren, J.; Zhou, L.; Lu, T.; Su, Y. Synthesis of Sn-Containing Nanosized Beta Zeolite as Efficient Catalyst for Transformation of Glucose to Methyl Lactate. ACS Sustain. Chem. Eng. 2018, 6, 8256–8265. [Google Scholar] [CrossRef]
- De Clercq, R.; Dusselier, M.; Christiaens, C.; Dijkmans, J.; Iacobescu, R.I.; Pontikes, Y.; Sels, B.F. Confinement Effects in Lewis Acid-Catalyzed Sugar Conversion: Steering Toward Functional Polyester Building Blocks. ACS Catal. 2015, 5, 5803–5811. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Groen, J.C.; Brückner, A.; Kumar, M.S.; Bentrup, U.; Debbagh, M.N.; Villaescusa, L.A. Evolution of isomorphously substituted iron zeolites during activation: Comparison of Fe-beta and Fe-ZSM-5. J. Catal. 2005, 232, 318–334. [Google Scholar] [CrossRef]
- Müller, M.; Harvey, G.; Prins, R. Comparison of the dealumination of zeolites beta, mordenite, ZSM-5 and ferrierite by thermal treatment, leaching with oxalic acid and treatment with SiCl4 by 1H, 29Si and 27Al MAS NMR. Micropor. Mesopor. Mater. 2000, 34, 135–147. [Google Scholar] [CrossRef]
- Wolf, P.; Hammond, C.; Conrad, S.; Hermans, I. Post-synthetic preparation of Sn-, Ti- and Zr-beta: A facile route to water tolerant, highly active Lewis acidic zeolites. Dalton Trans. 2014, 43, 4514–4519. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.; Conrad, S.; Hermans, I. Simple and Scalable Preparation of Highly Active Lewis Acidic Sn-β. Angew. Chem. Int. Ed. 2012, 51, 11736–11739. [Google Scholar] [CrossRef] [PubMed]
- Botti, L.; Navar, R.; Tolborg, S.; Martinez-Espin, J.S.; Padovan, D.; Taarning, E.; Hammond, C. Influence of Composition and Preparation Method on the Continuous Performance of Sn-Beta for Glucose-Fructose Isomerisation. Top. Catal. 2018, 62, 1178–1191. [Google Scholar] [CrossRef]
- Losch, P.; Hoff, T.C.; Kolb, J.F.; Bernardon, C.; Tessonnier, J.-P.; Louis, B. Mesoporous ZSM-5 Zeolites in Acid Catalysis: Top-Down vs. Bottom-Up Approach. Catalysts 2017, 7, 225. [Google Scholar] [CrossRef]
- Tang, B.; Dai, W.; Sun, X.; Wu, G.; Guan, N.; Hunger, M.; Li, L. Mesoporous Zr-Beta zeolites prepared by a post-synthetic strategy as a robust Lewis acid catalyst for the ring-opening aminolysis of epoxides. Green Chem. 2015, 17, 1744–1755. [Google Scholar] [CrossRef]
- Tang, B.; Dai, W.; Sun, X.; Guan, N.; Li, L.; Hunger, M. A procedure for the preparation of Ti-Beta zeolites for catalytic epoxidation with hydrogen peroxide. Green Chem. 2014, 16, 2281–2291. [Google Scholar] [CrossRef]
- Van der Graaff, W.N.P.; Tempelman, C.H.L.; Hendriks, F.C.; Ruiz-Martinez, J.; Bals, S.; Weckhuysen, B.M.; Pidko, E.A.; Hensen, E.J.M. Deactivation of Sn-Beta during carbohydrate conversion. Appl. Catal. A Gen. 2018, 564, 113–122. [Google Scholar] [CrossRef]
- Kucherov, A.V.; Slinkin, A.A. Solid-state reaction as a way to transition metal cation introduction into high-silica zeolites. J. Mol. Catal. 1994, 90, 323–354. [Google Scholar] [CrossRef]
- Van Bokhoven, J.A.; Koningsberger, D.C.; Kunkeler, P.; Van Bekkum, H.; Kentgens, A.P.M. Stepwise Dealumination of Zeolite Βeta at Specific T-Sites Observed with 27Al MAS and 27Al MQ MAS NMR. J. Am. Chem. Soc. 2000, 122, 12842–12847. [Google Scholar] [CrossRef]
- Escola, J.M.; Serrano, D.P.; Sanz, R.; Garcia, R.A.; Peral, A.; Moreno, I.; Linares, M. Synthesis of hierarchical Beta zeolite with uniform mesopores: Effect on its catalytic activity for veratrole acylation. Catalysts 2018, 304, 89–96. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, H.; Jiang, J.; Guan, Y.; Wu, P. Sn-Beta zeolite derived from a precursor synthesized via an organotemplate-free route as efficient Lewis acid catalyst. Appl. Catal. A Gen. 2018, 556, 52–63. [Google Scholar] [CrossRef]
- Koller, H.; Senapati, S.; Ren, J.; Uesbeck, T.; Siozios, V.; Hunger, M.; Lobo, R.F. Post-Synthesis Conversion of Borosilicate Zeolite Beta to an Aluminosilicate with Isolated Acid Sites: A Quantitative Distance Analysis by Solid-State NMR. J. Phys. Chem. C 2016, 120, 9811–9820. [Google Scholar] [CrossRef]
- Ranoux, A.; Djanashvili, K.; Arends, I.W.C.E.; Hanefeld, U. B-TUD-1: A versatile mesoporous catalyst. RSC Adv. 2013, 3, 21524–21534. [Google Scholar] [CrossRef][Green Version]
- De Ruiter, R.; Kentgens, A.P.M.; Grootendorst, J.; Jansen, J.C.; van Bekkum, H. Calcination and deboronation of [B]-MFI single crystals. Zeolites 1993, 13, 128–138. [Google Scholar] [CrossRef]
- Hammond, C.; Padovan, D.; Al-Nayili, A.; Wells, P.P.; Gibson, E.K.; Dimitratos, N. Identification of Active and Spectator Sn Sites in Sn-β Following Solid-State Stannation, and Consequences for Lewis Acid Catalysis. ChemCatChem 2015, 7, 3322–3331. [Google Scholar] [CrossRef]
- Kolyagin, Y.G.; Yakimov, A.V.; Tolborg, S.; Vennestrøm, P.N.R.; Ivanova, I.I. Application of 119Sn CPMG MAS NMR for Fast Characterization of Sn Sites in Zeolites with Natural 119Sn Isotope Abundance. J. Phys. Chem. Lett. 2016, 7, 1249–1253. [Google Scholar] [CrossRef]
- Yakimov, A.V.; Kolyagin, Y.G.; Tolborg, S.; Vennestrøm, P.N.R.; Ivanova, I.I. 119Sn MAS NMR Study of the Interaction of Probe Molecules with Sn-BEA: The Origin of Penta- and Hexacoordinated Tin Formation. J. Phys. Chem. C 2016, 120, 28083–28092. [Google Scholar] [CrossRef]
- Tolborg, S.; Katerinopoulou, A.; Falcone, D.D.; Sádaba, I.; Osmundsen, C.M.; Davis, R.J.; Taarning, E.; Fristrup, P.; Holm, M.S. Incorporation of tin affects crystallization, morphology, and crystal composition of Sn-Beta. J. Mater. Chem. A 2014, 2, 20252–20262. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Khatami, H.; Szymanski, H.A. Molecular Sieve Zeolites-I. In Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1974; pp. 16–201. [Google Scholar]
- Tomlinson, S.R.; McGown, T.; Schlup, J.R.; Anthony, J.L. Infrared Spectroscopic Characterization of CIT-6 and a Family of ∗BEA Zeolites. Int. J. Spectrosc. 2013, 2013, 961404. [Google Scholar] [CrossRef]
- Chalupka, K.; Sadek, R.; Valentin, L.; Millot, Y.; Calers, C.; Nowosielska, M.; Rynkowski, J.; Dzwigaj, S. Dealuminated Beta Zeolite Modified by Alkaline Earth Metals. J. Chem. 2018, 2018, 7071524. [Google Scholar] [CrossRef]
- Kiricsi, I.; Flego, C.; Pazzuconi, G.; Parker, W.O.; Millini, R.; Perego, C.; Bellussi, G. Progress toward Understanding Zeolite ß Acidity: An IR and 27Al NMR Spectroscopic Study. J. Phys. Chem. 1994, 98, 4627–4634. [Google Scholar] [CrossRef]
- Xia, Q.H.; Song, J.; Kawi, S.; Li, L.L. Characterization and morphological control of β zeolite synthesized in a fluoride medium. Stud. Surf. Sci. Catal. 2004, 154, 195–202. [Google Scholar] [CrossRef]
- Tong, H.T.T.; Koller, H. Control of Al for B framework substitution in zeolite Beta by counterions. Micropor. Mesopor. Mater. 2012, 148, 80–87. [Google Scholar] [CrossRef]
- Hamoudi, S.; Larachi, F.; Sayari, A. Synthesis and Characterization of Titanium-Substituted Large Pore SSZ-42 Zeolite. Catal. Lett. 2001, 77, 227–231. [Google Scholar] [CrossRef]
- Padovan, D.; Botti, L.; Hammond, C. Active Site Hydration Governs the Stability of Sn-Beta during Continuous Glucose Conversion. ACS Catal. 2018, 8, 7131–7140. [Google Scholar] [CrossRef]
- Botti, L.; Padovan, D.; Navar, R.; Tolborg, S.; Martinez-Espin, J.S.; Hammond, C. Thermal Regeneration of Sn-Containing Silicates and Consequences for Biomass Upgrading: From Regeneration to Preactivation. ACS Catal. 2020, 10, 11545–11555. [Google Scholar] [CrossRef]
- Padovan, D.; Tolborg, S.; Botti, L.; Taarning, E.; Sádaba, I.; Hammond, C. Overcoming catalyst deactivation during the continuous conversion of sugars to chemicals: Maximising the performance of Sn-Beta with a little drop of water. React. Chem. Eng. 2018, 3, 155–163. [Google Scholar] [CrossRef]
- Padovan, D.; Parsons, C.; Simplicio Grasina, M.; Hammond, C. Intensification and deactivation of Sn-beta investigated in the continuous regime. Green Chem. 2016, 18, 5041–5049. [Google Scholar] [CrossRef]
- Kalvachev, Y.; Jaber, M.; Mavrodinova, V.; Dimitrov, L.; Nihtianova, D.; Valtchev, V. Seeds-induced fluoride media synthesis of nanosized zeolite Beta crystals. Micropor. Mesopor. Mater. 2013, 177, 127–134. [Google Scholar] [CrossRef]
- Botti, L.; Navar, R.; Tolborg, S.; Martinez-Espin, J.S.; Hammond, C. High-Productivity Continuous Conversion of Glucose to α-Hydroxy Esters over Postsynthetic and Hydrothermal Sn-Beta Catalysts. ACS Sustain. Chem. Eng. 2022, 10, 4391–4403. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Deval, R.; Gounder, R.; Davis, M.E. Framework and Extraframework Tin Sites in Zeolite Beta React Glucose Differently. ACS Catal. 2012, 2, 2705–2713. [Google Scholar] [CrossRef]
- Wolf, P.; Valla, M.; Núñez-Zarur, F.; Comas-Vives, A.; Rossini, A.J.; Firth, C.; Kallas, H.; Lesage, A.; Emsley, L.; Copéret, C.; et al. Correlating Synthetic Methods, Morphology, Atomic-Level Structure, and Catalytic Activity of Sn-β Catalysts. ACS Catal. 2016, 6, 4047–4063. [Google Scholar] [CrossRef]
- Hwang, S.J.; Gounder, R.; Bhawe, Y.; Orazov, M.; Bermejo-Deval, R.; Davis, M.E. Solid State NMR Characterization of Sn-Beta Zeolites that Catalyze Glucose Isomerization and Epimerization. Top. Catal. 2015, 58, 435–440. [Google Scholar] [CrossRef]
- Kolyagin, Y.G.; Yakimov, A.V.; Tolborg, S.; Vennestrøm, P.N.R.; Ivanova, I.I. Direct Observation of Tin in Different T-Sites of Sn-BEA by One- and Two-Dimensional 119Sn MAS NMR Spectroscopy. J. Phys. Chem. Lett. 2018, 9, 3738–3743. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Nemeth, L.T.; Renz, M.; Valencia, S. Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer–Villiger oxidations. Nature 2001, 412, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, H.; Zhao, X.; Zhu, L.; Miao, G.; Wang, H.; Li, S.; Kong, L. Continuous Conversion of Glucose into Methyl Lactate over the Sn-Beta Zeolite: Catalytic Performance and Activity Insight. Ind. Eng. Chem. Res. 2020, 59, 17365–17372. [Google Scholar] [CrossRef]
- Scott, S.L. A Matter of Life(time) and Death. ACS Catal. 2018, 8, 8597–8599. [Google Scholar] [CrossRef]
- Hammond, C. Intensification studies of heterogeneous catalysts: Probing and overcoming catalyst deactivation during liquid phase operation. Green Chem. 2017, 19, 2711–2728. [Google Scholar] [CrossRef]
- Tolborg, S.; Sádaba, I.; Osmundsen, C.M.; Fristrup, P.; Holm, M.S.; Taarning, E. Tin-containing Silicates: Alkali Salts Improve Methyl Lactate Yield from Sugars. ChemSusChem. 2015, 8, 613–617. [Google Scholar] [CrossRef]
- Elliot, S.G.; Tolborg, S.; Madsen, R.; Taarning, E.; Meier, S. Effects of Alkali and Counter Ions in Sn-Beta Catalyzed Carbohydrate Conversion. ChemSusChem 2018, 11, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Elliot, S.G.; Tosi, I.; Meier, S.; Martinez-Espin, J.S.; Tolborg, S.; Taarning, E. Stoichiometric active site modification observed by alkali ion titrations of Sn-Beta. Catal. Sci. Technol. 2019, 9, 4339–4346. [Google Scholar] [CrossRef]
- Yang, Q.; Lan, W.; Runge, T. Salt-Promoted Glucose Aqueous Isomerization Catalyzed by Heterogeneous Organic Base. ACS Sustain. Chem. Eng. 2016, 4, 4850–4858. [Google Scholar] [CrossRef]
- Furushiro, Y.; Kobayashi, T. Reaction Behavior of Glucose and Fructose in Subcritical Water in the Presence of Various Salts. J. Appl. Glycosci. 2020, 67, 11–15. [Google Scholar] [CrossRef]
- Yoo, C.G.; Li, N.; Swannell, M.; Pan, X. Isomerization of glucose to fructose catalyzed by lithium bromide in water. Green Chem. 2017, 19, 4402–4411. [Google Scholar] [CrossRef]
- Vega-Vila, C.; Harris, J.W.; Gounder, R. Controlled insertion of tin atoms into zeolite framework vacancies and consequences for glucose isomerization catalysis. J. Catal. 2016, 344, 108–120. [Google Scholar] [CrossRef]
- Humplik, T.; Raj, R.; Maroo, S.C.; Laoui, T.; Wang, E.N. Effect of Hydrophilic Defects on Water Transport in MFI Zeolites. Langmuir 2014, 30, 6446–6453. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, H.; Jiang, J.; Wu, H.; Wu, P. Hydrophobic Nanosized All-Silica Beta Zeolite: Efficient Synthesis and Adsorption Application. ACS Appl. Mater. Interfaces 2017, 9, 27273–27283. [Google Scholar] [CrossRef]
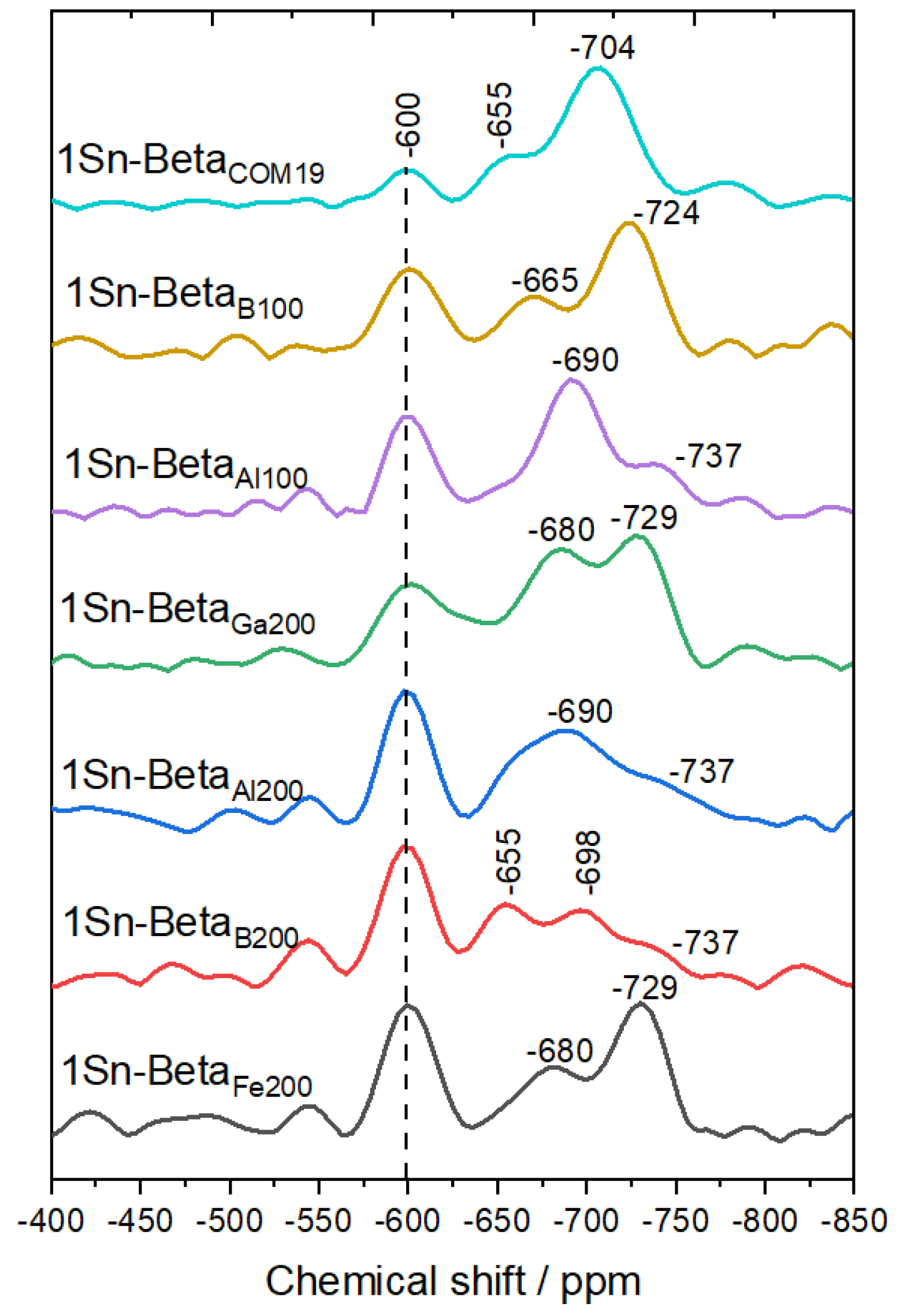
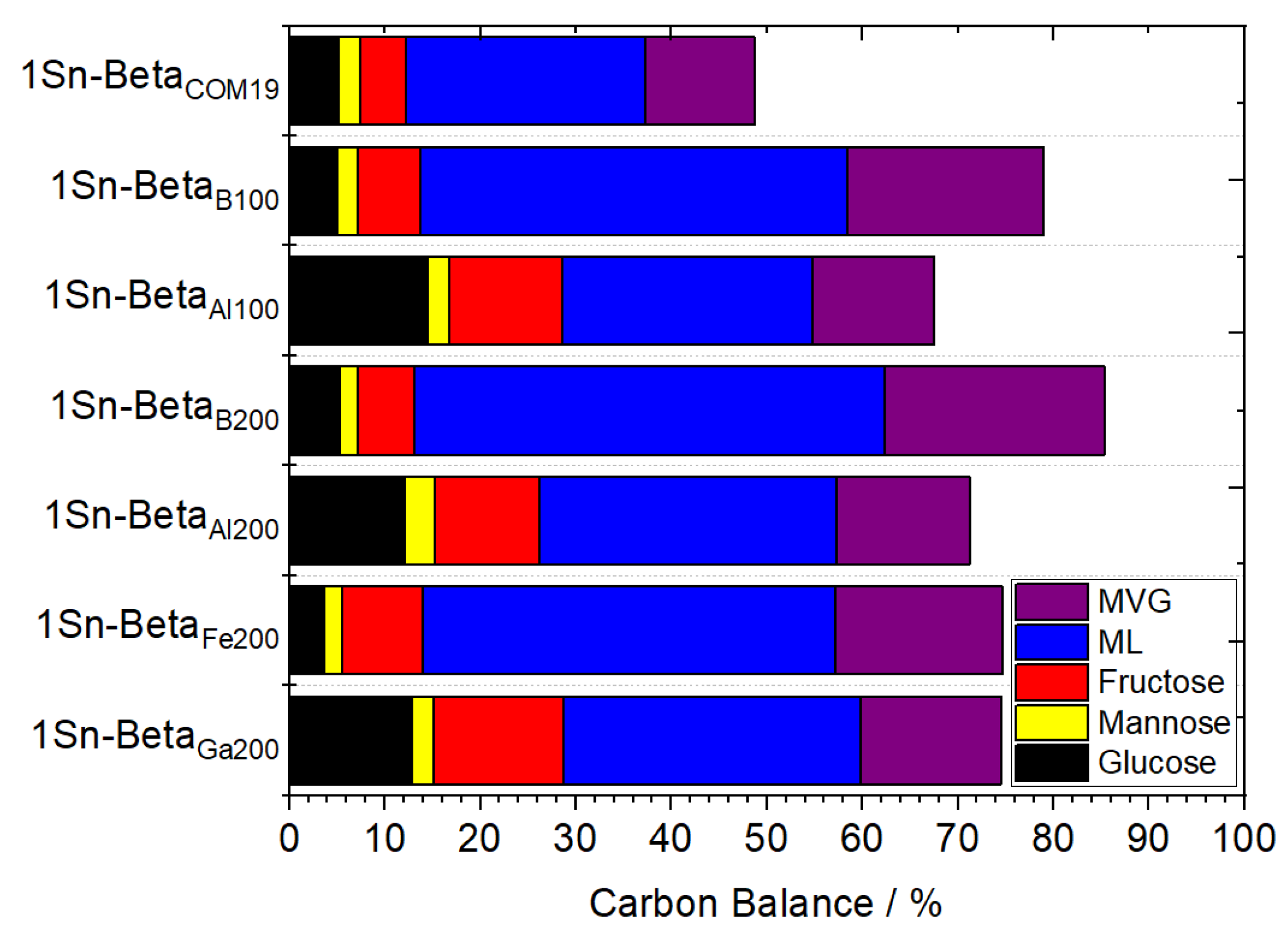

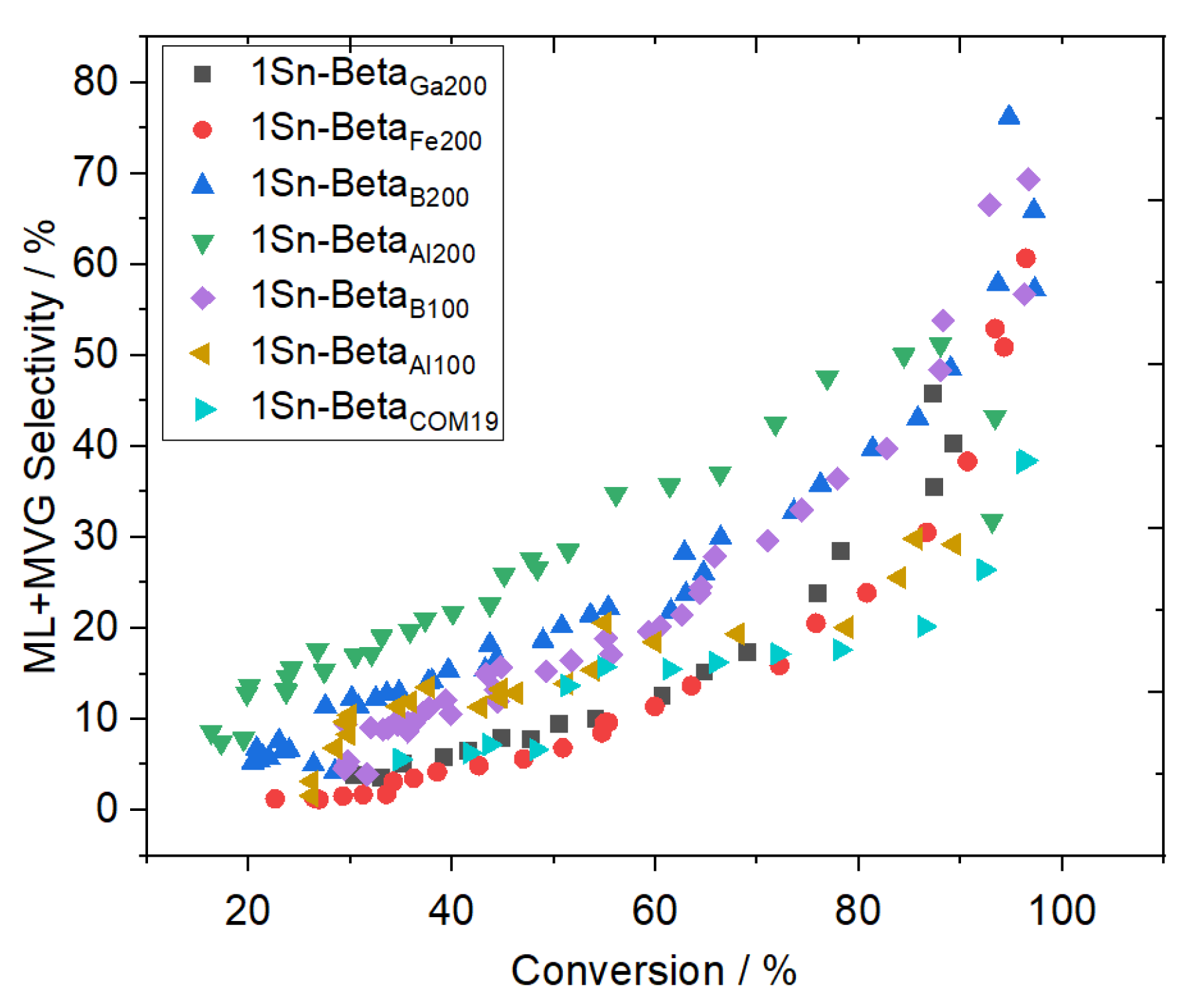
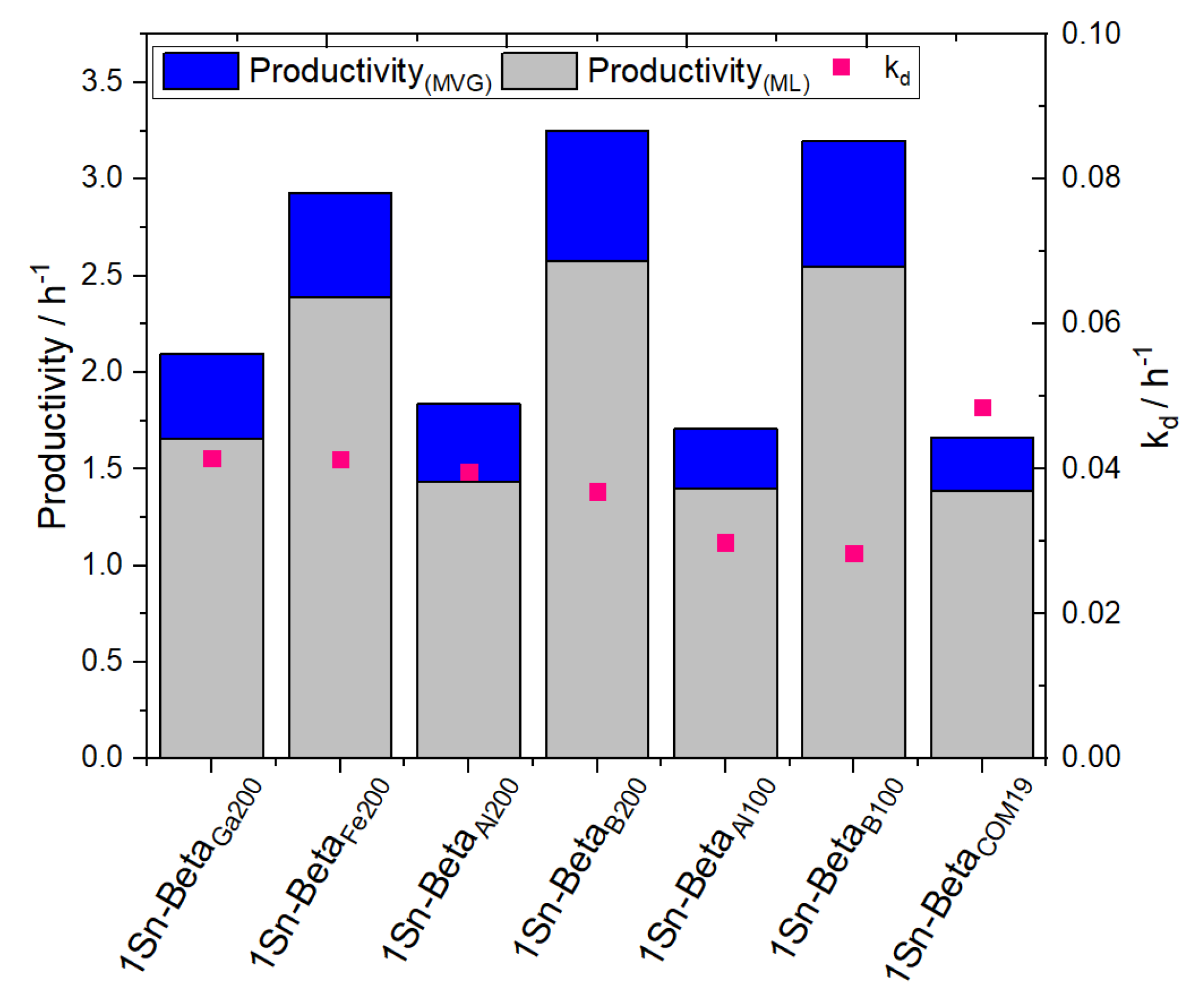
| Catalyst | Si/M a | SBET b (m2 g−1) | Smeso/ext c (m2 g−1) | Vtotal d (cm3 g−1) | Vmicro e (cm3 g−1) |
|---|---|---|---|---|---|
| Fe-Beta 200 f | 207 | 501 | 59 | 0.32 | 0.23 |
| Ga-Beta 200 f | 152 | 504 | 83 | 0.34 | 0.22 |
| Al-Beta 200 f | 220 | 463 | 87 | 0.34 | 0.20 |
| B-Beta 200 f | 204 | 468 | 13 | 0.32 | 0.22 |
| Al-Beta 100 g | 116 | 453 | 46 | 0.34 | 0.21 |
| B-Beta 100 g | 107 | 499 | 65 | 0.33 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navar, R.; Botti, L.; Tarantino, G.; Hammond, C. Catalytic Performances of Sn-Beta Catalysts Prepared from Different Heteroatom-Containing Beta Zeolites for the Retro-Aldol Fragmentation of Glucose. Reactions 2022, 3, 265-282. https://doi.org/10.3390/reactions3020020
Navar R, Botti L, Tarantino G, Hammond C. Catalytic Performances of Sn-Beta Catalysts Prepared from Different Heteroatom-Containing Beta Zeolites for the Retro-Aldol Fragmentation of Glucose. Reactions. 2022; 3(2):265-282. https://doi.org/10.3390/reactions3020020
Chicago/Turabian StyleNavar, Ricardo, Luca Botti, Giulia Tarantino, and Ceri Hammond. 2022. "Catalytic Performances of Sn-Beta Catalysts Prepared from Different Heteroatom-Containing Beta Zeolites for the Retro-Aldol Fragmentation of Glucose" Reactions 3, no. 2: 265-282. https://doi.org/10.3390/reactions3020020
APA StyleNavar, R., Botti, L., Tarantino, G., & Hammond, C. (2022). Catalytic Performances of Sn-Beta Catalysts Prepared from Different Heteroatom-Containing Beta Zeolites for the Retro-Aldol Fragmentation of Glucose. Reactions, 3(2), 265-282. https://doi.org/10.3390/reactions3020020






