1. Introduction
The global increase in human infrastructure is well known to affect ecosystems and the organisms that live in them. An important component of human activities associated with infrastructure is the production of substrate-borne vibrations. Road and railway traffic, as well as construction work, has led to an increase in subterranean vibratory noise levels, potentially influencing organisms that live in the soil [
1]. Wind energy turbines are a fast-growing potential source of anthropogenic vibrational noise. In Europe, wind energy remains the second-largest form of power generation capacity, with a total net installed capacity of 168.7 GW [
2]. Most inland wind energy turbines are located in agricultural fields, where plants and the insects that interact with them are potentially constantly exposed to vibrational noise.
Organisms ranging from animals [
3,
4] to plants [
5,
6,
7,
8,
9] to fungi [
10] are known to rely on vibratory signals and cues for their survival and reproduction [
11,
12,
13]. Human-induced vibratory noise levels are known to impact organisms across trophic levels. For example, Mortimer and colleagues [
14] showed that elephants exhibit risk-avoidance behavior in response to anthropogenic seismic noise [
14]. At the other end of the size spectrum, web-building spiders experienced a lower pre-detection ability when exposed to vibratory noise in anthropogenic habitats [
15]. Marine organisms like the hermit crab
Pagurus bernhardus showed behavioral changes in response to anthropogenic vibratory noise [
16]. Moreover, vibrational noise induced by wind energy turbines affected earthworm abundance, leading to a decrease in abundance of approximately 40% at closer distances from the wind turbine (8 m) versus further away (128 m) [
17]. Despite the ecological implications, most studies have focused on animals, and the few studies that have investigated the effects of sound or vibrations on plants have focused on pure tones and not a relevant source of noise.
Plants are naturally exposed to all sorts of vibrations, which might either directly influence their physiology and growth or affect the organisms living on them. Natural sources move either above-ground (e.g., wind or rain) or below-ground (roots) plant parts and, as such, influence plants’ morphology and physiology. Exposing
B. nigra plants to wind, e.g., leads to reduced growth, which indirectly affects their herbivores
P. brassicae and
P. xylostella [
18]. Although wind experiments also induce other changes to a plant’s physical environment, the induced vibrations are likely a key (but not exclusive) component driving plant changes. Understanding how plants are affected by vibratory noise—either directly, through changes in their morphology and physiology, or indirectly, via changes in their interactions with herbivores—would therefore be highly relevant, both from a fundamental and applied point of view.
The few studies on the effects of vibratory noise on plants suggest that vibrational cues are used across a range of different ecological contexts.
Zea mays roots, for example, grow towards vibrations associated with flowing water [
5], and
Arabidopsis thaliana increases its anti-herbivory chemical defense when exposed to the chewing vibrations induced by its herbivores [
11]. Moreover, stimulating plants with sounds and vibrations of different frequency ranges has been shown to affect germination timing [
9,
19,
20] and growth [
8].
Anthropogenic vibratory noise created by energy or transport infrastructure therefore has the potential to impact plants directly, through changes in their growth and development, or indirectly, via species interactions. For example, vibrations may deter beneficial soil fauna such as earthworms and, therefore, decrease soil fertility [
17], or it can affect organisms in the trophic cascade of herbivores, predators, and parasitoids.
In our current study we assessed whether vibrational noise generated by wind energy turbines influences plant–insect interactions. We first examined the effects of vibrational noise on plant developmental processes. Then, we tested for direct and indirect effects of vibrational noise on herbivory. To do this, we experimentally exposed Pisum sativum seeds to high and low vibrational noise and tracked their development on a daily basis. After fruiting of the plants, we carried out an herbivory experiment, using the generalist caterpillar Spodoptera exigua and a full-factorial design. For the herbivory experiment we were interested in two questions: (1) Does vibrational noise occurring at the moment of foraging affect herbivory? (2) Does prior plant exposure to vibrational noise have carry-over effects on herbivory (for example, via changes in secondary metabolites)? Caterpillars were placed on plants that developed during either low- or high-noise conditions, and they were exposed to either high or low vibrational noise during the foraging period.
2. Materials and Methods
2.1. Study Species
We decided to work with
P. sativum plants because we were interested in linking our vibrational noise field measurements to crop plants, and because of evidence indicating the sensitivity of this species to vibrational noise [
5]. Organic
P. sativum seeds were obtained from the company Intratuin (Nobelweg 10, 1097 AR Amsterdam, The Netherlands).
For our herbivory experiments we used the caterpillar S. exigua. We chose this species because it is a generalist herbivore, easy to rear and maintain in the lab. It is a species native to Europe, a common pest in The Netherlands (where this study took place), and it is found worldwide, which facilitates the replicability of our study. We obtained S. exigua eggs from the company Entocare (Haagsteeg 4, 6708 PM Wageningen, The Netherlands). The animals were reared in climate rooms at 26 °C ± 1 °C and 80% relative humidity on a 12D/12N light cycle. The caterpillars were fed ad libitum with a corn-based artificial diet. For our herbivory experiments we used caterpillars in the 5th instar. The caterpillars that took part in our experiments were fed with P. sativum the day before testing them to acclimatize them to the diet.
2.2. Experimental Procedures
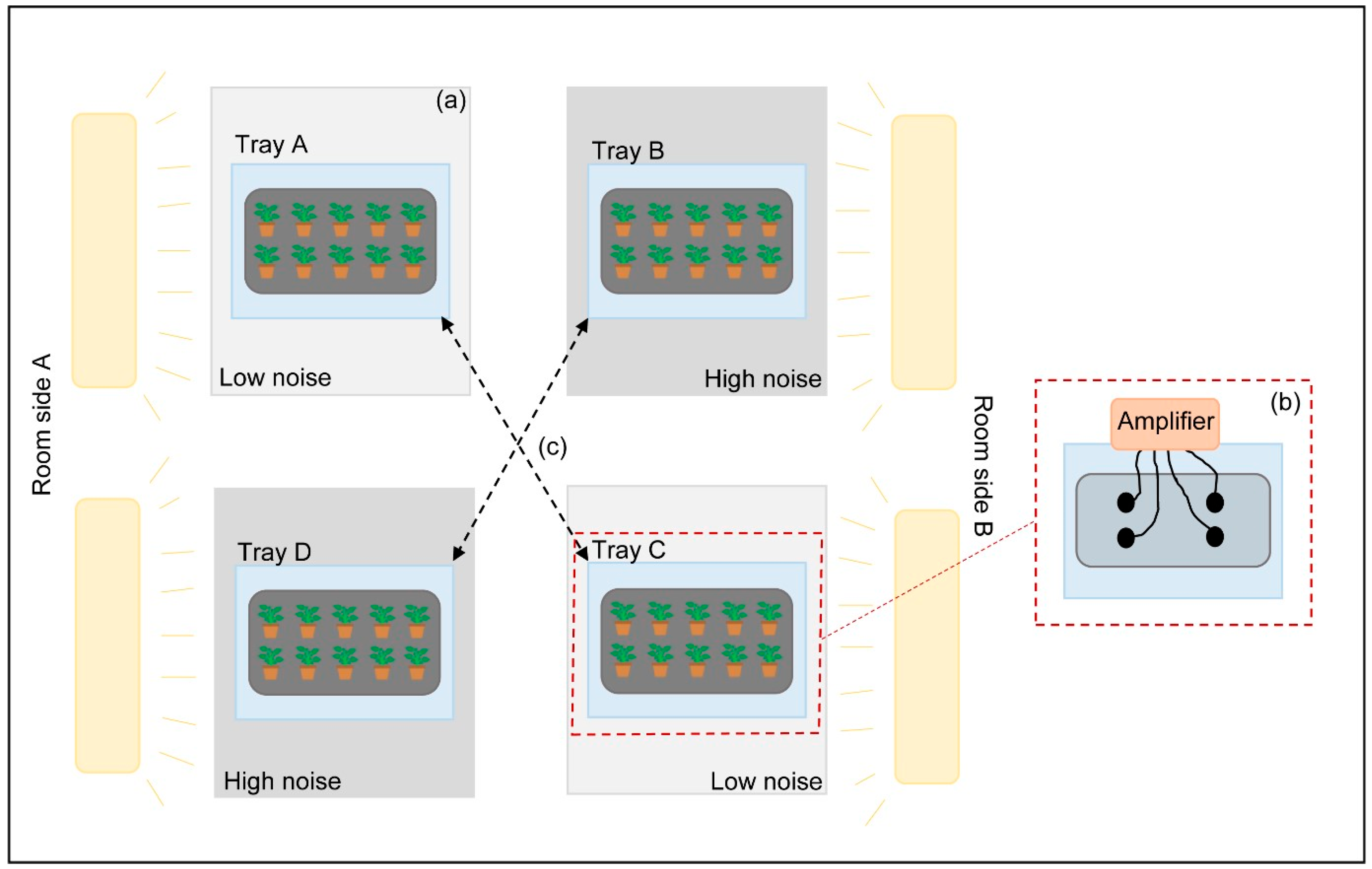
We exposed P. sativum plants to high and low levels of vibratory noise throughout their development (47 ± 3.5 days). Seeds were planted individually on 26 June 2019 in 6 cm diameter plastic plots containing organic potting soil (Horticoop, Kalppolder 150, Bleiswijk, The Netherlands). The entirety of this experiment took place in a climate chamber at 20 °C with an 8:16 (L:D) photoperiod and 70% relative humidity. The lighting in the experimental room contained Photosynthetically Active Radiation (PAR) in the range of 400–700 nm and some far-red lighting using LED lamps, in the range of 600–800 nm. The light level in the experimental room was 450 µmol/m2/s. We exposed a total of 40 plants to vibrational noise from the seed stage to the fruiting stage. Of the 40 plants, 20 were exposed to high vibrational noise, and the other 20 were exposed to low vibrational noise (see below). Ten pots were placed on a plastic tray (48.5 × 31 cm). Below the tray, four shakers (Monacor AR-30, Monacor International GmbH & Co. Zum Falsch 36, 28307 Bremen, Germany) connected to a constantly charging mp3 player (Lenco Xemio-200) and to a stereo amplifier (Renkforce SAP-702 2 × 20 W manufacturer No. RF-3511635 or Renkforce T21 2 × 50 W manufacturer No. RF-4602693) broadcasted vibrational pink noise.
Our playback stimulus (pink noise) was based on field recordings of subterranean vibrational noise induced by windmills (see [
17], for methods on field recordings of wind turbine-induced vibrations). Since windmill-induced vibrational noise was highly represented by energy in the low frequencies (<100 Hz), we decided to use pink noise as a standardized playback stimulus, also biased towards the low frequencies, albeit in a wider range.
We measured the ambient vibratory noise levels of our setup using a laser Doppler vibrometer, Polytec (Waldbronn, Germany) (LDV; Polytec PDV-100, set to 100 mm/s/V, sampling rate 22 kHz) connected to an oscilloscope (Rigol, DS1054, 4 Channel 50 MHz, 1 GSa/s). We adjusted the amplitude by changing the settings of the amplifier. For the low-noise treatment, we matched the RMS values obtained from the oscilloscope to the value obtained for the ambient conditions of the room. For the high-noise treatment, we set the amplitude 12 dB higher compared to the low-noise treatment. A difference of 12 dB corresponds to the difference obtained from recording windmill-induced soil vibrations at 8 m compared to 128 m. We chose 12 dB because this was the maximum intensity difference that we could obtain with our speaker setup without creating distortion. The amplitude of our vibrational noise stimuli was calibrated on several positions on the tray to ensure that all plants were exposed to equal vibrational noise amplitude (
Figure 1).
We divided the treatments into two trays, and the trays were positioned opposite to each other (on opposite sides of the room) to control for any lighting effects (
Figure 2). Halfway through the experiment (19 days after planting) we switched the tray positions so that the plants in one side of the room would be exposed to the other side of the room, thus avoiding confounding effects of location in the room.
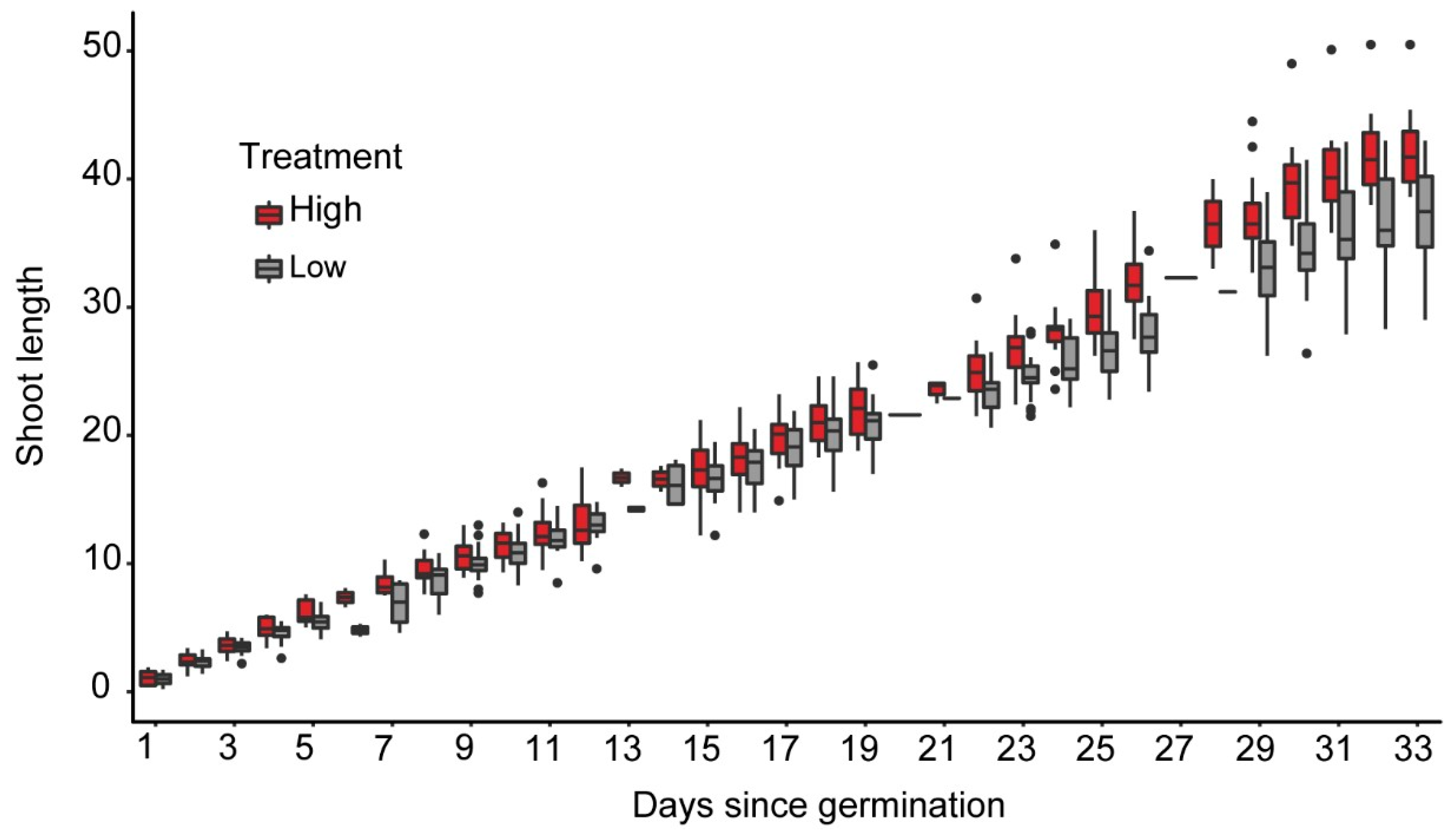
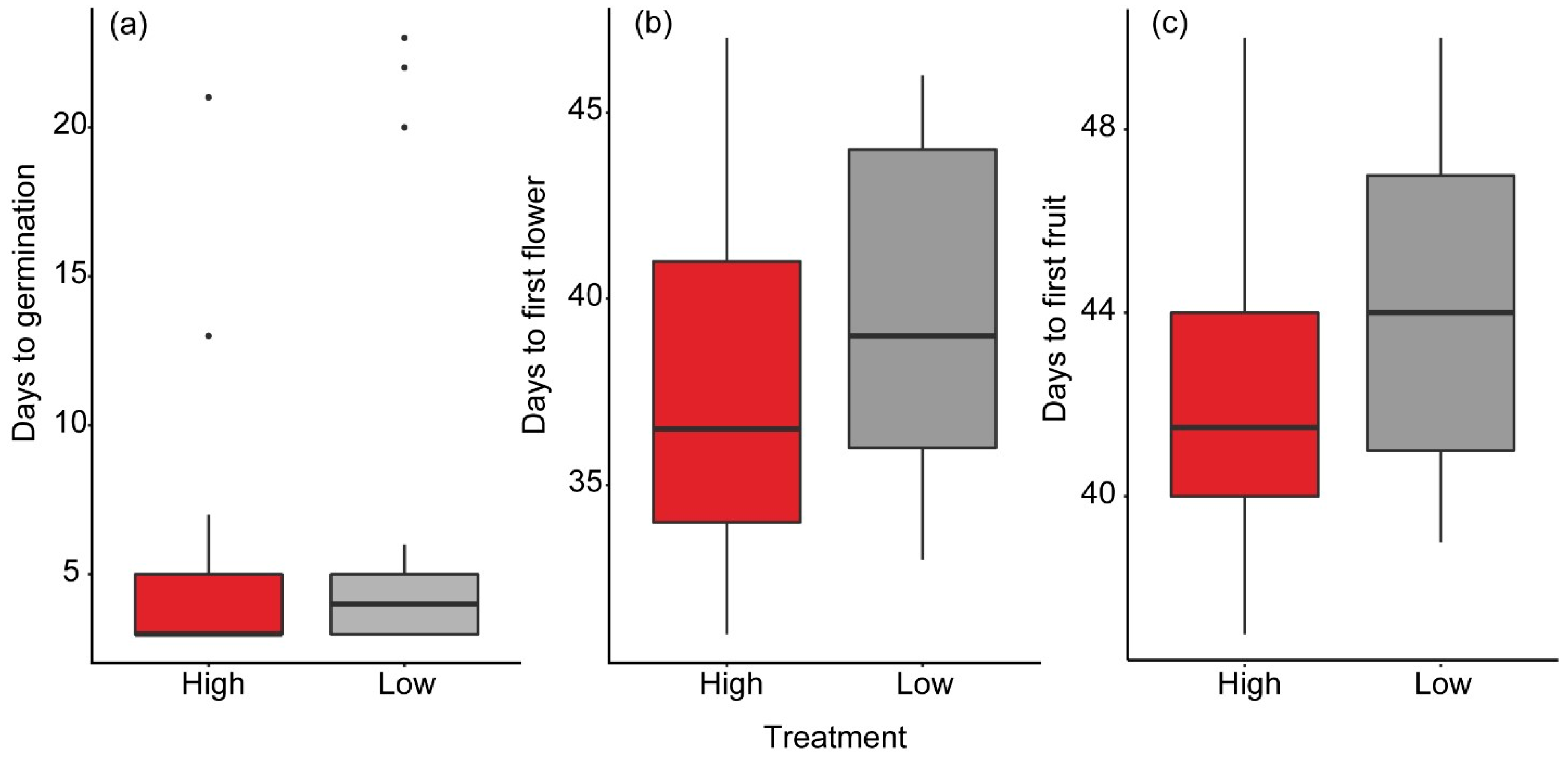
The seeds/plants were checked on a daily basis, except for weekends. We extrapolated based on developmental events recorded during the working week to calculate the day an event took place during the weekend. We noted the germination date, date of appearance of the first flower, and date of appearance of the first fruit. Additionally, shoot length was measured on a daily basis with a metallic 30 cm ruler. The plants were watered by adding water to their tray, allowing them to absorb as much water as needed.
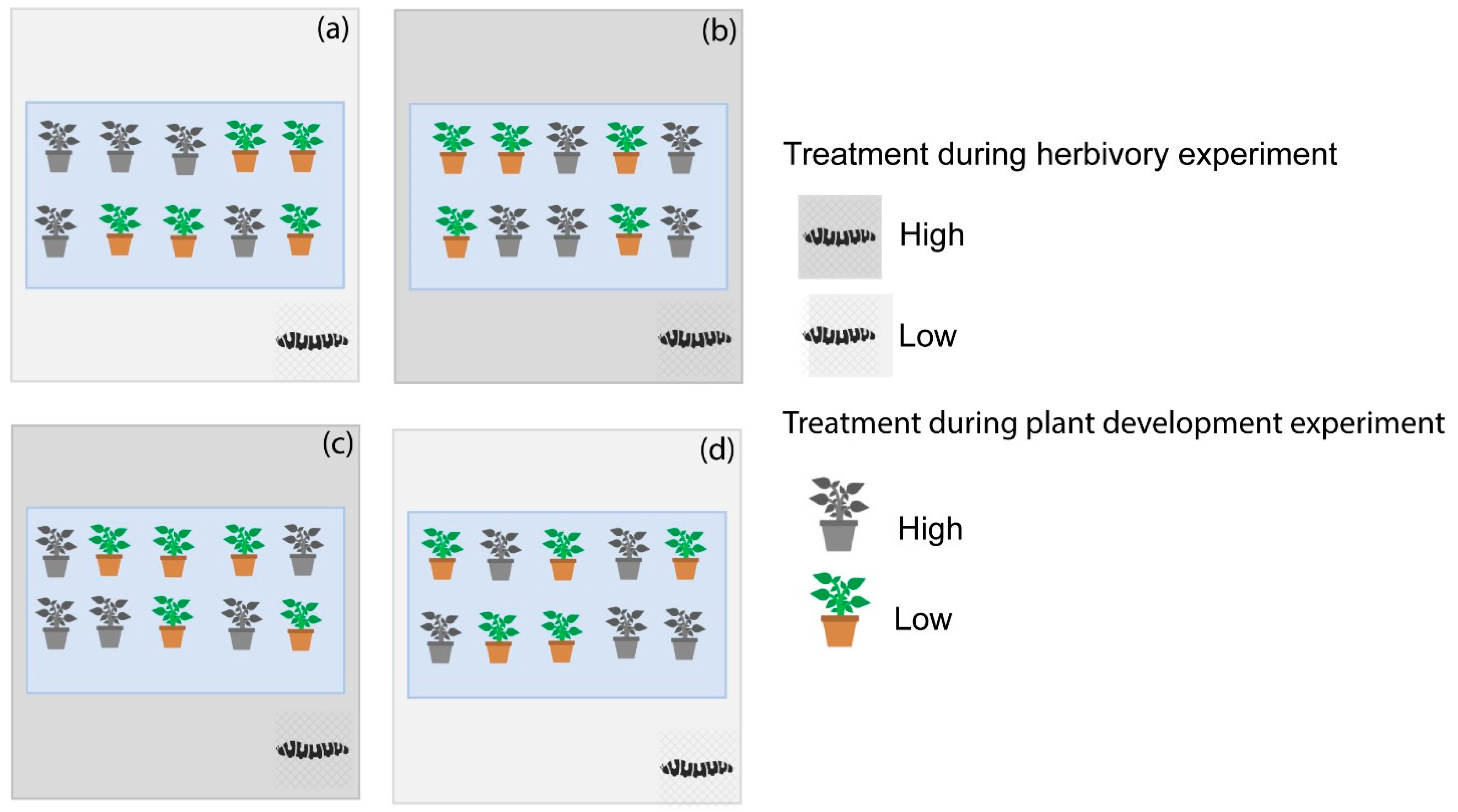
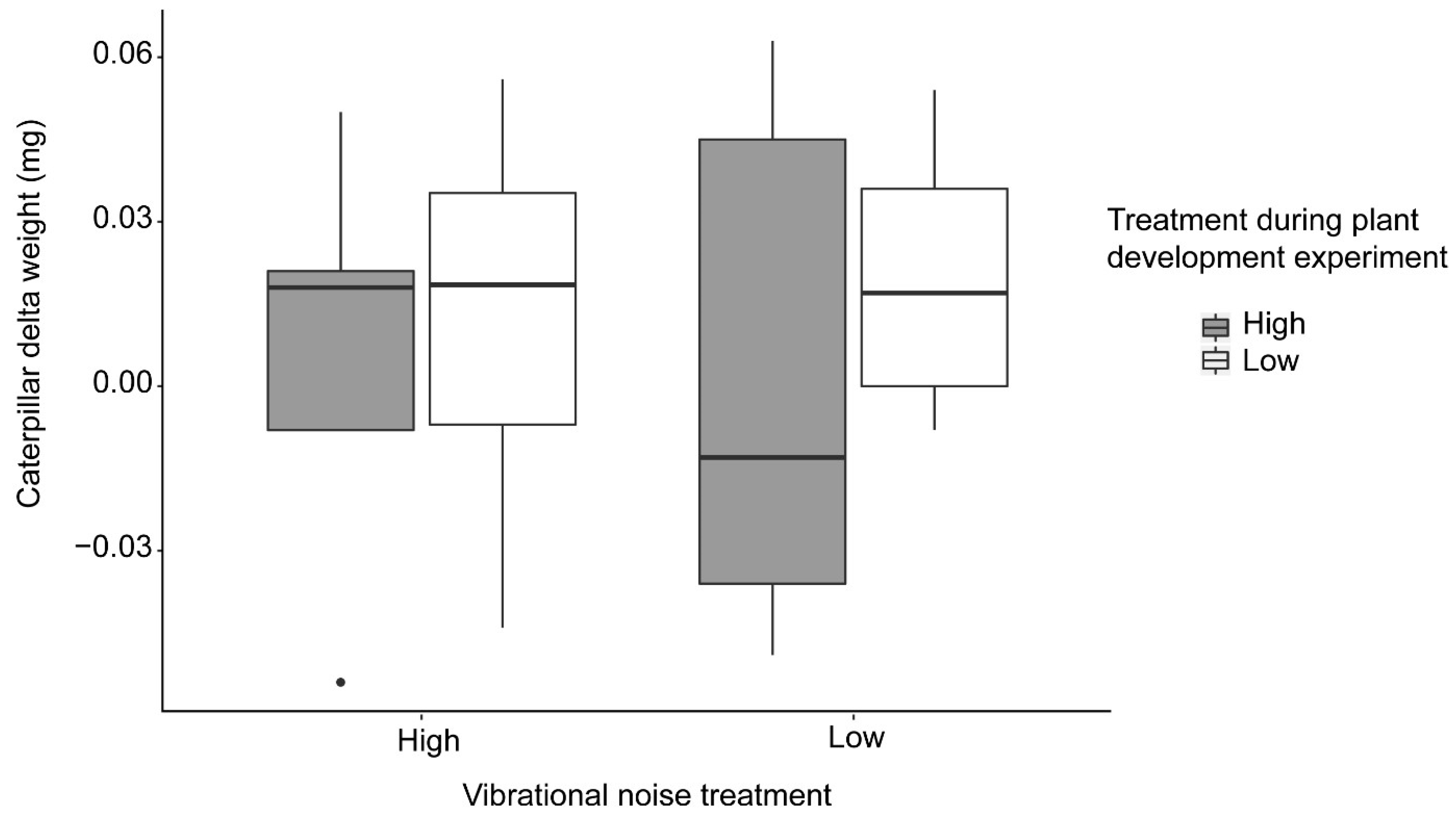
For the herbivory experiment we exposed caterpillars to high and low vibrational noise while they foraged on plants that had previously been exposed to either high or low vibrational noise during the plant development experiment (
Figure 3). Half of the plants that were exposed to high vibrational noise during the plant development experiment were exposed to low vibrational noise during the herbivory experiment, while the other half were exposed to high vibrational noise. The same applied for plants exposed to low vibrational noise during the plant development experiment, with half of the plants exposed to high vibrational noise during the herbivory experiment, and the other half exposed to low vibrational noise. We used the change in caterpillar weight (delta weight) as a proxy for herbivory intensity. Some caterpillars failed to forage at all and were excluded from the results. The caterpillars that failed to forage fell into the soil and did not climb the plant again. The number of caterpillars that failed to forage was distributed across treatments. In total we obtained data for 35 caterpillar–plant combinations.
We deprived the caterpillars (5th instar, 22–27 days old) of food for 2 h before testing them. The caterpillars were weighed just before placing them on a plant. Individual shakers were placed under individual plants. The caterpillars were allowed to forage for 24 h, after which they were removed and immediately weighed. The foraging intensity of caterpillars under different noise levels could differ because the caterpillars might be disturbed by noise, in which case we would expect their foraging intensity to be lower under high-noise conditions, with caterpillars showing a lower weight increase. Alternatively, caterpillars’ chewing vibrations, which can increase secondary metabolite emission in plants [
11], could be masked by our high-noise treatment. The masking of caterpillar-induced vibrations could result in lower emission of secondary metabolites, thus allowing the caterpillars to forage for a longer amount of time. In this case, we would expect a higher caterpillar weight increase. For logistical reasons we did not measure the surface eaten from the leaves. However, we would expect the surface eaten to be highly correlated with increases in caterpillar weight. Therefore, we used delta weight as a proxy for herbivory intensity.
2.3. Data Analyses
Statistical analyses were carried out with R version 3.3.1 (R Core Team, 2016), run in the R studio interface (RStudio Team, 2015).
We used a Kruskal–Wallis test to test the effects of high and low vibrational noise on the number of days it took for the seeds to germinate. We used a non-parametric test here, because our data did not follow a normal distribution, and no transformation or alternative choice of distribution family produced a satisfactory model. Furthermore, to test the effects of high and low vibrational noise on the number of days it took for the plants to produce their first flower and their first fruit, we used linear mixed-effects models following Gaussian distribution. We included tray ID as a random effect with a random intercept. Days to flowering and days to fruiting, which were our response variables, were both squared-root transformed to achieve a better model fit. The number of days it took to flower and produce fruit was calculated from the germination date and not from the planting date. To test the effects of high and low vibrational noise on plant growth, we used a linear mixed-effects model with repeated shoot length measurements as our response variable. We nested plant identity in tray as a random effect, with a random intercept.
We tested whether vibrational noise affected herbivory using a linear regression, with caterpillar delta weight as our response variable. Our main predictors were treatment during the herbivory experiment (high or low vibrational noise at the time of foraging), treatment during the plant development experiment (treatment to which the plants had been exposed in the previous experiment), and final shoot length. We tested the interaction between treatment and previous treatment. We included the plants’ final shoot length as a predictor to control for effects of plant size on the investment of plant defenses against herbivory.
All model residuals were inspected by means of Q-Q plots and histograms. There were no deviations from the normality or variance homogeneity assumptions from the linear models. The effects of individual model predictors were established by full-null model comparisons by means of Wald chi-squared statistics from the “Anova” function in the statistical package “Car” [
21].
4. Discussion
In this study we examined whether windmill-like vibrations (pink noise) affected plant development from the seed stage to the fruiting stage. We exposed garden pea plants (P. sativum) to vibrational pink noise at 12 dB above ambient sound levels, and we compared their developmental processes like germination, flowering time, fruiting time, and growth to those of plants exposed to low noise levels (undistinguishable from ambient sound levels). Our results show that plants exposed to high noise levels grew taller compared to plants exposed to low noise levels. Furthermore, we found that, on average, plants exposed to high noise germinated, flowered, and produced fruit slightly faster than plants exposed to low noise. The differences in timing for these developmental stages, however, were not statistically significant between the treatments.
Previous studies have shown that acoustic or vibrational stimuli can affect plant growth and other plant development characteristics. For example, mechanical stimulation with 50 Hz vibrations has been shown to promote germination in
Cucumis sativus and
Oryza sativa [
8]. Uchida and Yamamoto [
9] also showed that vibrational stimulation with monotone frequencies in the range of 40–120 Hz affected the germination of
Arabidopsis thaliana. Moreover, ethylene synthesis, which is necessary for seed germination [
22], is known to be induced by mechanical stresses like bending, rubbing, and shaking of plants [
23,
24], and was more recently shown to be induced by vibrations [
9]. Some studies have also provided evidence that vibrations of certain frequencies positively influence not only germination but also root elongation, callus growth, and cell cycling [
25,
26,
27].
Preliminary investigations indicate that plants exhibit frequency-selective sensitivity [
6]. A study from 2014 experimented with several plant species, exposing them to sounds ranging from 0.1 to 1 kHz with plant acoustic frequency technology (PAFT) [
28]. They found a significant increase in the yield of sweet pepper, cucumber, and tomato, by 30.05, 37.1, and 13.2%, respectively [
28]. Although the authors did not go into the physiological mechanisms behind the increases in crop yield, similar experiments on non-crop plants (
Dendradema morifolium) showed an increase in soluble protein and sugar in the cytoplasm, which indicates higher metabolism levels and a vigorous state of cell division [
29]. Interestingly, all of the studies discussed above used pure tone frequencies. In our study, we used pink noise to simulate windmill-induced noise, which is a broadband stimulus.
A study in 2001 tested the difference in growth in bean and impatiens plants when exposed to pure tones versus white noise (also a broadband noise), finding that plant growth was higher when the plants were exposed to pure tones instead of white noise [
30]. It is therefore possible that
P. sativum is sensitive to a particular frequency or frequencies, and that this is why we see a trend, but not enough to be statistically significant in all of the developmental characteristics that we measured. Because the effect of sound vibrations on plants can be frequency- and amplitude-dependent [
25], not all plant species’ development could be affected by windmill-induced vibrations in the same way, possibly altering competition rates between plant species. Although this would not necessarily be a problem for cultivated fields with monocultures, it could have consequences for natural plant communities, where interspecific competition is an important determinant of the structure and the dynamics of plant communities [
31]. Furthermore, investment in plant growth might come at the expense of plant defense activation, which is imperative for plant survival [
32], since plants count on a limited pool of resources that can be used for growth or for defense [
33,
34,
35]. Our results highlight the susceptibility of plants to vibratory noise. Future studies are needed to understand the sensitivity of plants to vibrations of various frequencies, as well as whether different developmental stages are affected differently by the different frequencies.
Vibrations are not only important for plants’ developmental processes, they can also be important for plant–animal interactions. For instance,
Arabidopsis thaliana increases its anti-herbivory defenses when stimulated with its herbivores’ chewing vibrations [
11], and
Oenothera drummondii increases its nectar sugar concentration when exposed to the buzzing vibrations of its pollinators [
13]. Plants that are constantly exposed to vibrational noise, like plants in a windmill field, could have a harder time detecting the chewing vibrations of their herbivores, making them more vulnerable to higher herbivory intensity. In our study we also tested whether vibrational noise affected herbivory by
Spodoptera exigua, a generalist caterpillar. We did not find any evidence that vibrational noise affected herbivory directly or indirectly (see our methods for the difference between direct and indirect exposure). Our results suggest that the stimulus to which we exposed our plants might not have elicited a chemical anti-herbivory response in the plants, such as shown by [
11]. However, in our experimental setup, we used caterpillar delta weight as a proxy for herbivory intensity, which might not be the most accurate measurement of effects on herbivory, since fluctuations in weight might also be related to the individual moving activity patterns of the caterpillars. Therefore, future studies should consider using the percentage of leaf consumed instead of caterpillar weight.
In a fast-changing world transitioning to renewable energy, it is important to investigate how vibrational noise induced by wind energy turbines might affect plant development and plant–insect interactions. In spite of our small sample size and methodological constraints, we believe that this study provides a first step towards understanding how plants respond to a relevant noise source. Finally, the positive effects on plant growth reported in this study may offset some of the potentially negative effects that we observed at the field sites from which we obtained the seismic noise recordings. At those organic crop fields, we observed that earthworm abundances decrease with increasing seismic noise levels [
17]. As earthworms are well-known ecosystem engineers influencing soil functioning, a negative effect on their populations may reverberate into reduced crop performances. Future studies should combine both direct and indirect effects on plants, animals, and perhaps even micro-organisms to obtain a better understanding of the impact of seismic noise on soil organisms.