Impacts of Syngas Composition on Anaerobic Fermentation
Abstract
:1. Introduction
2. Syngas
2.1. Gasification
2.1.1. Gasification of Biomass
2.1.2. Gasification Parameters
2.1.3. Biomass Composition
3. Syngas Fermentation
3.1. Microorganisms
3.2. Fermentation Pathways
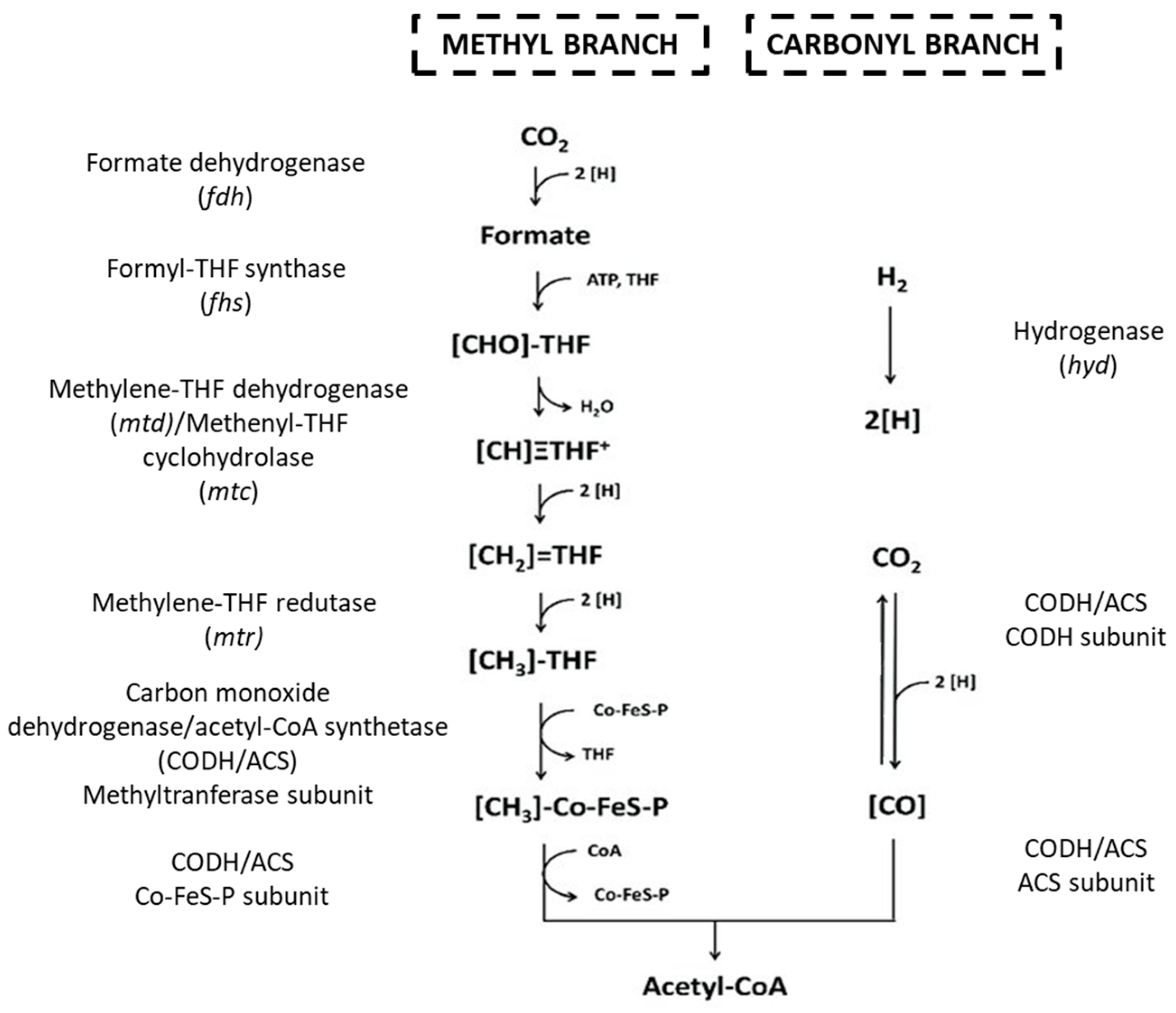
Wood–Ljungdahl Pathway
3.3. The Effect of Syngas Composition on Fermentation
3.3.1. Syngas Impurities
| Impurity (Conc.) | Process | Microorganism | Impurity Effect | Solution | Ref. |
|---|---|---|---|---|---|
| Benzene (327 mg/mL), toluene (117 mg/mL), ethylbenzene (131 mg/mL), p-xylene (92 mg/mL), and o-xylene and naphtha | Fermentation for ethanol/acetic acid production | Clostridium carboxidivorans P7 | Cause of cell dormancy and product redistribution (more ethanol, less acetic acid) | Addition of filter in the gas cleanup | [49] |
| Acetone (2 g/L) | Isopropanol production from producer gas treated by wet scrubbing techniques using acetone. | C. ragsdalei (Clostridium strain P11), and Clostridium carboxidivorans P7 | P11: Reduction of acetone to isopropanol; growth unaffected and ethanol concentrations increased by 55%; P7: no reduction of acetone; growth unaffected; 41% increase in ethanol and 79% decrease in acetic acid. | P11: opportunity for biological production of isopropanol from acetone with gaseous substrates | [60] |
| H2S (1.0 g/L) | Bioconversion of CO-rich waste gases into short- and medium-chain alcohols | C. carboxidivorans | Positive effect on both growth and alcohol formation (ethanol, 1-butanol, and 1-hexanol). | - | [61] |
| NaNO3 (0.1 g/L from 2.2 g/L thioacetamide) | Bioconversion of CO-rich waste gases into short- and medium-chain alcohols | C. carboxidivorans | Reduce growth and 25% reduction of ethanol concentration | Reduction of NOx components in syngas from the gasification and/or selectively removed | [61] |
| NaNO2 (0.5 and 0.1 g/L) | Bioconversion of CO-rich waste gases into short- and medium-chain alcohols | C. carboxidivorans | Strong toxic effect on the metabolism: no product formation | - | [61] |
| NH4Cl (5.0 g/L) | Bioconversion of CO-rich waste gases into short- and medium-chain alcohols | C. carboxidivorans | Positive effect on both growth and alcohol formation (ethanol, 1-butanol, and 1-hexanol). Cell growth: more than 50% increase; 2x ethanol concentration | .- | [61] |
| NO (150 ppm) | Fermentation for ethanol/acetic acid production | Clostridium carboxidivorans P7 | Inhibitor of the hydrogenase enzyme involved in H2 consumption | Filter does not eliminate inhibition | [49] |
| NO (0–160 ppm) | Fermentation of Biomass-Generated Synthesis Gas | Clostridium carboxidivorans P7 | NO < 40 ppm can be tolerated by cells in a syngas fermentation; NO > 40 ppm is a non-competitive inhibitor of hydrogenase activity (but it is reversible) | Use of syngas with NO < 40 ppm | [59] |
| NH3 (mole fraction of 0.37%) | Fermentation for biofuels production | Clostridium ragsdalei (Clostridium strain P11) | NH3 converts to ammonium ion (NH4+): inhibition of hydrogenase activity (at 650 mol/m3 of [NH4+]: 50% of V0) and cell growth (cell density: 23% of the control at 227 mol/m3 NH4+) | Remove NH3 impurity from raw syngas | [50] |
| O2 (400–26,000 ppm) | Syngas fermentation in a 100-L pilot scale fermentor | Clostridium ragsdalei (Clostridium strain P11) | Oxygen concentration in headspace (400 and 26,000 ppm): Clostridium strain P11 inoculum demonstrated growth and product formation. | - | [62] |
3.3.2. H2/CO Ratios
4. Challenges and Opportunities for Syngas Fermentation
5. Current Developments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wackernagel, M.; Hanscom, L.; Jayasinghe, P.; Lin, D.; Murthy, A.; Neill, E.; Raven, P. The importance of resource security for poverty eradication. Nat. Sustain. 2021, 4, 731–738. [Google Scholar] [CrossRef]
- Liberato, V.; Benevenuti, C.; Coelho, F.; Botelho, A.; Amaral, P.; Pereira, J.N.; Ferreira, T. Clostridium sp. as Bio-Catalyst for Fuels and Chemicals Production in a Biorefinery Context. Catalysts 2019, 9, 962. [Google Scholar] [CrossRef] [Green Version]
- Gunes, B. A critical review on biofilm-based reactor systems for enhanced syngas fermentation processes. Renew. Sustain. Energy Rev. 2021, 143, 110950. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.; Tanner, R.S. Syngas fermentation process development for production of biofuels and chemicals: A review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar] [CrossRef]
- Benevenuti, C.; Botelho, A.; Ribeiro, R.; Branco, M.; Pereira, A.; Vieira, A.C.; Ferreira, T.; Amaral, P. Experimental Design to Improve Cell Growth and Ethanol Production in Syngas Fermentation by Clostridium carboxidivorans. Catalysts 2020, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Daniell, J.; Köpke, M.; Simpson, S.D. Commercial Biomass Syngas Fermentation. Energies 2012, 5, 5372–5417. [Google Scholar] [CrossRef] [Green Version]
- Khushboo; Ankush; Yadav, K.; Mandal, M.K.; Pal, S.; Chaudhuri, H.; Dubey, K.K. Bioeconomy of municipal solid waste (MSW) using gas fermentation. Curr. Dev. Biotechnol. Bioeng. 2020, 289–304. [Google Scholar] [CrossRef]
- Speight, J.G. Synthesis gas and the Fischer–Tropsch process. Kirk-Othmer Encycl. Chem. Technol. 2020, 427–468. [Google Scholar] [CrossRef]
- Chan, Y.H.; Rahman, S.N.F.S.A.; Lahuri, H.M.; Khalid, A. Recent progress on CO-rich syngas production via CO2 gasification of various wastes: A critical review on efficiency, challenges and outlook. Environ. Pollut. 2021, 278, 116843. [Google Scholar] [CrossRef]
- Ramachandriya, K.; Kundiyana, D.K.; Sharma, A.M.; Kumar, A.; Atiyeh, H.K.; Huhnke, R.L.; Wilkins, M.R. Critical factors affecting the integration of biomass gasification and syngas fermentation technology. AIMS Bioeng. 2016, 3, 188–210. [Google Scholar] [CrossRef]
- Das, P.; Chandramohan, V.P.; Mathimani, T.; Pugazhendhi, A. Recent advances in thermochemical methods for the conversion of algal biomass to energy. Sci. Total Environ. 2021, 766, 144608. [Google Scholar] [CrossRef] [PubMed]
- Murugan, P.; Sekhar, S.J. Investigation on the yield of producer gas from tamarind shell (Tamarindus Indica) as feedstock in an Imbert type biomass gasifier. Fuel 2021, 292, 120310. [Google Scholar] [CrossRef]
- He, Q.; Guo, Q.; Umeki, K.; Ding, L.; Wang, F.; Yu, G. Soot formation during biomass gasification: A critical review. Renew. Sustain. Energy Rev. 2021, 139, 110710. [Google Scholar] [CrossRef]
- Li, X.; Grace, J.; Lim, C.; Watkinson, A.; Chen, H.; Kim, J. Biomass gasification in a circulating fluidized bed. Biomass-Bioenergy 2004, 26, 171–193. [Google Scholar] [CrossRef]
- Carvalho, M.M.; Cardoso, M.; Vakkilainen, E. Biomass gasification for natural gas substitution in iron ore pelletizing plants. Renew. Energy 2015, 81, 566–577. [Google Scholar] [CrossRef]
- Zhang, K.; Chang, J.; Guan, Y.; Chen, H.; Yang, Y.; Jiang, J. Lignocellulosic biomass gasification technology in China. Renew. Energy 2013, 49, 175–184. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kawamoto, K.; Fukushima, R.; Tanaka, S. Woody biomass and RPF gasification using reforming catalyst and calcium oxide. Chemosphere 2011, 83, 1273–1278. [Google Scholar] [CrossRef]
- Cao, W.; Guo, L.; Yan, X.; Zhang, D.; Yao, X. Assessment of sugarcane bagasse gasification in supercritical water for hydrogen production. Int. J. Hydrogen Energy 2018, 43, 13711–13719. [Google Scholar] [CrossRef]
- Michel, R.; Rapagnà, S.; Burg, P.; DI Celso, G.M.; Courson, C.; Zimny, T.; Gruber, R. Steam gasification of Miscanthus X Giganteus with olivine as catalyst production of syngas and analysis of tars (IR, NMR and GC/MS). Biomass Bioenergy 2011, 35, 2650–2658. [Google Scholar] [CrossRef]
- Siedlecki, M.; de Jong, W. Biomass gasification as the first hot step in clean syngas production process—Gas quality optimization and primary tar reduction measures in a 100 kW thermal input steam–oxygen blown CFB gasifier. Biomass Bioenergy 2011, 35, S40–S62. [Google Scholar] [CrossRef]
- Jayah, T.; Aye, L.; Fuller, R.; Stewart, D. Computer simulation of a downdraft wood gasifier for tea drying. Biomass-Bioenergy 2003, 25, 459–469. [Google Scholar] [CrossRef]
- Pedroso, D.T.; Aiello, R.C.; Conti, L.; Mascia, S. Biomass gasification on a new really tar free downdraft gasifier. Rev. Ciências Exatas 2005, 11, 59–62. [Google Scholar]
- Liakakou, E.T.; Infantes, A.; Neumann, A.; Vreugdenhil, B.J. Connecting gasification with syngas fermentation: Comparison of the performance of lignin and beech wood. Fuel 2021, 290, 120054. [Google Scholar] [CrossRef]
- Migliaccio, R.; Brachi, P.; Montagnaro, F.; Papa, S.; Tavano, A.; Montesarchio, P.; Ruoppolo, G.; Urciuolo, M. Sewage Sludge Gasification in a Fluidized Bed: Experimental Investigation and Modeling. Ind. Eng. Chem. Res. 2021, 60, 5034–5047. [Google Scholar] [CrossRef]
- Patel, V.R.; Patel, D.; Varia, N.; Patel, R. Co-gasification of lignite and waste wood in a pilot-scale (10 kWe) downdraft gasifier. Energy 2017, 119, 834–844. [Google Scholar] [CrossRef]
- Pio, D.T.; Tarelho, L.; Pinto, R.; Matos, M.; Frade, J.; Yaremchenko, A.; Mishra, G.; Pinto, P. Low-cost catalysts for in-situ improvement of producer gas quality during direct gasification of biomass. Energy 2018, 165, 442–454. [Google Scholar] [CrossRef]
- Sethuraman, S.; Van Huynh, C.; Kong, S.-C. Producer Gas Composition and NOxEmissions from a Pilot-Scale Biomass Gasification and Combustion System Using Feedstock with Controlled Nitrogen Content. Energy Fuels 2011, 25, 813–822. [Google Scholar] [CrossRef]
- Habibollahzade, A.; Ahmadi, P.; Rosen, M.A. Biomass gasification using various gasification agents: Optimum feedstock selection, detailed numerical analyses and tri-objective grey wolf optimization. J. Clean. Prod. 2021, 284, 124718. [Google Scholar] [CrossRef]
- Claassens, N.J.; Sousa, D.Z.; Dos Santos, V.A.P.M.; De Vos, W.M.; van der Oost, J. Harnessing the power of microbial autotrophy. Nat. Rev. Genet. 2016, 14, 692–706. [Google Scholar] [CrossRef]
- Berg, I.A. Ecological Aspects of the Distribution of Different Autotrophic CO2 Fixation Pathways. Appl. Environ. Microbiol. 2011, 77, 1925–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengelsdorf, F.R.; Straub, M.; Dürre, P. Bacterial synthesis gas (syngas) fermentation. Environ. Technol. 2013, 34, 1639–1651. [Google Scholar] [CrossRef]
- Dürre, P.; Eikmanns, B.J. C1-carbon sources for chemical and fuel production by microbial gas fermentation. Curr. Opin. Biotechnol. 2015, 35, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wan, N.; Sathish, A.; You, L.; Tang, Y.J.; Wen, Z. Deciphering Clostridium metabolism and its responses to bioreactor mass transfer during syngas fermentation. Sci. Rep. 2017, 7, 10090. [Google Scholar] [CrossRef] [Green Version]
- Sathish, A.; Sharma, A.; Gable, P.; Skiadas, I.V.; Brown, R.; Wen, Z. A novel bulk-gas-to-atomized-liquid reactor for enhanced mass transfer efficiency and its application to syngas fermentation. Chem. Eng. J. 2019, 370, 60–70. [Google Scholar] [CrossRef]
- Riegler, P.; Chrusciel, T.; Mayer, A.; Doll, K.; Weuster-Botz, D. Reversible retrofitting of a stirred-tank bioreactor for gas-lift operation to perform synthesis gas fermentation studies. Biochem. Eng. J. 2019, 141, 89–101. [Google Scholar] [CrossRef]
- Bertsch, J.; Müller, V. Bioenergetic constraints for conversion of syngas to biofuels in acetogenic bacteria. Biotechnol. Biofuels 2015, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.E.; Richter, H.; Saha, S.; Angenent, L.T. Traits of selected Clostridium strains for syngas fermentation to ethanol. Biotechnol. Bioeng. 2016, 113, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.W.; Chae, C.G.; Kwon, S.J.; Kim, Y.J.; Lee, J.H.; Lee, H.S. Domestication of the novel alcohologenic acetogen Clostridium sp. AWRP: From isolation to characterization for syngas fermentation. Biotechnol. Biofuels 2019, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.L.; Gößner, A.S.; Daniel, S.L. Old acetogens, new light. Ann. N. Y. Acad. Sci. 2008, 1125, 100–128. [Google Scholar] [CrossRef]
- Fast, A.G.; Schmidt, E.D.; Jones, S.W.; Tracy, B.P. Acetogenic mixotrophy: Novel options for yield improvement in biofuels and biochemicals production. Curr. Opin. Biotechnol. 2015, 33, 60–72. [Google Scholar] [CrossRef]
- Drake, H.L.; Küsel, K.; Matthies, C. Acetogenic Prokaryotes. Prokaryotes 2013, 3–60. [Google Scholar] [CrossRef]
- Wong, T.S.; Jajesniak, H.E.M.O.A.P. Carbon Dioxide Capture and Utilization using Biological Systems: Opportunities and Challenges. J. Bioprocess. Biotech. 2014, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.E.; Jang, Y.-S. Metabolic engineering of microorganisms for the production of ethanol and butanol from oxides of carbon. Appl. Microbiol. Biotechnol. 2019, 103, 8283–8292. [Google Scholar] [CrossRef]
- Fernández-Naveira, Á.; Veiga, M.C.; Kennes, C. Effect of pH control on the anaerobic H-B-E fermentation of syngas in bioreactors. J. Chem. Technol. Biotechnol. 2017, 92, 1178–1185. [Google Scholar] [CrossRef]
- Heiskanen, H.; Virkajärvi, I.; Viikari, L. The effect of syngas composition on the growth and product formation of Butyribacterium methylotrophicum. Enzym. Microb. Technol. 2007, 41, 362–367. [Google Scholar] [CrossRef]
- Han, Y.-F.; Xie, B.-T.; Wu, G.-X.; Guo, Y.-Q.; Li, D.-M.; Huang, Z.-Y. Combination of Trace Metal to Improve Solventogenesis of Clostridium carboxidivorans P7 in Syngas Fermentation. Front. Microbiol. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Doll, K.; Rückel, A.; Kämpf, P.; Wende, M.; Weuster-Botz, D. Two stirred-tank bioreactors in series enable continuous production of alcohols from carbon monoxide with Clostridium carboxidivorans. Bioprocess Biosyst. Eng. 2018, 41, 1403–1416. [Google Scholar] [CrossRef]
- Benevenuti, C.S.G.; Branco, M.; Nascimento-Correa, M.; Botelho, A.; Ferreira, T.; Amaral, P. Residual gas for ethanol production by Clostridium carboxidi-vorans in a dual impeller stirred tank bioreactor (STBR). Fermentation 2021, 7, 199. [Google Scholar] [CrossRef]
- Ahmed, A.; Cateni, B.G.; Huhnke, R.; Lewis, R.S. Effects of biomass-generated producer gas constituents on cell growth, product distribution and hydrogenase activity of Clostridium carboxidivorans P7T. Biomass Bioenergy 2006, 30, 665–672. [Google Scholar] [CrossRef]
- Xu, D.; Lewis, R.S. Syngas fermentation to biofuels: Effects of ammonia impurity in raw syngas on hydrogenase activity. Biomass Bioenergy 2012, 45, 303–310. [Google Scholar] [CrossRef]
- Infantes, A.; Kugel, M.; Raffelt, K.; Neumann, A. Side-by-Side Comparison of Clean and Biomass-Derived, Impurity-Containing Syngas as Substrate for Acetogenic Fermentation with Clostridium ljungdahlii. Fermentation 2020, 6, 84. [Google Scholar] [CrossRef]
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of supercritical water gasification with lignocellulosic real biomass as the feedstocks: Process parameters, biomass composition, catalyst development, reactor design and its challenges. Chem. Eng. J. 2021, 415, 128837. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef] [Green Version]
- Haryanto, A.; Fernando, S.D.; Pordesimo, L.O.; Adhikari, S. Upgrading of syngas derived from biomass gasification: A thermodynamic analysis. Biomass Bioenergy 2009, 33, 882–889. [Google Scholar] [CrossRef]
- Xu, D.; Tree, D.; Lewis, R.S. The effects of syngas impurities on syngas fermentation to liquid fuels. Biomass Bioenergy 2011, 35, 2690–2696. [Google Scholar] [CrossRef]
- Munasinghe, P.C.; Khanal, S.K. Biomass-derived Syngas Fermentation into Biofuels. Biofuels 2011, 101, 79–98. [Google Scholar] [CrossRef]
- Ciferno, J.P.; Marano, J. Benchmarking Biomass Gasification Technologies for Fuels, Chemicals and Hydrogen Production; US Dep. Energy. Natl. Energy Technol. Lab.: Pittsburgh, PA, USA, 2002. [Google Scholar]
- Monir, M.U.; Aziz, A.A.; Khatun, F.; Yousuf, A. Bioethanol production through syngas fermentation in a tar free bioreactor using Clostridium butyricum. Renew. Energy 2020, 157, 1116–1123. [Google Scholar] [CrossRef]
- Ahmed, A.; Lewis, R.S. Fermentation of biomass-generated synthesis gas: Effects of nitric oxide. Biotechnol. Bioeng. 2007, 97, 1080–1086. [Google Scholar] [CrossRef]
- Ramachandriya, K.D.; Wilkins, M.R.; Delorme, M.J.; Zhu, X.; Kundiyana, D.K.; Atiyeh, H.K.; Huhnke, R.L. Reduction of acetone to isopropanol using producer gas fermenting microbes. Biotechnol. Bioeng. 2011, 108, 2330–2338. [Google Scholar] [CrossRef]
- Rückel, A.; Hannemann, J.; Maierhofer, C.; Fuchs, A.; Weuster-Botz, D. Studies on Syngas Fermentation With Clostridium carboxidivorans in Stirred-Tank Reactors With Defined Gas Impurities. Front. Microbiol. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Kundiyana, D.K.; Huhnke, R.; Wilkins, M.R. Syngas fermentation in a 100-L pilot scale fermentor: Design and process considerations. J. Biosci. Bioeng. 2010, 109, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Lanzillo, F.; Ruggiero, G.; Raganati, F.; Russo, M.E.; Marzocchella, A. Batch Syngas Fermentation by Clostridium carboxidivorans for Production of Acids and Alcohols. Processes 2020, 8, 1075. [Google Scholar] [CrossRef]
- Hurst, K.M.; Lewis, R.S. Carbon monoxide partial pressure effects on the metabolic process of syngas fermentation. Biochem. Eng. J. 2010, 48, 159–165. [Google Scholar] [CrossRef]
- Maddipati, P.; Atiyeh, H.; Bellmer, D.D.; Huhnke, R.L. Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresour. Technol. 2011, 102, 6494–6501. [Google Scholar] [CrossRef]
- Valgepea, K.; de Souza Pinto Lemgruber, R.; Abdalla, T.; Binos, S.; Takemori, N.; Takemori, A.; Tanaka, Y.; Tappel, R.; Köpke, M.; Simpson, S.D.; et al. Biotechnology for Biofuels H2 drives metabolic rearrangements in gas—Fermenting Clostridium autoethanogenum. Biotechnol. Biofuels 2018, 11, 1–15. [Google Scholar] [CrossRef]
- Jack, J.; Lo, J.; Maness, P.-C.; Ren, Z.J. Directing Clostridium ljungdahlii fermentation products via hydrogen to carbon monoxide ratio in syngas. Biomass Bioenergy 2019, 124, 95–101. [Google Scholar] [CrossRef]
- Diender, M.; Stams, A.J.M.; Sousa, D.Z. Production of medium-chain fatty acids and higher alcohols by a synthetic co-culture grown on carbon monoxide or syngas. Biotechnol. Biofuels 2016, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, P.; Bowen, S.H.; Lewis, R.S. A thermodynamic analysis of electron production during syngas fermentation. Bioresour. Technol. 2011, 102, 8071–8076. [Google Scholar] [CrossRef]
- US Patent & Trademark Office. Available online: www.uspto.gov/ (accessed on 12 September 2021).
- Espacenet. Available online: Worldwide.espacenet.com/ (accessed on 12 September 2021).
- Chandolias, K.; Richards, T.; Taherzadeh, M.J. Combined Gasification-Fermentation Process in Waste Biorefinery. Waste Biorefinery 2018, 157–200. [Google Scholar] [CrossRef]
| Feedstock | Gasifying Agent | Equivalence Ratio (ER) | Gasifier | Temperature | Gas Composition | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2% | N2% | CO% | CH4% | CO2% | Others | ||||||
| Cypress sawdust | air | 0.54 | fluidized bed | 700–850 °C | 5.6 | 68.0 | 6.9 | 1.4 | 18.1 | [14] | |
| Mixed pine bark-spruce | 0.22 | 5.4 | 53.9 | 21.4 | 4.6 | 14.7 | tar: 15.3 g/Nm3 | ||||
| Wood chips | air | 0.30 | fluidized bed | 800 °C | 13.2 | 41.1 | 18.0 | 4.1 | 11.5 | [15] | |
| O2 | 0.30 | 27.9 | 0.9 | 35.7 | 0.76 | 10.5 | |||||
| steam | n/a | 31.1 | 0.0 | 14.0 | 7.3 | 14.1 | |||||
| steam (dry) | n/a | 41.0 | 0.06 | 18.5 | 9.7 | 18.6 | |||||
| Rice husk | air | - | bubbling fluidized bed | 702 °C | 4.4 | 57.1 | 21.3 | 4.3 | 11.3 | [16] | |
| - | 737 °C | 4.8 | 57.1 | 16.9 | 3.7 | 15.9 | |||||
| Wood biomass pellet | steam and O2 | 0.28 | fluidized bed | 750 °C | 40.0 | 5.0 | 20.0 | 5.0 | 30.0 | tar: 5 g/Nm3 | [17] |
| Refused paper and plastic fuel | 0.31 | 35.0 | 2.0 | 20.0 | 8.0 | 30.0 | tar > 10 g/Nm3 | ||||
| Sugar cane bagasse | super critical water | n/a | autoclave reactor | 750 °C | 45.0 | 5.0 | 5.0 | 10.0 | 40.0 | [18] | |
| Miscanthus | steam | n/a | fluidized bed | 815 °C | 41.7 | - | 25.6 | 9.3 | 23.4 | [19] | |
| Miscanthus | steam and O2 | 0.24 | fluidized bed | 800 °C | 22.8 | 4.6 | 31.4 | 9.5 | 31.7 | BTX:42 g/m3; phenolics: 4.6 g/m3 | [20] |
| Straw | 0.35 | 32.0 | 4.2 | 12.7 | 5.8 | 45.3 | BTX:23 g/m3; phenolics: 1.2 g/m3 | ||||
| Wood | 0.28 | 21.8 | 4.6 | 33.7 | 8.9 | 31.0 | BTX:38 g/m3; phenolics: 2.2 g/m3 | ||||
| Rubber wood | air | - | fixed bed downdraft | 600 °C | 17.2 | 51.9 | 19.6 | 1.4 | 9.9 | [21] | |
| Olive | air | fixed bed downdraft | 1190 °C | 13.2 | 54.9 | 17.4 | 0.8 | 12.4 | [22] | ||
| Peach | - | 1170 °C | 15.0 | 51.7 | 17.7 | 1.2 | 13.5 | ||||
| Pine | 1140 °C | 12.0 | 59.4 | 16.0 | 0.2 | 11.4 | |||||
| Beech wood | steam | n/a | bubbling fluidized bed | 850 °C | 33.4 | - | 28 | 8.8 | 23.8 | 13 g/Nm3 tar, 0.05% C2H6, 1.6% C2H4, 0.03% C2H2 | [23] |
| Lignin-rich feedstock | n/a | 35.5 | - | 19.8 | 11.4 | 24.4 | 21.2 g/Nm3, 0.67% C2H6, 3.3% C2H4, 0.11% C2H2 | ||||
| Sewage sludge * | Air and N2 | 0.10 | fluidized bed | 850 °C | 33.30 | - | 26.16 | 11.46 | 20.90 | C2H4, 7.99%; C2H6, 0.19%; tar; solid residues | [24] |
| 0.20 | 26.92 | - | 30.99 | 10.15 | 28.97 | C2H4, 2.77% C2H6, 0.19%; tar; solid residues | |||||
| Microorganism | Reactor | CO:H2:CO2:N2:CH4 | Impurities | Cell Growth (g Dry Weight Cell/L) | Products | Ref. |
|---|---|---|---|---|---|---|
| Butyribacterium methylotrophicum | Serum bottles | 100:0:0:0:0 | <0.4 | <0.6 g/L acetic acid; 0.4 g/L butyric acid; 0.07 g/L ethanol | [45] | |
| 70:0:30:0:0 | 0.4 | 1.3 g/L acetic acid; 0.4 g/L butyric acid; 0.08 g/L ethanol | ||||
| 70:30:0:0:0 | <0.4 | <0.6 g/L acetic acid 0.6 g/L butyric acid; <0.02 g/L ethanol | ||||
| 35:40:25:0:0 | 0.2 | 1 g/L acetic acid; 0.3 g/L butyric acid; 0.02 g/L ethanol | ||||
| Clostridium carboxidivorans | CSTR | 20:10:20:50:0 | 0.42 | 2.7 g/L ethanol; 1.9 g/L butanol; 0.85 g/L hexanol | [44] | |
| Clostridium carboxidivorans | CSTR | 50:15:35:0:0 | <0.6 | 2 g/L ethanol; 1 g/L butanol; 0.5 g/L hexanol | [46] | |
| Serum bottles | 50:15:35:0:0 | <0.6 | 2.59 g/L acetic acid; 0.32 g/L butyric acid; 1.19 g/L ethanol; 0.18 g/L butanol | |||
| Clostridium carboxidivorans | CSTR | 80:0:20:0:0 | 0.74 | 1.86 g/L acetic acid; 2.52 g/L ethanol; 0.5 g/L butanol | [47] | |
| 60:0:40:0:0 | 1.8 | 3.35 g/L acetic acid; 1.8 g/L ethanol; 0.66 g/L butanol; 0.38 g/L hexanol | ||||
| Clostridium carboxidivorans | Serum bottles | 25:44:10:10:11 | 0.47 | 2.3 g/L acetic acid; 1.9 g/L ethanol | [48] | |
| STBR | 25:44:10:10:11 | 1.93 | 1.32 g/L acetic acid; 1.76 g/L ethanol; 0.43 g/L butanol | |||
| Clostridium carboxidivorans | Serum bottles | 16.5:5:15.5:56:4.5 | no tar | 0.35 (7 days) | 2.2 g/L acetic acid; 0.12 g/L ethanol | [49] |
| 16.5:5:15.5:56:4.6 | tar (C2H2, C2H6, C2H4) | 0.4 (11 days) | 0.5 g/L acetic acid; 0.22 g/L ethanol | |||
| Clostridium ragsdalei | CSTR | 40:30:30:0:0 | no impurities | 0.52 | significant reduction in hydrogenase activity with NH3 in the syngas | [50] |
| 0.37% NH3 | 0.41 | |||||
| Clostridium ljungdahlii | CSTR | 32.5:32.5:16:19:0 | no impurities | 0.76 | 16.75 g/L acetic acid; 2.47 g/L ethanol | [51] |
| 32.5:32.5:16:19:0 | 150 ppm NH3; 54 ppb H2S | 0.71 | 10.27 g/L acetic acid; 3.29 g/L ethanol | |||
| Clostridium carboxidivorans | CSTR | 80:0:20:0:0 | no impurities | 0.4 | 0.96g/L acetic acid; 1.17 g/L ethanol; 0.56 g/L butanol; 0.16 g/L | [47] |
| 80:0:20:0:0 | 0.1 g/L H2S | 0.76 | 0.8 g/L acetic acid; 3.2 g/L ethanol; 0.38 g/L hexanoic acid | |||
| 80:0:20:0:0 | 0.1 g/L NaNO3 | 0.6 | 0.38 g/L acetic acid; 1.1 g/L ethanol; 2.04 g/L butyric acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benevenuti, C.; Amaral, P.; Ferreira, T.; Seidl, P. Impacts of Syngas Composition on Anaerobic Fermentation. Reactions 2021, 2, 391-407. https://doi.org/10.3390/reactions2040025
Benevenuti C, Amaral P, Ferreira T, Seidl P. Impacts of Syngas Composition on Anaerobic Fermentation. Reactions. 2021; 2(4):391-407. https://doi.org/10.3390/reactions2040025
Chicago/Turabian StyleBenevenuti, Carolina, Priscilla Amaral, Tatiana Ferreira, and Peter Seidl. 2021. "Impacts of Syngas Composition on Anaerobic Fermentation" Reactions 2, no. 4: 391-407. https://doi.org/10.3390/reactions2040025
APA StyleBenevenuti, C., Amaral, P., Ferreira, T., & Seidl, P. (2021). Impacts of Syngas Composition on Anaerobic Fermentation. Reactions, 2(4), 391-407. https://doi.org/10.3390/reactions2040025








