Fertilizers and Microorganisms Promote Strawberry Growth, Yield, and Quality in Peru
Abstract
1. Introduction
2. Materials and Methods
2.1. Localization
2.2. Strawberry Cultivars
2.3. Soil Characteristics
2.4. Experimental Design
2.5. Pre-Plant Practices and Strawberry Agronomic Management
2.6. Application of Microorganisms
2.7. Agronomic Measurements
2.8. Plant Sampling and Chemical Analysis
2.9. Fruit Quality and Nutrition Assessment
2.10. Statistical Analysis
3. Results
3.1. Effects on Agronomic Variables
3.2. Effects on Nutrient Concentrations
3.3. Effects on Fruit Quality
4. Discussion
4.1. Strawberry Growth and Yield
4.2. Strawberry Nutrition
4.3. Strawberry Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| RCBD | Randomized Complete Block Design |
| SE | Standard Error |
References
- Dirección General de Estadística—MIDAGRI. Seguimiento y Evaluación de Políticas. In Boletin estadistico Mensual: El Agro en Cifras. Diciembre 2024; MIDAGRI: Lima, Perú, 2025; p. 166. [Google Scholar]
- Fischer, G.; Miranda, D.; Magnitskiy, S.; Balaguera-López, H.E.; Molano, Z. (Eds.) Avances en el Cultivo de las Berries en el Trópico; Sociedad Colombiana de Ciencias Hortícolas: Bogotá, Colombia, 2021; ISBN 978-958-59886-1-3. [Google Scholar]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry As a Functional Food: An Evidence-Based Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef]
- Skaggs, R.K. Predicting Drip Irrigation Use and Adoption in a Desert Region. Agric. Water Manag. 2001, 51, 125–142. [Google Scholar] [CrossRef]
- Gamboa, N.R.; Marchese, A.B.; Tavares Corrêa, C.H. Salinization in Peruvian North Coast Soils: Case Study in San Pedro de Lloc. In Saline and Alkaline Soils in Latin America: Natural Resources, Management and Productive Alternatives; Taleisnik, E., Lavado, R.S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 141–159. ISBN 978-3-030-52592-7. [Google Scholar]
- Nestby, R.; Lieten, F.; Pivot, D.; Raynal Lacroix, C.; Tagliavini, M. Influence of Mineral Nutrients on Strawberry Fruit Quality and Their Accumulation in Plant Organs. Int. J. Fruit Sci. 2005, 5, 139–156. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.; Nagarajan, L.; Roy, A.; Rabbinge, R. Revisiting Fertilisers and Fertilisation Strategies for Improved Nutrient Uptake by Plants. Biol. Fertil. Soils 2015, 51, 897–911. [Google Scholar] [CrossRef]
- Tomić, J.; Pešaković, M.; Milivojević, J.; Karaklajić-Stajić, Ž. How to Improve Strawberry Productivity, Nutrients Composition, and Beneficial Rhizosphere Microflora by Biofertilization and Mineral Fertilization? J. Plant Nutr. 2018, 41, 2009–2021. [Google Scholar] [CrossRef]
- Bhattacharjee, R.B.; Singh, A.; Mukhopadhyay, S.N. Use of Nitrogen-Fixing Bacteria as Biofertiliser for Non-Legumes: Prospects and Challenges. Appl. Microbiol. Biotechnol. 2008, 80, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; McCormack, M.L.; Guo, D. Arbuscular Mycorrhizal Fungal Effects on Plant Competition and Community Structure. J. Ecol. 2015, 103, 1224–1232. [Google Scholar] [CrossRef]
- Cassán, F.; Coniglio, A.; López, G.; Molina, R.; Nievas, S.; de Carlan, C.L.N.; Donadio, F.; Torres, D.; Rosas, S.; Pedrosa, F.O.; et al. Everything You Must Know about Azospirillum and Its Impact on Agriculture and Beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial Effects of Trichoderma Secondary Metabolites on Crops. Phytother. Res. 2020, 34, 2835–2842. [Google Scholar] [CrossRef]
- Drobek, M.; Cybulska, J.; Gałązka, A.; Feledyn-Szewczyk, B.; Marzec-Grządziel, A.; Sas-Paszt, L.; Gryta, A.; Trzciński, P.; Zdunek, A.; Frąc, M. The Use of Interactions Between Microorganisms in Strawberry Cultivation (Fragaria x Ananassa Duch.). Front. Plant Sci. 2021, 12, 780099. [Google Scholar] [CrossRef]
- Todeschini, V.; AitLahmidi, N.; Mazzucco, E.; Marsano, F.; Gosetti, F.; Robotti, E.; Bona, E.; Massa, N.; Bonneau, L.; Marengo, E.; et al. Impact of Beneficial Microorganisms on Strawberry Growth, Fruit Production, Nutritional Quality, and Volatilome. Front. Plant Sci. 2018, 9, 1611. [Google Scholar] [CrossRef]
- Bona, E.; Lingua, G.; Manassero, P.; Cantamessa, S.; Marsano, F.; Todeschini, V.; Copetta, A.; D’Agostino, G.; Massa, N.; Avidano, L.; et al. AM Fungi and PGP Pseudomonads Increase Flowering, Fruit Production, and Vitamin Content in Strawberry Grown at Low Nitrogen and Phosphorus Levels. Mycorrhiza 2015, 25, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Gryndler, M.; Vosátka, M.; Hrŝelová, H.; Catská, V.; Chvátalová, I.; Jansa, J. Effect of Dual Inoculation with Arbuscular Mycorrhizal Fungi and Bacteria on Growth and Mineral Nutrition of Strawberry. J. Plant Nutr. 2002, 25, 1341–1358. [Google Scholar] [CrossRef]
- Cui, D.; Liang, S.; Wang, D. Observed and Projected Changes in Global Climate Zones Based on Köppen Climate Classification. WIREs Clim. Change 2021, 12, e701. [Google Scholar] [CrossRef]
- Richardson, M.L.; Arlotta, C.G.; Lewers, K.S. Yield and Nutrients of Six Cultivars of Strawberries Grown in Five Urban Cropping Systems. Sci. Hortic. 2022, 294, 110775. [Google Scholar] [CrossRef]
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods; CAB International: Wallingford, UK, 1993; Volume 78, ISBN 0-85198-821-0. [Google Scholar]
- Demirsoy, H.; Demirsoy, L.; Öztürk, A. Improved Model for the Non-Destructive Estimation of Strawberry Leaf Area. Fruits 2005, 60, 69–73. [Google Scholar] [CrossRef]
- EMBRAPA. Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Embrapa: Brasília, Brazil, 2009; ISBN 978-85-7383-430-7. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org/ (accessed on 10 September 2025).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Lme4: Linear Mixed-Effects Models Using “Eigen” and S4, version 1.1-37; R Foundation for Statistical Computing: Vienna, Austria, 2003. [Google Scholar]
- Faria, J.C.; Jelihovschi, E.G.; Allaman, I.B. ScottKnott: The ScottKnott Clustering Algorithm, version 1.3-3; R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Li, Q.; Zhang, D.; Song, Z.; Ren, L.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A. Organic Fertilizer Activates Soil Beneficial Microorganisms to Promote Strawberry Growth and Soil Health after Fumigation. Environ. Pollut. 2022, 295, 118653. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, K.; Ning, T.; Deng, C.; Wang, L.; Li, D.; Wang, T.; Li, J. Effects of Multiple N, P, and K Fertilizer Combinations on Strawberry Growth and the Microbial Community. PLoS ONE 2023, 18, e0293088. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, F.M.; de Assis Pereira, T.; Souza, T.P.; Guimarães, P.H.S.; Martins, A.D.; Schwan, R.F.; Pasqual, M.; Dória, J. Beneficial Effects of Inoculation of Growth-Promoting Bacteria in Strawberry. Microbiol. Res. 2019, 223, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Huasasquiche, L.; Alejandro, L.; Ccori, T.; Cántaro-Segura, H.; Samaniego, T.; Quispe, K.; Solórzano, R. Bacillus Subtilis and Rhizophagus Intraradices Improve Vegetative Growth, Yield, and Fruit Quality of Fragaria × Ananassa Var. San Andreas. Microorganisms 2024, 12, 1816. [Google Scholar] [CrossRef]
- Huasasquiche, L.; Ccori, T.; Alejandro, L.; Cántaro-Segura, H.; Samaniego, T.; Solórzano, R. Interaction between Trichoderma Sp., Pseudomonas Putida, and Two Organic Amendments on the Yield and Quality of Strawberries (Fragaria x Annanasa Cv. San Andreas) in the Huaral Region, Peru. Appl. Microbiol. 2024, 4, 1110–1123. [Google Scholar] [CrossRef]
- Pérez-Moncada, U.A.; Santander, C.; Ruiz, A.; Vidal, C.; Santos, C.; Cornejo, P. Design of Microbial Consortia Based on Arbuscular Mycorrhizal Fungi, Yeasts, and Bacteria to Improve the Biochemical, Nutritional, and Physiological Status of Strawberry Plants Growing under Water Deficits. Plants 2024, 13, 1556. [Google Scholar] [CrossRef]
- Flórez-Hernández, E.A.; Montes-Ciro, E.; Hurtado-Salazar, A.; Aristizábal, J.C.; Ceballos-Aguirre, N.; Flórez-Hernández, E.A.; Montes-Ciro, E.; Hurtado-Salazar, A.; Aristizábal, J.C.; Ceballos-Aguirre, N. Technical-Economic Evaluation of Bacterial Consortia in Strawberry Cultivation across Two Production Systems. Rev. Colomb. Cienc. Hortícolas 2023, 17, e16506. [Google Scholar] [CrossRef]
- Cruz, S.M.-D.L.; González-Fuentes, J.A.; Robledo-Olivo, A.; Mendoza-Villarreal, R.; Hernández-Pérez, A.; Dávila-Medina, M.D.; Alvarado-Camarillo, D. Humic Substances and Rhizobacteria Enhance the Yield, Physiology and Quality of Strawberries. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12578. [Google Scholar] [CrossRef]
- Calzavara, A.K.; Paiva, P.H.G.; Gabriel, L.C.; Oliveira, A.L.M.; Milani, K.; Oliveira, H.C.; Bianchini, E.; Pimenta, J.A.; de Oliveira, M.C.N.; Dias-Pereira, J.; et al. Associative Bacteria Influence Maize (Zea mays L.) Growth, Physiology and Root Anatomy under Different Nitrogen Levels. Plant Biol. 2018, 20, 870–878. [Google Scholar] [CrossRef]
- Paradiso, R.; Arena, C.; De Micco, V.; Giordano, M.; Aronne, G.; De Pascale, S. Changes in Leaf Anatomical Traits Enhanced Photosynthetic Activity of Soybean Grown in Hydroponics with Plant Growth-Promoting Microorganisms. Front. Plant Sci. 2017, 8, 674. [Google Scholar] [CrossRef] [PubMed]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Chaïbi, W.; Zarrouk, M. Impacts of Water Stress on Gas Exchange, Water Relations, Chlorophyll Content and Leaf Structure in the Two Main Tunisian Olive (Olea europaea L.) Cultivars. Sci. Hortic. 2009, 119, 257–263. [Google Scholar] [CrossRef]
- Acharya, B.R.; Gill, S.P.; Kaundal, A.; Sandhu, D. Strategies for Combating Plant Salinity Stress: The Potential of Plant Growth-Promoting Microorganisms. Front. Plant Sci. 2024, 15, 1406913. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, J.; Wei, H.; Zhang, H.; Zhu, J. Relationship between Plant Roots, Rhizosphere Microorganisms, and Nitrogen and Its Special Focus on Rice. Agriculture 2021, 11, 234. [Google Scholar] [CrossRef]
- Dellagi, A.; Quillere, I.; Hirel, B. Beneficial Soil-Borne Bacteria and Fungi: A Promising Way to Improve Plant Nitrogen Acquisition. J. Exp. Bot. 2020, 71, 4469–4479. [Google Scholar] [CrossRef]
- Guerrero-Molina, M.F.; Winik, B.C.; Pedraza, R.O. More than Rhizosphere Colonization of Strawberry Plants by Azospirillum brasilense. Appl. Soil Ecol. 2012, 61, 205–212. [Google Scholar] [CrossRef]
- Naqqash, T.; Malik, K.A.; Imran, A.; Hameed, S.; Shahid, M.; Hanif, M.K.; Majeed, A.; Iqbal, M.J.; Qaisrani, M.M.; van Elsas, J.D. Inoculation with Azospirillum spp. Acts as the Liming Source for Improving Growth and Nitrogen Use Efficiency of Potato. Front. Plant Sci. 2022, 13, 929114. [Google Scholar] [CrossRef]
- Castellanos-Morales, V.; Villegas-Moreno, J.; Vierheilig, H.; Cárdenas-Navarro, R. Nitrogen Availability Drives the Effect of Glomus Intraradices on the Growth of Strawberry (Fragaria x Ananassa Duch.) Plants. J. Sci. Food Agric. 2012, 92, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Dwivedi, P.; Sarma, B.K.; Singh, G.S.; Singh, H.B. Trichoderma Asperellum T42 Reprograms Tobacco for Enhanced Nitrogen Utilization Efficiency and Plant Growth When Fed with N Nutrients. Front. Plant Sci. 2018, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Caira, S.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Salzano, A.M.; Lorito, M.; Woo, S.L. Trichoderma Applications on Strawberry Plants Modulate the Physiological Processes Positively Affecting Fruit Production and Quality. Front. Microbiol. 2020, 11, 1364. [Google Scholar] [CrossRef]
- Paliwoda, D.; Mikiciuk, G.; Chudecka, J.; Tomaszewicz, T.; Miller, T.; Mikiciuk, M.; Kisiel, A.; Sas-Paszt, L. Effects of Inoculation with Plant Growth-Promoting Rhizobacteria on Chemical Composition of the Substrate and Nutrient Content in Strawberry Plants Growing in Different Water Conditions. Agriculture 2024, 14, 46. [Google Scholar] [CrossRef]
- Ghosh, S.; Bheri, M.; Bisht, D.; Pandey, G.K. Calcium Signaling and Transport Machinery: Potential for Development of Stress Tolerance in Plants. Curr. Plant Biol. 2022, 29, 100235. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of Microorganisms in Adaptation of Agriculture Crops to Abiotic Stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Agehara, S. Characterizing Early-Season Nitrogen Fertilization Rate Effects on Growth, Yield, and Quality of Strawberry. Agronomy 2021, 11, 905. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Li, M.; Zhang, M.; Sun, H.; Sigrimis, N. Optimal Fertigation for High Yield and Fruit Quality of Greenhouse Strawberry. PLoS ONE 2020, 15, e0224588. [Google Scholar] [CrossRef]
- Hawkins, H.-J.; Cargill, R.I.M.; Nuland, M.E.V.; Hagen, S.C.; Field, K.J.; Sheldrake, M.; Soudzilovskaia, N.A.; Kiers, E.T. Mycorrhizal Mycelium as a Global Carbon Pool. Curr. Biol. 2023, 33, R560–R573. [Google Scholar] [CrossRef] [PubMed]
- Cecatto, A.P.; Ruiz, F.M.; Calvete, E.O.; Martínez, J.; Palencia, P. Mycorrhizal Inoculation Affects the Phytochemical Content in Strawberry Fruits. Acta Sci. Agron. 2016, 38, 227–237. [Google Scholar] [CrossRef]
- Cordeiro, E.C.N.; de Resende, J.T.V.; Córdova, K.R.V.; Nascimento, D.A.; Saggin, O.J.; Zeist, A.R.; Favaro, R. Arbuscular Mycorrhizal Fungi Action on the Quality of Strawberry Fruits. Hortic. Bras. 2019, 37, 437–444. [Google Scholar] [CrossRef]
- Nam, J.H.; Thibodeau, A.; Qian, Y.L.; Qian, M.C.; Park, S.H. Multidisciplinary Evaluation of Plant Growth Promoting Rhizobacteria on Soil Microbiome and Strawberry Quality. AMB Express 2023, 13, 18. [Google Scholar] [CrossRef]
- Berger, B.; Baldermann, S.; Ruppel, S. The Plant Growth-promoting Bacterium Kosakonia Radicincitans Improves Fruit Yield and Quality of Solanum Lycopersicum. J. Sci. Food Agric. 2017, 97, 4865–4871. [Google Scholar] [CrossRef]


| Treatments | Number of Leaves | Foliar Area—cm2 | Number of Crowns | Number of Flowers | Stolons | Petiole Length—cm | Petiole Diameter—cm | Crown Diameter—cm | Number of Fruits—106 | Yield—Mg/ha |
|---|---|---|---|---|---|---|---|---|---|---|
| Cultivars (n = 3) | ||||||||||
| Sabrina | 49.32 (10.32) a | 108.9 (15.18) a | 2.18 (0.81) a | 1.83 (0.58) a | 0.09 (0.07) b | 17.50 (2.33) a | 2.49 (0.32) | 33.79 (1.70) a | 3.47 (0.08) a | 62.5 (15.7) a |
| San Andreas | 30.21 (6.15) b | 93.2 (14.55) b | 1.82 (0.46) b | 1.51 (0.44) b | 0.16 (0.09) a | 15.16 (2.70) b | 2.46 (0.24) | 30.70 (1.62) b | 3.08 (0.10) b | 54.2 (11.5) b |
| Pv | ** | ** | * | * | ** | * | ns | ** | * | * |
| Doses % (n = 3) | ||||||||||
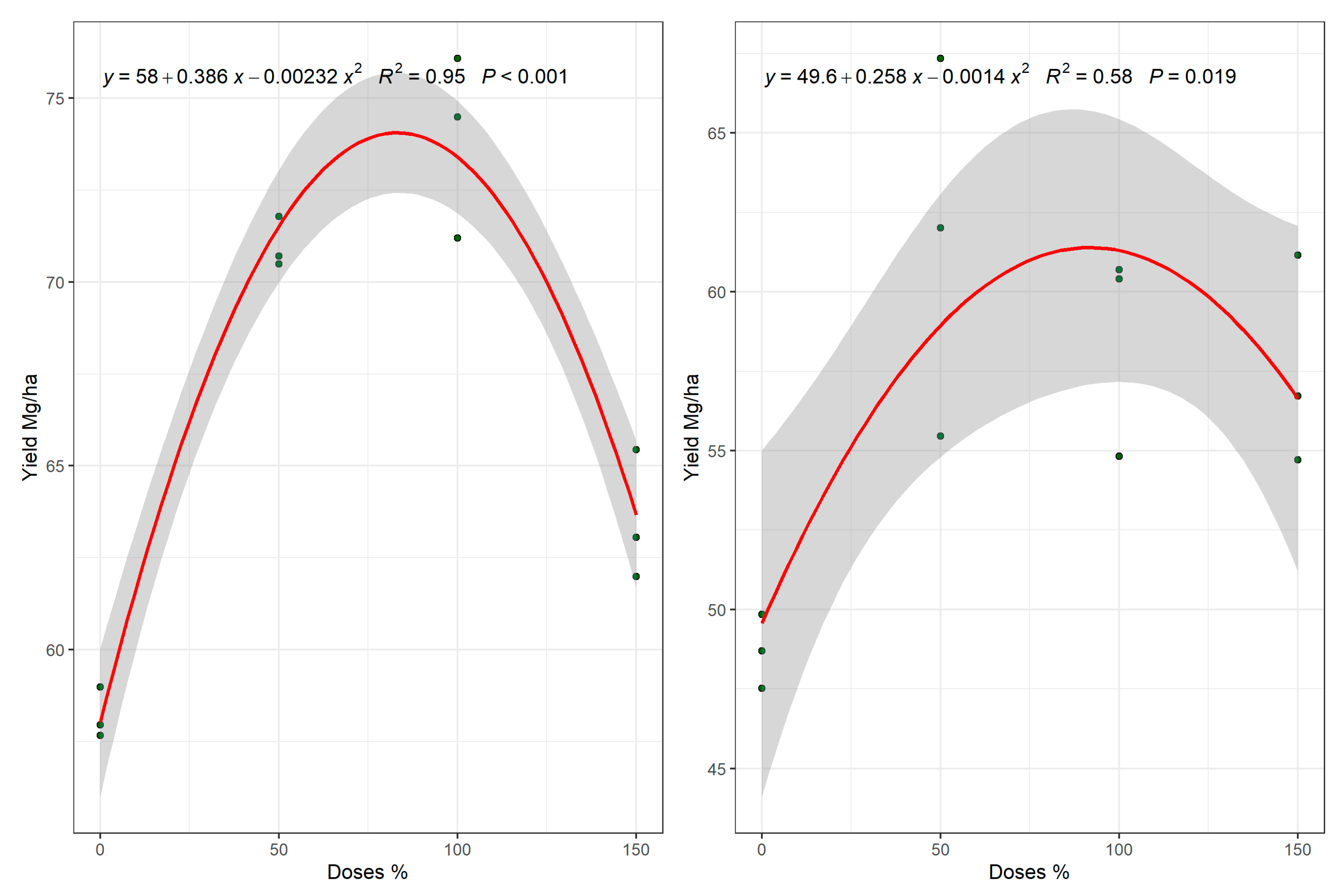
| 0 | 42.28 (10.21) | 84.3 (8.72) c | 2.54 (0.61) | 1.40 (0.41) | 0.15 (0.08) | 15.42 (2.63) | 2.62 (0.34) | 33.76 (1.63) | 2.79 (0.3) c | 49.9 (12.0) c |
| 50 | 36.94 (6.89) | 121.7 (17.96) a | 1.25 (0.26) | 1.71 (0.47) | 0.13 (0.08) | 16.51 (2.51) | 2.27 (0.15) | 32.18 (2.10) | 3.63 (0.17) a | 62.7 (13.8) a |
| 100 | 41.40 (11.51) | 108.2 (12.93) a | 1.55 (0.58) | 1.62 (0.55) | 0.06 (0.07) | 15.29 (2.48) | 2.45 (0.33) | 31.25 (1.68) | 3.45 (0.16) a | 64.6 (17.1) a |
| 150 | 39.55 (10.64) | 90.0 (10.45) b | 2.61 (0.70) | 1.91 (0.61) | 0.18 (0.09) | 17.94 (2.63) | 2.57 (0.21) | 31.57 (1.83) | 3.24 (0.12) b | 56.4 (13.0) b |
| Pv | ns | ** | * | * | ns | ns | ns | ns | ** | * |
| Microorganisms (n = 3) | ||||||||||
| Azospirillum brasilense | 47.58 (11.43) a | 103.0 (14.43) a | 2.1 (0.80) | 1.65 (0.64) b | 0.11 (0.08) | 15.82 (2.60) | 2.41 (0.25) | 32.74 (1.77) a | 3.54 (0.06) a | 62.2 (11.8) a |
| Rhizophagus sp. | 42.63 (8.79) a | 112.7 (18.48) a | 1.97 (0.71) | 1.69 (0.43) b | 0.12 (0.09) | 16.17 (2.82) | 2.44 (0.26) | 33.15 (1.88) a | 3.25 (0.08) b | 59.6 (10.1) a |
| Trichoderma sp. | 41.17 (7.74) a | 102.9 (15.13) a | 2.13 (0.73) | 1.94 (0.62) a | 0.11 (0.09) | 15.6 (2.62) | 2.56 (0.36) | 33.0 (1.55) a | 3.52 (0.11) a | 64.2 (8.59) a |
| Control | 26.09 (7.62) b | 85.6 (8.08) b | 1.78 (0.29) | 1.36 (0.30) c | 0.17 (0.08) | 17.53 (2.38) | 2.49 (0.24) | 29.84 (1.69) b | 2.13 (0.08) c | 40.9 (7.13) b |
| Pv | ** | ** | ns | * | ns | ns | ns | ** | ** | * |
| Treatments | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| % | |||||
| Cultivars (n = 3) | |||||
| Sabrina | 2.88 (0.13) b | 0.43 (0.03) b | 1.83 (0.16) b | 0.94 (0.15) | 0.37 (0.04) |
| San Andreas | 2.95 (0.15) a | 0.46(0.05) a | 1.98 (0.21) a | 0.92 (0.14) | 0.36 (0.04) |
| Pv | * | * | ** | ns | ns |
| Doses (n = 3) | |||||
| 0 | 2.90 (0.13) | 0.42 (0.06) b | 1.77 (0.20) c | 0.87 (0.15) b | 0.32 (0.03) b |
| 50 | 2.89 (0.18) | 0.45 (0.03) a | 2.01 (0.15) a | 0.97 (0.14) a | 0.39 (0.04) a |
| 100 | 2.95 (0.16) | 0.45 (0.05) a | 1.95 (0.19) a | 0.91 (0.14) b | 0.37 (0.04) a |
| 150 | 2.91 (0.11) | 0.44 (0.03) a | 1.85 (0.15) b | 0.98 (0.11) a | 0.37 (0.03) a |
| Pv | ns | * | ** | * | ** |
| Microorganisms (n = 3) | |||||
| Azospirillum brasilense | 2.92 (0.16) b | 0.44 (0.05) | 1.89 (0.17) b | 0.99 (0.14) a | 0.37 (0.03) |
| Rhizophagus sp. | 2.88 (0.09) b | 0.46 (0.04) | 1.87 (0.19) b | 0.88 (0.13) b | 0.35 (0.05) |
| Trichoderma sp. | 2.87 (0.12) b | 0.43 (0.04) | 1.88 (0.19) b | 0.89 (0.12) b | 0.36 (0.04) |
| Control | 3.01 (0.15) a | 0.45 (0.06) | 1.99 (0.24) a | 0.97 (0.17) a | 0.37 (0.04) |
| Pv | * | ns | ** | * | ns |
| Treatments | pH | Acidity | Brix Grade | Firmness—g-f | Circumference—mm |
|---|---|---|---|---|---|
| Cultivars (n = 3) | |||||
| Sabrina | 3.35 (0.07) | 0.95 (0.03) | 7.08 (0.95) | 386 (75.1) | 24.95 (9.5) |
| San Andreas | 3.30 (0.03) | 1.03 (0.06) | 7.18 (0.88) | 394 (81.2) | 24.1 (8.88) |
| Pv | ns | ns | ns | ns | ns |
| Doses % (n = 3) | |||||
| 0 | 3.35 (0.10) | 1.00 (0.03) | 7.31 (0.97) a | 363 (82.3) b | 23.7 (8.51) b |
| 50 | 3.29 (0.03) | 0.99 (0.05) | 7.04 (0.89) b | 437 (65.5) a | 26.4 (10.3) a |
| 100 | 3.34 (0.02) | 0.96 (0.07) | 6.96 (0.94) b | 396 (59.5) a | 25.5 (9.43) a |
| 150 | 3.33 (0.04) | 0.99 (0.09) | 7.13 (0.86) b | 364 (77.8) b | 23.9 (9.55) b |
| Pv | ns | ns | * | * | ** |
| Microorganisms (n = 3) | |||||
| Azospirillum | 3.33 (0.04) | 0.95 (0.04) | 7.25 (0.93) a | 387 (80.2) b | 25.1 (9.94) |
| Rhizophagus sp. | 3.30 (0.04) | 0.99 (0.06) | 6.86 (0.9) b | 385 (71.2) b | 25.6 (10.1) |
| Trichoderma | 3.32 (0.05) | 1.01 (0.08) | 7.20 (0.86) a | 374 (84.0) c | 24.9 (9.47) |
| Control | 3.34 (0.1) | 1.01 (0.08) | 7.18 (0.96) a | 397 (80.1) a | 23.2 (8.49) |
| Pv | ns | ns | * | * | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ttacca, B.L.; Peña Meneses, A.J.; Chipana Manrique, R.L.; Ñique Alvarez, M.A.; Arévalo-Hernández, C.O. Fertilizers and Microorganisms Promote Strawberry Growth, Yield, and Quality in Peru. AgriEngineering 2025, 7, 381. https://doi.org/10.3390/agriengineering7110381
Ttacca BL, Peña Meneses AJ, Chipana Manrique RL, Ñique Alvarez MA, Arévalo-Hernández CO. Fertilizers and Microorganisms Promote Strawberry Growth, Yield, and Quality in Peru. AgriEngineering. 2025; 7(11):381. https://doi.org/10.3390/agriengineering7110381
Chicago/Turabian StyleTtacca, Betsabe Leon, Ariana Jossety Peña Meneses, Reyno Leonardo Chipana Manrique, Manuel Alfredo Ñique Alvarez, and César Oswaldo Arévalo-Hernández. 2025. "Fertilizers and Microorganisms Promote Strawberry Growth, Yield, and Quality in Peru" AgriEngineering 7, no. 11: 381. https://doi.org/10.3390/agriengineering7110381
APA StyleTtacca, B. L., Peña Meneses, A. J., Chipana Manrique, R. L., Ñique Alvarez, M. A., & Arévalo-Hernández, C. O. (2025). Fertilizers and Microorganisms Promote Strawberry Growth, Yield, and Quality in Peru. AgriEngineering, 7(11), 381. https://doi.org/10.3390/agriengineering7110381







