Improving the Efficiency of Essential Oil Distillation via Recurrent Water and Steam Distillation: Application of a 500-L Prototype Distillation Machine and Different Raw Material Packing Grids
Abstract
1. Introduction
2. Related Principles and Theories
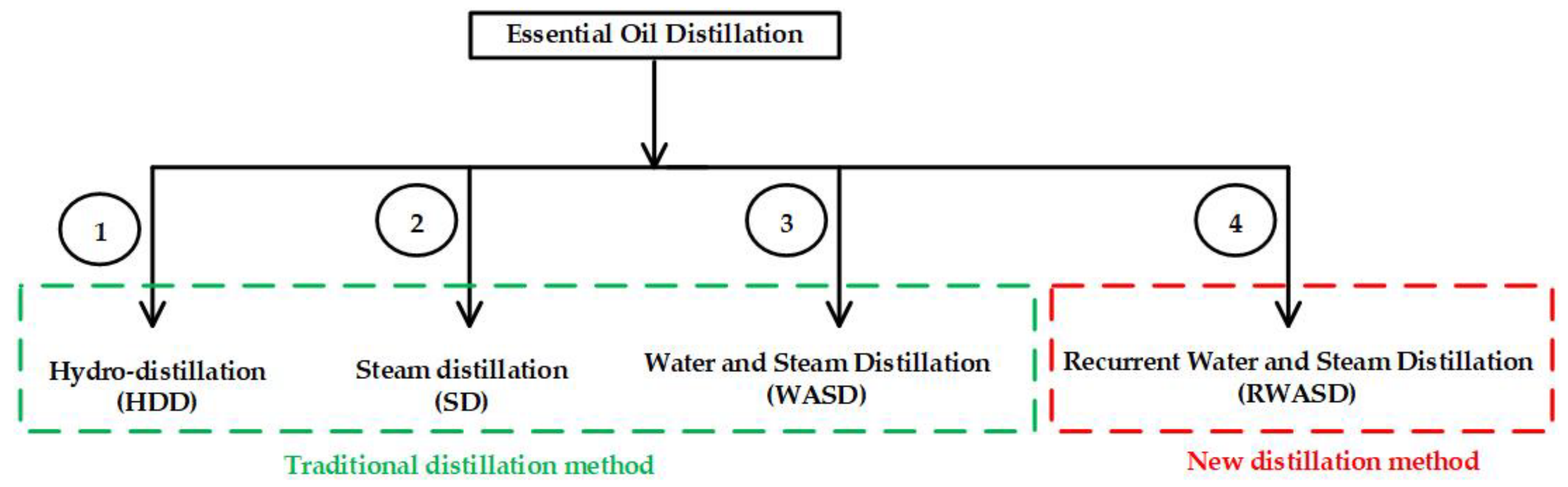
2.1. Extraction of Essential Oils by Distillation
- Hydro-distillation (HDD)
- 2.
- Steam distillation (SD)
- 3.
- Water and steam distillation (WASD)
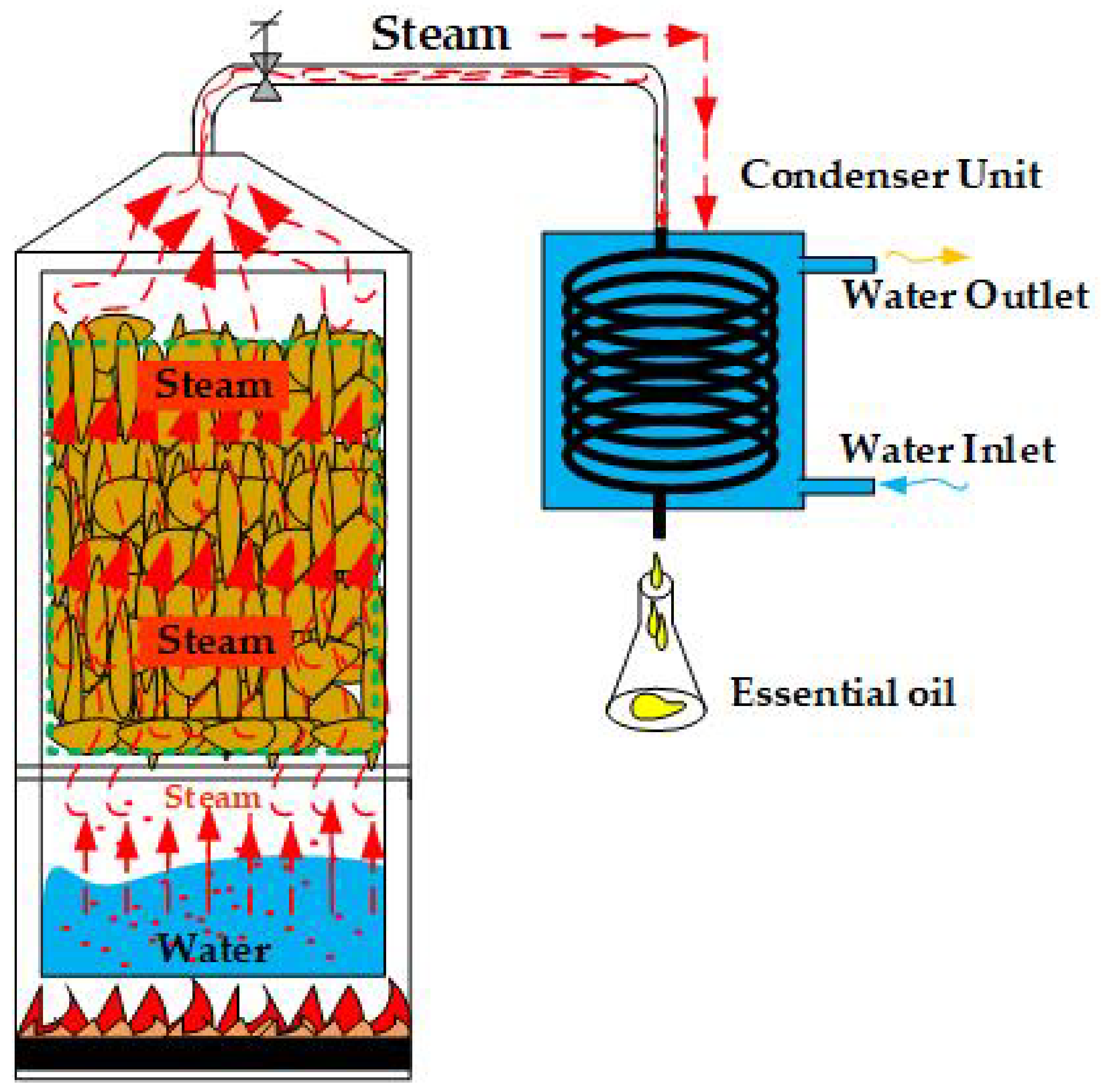
2.2. Recurrent Water and Steam Distillation (RWASD)
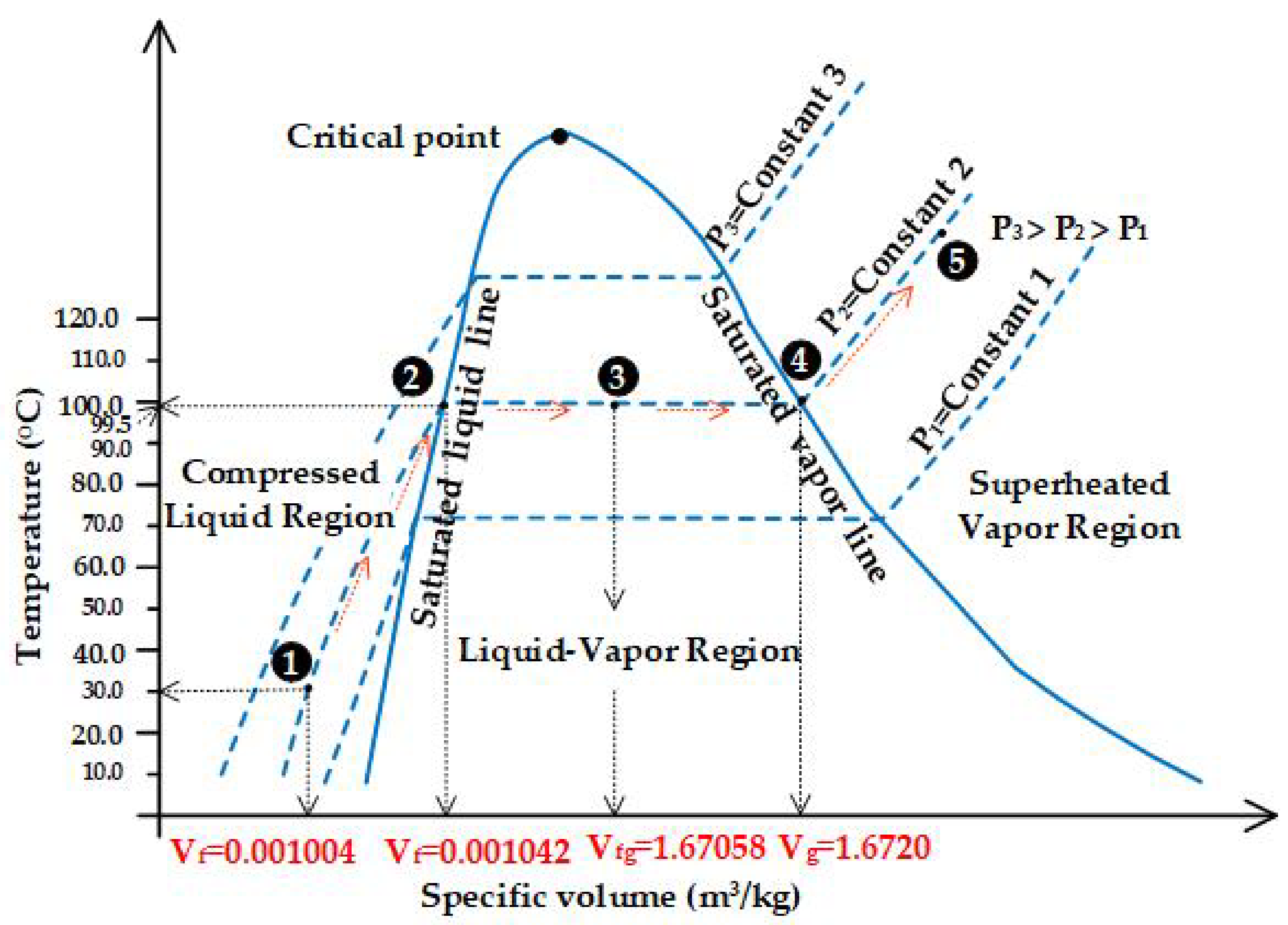
2.3. Status and Properties of Steam Used in Recurrent Distillation with Water and Steam
| Types of Steam | Characteristics of Steam/ Heat Transfer | Temperature of Steam |
|---|---|---|
| Saturated steam | - Saturated steam that still contains moisture, sometimes called wet steam, forms when water begins to boil and turns into steam. The amount of heat in saturated steam will have a greater or lesser value depending on the pressure. Steam with low pressure has more energy to transfer heat (latent heat). - When heat is transferred, the temperature does not decrease. The amount of heat transferred is equal to the latent heat of vaporization. This is heat transfer by condensation into a condensate with the same temperature and pressure as saturated steam. - Saturated steam will immediately become superheated steam when the pressure is lowered. | 99.15 °C ≤ Saturated steam ≤ 100 °C |
| Superheated steam | - Superheated steam occurs when saturated steam is further heated at constant pressure until it reaches a temperature higher than the boiling temperature of water at that pressure. - When superheated steam transfers heat, the temperature drops to the saturation point. The heat is transferred from the gas, so there is no condensation of water. - Saturated steam becomes superheated steam when the pressure is lowered. | Above 99.15 °C, but not exceeding 1100 °C |
2.4. The Amount of Essential Oil Obtained by Recurrent Distillation with Water and Steam (RWASD)
2.5. GC-MS Analysis of Volatile Substances
2.6. Design and Manufacturing of Distillers
| Details | Equation |
|---|---|
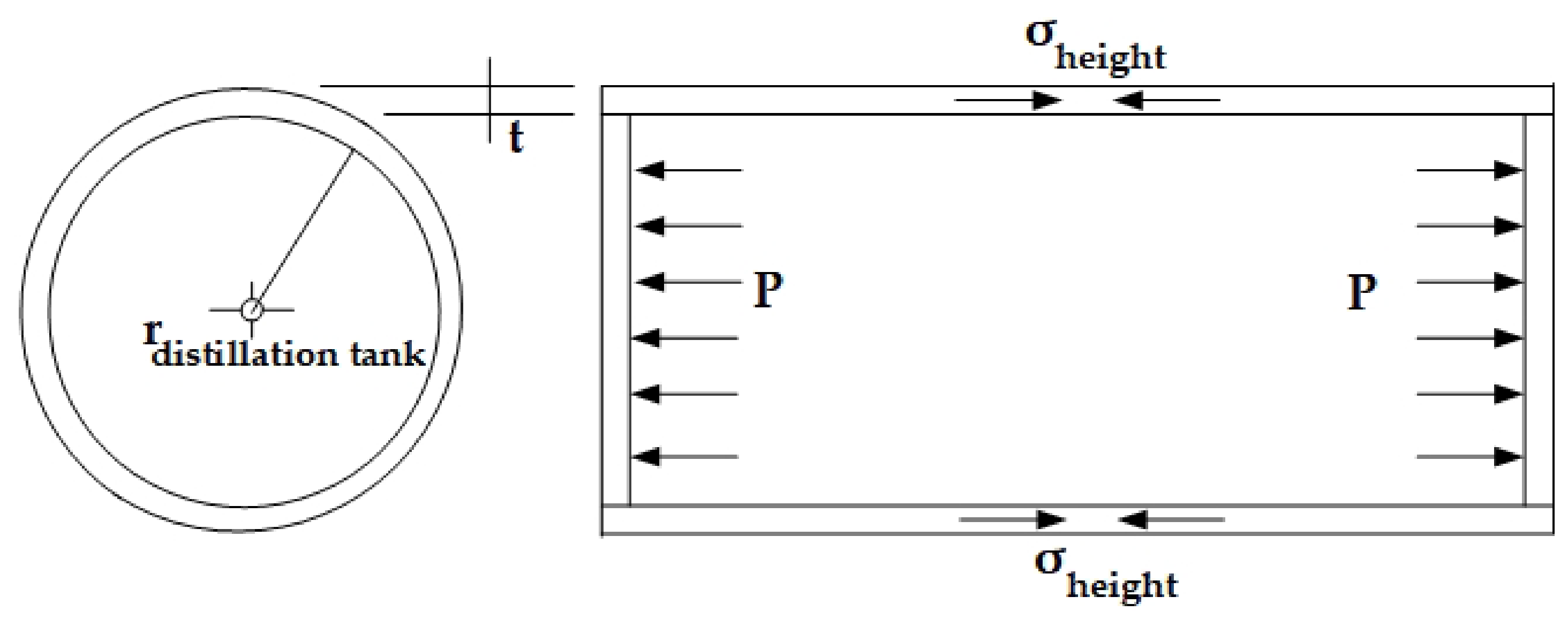
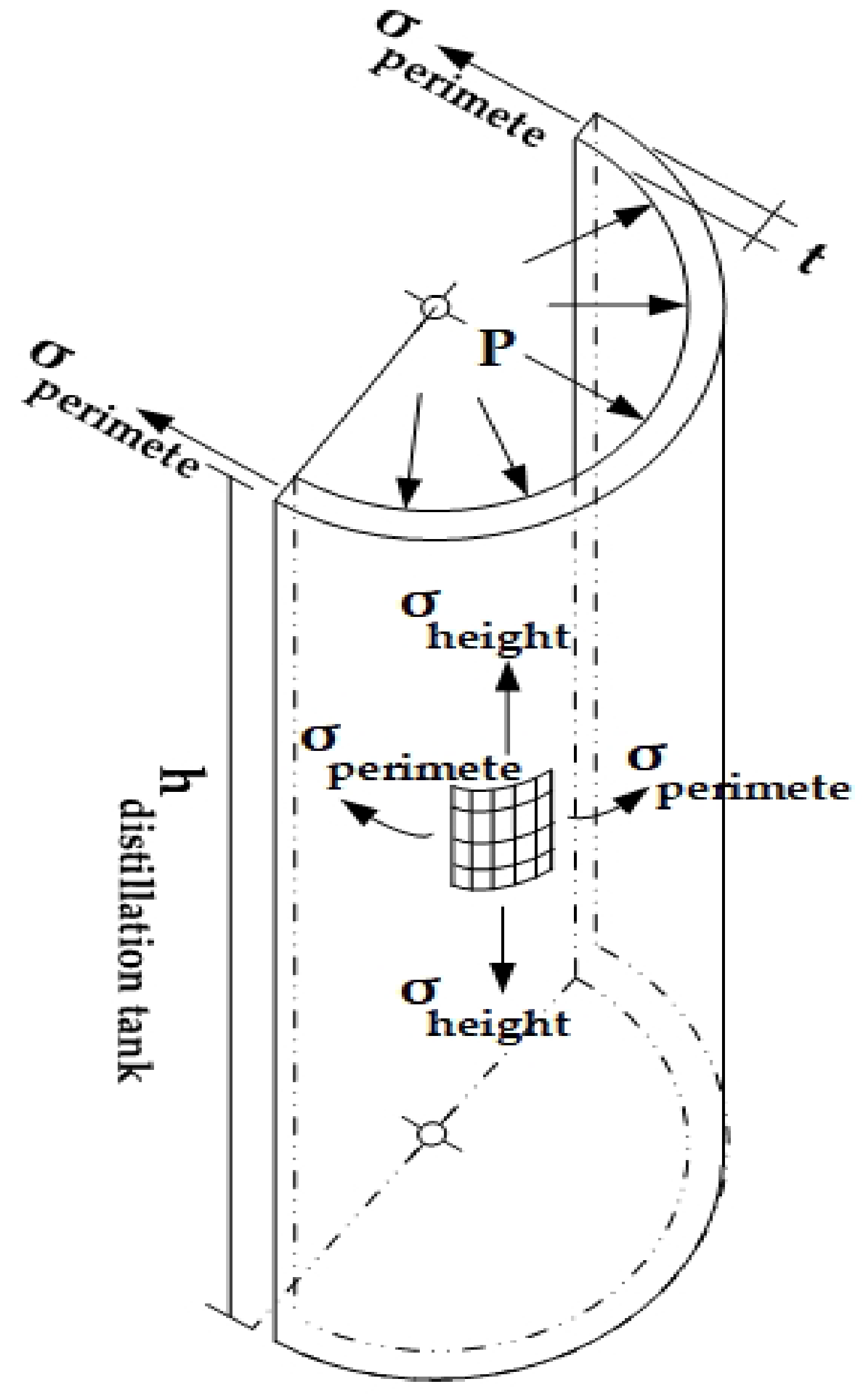
| For the design of the distillation tank volume (Vdistillation tank, m3), this section typically addresses applications involving pressure capacity under specified design conditions. The volume is calculated using Equation (5). | hdistillation tank is the height of the holding tank (m), as illustrated in Figure 7 and Figure 8, respectively. |
| The design and construction of the distillation tank take into account the internal pressure (P) generated during operation. This pressure can be determined using Equation (6). | ρ is the liquid density (Kg/m3). g is the gravitational force (m/s2). h is the liquid height (m). |
| Another significant force acting on the pipe wall arises from the internal stress within the tank, which induces tension along both the circumferential and longitudinal directions. These stresses are calculated using Equations (7) and (8). | denotes the longitudinal stress (N). L is the length measured along the tank wall (m). tdistillation tank is the wall thickness of the distillation tank (m). rdistillation tank is the radius of the distillation tank (m). σheight refers to the axial stress acting along the vertical of the tank (MPa). P represents the internal pressure within the designed tank (Pa), as illustrated in Figure 7 and Figure 8, respectively. |
| The determination of the thicknesses of the distillation tank and its cap is based on the specified design pressure. In this section, the required thicknesses for both the tank and the cap are calculated using the formulas presented in Equations (9) and (10), respectively. | |
| For the cooling system, the design process involved determining the volumes of both the condensation tank and the internal coiled pipe (Vcondensation tank and Vcoiled tube, in m3), which were calculated using Equations (11) and (12). | |
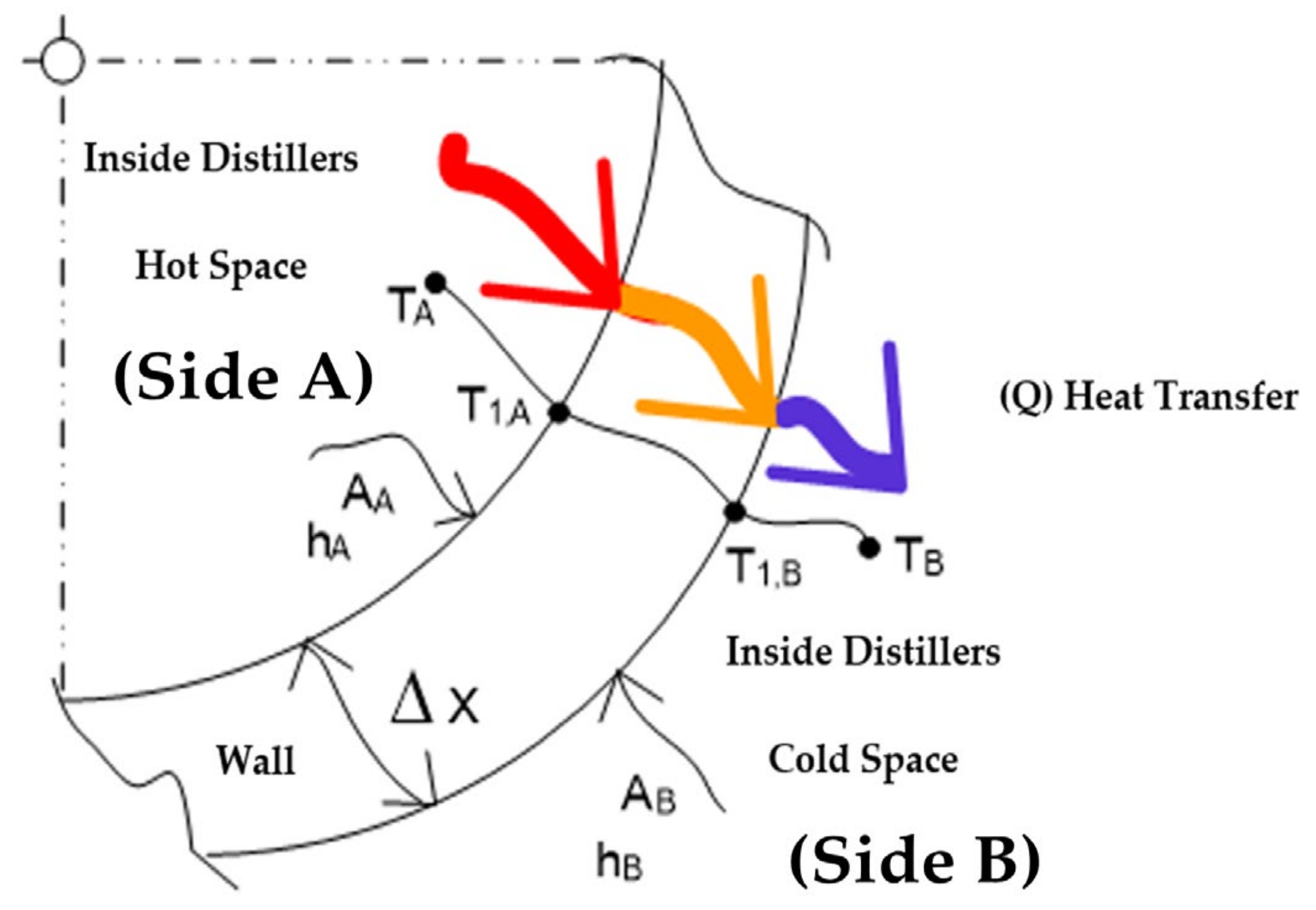
| The heat transfer of thermal conductivity of distiller (Qcond, distiller, Qcond), (W). It is calculated from Equation (13). |
Qcond, distiller = Qcond = −kAcond(dT/dx)
Figure 9. |
| The heat transfer by convection from the outer surface of the distillation tank wall to the surrounding environment, denoted as Qcond, disstiller, Qconv distiller, or Qconv (W), is calculated using Equation (14) |
Qconv, distiller = Qconv = hconvAconv(Ts − Tf)
|
| The heat transfer in the condensing units (Qconden, Qc), (W) is calculated from Equation (15). |
Qconden = Qc = As UΔTm
It may also be further categorized as follows: , (°C) is the temperature differencebetween the hot essential oil inlet and cold-water outlet as shown in Figure 9; , (°C) is the temperature difference between the hot essential oil outlet and cold water inlet as shown in Figure 9; the overall heat transfer coefficient, U; U, can be determined from the following component expressions: , is the total heat transfer coefficient (W/m2 °C). , The thermal resistance of the tube is negligible (Rwall = 0). Furthermore, Ai ≈ Ao ≈ As denotes the inner and outer surface of the heat transfer coiled tube (m2), and hi and ho are determined using the heat convection coefficients of the inner and outer fluids (W/m2 °C), as shown in Figure 8 and Figure 9. |
2.7. Energy and Exergy Analysis of Recurrent Water and Steam Distillation (RWASD) Process
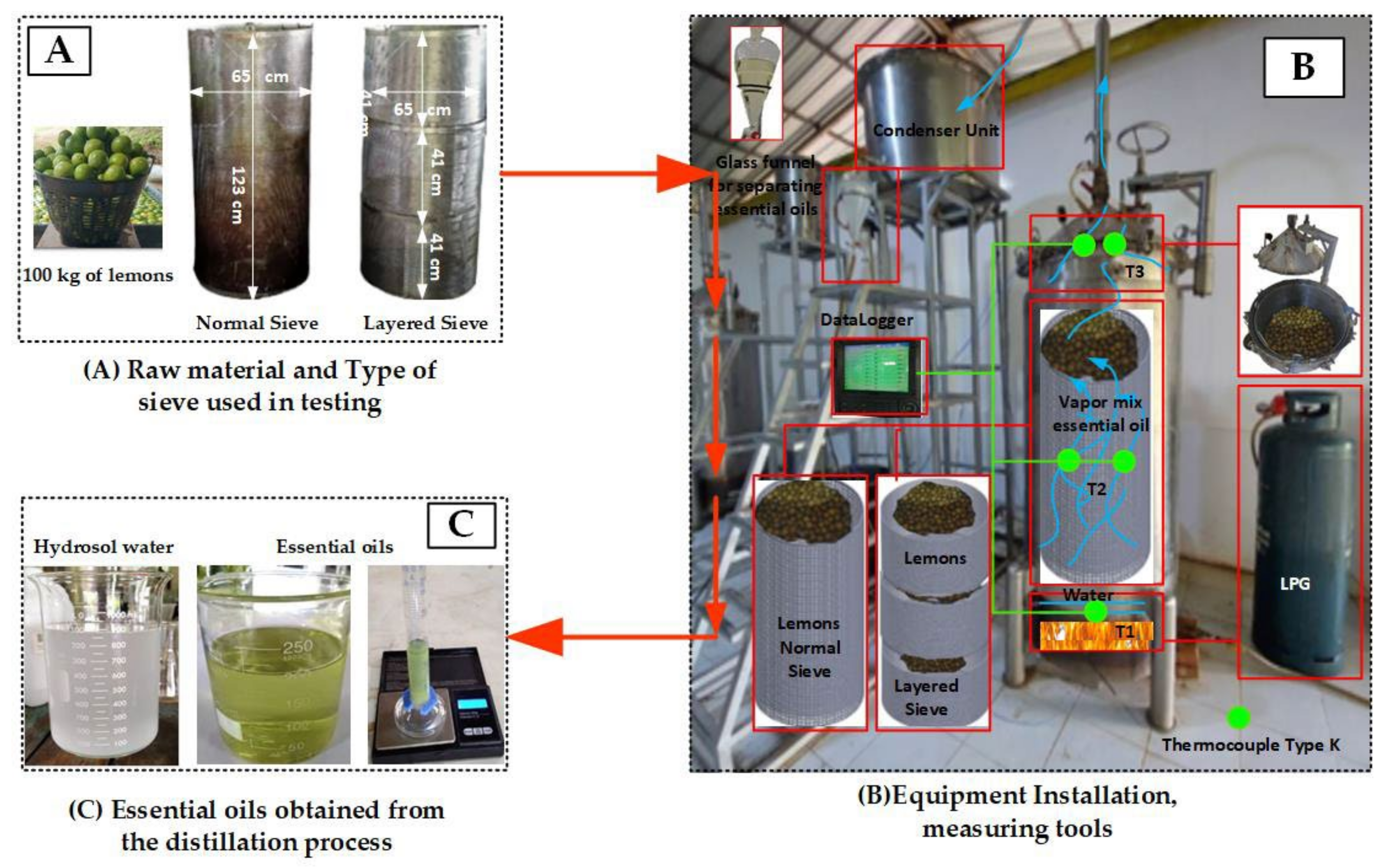
3. Test Procedure
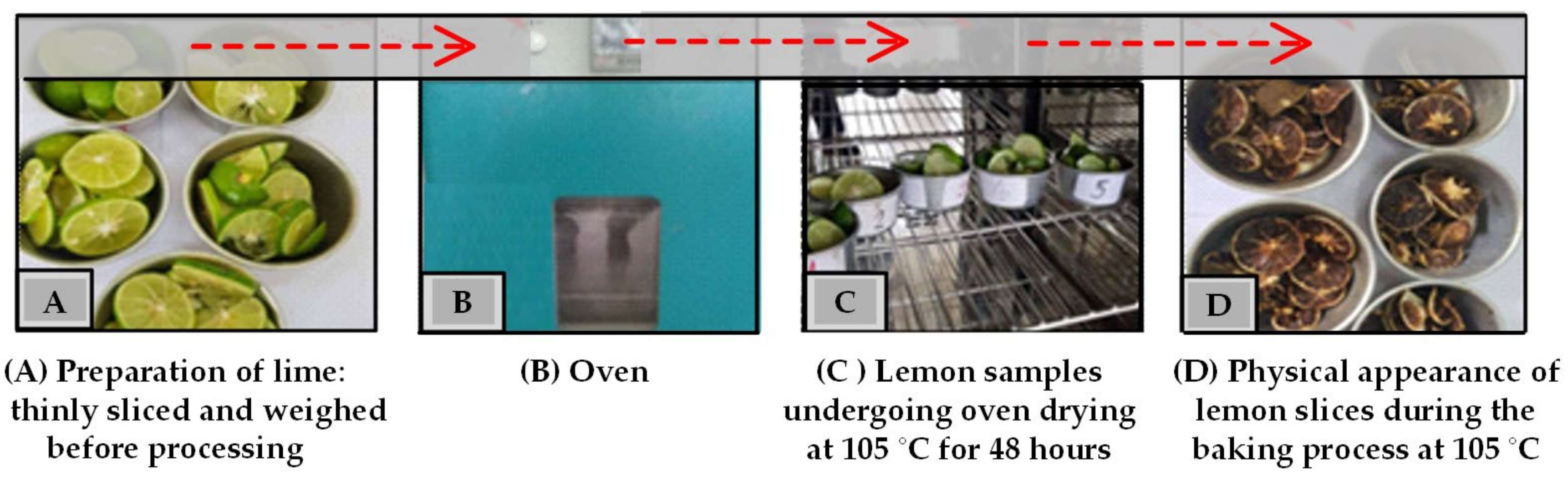
- A hundred kilograms of fresh lime fruit was thoroughly washed, as shown in Figure 12A.
- Each of the washed samples was then placed into a specific sieve according to the test conditions, as shown in Figure 12A.
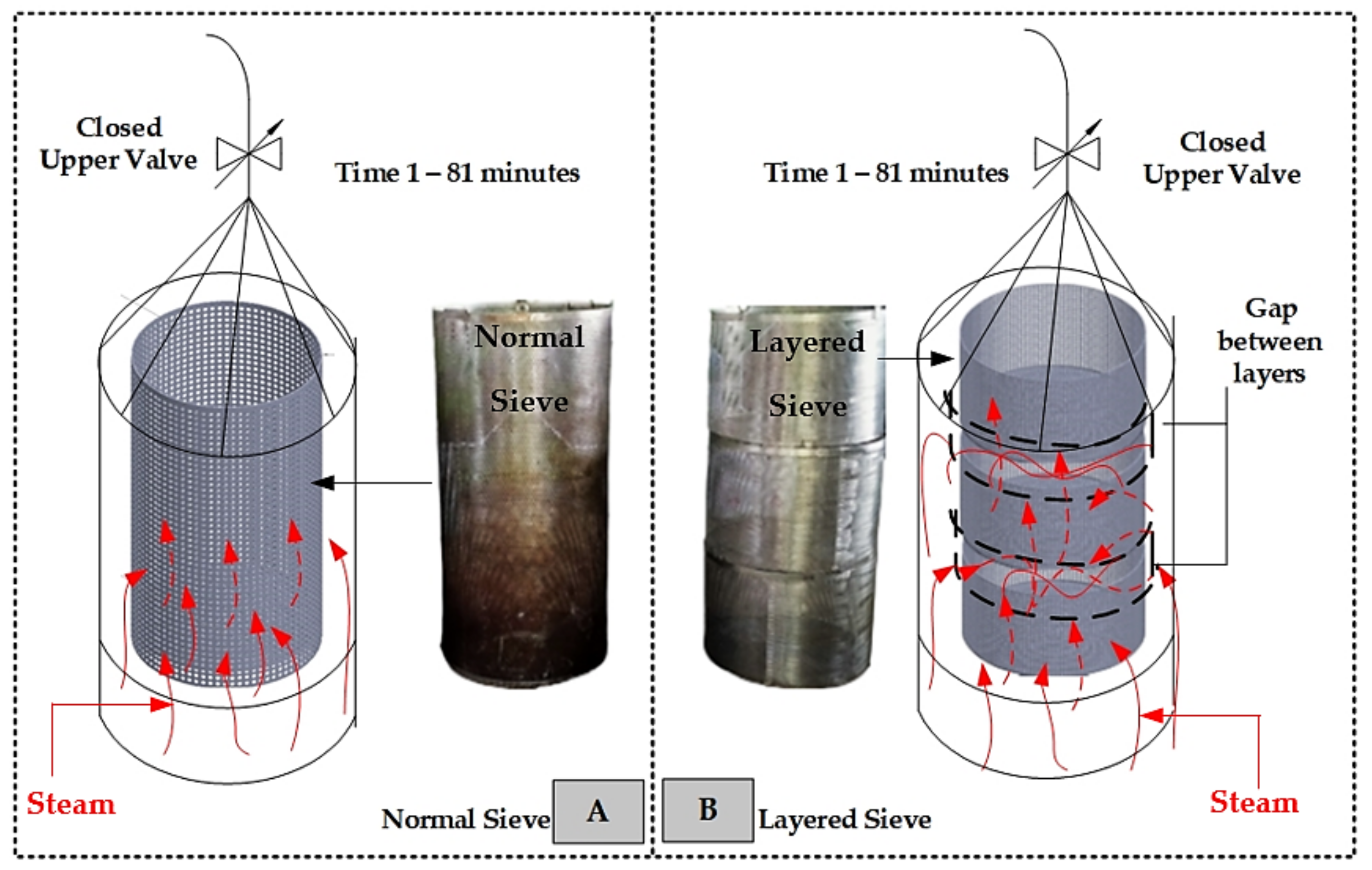
- The sieves filled with the samples were then hung and lowered into the distillation pot. For conventional sieves and layer sieves made of 304 stainless steel sieve sheets with a thickness of 1 mm, the sieves are porous sheets with a hole diameter of 3 mm and a thickness of 1 mm, with a total volume of 0.084 m3 and a total volume of 0.084 m3 in the section of the laminated sieve with a total volume of 0.084 m3 divided into three layers with the layers having a volume of 0.028 m3, as shown in Figure 12A.
- The thermocouple cables and measuring instruments related to the test were installed accordingly. As specified in Figure 12B, the lid of the distillation pot and the upper valve on top of the lid of the distillation pot were closed. The steam temperature distribution measurement points in the distillation tank and condenser were as follows: T1 was the temperature of the water in the combustion chamber, T2 was the temperature of the steam dissipated in the middle of the distillation tank, and T3 was the temperature of the steam dissipated at the top of the distillation tank. The steam inlet and outlet of the condensing unit were at positions T4 and T5, respectively.
- The temperatures in different areas were recorded using a data logging device.
- The data were analyzed, and the variables were adjusted according to the experimental conditions.
- Each experiment was conducted a minimum of three times. The mean value was calculated by averaging all recorded data points, and a 95% confidence interval was subsequently determined to ensure the statistical reliability of the results.
4. Results and Discussion
- The effect of recurrent water and steam distillation on the amount of essential oil;
- The effects of time and steam heating temperature on the recurrent water and steam distillation method;
- Determination of the quality of essential oils obtained through recurrent water and steam distillation via GC-MS analysis;
- Useful energy efficiency and exergy of essential oil distillation via recurrent water and steam distillation method;
- The effects of different raw material packing screens on the yield of essential oil;
- Analysis of the design and construction of a 500 L prototype distillation apparatus.
- Essential oil distillation cost.
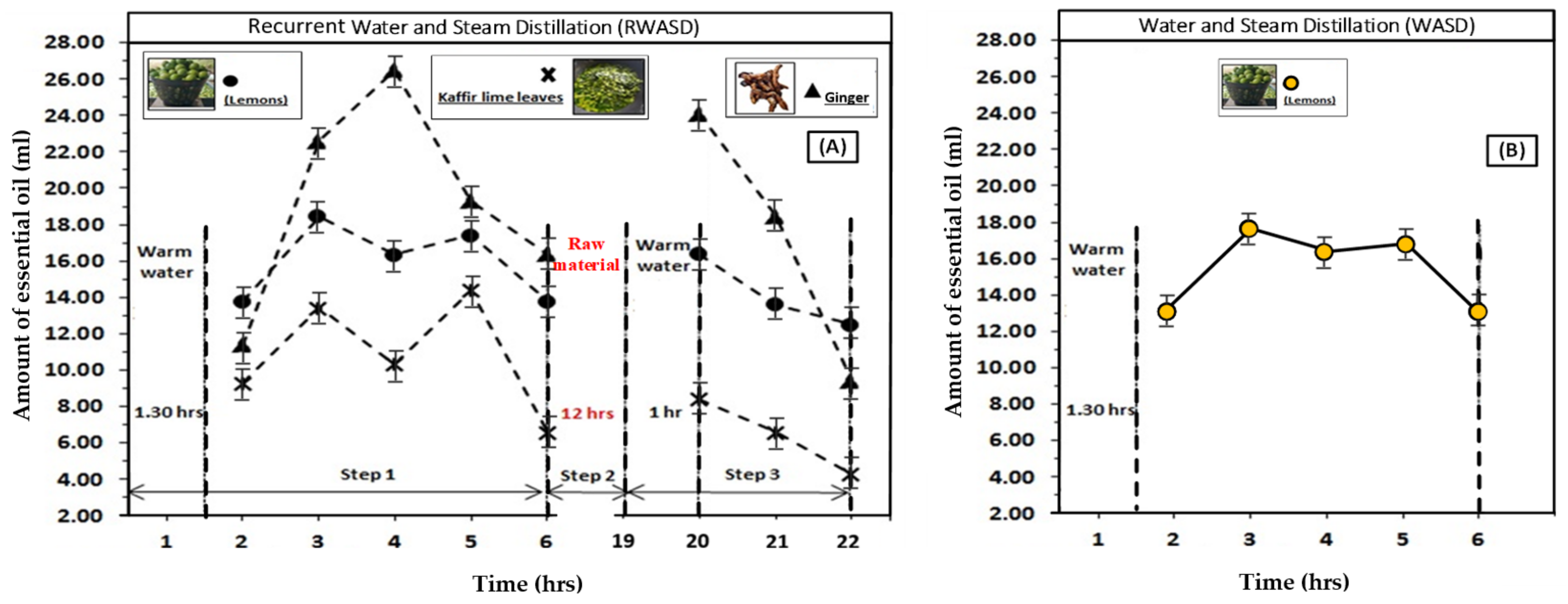
4.1. The Effect of Recurrent Water and Steam Distillation on the Amount of Essential Oil
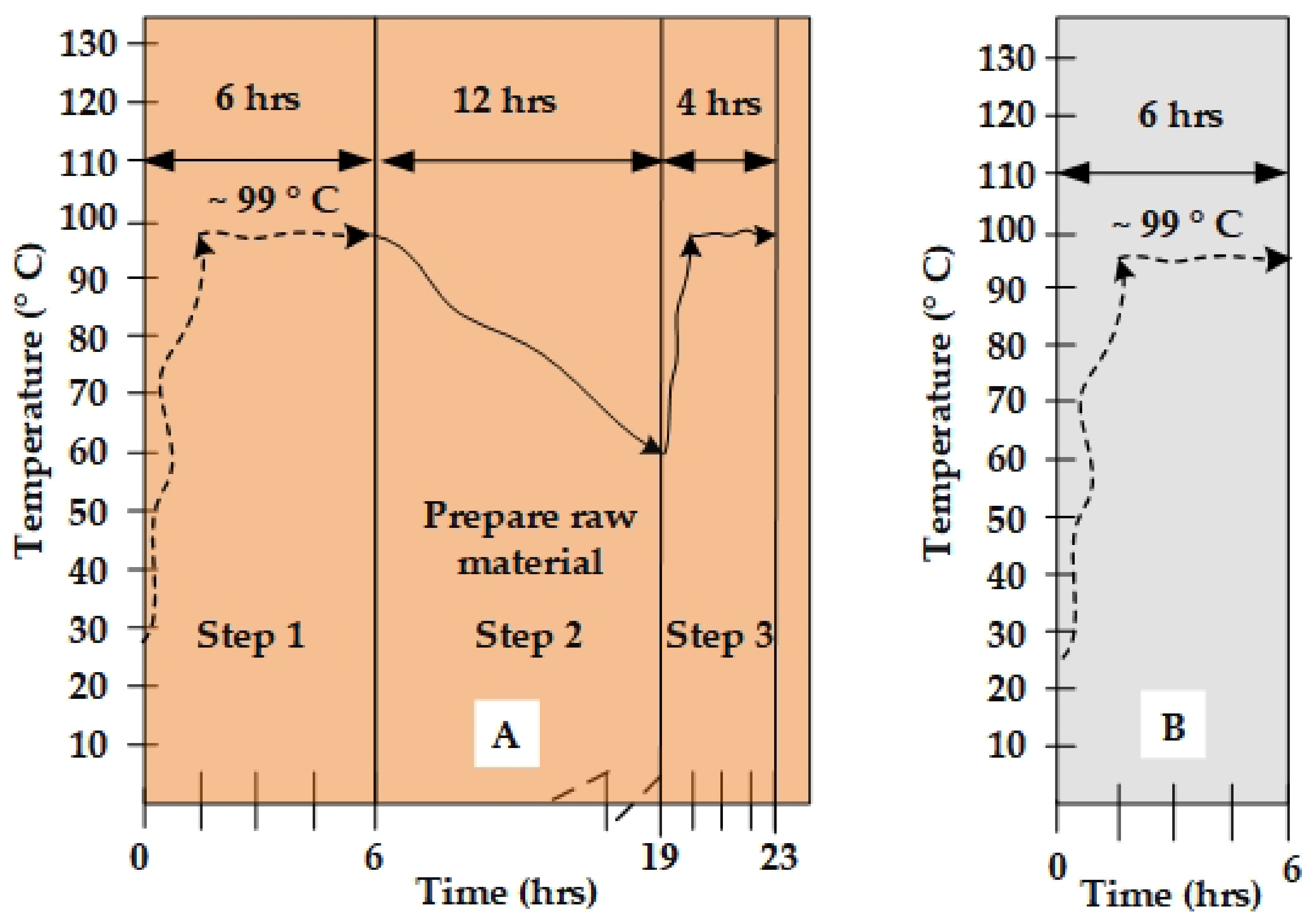
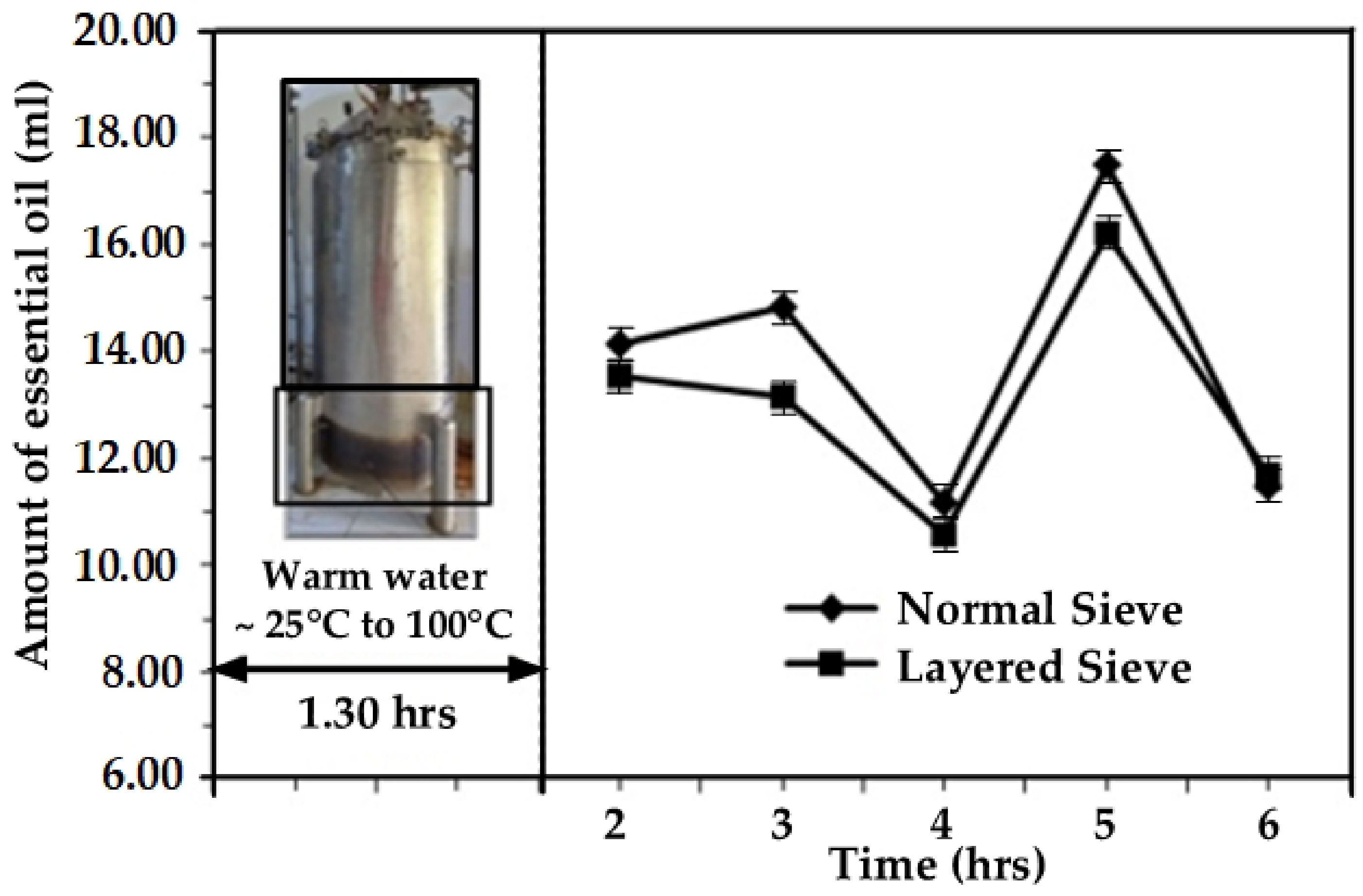
4.2. The Effects of Time and Steam Heating Temperature on the Recurrent Water and Steam Distillation Method
4.3. Determination of the Quality of Essential Oils Obtained Through Recurrent Water and Steam Distillation via GC-MS Analysis
- -
- -
- Limonene is used as a bittering agent, as a seasoning in the pharmaceutical industry, and as a wood paint to kill moths and termites. Limonene is also used as an ingredient in essential oils for medicinal purposes (also known as aromatherapy). In terms of its pharmacological properties, it is beneficial to health, such as its antioxidant properties, its ability to treat Alzheimer’s disease, and its ability to lower blood [56,57].
- -
- -
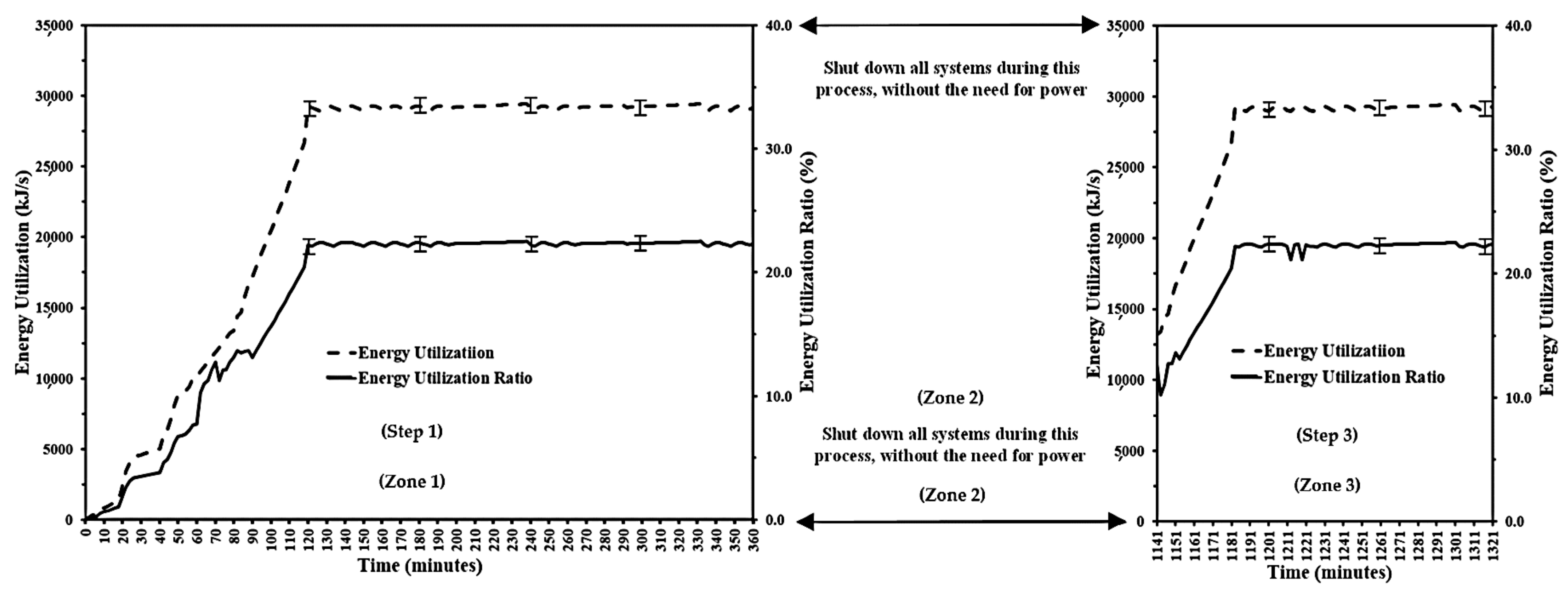
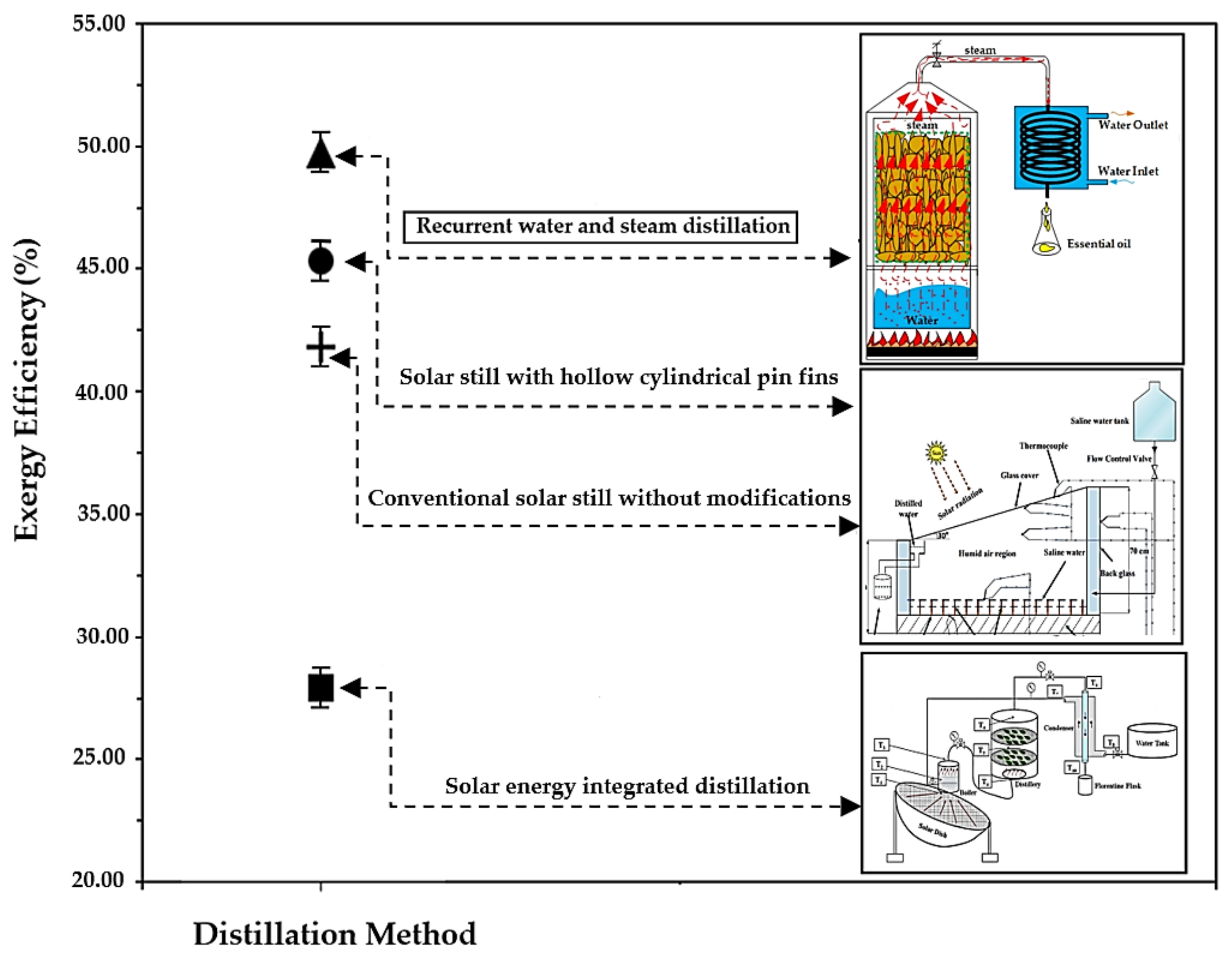
4.4. Useful Energy Efficiency and Exergy of Essential Oil Distillation via Recurrent Water and Steam Distillation Method
4.5. The Effects of Different Raw Material Packing Screens on the Yield of Essential Oil
4.6. Analysis of the Design and Construction of a 500 L Prototype Distillation Apparatus
4.7. Essential Oil Distillation Cost
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 500 L PDM | 500 Liter Prototype Distillation Machine |
| EO | Essential Oil |
| SHSD | Superheated steam distillation |
| CE | Cold Extraction |
| RWASD | Recurrent Water and Steam Distillation |
| WASD | Water and Steam Distillation |
| HD | Hydro-Distillation |
| SD | Steam Distillation |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| v | vapor |
| l | liquid |
| SMEs | Small- and Medium-sized Enterprises |
| SCFE | Supercritical Fluid Extraction |
| UE | Ultrasonic Extraction |
| SE | Solvent Extraction |
| NS | Normal Sieve |
| LS | Layered Sieve |
| i | in |
| o | out |
| e | Energy |
| Ex | Exergy |
| LHV | Lower Heating Value |
| EERU/EO | Energy Efficiency Ratio Useful for Essential Oils |
References
- Carvalho, R.N., Jr.; Moura, L.S.; Rosa, P.T.V.; Meireles, M.A.A. Supercritical fluid extraction from rosemary (Rosmarinus officinalis): Kinetic data, extract’s global yield, composition, and antioxidant activity. J. Supercrit. Fluids 2005, 35, 197–204. [Google Scholar] [CrossRef]
- Mekonnen, S.; Kassahun, B. Bringing Aromatic Plants into Cultivation by Smallholder Farmers of Ethiopia: Opportunities and Challenges, Improving Quality Production of Horticultural Crops for Sustainable Development Proceedings; Jimma University College of Agriculture and Veterinary Medicine: Jimma, Ethiopia, 2011. [Google Scholar]
- Sichamnan, S.; Trongthiang, S.; Chimnin, T.; Pipatpaiboon, N. Efficiency of Essential Oil Distillers by a Convection Rod Sieve. J. Southwest Jiaotong Univ. 2022, 57, 562–571. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, A. Process optimization of conventional steam distillation system for peppermint oil extraction. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 3960–3980. [Google Scholar] [CrossRef]
- Arranz, E.; Jaime, L.; Hazas, M.L.d.L.; Reglero, G.; Santoyo, S. Supercriticalfluid extraction as an alternative process to obtain essential oils with anti-inflammatory propertiesfrom marjoram and sweet basil. Ind. Crop. Prod. 2015, 67, 121–129. [Google Scholar] [CrossRef]
- Deineka, V.I.; Salasina, Y.Y. Determination of Chamazulene by Reversed Phase Hplc. ChemChemTech 2024, 67, 14–20. [Google Scholar] [CrossRef]
- Udalova, V. Leveraging Administrative Records and Record Linkages to Enhance Health Data at the U.S. Census Bureau. Int. J. Popul. Data Sci. 2024, 9, 5. [Google Scholar] [CrossRef]
- Figoli, A.; Donato, L.; Carnevale, R.; Tundis, R.; Statti, G.; Menichini, F.; Drioli, E. Bergamot essential oil extraction by pervaporation. Desalination 2006, 193, 160–165. [Google Scholar] [CrossRef]
- Naz, S.; Hanif, M.A.; Bhatti, H.N.; Ansari, T.M. Impact of Supercritical Fluid Extraction and Traditional Distillation on the Isolation of Aromatic Compounds from Cannabis indica and Cannabis sativa. J. Essent. Oil Bear. Plants 2017, 20, 175–184. [Google Scholar] [CrossRef]
- Masondo, N.A.; Makunga, N.P. Advancement of analytical techniques in some South African commercialized medicinal plants: Current and future perspectives. S. Afr. J. Bot. 2019, 126, 40–57. [Google Scholar] [CrossRef]
- Puwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- Silva, M.G.d.V.; Matos, F.J.d.A.; Lopes, P.R.O.; Silva, F.O.; Holanda, M.T. Composition of essential oils from three Ocimum species obtained by steam and microwave distillation and supercritical CO2 extraction. Arkivoc 2003, 2004, 66–71. [Google Scholar] [CrossRef]
- Ayub, M.A.; Hanif, M.A.; Blanchfield, J.; Zubair, M.; Abid, M.A.; Saleh, M.T. Chemical composition and antimicrobial activity of Boswellia serrata oleo-gum-resin essential oil extracted by superheated steam. Nat. Prod. Res. 2023, 37, 2451–2456. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Guo, J.; Zhu, H.; Wen, J. Optimization of superheated steam treatment conditions for wheat aleurone layer flour. Food Sci. Technol. 2021, 42, 1–8. [Google Scholar] [CrossRef]
- Smith, R.M. Extractions with Superheated Water. J. Chromatogr. A 2002, 975, 31–46. [Google Scholar] [CrossRef]
- Jayawardena, B.; Smith, R.M. Superheated water extraction of essential oils from Cinnamomum zeylanicum (L.). Phytochem. Anal. 2010, 21, 470–472. [Google Scholar] [CrossRef]
- Raza, M.H.; Ayub, M.A.; Zubair, M.; Hussain, A.; Saleem, S.; Azam, M.T.; Hussain, M.; Memon, A.G.; Abdelgawad, M.A.; Ghoneim, M.M.; et al. Comparative Study of Essential Oils Extracted from Foeniculum vulgare Miller Seeds Using Hydrodistillation, Steam Distillation, and Superheated Steam Distillation. Food Sci. Nutr. 2024, 12, 10535–10549. [Google Scholar] [CrossRef]
- Makeri, M.; Salihu, A. Jasmine essential oil: Production, extraction, characterization, and applications. In Essential Oils; Elsevier: Amsterdam, The Netherlands, 2023; pp. 147–177. [Google Scholar]
- Katekar, V.P.; Rao, A.B.; Sardeshpande, V.R. Review of the rose essential oil extraction by hydrodistillation: An investigation for the optimum operating condition for maximum yield. Sustain. Chem. Pharm. 2022, 29, 100783. [Google Scholar] [CrossRef]
- Abbas, A.; Abdulazeez, M.A.; Abdullahi, A.S.; Abusamra, Y.; Adegoke, G.O.; Adelakun, O.E.; Adjou, E.S.; Afolabi, M.O.; Ahmad, N.; Ahmed, N.; et al. Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 23–29. [Google Scholar]
- Preedy, V.R. (Ed.) Preface. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: San Diego, CA, USA, 2016; p. 33. [Google Scholar] [CrossRef]
- Hassanzadeh, M.K.; Tayarani, N.Z.; Nasery, M.; Emami, S.A. Essential Oils in Food Preservation, Flavor and Safety; Academic Press: San Diego, CA, USA, 2016; pp. 757–764. [Google Scholar]
- Cengel, Y.A. Steady Versus Transient Heat Transfer 63 Multidimensional Heat Transfer 64 Heat Generation 66 Generation 6. 2003. Available online: https://www.amirajcollege.in/wp-content/uploads/2020/10/3151909-heat-transfer-a-practical-approach-by-y-a-cengel.pdf (accessed on 1 April 2025).
- Shepherd, P. Fundamentals of Thermodynamics; John Wiley and Sons: New York, NY, USA, 2013; pp. 97–123. [Google Scholar] [CrossRef]
- Mutair, S.; Ikegami, Y. Experimental investigation on the characteristics of flash evaporation from superheated water jets for desalination. Desalination 2010, 251, 103–111. [Google Scholar] [CrossRef]
- Boppena, K.; Mekala, M.; Mallarapu, L.P.; Mohammed, R.W.; Uttaravalli, A.N.; Gidla, B.R.; Addanki, V.K. Extraction of rain water tree seed oil: Sustainable applications and management. Bioresour. Technol. Rep. 2023, 24, 101657. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Rezaei, K. Comparison of microwave-assisted hydrodistillation withthe traditional hydrodistillation method in the extractionof essential oils from Thymus vulgaris L. Food Chem. 2008, 109, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Ayub, M.A.; Goksen, G.; Fatima, A.; Zubair, M.; Abid, M.A.; Starowicz, M. Comparison of Conventional Extraction Techniques with Superheated Steam Distillation on Chemical Characterization and Biological Activities of Syzygium aromaticum L. Essential Oil. Separations 2023, 10, 27. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Loganayagan, S.; Kowshika, G.; Ramprakash, C.; Aruneshwaran, M. Adobe blocks reinforced with natural fibres: A review. Mater. Today Proc. 2021, 45, 6493–6499. [Google Scholar] [CrossRef]
- Lal, A.N.; Krishnamurthy, S.; Girinandagopal, M.; Kothakota, A.; Kumar, R.; Venugopalan, V.; Ishwarya, S.P.; Venkatesh, T. A comparison of the Refrigerated Adsorption Drying of Daucus carota with fluidized bed drying. LWT 2022, 154, 112749. [Google Scholar]
- Akdağ, A.; Ozturk, E. Distillation Methods of Essential Oils. Agric. Eng. 2019, 45, 22–31. [Google Scholar]
- Shamspur, T.; Mohamadi, M.; Mostafavi, A. The effects of onion and salt treatments on essential oil content and composition of Rosa damascena Mill. Ind. Crop. Prod. 2012, 37, 451–456. [Google Scholar] [CrossRef]
- El-Shazly, M.; Mansour, M.; Elsayed, E.; Elgindi, M.; Singab, A.-N. Characterization of Essential Oils of the Leaves and Fruits of Adenanthera pavonina L. by GC/MS. Arch. Pharm. Sci. Ain Shams Univ. 2020, 4, 63–69. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, V.T.; Dao, T.P.; Lam, T.D.; Toan, T.Q.; Nguyen, T.D.; Vo, D.-V.N.; Vy, T.A.; Bui, L.M. New direction in research on extraction of Citrus aurantifolia (Lemon fruit) essential oil grown in Mekong Delta—Vietnam via microwave-assisted hydrodistillation. In IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2019; Volume 542, p. 012038. [Google Scholar]
- Talati, A. Extraction Methods of Natural Essential Oils. J. Method 2017, 1, 1–15. [Google Scholar]
- Van Rompaey, R. Distribution and Ecology of Allanblackia spp. (Clusiaceae) in African Rain Forests with Special Attention to the Development of a Wild Picking System of the Fruits (Clusiaceae) in African Rain Forests. 2003. Available online: https://scholar.google.com/scholar?hl=sr&as_sdt=0%2C5&q=37.%09Van+Rompaey%2C+R.+Distribution+and+ecology+of+Allanblackia+spp+%28Clusiaceae%29+in+African+rain+forests+with+special+attention+to+the+development+of+a+wild+picking+system+of+the+fruits+%28Clusiaceae%29+in+African+rain+forests.+2003.+https%3A%2F%2Fdoi.org%2F10.13140%2FRG.2.2.19915.59683&btnG= (accessed on 1 April 2025).
- Vora, L.K.; Gholap, A.D.; Hatvate, N.T.; Naren, P.; Khan, S.; Chavda, V.P.; Balar, P.C.; Gandhi, J.; Khatri, D.K. Essential oils for clinical aromatherapy: A comprehensive review. J. Ethnopharmacol. 2024, 330, 118180. [Google Scholar] [CrossRef]
- Tancon, M.; Abbatecola, A.; Mirafiori, M.; Bortolin, S.; Colusso, E.; Martucci, A.; Del Col, D. Investigation of surface inclination effect during dropwise condensation of flowing saturated steam. Int. J. Therm. Sci. 2024, 196, 108738. [Google Scholar] [CrossRef]
- Mousa, M.H.; Yang, C.-M.; Nawaz, K.; Miljkovic, N. Review of heat transfer enhancement techniques in two-phase flows for highly efficient and sustainable cooling. Renew. Sustain. Energy Rev. 2022, 155, 111896. [Google Scholar] [CrossRef]
- Al-Muslim, H.; Dincer, I. Thermodynamic analysis of crude oil distillation systems. Int. J. Energy Res. 2005, 29, 637–655. [Google Scholar] [CrossRef]
- Doppalapudi, A.T.; Azad, A.K.; Khan, M.M.K. Exergy, energy, performance, and combustion analysis for biodiesel NOx reduction using new blends with alcohol, nanoparticle, and essential oil. J. Clean. Prod. 2024, 467, 142968. [Google Scholar] [CrossRef]
- Jamnani, M.B.; Ting, D.S.-K.; Carriveau, R.; Kardgar, A. Energy, Exergy, Environmental (3E) and Parametric Assessment of a Triple-Pressure Reheat Combined-Cycle Power Plant. J. Energy Resour. Technol. 2021, 143, 1–27. [Google Scholar] [CrossRef]
- Krishnan, M.G.; Rajkumar, S. Effects of dual fuel combustion on performance, emission and energy-exergy characteristics of diesel engine fuelled with diesel-isobutanol and biodiesel-isobutanol. Energy 2022, 252, 124022. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Boles, M.A. Thermodynamics: An Engineering Approach/Yunus A. Çengel, Michael Boles; McGraw-Hill Higher Education: London, UK, 2007. [Google Scholar]
- Kumar, R.; Sharma, S.; Sharma, S.; Kumar, N. Drying methods and distillation time affects essential oil content and chemical compositions of Acorus calamus L. in the western Himalayas. J. Appl. Res. Med. Aromat. Plants 2016, 3, 136–141. [Google Scholar] [CrossRef]
- Kumar, D.; Suryavanshi, P.; Padalia, R.C.; Chauhan, A.; Venkatesha, K.T.; Tiwari, A.K.; Singh, V.R.; Singh, S.; Upadhyay, R.K. Evaluation of harvesting time and standardization of distillation duration for higher essential oil content and quality in German chamomile (Chamomilla recutita L.). J. Spices Aromat. Crop. 2021, 29, 140–147. [Google Scholar] [CrossRef]
- Indira, T.I.; Burhan, K.H.; Manurung, R.; Widiana, A. Enhancement of Essential Oil Yield from Melaleuca leucadendra L. Leaves by Lignocellulose Degradation Pre-Treatment Using Filamentous Fungi. J. Bioresour. Bioprod. 2021, 6, 379–386. [Google Scholar] [CrossRef]
- Kwanga, S.N.; Djuffo, D.T.; Boum, A.T.; Anoh, F.A.; Dongmo, P.M.J. Effect of Solid-State Fermentation on the Essential Oil Yield of Curcuma longa Residues. Waste Biomass Valorization 2022, 13, 4565–4573. [Google Scholar] [CrossRef]
- Hamidi, N. The role of pre-destilation fermentation in increasing patchouli oil productivity (Pogostemon cablin benth). In IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2019. [Google Scholar]
- NAl-Jabri, N.N.; Hossain, M.A. Chemical composition and antimicrobial potency of locally grown lemon essential oil against selected bacterial strains. J. King Saud Univ. Sci. 2018, 30, 14–20. [Google Scholar] [CrossRef]
- Jain, N.; Sharma, M. Evaluation of Citrus lemon essential oil for its chemical and biological properties against fungi causing dermatophytic infection in human beings. Anal. Chem. Lett. 2017, 7, 402–409. [Google Scholar] [CrossRef]
- Yoo, Z.-W.; Kim, N.-S.; Lee, D.-S. Analyses of Hallabong Flavor by SPTE and HS-SPME with GC-MS. 2004. Available online: https://koreascience.kr/article/JAKO200402727444416.pdf (accessed on 1 April 2025).
- Murali, R.; Saravanan, R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2012, 2, 269–275. [Google Scholar] [CrossRef]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Sun, H.-L.; Chen, S.-Y.; Zeng, L.; Wang, T.-T. Anti-fungal activity, mechanism studies on α-Phellandrene and Nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 1–9. [Google Scholar] [CrossRef]
- Lima, D.F.; Brandão, M.S.; Moura, J.B.; Leitão, J.M.R.S.; Carvalho, F.A.A.; Miúra, L.M.C.V.; Leite, J.R.S.A.; Sousa, D.P.; Almeida, F.R.C. Antinociceptive activity of the monoterpene α-phellandrene in rodents: Possible mechanisms of action. J. Pharm. Pharmacol. 2012, 64, 283–292. [Google Scholar] [CrossRef]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Cassone, A.; Salvatore, G. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect. Dis. 2006, 6, 158. [Google Scholar] [CrossRef]
- Hart, P.; Brand, C.; Carson, C.; Riley, T.; Prager, R.; Finlay-Jones, J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000, 49, 619–626. [Google Scholar] [CrossRef]
- Cavina, V.; Mari, A.; Carlini, A.; Giovannetti, V. Optimal thermodynamic control in open quantum systems. Phys. Rev. A 2017, 98, 012139. [Google Scholar] [CrossRef]
- Hsiang, J.-T.; Chou, C.H.; Subaşı, Y.; Hu, B.L. Quantum Thermodynamics from the Nonequilibrium Dynamics of Open Systems: Energy, Heat Capacity, and the Third Law. Phys. Rev. E 2017, 97, 012135. [Google Scholar] [CrossRef]
- Kuhl, E.; Steinmann, P. Mass– and volume–specific views on thermodynamics for open systems. Proc. R. Soc. A Math. Phys. Eng. Sci. 2003, 459, 2547–2568. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, A. Energy-economic and exergy-environment performance evaluation of solar energy integrated essential oil extraction system. Sol. Energy 2023, 265, 112101. [Google Scholar] [CrossRef]
- Yousef, M.S.; Hassan, H.; Sekiguchi, H. Energy, exergy, economic and enviroeconomic (4E) analyses of solar distillation system using different absorbing materials. Appl. Therm. Eng. 2019, 150, 30–41. [Google Scholar] [CrossRef]
- Andrade, F.; Moita, A.; Nikulin, A.; Moreira, A.; Santos, H. Experimental investigation on heat transfer and pressure drop of internal flow in corrugated tubes. Int. J. Heat Mass Transf. 2019, 140, 940–955. [Google Scholar] [CrossRef]
- Yu, C.; Shao, M.; Zhang, W.; Huang, M.; Wang, G. Enhancing heat transfer efficiency in corrugated tube heat exchangers: A comprehensive approach through structural optimization and field synergy analysis. Heliyon 2024, 10, e30113. [Google Scholar] [CrossRef]
- Lopes, E.F.; Salviano, L.O. Investigation of the Enhancement of Heat Transfer in a Flat Plate Solar Collector with a Corrugated Tube Under Thermosiphon Effect. J. Sol. Energy Eng. 2024, 146, 1–26. [Google Scholar] [CrossRef]







| Recurrent Water and Steam Distillation/GC-MS (%) | GC-MS (%) [51] | GC-MS (%) [52] |
|---|---|---|
| Limonene (20.72%) β-myrcene (2.72%) α-Phellandrene (1.27%) Terpinen-4-ol (3.04%) | Limonene (43.07%) β-myrcene (1.87%) α-Phellandrene (-) Terpinen-4-ol (2.85%) | Limonene (54.82%) β-myrcene (-) α-Phellandrene (-) Terpinen-4-ol (2.85%) |
| Parameter Design and Construction | True Value (Design/Calculation) | Measure Values | Error (%) |
|---|---|---|---|
| Thickness of distillation pot and lid (SS 304) | 6 mm | 6 mm | - |
| Volume of distillation pot | 0.3171 m3 | 0.3020 m3 | ±5.00% |
| Volume of boiled water | 0.105 m3/105 L | 0.110 m3/110 L | ±4.76% |
| Condensing unit volume | 0.23 m3 | 0.25 m3 | ±8.69% |
| Volume of coiled pipe | 0.0057 | 0.0060 | ±5.26% |
| (%) Total design and construction error | ±5.90% | ||
| Parameter Heat Loss | True value (Design/Calculation) | Measure values | Error (%) |
| The rate of heat conduction of the distillation pot | 47.07 kW | 43.45 kW | ±7.69% |
| Convection of the distillation pot | 62.64 kW | 57.60 kW | ±7.79% |
| Total heat transfer coefficient | 37.21 W/m2 °C | 34.35 W/m2 °C | ±7.68% |
| Heat energy used for boiling | 6221.76 W | 5694.88 W | ±8.46% |
| Heat transfer rate | 7.69 kW | 7.11 kW | ±7.54% |
| (%) Total heat loss error | ±7.83% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pipatpaiboon, N.; Parametthanuwat, T.; Bhuwakietkumjohn, N.; Ding, Y.; Li, Y.; Sichamnan, S. Improving the Efficiency of Essential Oil Distillation via Recurrent Water and Steam Distillation: Application of a 500-L Prototype Distillation Machine and Different Raw Material Packing Grids. AgriEngineering 2025, 7, 175. https://doi.org/10.3390/agriengineering7060175
Pipatpaiboon N, Parametthanuwat T, Bhuwakietkumjohn N, Ding Y, Li Y, Sichamnan S. Improving the Efficiency of Essential Oil Distillation via Recurrent Water and Steam Distillation: Application of a 500-L Prototype Distillation Machine and Different Raw Material Packing Grids. AgriEngineering. 2025; 7(6):175. https://doi.org/10.3390/agriengineering7060175
Chicago/Turabian StylePipatpaiboon, Namphon, Thanya Parametthanuwat, Nipon Bhuwakietkumjohn, Yulong Ding, Yongliang Li, and Surachet Sichamnan. 2025. "Improving the Efficiency of Essential Oil Distillation via Recurrent Water and Steam Distillation: Application of a 500-L Prototype Distillation Machine and Different Raw Material Packing Grids" AgriEngineering 7, no. 6: 175. https://doi.org/10.3390/agriengineering7060175
APA StylePipatpaiboon, N., Parametthanuwat, T., Bhuwakietkumjohn, N., Ding, Y., Li, Y., & Sichamnan, S. (2025). Improving the Efficiency of Essential Oil Distillation via Recurrent Water and Steam Distillation: Application of a 500-L Prototype Distillation Machine and Different Raw Material Packing Grids. AgriEngineering, 7(6), 175. https://doi.org/10.3390/agriengineering7060175









