Abstract
Introduction: Many patients with Crohn’s Disease (CD) require surgical resection during their lifetime. Nevertheless, postoperative recurrence (POR) is common. Risk factors for POR are still yet to be completely established, but some prognostic factors have already been widely recognized. Patients that undergo early postoperative immunomodulators (EPI) (azathioprine or biological therapy) seem to have a lower risk of recurrence. We aimed at assessing whether or not EPI is effective in preventing POR and at the same time validating traditional and new risk factors for POR. Methods: A single-center retrospective cohort study was performed. Review of clinical, demographic and histopathological characteristics of patients undergoing ileocolonic resection for CD between 2015 and 2020 was performed. EPI was defined as the restarting or introduction of azathioprine or biologics within 8 weeks after surgery. Presence of recurrence was defined as Rutgeerts score ≥ i2. Results: Sixty-five patients were included. The median age at diagnosis was 25 years (inter-quartile range 20–33 years). POR was present in 44.6% of patients, and the median time from surgery to recurrence was 2 years. EPI was the therapeutic option in 36 patients (55.4%). Univariate analysis identified as predictors of POR within 2 years: the behavior of the disease and not taking azathioprine or biologics prior to the surgery. Moreover, univariate analysis identified as predictors of time to POR: behavior of disease, less than 5 years between diagnosis and surgery and the absence of immunomodulatory therapy prior to the surgery. Multivariable analysis revealed that EPI, after adjusting for other predictors, was not associated with a reduction in POR. Conclusions: EPI may not have a protective effect against recurrence. The strength of prognostic factors for POR may not be modified by EPI.
1. Introduction
Inflammatory bowel disease (IBD) is one of the most common immune-mediated diseases of the gastrointestinal (GI) tract. Globally, IBD affects almost 68 million people [1], and the incidence has been progressively increasing [2].
Crohn’s disease (CD) is characterized by relapsing and remitting symptoms [3]. In a major proportion of patients with CD, surgical treatment is needed either to solve complications or to tackle short segments of disease [4,5]. Nevertheless, postoperative recurrence (POR) is common, with around 50% of patients who undergo surgical resection need re-operation [6,7,8].
Endoscopic recurrence (ER) seems to precede symptomatic relapse and may be observed in almost 80% of the patients undergoing ileocolonic resection [9]. The Rutgeerts score was created to predict the postoperative course [10]. Generally, POR is defined as the endoscopic presence of more than five ulcers or diffuse inflammation after the surgery, in accordance with the above-mentioned score [11,12].
The physiopathology and prognostic factors associated with this phenomenon are not completely established. POR is considered to be a major challenge in clinical practice, and it is significantly detrimental to patients’ quality of life [13]. Several studies have tried to detect risk factors for POR. Patient-related risk factors such as active smoking [14,15,16] and a history of surgical resection for CD [17] are commonly identified. As far as disease-related risk factors are concerned, penetrating behavior [18], a shorter disease duration before surgery [19], and a longer intestinal resection [20] have also been correlated with higher rates of POR. Ryan et al. [15] recognized that smoking cessation was protective against POR. The same conclusion was reached in a meta-analysis that involved 2692 individuals with CD [16]. Regarding penetrating behavior, a meta-analysis [18] and even the European Crohn’s and Colitis organization consensus [21] further confirm its status as a poor prognostic factor. Regarding disease duration before surgical resection, conclusions are still under debate [22]. Moreover, the effect of a wider length of intestinal resection has been controversial [23]. Finally, as far as clinical factors are concerned, a history of surgical resection for CD and a shorter disease duration before the surgery have been associated with an increased risk of POR [17,24,25,26].
Furthermore, there are some histopathological-related factors predictive of POR, such as positive ileal margins, plexitis, epithelioid granulomas and lymphatic density [27,28,29]. A recent meta-analysis showed that positive resection margins increased the risk of clinical, endoscopic and surgical recurrence [29]. The same report showed that plexitis was a prognostic factor predictive of ER. Concerning the presence of granulomas, another meta-analysis demonstrated that granulomas were a risk factor for POR [30]. Moreover, recently, transmural involvement of the resection margin has been proposed as a further risk factor for POR [31].
Patients at a higher risk of a poorer prognosis may benefit from the implementation of post-surgical immunomodulators. The introduction of immunomodulators has been performed taking the proposed risk factors into consideration. Nevertheless, these decisions are empirical and subjective. More importantly, in the context of early postoperative immunomodulators (EPI), whether treatment initiation (either sustained or initiated) has been a matter of controversy [32,33]. A recent meta-analysis showed that the timing of initiating biologic therapy (BT) after surgery does not have a significant impact on POR [34]. Therefore, further clarification is needed to determine whether EPI is effective in preventing POR when compared to an introduction of these therapies after endoscopic recurrence.
Furthermore, recent studies have shown that ileal segments of the disease tend to respond worse to BT than colonic segments [35,36]. This fact poses an additional challenge in the introduction of EPI in patients who have undergone ileocolonic resection.
Clearly, identifying the lowest POR risk patients is critical, as this will enable the prevention of unnecessary use of immunomodulators. Additionally, knowing the most effective time to introduce immunomodulators is extremely important. This will certainly lead to savings in terms of health-related quality of life, healthcare expenditure and treatment adverse effects.
Overall, the primary aim of this study is to assess the putative benefit of EPI in preventing POR in patients with CD who underwent ileocolonic resection. Moreover, our secondary aim is the validation of the already described and recently proposed risk factors for POR.
2. Methods
2.1. Study Design
We have conducted a single-center retrospective observational study in the Gastroenterology department of a tertiary hospital. Clinical, endoscopic and pathological factors of patients who underwent ileocolonic resection between January 2015 and November 2020 were reviewed. The indications for surgery included a poor response to medical therapy or the presence of complications (either abscesses, fistulas or perforation or symptomatic strictures). Clinical information was collected from patients’ medical records the hospital’s database.
2.2. Study Population
The study included patients who underwent surgical resection for ileocolonic CD between the ages of 18 and 75. Categories of patients regarding behavior of disease were created according to Montreal classification to homogenize patients’ subgroups characteristics [29].
The patients were followed-up with regular consultations with clinical and analytical evaluations, including inflammatory biomarkers (fecal calprotectin and C-reactive protein), every six months. Postoperative colonoscopy was performed after a minimum follow-up period of 6 months. Patients without an endoscopic evaluation within 2 years after surgery were excluded. EPI was defined as the initiation of immunomodulators within 8 weeks after surgery. Patients who initiated EPI were compared with patients who did not receive postoperative medical therapy during the follow-up period and patients who initiated immunomodulators later, in case endoscopic signs of recurrence were detected.
A follow-up of less than 2 years was an exclusion criterion. All patients were followed until the end of the study period.
3. Outcomes
The primary outcome evaluated during postoperative colonoscopies was the presence of recurrence, defined as more than five aphthous ulcers or diffuse inflammation of mucosa or the presence of larger ulcers, nodules or narrowing (Rutgeerts score ≥ i2) [8]. The time to recurrence was defined from the date of surgery until the end of the follow-up period or until a colonoscopy with Rutgeerts score ≥ i2 was recorded.
3.1. Surgical Procedures
The surgical procedure performed was ileocolonic resection. Appendectomy and perianal surgery were not considered surgical resection for CD. Resection of other segments of the disease (other than ileum or colon) led to the exclusion of patients. Patients who underwent segmentary enterectomy or colectomy were also excluded from the study.
3.2. Histopathological Analysis
Archival material (resection samples) was examined by two pathologists with extensive experience in IBD, and they were blinded to clinical data and outcomes.
The histological characteristics analyzed were the presence of plexitis (defined as inflammation of nerve bundles), epithelial granulomas and inflammation in the margin of the resection, either transmural (defined as involvement of several layers of the bowel wall) or mucosal.
3.3. Statistical Analysis
Data were analyzed using Stata (Stata Corp LP®, College Station, TX, USA) (version 16.0). Descriptive statistics were used in the description of clinical and analytical data at baseline and throughout the follow-up. Continuous variables were described with median and inter-quartile range (IQR), and categorical variables were presented as frequencies. The primary outcome analyzed was recurrence at the end of a two-year follow-up. A logistic regression was created to assess whether demographic and clinical characteristics of the study population were predictive of POR. Moreover, a logistic regression model was also used to perform univariate comparisons between groups that initiated EPI and those that did not. Furthermore, a survival analysis was conducted to compare the time to recurrence within subgroups of patients. Survival studies were performed using the Kaplan–Meier method and compared with Log-rank test. An analysis of independent risk factors for POR after a two-year follow-up and for time to POR was performed with a multivariable logistic regression and a Cox regression, respectively. Factors included in this multivariable analysis were those with a p-value < 0.2 in the univariate analysis, as well as EPI. The discriminative ability of each predictive factor identified in the multivariable analysis was measured using area under the curve [AUC] analysis. A p-value < 0.05 was considered statistically significant.
4. Results
4.1. Study’s Population Characteristics
The study population included 65 patients (Figure 1). The majority of the patients were male (33; 50.8%). The median age at diagnosis was 25 years (IQR 20–33 years). Smoking habits were present in 23 patients (41.1%). A family history of IBD was reported by eight patients (17%). Regarding the behavior of the disease, seven patients (10.8%) had non-stricturing non-penetrating behavior, while 27 (41.5%) had stricturing behavior, and 31 (47.7%) had penetrating behavior. A minor proportion of patients (11; 16.9%) had perianal involvement due to the disease. Previous abdominal surgery for CD had been performed in eight cases (12.3%). Immunomodulatory therapy (either as biological therapy or as azathioprine) was present in the majority (35; 53.9%) of patients prior to the surgical procedure. The therapies performed before the surgery were as follows: corticosteroids in eight patients (12.3%); biological therapy either in monotherapy or in combination in 20 patients (30.8%); azathioprine as monotherapy in 15 patients (23.1%), and messalazine in five (7.7%) patients. Clinical, demographic and histopathological characteristics of the study population are further demonstrated in Table 1. The median age at the time of surgical resection was 34 years (IQR 27–42 years).
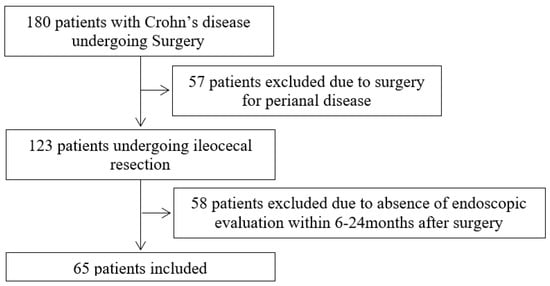
Figure 1.
Flowchart of the patients included in the study.

Table 1.
Demographic and clinical characteristics of study population (N = 65 patients) undergoing surgical resection for Crohn’s Disease. Assessment of predictor of POR (Logistic regression; statistical significance with p < 0.05). CD: Crohn’s Disease; IBD: Inflammatory Bowel Disease; IQR: interquartile range; OR: Odds-ratio; POR: Postoperative recurrence after a 2 year follow-up. * significant p values.
Indications for surgery were as follows: poor response to medical therapy in 18 patients (27.7%) or he presence of complications—either abscesses, fistulas, perforations or occlusive symptoms in 47 patients (72.3%). Abscesses were present in seven patients (10.8%), fistulas in 16 patients (24.6%), perforation in four patients (6.2%) and symptomatic strictures in 20 patients (30.8%). The median duration from diagnosis until the surgery was 7 years (IQR 1–13 years). The median extension of the resection specimen was 32 cm (IQR 28–45 cm). The median extension of ileum resected was 23 cm (IQR 17–35 cm). Anastomosis performed was laterolateral in 61 patients (93.8%), end-to-end in two patients (3.1%) and terminal ileostomy two patients (3.1%).
Regarding histopathological parameters, plexitis was detected in 61 resection specimens (93.9%), while granulomas in 20 (30.8%). Inflammation of the resection margin was present in 12 specimens (18.5%), with seven of them showing transmural involvment (10.8%).
The median follow-up period was 5 years. POR after a 2 year follow-up occurred in 29 patients (44.6%). The Rutgeerts score after this follow-up period was as follows: 0 in 26 patients, 1 in 10 patients, 2 in 15 patients, 3 in 10 patients and 4 in 4 patients. Over the entire follow-up period, POR occurred in 32 patients (49.2%). The median time from surgery to recurrence was two years (IQR 1–4 years).
EPI was the therapeutic option in 36 patients (55.4%). Azathioprine was initiated in 18 patients (27.7%) and BT in 19 patients (29.2%). One patient initiated both infliximab and azathioprine concomitantly. Clinical and histological characteristics of the groups that did or did not receive EPI are demonstrated in Table 2. Patients who initiated EPI were younger at diagnosis, had a more aggressive phenotype of CD, and a longer period of disease evolution (p < 0.05). Moreover, the use of biological therapy or azathioprine before the surgery was more common in the group that initiated EPI (p = 0.023).

Table 2.
Comparison of characteristics between patients initiating biological therapy or azathioprine in the 8 first postoperative weeks and the patients who did not. Study population (N = 65 patients) undergoing surgical resection for Crohn’s Disease. CD: Crohn’s Disease; EPI: early postoperative immunomodulators (biological therapy or azathioprine); IBD: Inflammatory Bowel Disease; IQR: interquartile range. * significant p values.
EPI did not have a significant impact on POR at 2 years. POR developed in 13 patients (44.8%) who did not initiate EPI and in 16 patients (44.4%) that underwent EPI (p = 0.975).
4.2. Univariate Analysis
The prognostic factors associated with POR in the logistic univariate analysis are presented in Table 1. Penetrating and stricturing behavior of CD and the absence of biologics or azathioprine prior to the surgery were predictive of higher rates of POR after a 2 year follow-up. Patients who had previously undergone abdominal surgery for CD had higher rates of biologics or azathioprine use before this latter surgery (p = 0.06).
The predictors for the time to POR, as assessed in the univariate analysis using the Kaplan–Meyer method, are presented in Table 3. Penetrating and stricturing behavior of CD, the absence of biologics or azathioprine before the surgery and a time interval from diagnosis to surgery shorter than five years were predictive of a shorter time to recurrence.

Table 3.
Clinical predictors of time to recurrence in the study population (N = 65 patients) undergoing surgical resection for Crohn’s Disease (Log-rank test; * statistical significance with p < 0.05).
4.3. Multivariable Analysis
The independent prognostic factors for POR at 2 years, as identified by the logistic multivariable analysis, are reported in Table 4. Previous abdominal surgery for CD and the absence of a history of immunomodulators prior to the surgery were associated with higher rates of POR. The AUC for the absence of either biological therapy or azathioprine prior to surgery was 0.66 (CI 0.54–0.78). The AUC of previous abdominal surgery for CD was 0.67 (CI 0.49–0.84). After adjusting for significant predictors in the univariate analysis, EPI was not associated with POR.

Table 4.
Multivariable analysis: Clinical independent predictors of Postoperative Recurrence after a 2 year follow-up and of time to Postoperative Recurrence a in the study population (N = 65 patients) undergoing surgical resection for Crohn’s Disease (Logistic Regression); * statistical significance with p < 0.05; CD: Crohn’s disease; OR: Odds ratio; CI: Confidence Interval; HR: Hazard-ratio.
The independent factors for time to POR, as identified by the Cox multivariable analysis, are also reported in Table 4. A time interval from diagnosis to surgery shorter than five years, previous abdominal surgery for CD and the absence of biologics or azathioprine prior to the surgery were independent predictors of shorter disease-free survival.
5. Discussion
CD is a chronic, relapsing condition requiring surgery in case of refractivity to medical therapy or due to complications. A significant proportion of patients with CD will need surgical resection during their lifetime. However, surgery is not curative, and recurrence is common [2,6]. In fact, throughout the postoperative course, the 10 year risk of recurrence is almost 50% [8]. The rates of POR in our study were in accordance with previous works [6]. A meta-analysis of Renna et al. [37] revealed that rates of postoperative endoscopic recurrence in patients not undergoing medical postoperative therapies were around 50%. Our rates of POR were similar in both groups of patients: the group that initiated EPI and the group that did not. However, differences existed between these groups, as discussed below.
Endoscopic recurrence takes precedence over symptomatic recurrence. In fact, an endoscopic evaluation with ileocolonoscopy is recommended 6 to 12 months after surgery [25]. Nevertheless, whether endoscopic monitoring and prophylactic therapy guided by colonoscopy are beneficial compared to empirical initiation shortly after surgery is still a matter of debate [38].
With this study, we aimed at assessing the role of EPI in reducing rates of POR. We also aimed at assessing traditional and recently proposed risk factors for POR.
Biological therapies and other immunomodulators seem to be the most effective prophylaxis for POR compared to less effective salicylates and steroids [25,30,31]. Studies have demonstrated that the introduction of postoperative BT is associated with lower rates of POR [10,32]. For example, the PREVENT trial, which included 297 patients with CD in the postoperative period, showed that Infliximab led to lower rates of endoscopic recurrence compared to a placebo [39]. The results are similar with Adalimumab [40]. Nevertheless, the benefits of the introduction of these therapies must be balanced with their risks and economic burden [41]. In fact, regardless of their efficacy, biological therapies may have limited durability of effect and can lead to serious adverse effects such as infections and malignancies. Moreover, in some cases, surgery leads to long-term remission, and patients may not need any more therapies throughout lifetime. As a result, postoperative medical therapies must only be initiated in highly selected patients who carry a greater risk of POR. As a matter of fact, identifying high POR risk patients will enable physicians to select the appropriate prophylactic and postoperative therapy. However, the optimal timing for postoperative treatment onset is still also controversial.
Histological recurrence has been identified as the first form of recurrence, with inflammatory infiltrate being present in the first postoperative days [42]. This is part of the rational for considering EPI as advantageous.
EPI has been introduced according to the already recognized predictors of recurrence, namely: younger age, active smoking, previous surgeries performed for CD, perianal disease and a penetrating behavior of the disease [10,33]. After a postoperative period of at least six months, an endoscopic assessment is performed.
In this study, concerning immunomodulatory treatment, EPI tended to be introduced in younger patients with a penetrating behavior and a longer period of time between the diagnosis and the indication for surgery. This last data may be due to higher rates of previous abdominal surgery for CD in the group of individuals in which EPI was initiated. Moreover, there was a higher proportion of patients who had previously undergone biological or other immunomodulatory therapies in the group that initiated EPI.
Nonetheless, it is important to emphasize that the outcome of the predictors was not modified by EPI. The univariate analysis showed that POR rates in the EPI-treated group were not different from those observed in the group with a conservative approach. The multivariable analysis further showed that, when other predictors were controlled, EPI did not offer protection against POR.
Nevertheless, it is worth noting that, in fact, rates of POR were similar between the patients who initiated EPI and those who did not. Given the statistical differences in some POR risk factors between these groups, it may be inferred that these therapies may be effective in a very high-risk group. However, this conclusion cannot be definitively drawn from our study. Taking into consideration such results, whether EPI therapy represents a substantial benefit or not remains to be elucidated. Additionally, the potential benefit of EPI may also depend on specific patient subsets.
Concerning the POR risk factors assessment, the univariate analysis identified disease phenotype and immunomodulators, either biologics or azathioprine used surgery, as the only factors associated with POR. The penetrating and stricturing phenotypes have already been largely associated with worse prognosis [25].
In the logistic multivariable analysis, consistent with prior findings [12,15], the use of immunomodulators already taken prior to the surgery and the absence of previous abdominal surgery for CD were associated with lower rates of POR. Therefore, pre-surgery immunomodulatory treatment may pose an additional advantage in recurrence prevention. Previous abdominal surgery for CD as a risk factor for recurrence may indicate that the disease is more severe regardless of the resections performed. This finding is in accordance with previous research [17,24,25]. This result must be an alert for the monitoring of these patients to be carried out even more closely. Repeated resections may have serious complications such as short bowel syndrome or complication inherent to the surgical procedures such ad infections or dehiscence.
In the Cox regression analysis, a time interval between diagnosis and surgery shorter than five years, previous abdominal surgery for CD and the absence of biologics or azathioprine used before surgery were independent predictors of a shorter time to POR. A shorter time interval between the diagnosis of CD and ileocecal regression being a risk factor for earlier recurrence is in accordance with other studies [26]. A more aggressive evolution with complications or poor response to medical therapy early in the course of the disease may be associated with higher chances of CD recurrence following ileocecal resection.
Histopathological predictors did not reach statistical significance in our analysis, in contrast to the findings observed in previous studies [20,21,22,23,34]. However, in the univariate analysis, the presence of granulomas and transmural inflammation at the margin of resection showed numerical differences between the POR and recurrence-free groups. Nevertheless, the numerical differences were not in accordance with what was proposed by previous works. Even though these differences were not statistically significant, these factors must be taken into consideration in future studies. Prospective studies including a larger number of patients and standardized resection and analysis techniques are the path to pursue.
It has already been identified that luminal content may be underlying POR [42,43]. Microbiota, dietary components and bile salts may lead to ileal inflammation in the first postoperative days [43]. These findings reinforce the necessity of EPI. Moreover, luminal mechanisms must be taken into consideration in future research. The roles of dietary and microbiota as contributors to POR should be thoroughly studied, and a putative role of enteral nutrition as a preventive therapy of POR should thoroughly investigation.
However, there are limitations to this study that should be noted. The study population is from a single institution, and the design is retrospective, which may reflect some variation in patient selection for surgery and in BT onset. This fact may have been associated with the long follow-up period. Moreover, the study has a small sample size, which may have led to a suboptimal power.
More studies are needed to address these limitations. Results from randomized controlled trials to assess this clinical question, or even from prospective studies, will be crucial for informing clinical decision-making. As future perspectives, a randomized controlled trial comparing EPI with a “wait and see” postoperative approach would be the most accurate way to assess whether EPI is protective against recurrence or not. This comparison should be made across subgroups with higher and lower risk of POR. In fact, even in patients considered to have a higher risk of recurrence, the role of EPI is not yet established.
Moreover, the putative protective role of newer biological therapies such as Ustekinumab and Vedolizumab should be more profoundly investigated. While these therapies have demonstrated efficacy in other indications, their effectiveness in the context of postoperative CD has not been well-established.
The major strength of our study is its innovative approach to comparing the introduction of EPI with its absence in patients with CD undergoing ileocolonic resection.
6. Conclusions
Early postoperative immunomodulators did not seem to effectively prevent postoperative recurrence in CD. The strength of the previously identified risk factors for POR may be preponderant when compared to the power of immunomodulators to mitigate this effect.
Author Contributions
M.J.T. performed study concepts, data acquisition, analysis and interpretation, statistical analysis and manuscript preparation. R.C.O., A.S. and G.N.F. performed study concept and design, data acquisition, analysis and interpretation and manuscript preparation. S.M.F.L., P.F. and F.P. were responsible for study concept and design, manuscript editing and preparation, analysis and interpretation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the institutional ethics committee of Comissão de Ética CHUC (OBS.SF.170-2021).
Statement of Ethics
The project adhered to the standards of good clinical practice and always complied with the ethical precepts of the Helsinki’s Declaration.
Informed Consent Statement
Informed consent was asked of each patient and signed by either the patient or their legal representative.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Conflicts of Interest
Francisco Portela received speaker fees from Abbvie, Falk, Ferring, Janssen, Pfizer, Pharmakern, Takeda and Tillotts. The other authors of this manuscript report no conflicts of interest.
References
- Centers for Disease Control and Prevention. Inflammatory Bowel Disease (IBD) 2021. Available online: www.cdc.gov/ibd/#epidIBD (accessed on 1 January 2023).
- Azevedo, L.F.; Magro, F.; Portela, F. Estimating the prevalence of inflammatory bowel disease in Portugal using a pharmaco-epidemiological approach. Pharmacoepidemiol. Drug Saf. 2010, 19, 499–510. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Buisson, A.; Chevaux, J.B.; Allen, P.B.; Bommelaer, G.; Peyrin-Biroulet, L. Review article: The natural history of postoperative Crohn’s disease recurrence. Aliment. Pharmacol. Ther. 2012, 35, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Adamina, M.; Bonovas, S.; Raine, T.; Spinelli, A.; Warusavitarne, J.; Armuzzi, A.; Bachmann, O.; Bager, P.; Biancone, L.; Bokemeyer, B.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J. Crohn’s Colitis 2020, 14, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T. Factors affecting recurrence after surgery for Crohn’s disease. World J. Gastroenterol. 2005, 11, 3971–3979. [Google Scholar] [CrossRef]
- Rivière, P.; Vermeire, S.; Irles-Depe, M.; Van Assche, G.; Rutgeerts, P.; Denost, Q.; Wolthuis, A.; D’hoore, A.; Laharie, D.; Ferrante, M. Rates of Postoperative Recurrence of Crohn’s Disease and Effects of Immunosuppressive and Biologic Therapies. Clin. Gastroenterol. Hepatol. 2021, 19, 713–720.e1. [Google Scholar] [CrossRef]
- Frolkis, A.D.; Dykeman, J.; Negrón, M.E.; Debruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef]
- O’Connor, A.; Hamlin, P.J.; Taylor, J.; Selinger, C.; Scott, N.; Ford, A.C. Postoperative prophylaxis in Crohn’s disease after intestinal resection: A retrospective analysis. Frontline Gastroenterol. 2017, 8, 203–209. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Geboes, K.; Vantrappen, G.; Beyls, J.; Kerremans, R.; Hiele, M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990, 99, 956–963. [Google Scholar] [CrossRef]
- Rivière, P.; Vermeire, S.; Irles-Depe, M.; Van Assche, G.; Rutgeerts, P.; de Buck van Overstraeten, A.; Denost, Q.; Wolthuis, A.; D’hoore, A.; Laharie, D.; et al. No Change in Determining Crohn’s Disease Recurrence or Need for Endoscopic or Surgical Intervention with Modification of the Rutgeerts’ Scoring System. Clin. Gastroenterol. Hepatol. 2019, 17, 1643–1645. [Google Scholar] [CrossRef]
- Dasharathy, S.S.; Limketkai, B.N.; Sauk, J.S. What’s New in the Postoperative Management of Crohn’s Disease? Dig. Dis. Sci. 2021, 67, 3508–3517. [Google Scholar] [CrossRef] [PubMed]
- Ha, F.J.; Thong, L.; Khalil, H. Quality of Life after Intestinal Resection in Patients with Crohn Disease: A Systematic Review. Dig. Surg. 2017, 34, 355–363. [Google Scholar] [CrossRef]
- Auzolle, C.; Nancey, S.; Tran-Minh, M.L.; Buisson, A.; Pariente, B.; Stefanescu, C.; Fumery, M.; Marteau, P.; Treton, X.; Hammoudi, N.; et al. Male gender, active smoking and previous intestinal resection are risk factors for post-operative endoscopic recurrence in Crohn’s disease: Results from a prospective cohort study. Aliment. Pharmacol. Ther. 2018, 48, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Ryan, W.R.; Allan, R.N.; Yamamoto, T.; Keighley, M.R.B. Crohn’s disease patients who quit smoking have a reduced risk of reoperation for recurrence. Am. J. Surg. 2004, 187, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Reese, G.E.; Nanidis, T.; Borysiewicz, C.; Yamamoto, T.; Orchard, T.; Tekkis, P.P. The effect of smoking after surgery for Crohn’s disease: A meta-analysis of observational studies. Int. J. Color. Dis. 2008, 23, 1213–1221. [Google Scholar] [CrossRef]
- Colombo, F.; Frontali, A.; Baldi, C.; Cigognini, M.; Lamperti, G.; Manzo, C.A.; Maconi, G.; Ardizzone, S.; Foschi, D.; Sampietro, G.M. Repeated surgery for recurrent Crohn’s disease: Does the outcome keep worsening operation after operation? A comparative study of 1224 consecutive procedures. Updates Surg. 2021, 74, 73–80. [Google Scholar] [CrossRef]
- Simillis, C.; Yamamoto, T.; Reese, G.E.; Umegae, S.; Matsumoto, K.; Darzi, A.W.; Tekkis, P.P. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am. J. Gastroenterol. 2008, 103, 196–205. [Google Scholar] [CrossRef]
- Raab, Y.; Bergström, R.; Ejerblad, S.; Graf, W.; Påhlman, L. Factors influencing recurrence in Crohn’s disease. An analysis of a consecutive series of 353 patients treated with primary surgery. Dis. Colon Rectum 1996, 39, 918–925. [Google Scholar] [CrossRef]
- Krause, U.; Ejerblad, S.; Bergman, L. A long-term study of the clinical course in 186 patients. Scand. J. Gastroenterol. 1985, 20, 516–524. [Google Scholar] [CrossRef]
- Gionchetti, P.; Dignass, A.; Danese, S.; Dias, F.J.M.; Rogler, G.; Lakatos, P.L.; Adamina, M.; Ardizzone, S.; Buskens, C.J.; Sebastian, S.; et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 2: Surgical management and special situations. J. Crohn’s Colitis 2017, 11, 135–149. [Google Scholar] [CrossRef]
- Fornaro, R.; Caratto, E.; Caratto, M.; Fornaro, F.; Caristo, G.; Frascio, M.; Sticchi, C. Post-operative recurrence in Crohn’s disease: Critical analysis of potential risk factors. An update. Surgeon 2015, 13, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Fazio, V.W.; Marchetti, F.; Church, J.M.; Goldblum, J.R.; Lavery, L.C.; Hull, T.L.; Milsom, J.W.; Strong, S.A.; Oakley, J.R.; Secic, M. Effect of resection margins on the recurrence of Crohn’s disease in the small bowel: A randomized controlled trial. Ann. Surg. 1996, 224, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Bernell, O.; Lapidus, A.; Hellers, G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann. Surg. 2000, 231, 38–45. [Google Scholar] [CrossRef]
- Regueiro, M.; Velayos, F.; Greer, J.B.; Bougatsos, C.; Chou, R.; Sultan, S.; Singh, S. American Gastroenterological Association Institute Technical Review on the Management of Crohn’s Disease after Surgical Resection. Gastroenterology 2017, 152, 277–295.e3. [Google Scholar] [CrossRef] [PubMed]
- Sachar, D.B.; Wolfson, D.M.; Greenstein, A.J.; Goldberg, J.; Styczynski, R.; Janowitz, H.D. Risk factors for postoperative recurrence of Crohn’s disease. Gastroenterology 1983, 85, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Bressenot, A.; Peyrin-Biroulet, L. Histologic features predicting postoperative Crohn’s disease recurrence. Inflamm. Bowel Dis. 2015, 21, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Valibouze, C.; Desreumaux, P.; Zerbib, P. Post-surgical recurrence of Crohn’s disease: Situational analysis and future prospects. J. Visc. Surg. 2021, 158, 401–410. [Google Scholar] [CrossRef]
- Tandon, P.; Malhi, G.; Abdali, D.; Pogue, E.; Marshall, J.K.; de Buck van Overstraeten, A.; Riddell, R.; Narula, N. Active Margins, Plexitis, and Granulomas Increase Postoperative Crohn’s Recurrence: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 451–462. [Google Scholar] [CrossRef]
- Simillis, C.; Jacovides, M.; Reese, G.E.; Yamamoto, T.; Tekkis, P.P. Meta-analysis of the role of granulomas in the recurrence of crohn disease. Dis. Colon Rectum 2010, 53, 177–185. [Google Scholar] [CrossRef]
- Hammoudi, N.; Cazals-Hatem, D.; Auzolle, C.; Gardair, C.; Ngollo, M.; Bottois, H.; Nancey, S.; Pariente, B.; Buisson, A.; Treton, X.; et al. Association between Microscopic Lesions at Ileal Resection Margin and Recurrence after Surgery in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 141–149.e2. [Google Scholar] [CrossRef]
- Barreiro-de Acosta, M.; Marín-Jimenez, I.; Rodríguez-Lago, I.; Guarner, F.; Espín, E.; Ferrer Bradley, I.; Gutiérrez, A.; Beltrán, B.; Chaparro, M.; Gisbert, J.P.; et al. Recommendations of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) on pouchitis in ulcerative colitis. Part 2: Treatment. Gastroenterol. Hepatol. 2020, 43, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Loftus, E.V.; Hirano, I.; Falck-Ytter, Y.; Singh, S.; Sultan, S.; Flamm, S.L.; Lim, J.K.; Rubenstein, J.H.; Smalley, W.E.; et al. American Gastroenterological Association Institute Guideline on the Management of Crohn’s Disease after Surgical Resection. Gastroenterology 2017, 152, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.R.; Ow, Z.G.W.; Chin, Y.H.; Lim, W.H.; Kong, G.; Tham, H.Y.; Wong, N.W.; Chong, C.S.; Foo, F.J.; Chan, W.P.W. Quantifying the rate of recurrence of postoperative Crohn’s disease with biological therapy. A meta-analysis. J. Dig. Dis. 2021, 22, 399–407. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Vande Casteele, N.; Boland, B.S.; Rivera-Nieves, J.; Ernst, P.B.; Eckmann, L.; Barrett, K.E.; Chang, J.T.; Sandborn, W.J. Should We Divide Crohn’s Disease into Ileum-Dominant and Isolated Colonic Diseases? Clin. Gastroenterol. Hepatol. 2019, 17, 2634–2643. [Google Scholar] [CrossRef]
- Atreya, R.; Siegmund, B. Location is important: Differentiation between ileal and colonic Crohn’s disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Renna, S.; Cammà, C.; Modesto, I.; Cabibbo, G.; Scimeca, D.; Civitavecchia, G.; Mocciaro, F.; Orlando, A.; Enea, M.; Cottone, M. Meta-Analysis of the Placebo Rates of Clinical Relapse and Severe Endoscopic Recurrence in Postoperative Crohn’s Disease. Gastroenterology 2008, 135, 1500–1509. [Google Scholar] [CrossRef]
- Candia, R.; Bravo-Soto, G.; Monrroy, H.; Hernandez, C.; Nguyen, G.C. Colonoscopy-guided therapy for the prevention of post-operative recurrence of Crohn’s disease. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Regueiro, M.; Feagan, B.G.; Zou, B.; Johanns, J.; Blank, M.A.; Chevrier, M.; Plevy, S.; Popp, J.; Cornillie, F.J.; Lukas, M.; et al. Infliximab Reduces Endoscopic, but Not Clinical, Recurrence of Crohn’s Disease after Ileocolonic Resection. Gastroenterology 2016, 150, 1568–1578. [Google Scholar] [CrossRef]
- Kotze, P.G.; Yamamoto, T.; Danese, S.; Suzuki, Y.; Teixeira, F.V.; De Albuquerque, I.C.; Saad-Hossne, R.; de Barcelos, I.F.; da Silva, R.N.; Kotze, L.M.d.S.; et al. Direct retrospective comparison of adalimumab and infliximab in preventing early postoperative endoscopic recurrence after ileocaecal resection for Crohn’s disease: Results from the MULTIPER database. J. Crohn’s Colitis 2015, 9, 541–547. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Surgery for Crohn’s disease: Look harder, act faster. Lancet 2015, 385, 1370–1371. [Google Scholar] [CrossRef]
- Haens, G.R.D.; Geboes, K.; Peeters, M.; Baert, F.; Penninckx, F.; Rutgeerts, P. Early Lesions of Recurrent Crohn’s Disease Caused by Infusion. Gastroenterology 1998, 114, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Goboes, K.; Peeters, M.; Hiele, M.; Penninckx, F.; Aerts, R.; Kerremans, R.; Goboes, K. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum Histological techniques. Lancet 1991, 338, 771–774. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).