Hydrocracking of Octacosane and Cobalt Fischer–Tropsch Wax over Nonsulfided NiMo and Pt-Based Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Catalyst Preparation
2.2. BET Measurement
2.3. X-ray Diffraction (XRD)
2.4. Scanning Electron Microscopy (SEM-EDX)
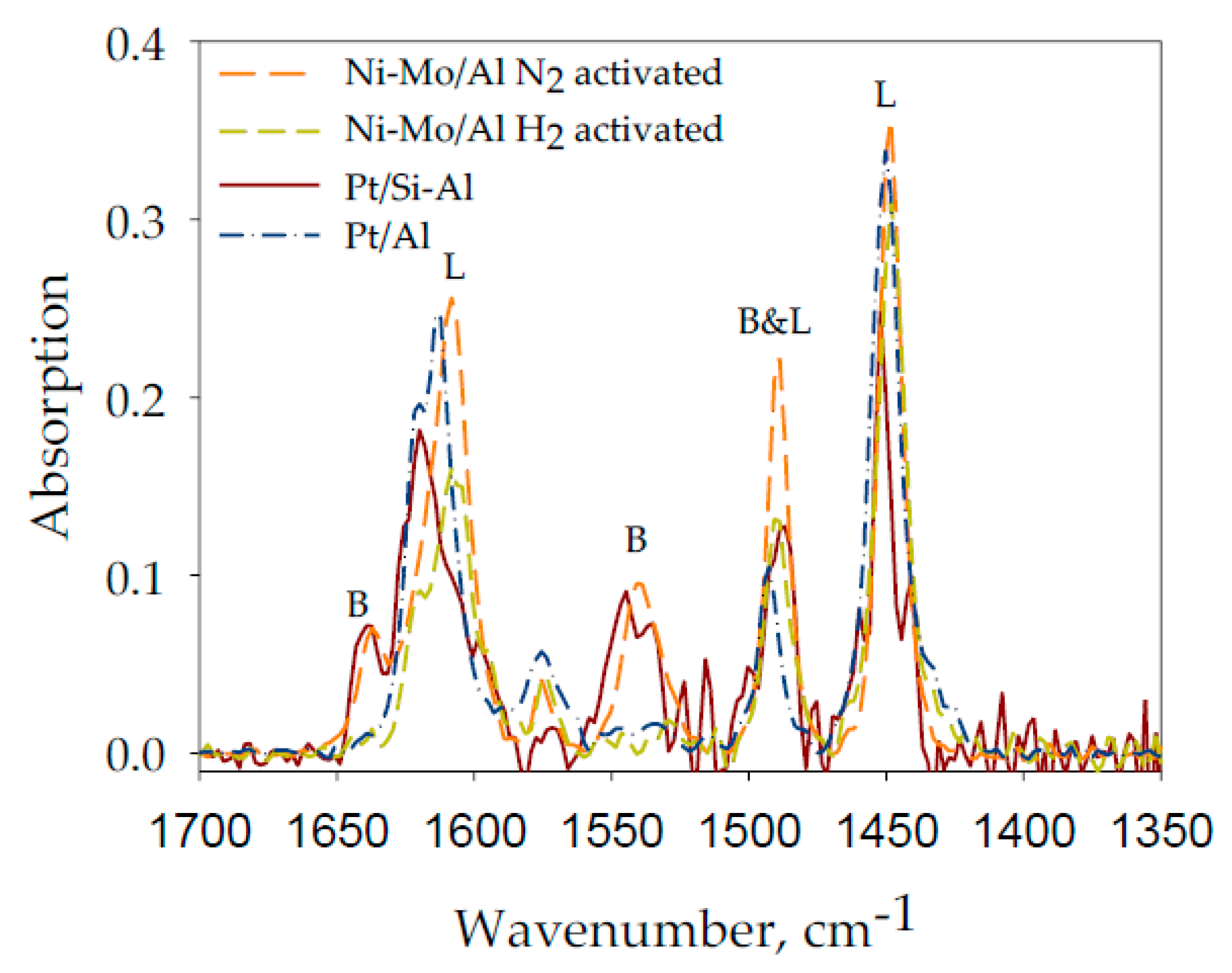
2.5. FTIR Pyridine Adsorption
2.6. Hydrocracking of C28 and Cobalt FT Wax in a Fixed-Bed Reactor
- C28 conversion = 100 × (C28 in feed − C28 in product)/C28 in feed
- C1 = 100 × C1 in product/(C28 in feed − C28 in product)
- C2–C4 = 100 × C2–C4 in product/(C28 in feed − C28 in product)
- C5–C11 = 100 × C5–C11 in product/(C28 in feed − C28 in product)
- C12–C19 = 100 × C12 in product/(C28 in feed − C28 in product)
- C20–C27 = 100 × (C20–C27 in product − C20–C27 in feed)/(C28 in feed − C28 in product)
- C20+ conversion = 100 × (C20+ in feed − C20+ in product)/C20+ in feed
- C1 = 100 × C1 in product/(C20 in feed − C20 in product)
- C2–C4 = 100 × C2–C4 in product/(C20 in feed − C20 in product)
- C5–C11 = 100 × (C5–C11 in product − C5–C11 in feed)/(C20 in feed − C20 in product)
- C12–C19 = 100 × (C12–C19 in product − C12–C19 in feed)/ (C20 in feed − C20 in product)
3. Results
3.1. Characterization Results
3.1.1. BET Measurement Results
3.1.2. X-ray Diffraction (XRD)
3.1.3. Scanning Electron Microscopy (SEM-EDX)
3.1.4. FTIR Pyridine Adsorption
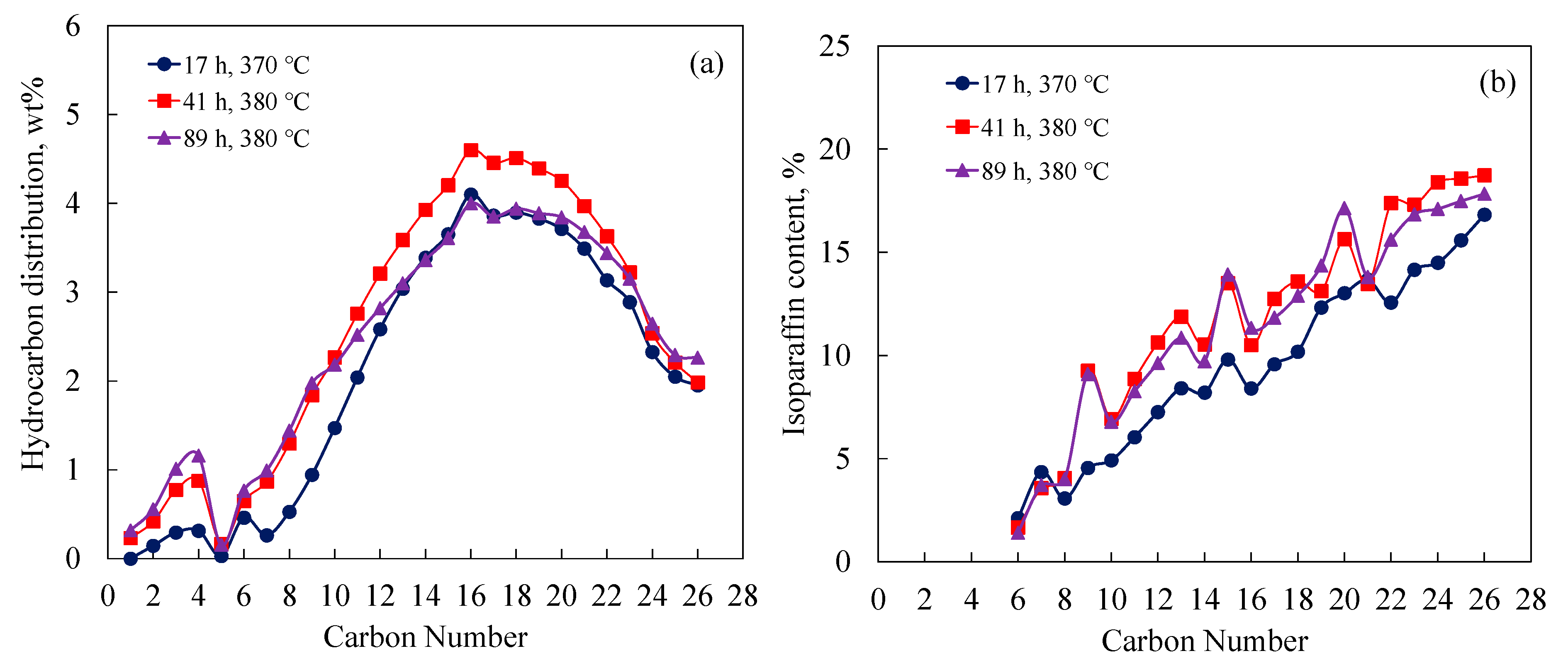
3.2. Hydrocracking C28 over Nonsulfided NiMo/Al and NiMo/Si-Al Catalysts
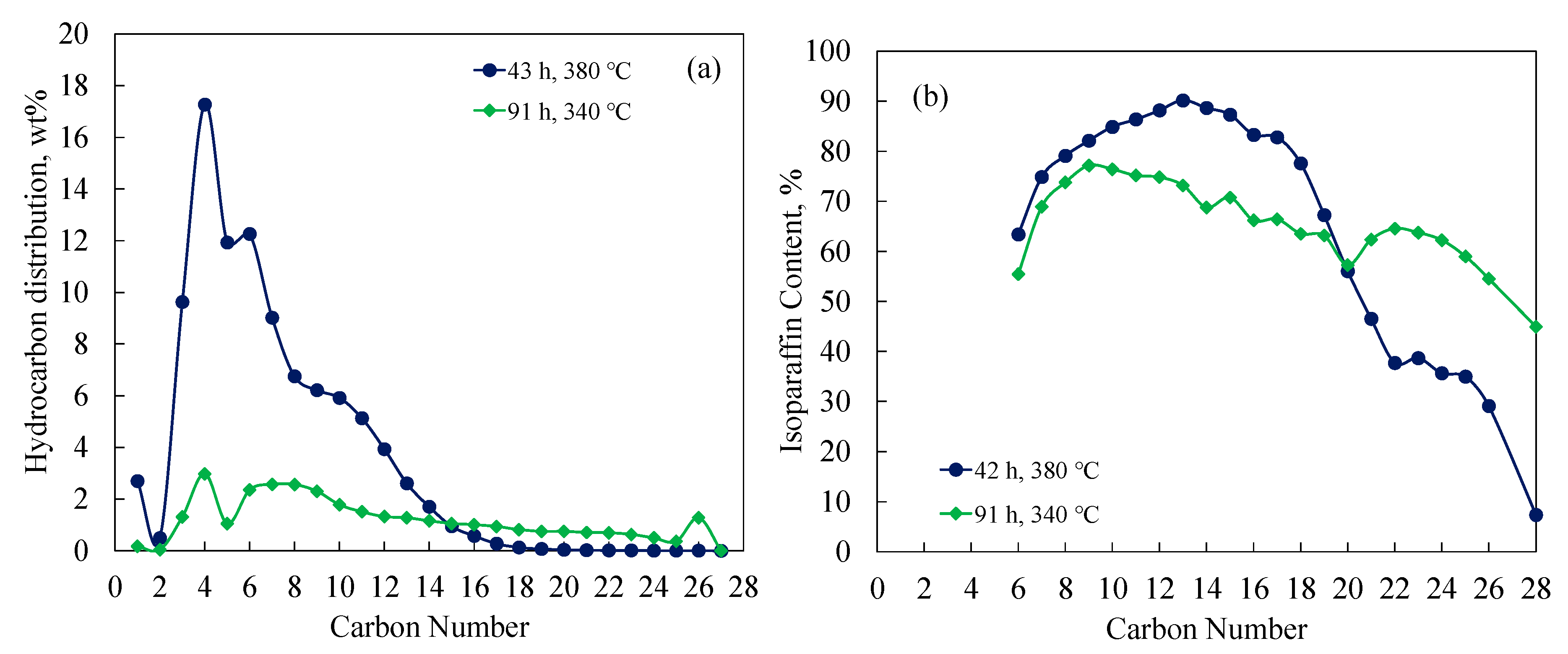
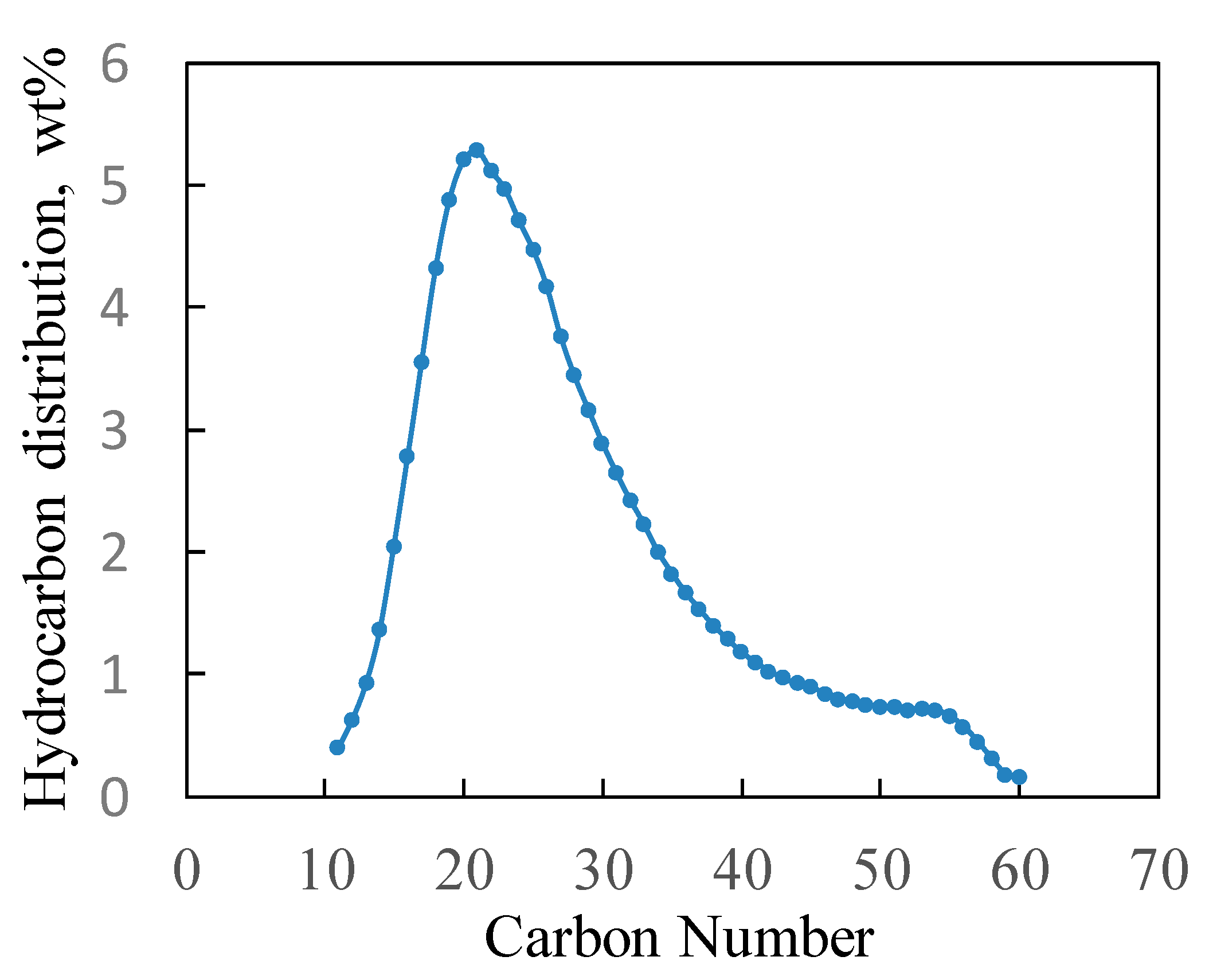
3.3. Hydrocracking FT Wax over Pt/Al and Pt/Si-Al Catalysts
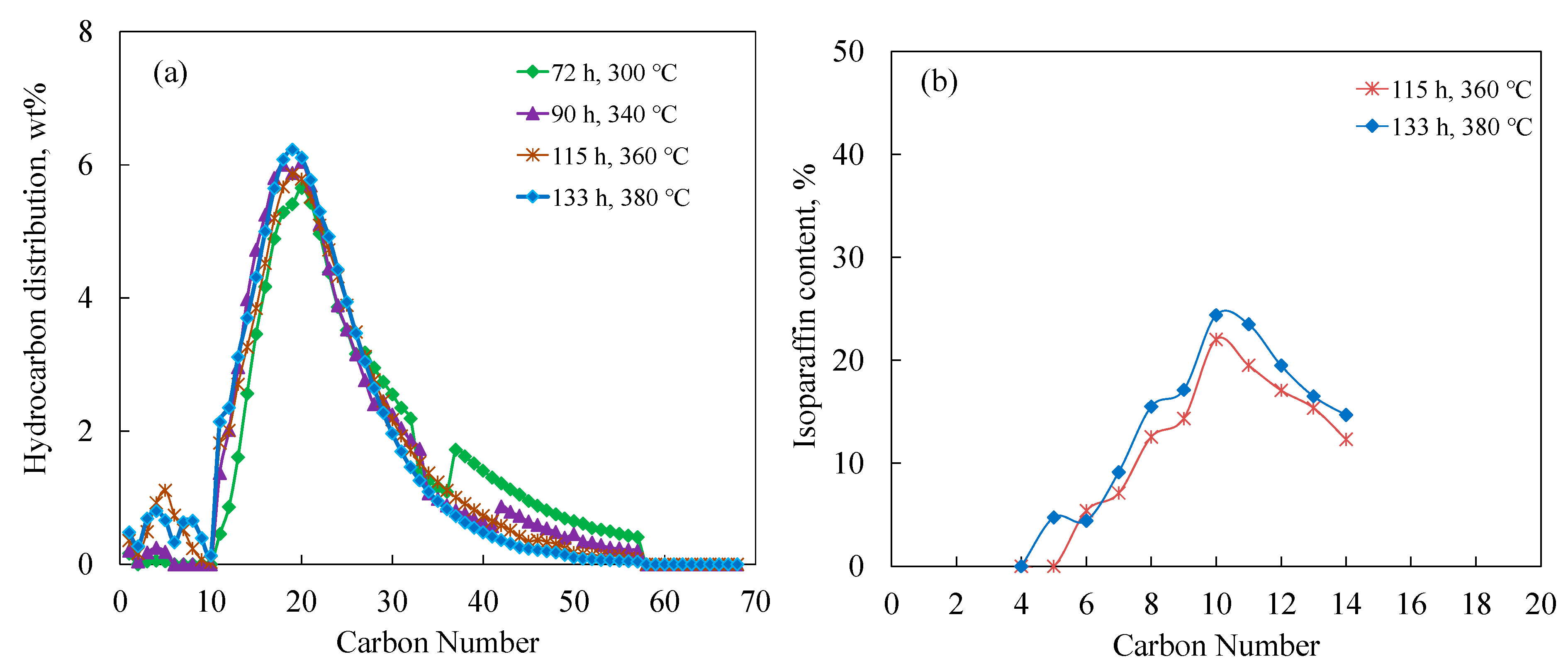
3.4. Isoparaffin Content versus Carbon Number
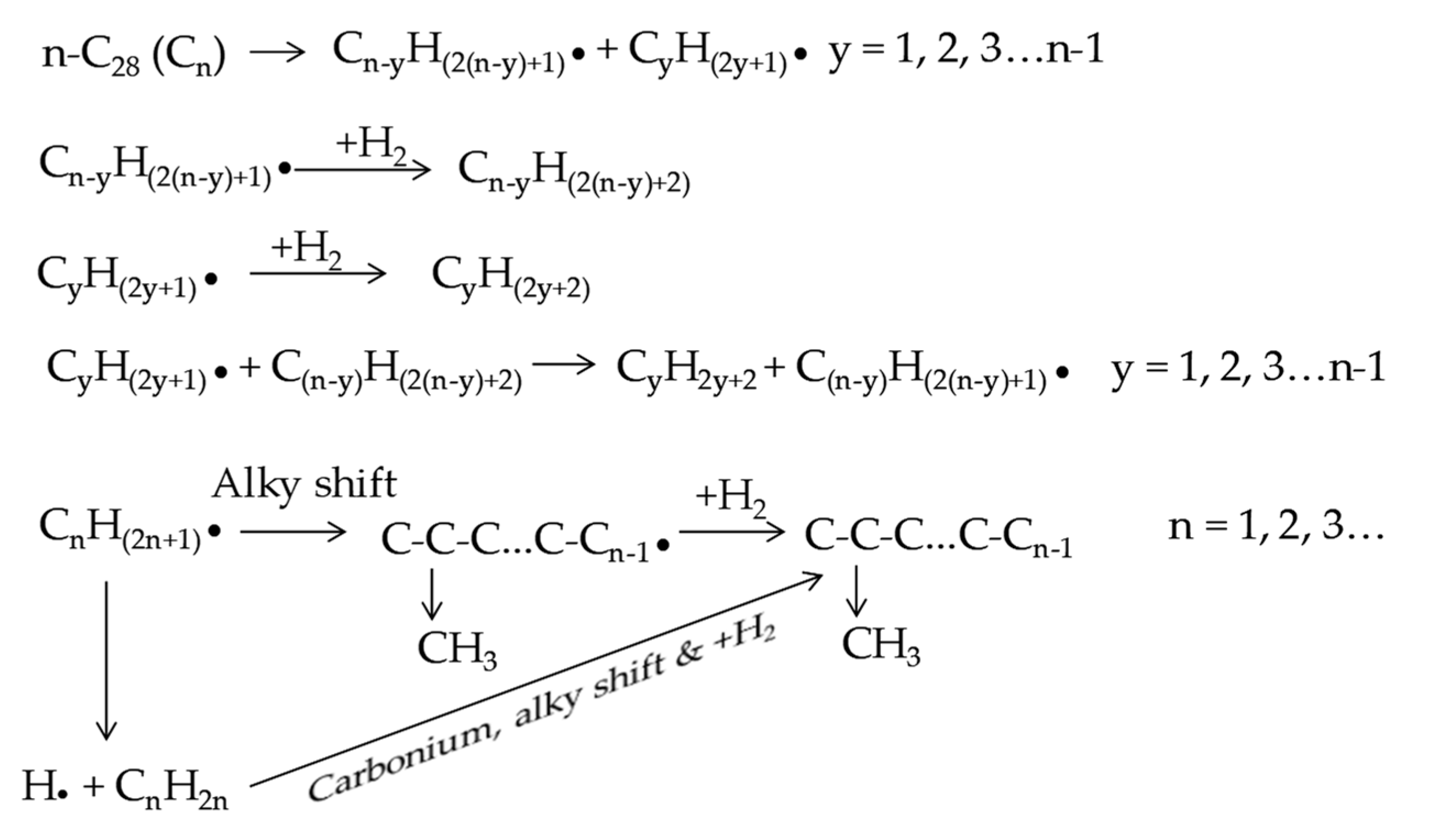
3.5. Mechanistic Considerations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hwang, S.; Lee, J.; Park, S.; Park, D.R.; Jung, J.C.; Lee, S.B.; Song, I.K. Production of Middle Distillate Through Hydrocracking of Paraffin Wax over NiMo/SiO2–Al2O3 Catalysts: Effect of SiO2–Al2O3 Composition on Acid Property and Catalytic Performance of NiMo/SiO2–Al2O3 Catalysts. Catal. Lett. 2009, 129, 163–169. [Google Scholar] [CrossRef]
- Leckel, D. Low-Pressure Hydrocracking of Coal-Derived Fischer-Tropsch Waxes to Diesel. Energy Fuels 2007, 21, 1425–1431. [Google Scholar] [CrossRef]
- Calemma, V.; Peratello, S.; Perego, C. Hydroisomerization and hydrocracking of long chain n-alkanes on Pt/amorphous SiO2–Al2O3 catalyst. Appl. Catal. 2000, 190, 207–218. [Google Scholar] [CrossRef]
- Park, K.C.; Ihm, S.K. Comparison of Pt/zeolite catalysts for n-hexadecane hydroisomerization. Appl. Catal. 2000, 203, 201–209. [Google Scholar] [CrossRef]
- Valery, E.; Guillaume, D.; Surla, K.; Galtier, P.; Verstraete, J.; Schweich, D. Kinetic Modeling of Acid Catalyzed Hydrocracking of Heavy Molecules: Application to Squalane. Ind. Eng. Chem. Res. 2007, 46, 4755–4763. [Google Scholar] [CrossRef]
- Leite, L.; Benazzi, E.; Marcheal-George, N. Hydrocracking of phenanthrene over bifunctional Pt catalysts. Catal. Today 2001, 65, 241–247. [Google Scholar] [CrossRef]
- Sato, K.; Iwata, Y.; Yoneda, T.; Nishijima, A.; Miki, Y.; Shimada, H. Hydrocracking of diphenylmethane and tetralin over bifunctional NiW sulfide catalysts supported on three kinds of zeolites. Catal. Today 1998, 45, 367–374. [Google Scholar] [CrossRef]
- Dupain, X.; Krul, R.A.; Makkee, M.; Moulijin, J.A. Are Fischer–Tropsch waxes good feedstocks for fluid catalytic cracking units? Catal. Today 2005, 106, 288–292. [Google Scholar] [CrossRef]
- Qiu, B.; Yi, X.; Lin, L.; Fang, W.; Wan, H. The hydrocracking of n-decane over bifunctional Ni-H3PW12O40/SiO2 catalysts. Catal. Today 2008, 131, 464–471. [Google Scholar] [CrossRef]
- Gamba, S.; Pellegrini, L.A.; Calemma, V.; Gambaro, C. Liquid fuels from Fischer–Tropsch wax hydrocracking: Isomer distribution. Catal. Today 2010, 156, 58–64. [Google Scholar] [CrossRef]
- Rezgui, Y.; Guemini, M. Effect of acidity and metal content on the activity and product selectivity for n-decane hydroisomerization and hydrocracking over nickel–tungsten supported on silica–alumina catalysts. Appl. Catal. 2005, 282, 45–53. [Google Scholar] [CrossRef]
- Chiranjeevi, W.T.; Gnanamani, M.G.; Gupta, J.K.; Dhar, M.G. Effect of Si/Al ratio of HMS support on catalytic functionalities of Mo, CoMo, NiMo hydrotreating catalysts. Catal. Commun. 2005, 6, 101–106. [Google Scholar] [CrossRef]
- de Haan, R.; Joorst, G.; Mokoena, E.; Nicolaides, C.P. Nonsulfided nickel supported on silicated alumina as catalyst for the hydrocracking of n-hexadecane and of iron-based Fischer-Tropsch wax. Appl. Catal. 2007, 327, 247–254. [Google Scholar] [CrossRef]
- Hanaoka, T.; Miyazawa, T.; Shimura, K.; Hirata, S. Effects of Catalyst Preparation on Hydrocarbon Product Distribution in Hydrocracking of the Fischer-Tropsch Product with Low Pt-Loaded Catalysts. Catalysts 2015, 5, 1983–2000. [Google Scholar] [CrossRef]
- Rezgui, Y.; Guemini, M. Hydroisomerization of n-octane over nickel-tungsten supported on silica-alumina catalysts. Energy & Fuels 2007, 21, 602–609. [Google Scholar] [CrossRef]
- Leckel, D. Noble Metal Wax Hydrocracking Catalysts Supported on High-Siliceous Alumina. Ind. Eng. Chem. Res. 2007, 46, 3505–3512. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Hanaoka, T.; Murata, K.; Sakanishi, K. Hydroisomerization and hydrocracking of long chain n-alkane and Fischer-Tropsch wax over bifunctional Pt-promoted Al-HMS catalysts. In Recent Progress in Mesostructured Materials Proceedings of the 5th International Mesostructured Materials Symposium (IMMS2006). Book Ser. Stud. Surf. Sci. Catal. 2007, 165, 781–785. [Google Scholar]
- Maxwell, I.E.; Minderhoud, J.K.; Stork, W.H.J.; van Veen, J.A.R. Handbook of Heterogeneous Catalysis; Ertl, G., Knözinger, H., Weitkamp, J., Eds.; Wiley-VCH: Weinheim, Germany, 1997; Volume 4, p. 2017. [Google Scholar]
- van Veen, J. Zeolites for Cleaner Technologies, Catalytic Science Series; Guisnet, M., Gilson, J.-P., Eds.; Imperial College Press: London, UK, 2002; Volume 3, p. 131. [Google Scholar]
- Weitkamp, J.; Ernst, S. Guideliness for Mastering the Properties of Molecular Sieves; Barthomeuf, D., Ed.; Plenum Press: New York, NY, USA, 1990; p. 343. [Google Scholar]
- Trombetta, M.; Busca, G.; Rossini, S.; Piccoli, V.; Cornaro, U.; Guercio, A.; Catani, R.; Willey, R.J. FT-IR Studies on Light Olefin Skeletal Isomerization Catalysis: III. Surface Acidity and Activity of Amorphous and Crystalline Catalysts Belonging to the SiO2–Al2O3System. J. Catal. 1998, 179, 581–596. [Google Scholar] [CrossRef]
- Calemma, V.; Correra, S.; Perego, C.; Pollesel, P.L. Pellegrini Hydroconversion of Fischer–Tropsch waxes: Assessment of the operating conditions effect by factorial design experiments. Catal. Today 2005, 106, 282–287. [Google Scholar] [CrossRef]
- Leckel, D.; Liwanga-Ehumbu, M. Diesel-Selective Hydrocracking of an Iron-Based Fischer-Tropsch Wax Fraction (C15-C45) Using a MoO3-Modified Noble Metal Catalyst. Energy Fuels 2006, 20, 2330–2336. [Google Scholar] [CrossRef]
- Rice, F.O.; Herzfeld, K.F. The Thermal Decomposition of Organic Compounds from the Standpoint of Free Radicals. VI. The Mechanism of Some Chain Reactions. J. Am. Chem. Soc. 1934, 56, 284. [Google Scholar] [CrossRef]
- Kossiakoff, A.; Rice, F.O. Thermal Decomposition of Hydrocarbons, Resonance Stabilization and Isomerization of Free Radicals. J. Am. Chem. Soc. 1943, 65, 590. [Google Scholar] [CrossRef]
- Thomas, C.L. Chemistry of Cracking Catalyst. Ind. Eng. Chem. 1949, 41, 2564–2573. [Google Scholar] [CrossRef]
- Greensfelder, B.S.; Voge, H.H.; Good, G.M. Catalytic cracking of pure hydrocarbons. Ind. Eng. Chem. 1949, 41, 2573–2584. [Google Scholar] [CrossRef]
- Blomsma, E.; Martens, J.A.; Jacobs, P.A. Isomerization and Hydrocracking of Heptane over Bimetallic Bifunctional PtPd/H-Beta and PtPd/USY Zeolite Catalysts. J. Catal. 1997, 165, 241–248. [Google Scholar] [CrossRef]
- Rezgui, Y.; Guemini, M. Hydroisomerization of n-decane over Ni-Pt-W supported on amorphous silica-alumina catalysts. Appl. Catal. 2010, 374, 31–40. [Google Scholar] [CrossRef]
- Fang, K.; Wei, W.; Ren, J.; Sun, Y. n-Dodecane Hydroconversion over Ni/AlMCM-41 Catalysts. Catal. Lett. 2004, 93, 235–242. [Google Scholar] [CrossRef]
- Demirel, B.; Givens, E.N. Fuel Process. Technolology 2000, 79, 1975–1980. [Google Scholar] [CrossRef]
- Zhang, H.J.; Chen, Z.Q.; Wang, S.J. Spin conversion of positronium in NiO/Al2O3 catalysts observed by coincidence Doppler broadening technique. Phys. Rev. B 2010, 82, 035439. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.H.; Zhu, J.H.; Tang, K.J.; Cheng, Y.L.; Xu, Z.Q.; Yang, W.M. Formation and Properties of 1-D Alumina Nanostructures Prepared via a Template-free Thermal Reaction. Procedia Eng. 2015, 102, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Alemán-Vázquez, L.O.; Torres-García, E.; Villagómez-Ibarra, J.R.; Cano-Domínguez, J.L. Effect of the particle size on the activity of MoOxCy catalysts for the isomerization of heptane. Catal. Lett. 2005, 100, 219–225. [Google Scholar] [CrossRef]
- Oloye, F.F. Raman spectroscopy and XRD study on molybdenum oxide supported titania. Results Mater. 2020, 5, 100064. [Google Scholar] [CrossRef]
- Li, B.; Jiang, L.; Li, X.; Ran, P.; Zuo, P.; Wang, A.D.; Qu, L.T.; Zhao, Y.; Cheng, Z.H.; Lu, Y.F. Preparation of Monolayer MoS2 Quantum Dots using Temporally Shaped Femtosecond Laser Ablation of Bulk MoS2 Targets in Water. Sci. Rep. 2017, 7, 11182. [Google Scholar] [CrossRef]
- Han, Y.; Wen, B.; Zhu, M.Y. Core-Shell Structured Ni@SiO2 Catalysts Exhibiting Excellent Catalytic Performance for Syngas Methanation Reactions. Catalysts 2017, 7, 21. [Google Scholar] [CrossRef]
- Keshavarzaand, A.; Salabat, A. Effect of HCl on the structure and catalytic activity of Pt/Al2O3 nanocatalyst prepared in microemulsion system. Sci. Iran. 2019, 26, 1925–1930. [Google Scholar] [CrossRef] [Green Version]
- Chandraboss, V.L.; Kamalakkannan, J.; Senthilvelan, S. Synthesis of AC-Bi@SiO2 Nanocomposite Sphere for Superior Photocatalytic Activity Towards the Photodegradation of Malachite Green. Canad. Chem. Trans. 2015, 3, 410–429. [Google Scholar] [CrossRef]
- Puello-Polo, E.; Marquez, E.; Brito, J.L. One-pot synthesis of Nb-modified Al2O3 support for NiMo hydrodesulfurization catalysts. J. Sol-Gel Sci. Tech. 2018, 88, 90–99. [Google Scholar] [CrossRef]
- Pimerzin, A.; Savinov, A.; Vutolkina, A.; Makova, A.; Glotov, A.; Vinokurov, V.; Pimerzin, A. Transition Metal Sulfides- and Noble Metal-Based Catalysts for N-Hexadecane Hydroisomerization: A Study of Poisons Tolerance. Catalysts 2020, 10, 594. [Google Scholar] [CrossRef]
- Kang, J.; Ma, W.; Keogh, R.A.; Shafer, W.D.; Jacobs, G.; Davis, B.H. Hydrocracking and hydroisomerization of n-hexadecane, n-octacosane and Fischer-Tropsch wax over a Pt/SiO2-Al2O3 catalyst. Catal. Lett. 2012, 142, 1295–1305. [Google Scholar] [CrossRef]
- Calemma, V.; Peratello, S.; Stroppa, F.; Giardano, R.; Perego, C. Hydrocracking and Hydroisomerization of Long-Chain n-Paraffins. Reactivity and Reaction Pathway for Base Oil Formation. Ind. Eng. Chem. Res. 2004, 43, 934–940. [Google Scholar] [CrossRef]
- Rossetti, I.; Gambaro, C.; Calemm, V. Hydrocracking of long chain linears paraffins. Chem. Eng. J. 2009, 154, 295–301. [Google Scholar] [CrossRef]
- Rausch, P.; Jess, A.; Kern, C.; Korth, W.; Kraft, N. Hydrocracking of Fischer-Tropsch Wax with Tungstovanadophosphoric Salts as Catalysts. Chem. Eng. Tech. 2018, 41, 469–478. [Google Scholar] [CrossRef]
- Li, B.; Calemma, V.; Gambaro, C.; Baron, G.V.; Denayer, J.F.M. Competitive Adsorption of C20−C36 Linear Paraffins on the Amorphous Microporous Silica−Alumina ERS-8 in Vapor Phase and Liquid Phase. Ind. Eng. Chem. Res. 2010, 49, 7541–7549. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Farrauto, R.J. Fundamentals of Industrial Catalytic Processes, 2nd ed.; Wiley-Interscience: New York, NY, USA, 2005; Chapter 9. [Google Scholar]






| Description | N2 Physisorption | ||
|---|---|---|---|
| BET Surf. Area | Pore Volume | Average Pore | |
| m2/g | cm3/g | Diameter, nm | |
| Catalox-150 γ-Al2O3 (Al) | 150 | 0.5 | 10.5 |
| SiO2-Al2O3 (Si-Al) | 395 | 0.66 | 7.0 |
| 0.5%Pt/Al | 145 | 0.42 | 9.6 |
| 0.5%Pt/Si-Al | 358 | 0.60 | 6.5 |
| 2.5%Ni12.9%Mo/Al (a) | 192 | 0.40 | 7.6 |
| 3%Ni12%Mo/Si-Al | 289 | 0.52 | 6.8 |
| Catalyst | NiMo/Al | NiMo/Si-Al | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Activation | N2 | N2 | N2 | H2S | H2S | H2 | H2 | H2 | H2 |
| Time on stream, h | 17 | 41 | 89 | 17 | 41 | 40 | 43 | 91 | 145 |
| Temp. °C | 370 | 380 | 380 | 380 | 380 | 380 | 380 | 340 | 320 |
| C28 conversion = 100 × (C28 in feed − C28 in product)/C28 in feed | |||||||||
| C28 conversion, % | 78.1 | 75.0 | 68.1 | 3.1 | 5.5 | 6.1 | 99.1 | 29.9 | 14.4 |
| Product distribution, wt% | |||||||||
| C1 | 0.1 | 0.2 | 0.3 | 0.0 | 0.0 | 0.0 | 2.7 | 0.2 | 0.0 |
| C2–C4 | 0.7 | 2.1 | 2.7 | 0.1 | 0.2 | 0.6 | 27.4 | 4.3 | 0.9 |
| C5–C11 | 5.7 | 9.8 | 10.0 | 1.2 | 1.3 | 0.6 | 57.2 | 14.1 | 2.4 |
| C12–C19 | 28.8 | 37.6 | 28.6 | 1.5 | 1.4 | 1.1 | 10.3 | 8.3 | 2.4 |
| C20+ | 64.7 | 50.2 | 58.3 | 97.2 | 97.1 | 97.8 | 2.4 | 73.0 | 94.3 |
| Selectivity, % | |||||||||
| C1–C4 | 1.4 | 3.1 | 4.6 | 1.7 | 3.9 | 15.2 | 30.8 | 14.1 | 11.2 |
| C5–C11 | 10.5 | 13.2 | 15.0 | 24.9 | 28.6 | 16.1 | 58.6 | 44.3 | 31.1 |
| C12–C19 | 52.5 | 50.4 | 42.9 | 32.2 | 31.7 | 30.0 | 10.5 | 26.1 | 31.5 |
| C20–C27 | 35.6 | 33.3 | 37.5 | 41.2 | 35.8 | 38.8 | 0.1 | 15.5 | 26.2 |
| Catalyst | Pt/Al | Pt/Si-Al | |||||
|---|---|---|---|---|---|---|---|
| Activation | H2 | ||||||
| Time on stream, h | 72 | 90 | 115 | 133 | 94 | 118 | 140 |
| Temp. °C | 320 | 340 | 360 | 380 | 280 | 290 | 300 |
| C20+ conversion = 100 × (C20+ in feed − C20+ in product)/C20+ in feed | |||||||
| C20+ conversion, % | 10.97 | 25.45 | 42.89 | 32.73 | 39.43 | 82.74 | 99.85 |
| Product distribution, wt% | |||||||
| C1 | 0.16 | 0.21 | 0.34 | 0.48 | 0.02 | 0.03 | 0.03 |
| C2–C4 | 0.10 | 0.47 | 1.29 | 1.76 | 1.45 | 4.23 | 4.71 |
| C5–C11 | 0.50 | 1.56 | 3.59 | 4.94 | 2.95 | 32.64 | 63.76 |
| C12–C19 | 28.24 | 36.61 | 46.43 | 36.42 | 47.61 | 49.73 | 31.38 |
| C20+ | 71.00 | 61.15 | 48.34 | 56.39 | 47.96 | 13.36 | 0.12 |
| Selectivity, % | |||||||
| C1–C4 | 1.89 | 1.17 | 1.14 | 2.16 | 0.08 | 0.05 | 0.03 |
| C5–C11 | 1.18 | 2.65 | 4.28 | 7.95 | 4.66 | 6.38 | 5.92 |
| C12–C19 | 4.54 | 8.10 | 11.59 | 21.78 | 8.22 | 48.69 | 79.94 |
| C20–C27 | 92.40 | 88.07 | 82.98 | 68.11 | 87.05 | 44.87 | 14.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Kang, J.; Jacobs, G.; Hopps, S.D.; Davis, B.H. Hydrocracking of Octacosane and Cobalt Fischer–Tropsch Wax over Nonsulfided NiMo and Pt-Based Catalysts. Reactions 2021, 2, 374-390. https://doi.org/10.3390/reactions2040024
Ma W, Kang J, Jacobs G, Hopps SD, Davis BH. Hydrocracking of Octacosane and Cobalt Fischer–Tropsch Wax over Nonsulfided NiMo and Pt-Based Catalysts. Reactions. 2021; 2(4):374-390. https://doi.org/10.3390/reactions2040024
Chicago/Turabian StyleMa, Wenping, Jungshik Kang, Gary Jacobs, Shelley D. Hopps, and Burtron H. Davis. 2021. "Hydrocracking of Octacosane and Cobalt Fischer–Tropsch Wax over Nonsulfided NiMo and Pt-Based Catalysts" Reactions 2, no. 4: 374-390. https://doi.org/10.3390/reactions2040024
APA StyleMa, W., Kang, J., Jacobs, G., Hopps, S. D., & Davis, B. H. (2021). Hydrocracking of Octacosane and Cobalt Fischer–Tropsch Wax over Nonsulfided NiMo and Pt-Based Catalysts. Reactions, 2(4), 374-390. https://doi.org/10.3390/reactions2040024






