Infrared Thermographic Signal Analysis of Bioactive Edible Oils Using CNNs for Quality Assessment
Abstract
1. Introduction
2. Nutritional and Health Challenges of Edible Oils
2.1. Olive Oil: Nutritional and Functional Profile
2.2. Sunflower Seed Oil: Nutritional and Functional Profile
2.3. Tomato Seed Oil: Nutritional and Functional Profile
2.4. Pumpkin Seed Oil: Nutritional and Functional Profile
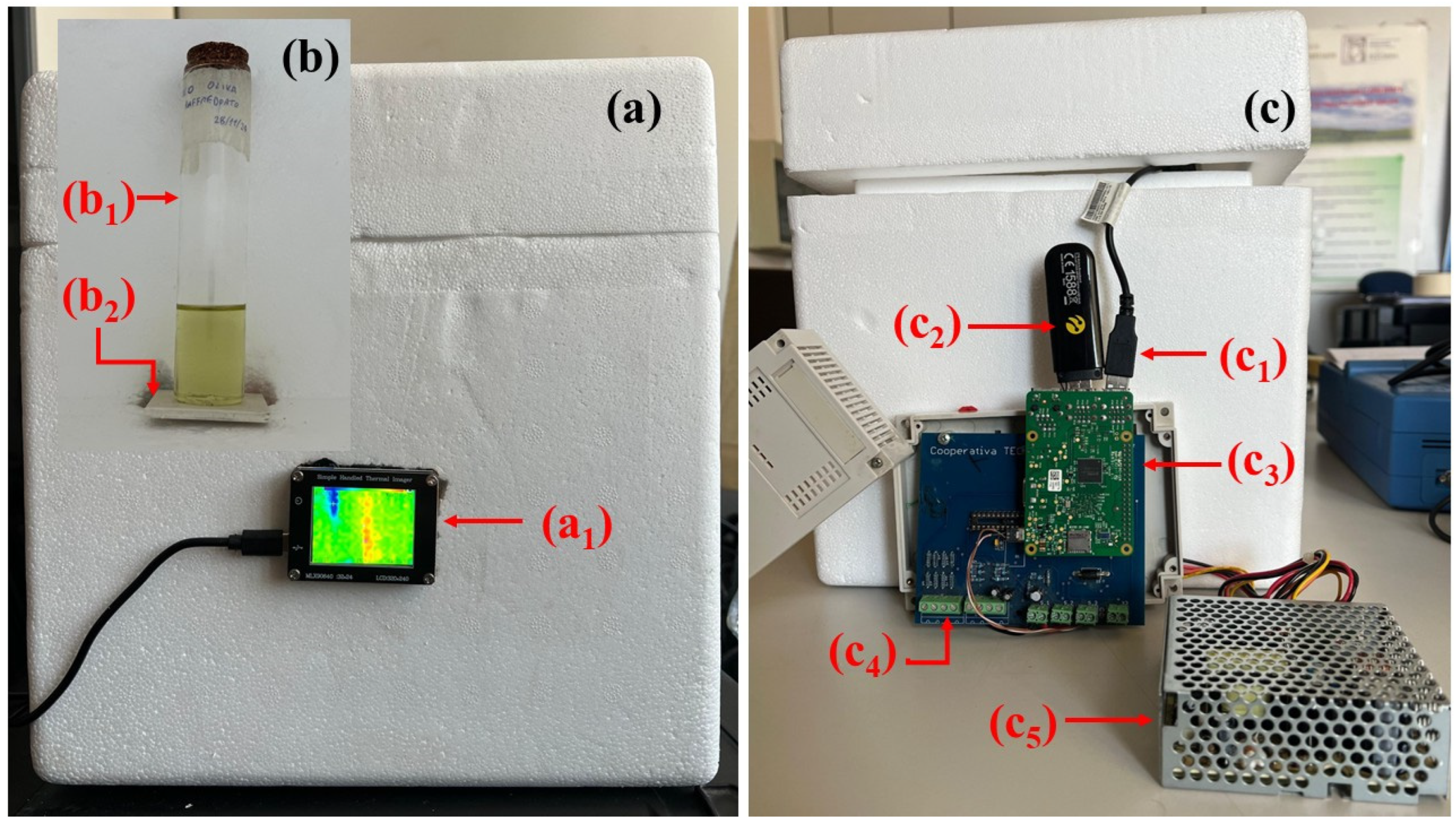
3. Materials and Methods: Thermographic Signal Acquisition System
4. Post-Processing of IR Signals: Proposed Supervised Learning Model
4.1. Data Assessment
4.2. Proposed Model Architecture
5. Results and Performance Evaluation
5.1. Classification Performance
5.2. Ablation Studies
5.3. Implications and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef] [PubMed]
- Ambroselli, D.; Masciulli, F.; Romano, E.; Catanzaro, G.; Besharat, Z.M.; Massari, M.C.; Ferretti, E.; Migliaccio, S.; Izzo, L.; Ritieni, A.; et al. New Advances in Metabolic Syndrome, from Prevention to Treatment: The Role of Diet and Food. Nutrients 2023, 15, 640. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Bai, Y.; Tian, H.; Zhao, X. The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review. Molecules 2023, 28, 6393. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, Y.; Wang, X.; Bai, J.; Lin, L.; Luo, F.; Zhong, H. A Comprehensive Review of Health-Benefiting Components in Rapeseed Oil. Nutrients 2023, 15, 999. [Google Scholar] [CrossRef]
- Bakhshabadi, H.; Ganje, M.; Gharekhani, M.; Mohammadi-Moghaddam, T.; Aulestia, C.; Morshedi, A. A Review of New Methods for Extracting Oil from Plants to Enhance the Efficiency and Physicochemical Properties of the Extracted Oils. Processes 2025, 13, 1124. [Google Scholar] [CrossRef]
- Masoodi, L.; Gull, A.; Masoodi, F.A.; Gani, A.; Nissar, J.; Ahad, T.; Nayik, G.A.; Mukarram, S.A.; Kovács, B.; Prokisch, J.; et al. An Overview on Traditional vs. Green Technology of Extraction Methods for Producing High Quality Walnut Oil. Agronomy 2022, 12, 2258. [Google Scholar] [CrossRef]
- Fatima, S.; Kumar, V.; Bhadauria, G.; Verma, H. Quality indicators based rapid test kits for detection of frying oil quality: A review. Food Chem. Adv. 2023, 2, 100305. [Google Scholar] [CrossRef]
- Dhotre, I. A comprehensive review on progression and innovations in microwave assisted extraction technology for essential oils. J. Chem. Technol. Biotechnol. 2025, 100, 894–907. [Google Scholar] [CrossRef]
- Altaf, M.A.; Ahn, J.; Khan, D.; Kim, M.Y. Usage of IR sensors in the HVAC systems, vehicle and manufacturing industries: A review. IEEE Sens. J. 2022, 22, 9164–9176. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Khabibullin, V.R.; Usoltseva, L.O.; Vyrko, E.A.; Mikheev, I.V.; Volkov, D.S. Photothermal and optoacoustic spectroscopy: State of the art and prospects. Phys.-Uspekhi 2022, 65, 270. [Google Scholar] [CrossRef]
- Li, K.; Kinoshita, Y.; Sakai, D.; Kawano, Y. Recent Progress in Development of Carbon-Nanotube-Based Photo-Thermoelectric Sensors and Their Applications in Ubiquitous Non-Destructive Inspections. Micromachines 2023, 14, 61. [Google Scholar] [CrossRef]
- Pratticò, D.; Laganà, F.; Oliva, G.; Fiorillo, A.S.; Pullano, S.A.; Calcagno, S.; De Carlo, D.; La Foresta, F. Integration of LSTM and U-Net models for monitoring electrical absorption with a system of sensors and electronic circuits. IEEE Trans. Instrum. Meas. 2025, 74, 253331. [Google Scholar] [CrossRef]
- Pratticò, D.; Laganà, F.; Oliva, G.; Fiorillo, A.S.; Pullano, S.A.; Calcagno, S.; De Carlo, D.; La Foresta, F. Sensors and Integrated Electronic Circuits for Monitoring Machinery On Wastewater Treatment: Artificial Intelligence Approach. In Proceedings of the 2024 IEEE Sensors Applications Symposium (SAS), Naples, Italy, 23–25 July 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Im, S.K.; Pearmain, A.J. Unequal error protection with the H.264 flexible macroblock ordering. In Proceedings of the Visual Communications and Image Processing 2005, Beijing, China, 24 June 2005; Volume 5960, pp. 1033–1040. [Google Scholar] [CrossRef]
- Chan, K.H.; Im, S.K. Sentiment analysis by using Naïve-Bayes classifier with stacked CARU. Electron. Lett. 2022, 58, 411–413. [Google Scholar] [CrossRef]
- Melnikov, P.; Bobrov, A.; Marfin, Y. On the Use of Polymer-Based Composites for the Creation of Optical Sensors: A Review. Polymers 2022, 14, 4448. [Google Scholar] [CrossRef]
- Chen, W.; Ball, A.S.; Cole, I.; Yin, H. Metal-Doped Carbon Dots as Fenton-like Catalysts and Their Applications in Pollutant Degradation and Sensing. Sustainability 2025, 17, 3642. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Strani, L.; Cocchi, M. Toward the Non-Targeted Detection of Adulterated Virgin Olive Oil with Edible Oils via FTIR Spectroscopy & Chemometrics: Research Methodology Trends, Gaps and Future Perspectives. Molecules 2023, 28, 337. [Google Scholar] [CrossRef]
- Tarapoulouzi, M.; Agriopoulou, S.; Koidis, A.; Proestos, C.; Enshasy, H.A.E.; Varzakas, T. Recent Advances in Analytical Methods for the Detection of Olive Oil Oxidation Status during Storage along with Chemometrics, Authenticity and Fraud Studies. Biomolecules 2022, 12, 1180. [Google Scholar] [CrossRef]
- Pielesz, A.; Biniaś, D.; Waksmańska, W.; Bobiński, R. Lipid bands of approx. 1740 cm−1 as spectral biomarkers and image of tissue oxidative stress. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 121926. [Google Scholar] [CrossRef]
- Pullano, S.A.; Marcianò, G.; Bianco, M.G.; Oliva, G.; Rania, V.; Vocca, C.; Cione, E.; De Sarro, G.; Gallelli, L.; Romeo, P.; et al. FT-IR Analysis of Structural Changes in Ketoprofen Lysine Salt and KiOil Caused by a Pulsed Magnetic Field. Bioengineering 2022, 9, 503. [Google Scholar] [CrossRef]
- Sivakumar, V. Towards environmental protection and process safety in leather processing—A comprehensive analysis and review. Process Saf. Environ. Prot. 2022, 163, 703–726. [Google Scholar] [CrossRef]
- Tong, P.H.; Wu, T.Y.; Li, M.; Wang, H.B.; Zheng, F.; Xu, L.; Dou, W.T. Fluorescent probes for the visualization of membrane microdomain, deformation, and fusion. Smart Mol. 2025, 3, e20240059. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.; Sappa, A.D. Multispectral semantic segmentation for land cover classification: An overview. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2024, 17, 14295–14336. [Google Scholar] [CrossRef]
- Menniti, M.; Laganà, F.; Oliva, G.; Bianco, M.; Fiorillo, A.S.; Pullano, S.A. Development of Non-Invasive Ventilator for Homecare and Patient Monitoring System. Electronics 2024, 13, 790. [Google Scholar] [CrossRef]
- Menniti, M.; Oliva, G.; Laganà, F.; Bianco, M.G.; Fiorillo, A.S.; Pullano, S.A. Portable Non-Invasive Ventilator for Homecare and Patients Monitoring System. In Proceedings of the 2023 IEEE International Symposium on MeMeA, Jeju, Republic of Korea, 14–16 June 2023; pp. 1–5. [Google Scholar] [CrossRef]
- Laganà, F.; Bibbò, L.; Calcagno, S.; De Carlo, D.; Pullano, S.A.; Pratticò, D.; Angiulli, G. Smart Electronic Device-Based Monitoring of SAR and Temperature Variations in Indoor Human Tissue Interaction. Appl. Sci. 2025, 15, 2439. [Google Scholar] [CrossRef]
- Zhang, W.; Kasun, L.C.; Wang, Q.J.; Zheng, Y.; Lin, Z. A Review of Machine Learning for Near-Infrared Spectroscopy. Sensors 2022, 22, 9764. [Google Scholar] [CrossRef]
- Mohd Hilmi Tan, M.I.S.; Jamlos, M.F.; Omar, A.F.; Dzaharudin, F.; Chalermwisutkul, S.; Akkaraekthalin, P. Ganoderma boninense Disease Detection by Near-Infrared Spectroscopy Classification: A Review. Sensors 2021, 21, 3052. [Google Scholar] [CrossRef]
- Mabrouk, M.; Das, D.B.; Salem, Z.A.; Beherei, H.H. Nanomaterials for Biomedical Applications: Production, Characterisations, Recent Trends and Difficulties. Molecules 2021, 26, 1077. [Google Scholar] [CrossRef]
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef]
- Laganà, F.; Pellicanò, D.; Arruzzo, M.; Pratticò, D.; Pullano, S.A.; Fiorillo, A.S. FEM-Based Modelling and AI-Enhanced Monitoring System for Upper Limb Rehabilitation. Electronics 2025, 14, 2268. [Google Scholar] [CrossRef]
- Versaci, M.; Laganà, F.; Morabito, F.C.; Palumbo, A.; Angiulli, G. Adaptation of an Eddy Current Model for Characterizing Subsurface Defects in CFRP Plates Using FEM Analysis Based on Energy Functional. Mathematics 2024, 12, 2854. [Google Scholar] [CrossRef]
- Laganà, F.; De Carlo, D.; Calcagno, S.; Pullano, S.A.; Critello, D.; Falcone, F.; Fiorillo, A.S. Computational model of cell deformation under fluid flow-based rolling. In Proceedings of the 2019 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 21–23 November 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Kupikowska-Stobba, B.; Domagała, J.; Kasprzak, M.M. Critical Review of Techniques for Food Emulsion Characterization. Appl. Sci. 2024, 14, 1069. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, J.; Yang, Y.; Chen, Y. Infrared Thermographic Detection of Vegetable Oils under Controlled Thermal Excitation. Sensors 2023, 23, 2105. [Google Scholar]
- De Luca, M.; D’Accolti, L.; Montagna, G.; Biasioli, F.; Schuhfried, E.; Ferri, G. Use of Infrared Thermography for the Non-Destructive Evaluation of Food Quality: A Review. Foods 2022, 11, 3120. [Google Scholar]
- Rahman, M.M.; Uddin, M.T.; Hasan, M.; Khatun, R.; Hoque, M.M. Effect of Fatty Acid Composition on the Thermal Properties of Edible Oils. J. Food Process. Preserv. 2021, 45, e15417. [Google Scholar]
- Nguyen, M.H.; Tran, L.T.; Phan, T.N.; Le, D.H. Thermophysical Properties and Oxidative Stability of Edible Oils as Related to Fatty Acid Composition. Processes 2023, 11, 2190. [Google Scholar]
- Yang, X.; Niu, Z.; Wang, X.; Lu, X.; Sun, J.; Carpena, M.; Li, N. The nutritional and bioactive components, potential health function and comprehensive utilization of pomegranate: A review. Food Rev. Int. 2023, 39, 6420–6446. [Google Scholar] [CrossRef]
- Purohit, S.; Kalita, D.; Barik, C.R.; Sahoo, L.; Goud, V.V. Evaluation of thermophysical, biochemical and antibacterial properties of unconventional vegetable oil from Northeast India. Mater. Sci. Energy Technol. 2021, 4, 81–91. [Google Scholar] [CrossRef]
- Sorrenti, V.; Fortinguerra, S.; Lucarini, M.; De Lisi, A.; Malaguarnera, M. Oxidative Stability of Vegetable Oils: The Role of Natural Antioxidants and Thermophysical Characterisation. Antioxidants 2023, 12, 1101. [Google Scholar]
- Adedokun, O.A.; Olayanju, T.M.A.; Obafemi, T.E.; Omodara, O.J. Use of Thermal Imaging in Evaluating Oil Quality Degradation During Frying. Foods 2022, 11, 4221. [Google Scholar]
- Barbinta-Patrascu, M.-E.; Bita, B.; Negut, I. From Nature to Technology: Exploring the Potential of Plant-Based Materials and Modified Plants in Biomimetics, Bionics, and Green Innovations. Biomimetics 2024, 9, 390. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate (CaPO4) Containing Composites for Biomedical Applications: Formulations, Properties, and Applications. J. Compos. Sci. 2024, 8, 218. [Google Scholar] [CrossRef]
- Ashiagbor, K.; Jayan, H.; Gao, S.; Amaglo, N.K.; Adade, S.Y.S.S.; El-Seedi, H.R.; Guo, Z. Recent advances in photoelectric methods application for cooking oil quality and safety evaluation: A review. J. Sci. Food Agric. 2025. [Google Scholar] [CrossRef]
- Rahman, M.A.; Islam, M.J.; Park, J.Y. Hybrid CNN-LSTM Network for Automated Diagnosis of Thermal Imaging Data in Medical Applications. Sensors 2022, 22, 1501. [Google Scholar]
- Zhang, Y.; Li, L.; Wang, R. Spatiotemporal Deep Learning Framework for Thermal Signal Classification in Non-Destructive Testing. Appl. Sci. 2021, 11, 8463. [Google Scholar]
- Mannucci, P.M.; Jolliet, O.; Meijaard, E.; Slavin, J.; Rasetti, M.; Aleta, A.; Agostoni, C. Sustainable nutrition and the case of vegetable oils to match present and future dietary needs. Front. Public Health 2023, 11, 1106083. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Zhang, X.; Qu, Z.; Gao, Y.; Li, Q.; Yu, X. Mechanism, indexes, methods, challenges, and perspectives of edible oil oxidation analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 4901–4915. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.; Hu, S.; Wang, H.; Zhang, Y.; Ming, W. Progress in Active Infrared Imaging for Defect Detection in the Renewable and Electronic Industries. Sensors 2023, 23, 8780. [Google Scholar] [CrossRef]
- Cravotto, C.; Claux, O.; Bartier, M.; Fabiano-Tixier, A.-S.; Tabasso, S. Leading Edge Technologies and Perspectives in Industrial Oilseed Extraction. Molecule 2023, 28, 5973. [Google Scholar] [CrossRef]
- Marx, Í.M.G. Co-Extraction Technique Improves Functional Capacity and Health-Related Benefits of Olive Oils: A Mini Review. Foods 2023, 12, 1667. [Google Scholar] [CrossRef]
- Žugić, A.; Martinović, M.; Tadić, V.; Rajković, M.; Racić, G.; Nešić, I.; Koren, A. Comprehensive Insight into Cutaneous Application of Hemp. Pharmaceutics 2024, 16, 748. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Ayub, H.; Sehrish, A.; Ambreen, S.; Khan, F.A.; Itrat, N.; Nazir, A.; Shoukat, A.; Shoukat, A.; Ejaz, A.; et al. Essential Components from Plant Source Oils: A Review on Extraction, Detection, Identification, and Quantification. Molecules 2023, 28, 6881. [Google Scholar] [CrossRef] [PubMed]
- Campo, C.; Gangemi, S.; Pioggia, G.; Allegra, A. Beneficial Effect of Olive Oil and Its Derivates: Focus on Hematological Neoplasm. Life 2024, 14, 583. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; López-Maldonado, E.A.; Landázuri, A.C.; Ovuoraye, P.E.; Ogbu, A.I.; Vela-García, N.; Białowiec, A. Sustainable municipal landfill leachate management: Current practices, challenges, and future directions. Desalin. Water Treat. 2024, 320, 100709. [Google Scholar] [CrossRef]
- Elliott, P.S.; Kharaty, S.S.; Phillips, C.M. Plant-Based Diets and Lipid, Lipoprotein, and Inflammatory Biomarkers of Cardiovascular Disease: A Review of Observational and Interventional Studies. Nutrients 2022, 14, 5371. [Google Scholar] [CrossRef]
- Shaya, G.E.; Leucker, T.M.; Jones, S.R.; Martin, S.S.; Toth, P.P. Coronary heart disease risk: Low-density lipoprotein and beyond. Trends Cardiovasc. Med. 2022, 32, 181–194. [Google Scholar] [CrossRef]
- Pereira, Q.C.; dos Santos, T.W.; Fortunato, I.M.; Ribeiro, M.L. The Molecular Mechanism of Polyphenols in the Regulation of Ageing Hallmarks. Int. J. Mol. Sci. 2023, 24, 5508. [Google Scholar] [CrossRef]
- Huseynli, L.; Walser, C.; Blumenthaler, L.; Vene, K.; Dawid, C. Toward a Comprehensive Understanding of Flavor of Sunflower Products: A Review of Confirmed and Prospective Aroma- and Taste-Active Compounds. Foods 2025, 14, 1940. [Google Scholar] [CrossRef]
- Axentii, M.; Codină, G.G. Exploring the Nutritional Potential and Functionality of Hemp and Rapeseed Proteins: A Review on Unveiling Anti-Nutritional Factors, Bioactive Compounds, and Functional Attributes. Plants 2024, 13, 1195. [Google Scholar] [CrossRef]
- Frasinariu, O.; Serban, R.; Trandafir, L.M.; Miron, I.; Starcea, M.; Vasiliu, I.; Alisi, A.; Temneanu, O.R. The Role of Phytosterols in Nonalcoholic Fatty Liver Disease. Nutrients 2022, 14, 2187. [Google Scholar] [CrossRef]
- Poljšak, N.; Kočevar Glavač, N. Vegetable butters and oils as therapeutically and cosmetically active ingredients for dermal use: A review of clinical studies. Front. Pharmacol. 2022, 13, 868461. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.P.; Kumar, P.; Basak, P.; Goel, A.; Ohia, S.E.; Bagchi, M.; Bagchi, D. A critical insight into the physicochemical stability of macular carotenoids with respect to their industrial production, safety profile, targeted tissue delivery, and bioavailability. Toxicol. Mech. Methods 2025, 35, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chandran, D.; Tomar, M.; Bhuyan, D.J.; Grasso, S.; Sá, A.G.A.; Carciofi, B.A.M.; Radha; Dhumal, S.; Singh, S.; et al. Valorization Potential of Tomato (Solanum lycopersicum L.) Seed: Nutraceutical Quality, Food Properties, Safety Aspects, and Application as a Health-Promoting Ingredient in Foods. Horticulturae 2022, 8, 265. [Google Scholar] [CrossRef]
- Pandey, V.K.; Singh, K.; Suthar, T.; Srivastava, S.; Rustagi, S.; Ungai, D.; Kovács, B.; Shaikh, A.M. Current Strategies to Modify the Functional Properties of Proteins Extracted from Pumpkin Seeds: A Comprehensive Review. Horticulturae 2024, 10, 1194. [Google Scholar] [CrossRef]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef]
- Laganà, F.; Pellicanò, D.; De Carlo, D.; Pratticò, D.; Calcagno, S. Evaluation and monitoring of SAR and temperature during an indoor Wi-Fi call. In Proceedings of the 2024 International Workshop on Q-BATS, Durrës, Albania, 10–11 October 2024; pp. 105–110. [Google Scholar] [CrossRef]
- Bibbò, L.; Angiulli, G.; Laganà, F.; Pratticò, D.; Cotroneo, F.; La Foresta, F.; Versaci, M. MEMS and IoT in HAR: Effective Monitoring for the Health of Older People. Appl. Sci. 2025, 15, 4306. [Google Scholar] [CrossRef]
- Noman, H.A.; Abu-Sharkh, O.M.F. Code Injection Attacks in Wireless-Based Internet of Things (IoT): A Comprehensive Review and Practical Implementations. Sensors 2023, 23, 6067. [Google Scholar] [CrossRef]
- Laganà, F.; Pratticò, D.; Angiulli, G.; Oliva, G.; Pullano, S.A.; Versaci, M.; La Foresta, F. Development of an Integrated System of sEMG Signal Acquisition, Processing, and Analysis with AI Techniques. Signals 2024, 5, 476–493. [Google Scholar] [CrossRef]
- Sesidhar, D.V.S.R.; Badachi, C.; Green, R.C., II. A review on data-driven SOC estimation with Li-Ion batteries: Implementation methods & future aspirations. J. Energy Storage 2023, 72, 108420. [Google Scholar]



| Component | Per 100 g | % RDA/AI |
|---|---|---|
| Energy | 884 kcal | 44% (2000 kcal) |
| Total Fat | 100 g | 129% (77 g) |
| Saturated Fat | 13.8 g | 69% (20 g max) |
| Monounsaturated Fats | 73.0 g | - |
| Polyunsaturated Fats | 10.5 g | - |
| Sodium | 2 mg | <0.1% (2300 mg) |
| Vitamin E | 14 mg | 93% (15 mg) |
| Vitamin K | 60.2 μg | 75% (80 μg) |
| Polyphenols (approx.) | Variable (50–500 mg) | No RDA |
| Component | Per 100 g | % RDA/AI |
|---|---|---|
| Energy | 884 kcal | 44.2% (2000 kcal) |
| Total Fat | 100 g | 153.8% (based on 65 g/day) |
| Saturated Fat | 10 g | 50% (20 g/day max) |
| Monounsaturated Fats | 20.0 g | - |
| Polyunsaturated Fats | 70 g | - |
| Sodium | 2 mg | <0.1% (2300 mg) |
| Vitamin E | 41 mg | 273% (15 mg RDA) |
| Vitamin K | 0.6 mg | 0.5% (120 μg RDA) |
| Component | Per 100 g | % RDA/AI |
|---|---|---|
| Energy | 900 kcal | 45% (2000 kcal) |
| Total Fat | 100 g | 154% (based on 65 g/day) |
| Saturated Fat | 12 g | 60% |
| Monounsaturated Fats | 13 g | - |
| Polyunsaturated Fats | 70 g | - |
| Omega-3 Fatty Acids | 6 g | 375% |
| Omega-6 Fatty Acids | 67 g | 558% |
| Phytosterols (total) | 180 mg | 60% |
| Lycopene | 1.2 mg | - |
| Β-carotene | 0.8 mg | 10% (as vitamin A equivalent) |
| Vitamin E (α-tocopherol) | 24 mg | 160% |
| Component | Per 100 g | % RDA/AI |
|---|---|---|
| Energy | 814 kcal | 40.7% |
| Total Fat | 90 g | 138.5% |
| Saturated Fat | 18 g | 90% |
| Monounsaturated Fats | 30 g | - |
| Polyunsaturated Fats | 42 g | - |
| Phytosterols | 230 mg | - |
| Vitamin E | 10 mg | 66.7% |
| Sample | Room Temperature (°C) | High Temperature (°C) | Low Temperature (°C) |
|---|---|---|---|
| Olive oil | 20–24 | 28–73 | −17–16 |
| Sunflower seed oil | 22–26 | 28–71 | −14–16.5 |
| Tomato seed oil | 22–26 | 30–65 | −14–15.3 |
| Pumpkin seed oil | 22–26 | 30–51 | −13.5–15.7 |
| Layer Number | Layer Name | Input Shape | Output Shape | Kernel Size | Stride |
|---|---|---|---|---|---|
| 1 | Conv2D + ReLU | (128, 128, 1) | (126, 126, 32) | (3, 3) | (1, 1) |
| 2 | MaxPooling2D | (126, 126, 32) | (63, 63, 32) | (2, 2) | (2, 2) |
| 3 | Conv2D + ReLU | (63, 63, 32) | (61, 61, 64) | (3, 3) | (1, 1) |
| 4 | MaxPooling2D | (61, 61, 64) | (30, 30, 64) | (2, 2) | (2, 2) |
| 5 | Flatten | (30, 30, 64) | (57,600) | – 1 | – |
| Layer Number | Layer Name | Input Shape | Output Shape | Notes |
|---|---|---|---|---|
| 6 | LSTM | (Num frames, 57,600) | (64) | Captures temporal dynamics |
| 7 | Dense + ReLU | (64) | (128) | Fully connected |
| 8 | Dropout (rate = 0.3) | (128) | (128) | Regularisation |
| 9 | Dense + SoftMax | (128) | (4) | 4-class oil classification |
| Metric | Mean (%) | Std. Dev (%) |
|---|---|---|
| Accuracy | 93.25 | 1.45 |
| Precision | 94.55 | 1.22 |
| Recall | 92.80 | 1.78 |
| F1-score | 93.66 | 1.60 |
| Model Variant | Accuracy | Precision | Recall |
|---|---|---|---|
| CNN-only (spatial features only) | 85.23% | 86.14% | 84.03% |
| LSTM-only (temporal only) | 70.11% | 70.24% | 70.48% |
| CNN-LSTM (proposed model) | 93.25% | 94.55% | 92.80% |
| CNN-LSTM w/o dropout | 90.22% | 90.14% | 88.78% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratticò, D.; Laganà, F. Infrared Thermographic Signal Analysis of Bioactive Edible Oils Using CNNs for Quality Assessment. Signals 2025, 6, 38. https://doi.org/10.3390/signals6030038
Pratticò D, Laganà F. Infrared Thermographic Signal Analysis of Bioactive Edible Oils Using CNNs for Quality Assessment. Signals. 2025; 6(3):38. https://doi.org/10.3390/signals6030038
Chicago/Turabian StylePratticò, Danilo, and Filippo Laganà. 2025. "Infrared Thermographic Signal Analysis of Bioactive Edible Oils Using CNNs for Quality Assessment" Signals 6, no. 3: 38. https://doi.org/10.3390/signals6030038
APA StylePratticò, D., & Laganà, F. (2025). Infrared Thermographic Signal Analysis of Bioactive Edible Oils Using CNNs for Quality Assessment. Signals, 6(3), 38. https://doi.org/10.3390/signals6030038






