Assessing the Relationship between Gastrointestinal and Pancreatic Neuroendocrine Tumor Grade and Overall Survival: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
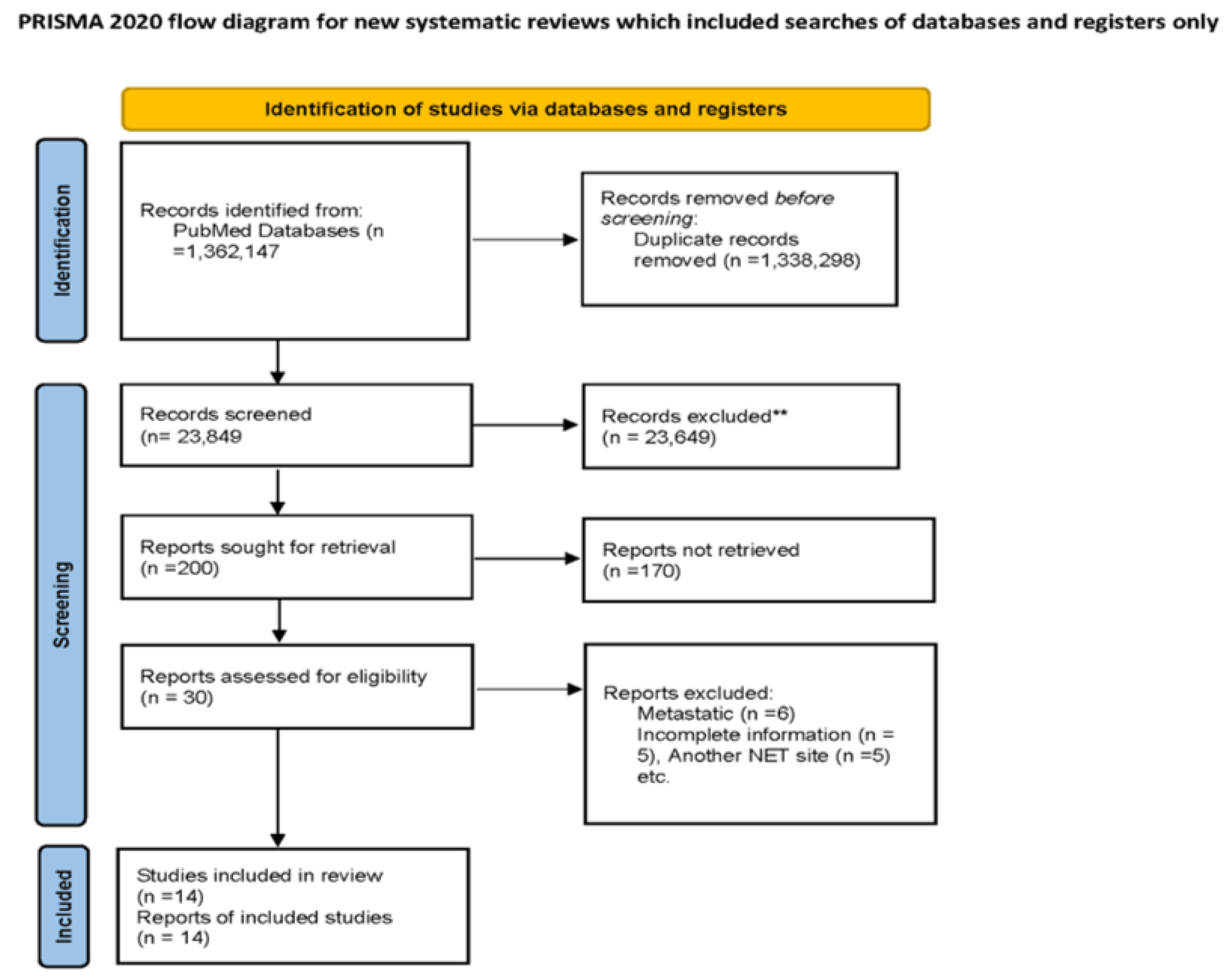
2.1. Aim and Literature Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
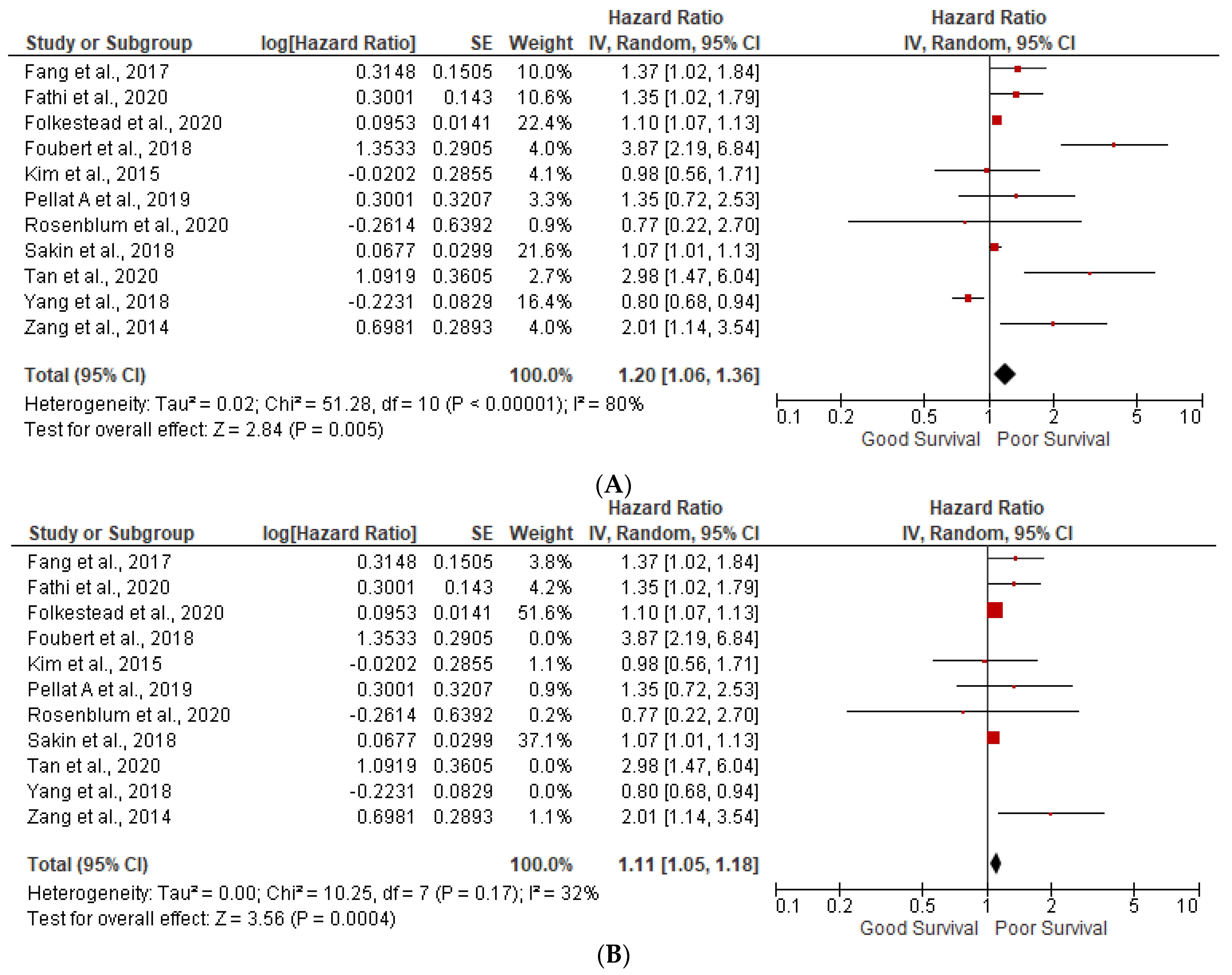
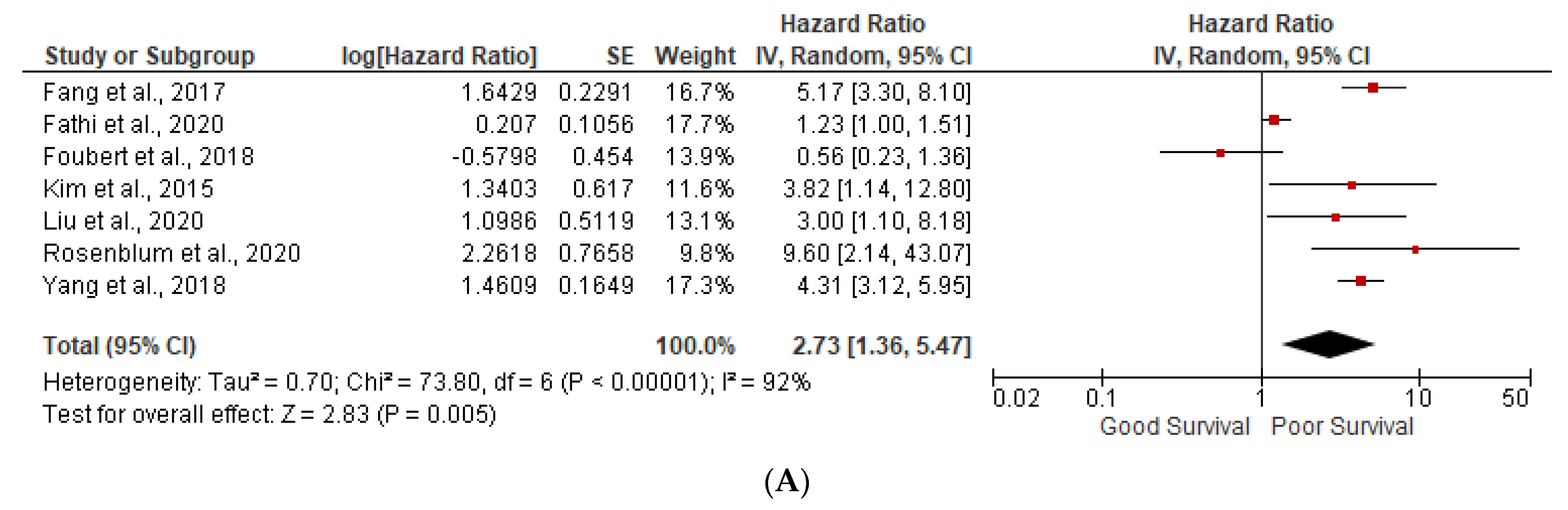
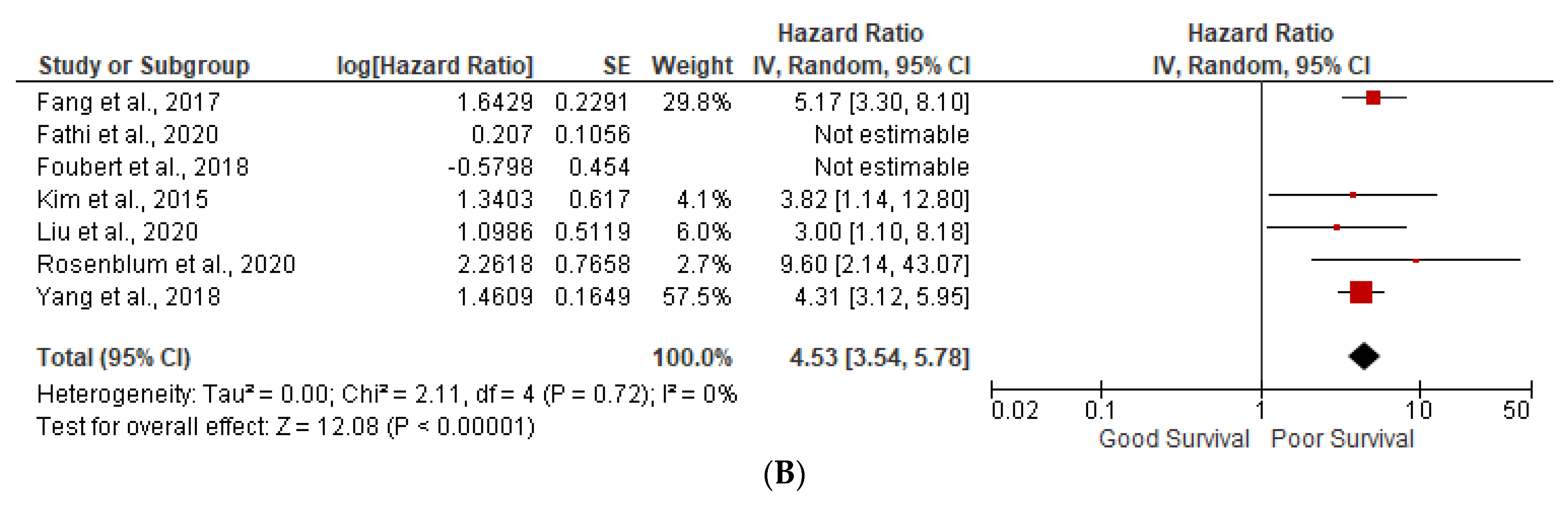
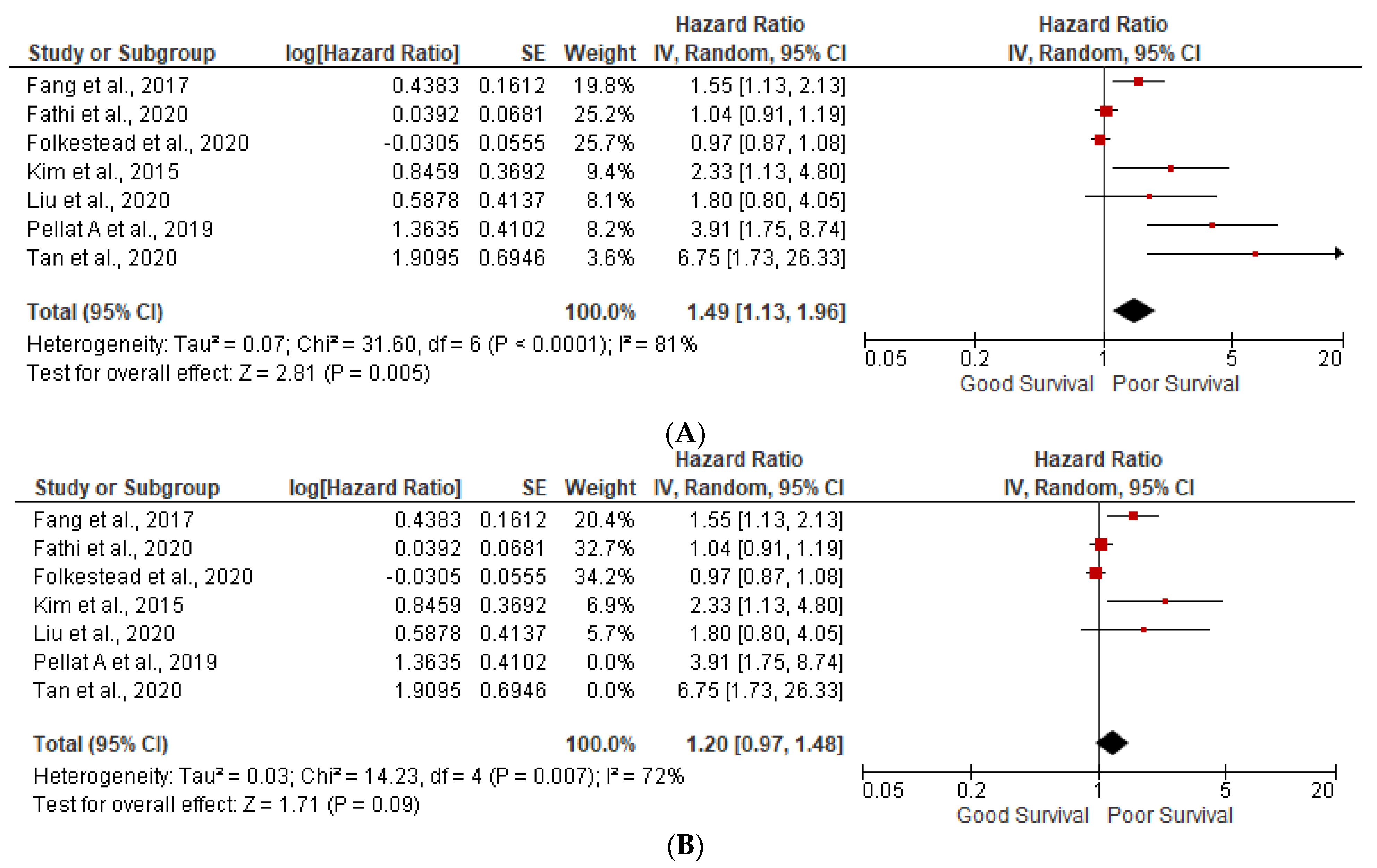
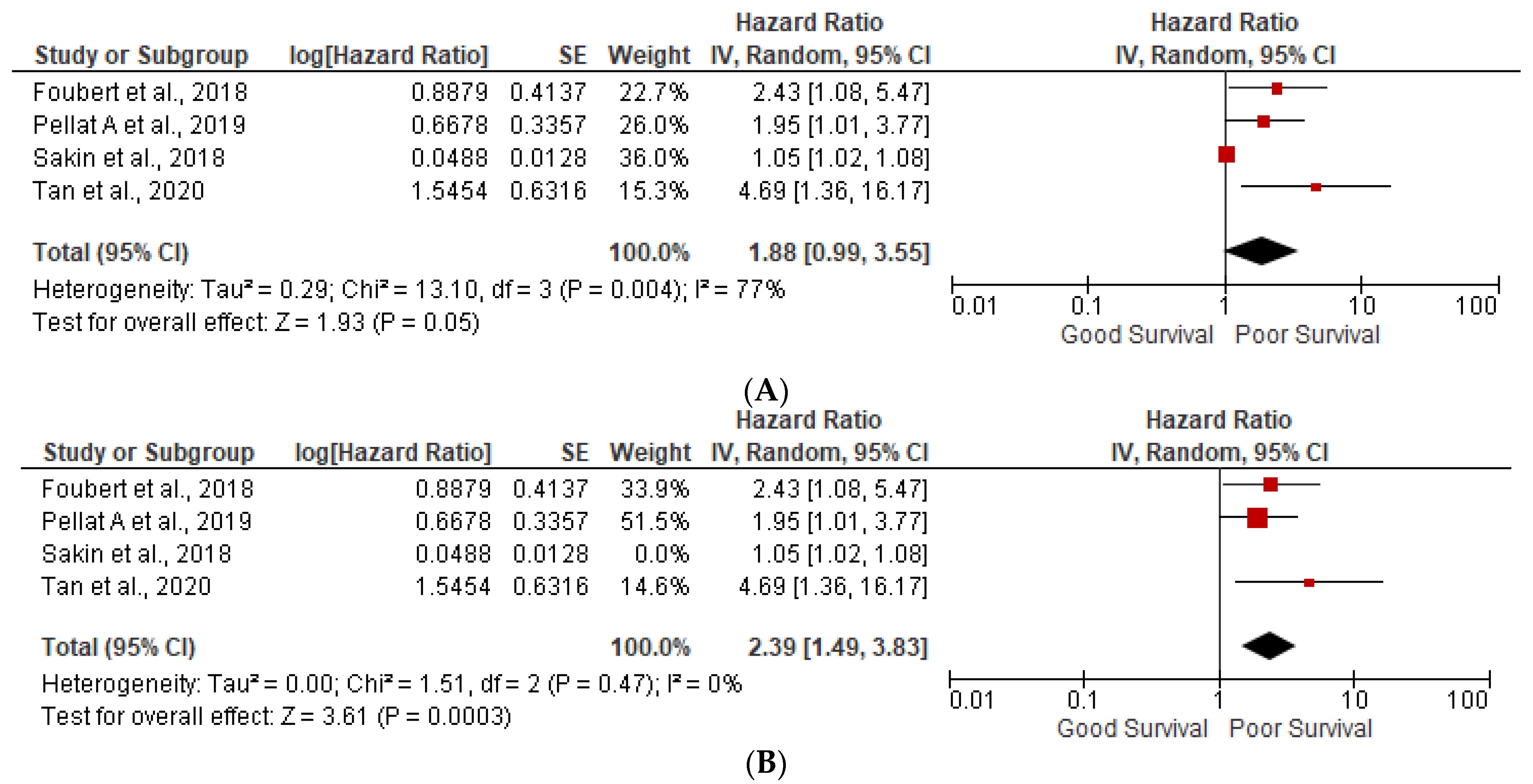
3.1. Prognostic Factors
3.2. Lymph Node Positivity
3.3. Ki-67 More than 5%
3.4. Table 2 Describes the Overall Findings of This Study
3.5. Heterogeneity (I2) Statistics and NOS Scale
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Wang, W.; Lu, J.; Pan, G.; Pan, Z.; Chen, Q.; Liu, W.; Zhao, Y. Gastric Neuroendocrine Tumors (G-Nets): Incidence, Prognosis and Recent Trend Toward Improved Survival. Cell Physiol. Biochem. 2018, 45, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Cancer.net: Neuroendocrine Tumors/Introduction [Internet]. Available online: https://www.cancer.net/cancer-types/neuroendocrine-tumors/introduction (accessed on 1 August 2023).
- Neuroendocrine Tumors: Statistics [Internet]. It Is Estimated That, Has Been Increasing for Years. Available online: https://www.cancer.net/cancer-types/neuroendocrine-tumors/statistics#:~:text=Overall%2C (accessed on 1 August 2023).
- Robertson, R.G.; Geiger, W.J.; Davis, N.B. Carcinoid tumors. Am. Fam. Physician 2006, 74, 429–434. [Google Scholar] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Kaderli, R.M.; Spanjol, M.; Kollár, A.; Bütikofer, L.; Gloy, V.; Dumont, R.A.; Seiler, C.A.; Christ, E.R.; Radojewski, P.; Briel, M.; et al. Therapeutic Options for Neuroendocrine Tumors: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2019, 5, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Horton, K.M.; Kamel, I.; Hofmann, L.; Fishman, E.K. Carcinoid Tumors of the Small Bowel: A Multitechnique Imaging Approach. Am. J. Roentgenol. 2004, 182, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.S.; Tsai, C.L.; Chu, Y.Y.; Chen, K.C.; Lin, J.C.; Chen, B.C.; Sun, W.C.; Yen, H.H.; Chen, C.Y.; Wu, I.C.; et al. Clinical features and outcomes of gastric neuroendocrine tumors after endoscopic diagnosis and treatment a digestive endoscopy society of tawian (dest). Medicine 2018, 97, e12101. [Google Scholar] [CrossRef] [PubMed]
- Folkestad, O.; Wasmuth, H.H.; Mjønes, P.; Fougner, R.; Hauso, Ø.; Fossmark, R. Survival and disease recurrence in patients operated for small intestinal neuroendocrine tumors at a referral hospital. Surg. Oncol. 2020, 35, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Park, Y.S.; Yook, J.H.; Oh, S.T.; Kim, B.S. Differing Clinical Courses and Prognoses in Patients with Gastric Neuroendocrine Tumors Based on the 2010-WHO Classification Scheme. Medicine 2015, 94, e1748. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, R.E.; Harris, C.K.; Baeg, K.J.; Starr, J.A.; Brais, L.K.; Stashek, K.M.; Ward, S.C.; Katona, B.W.; Clancy, T.E.; Wisnivesky, J.P.; et al. Predictors of Recurrence and Survival in Patients with Surgically Resected Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Prisma Guidelines [Internet]. Available online: http://www.prisma-statement.org/Extensions/NetworkMetaAnalysis (accessed on 1 August 2023).
- IJNS. MOOSE (Meta-Analyses of Observational Studies in Epidemiology) Checklist. 2019. Available online: https://www.elsevier.com/__data/promis_misc/ISSM_MOOSE_Checklist.pdf (accessed on 1 August 2023).
- Aging Changes in Immunity [Internet]. Available online: https://medlineplus.gov/ency/article/004008.htm#:~:text=As you grow older%2C (accessed on 1 August 2023).
- Niederle, M.B.; Hackl, M.; Kaserer, K.; Niederle, B. Gastroenteropancreatic neuroendocrine tumours: The current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: An analysis based on prospectively collected parameters. Endocr. Relat. Cancer 2010, 17, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.M.; Tang, L.; Qiao, X.W.; Wolin, E.; Nissen, N.N.; Dhall, D.; Chen, J.; Shen, L.; Chi, Y.; Yuan, Y.Z.; et al. Differences and similarities in the clinicopathological features of pancreatic neuroendocrine tumors in China and the United States: A multicenter study. Medicine 2016, 95, e2836. [Google Scholar] [CrossRef] [PubMed]
- Coriat, R.; Walter, T.; Terris, B.; Couvelard, A.; Ruszniewski, P. Gastroenteropancreatic Well-Differentiated Grade 3 Neuroendocrine Tumors: Review and Position Statement. Oncologist 2016, 21, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P.C.; Neifeld, J.P.; Lawrence, W., Jr.; Kay, S.A.U.L.; Horsley, J.S., 3rd; Parker, G.A. Gastrointestinal sarcomas: Analysis of prognostic factors. Ann. Surg. 1987, 206, 706–710. [Google Scholar] [CrossRef] [PubMed]
- NIH: Cancer Staging [Internet]. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/staging (accessed on 1 August 2023).
- Zaidi, M.Y.; Lopez-Aguiar, A.G.; Dillhoff, M.; Beal, E.; Poultsides, G.; Makris, E.; Rocha, F.; Crown, A.; Idrees, K.; Smith, P.M.; et al. Prognostic Role of Lymph Node Positivity and Number of Lymph Nodes Needed for Accurately Staging Small-Bowel Neuroendocrine Tumors. JAMA Surg. 2019, 154, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Blair, R. HHS Public Access. Physiol. Behav. 2017, 176, 139–148. [Google Scholar]
- Ahmed, M. Gastrointestinal neuroendocrine tumors in 2020. World J. Gastrointest. Oncol. 2020, 12, 791–807. [Google Scholar] [PubMed]
- Ellis, L.; Shale, M.J.; Coleman, M.P. Carcinoid tumors of the gastrointestinal tract: Trends in incidence in England since 1971. Am. J. Gastroenterol. 2010, 105, 2563–2569. [Google Scholar] [CrossRef] [PubMed]
| Study Name, Year | Country | Study Period | Sample Size | NET Site | Overall Survival | Prognostic Factors |
|---|---|---|---|---|---|---|
| Folkestead et al., 2020 | Norway | January 1998–May 2018 | 186 | Intestinal | 5-year survival: 75.8% 9.7 years (95%CI 7.7–11.6) | Age, gender, and Positive Lymph node |
| Tan et al., 2020 | China | January 2009 to December 2017 | 88 | Pancreatic | NA | Age, Ki-67, and positive lymph nodes |
| Liu et al., 2020 | China | February 2003–February 2014 | 155 | Gastroenteropancreatic (GEP) NENs | 1-year = 82%, 3-year = 72%, 5-year = 51% | Grade and positive lymph nodes |
| Kim et al., 2015 | Korea | 1996–2014 | 175 | Gastric | NA | Age, gender, grade, and positive lymph node |
| Ptasnuka et al., 2019 | Latvia | 2006–2018 | 205 | Gastropancreatic | 1-year: 88.0% (95%CI 83.3–92.7) 3 year: 77.1% (95%CI 70.4–83.8) | NA |
| Pellat A et al., 2019 | France | 2000–2016 | 73 | GI pancreatic | 5-year OS 50% (25–50%) | Age, gender, Ki-67, and positive lymph nodes |
| Yang et al., 2018 | China | 1973–2014 | 3740 | Gastric | NA | Age, gender, and grade |
| Fathi et al., 2020 | USA | 1988–2012 | 1787 | Pancreatic | 5-year OS: 24.4%. | Age, gender, grade, and positive lymph nodes |
| Cetinkaya et al., 2014 | Norway | 1982–2010 | 114 | Pancreatic | 5-year OS: 53.9% (95% CI: 43.4–63.3) | NA |
| Zang et al., 2014 | China | 2003–2012 | 168 | Gastroenteropancreatic (GEP-NENs) | 8.94 years (95% confidence interval (CI): 8.40–9.48) | Age and gender |
| Fang et al., 2017 | South China | 2005–2015 | 1183 | GEP- NENs | 28 months (range, 4–135 months) | Age, gender, grade, and positive lymph nodes |
| Foubert et al., 2018 | France | October 1994–October 2013 | 151 | Intestinal and Pancreatic NET | NA | Age, grade, and Ki-67 |
| Sakin et al., 2018 | Turkey | 2000–2016 | 85 | GEP NET | NA | Age and Ki-67 |
| Rosenblum et al., 2020 | USA | 1990–2017 | 501 | Pancreatic | NA | Age, gender, and grade |
| Prognostic Factors of Poor Outcomes |
|
| Sensitivity Analysis
| Sensitivity Analysis
|
|---|---|---|---|---|
| Age |
|
|
|
|
| Grade 3 |
|
|
|
|
| Lymph node positivity |
|
|
|
|
| Ki-67 more than 5% |
|
|
|
|
| Studies | Selection | Comparability | Outcome | Overall Quality/Overall Risk of Bias |
|---|---|---|---|---|
| Folkestead et al., 2020 | ++ | + | ++ | High |
| Tan et al., 2020 | ++ | + | ++ | Moderate |
| Liu et al., 2020 | +++ | ++ | +++ | Low |
| Kim et al., 2015 | +++ | ++ | +++ | Low |
| Ptasnuka et al., 2019 | +++ | ++ | ++ | Low |
| Pellat A et al., 2019 | ++ | ++ | ++ | Moderate |
| Yang et al., 2018 | +++ | ++ | +++ | Low |
| Fathi et al., 2020 | ++ | ++ | ++ | High |
| Cetinkaya et al., 2014 | ++ | + | +++ | Moderate |
| Zang et al., 2014 | +++ | ++ | +++ | Low |
| Fang et al., 2017 | +++ | ++ | +++ | Low |
| Foubert, 2018 | ++ | + | ++ | High |
| Sakin, 2018 | +++ | ++ | + | Moderate |
| Rosenblum et al., 2020 | +++ | ++ | +++ | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, P.; Patel, N.; Khayyat, A.; Asad, M.; Dawoodi, S.; Chandramohan, S.; Unachukwu, N.; Nasyrlaeva, B.; Vaisyambath, L.; Chowdary, S.B.; et al. Assessing the Relationship between Gastrointestinal and Pancreatic Neuroendocrine Tumor Grade and Overall Survival: A Systematic Review and Meta-Analysis. Gastrointest. Disord. 2023, 5, 408-418. https://doi.org/10.3390/gidisord5030033
Malik P, Patel N, Khayyat A, Asad M, Dawoodi S, Chandramohan S, Unachukwu N, Nasyrlaeva B, Vaisyambath L, Chowdary SB, et al. Assessing the Relationship between Gastrointestinal and Pancreatic Neuroendocrine Tumor Grade and Overall Survival: A Systematic Review and Meta-Analysis. Gastrointestinal Disorders. 2023; 5(3):408-418. https://doi.org/10.3390/gidisord5030033
Chicago/Turabian StyleMalik, Preeti, Neel Patel, Azadeh Khayyat, Muhammad Asad, Sameer Dawoodi, Sangeetha Chandramohan, Nkechi Unachukwu, Bibimariyam Nasyrlaeva, Laseena Vaisyambath, Sriram B. Chowdary, and et al. 2023. "Assessing the Relationship between Gastrointestinal and Pancreatic Neuroendocrine Tumor Grade and Overall Survival: A Systematic Review and Meta-Analysis" Gastrointestinal Disorders 5, no. 3: 408-418. https://doi.org/10.3390/gidisord5030033
APA StyleMalik, P., Patel, N., Khayyat, A., Asad, M., Dawoodi, S., Chandramohan, S., Unachukwu, N., Nasyrlaeva, B., Vaisyambath, L., Chowdary, S. B., Venkata, V. S., & Patel, U. (2023). Assessing the Relationship between Gastrointestinal and Pancreatic Neuroendocrine Tumor Grade and Overall Survival: A Systematic Review and Meta-Analysis. Gastrointestinal Disorders, 5(3), 408-418. https://doi.org/10.3390/gidisord5030033







