Abstract
The gas-phase reactivity of vanadium-containing dianions, NaV3O92− and its hydrated form H2NaV3O102−, were probed towards sulphur dioxide at room temperature by ion-molecule reaction (IMR) experiments in the collision cell of an ion trap mass spectrometer. The sequential addition of two SO2 molecules to the NaV3O92− dianion leads to the breakage of the stable V3O9 backbone, resulting in a charge separation process with the formation of new V-O and S-O bonds. On the contrary, the H2NaV3O102− hydroxide species reacts with SO2, promoting regioselective hydrolysis and bond-forming processes, the latter similar to that observed for the NaV3O92− reactant anion. Kinetic analysis shows that these reactions are fast and efficient with rate constants of the 10−9 (±30) cm3 s−1 molecule−1 order of magnitude.
1. Introduction
Vanadium-based compounds find several applications in a wide range of research fields. For example, supported vanadium oxides are largely employed in the metallurgic industry as heterogeneous catalysts for the manufacture of important chemicals [1]. Among these, sulphuric acid is considered an excellent indicator of the “industrial strength” of a nation, being one of the most produced chemicals worldwide [2]. More recently, vanadium hydroxides have also attracted interest as secondary electrode materials in the design of Li-ion and redox flow batteries [3,4,5,6].
Considering the significant potential of vanadium species, the chemical features of these compounds have been extensively investigated both in the solution and in the gas-phase environments. The latter represents a designable arena in which a variety of different experiments performed by means of mass spectrometric techniques allow one to elucidate structural and thermochemical features of ionic clusters. The refined control of the cluster size, charge, and stoichiometry in the absence of perturbing effects can indeed contribute to assessing the elementary steps of a complex process at a strictly molecular level, obtaining detailed mechanistic information [7,8,9,10,11,12,13]. Accordingly, the reactivity of vanadium oxide mono-cations and anions was probed towards selected hydrocarbons, water, and SO2 by ion-molecule reaction (IMR) experiments [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. In particular, it has been reported that the vanadium-oxide kernel of the V4O10− anion can effectively incorporate SO2, leading to a [V4O10-SO2]− complex with a square pyramidal structure [29], whereas neutral vanadium oxides only give association intermediates or oxidation and reduction products by the reaction with SO2 [32,33].
Although many studies in the literature describe the gas-phase reactivity of mono-charged vanadium oxide ions, the chemistry of the corresponding multi-charged species has been only partially explored. These ions can be generated by electrospray ionization (ESI) processes by the direct transfer of charged species formed in solution under mild conditions into the gas-phase environment [34]. In particular, we refer both to small doubly-charged species (e.g., VO2+, VOH2+, VOH2+) stabilized as water adducts [35] and large multiply-charged polyoxovanadates in which vanadium can assume different oxidation states and a variety of intriguing geometries [36].
The typical gas-phase reactions of dications are the so-called bond-forming processes where the simultaneous breakage and formation of chemical bonds lead to two singly-charged ions and/or a new doubly-charged species [37,38,39,40,41,42,43,44]. On the contrary, dianions are commonly involved in proton-transfer and substitution/elimination reactions always resulting in the incorporation of the intact dianion in the final product scaffold [45,46,47]. Recently, we reported the unprecedented bond-forming reactivity of doubly-charged vanadium hydroxoanions in the gas phase studied by IMR experiments and theoretical calculations [48,49]. H2V2O72− and HNaV4O122− react with SO2, leading to H2VO4− and VO3SO2−, and to NaV4O11− and HOSO2−, respectively [48]. These singly-charged products are formed through the action of SO2, which effectively favors the breakage of the stable V2O7 kernel of H2V2O72− and the terminal V-OH bond of HNaV4O122− dianions. Furthermore, a cooperative effect was evidenced in complexes of the V2O62− and HV3O102− formula with SO2 in favoring a hydrolysis reaction, giving rise to the charge separation of the dianion along with the formation of the HOSO2− bisulfite anion [49].
Continuing with our studies focused on the gas-phase activation of SO2 [50,51,52,53,54,55,56], here we report on the reactivity of two polyoxovanadate dianions, namely NaV3O92− and H2NaV3O102−, towards SO2 by using IMR mass spectrometry. The reactions are very fast and efficient and lead to singly charged products that do not contain the reactants in the molecular scaffold. Cooperative effects and regioselectivity were demonstrated when addressing different reaction outcomes, providing new interesting examples of bond-forming and hydrolysis reactions in the gas phase.
2. Materials and Methods
2.1. Materials
The research-grade chemicals and solvents employed in this study were purchased from Sigma-Aldrich and used without further purification. The stated purities of the chemicals, as reported by the vendor, are as follows: NaVO3 (99.9%), SO2 (99.9%), whereas H2O and CH3CN solvents are at the HPLC grade.
Pure N2 (99.995%) and He (99.995%) gases were purchased from Nippon Gases.
2.2. Mass Spectrometry
All the experiments were carried out on an LTQ XL linear ion trap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) that was in-house modified to perform ion-molecule reactions and study the kinetic profiles of gas-phase processes [57]. The instrument was equipped with an electrospray ionization (ESI) source operating in negative ion mode, under the following experimental conditions: source voltage of 2–3 kV and capillary temperature of 275 °C. Nitrogen (N2) was used as sheath and auxiliary gas at a flow rate of 3.7 and 0.74 L min−1, respectively [58]. Capillary and tube lens voltages were set to low values (in the range of −1 and −10 V) to maximize the formation of multiply-charged ions at the expense of singly-charged species [48,49].
Millimolar solutions of NaVO3 in H2O/CH3CN (1/3 v/v) were infused into the ESI source via the on-board syringe pump at a flow rate of 5 μL min−1, whereas neutral sulphur dioxide (SO2) was separately introduced into the ion trap through a deactivated fused-silica capillary that entered the vacuum chamber from a 6.25 mm hole placed in the backside of the mass spectrometer. The pressure of the neutral gas was kept constant by a metering valve and measured by a Granville−Phillips Series 370 Stabil Ion Vacuum Gauge. Owing to the position of the Pirani gauge, the actual SO2 pressure was estimated based on a calibration of the pressure reading [59]. Typical pressures of SO2 ranged between 1.1 × 10−7 and 7.6 × 10−7 Torr.
The NaV3O92− and H2NaV3O102− reactant ions were generated in the ESI source and thermalized by collisions with background helium during the transfer and inside the trap (collision frequency ≈ 105·s−1). After 0.1–0.2 s, they were mass-to-charge isolated and reacted with SO2. The signals of the ionic reactant and products were monitored over time as a function of the SO2 concentration, and for each reaction time an average of 10 scan acquisitions were acquired. The normalized collision energy was set to zero, and the activation Q value was optimized to ensure stable trapping fields for all the ions under investigation.
To measure the rate constants, logarithmic plots of the reactant species concentration vs. time were constructed. Accordingly, all the studied reactions display a pseudo-first-order decay. Experimental data obtained from the kinetic analysis were fitted to a mathematical model built on the basis of the hypothesized reaction mechanism by using Dynafit 4 package [60]. Bimolecular rate constants k (cm3 molecule−1 s−1) were derived as a product of the pseudo-first-order constants (s−1) divided by the concentration of neutral reagent gas. To ensure the accuracy of the k values, ca. 10–15 independent measurements for each precursor ion were performed on different days. All rates were measured over a 7-fold SO2 pressure range, showing a linear correlation with the neutral density and a standard deviation <15%. Nevertheless, a conservative error of 30% was given, due to the uncertainties of the neutral pressure measurements. The reaction efficiencies were expressed as the ratio of the bimolecular rate constants k to the collision rates that were calculated according to the average dipole orientation (ADO) theory [61].
Collision-induced dissociation (CID) experiments were carried out with the MSn function of the LTQ XL mass spectrometer. Helium was used as the collision gas at a pressure of ca. 3 × 10−3 Torr. Typical normalized collision energies (NCE) were in the range of 10% and 50%, depending on the isolated ion, whereas the activation time and Q value were set to 30 ms and 0.250, respectively.
All spectra were recorded and elaborated using the Xcalibur software (version 2.0.6, Thermo Fisher Scientific, Bremen, Germany) supplied with the instrument.
3. Results and Discussion
3.1. The NaV3O92− and H2NaV3O102− Reactant Dianions
Trimeric species of NaV3O92− and H2NaV3O102− general formula were generated in the gas phase by electrospraying aqueous solutions of sodium metavanadate. The speciation of this salt was deeply investigated both in solution [62,63] and in the gas phase [64,65,66,67], highlighting the formation of discrete multi-charged anions containing several vanadium atoms organized in higher structures (e.g., dimer, trimer, tetramer).
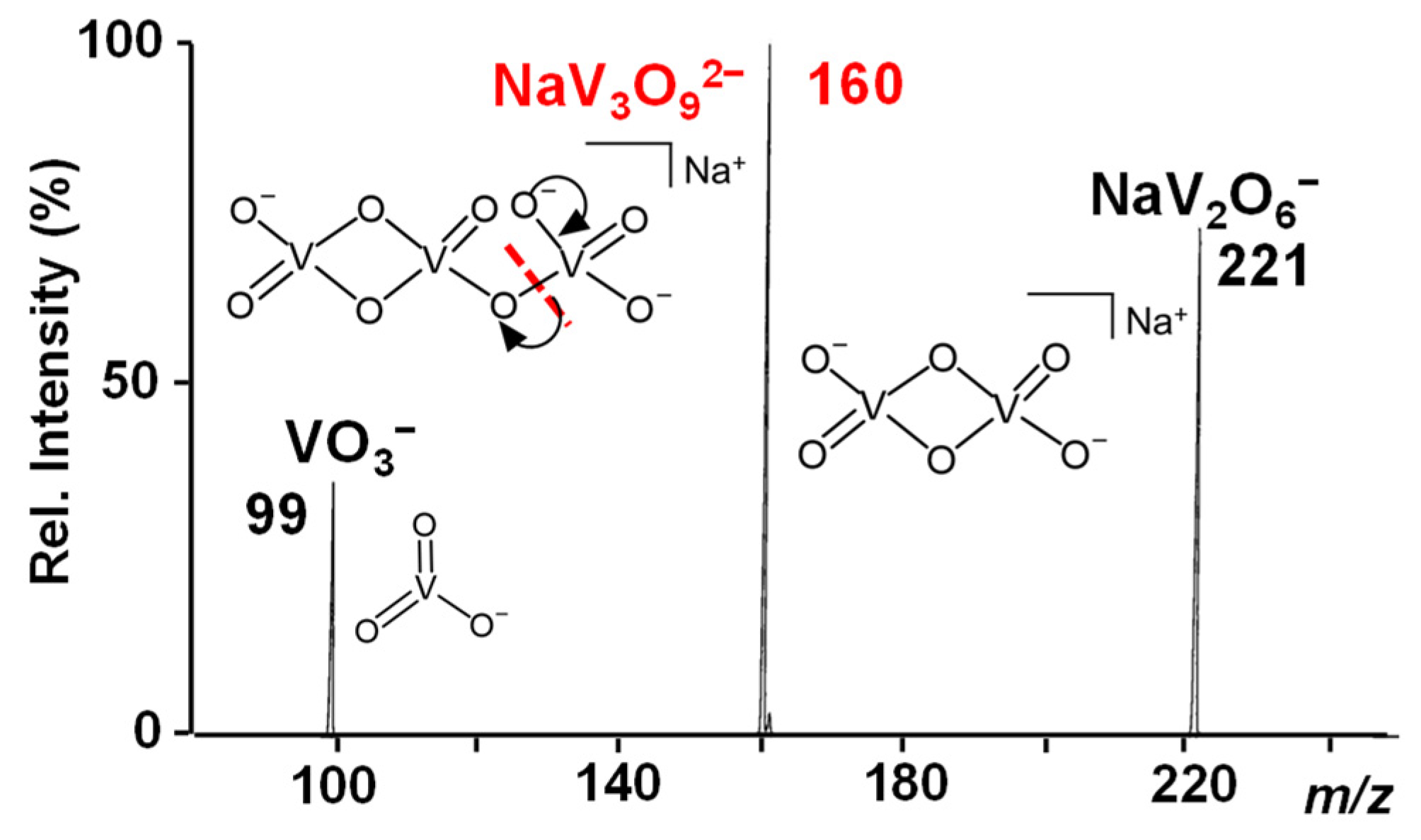
Before introducing SO2 into the trap, both NaV3O92− and H2NaV3O102− reactant ions were mass-to-charge selected and exposed to helium buffer gas over long activation times (maximum a. t. = 10 s). Since no remarkable signal loss occurred as a result of the isolation and subsequent accumulation, these doubly-charged species can be considered stable towards spontaneous dissociation in the gas phase. Hence, CID experiments were performed to obtain salient structural information that was interpreted in the light of our previous calculations on similar dianionic species [48,49]. In particular, the NaV3O92− dianion can be associated with the analogous HV3O92− hydroxovanadate species characterized by a V2O6 four-membered ring connected to a VO3 moiety [49]. Indeed, pentavalent vanadium derivatives form polyoxovanadates showing repetitive units of VO3 revealed at m/z 99 by mass spectrometry [67]. Accordingly, the NaV3O92− parent ion at m/z 160 dissociates into two singly-charged fragments at m/z 221 and 99 that are respectively consistent with the anions of the general formula: NaV2O6− and VO3− (Figure 1).
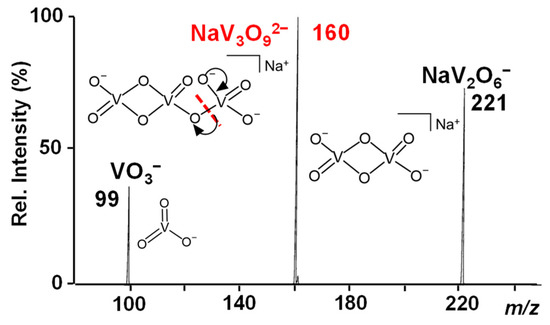
Figure 1.
IT CID mass spectrum of the NaV3O92− reactant ion at m/z 160.
We can thus suppose that the VO3 moiety is released as a consequence of a V-O cleavage induced by the collision of the parent species with the background helium gas forming a NaV2O6− counterpart. NaV2O6− maintains a V2O6-closed structure in agreement with the CID mass spectrum of the similar HV3O92− species, which dissociates into VO3− and HV2O6−, as theoretically described elsewhere [49].
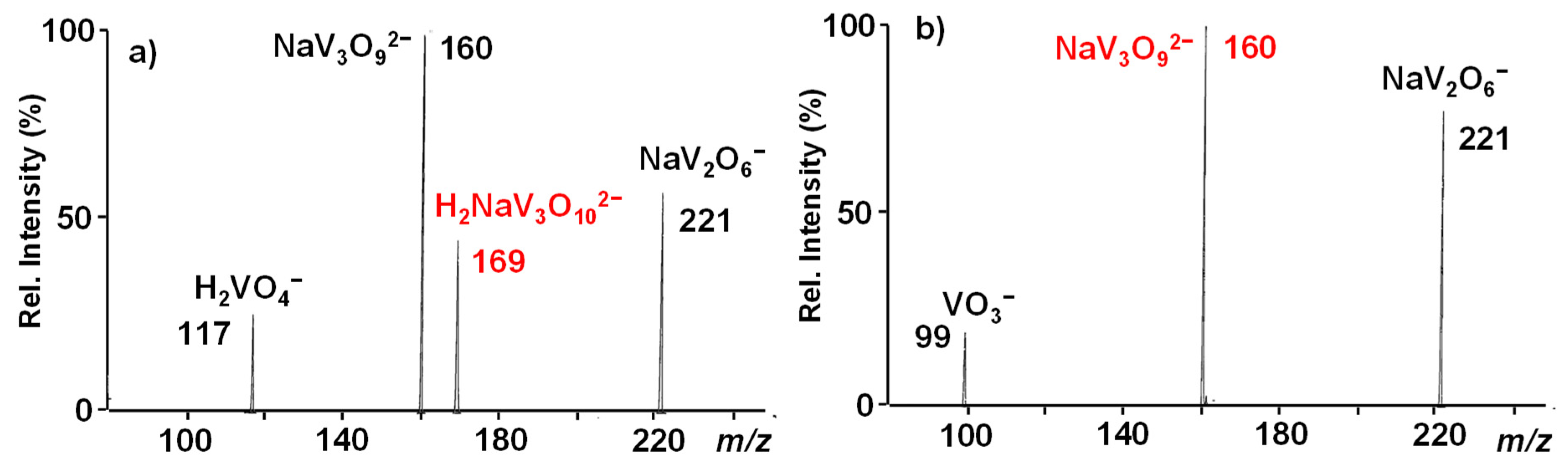
Passing to the H2NaV3O102− ion at m/z 169, it dissociates either by breaking into two singly-charged fragments, respectively corresponding to H2VO4− (m/z = 117) and NaV2O6− (m/z = 221) anions, or by losing an H2O molecule with the formation of the doubly-charged daughter species at m/z 160 (Figure 2a).
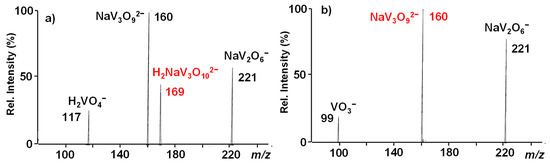
Figure 2.
IT CID mass spectra of (a) the H2NaV3O102− reactant ion at m/z 169 and (b) the NaV3O92− daughter ion at m/z 160 isolated from the MS3 sequence 169→160.
The latter was, in turn, isolated through the sequence 169 → 160 and submitted to MS3 dissociation, giving rise to the same fragmentation pattern already observed for the NaV3O92− dianion (Figure 2b vs. Figure 1), thus pointing to the same structure.
The doubly-charged species at m/z 169 could be thus composed of a mixture of H2NaV3O102− and the isomeric hydrated electrostatic complex ion [NaV3O9·H2O]2−. However, the loss of a water molecule forming a daughter ion with a V2O62− four-membered ring scaffold was also observed for the covalent open-chain pirovanadate dianion, H2V2O72−. The theoretical structure predicted for this species is characterized by two vicinal OH groups that are involved in the H2O release resulting from a fast proton transfer reaction [48]. In addition, when exposed to H2O in the trap, a naked NaV3O92− dianion proved, even for the longest possible time, to be unreactive towards the addition of a water molecule.
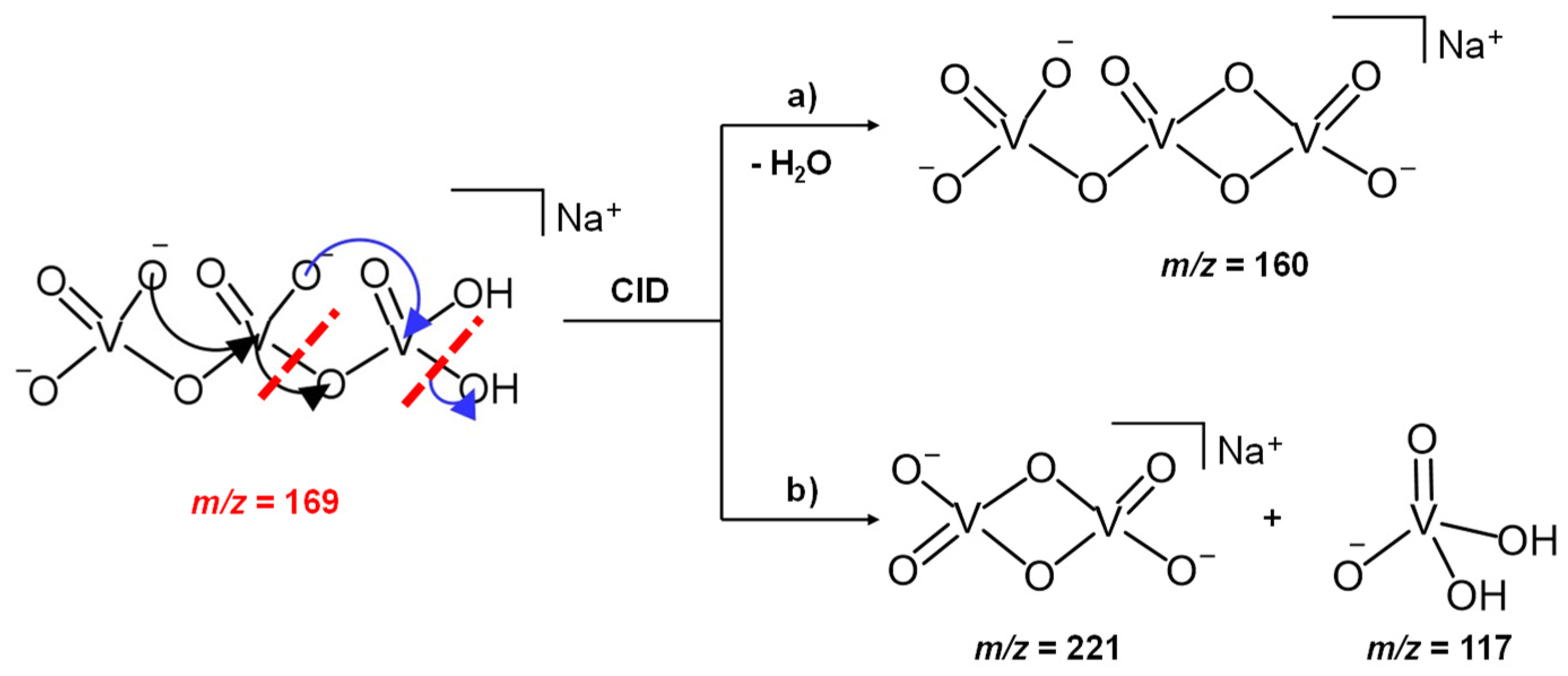
In light of this experimental evidence and always keeping a +5 oxidation state for the vanadium atoms, we propose that the H2NaV3O102− parent ion at m/z 169, presumably formed in solution by a hydrolysis reaction at the expense of the NaV3O92− dianion, may be characterized by an open structure, showing an H2VO4 terminal moiety which is released as the mono-anion of the vanadic acid (H2VO4−) through an intracluster rearrangement, according to Scheme 1. The singly charged vanadate, H2VO4−, is indeed a stable and deeply characterized species, previously obtained as a product of an ion-molecule reaction between the H2V2O72− dianions and SO2 [48].
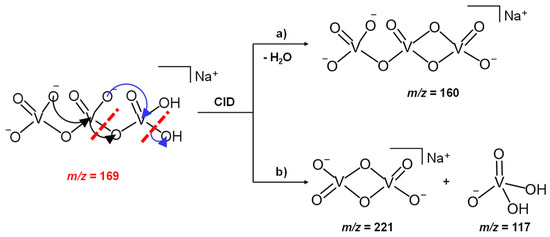
Scheme 1.
CID mechanism of the H2NaV3O102− reactant ion at m/z 169.
3.2. Reactivity of NaV3O92− and H2NaV3O102− Dianions towards SO2
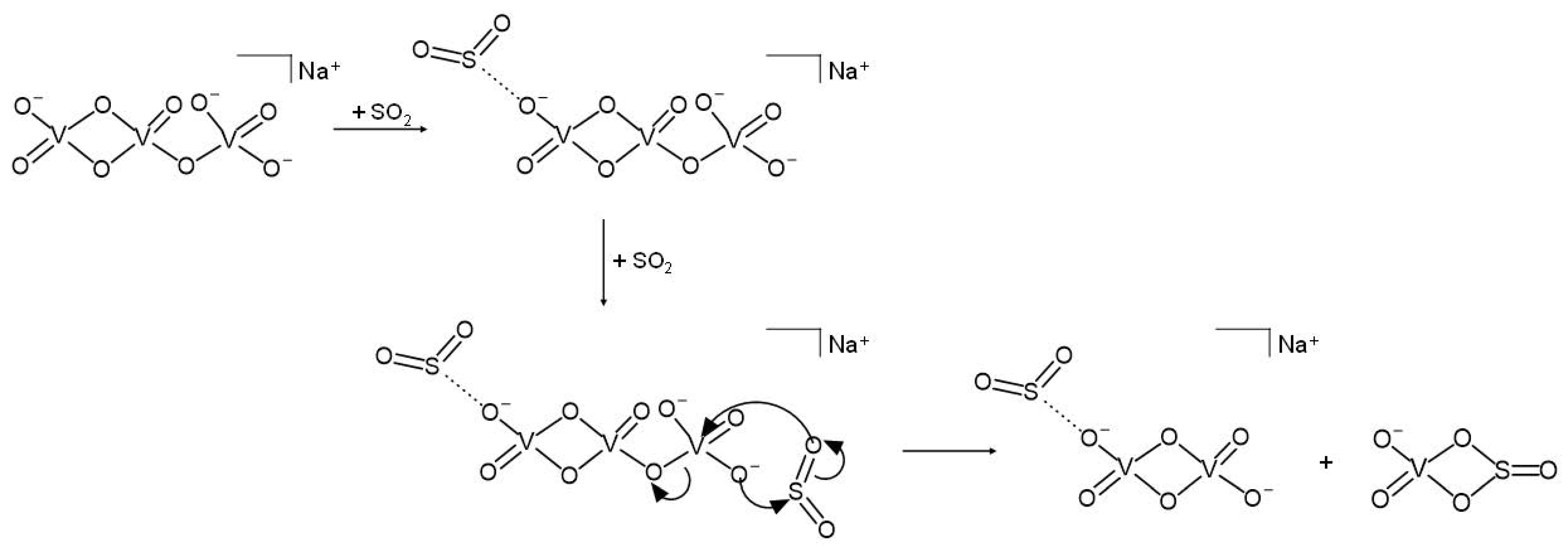
In the presence of sulphur dioxide in the ion trap, a thermal NaV3O92− dianion binds an SO2 molecule, giving the ligated [NaV3O9·SO2]2− addition product (Equation (1)). It in turn reacts with a second SO2, forming two singly-charged product ions according to Equation (2).
NaV3O92− + SO2 → [NaV3O9·SO2]2−
[NaV3O9·SO2]2− + SO2 → [NaV2O6·SO2]− + VO3SO2−
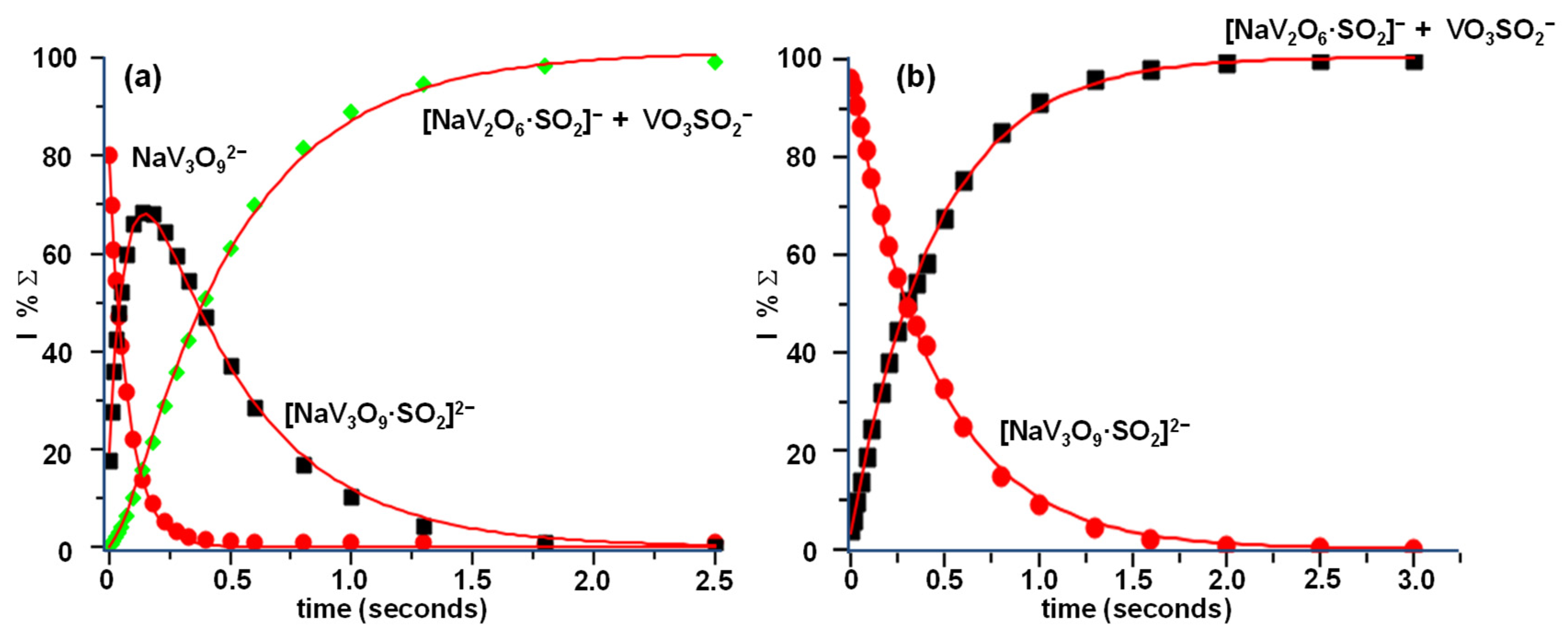
The reaction sequence was confirmed by the kinetic analysis reported in Figure 3 showing both the reactivity of the NaV3O92− parent ion (Figure 3a) and that of its ligated product, [NaV3O9·SO2]2−, obtained by the direct addition of the first SO2 molecule (Figure 3b).
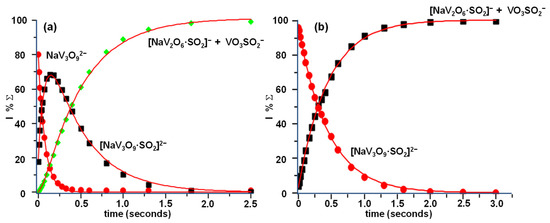
Figure 3.
Kinetic plot and best fit lines of the reactions of thermal (a) NaV3O92− ions with SO2, P(SO2) = 2.1 × 10−7 torr, R2 (NaV3O92−) = 0.9991, R2 (NaV3O9·SO22−) = 0.9980, R2 (NaV2O6·SO2− + VO3SO2−) = 0.9992 and (b) [NaV3O9·SO2]2− ions with SO2 P(SO2) = 2.2 × 10−7 torr, R2 (NaV3O9·SO22−) = 0.9997, R2 (NaV2O6·SO2− + VO3SO2−) = 0.9989.
The rate constants of the two reactions at 298 K, as measured from the kinetic plots, amount to k1 = 1.40 × 10−9 and k2 = 0.39 × 10−9 (±30%) cm3 s−1 molec−1, whereas the reactions’ efficiencies are estimated to be slightly > 100% and 34.5%, respectively.
A possible reaction mechanism can be postulated based on the borderline acid nature of the SO2 neutral reagent and on the heteronuclear four-membered stable structure predicted for the VO3SO2− product ion [48]. According to Scheme 2, in fact, after the addition of an SO2 molecule, the attack of the second SO2 to the opposite side of the dianionic species triggers the breaking of a V-O bond containing a bridged oxygen atom and the formation of new V-O and S-O bonds, eventually leading to two singly charged products, [NaV2O6·SO2]− and VO3SO2−. Hence, two SO2 molecules are needed to activate the bond-forming reaction, one of which only plays a spectator role, remaining non-covalently attached to the NaV2O6− anion and forming the [NaV2O6·SO2]− product ion. In support of this hypothesis, an analogous [HV2O6·SO2]− electrostatic structure was predicted for the product of the reaction of V2O62− with SO2 and H2O [49].
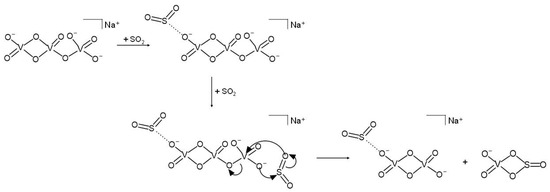
Scheme 2.
Plausible reaction mechanism of NaV3O92− ions with SO2 based on the corresponding kinetic plot.
When moving on to the reactivity of the H2NaV3O102− dianionic species to sulphur dioxide, two parallel reaction channels were highlighted: an OH anion transfer to SO2 leading to HOSO2− and HNaV3O9− product ions (Equation (3a)), and a V-O cleavage process assisted by the SO2 molecule (Equation (3b)), forming [NaV2O6·SO2]− and the H2VO4− vanadate anion.
H2NaV3O102− + SO2 → HOSO2− + HNaV3O9−
H2NaV3O102− + SO2 → [NaV2O6·SO2]− + H2VO4−
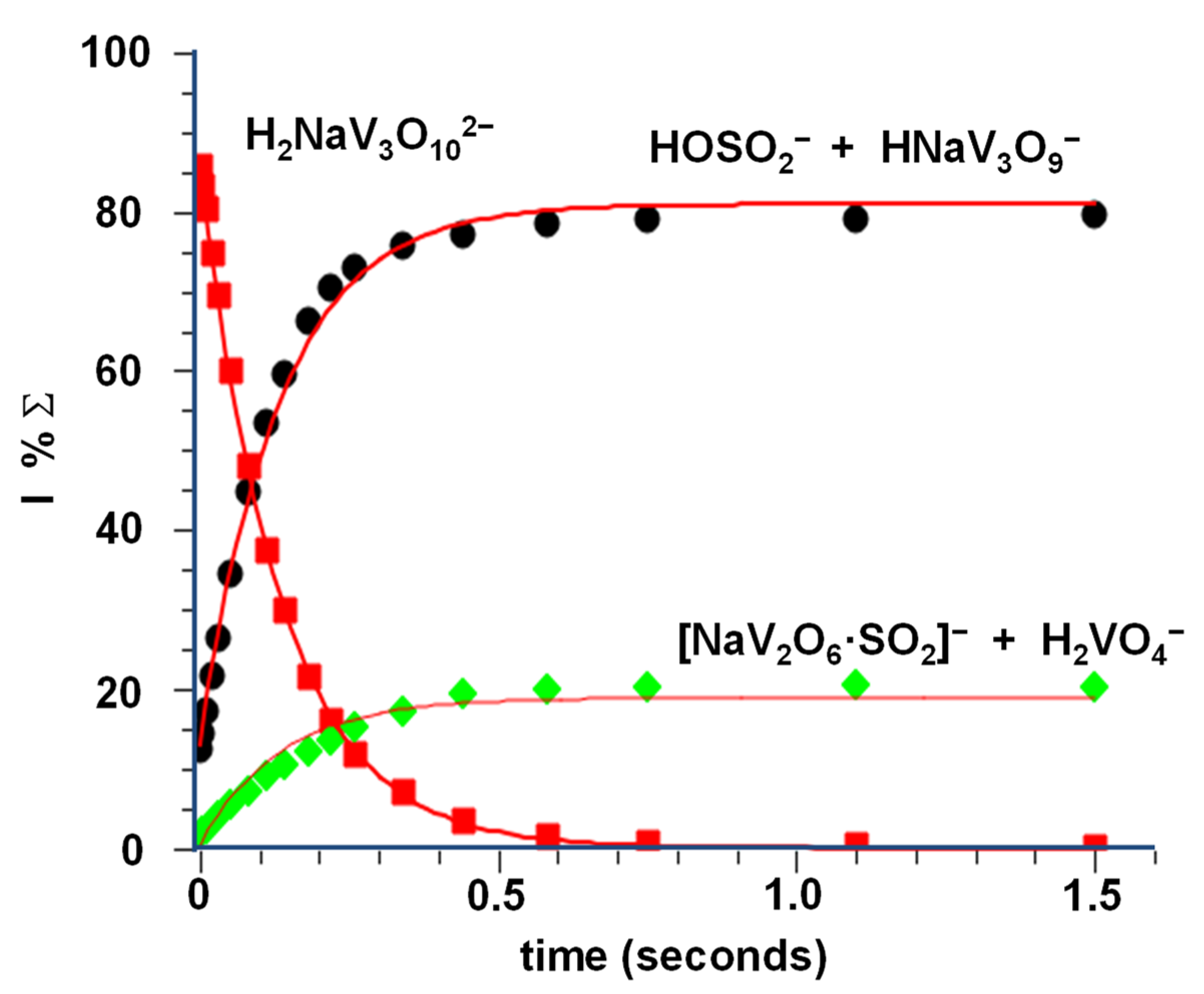
As shown in the kinetic plot of Figure 4, the whole process is fast and efficient, showing a k3 = 1.15 × 10−9 (±30%) cm3 s−1 molec−1 and an efficiency of 100%. The OH− transfer (Equation (3a)) predominates over the V-O cleavage, as demonstrated by the branching ratio between the two reaction channels (Equation (3a) vs. Equation (3b)), amounting to 3.7. The high rate constant of reaction 3.1, very close to that observed for a similar system [48], seems to confirm our previous experimental evidence of a hydroxide structure for the H2NaV3O102− dianion, rather than a hydrated one. Hydrolysis reactions, in fact, occur at a much slower rate [49].
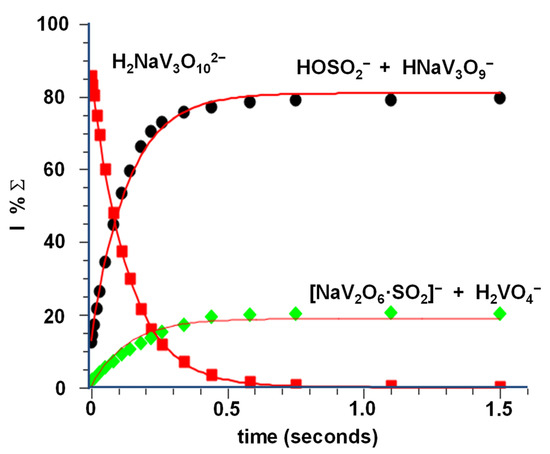
Figure 4.
Kinetic plot and best fit lines of the reaction of thermal H2NaV3O102− ions with SO2, P(SO2) = 2.1 × 10−7 torr, R2 (H2NaV3O102−) = 0.9998, R2 (H2VO4− + NaV2O6·SO2−) = 0.9652, R2 (HOSO2− + HNaV3O9−) = 0.9964.
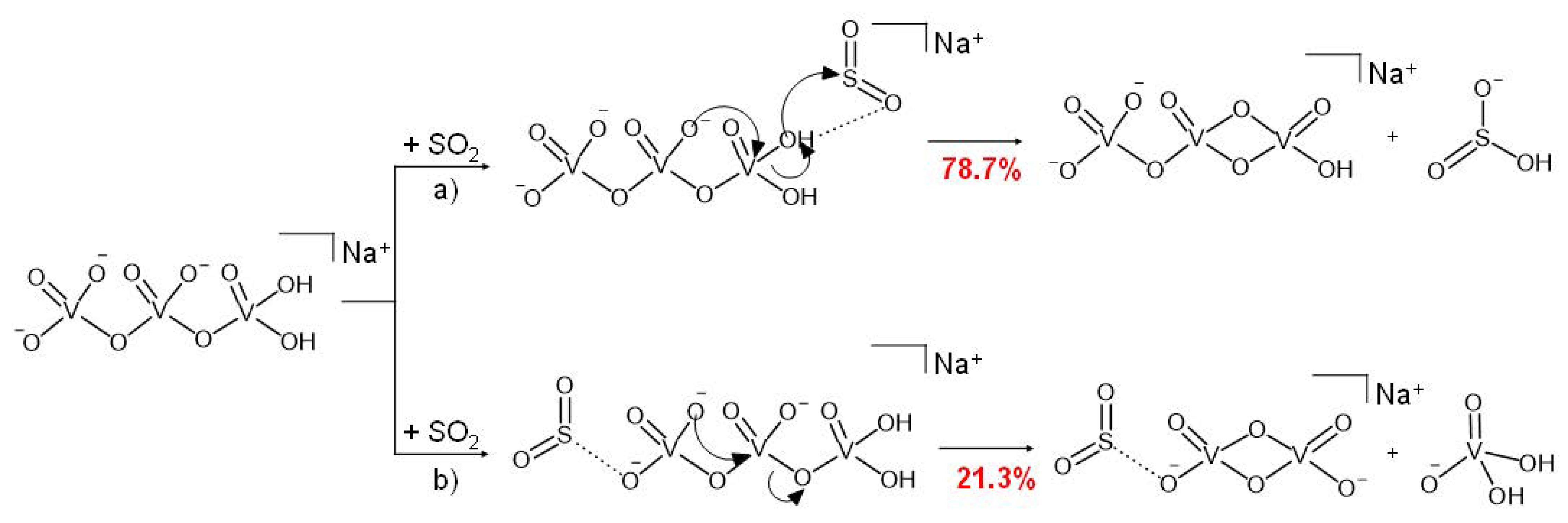
Interestingly, reaction 3 is a regioselective process, the outcome of which depends on where the SO2 molecule approaches the H2NaV3O102− dianion (Scheme 3). If sulphur dioxide coordinates the O-H bond, then pathway (a) of Scheme 3 takes place (Equation (3a)). Hence, the H2NaV3O102− parent ion behaves as an effective HO− donor, forming the bisulfite ionic species HOSO2− and a second product, HNaV3O9−, reasonably consisting of a V2O6 four-membered ring connected to a VO3 moiety, as in the case of the similar H2V3O9− dianion obtained by the reaction of the HV3O92− species with SO2 and H2O [49].
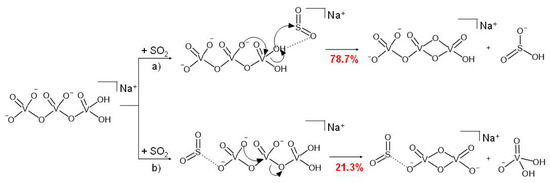
Scheme 3.
Plausible reaction mechanism of H2NaV3O102− ions with SO2 based on the corresponding kinetic plot.
Conversely, when the SO2 molecule attacks the H2NaV3O102− dianion on the opposite side, or at least far from the –OH groups, we observe the bond-forming reaction illustrated in Scheme 3b (Equation (3b)). Accordingly, the stable V3O10 kernel is broken with the formation of two singly charged products, the H2VO4− vanadate and the [NaV2O6·SO2]− anions, the latter being also formed in Equation (2) (Scheme 2).
The asymmetric structure of the H2NaV3O102− reactant ion may reasonably explain the occurrence of the regioselective process, as the SO2 molecule can approach the dianion from two chemically different extremities. Similar reactions have already been observed in the gas phase, where a plethora of different factors (e.g., thermodynamic, kinetic, electronic, steric, and orbital) can act by affecting the branching ratios of alternative reaction products [68,69,70]. As to the reactivity of the OH terminal of H2NaV3O102−, it is worth recalling that hydroxylation of acidic sites of vanadium-oxide-based materials, due to the reaction of the ubiquitous water, is known to change the electronic properties and the structure of the catalytic active sites of such materials [71].
Moreover, the open H2NaV3O102− dianion is substantially less constrained compared to the other NaV3O92− reactant species characterized by a V2O6 four-membered ring. Hence, when SO2 coordinates the H2NaV3O102− dianion from the VO3 side, a free terminal O− can play a nucleophilic attack on the vicinal vanadium atom, driving the formation of the two mono-charged products (Scheme 3, pathway (b)).
4. Conclusions
Fast and efficient reactions between polyvanadate dianions and sulphur dioxide have been reported in this study. Two SO2 molecules, once sequentially added to the doubly charged vanadium oxide anion, NaV3O92−, lead to the breakage of the stable V3O9 kernel.
Otherwise, the hydroxide H2NaV3O102− dianion reacts with sulphur dioxide in a regioselective process by following two different pathways according to which an SO2 molecule can promote either the breakage of a terminal V-OH bond or the V3O10 kernel.
All these reactions are associated with charge separation processes that result in two singly charged product ions by the formation of new V-O and S-O bonds. The charge separation occurs exclusively as a result of the chemical reaction with sulfur dioxide, in which the dipole moment plays a crucial role in promoting the ion-neutral bonding.
Author Contributions
Conceptualization, A.T. and C.S.; methodology, A.T.; validation, A.T., F.P. and C.S.; Funding acquisition, G.d.P.; investigation, C.S.; data curation, C.S.; Supervision G.d.P.; Visualization, C.S.; writing—original draft preparation, A.T. and C.S.; writing—review and editing, A.T., C.S., F.P. and G.d.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sapienza Rome University “Progetti di Ateneo” (Projects n. RM11816428291DFF, RM11715C81A54060, AR21916B746C06BB). C.S. thanks the Dipartimento di Chimica e Tecnologie del Farmaco, Sapienza Rome University, for a post-doc position within the project Dipartimenti di Eccellenza–L. 232/2016.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Weckhuysen, B.M.; Keller, D.E. Chemistry, Spectroscopy and the role of supported vanadium oxides. Catal. Today 2003, 78, 25–46. [Google Scholar] [CrossRef]
- Chenier, J.P. Survey of Industrial Chemistry; John Wiley & Sons: New York, NY, USA, 1987; pp. 45–57. [Google Scholar]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Song, H.; Zhang, C.; Liu, C.; Liu, Y.; Cao, G. Interface reduction synthesis of H2V2O3 nanobelts-graphene for high-rate Li-ion batteries. J. Phys. Chem. C 2015, 119, 11399. [Google Scholar]
- Xu, X.; Yan, M.; Tian, X.; Yang, C.; Shi, M.; Wei, Q.; Xu, L.; Mai, L. In situ investigation of Li and Na transport with single nanowire electrochemical devices. Nano Lett. 2015, 15, 3879–3884. [Google Scholar] [CrossRef]
- Anjass, M.; Lowe, G.A.; Streb, C. Molecular vanadium oxides for energy conversion and energy storage: Current trends and emerging opportunities. Angew. Chem. Int. Ed. 2021, 60, 7522–7532. [Google Scholar] [CrossRef]
- Johnson, G.; Tyo, C.C.; Castleman, A.W. Cluster reactivity experiments: Employing mass spectrometry to investigate the molecular level details of catalytic oxidation reactions. Proc. Natl. Acad. Sci. USA 2008, 105, 18108–18113. [Google Scholar] [CrossRef]
- Johnson, G.E.; Mitrić, R.; Bonačić-Koutecký, V.; Castleman, A. Clusters as model systems for investigating nanoscale oxidation catalysis. Chem. Phys. Lett. 2009, 475, 1–9. [Google Scholar] [CrossRef]
- Schlangen, M.; Schwarz, H. Effects of ligands, cluster size, and charge state in gas-phase catalysis: A happy marriage of experimental and computational studies. Catal. Lett. 2012, 142, 1265–1278. [Google Scholar] [CrossRef]
- Dietl, D.-C.N.; Troiani, A.; Schlangen, M.; Ursini, O.; Angelini, G.; Apeloig, Y.; de Petris, G.; Schwarz, H. Mechanistic aspects of gas-phase hydrogen-atom transfer from methane to [CO]+ and [SiO]+: Why do they differ? Chem. Eur. J. 2013, 19, 6662–6669. [Google Scholar] [CrossRef]
- Schlangen, M.; Schwarz, H. Probing elementary steps of nickel-mediated bond activation in gas-phase reactions: Ligand- and cluster-size effects. J. Catal. 2011, 284, 126–137. [Google Scholar] [CrossRef]
- Vikse, K.L.; McIndoe, J.S. Mechanistic insights from mass spectrometry: Examination of the elementary steps of catalytic reactions in the gas phase. Pure Appl. Chem. 2015, 87, 361–377. [Google Scholar] [CrossRef]
- Vikse, K.L.; Ahmadi, Z.; McIndoe, J.S. The application of electrospray ionization mass spectrometry to homogeneous catalysis. Coord. Chem. Rev. 2014, 279, 96–114. [Google Scholar] [CrossRef]
- Bell, R.C.; Zemski, K.A.; Kerns, K.P.; Deng, H.T.; Castleman, A.W. Reactivities and collision-induced dissociation of Vanadium oxide clusters cations. J. Phys. Chem. A 1998, 102, 1733–1742. [Google Scholar] [CrossRef]
- Zemski, K.A.; Justes, D.R.; Castleman, A.W. Reactions of group V transition metal oxide cluster ions with ethane and ethylene. J. Phys. Chem. A 2001, 105, 10237–10245. [Google Scholar] [CrossRef]
- Feyel, S.; Schröder, D.; Rozanska, X.; Sauer, J.; Schwarz, H. Gas-phase oxidation of propane and 1-butene with [V3O7]+: Experiment and theory in concert. Angew. Chem. Int. Ed. 2006, 45, 4677–4681. [Google Scholar] [CrossRef] [PubMed]
- Feyel, S.; Döbler, J.; Schröder, D.; Sauer, J.; Schwarz, H. Thermal activation of methane by tetranuclear [V4O10]+. Angew. Chem. Int. Ed. 2006, 45, 4681–4685. [Google Scholar] [CrossRef] [PubMed]
- Dietl, N.; Engeser, M.; Schwarz, H. Competitive hydrogen-atom abstraction versus oxygen-atom and electron transfers in gas-phase reactions of [X4O10]+ (X = P, V) with C2H4. Chem. Eur. J. 2010, 16, 4452–4456. [Google Scholar] [CrossRef]
- Zhou, S.; Li, J.; Schlangen, M.; Schwarz, H. Differences and commonalities in the gas-phase reactions of closed-shell metal dioxide clusters [MO2]+ (M = V, Nb, and Ta) with methane. Chem. Eur. J. 2016, 22, 7225–7228. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Wu, X.N.; Zhao, Y.X.; Ma, J.B.; Ding, X.L.; He, S.G. Room-temperature methane activation by a bimetallic oxide cluster AlVO4+. Chem. Phys. Lett. 2010, 489, 25–29. [Google Scholar] [CrossRef]
- Dietl, N.; Hockendorf, R.F.; Schlangen, M.; Lerch, M.; Beyer, M.K.; Schwarz, H. Generation, reactivity towards hydrocarbons, and electronic structure of heteronuclear vanadium phosphorous oxygen cluster ions. Angew. Chem. Int. Ed. 2011, 50, 1430–1434. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Liu, J.-W.; Schlangen, M.; Weiske, T.; Schröder, D.; Sauer, J.; Schwarz, H. Thermal methane activation by a binary V-Nb transition-metal oxide cluster cation: A further example for the crucial role of oxygen-centered radical. Chem. Eur. J. 2013, 19, 11496–11501. [Google Scholar] [CrossRef] [PubMed]
- Dietl, N.; Wende, T.; Chen, K.; Jiang, L.; Schlangen, M.; Zhang, X.; Asmis, K.R.; Schwarz, H. Structure and chemistry of the heteronuclear oxo-cluster [VPO4]·+: A model system for the gas-phase oxidation of small hydrocarbons. J. Am. Chem. Soc. 2013, 135, 3711–3721. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Li, H.F.; Zhao, Y.X.; He, S.G. Gold(III) mediated activation and transformation of methane on Au1-doped vanadium oxide cluster cations AuV2O6+. J. Am. Chem. Soc. 2016, 138, 9437–9443. [Google Scholar] [CrossRef]
- Bell, R.C.; Castleman, A.W. Reactions of vanadium oxide cluster ions with 1,3-butadiene and isomers of butene. J. Phys. Chem. A 2002, 106, 9893–9899. [Google Scholar] [CrossRef]
- Li, X.-N.; Xu, B.; Ding, X.-L.; He, S.-G. Interaction of vanadium oxide cluster anions with water: An experimental and theoretical study on the reactivity and mechanism. Dalton Trans. 2012, 41, 5562–5570. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mirabal, A.; Demuth, J.; Wöste, L.; Siebert, T. A complete reactant-product analysis of the oxygen transfer reaction in [V4O11·C3H6]−: A cluster complex for modelling surface activation and reactivity. J. Am. Chem. Soc. 2008, 130, 16832–16833. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Wu, X.-N.; Ma, J.-B.; He, S.-G.; Ding, X.-L. Experimental and theoretical study of the reactions between vanadium-silicon heteronuclear oxide cluster anions with n-butane. J. Phys. Chem. C 2010, 114, 12271–12279. [Google Scholar] [CrossRef]
- Janssens, E.; Lang, S.M.; Brümmer, M.; Niedziela, A.; Santambrogio, G.; Asmis, K.R.; Sauer, J. Kinetic study of the reaction of vanadium and vanadium-titanium oxide cluster anions with SO2. Phys. Chem. Chem. Phys. 2012, 14, 14344–14353. [Google Scholar] [CrossRef]
- Dinca, A.; Davis, T.P.; Fisher, K.J.; Smith, D.R.; Willett, G.D. Vanadium oxide anion cluster reactions with methyl isobutyrate and methyl methacrylate monomer and dimer: A study by FT/ICR mass spectrometry. Int. J. Mass Spectrom. 1999, 182–183, 73–84. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Z.-Y.; Zhou, Z.-X.; Liu, Q.-Y.; He, S.-G. Thermal reactions of (V2O5)nO− (n = 1–3) cluster anions with ethylene and propylene: Oxygen atom transfer versus molecular association. J. Phys. Chem. C 2014, 118, 14967–14976. [Google Scholar] [CrossRef]
- Jakubikova, E.; Bernestein, E.R. Reactions of sulfur dioxide with neutral vanadium oxide clusters in the gas phase. I. Density functional theory study. J. Phys. Chem. A 2007, 111, 13339–13346. [Google Scholar] [CrossRef] [PubMed]
- He, S.-G.; Xie, Y.; Dong, F.; Heinbuch, S.; Jakubikova, E.; Rocca, J.J.; Bernstein, E.R. Reactions of sulfur dioxide with neutral vanadium oxide clusters in the gas phase. II. Experimental study employing single-photon ionization. J. Phys. Chem. A 2008, 112, 11067–11077. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coelho, F.; Eberlin, M.N. The bridge connecting gas-phase and solution chemistries. Angew. Chem. Int. Ed. 2011, 50, 5261–5263. [Google Scholar] [CrossRef] [PubMed]
- Schröder, D.; Engeser, M.; Schwarz, H.; Harvey, J.N. Energetics of the ligated vanadium dications VO2+, VOH2+, and [V,O,H2]2+. ChemPhysChem 2002, 3, 584–591. [Google Scholar] [CrossRef]
- Warzok, U.; Mahnke, L.K.; Bensch, W. Soluble hetero-polyoxovanadates and their solution chemistry analysed by electrospray ionization mass spectrometry. Chem. Eur. J. 2019, 25, 1405–1419. [Google Scholar] [CrossRef]
- Ranasinghe, Y.A.; MacMahon, T.J.; Freiser, B.S. Gas-phase reactions of lanthanum dication with small alkanes and the photodissociation of LaC2H4n+ and LaC3H6n+ (n = 1 and 2). J. Am. Chem. Soc. 1992, 114, 9112–9118. [Google Scholar] [CrossRef]
- Petrie, S.; Javahery, G.; Wang, J.; Bohme, D.K. Derivatization of the fullerene dications C602+ and C702+ by ion-molecule reactions in the gas phase. J. Am. Chem. Soc. 1992, 114, 9177–9181. [Google Scholar] [CrossRef]
- Price, S.D.; Manning, M.; Leone, S.R. Bond-forming reactions of gas-phase molecular dications. J. Am. Chem. Soc. 1994, 116, 8673–8680. [Google Scholar] [CrossRef]
- Roithová, J.; Herman, Z.; Schröder, D.; Schwarz, H. Competition of proton and electron transfers in gas-phase reactions of hydrogen-containing dications CHX2+ (X = F, Cl, Br, I) with atoms, nonpolar and polar molecules. Chem. Eur. J. 2006, 12, 2465–2471. [Google Scholar] [CrossRef]
- Roithová, J.; Schröder, D. Bond-forming reactions of molecular dications as a new route to polyaromatic hydrocarbons. J. Am. Chem. Soc. 2006, 128, 4208–4209. [Google Scholar] [CrossRef]
- Roithová, J.; Žabka, J.; Herman, Z.; Thissen, R.; Schröder, D.; Schwarz, H. Reactivity of the CHBr2+ dication toward molecular hydrogen. J. Phys. Chem. A 2006, 110, 6447–6453. [Google Scholar] [CrossRef]
- Roithová, J.; Schröder, D. Bimolecular reactions of molecular dications: Reactivity paradigms and bond-forming processes. Phys. Chem. Chem. Phys. 2007, 9, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Roithová, J.; Schröder, D.; Schwarz, H. The SiF32+ dication: Chemistry counts! Chem. Eur. J. 2009, 15, 9995–9999. [Google Scholar] [CrossRef] [PubMed]
- Blades, A.; Peschke, M.; Verkerk, U.; Kebarle, P. Rates of proton transfer from carboxylic acids to dianions, CO2(CH2)pCO22−, and their significance to observed negative charge states of proteins in the gas phase. J. Phys. Chem. A 2002, 106, 10037–10042. [Google Scholar] [CrossRef]
- Flores, A.E.; Gronert, S. The gas-phase reactions of dianions with alkyl bromides: Direct identification of SN2 and E2 products. J. Am. Chem. Soc. 1999, 121, 2627–2628. [Google Scholar] [CrossRef]
- Gronert, S. Gas phase studies of the competition between substitution and elimination reactions. Acc. Chem. Res. 2003, 36, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Troiani, A.; Rosi, M.; Garzoli, S.; Salvitti, C.; de Petris, G. Vanadium hydroxyde cluster ions in the gas-phase: Bond-forming reactions of doubly-charged negative ions by SO2-promoted V-O activation. Chem. Eur. J. 2017, 23, 11752–11756. [Google Scholar] [CrossRef] [PubMed]
- Troiani, A.; Rosi, M.; Garzoli, S.; Salvitti, C.; de Petris, G. Sulphur dioxide cooperation in hydrolysis of vanadium oxide and hydroxide cluster dianions. New J. Chem. 2018, 42, 4008–4016. [Google Scholar] [CrossRef]
- de Petris, G.; Cartoni, A.; Troiani, A.; Angelini, G.; Ursini, O. Water activation by SO2+ ions: An effective source of OH radicals. PhysChemChemPhys 2009, 11, 9976–9978. [Google Scholar] [CrossRef]
- de Petris, G.; Troiani, A.; Rosi, M.; Angelini, G.; Ursini, O. Selective Activation of C-Cl and C-F Bonds by SO+ Radical Cations: An Experimental and Computational Study. ChemPlusChem 2013, 78, 1065–1072. [Google Scholar] [CrossRef]
- Troiani, A.; Rosi, M.; Salvitti, C.; de Petris, G. The oxidation of sulfur dioxide by single and double oxygen transfer paths. ChemPhysChem 2014, 15, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Troiani, A.; Salvitti, C.; de Petris, G. Gas-phase reactivity of carbonate ions with sulfur dioxide: An experimental study of cluster reactions. J. Am. Chem. Soc. Mass Spectrom. 2019, 30, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Troiani, A.; Rosi, M.; Garzoli, S.; Salvitti, C.; de Petris, G. Effective redox reactions by chromium oxide anions: Sulfur dioxide oxidation in the gas phase. Int. J. Mass Spectrom. 2019, 436, 18–22. [Google Scholar] [CrossRef]
- Salvitti, C.; Rosi, M.; Pepi, F.; Troiani, A.; de Petris, G. Reactivity of transition metal dioxide anions MO2− (M = Co, Ni, Cu, Zn) with sulfur dioxide in the gas phase: An experimental and theoretical study. Chem. Phys. Lett. 2021, 776, 138555. [Google Scholar] [CrossRef]
- Salvitti, C.; Pepi, F.; Troiani, A.; de Petris, G. Intracluster sulphur dioxide oxidation by sodium chlorite anions: A mass spectrometric study. Molecules 2021, 26, 7114. [Google Scholar] [CrossRef] [PubMed]
- Troiani, A.; Rosi, M.; Garzoli, S.; Salvitti, C.; de Petris, G. Iron-Promoted C-C Bond Formation in the Gas Phase. Angew. Chem. Int. Ed. 2015, 54, 14359–14362. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, C.M.; Bryantsev, V.S.; de Jong, W.A.; Diallo, M.S.; Goddard, W.A., III; Groenewold, G.S.; Van Stipdonk, M.J. Addition of H2O and O2 to acetone and dimethylsulfoxide ligated Uranyl(V) dioxocations. J. Phys. Chem. A 2009, 113, 2350–2358. [Google Scholar] [CrossRef]
- Bartmess, J.E.; Georgiadis, R.M. Empirical methods for determination of ionization gauge relative sensitivities for different gases. Vacuum 1983, 33, 149. [Google Scholar] [CrossRef]
- Kuzmic, P. Program DYNAFIT for the analysis of enzyme kinetic data: Application to HIV proteinase. Anal. Biochem. 1996, 237, 260–273. [Google Scholar] [CrossRef]
- Bowers, M.T.; Su, T. Interactions between Ions and Molecules; Plenum Press: New York, NY, USA, 1975. [Google Scholar]
- Habayeb, M.A.; Hileman, O.E., Jr. 51V- FT-nmr investigations of metavanadate ions in aqueous solutions. Can. J. Chem. 1980, 58, 2255–2261. [Google Scholar] [CrossRef]
- Aureliano, M.; Cran, D.C. Decavanadate (V10O286−) and oxovanadates: Oxometalates with many biological activities. J. Inorg. Biochem. 2009, 103, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Walanda, D.K.; Burns, R.C.; Lawrance, G.A.; Von Nagy-Felsobuki, E.I. New isopolyoxovanadate ions identified by electrospray mass spectrometry. Inorg. Chem. Commun. 1999, 2, 487–489. [Google Scholar] [CrossRef]
- Walanda, D.K.; Burns, R.C.; Lawrance, G.A.; Von Nagy-Felsobuki, E.I. Unknown isopolyoxovanadate species detected by electrospray mass spectrometry. Inorg. Chim. Acta 2000, 305, 118–126. [Google Scholar] [CrossRef]
- Al Hasan, N.M.; Johnson, G.E.; Laskin, J. Gas-phase synthesis of singly and multiply charged polyoxovanadate anions employing electrospray ionization and collision induced dissociation. J. Am. Soc. Mass Spectrom. 2013, 24, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.; Cristy, T.; Graves, S.W.; Hooth, M.J.; Waidyanatha, S. Characterization of aqueous formulations of tetra- and pentavalent forms of vanadium in support of test article selection in toxicology studies. Environ. Sci. Pollut. Res. 2017, 24, 405–416. [Google Scholar] [CrossRef]
- O’Hair, R.A.J.; Freitas, M.A.; Gronert, S.; Schmidt, J.A.R.; Williams, T.D. Concerning the regioselectivity of gas phase reactions of glycine with electrophiles. The cases of the dimethylchlorinium ion and the methoxymethal cation. J. Org. Chem. 1995, 60, 1990–1998. [Google Scholar] [CrossRef]
- Van der Wel, H.; Nibbering, N.M.M. A gas phase study of the regioselective BH4− reduction of some 2-substituted maleic anhydrides. Can. J. Chem. 1998, 66, 2587–2594. [Google Scholar]
- Wernik, M.; Hartmann, P.E.; Sipos, G.; Darvas, F.; Boese, A.D.; Dallinger, D.; Kappe, C.O. On the regioselectivity of the Gould-Jacobs reaction: Gas-phase versus solution-phase thermolysis. Eur. J. Org. Chem. 2020, 2020, 7051–7061. [Google Scholar] [CrossRef]
- Avdeed, V.I.; Tapilin, V.M. Water effect on the electronic structure of active sites of supported Vanadium oxide catalyst VOx/TiO2(001). J. Phys. Chem. C 2010, 114, 3609–3613. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).