Beyond Frequency Band Constraints in EEG Analysis: The Role of the Mode Decomposition in Pushing the Boundaries
Abstract
1. Introduction
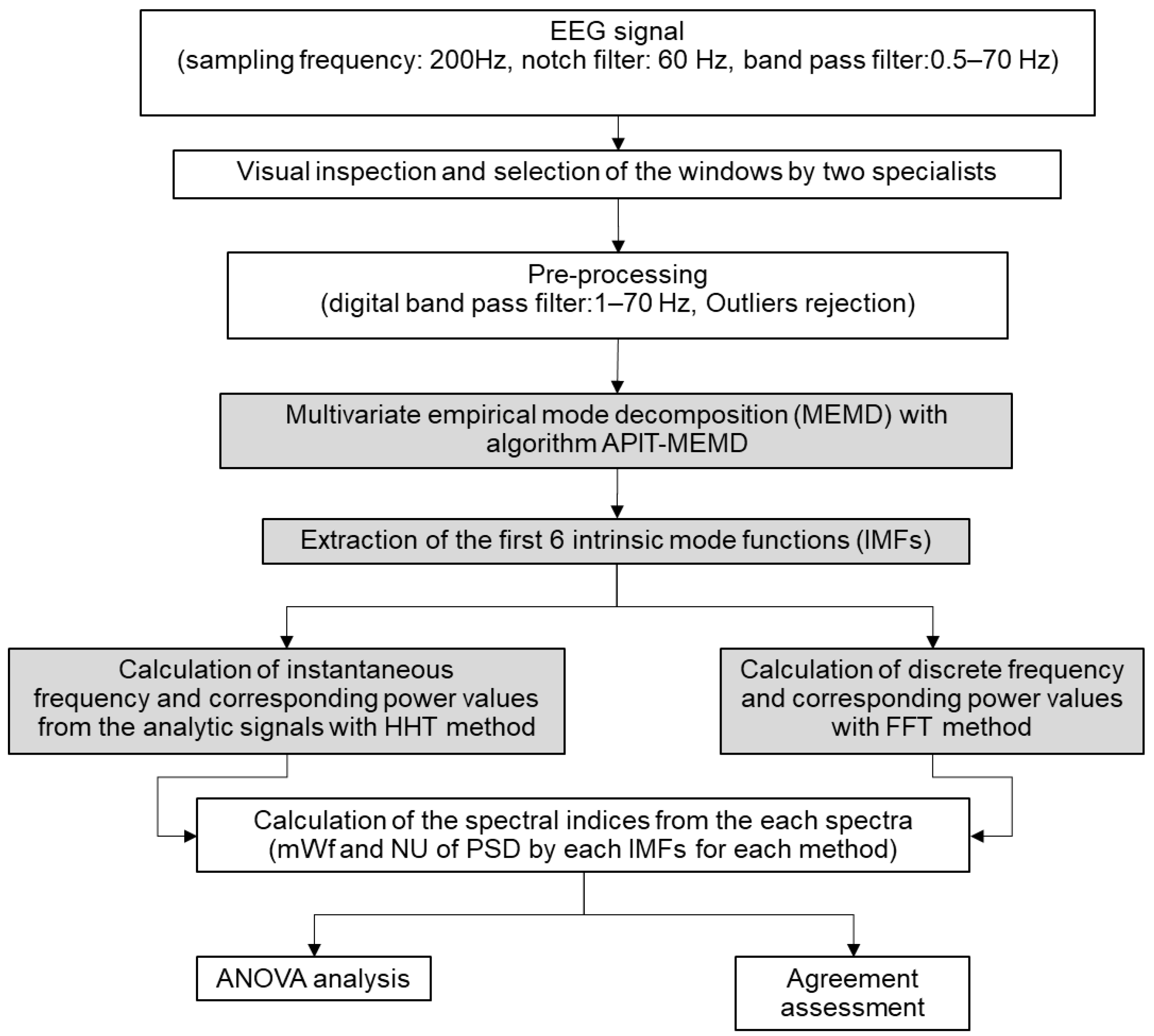
2. Materials and Methods
2.1. Participants and General Experimental Profile
2.2. EEG Recordings
2.3. EEG Pre-Processing
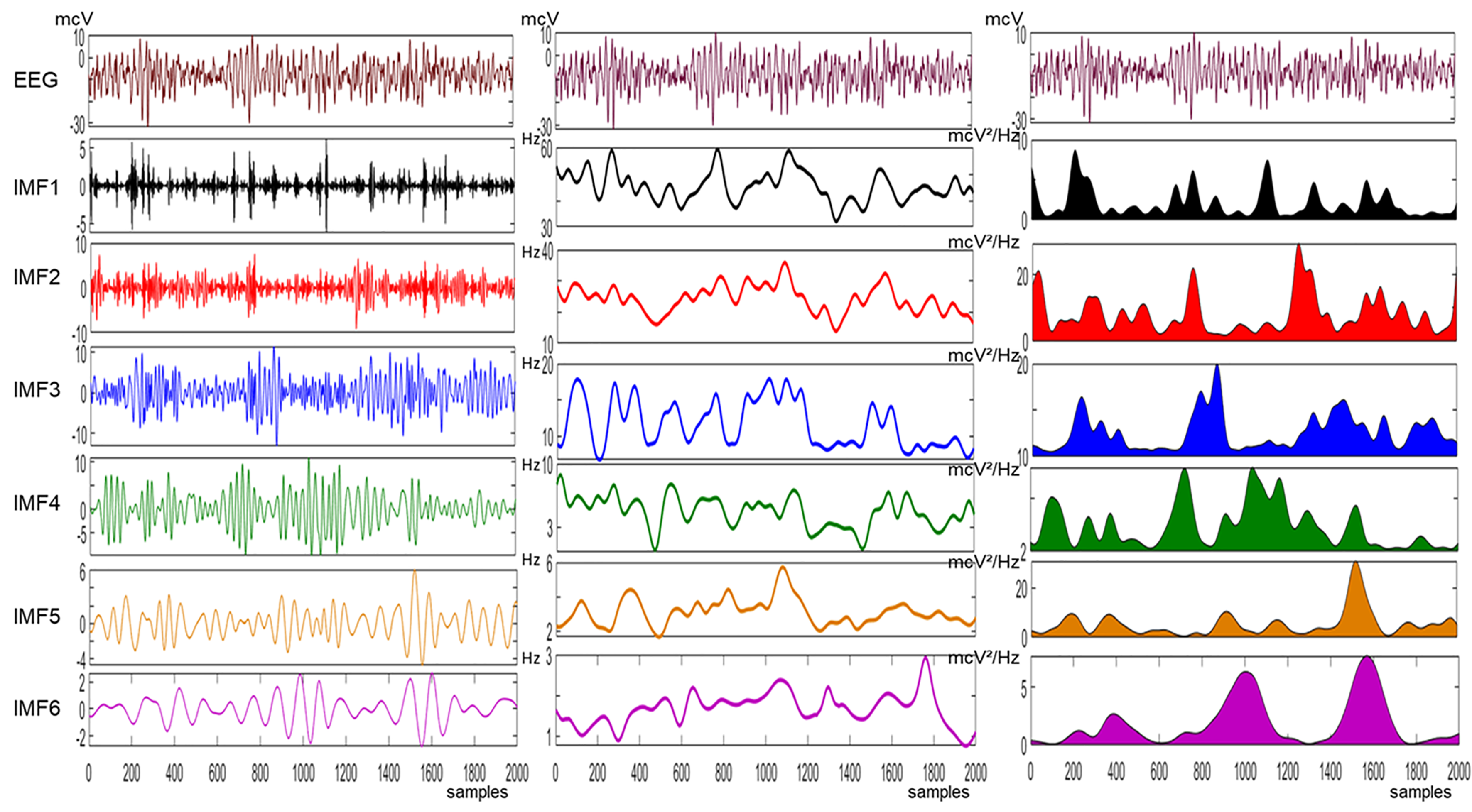
2.4. Empirical Mode Decomposition (EMD)
2.5. Multivariate Empirical Mode Decomposition (MEMD)
2.6. Adaptive-Projection Intrinsically Transformed MEMD (APIT-MEMD)
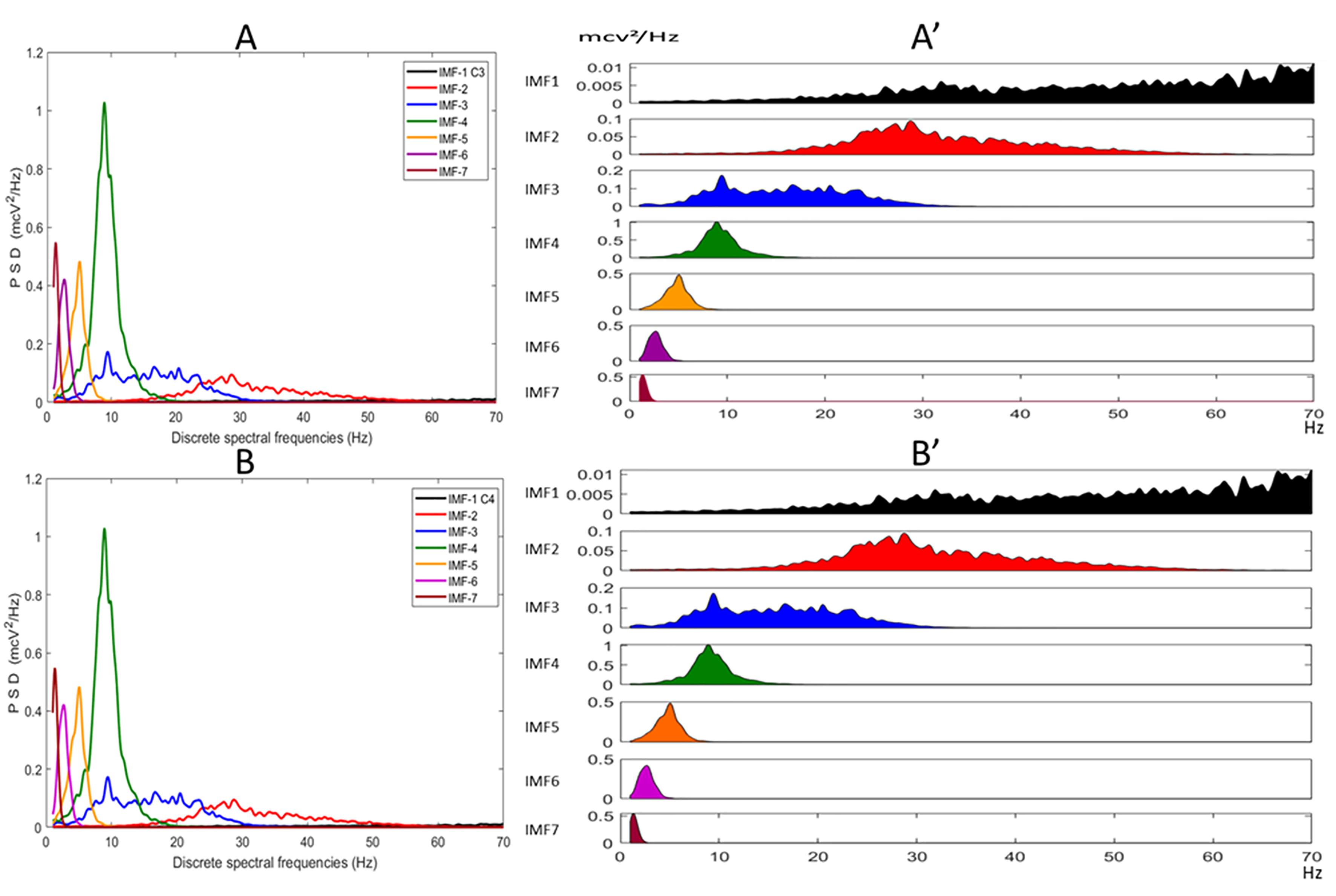
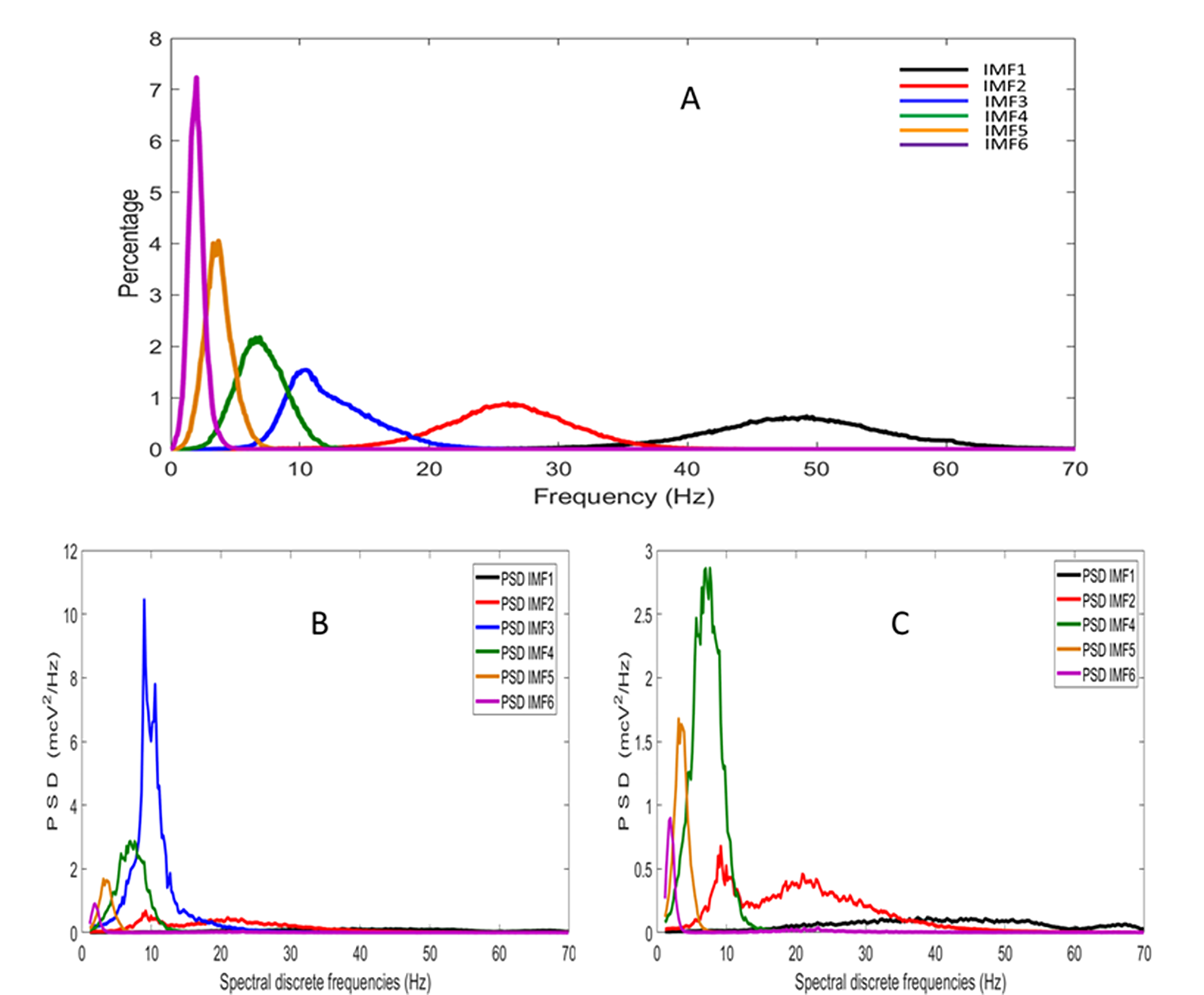
2.7. Estimation of the PSD of the IMFs Using the FFT Method and Spectral Indices (FFT)
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, S.W.; Lorincz, M.L.; Parri, H.R.; Crunelli, V. Infraslow (<0.1 Hz) oscillations in thalamic relay nuclei basic mechanisms and significance to health and disease states. Prog. Brain Res. 2011, 193, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh-Arasteh, O.; Pu, H.; Lim, J.; Liu, C.Y.; Do, A.H.; Nenadic, Z.; Heydari, P. An Energy-Efficient CMOS Dual-Mode Array Architecture for High-Density ECoG-Based Brain-Machine Interfaces. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 332–342. [Google Scholar] [CrossRef] [PubMed]
- van Putten, M.J.; Tjepkema-Cloostermans, M.C.; Hofmeijer, J. Infraslow EEG activity modulates cortical excitability in postanoxic encephalopathy. J. Neurophysiol. 2015, 113, 3256–3267. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, S.; Voipio, J.; Kaila, K. Full-band EEG (FbEEG): An emerging standard in electroencephalography. Clin. Neurophysiol. 2005, 116, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dinares-Ferran, J.; Ortner, R.; Guger, C.; Sole-Casals, J. A New Method to Generate Artificial Frames Using the Empirical Mode Decomposition for an EEG-Based Motor Imagery BCI. Front. Neurosci. 2018, 12, 308. [Google Scholar] [CrossRef]
- Berger, H. Über das Elektrenkephalogramm des Menschen. Arch. Psychiat. Nervenkr. 1929, 87, 527–570. [Google Scholar] [CrossRef]
- Amo, C.; de Santiago, L.; Barea, R.; Lopez-Dorado, A.; Boquete, L. Analysis of Gamma-Band Activity from Human EEG Using Empirical Mode Decomposition. Sensors 2017, 17, 989. [Google Scholar] [CrossRef]
- Park, C.; Plank, M.; Snider, J.; Kim, S.; Huang, H.C.; Gepshtein, S.; Coleman, T.P.; Poizner, H. EEG gamma band oscillations differentiate the planning of spatially directed movements of the arm versus eye: Multivariate empirical mode decomposition analysis. IEEE Trans. Neural Syst. Rehabil. Eng. A Publ. IEEE Eng. Med. Biol. Soc. 2014, 22, 1083–1096. [Google Scholar] [CrossRef]
- Buzsaki, G. Rhythms of the Brain; Oxford University Press, Inc.: Oxford, UK, 2006. [Google Scholar]
- Buzsaki, G.; Watson, B.O. Brain rhythms and neural syntax: Implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin. Neurosci. 2012, 14, 345–367. [Google Scholar] [CrossRef]
- Smyk, M.K.; van Luijtelaar, G. Circadian Rhythms and Epilepsy: A Suitable Case for Absence Epilepsy. Front. Neurol. 2020, 11, 245. [Google Scholar] [CrossRef]
- Soltani Zangbar, H.; Ghadiri, T.; Seyedi Vafaee, M.; Ebrahimi Kalan, A.; Fallahi, S.; Ghorbani, M.; Shahabi, P. Theta Oscillations Through Hippocampal/Prefrontal Pathway: Importance in Cognitive Performances. Brain Connect. 2020, 10, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Penttonen, M.; Buzsaki, G. Natural logarithmic relationship between brain oscillators. Thalamus Relat. Syst. 2003, 2, 145–152. [Google Scholar] [CrossRef]
- Cooley, J.W.; Tukey, J.W. An algorithm for the machine calculation of complex Fourier series. Math. Comput. 1965, 19, 297–301. [Google Scholar] [CrossRef]
- Noshadi, S.; Vahid Abootalebi, V.; Taghi Sadeghi, M.; Shahab Shahvazian, M. Selection of an efficient feature space for EEG-based mental task discrimination. Biocybern. Biomed. Eng. 2014, 34, 159–168. [Google Scholar] [CrossRef]
- Abdulhay, E.; Alafeef, M.; Abdelhay, A.; Al-Bashir, A. Classification of Normal, Ictal and Inter-ictal EEG via Direct Quadrature and Random Forest Tree. J. Med. Biol. Eng. 2017, 37, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Alegre-Cortes, J.; Soto-Sanchez, C.; Piza, A.G.; Albarracin, A.L.; Farfan, F.D.; Felice, C.J.; Fernandez, E. Time-frequency analysis of neuronal populations with instantaneous resolution based on noise-assisted multivariate empirical mode decomposition. J. Neurosci. Methods 2016, 267, 35–44. [Google Scholar] [CrossRef]
- Soler, A.; Munoz-Gutierrez, P.A.; Bueno-Lopez, M.; Giraldo, E.; Molinas, M. Low-Density EEG for Neural Activity Reconstruction Using Multivariate Empirical Mode Decomposition. Front. Neurosci. 2020, 14, 175. [Google Scholar] [CrossRef]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. Lond. 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Al-Subari, K.; Al-Baddai, S.; Tome, A.M.; Volberg, G.; Ludwig, B.; Lang, E.W. Combined EMD-sLORETA Analysis of EEG Data Collected during a Contour Integration Task. PLoS ONE 2016, 11, e0167957. [Google Scholar] [CrossRef]
- Al-Subari, K.; Al-Baddai, S.; Tome, A.M.; Volberg, G.; Hammwohner, R.; Lang, E.W. Ensemble Empirical Mode Decomposition Analysis of EEG Data Collected during a Contour Integration Task. PLoS ONE 2015, 10, e0119489. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Detection of focal electroencephalogram signals using higher-order moments in EMD-TKEO domain. Heal. Technol. Lett. 2019, 6, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Carella, T.; De Silvestri, M.; Finedore, M.; Haniff, I.; Esmailbeigi, H. Emotion Recognition for Brain Machine Interface: Non-linear Spectral Analysis of EEG Signals Using Empirical Mode Decomposition. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference, Honolulu, HI, USA, 18 July 2018; Volume 2018, pp. 223–226. [Google Scholar] [CrossRef]
- Chuang, K.Y.; Chen, Y.H.; Balachandran, P.; Liang, W.K.; Juan, C.H. Revealing the Electrophysiological Correlates of Working Memory-Load Effects in Symmetry Span Task With HHT Method. Front. Psychol. 2019, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Baez, M.; Machado, C.; Arrufat-Pie, E.; Santos, A. Aplicación del Método de Hilbert-Huang a Señales Biológicas en el Campo de la Neurología: Descripción y Aspectos Metodológicos; Technical Reports of the Institute of Neurology and Neurosurgery: Havana, Cuba, 2017; Available online: http://dx.doi.org/10.13140/RG.2.2.18537.83047 (accessed on 14 January 2023).
- Estevez-Baez, M.; Machado, C.; Arrufat-Pie, E.; Santos, A. El Método de Hilbert-Huang Aplicado al Estudio de Algunas Señales Biológicas en el Campo de la Neurología: Revisión Bibliográfica: Technical Reports of the Institute of Neurology and Neurosurgery: Havana, Cuba. 2017. Available online: http://dx.doi.org/10.13140/RG.2.2.25248.71683 (accessed on 14 January 2023).
- Estevez-Baez, M.; Machado, C.; Arrufat-Pie, E.; Santos, A. El método de Hilbert-Huang en el Análisis del EEG: Fundamentos y Perspectivas; Technical Reports of the Institute of Neurology and Neurosurgery: Havana, Cuba, 2017; Available online: http://dx.doi.org/10.13140/RG.2.2.11826.94401 (accessed on 14 January 2023).
- Hassan, A.R.; Bhuiyan, M.I.H. Automated identification of sleep states from EEG signals by means of ensemble empirical mode decomposition and random under sampling boosting. Comput. Methods Programs Biomed. 2017, 140, 201–210. [Google Scholar] [CrossRef]
- Hansen, S.T.; Hemakom, A.; Gylling Safeldt, M.; Krohne, L.K.; Madsen, K.H.; Siebner, H.R.; Mandic, D.P.; Hansen, L.K. Unmixing Oscillatory Brain Activity by EEG Source Localization and Empirical Mode Decomposition. Comput. Intell. Neurosci. 2019, 2019, 5618303. [Google Scholar] [CrossRef]
- Hou, F.; Yu, Z.; Peng, C.K.; Yang, A.; Wu, C.; Ma, Y. Complexity of Wake Electroencephalography Correlates With Slow Wave Activity After Sleep Onset. Front. Neurosci. 2018, 12, 809. [Google Scholar] [CrossRef]
- Javed, E.; Croce, P.; Zappasodi, F.; Gratta, C.D. Hilbert Spectral Analysis of EEG Data reveals Spectral Dynamics associated with Microstates. J. Neurosci. Methods 2019, 325, 108317. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Fattah, S.A. Mental Task Classification Scheme Utilizing Correlation Coefficient Extracted from Interchannel Intrinsic Mode Function. BioMed Res. Int. 2017, 2017, 3720589. [Google Scholar] [CrossRef]
- Ullal, A.; Pachori, R.B. EEG signal classification using variational mode decomposition. arXiv 2020, arXiv:arXiv:2003.12690v1. [Google Scholar]
- Mahmoudi, M.; Shamsi, M. Multi-class EEG classification of motor imagery signal by finding optimal time segments and features using SNR-based mutual information. Australas. Phys. Eng. Sci. Med. 2018, 41, 957–972. [Google Scholar] [CrossRef]
- Peng, C.J.; Chen, Y.C.; Chen, C.C.; Chen, S.J.; Cagneau, B.; Chassagne, L. An EEG-Based Attentiveness Recognition System Using Hilbert–Huang Transform and Support Vector Machine. J. Med. Biol. Eng. 2019, 40, 230–238. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Z.; Gong, A.; Qian, Q.; Su, L.; Zhao, L. Identification of Visual Imagery by Electroencephalography Based on Empirical Mode Decomposition and an Autoregressive Model. Comput. Intell. Neurosci. 2022, 2022, 1038901. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Atal, K.; Xie, S.Q.; Liu, Q. A new multivariate empirical mode decomposition method for improving the performance of SSVEP-based brain-computer interface. J. Neural Eng. 2017, 14, 046028. [Google Scholar] [CrossRef]
- ElSayed, N.E.; Tolba, A.S.; Rashad, M.Z.; Belal, T.; Sarhan, S. Multimodal analysis of electroencephalographic and electrooculographic signals. Comput. Biol. Med. 2021, 137, 104809. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, Y.; Cammon, J.; Zhang, S.; Zhang, J.; Zhang, Y. A new feature selection approach for driving fatigue EEG detection with a modified machine learning algorithm. Comput. Biol. Med. 2022, 147, 105718. [Google Scholar] [CrossRef]
- Chen, S.J.; Peng, C.J.; Chen, Y.C.; Hwang, Y.R.; Lai, Y.S.; Fan, S.Z.; Jen, K.K. Comparison of FFT and marginal spectra of EEG using empirical mode decomposition to monitor anesthesia. Comput. Methods Programs Biomed. 2016, 137, 77–85. [Google Scholar] [CrossRef]
- Schiecke, K.; Schumann, A.; Benninger, F.; Feucht, M.; Baer, K.; Schlattmann, P. Brain-Heart interactions considering complex physiological data: Processing schemes for timevariant, frequency-dependent, topographical and statistical examination of directed interactions by Convergent Cross Mapping. Physiol. Meas. 2019, 40, 114001. [Google Scholar] [CrossRef]
- Schiecke, K.; Schmidt, C.; Piper, D.; Putsche, P.; Feucht, M.; Witte, H.; Leistritz, L. Assignment of Empirical Mode Decomposition Components and Its Application to Biomedical Signals. Methods Inf. Med. 2015, 54, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.F.; Fan, S.Z.; Lin, Y.S.; Huang, N.E.; Yeh, J.R. Investigating Power Density and the Degree of Nonlinearity in Intrinsic Components of Anesthesia EEG by the Hilbert-Huang Transform: An Example Using Ketamine and Alfentanil. PLoS ONE 2016, 11, e0168108. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Cao, J.; Shi, Q.; Mandic, D.P.; Tanaka, T.; Wang, R. Analyzing the EEG Energy of Quasi Brain Death using MEMD. In Proceedings of the Asia-Pacific Signal and Information Processing Association Annual Summit and Conference, APSIPA ASC, Xian, China, 18–21 October 2011. [Google Scholar]
- Shi, Q.; Yang, J.; Cao, J.; Tanaka, T.; Wang, R.; Zhu, H. EEG data analysis based on EMD for coma and quasi-brain-death patients. J. Exp. Theor. Artif. Intell. 2011, 23, 97–110. [Google Scholar] [CrossRef]
- Hemakom, A.; Goverdovsky, V.; Looney, D.; Mandic, D.P. Adaptive-projection intrinsically transformed multivariate empirical mode decomposition in cooperative brain-computer interface applications. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2016, 374, 20150199. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xu, G. Quantifying mode mixing and leakage in multivariate empirical mode decomposition and application in motor imagery-based brain-computer interface system. Med. Biol. Eng. Comput. 2019, 57, 1297–1311. [Google Scholar] [CrossRef]
- Zhuang, N.; Zeng, Y.; Tong, L.; Zhang, C.; Zhang, H.; Yan, B. Emotion Recognition from EEG Signals Using Multidimensional Information in EMD Domain. BioMed Res. Int. 2017, 2017, 8317357. [Google Scholar] [CrossRef]
- Scarinci, N.; Priel, A.; Cantero, M.D.R.; Cantiello, H.F. Brain Microtubule Electrical Oscillations-Empirical Mode Decomposition Analysis. Cell Mol. Neurobiol. 2023, 43, 2089–2104. [Google Scholar] [CrossRef]
- Oppenheim, A.V.; Schafer, R.W. Discrete-Time Signal Processing, 3rd ed.; Perason: London, UK, 2010. [Google Scholar]
- Arrufat-Pie, E. Application of the Hilbert-Huang Method to the Development of a Platform for Quantitative Analysis of the Electroencephalographic Signal; Medical Physiology Specialty degree, Medical University of Havana: Havana, Cuba, 2019. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Z. Mean frequency derived via Hilbert-Huang transform with application to fatigue EMG signal analysis. Comput. Methods Programs Biomed. 2006, 82, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Arrufat-Pié, E.; Estévez-Báez, M.; Estévez-Carreras, J.M.; Machado-Curbelo, C.; Leisman, G.; Beltrán, C. Comparison between traditional fast Fourier transform and marginal spectra using the Hilbert–Huang transform method for the broadband spectral analysis of the electroencephalogram in healthy humans. Eng. Rep. 2021, 3, e12367. [Google Scholar] [CrossRef]
- Zou, G.Y. Confidence interval estimation for the Bland-Altman limits of agreement with multiple observations per individual. Stat. Methods Med. Res. 2013, 22, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Agreement Between Methods of Measurement with Multiple Observations Per Individual. J. Biopharm. Stat. 2007, 17, 571–582. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Bikfalvi, A. Advanced Matlab Boxplot. Available online: http://alex.bikfalvi.com/research/advanced_matlab_boxplot (accessed on 1 February 2020).
- Lucey, B.P.; McLeland, J.S.; Toedebusch, C.D.; Boyd, J.; Morris, J.C.; Landsness, E.C.; Yamada, K.; Holtzman, D.M. Comparison of a single-channel EEG sleep study to polysomnography. J. Sleep Res. 2016, 25, 625–635. [Google Scholar] [CrossRef]
- Durstewitz, D.; Deco, G. Computational significance of transient dynamics in cortical networks. Eur. J. Neurosci. 2008, 27, 217–227. [Google Scholar] [CrossRef]
- Munoz-Gutierrez, P.A.; Giraldo, E.; Bueno-Lopez, M.; Molinas, M. Localization of Active Brain Sources From EEG Signals Using Empirical Mode Decomposition: A Comparative Study. Front. Integr. Neurosci. 2018, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Baez, M.; Machado, C.; Montes-Brown, J.; Jas-Garcia, J.; Leisman, G.; Schiavi, A.; Machado-Garcia, A.; Carricarte-Naranjo, C.; Carmeli, E. Very High Frequency Oscillations of Heart Rate Variability in Healthy Humans and in Patients with Cardiovascular Autonomic Neuropathy. Adv. Exp. Med. Biol. 2018, 1070, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Baez, M.; Machado, C.; Garcia-Sanchez, B.; Rodriguez, V.; Alvarez-Santana, R.; Leisman, G.; Carrera, J.M.E.; Schiavi, A.; Montes-Brown, J.; Arrufat-Pie, E. Autonomic impairment of patients in coma with different Glasgow coma score assessed with heart rate variability. Brain Inj. 2019, 33, 496–516. [Google Scholar] [CrossRef]
- Dragomiretskiy, K.; Zosso, D. Variational Mode Decomposition. IEEE Trans. Signal Process. 2014, 62, 531–544. [Google Scholar] [CrossRef]
- Rehman, N.; Mandic, D.P. Multivariate empirical mode decomposition. Proc. R. Soc. A 2010, 466, 1291–1302. [Google Scholar] [CrossRef]
- Ur Rehman, N.; Xia, Y.; Mandic, D.P. Application of multivariate empirical mode decomposition for seizure detection in EEG signals. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE Engineering in Medicine and Biology Society, Annual Conference, Buenos Aires, Argentina, 31 August–4 September 2010; Volume 2010, pp. 1650–1653. [Google Scholar] [CrossRef]


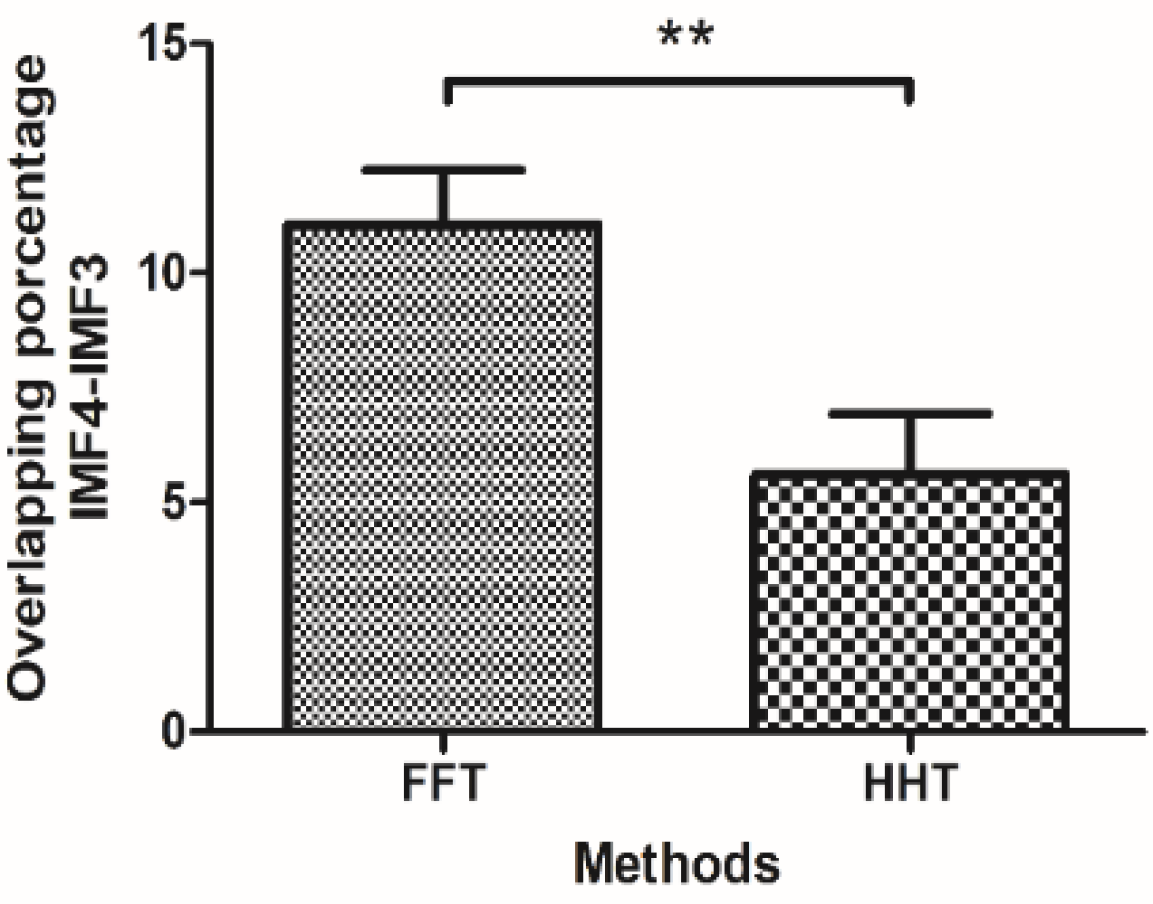
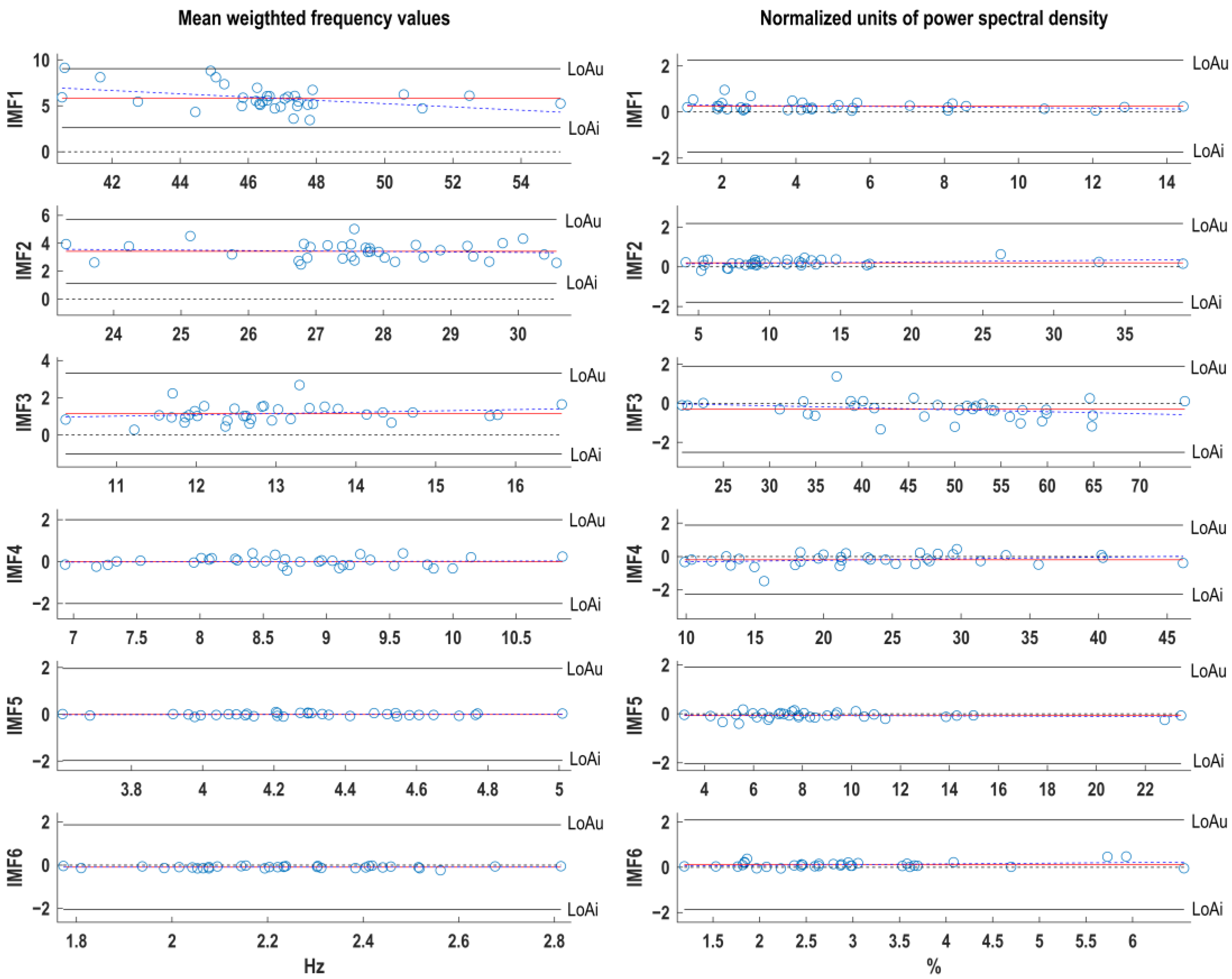
| Indices | IMF-1 | IMF-2 | IMF-3 | IMF-4 | IMF-5 | IMF-6 |
| B[Ul/Ll] | B[Ul/Ll] | B[Ul/Ll] | B[Ul/Ll] | B[Ul/Ll] | B[Ul/Ll] | |
| mWf (Hz) | 5.83 [9.0/2.6] | 3.42 [5.7/1.1] | 1.15 [3.3/−1.0] | 0.00 [2.0/−2.0] | −0.01 [2.0/−2.0] | −0.09 [1.9/−2.1] |
| nU (%) | 0.25 [2.2/−1.7] | 0.20 [2.2/−1.8] | −0.30 [1.9/−2.5] | −0.19 [1.9/−2.3] | 0.07 [1.9/−2.0] | 0.12 [2.1/−1.9 ] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrufat-Pié, E.; Estévez-Báez, M.; Estévez-Carreras, J.M.; Leisman, G.; Machado, C.; Beltrán-León, C. Beyond Frequency Band Constraints in EEG Analysis: The Role of the Mode Decomposition in Pushing the Boundaries. Signals 2023, 4, 489-506. https://doi.org/10.3390/signals4030026
Arrufat-Pié E, Estévez-Báez M, Estévez-Carreras JM, Leisman G, Machado C, Beltrán-León C. Beyond Frequency Band Constraints in EEG Analysis: The Role of the Mode Decomposition in Pushing the Boundaries. Signals. 2023; 4(3):489-506. https://doi.org/10.3390/signals4030026
Chicago/Turabian StyleArrufat-Pié, Eduardo, Mario Estévez-Báez, José Mario Estévez-Carreras, Gerry Leisman, Calixto Machado, and Carlos Beltrán-León. 2023. "Beyond Frequency Band Constraints in EEG Analysis: The Role of the Mode Decomposition in Pushing the Boundaries" Signals 4, no. 3: 489-506. https://doi.org/10.3390/signals4030026
APA StyleArrufat-Pié, E., Estévez-Báez, M., Estévez-Carreras, J. M., Leisman, G., Machado, C., & Beltrán-León, C. (2023). Beyond Frequency Band Constraints in EEG Analysis: The Role of the Mode Decomposition in Pushing the Boundaries. Signals, 4(3), 489-506. https://doi.org/10.3390/signals4030026








