Effects of Moisture Content and Lime Concentrate on Physiochemical, Mechanical, and Sensory Properties of Quinoa Snacks: An Ancient Andean Crop in Puno, Peru
Abstract
1. Introduction
2. Materials and Methods
2.1. Row Materials
2.2. Methods and Procedures of Analysis
2.2.1. Proximal Chemical
2.2.2. Minerals
2.2.3. Amino Acid Profile in Salcedo Quinoa, Scarified, Washed, and Dried
2.2.4. Starch, Amylopectin, and Particle Size Determination
2.2.5. Instrumental Texture or Hardness (N)
2.2.6. Net Color Variation (∆E)
2.2.7. Sectional Expansion Index (SEI)
2.2.8. Bulk Density (BD)
2.2.9. Degree of Starch Gelatinization (DSG)
2.2.10. Water Absorption Index (WAI) and Water Solubility Index (WSI)
2.2.11. Wetting
2.2.12. Sensory Evaluation of the Extruded
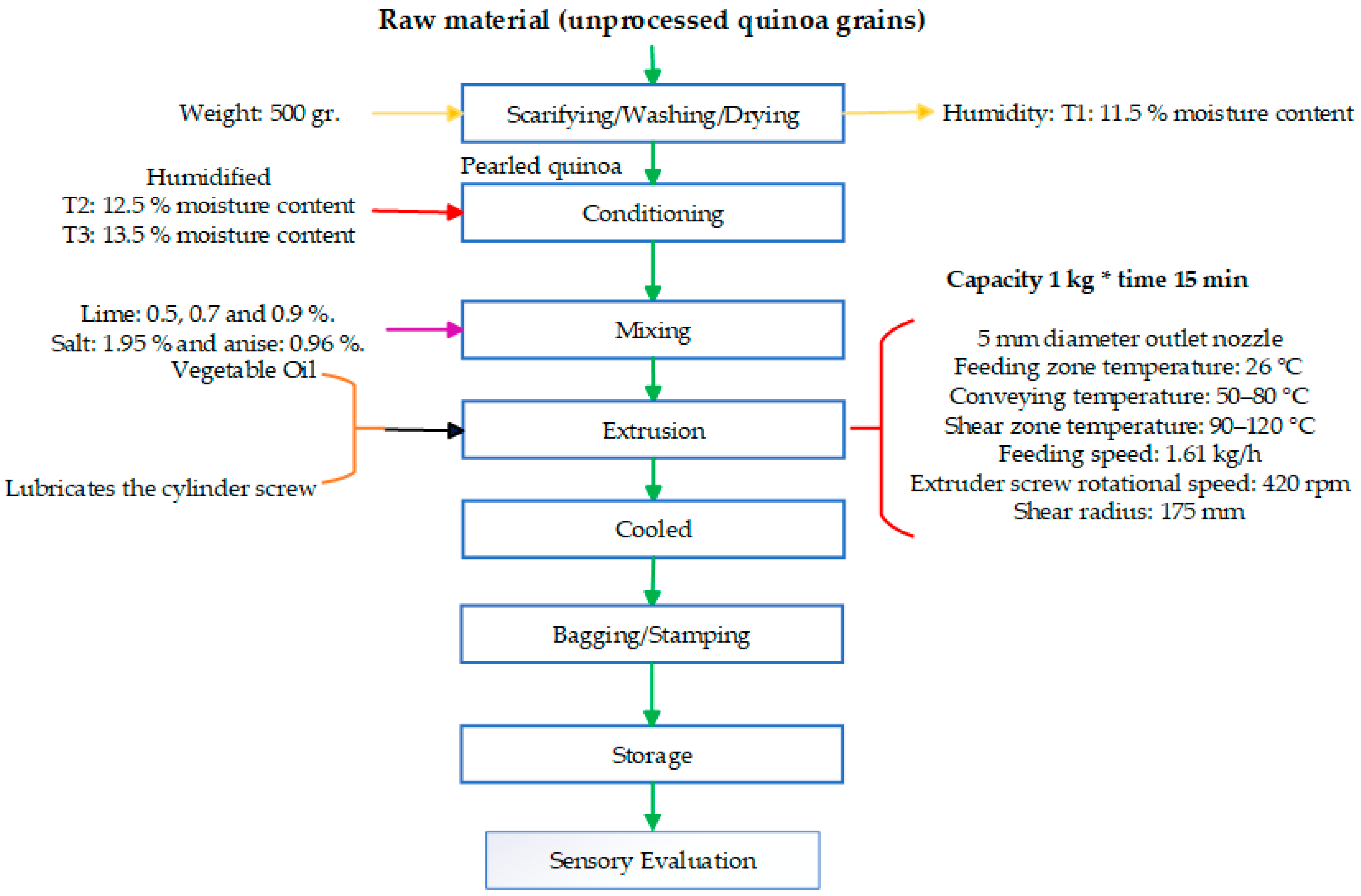
2.3. Experimental Methodology
2.3.1. Pearled Quinoa
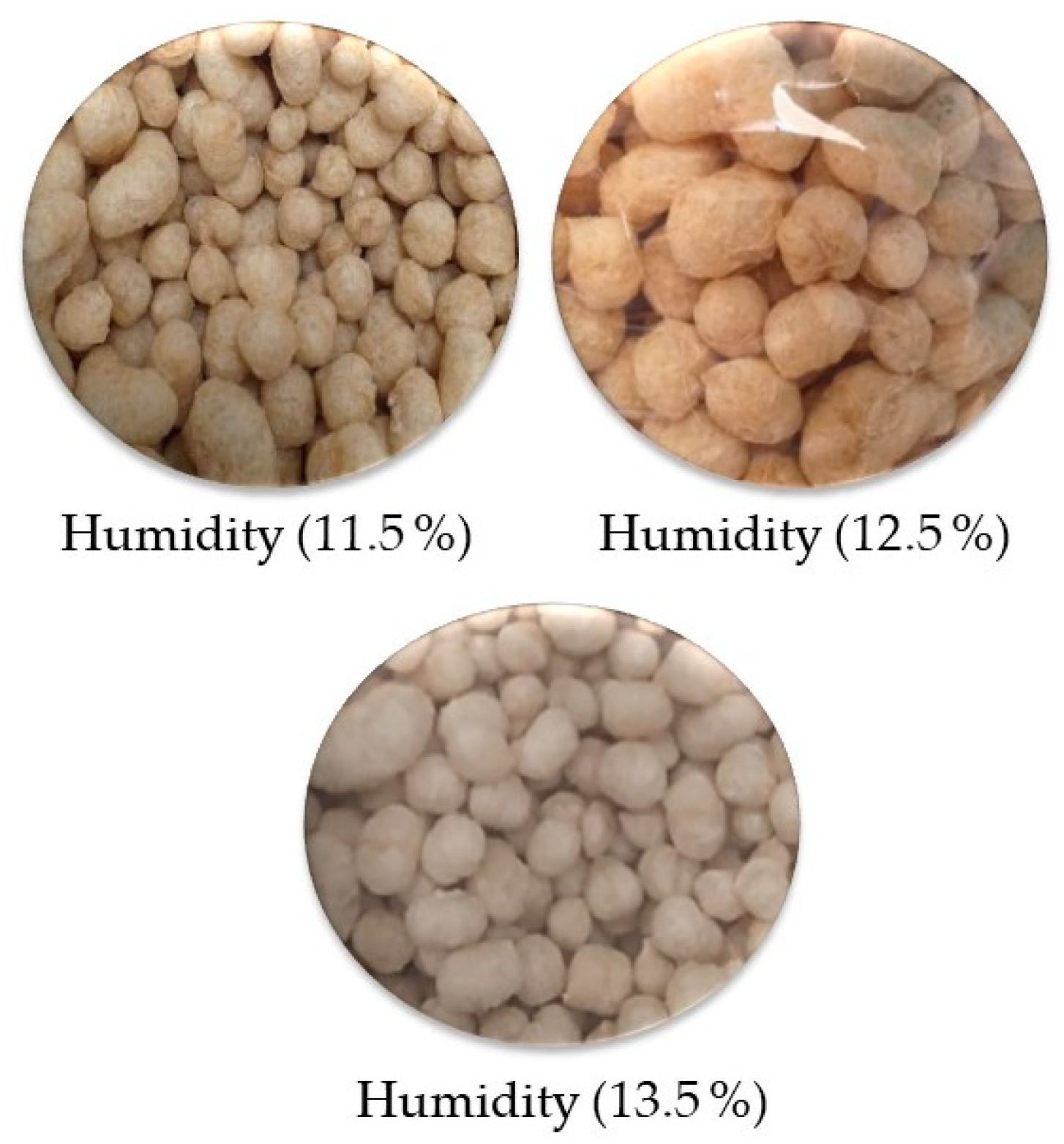
2.3.2. Moisture Adjustment of Quinoa Grains
2.3.3. Mixing of Ingredients
2.3.4. Extrusion Process
2.3.5. Cooling of Extrudates, Packaging and Storage
2.3.6. Evaluation and Optimization of the Extrusion of Quinoa, Lime, Salt, Anise, and Vegetable Oil
2.4. Application of Inferential Statistical Test
3. Results and Discussion
3.1. Physicochemical Composition of Quinoa and Extrudates
3.2. Evaluating the Physical Characteristics of Net Variation of the Extruded Quispiño
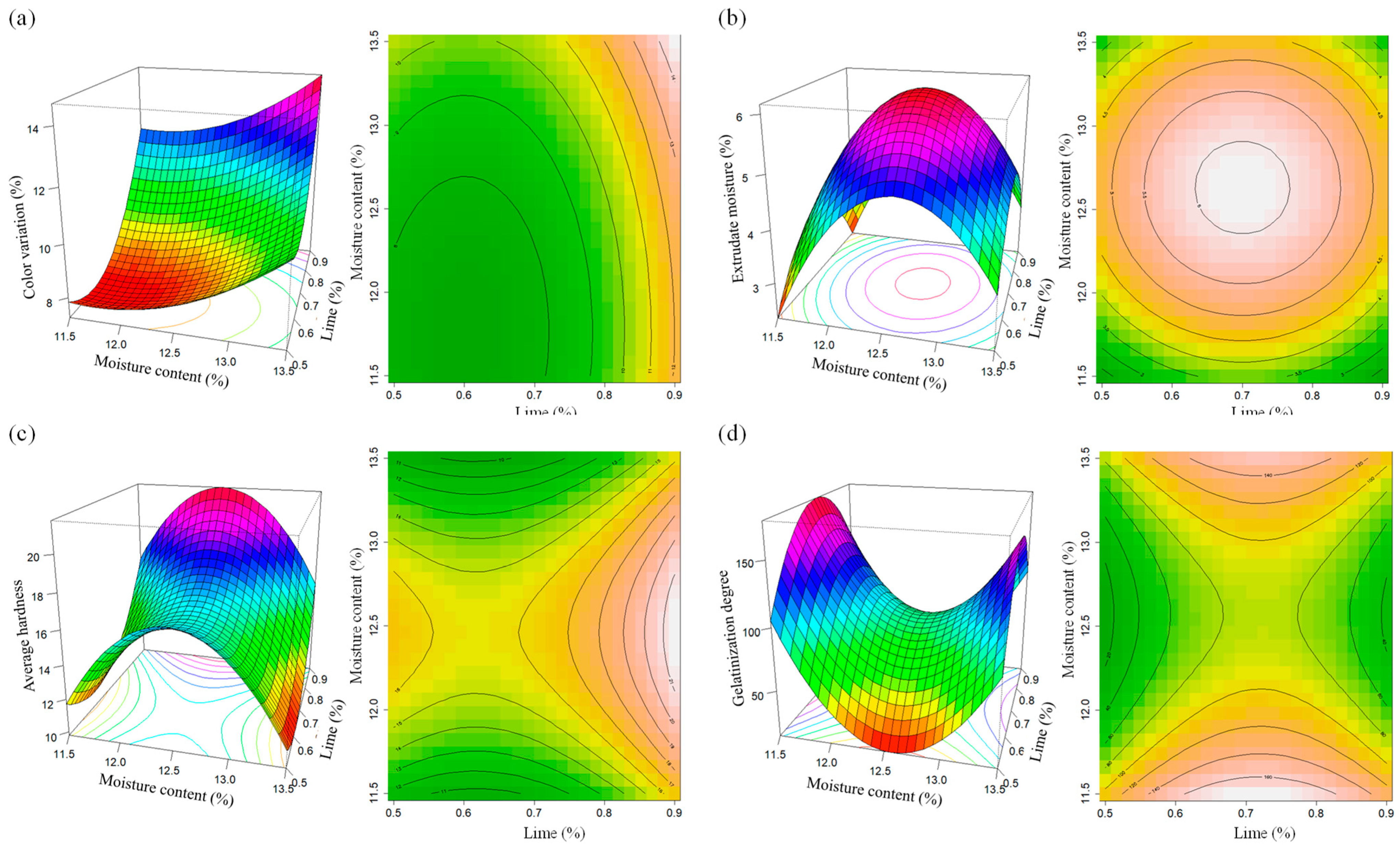
3.2.1. The Color of the Extruded Quispiño
3.2.2. The Moisture of the Extrudates
3.2.3. Hardness
3.2.4. Degree of Gelatinization
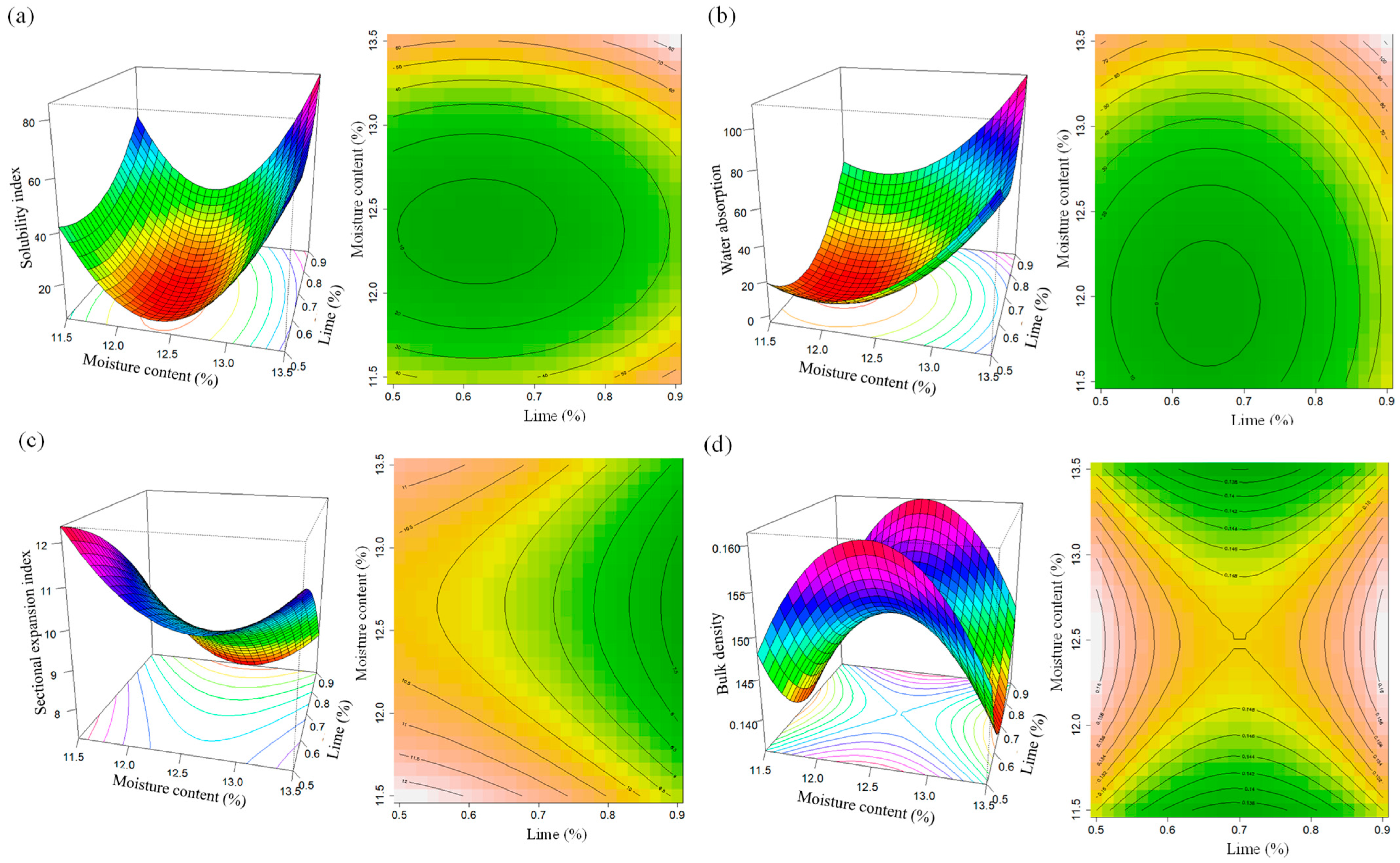
3.2.5. Water Solubility Index
3.2.6. Water Absorption Index
3.2.7. Sectional Expansion Index
3.2.8. Apparent Density
3.3. Optimization of Extruded Quispiño
3.4. Sensory Evaluation of Extruded Quispiño
3.5. Components of Acceptability of Extruded Quispiño
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haros, C.M.; Repo-Carrasco-Valencia, R.; Basilio-Atencio, J.; Luna-Mercado, G.I.; Pilco-Quesada, S.; Vidaurre-Ruiz, J.; Molina, L. Andean Ancient Grains: Nutritional Value and Novel Uses. Biol. Life Sci. Forum 2021, 8, 15. [Google Scholar] [CrossRef]
- Maldonado-Alvarado, P.; Abarca-Robles, J.; Pavón-Vargas, D.J.; Valencia-Chamorro, S.; Haros, C.M. Effect of Quinoa Germination on Its Nutritional Properties. Biol. Life Sci. Forum 2022, 17, 7. [Google Scholar] [CrossRef]
- Calizaya, F.; Gómez, L.; Zegarra, J.; Pozo, M.; Mindani, C.; Caira, C.; Calizaya, E. Unveiling Ancestral Sustainability: A Comprehensive Study of Economic, Environmental, and Social Factors in Potato and Quinoa Cultivation in the Highland Aynokas of Puno, Peru. Sustainability 2023, 15, 13163. [Google Scholar] [CrossRef]
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.D.S.; Pinheiro Sant’Ana, H.M.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, Functional, and Antinutritional Aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef]
- Maradini-Filho, A. Quinoa: Nutritional Aspects. J. Nutraceuticals Food Sci. 2017, 2, 3. [Google Scholar]
- Bastidas, E.G.; Roura, R.; Rizzolo, D.A.D.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd), from Nutritional Value to Potential Health Benefits: An Integrative Review. J. Nutr. Food Sci. 2016, 6. [Google Scholar] [CrossRef]
- Abugoch, J.L.E. Quinoa (Chenopodium quinoa Willd.): Composition, Chemistry, Nutritional, and Functional Properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar] [CrossRef]
- Valcárcel-Yamani, B.; Caetano Da, S.; Lannes, S. Applications of Quinoa (Chenopodium quinoa Willd.) and Amaranth (Amaranthus spp.) and Their Influence in the Nutritional Value of Cereal Based Foods. Food Public Health 2012, 2012, 265–275. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R. Andean Indigenous Food Crops: Nutritional Value and Bioactive Compounds; Publisher TURUN YLIOPISTO: Turku, Finland, 2011; ISBN 9789512946044. [Google Scholar]
- Repo-Carrasco, R.; Pilco, J.J.; Rene, C.; Zelada, E. Desarrollo y Elaboración de Un Snack Extruido a Partir de Quinua (Chenopodium quinoa Willd.) y Maíz (Zea mays L.); Universidad Nacional Agraria La Molina: Lima, Peru, 2011; Volume 29. [Google Scholar]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional Value and Use of the Andean Crops Quinoa (Chenopodium quinoa Willd.) and Kañiwa (Chenopodium pallidicaule). In Proceedings of the Food Reviews International; Haworth Press, Inc.: Binghamton, NY, USA, 2003; Volume 19, pp. 179–189. [Google Scholar]
- Berti, C.; Riso, P.; Monti, L.D.; Porrini, M. In Vitro Starch Digestibility and in Vivo Glucose Response of Gluten-Free Foods and Their Gluten Counterparts. Eur. J. Nutr. 2004, 43, 198–204. [Google Scholar] [CrossRef]
- Ruíz, F.; Nelina, A. Efectos Beneficiosos de Una Dieta Rica En Granos Enteros. Rev. Chil. Nutr. 2005, 32, 191–199. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Vidaurre-Ruiz, J.; Luna-Mercado, G.I. Development of Gluten-Free Breads Using Andean Native Grains Quinoa, Kañiwa, Kiwicha and Tarwi. Proceedings 2020, 53, 15. [Google Scholar] [CrossRef]
- Jaimes, A.; Ignacio, P.; Rivas, H. Glycemic Index of Quinoa (Chenopodium quinoa) in Healthy Subjects of Venezuela Venezuelan Nutrition and Health Study View Project Fruits and Vegetables in Venezuela View Project; Instituto Nacional de Nutrición: Caracas, Venezuela, 2017. [Google Scholar]
- Mindani, C.C.G.; Calizaya, L.; Fredy, G.; Cahuana, J.J.L.; Baldeón, E.O. Evaluación Del Cambio En El Quispiño Por La Adición de Hidróxido de Calcio. Rev. Soc. Quím. Perú 2023, 1, 1–10. [Google Scholar]
- Miguel, M. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef]
- Ortiz, M.R. Platos Típicos en el Aprendizaje de la Segunda Lengua en Educación Intelectual Bibligue de los Niños del Tercer Grado de Educación Primaria de Chucahuacas Chupa; Tesina; Universidad Nacional del Altiplano: Puno, Peru, 2011. [Google Scholar]
- Baker, P.T.; Mazess, R.B.; Collazos, C.C.; Moscoso, I.; Bravo, Y.; Castellanos, A.; Caceres, C.; Rosa, A.; Bradfield, R.B.; White, S.; et al. References and Notes; American Journal of Physical Anthropology: New York, NY, USA, 1962; Volume 102. [Google Scholar]
- Ofstehage, A. The Gift of the Middleman: An Ethnography of Quinoa Trading Networks in Los Lipez of Bolivia; University of New Mexico Press: Albuquerque, NM, USA, 2012. [Google Scholar]
- Burey, P.; Bhandari, B.R.; Rutgers, R.P.G.; Halley, P.J.; Torley, P.J.J. Confectionery Gels: A Review on Formulation, Rheological and Structural Aspects. Int. J. Food Prop. 2009, 12, 176–210. [Google Scholar] [CrossRef]
- Gomez, M.H.; Aguilera, J.M. Changes in the Starch Fraction During Extrusion-Cooking of Corn. J. Food Sci. 1983, 48, 378–381. [Google Scholar] [CrossRef]
- Kirby, A.R.; Ollett, A.-L.; Parker, R.; Smith, A.C. An Experimental Study of Screw Configuration Effects in the Twin-Screw Extrusion-Cooking of Maize Grits; The Institute of Food Science and Technology: London, UK, 1988; Volume 8. [Google Scholar]
- Kowalski, R.J.; Medina-Meza, I.G.; Thapa, B.B.; Murphy, K.M.; Ganjyal, G.M. Extrusion Processing Characteristics of Quinoa (Chenopodium quinoa Willd.) Var. Cherry Vanilla. J. Cereal Sci. 2016, 70, 91–98. [Google Scholar] [CrossRef]
- Ragaee, S.; Seetharaman, K.; Abdel-Aal, E.S.M. The Impact of Milling and Thermal Processing on Phenolic Compounds in Cereal Grains. Crit. Rev. Food Sci. Nutr. 2014, 54, 837–849. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Y.; Wang, Z.; Chen, F.; Ojokoh, A.O. Reaction Kinetics in Food Extrusion: Methods and Results. Crit. Rev. Food Sci. Nutr. 2011, 51, 835–854. [Google Scholar] [CrossRef]
- Song, J.; Shao, Y.; Chen, X.; Li, X. Release of Characteristic Phenolics of Quinoa Based on Extrusion Technique. Food Chem. 2022, 374, 128780. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Naczk, M.; Shahidi, F. Effect of Processing on the Antioxidant Activity of Millet Grains. Food Chem. 2012, 133, 1–9. [Google Scholar] [CrossRef]
- Pitts, K.F.; Favaro, J.; Austin, P.; Day, L. Co-Effect of Salt and Sugar on Extrusion Processing, Rheology, Structure and Fracture Mechanical Properties of Wheat-Corn Blend. J. Food Eng. 2014, 127, 58–66. [Google Scholar] [CrossRef]
- Bergesse, A.E.; Boiocchi, P.N.; Calandri, E.L.; Cervilla, N.S.; Gianna, V.; Guzmán, C.A.; Miranda, V.; Patricia, P.; Montoya, P.A.; Mufari, J.R. Aprovechamiento Integral del Grano de Quinua; Editorial Universitaria: La Paz, Bolivia, 2015. [Google Scholar]
- Uauy, R.; Teresa, M. Estimación de Las Necesidades de Energía a Nivel Nacional: Uso Del Enfoque FAO/OMS/UNU 1985. Arch. Latinoam. Nutr. 1988, 38, 466–482. [Google Scholar] [PubMed]
- INEM 517:2012; Norma Técnica Ecuatoriana. Instituto Ecuatoriano de Normalizacion: Quito, Ecuador, 2012; pp. 1–6.
- Paula, A.M.; Conti-Silva, A.C. Texture Profile and Correlation between Sensory and Instrumental Analyses on Extruded Snacks. J. Food Eng. 2014, 121, 9–14. [Google Scholar] [CrossRef]
- Coulter, L.A.; Lorenz, K. Extruded Corn Grits Quinoa Blends: II. Physical Characteristics of Extruded Products. J. Food Process. Preserv. 1991, 15, 243–259. [Google Scholar] [CrossRef]
- Ilo, S.; Liu, Y.; Berghofer, E. Extrusion Cooking of Rice Flour and Amaranth Blends. LWT-Food Sci. Technol. 1999, 32, 79–88. [Google Scholar] [CrossRef]
- Nayak, B.; Berrios, J.D.J.; Powers, J.R.; Tang, J.; Ji, Y. Colored Potatoes (Solanum tuberosum L.) Dried for Antioxidant-Rich Value-Added Foods. J. Food Process. Preserv. 2011, 35, 571–580. [Google Scholar] [CrossRef]
- Saeleaw, M.; Dürrschmid, K.; Schleining, G. The Effect of Extrusion Conditions on Mechanical-Sound and Sensory Evaluation of Rye Expanded Snack. J. Food Eng. 2012, 110, 532–540. [Google Scholar] [CrossRef]
- Menis, M.E.C.; Milani, T.M.G.; Jordano, A.; Boscolo, M.; Conti-Silva, A.C. Extrusion of Flavored Corn Grits: Structural Characteristics, Volatile Compounds Retention and Sensory Acceptability. LWT 2013, 54, 434–439. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Luo, S.; Li, C.; Ye, J.; Liu, C.; Gilbert, R.G. Physicochemical and Structural Properties of Pregelatinized Starch Prepared by Improved Extrusion Cooking Technology. Carbohydr. Polym. 2017, 175, 265–272. [Google Scholar] [CrossRef]
- Anderson, R.A.; Conway, H.F.; Peplinski, A. 1 Gelatinization of Corn Grits by Roll Cooking, Extrusion Cooking and Steaming. Starch-Stärke 1970, 22, 130–135. [Google Scholar] [CrossRef]
- Vílchez, T.L.M.; Guevara, P.A.; Zelada, C.R.E. Influencia Del Tamaño de Partícula, Humedad y Temperatura En El Grado de Gelatinización Durante El Proceso de Extrusión de Maca (Lepidium Meyenii Walp). Rev. Soc. Quím. Perú 2018, 78, 126–137. [Google Scholar]
- Akande, O.A.A.; Nakimbugwe, D.; Mukisa, I.M. Optimization of Extrusion Conditions for the Production of Instant Grain Amaranth-Based Porridge Flour. Food Sci. Nutr. 2017, 5, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Pabon, K.S.; Roa-Acosta, D.F.; Hoyos-Concha, J.L.; Bravo-Gómez, J.E.; Ortiz-Gómez, V. Quinoa Snack Production at an Industrial Level: Effect of Extrusion and Baking on Digestibility, Bioactive, Rheological, and Physical Properties. Foods 2022, 11, 3383. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Alvarado, P.; Trujillo, M. Gluten-Free Couscous Made from Quinoa Sprouts: Study of Shelf Life. Biol. Life Sci. Forum 2022, 17, 9. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, Y.-J.; Lim, J.-G.; Jeon, J.-H.; Yoon, K.-S.; Fernández-López, J.; Pateiro, M.; Victoria, M.; Martínez, S. Effect of Quinoa (Chenopodium quinoa Willd.) Starch and Seeds on the Physicochemical and Textural and Sensory Properties of Chicken Meatballs during Frozen Storage. Foods 2021, 10, 1601. [Google Scholar] [CrossRef]
- Solaesa, Á.G.; Villanueva, M.; Beltrán, S.; Ronda, F. Characterization of Quinoa Defatted by Supercritical Carbon Dioxide. Starch Enzymatic Susceptibility and Structural, Pasting and Thermal Properties. Food Bioprocess Technol. 2019, 12, 1593–1602. [Google Scholar] [CrossRef]
- Ward, S.M. Response to Selection for Reduced Grain Saponin Content in Quinoa (Chenopodium quinoa Willd.). Field Crops Res. 2000, 68, 157–163. [Google Scholar] [CrossRef]
- Mezzatesta, P.; Farah, S.; Di Fabio, A.; Emilia, R. Variation of the Nutritional Composition of Quinoa According to the Processing Used. Proceedings 2020, 53, 4. [Google Scholar] [CrossRef]
- Coţovanu, I.; Ungureanu-Iuga, M.; Mironeasa, S. Investigation of Quinoa Seeds Fractions and Their Application in Wheat Bread Production. Plants 2021, 10, 2150. [Google Scholar] [CrossRef]
- De Bock, P.; Van Bockstaele, F.; Muylle, H.; Quataert, P.; Vermeir, P.; Eeckhout, M.; Cnops, G. Yield and Nutritional Characterization of Thirteen Quinoa (Chenopodium quinoa Willd.) Varieties Grown in North-west Europe—Part i. Plants 2021, 10, 2689. [Google Scholar] [CrossRef]
- Amiryousefi, M.; Tadayon, M.R.; Ebrahimi, R. Energy and Exergy Efficiencies Assessment for Two Quinoa Cultivars Productions. Energy Rep. 2021, 7, 2324–2331. [Google Scholar] [CrossRef]
- NTP 205.062; Norma Técnica Peruana: Métodos de Ensayo de Productos de Panadería. Instituto Nacional de Calidad (INACAL): Lima, Peru, 2014; pp. 1–31.
- Koziol, M.J. Chemical Composition and Nutritional Evaluation of Quinoa (Chenopodium quinoa Willd.). J. Food Compos. Anal. 1992, 5, 35–68. [Google Scholar] [CrossRef]
- Rafik, S.; Rahmani, M.; Rodriguez, J.P.; Andam, S.; Ezzariai, A.; El Gharous, M.; Karboune, S.; Choukr-Allah, R.; Hirich, A. How Does Mechanical Pearling Affect Quinoa Nutrients and Saponin Contents? Plants 2021, 10, 1133. [Google Scholar] [CrossRef] [PubMed]
- Basilio, A.J. Obtención de Una Mezcla Alimenticia a Partir de Quinua (Chenopodium quinoa) y Kiwicha (Amaranthus caudatus) Extruidas y Molidas Lupinus Mutabilis); Universidad Nacional Agraria La Molina: Lima, Peru, 2020. [Google Scholar]
- Menegassi, B.; Pilosof, A.M.R.; Arêas, J.A.G. Comparison of Properties of Native and Extruded Amaranth (Amaranthus cruentus L.—BRS Alegría) Flour. LWT 2011, 44, 1915–1921. [Google Scholar] [CrossRef]
- Kaspchak, E.; de Oliveira, M.A.S.; Simas, F.F.; Franco, C.R.C.; Silveira, J.L.M.; Mafra, M.R.R.; Igarashi-Mafra, L. Determination of Heat-Set Gelation Capacity of a Quinoa Protein Isolate (Chenopodium quinoa) by Dynamic Oscillatory Rheological Analysis. Food Chem. 2017, 232, 263–271. [Google Scholar] [CrossRef]
- Chávez-Jáuregui, R.N.; Silva, M.E.M.P.; Arêas, J.A.G. Extrusion Cooking Process for Amaranth (Amaranthus caudatus L.); Institute of Food Technologists: Chicago, IL, USA, 2000; Volume 65. [Google Scholar]
- Ramos, D.J.M.; Kirjoranta, S.; Tenitz, S.; Penttilä, P.A.; Serimaa, R.; Lampi, A.M.; Jouppila, K. Use of Amaranth, Quinoa and Kañiwa in Extruded Corn-Based Snacks. J. Cereal Sci. 2013, 58, 59–67. [Google Scholar] [CrossRef]
- Doğan, H.; Karwe, M. V Physicochemical Properties of Quinoa Extrudates. Food Sci. Technol. Int. 2003, 9, 101–114. [Google Scholar] [CrossRef]
- Ruales, J.; Valencia, S.; Nair, B. Effect of Processing on the Physico-Chemical Characteristics of Quinoa Flour (Chenopodium quinoa, Willd). Starch-Stärke 1993, 45, 13–19. [Google Scholar] [CrossRef]
- Robin, F.; Théoduloz, C.; Srichuwong, S. Properties of Extruded Whole Grain Cereals and Pseudocereals Flours. Int. J. Food Sci. Technol. 2015, 50, 2152–2159. [Google Scholar] [CrossRef]
- Ramos-Diaz, J.M.; Rinnan, Å.; Jouppila, K. Application of NIR Imaging to the Study of Expanded Snacks Containing Amaranth, Quinoa and Kañiwa. LWT 2019, 102, 8–14. [Google Scholar] [CrossRef]
- González, R.; Carrara, C.; Tosi, E.; Añón, M.C.; Pilosof, A. Amaranth Starch-Rich Fraction Properties Modified by Extrusion and Fluidized Bed Heating. LWT 2007, 40, 136–143. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Schmiele, M.; Steel, C.J. Development of Whole Grain Wheat Flour Extruded Cereal and Process Impacts on Color, Expansion, and Dry and Bowl-Life Texture. LWT 2017, 75, 261–270. [Google Scholar] [CrossRef]
- Pérez, K.; Elías, C.; Delgado, V. High-Protein Snack: An Extruded from Quinoa (Chenopodium quinoa Willd.), Tarwi (Lupinus mutabilis Sweet), and Sweet Potato (Ipomoea batatas L.). Sci. Agropecu. 2017, 8, 377–388. [Google Scholar] [CrossRef]
- Dushkova, M.; Simitchiev, A.; Petrova, T.; Menkov, N.; Desseva, I.; Mihaylova, D. Physical and Functional Characteristics of Extrudates Prepared from Quinoa Enriched with Goji Berry. Appl. Sci. 2023, 13, 3503. [Google Scholar] [CrossRef]
- Zambrano, Y.; Contardo, I.; Moreno, M.C.; Bouchon, P. Effect of Extrusion Temperature and Feed Moisture Content on the Microstructural Properties of Rice-Flour Pellets and Their Impact on the Expanded Product. Foods 2022, 11, 198. [Google Scholar] [CrossRef]
- Granato, D.; Masson, M.L. Instrumental Color and Sensory Acceptance of Soy-Based Emulsions: A Response Surface Approach Cor Instrumental e Aceitação Sensorial de Emulsões à Base de Soja: Uma Abordagem Através Da Metodologia de Superfície de Resposta. Cienc. Tecnol. Aliment. 2010, 30, 1090–1096. [Google Scholar] [CrossRef]
- Aguiar, E.V.; Santos, F.G.; Centeno, A.; Capriles, V. Defining Amaranth, Buckwheat and Quinoa Flour Levels in Gluten-Free Bread: A Simultaneous Improvement on Physical Properties, Acceptability and Nutrient Composition through Mixture Design. Foods 2022, 11, 848. [Google Scholar] [CrossRef]

| Component | Quinoa Grains (g/100 g) | Quinoa Scarified Washed and Dried (g/100 g) |
|---|---|---|
| Carbohydrates (g/100 g) | 62.97 ± 0.06 | 64.02 ± 0.22 |
| Starch (g/100 g) | NR | 60.7 ± 0.02 4 |
| Amylopectin (%) | NR | 20.65 |
| Protein (g/100 g) | 13.27 ± 0.06 | 12.90 ± 0.10 |
| Moisture content (%) | 11.2 ± 0.01 | 11.81 ± 0.03 |
| Fiber (g/100 g) | 4.39 ± 0.01 | 2.76 ± 0.14 |
| Fat (g/100 g) | 5.53 ± 0.01 | 6.18 ± 0.03 |
| Ash (g/100 g. dry basis) | 2.66 ± 0.01 | 2.34 ± 0.03 |
| Energy (kJ/100 g) | 1485.31 ± 0.38 | 1520.55 ± 1.80 |
| Calcium (mg/100 g) | NR | 9.49 ± 0.01 3 |
| Iron (mg/100 g) | NR | 2.26 ± 0.01 3 |
| Parameters | g/100 g of Proteins | mg/kg | 1 mg/kg/day |
|---|---|---|---|
| Glutamic Ac. | 13.02 | 130,200 | - |
| Arginine | 8.14 | 81,400 | - |
| Aspartic acid | 7.78 | 77,800 | - |
| Leucine | 6.52 | 65,200 | 14 |
| Phenylalanine and Tyrosine | 6.45 | 64,500 | 14 |
| Isoleucine | 5.94 | 59,400 | 10 |
| Lysine | 5.88 | 58,800 | 12 |
| Glycine | 4.92 | 49,200 | - |
| Methionine ** and Cystine | 4.51 | 45,100 | 13 |
| Alanine | 3.91 | 39,100 | - |
| Serine | 3.72 | 37,200 | - |
| Threonine | 3.51 | 35,100 | 7 |
| Valine | 3.45 | 34,500 | 10 |
| Proline | 3.26 | 32,600 | - |
| Histidine | 2.82 | 28,200 | - |
| Tryptophan | 1.24 | 12,400 | 3.5 |
| Moisture Content | 12.5 (%) | 13.5 (%) |
|---|---|---|
| ml (g) | Mass of Water (g) | Mass of Water (g) |
| 1000 | 0.1001 | 0.2003 |
| 2000 | 0.2003 | 0.4005 |
| 4000 | 0.4005 | 0.8011 |
| 6000 | 0.6008 | 1.2016 |
| Run | Extrusion Parameters | Color Parameters | |||||
|---|---|---|---|---|---|---|---|
| Lime (%) | Moisture (%) | L* | a* | b* | C* | °H | |
| 1 | 0.5 | 11.5 | 69.12 ± 0.64 | 4.52 ± 0.09 | 23.80 ± 0.86 | 24.23 ± 0.83 | 79.23 ± 0.59 |
| 2 | 0.7 | 11.5 | 68.84 ± 0.28 | 4.97 ± 0.21 | 23.77 ± 0.84 | 24.29 ± 0.86 | 78.19 ± 0.08 |
| 3 | 0.9 | 11.5 | 67.36 ± 0.35 | 4.45 ± 0.20 | 24.69 ± 0.52 | 25.09 ± 0.54 | 79.78 ± 0.33 |
| 4 | 0.5 | 12.5 | 66.66 ± 0.50 | 5.38 ± 0.02 | 23.98 ± 0.30 | 24.57 ± 0.29 | 77.34 ± 0.15 |
| 5 | 0.7 | 12.5 | 67.53 ± 0.71 | 4.16 ± 0.43 | 24.54 ± 0.82 | 24.90 ± 0.77 | 80.37 ± 1.18 |
| 6 | 0.9 | 12.5 | 63.70 ± 0.65 | 5.21 ± 0.10 | 25.34 ± 0.80 | 25.87 ± 0.80 | 78.37 ± 0.23 |
| 7 | 0.5 | 13.5 | 66.96 ± 1.31 | 5.79 ± 0.17 | 24.92 ± 0.23 | 25.59 ± 0.20 | 76.92 ± 0.46 |
| 8 | 0.7 | 13.5 | 63.82 ± 0.74 | 6.35 ± 0.15 | 24.55 ± 0.64 | 25.36 ± 0.63 | 75.50 ± 0.39 |
| 9 | 0.9 | 13.5 | 64.17 ± 0.26 | 7.59 ± 0.29 | 24.55 ± 0.63 | 25.69 ± 0.69 | 72.81 ± 0.24 |
| Unextruded quinoa grains | 75.28 ± 0.43 | 3.41 ± 0.16 | 22.09 ± 0.26 | 22.35 ± 0.23 | 81.23 ± 0.50 | ||
| Run | Extrusion Parameters | Physical Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lime (%) | Moisture (%) | ΔE | Hf (%) | Hardness (N) | DG | WSI (%) | WAI (g/g) | SEI | AD (g/cm3) | |
| 1 | 0.5 | 11.5 | 6.54 ± 0.89 | 4.51 ± 0.02 | 11.60 ± 3.06 | 47 ± 41.68 | 28.06 ± 7.27 | 3.73 ± 0.63 | 13.08 ±3.92 | 0.13 ± 0.03 |
| 2 | 0.7 | 11.5 | 6.90 ± 0.41 | 3.08 ± 0.03 | 9.94 ± 2.03 | 237 ± 21.51 | 27.33 ± 2.71 | 4.68 ± 0.98 | 11.41 ± 2.97 | 0.11 ± 0.06 |
| 3 | 0.9 | 11.5 | 8.41 ± 0.88 | 3.33 ± 0.06 | 12.42 ± 2.64 | 247 ± 33.73 | 60.73 ± 4.64 | 66.62 ± 0.07 | 9.00 ± 2.43 | 0.17 ± 0.07 |
| 4 | 0.5 | 12.5 | 9.07 ± 0.84 | 6.12 ± 0.13 | 13.25 ± 2.95 | 22 ± 3.54 | 8.94 ± 0.11 | 6.94 ± 0.64 | 8.41 ± 1.73 | 0.16 ± 0.03 |
| 5 | 0.7 | 12.5 | 8.24 ± 0.84 | 6.12 ± 0.16 | 13.16 ± 4.20 | 84 ± 7.07 | 8.70 ± 0.43 | 6.35 ± 0.39 | 9.20 ± 2.00 | 0.15 ± 0.09 |
| 6 | 0.9 | 12.5 | 12.20 ± 0.24 | 6.00 ± 0.06 | 25.86 ± 8.85 | 69 ± 15.41 | 31.83 ± 17.25 | 66.89 ± 0.03 | 8.24 ± 1.62 | 0.16 ± 0.07 |
| 7 | 0.5 | 13.5 | 9.14 ± 1.47 | 5.66 ± 0.15 | 7.25 ± 1.60 | 133 ± 103.62 | 28.29 ± 1.28 | 4.18 ± 0.51 | 9.27 ± 2.14 | 0.09 ± 0.06 |
| 8 | 0.7 | 13.5 | 12.12 ± 0.93 | 5.04 ± 0.09 | 9.97 ± 2.46 | 137 ± 62.85 | 71.61 ± 9.60 | 66.51 ± 0.06 | 9.43 ± 2.70 | 0.12 ± 0.06 |
| 9 | 0.9 | 13.5 | 12.14 ± 0.22 | 4.63 ± 0.06 | 11.92 ± 2.99 | 102 ± 49.50 | 47.09 ± 16.39 | 66.65 ± 0.26 | 7.33 ± 1.23 | 0.17 ± 0.09 |
| Quinoa flour | - | - | - | 4 ± 3.54 | 0.17 ± 7.88 | - | - | - | ||
| Extruded chocolate-flavored corn | 11.65 ± 0.47 | |||||||||
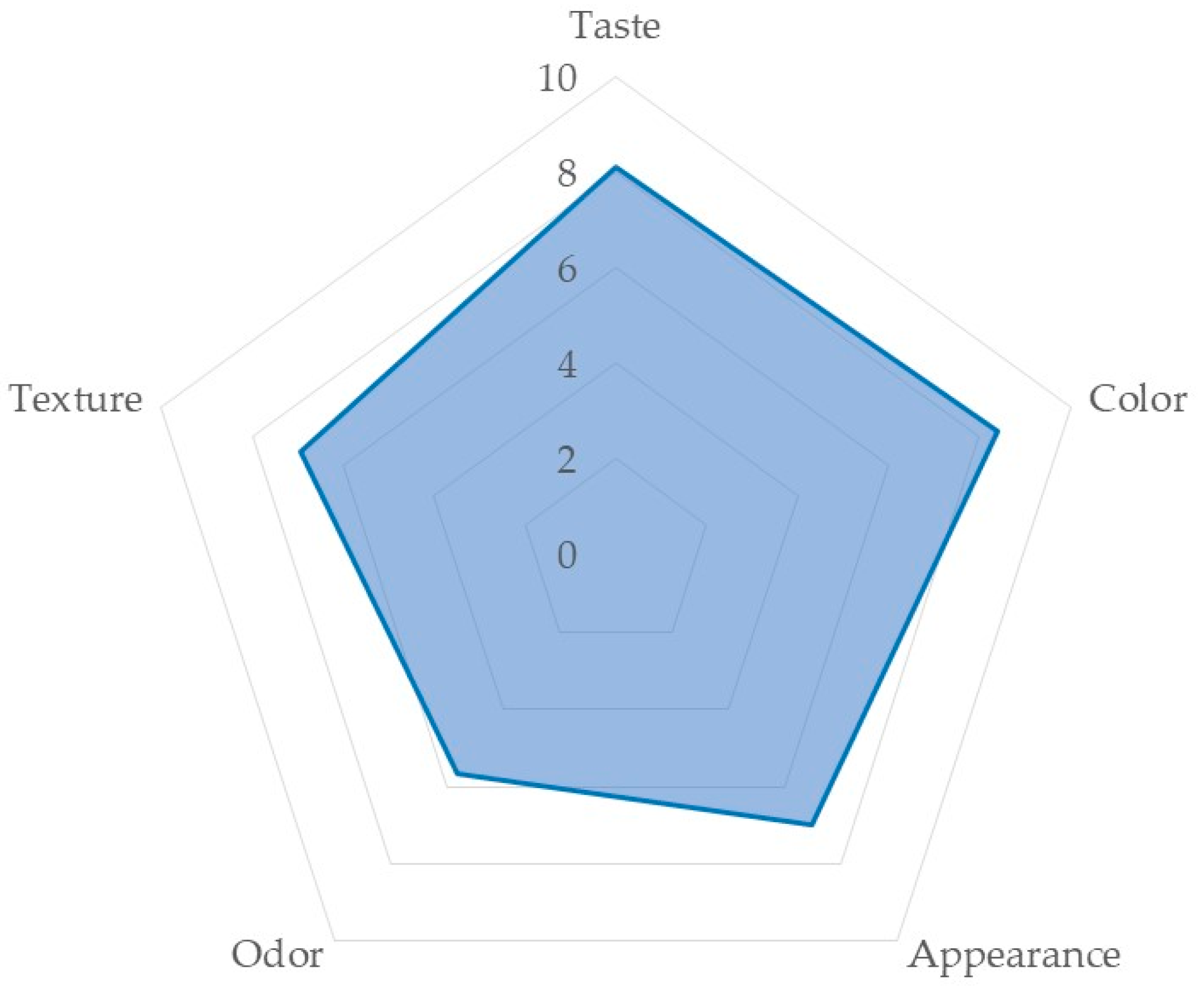
| Aspects | Taste | Color | Appearance | Odor | Texture |
|---|---|---|---|---|---|
| Average | 7 ± 2.06 | 8 ± 3.00 | 6 ± 2.59 | 4 ± 3.29 | 6 ± 3.19 |
| Coefficient of variation | 27.74 | 39.86 | 42.73 | 86.69 | 57.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mindani, C.; Baldeón, E.O.; Ibáñez, V.; Calizaya, F.; Taipe, C.; Zegarra, J.; Pozo, M. Effects of Moisture Content and Lime Concentrate on Physiochemical, Mechanical, and Sensory Properties of Quinoa Snacks: An Ancient Andean Crop in Puno, Peru. AgriEngineering 2024, 6, 3931-3951. https://doi.org/10.3390/agriengineering6040223
Mindani C, Baldeón EO, Ibáñez V, Calizaya F, Taipe C, Zegarra J, Pozo M. Effects of Moisture Content and Lime Concentrate on Physiochemical, Mechanical, and Sensory Properties of Quinoa Snacks: An Ancient Andean Crop in Puno, Peru. AgriEngineering. 2024; 6(4):3931-3951. https://doi.org/10.3390/agriengineering6040223
Chicago/Turabian StyleMindani, Carmen, Edwin O. Baldeón, Vladimiro Ibáñez, Fredy Calizaya, Carmen Taipe, Jorge Zegarra, and Melvin Pozo. 2024. "Effects of Moisture Content and Lime Concentrate on Physiochemical, Mechanical, and Sensory Properties of Quinoa Snacks: An Ancient Andean Crop in Puno, Peru" AgriEngineering 6, no. 4: 3931-3951. https://doi.org/10.3390/agriengineering6040223
APA StyleMindani, C., Baldeón, E. O., Ibáñez, V., Calizaya, F., Taipe, C., Zegarra, J., & Pozo, M. (2024). Effects of Moisture Content and Lime Concentrate on Physiochemical, Mechanical, and Sensory Properties of Quinoa Snacks: An Ancient Andean Crop in Puno, Peru. AgriEngineering, 6(4), 3931-3951. https://doi.org/10.3390/agriengineering6040223






