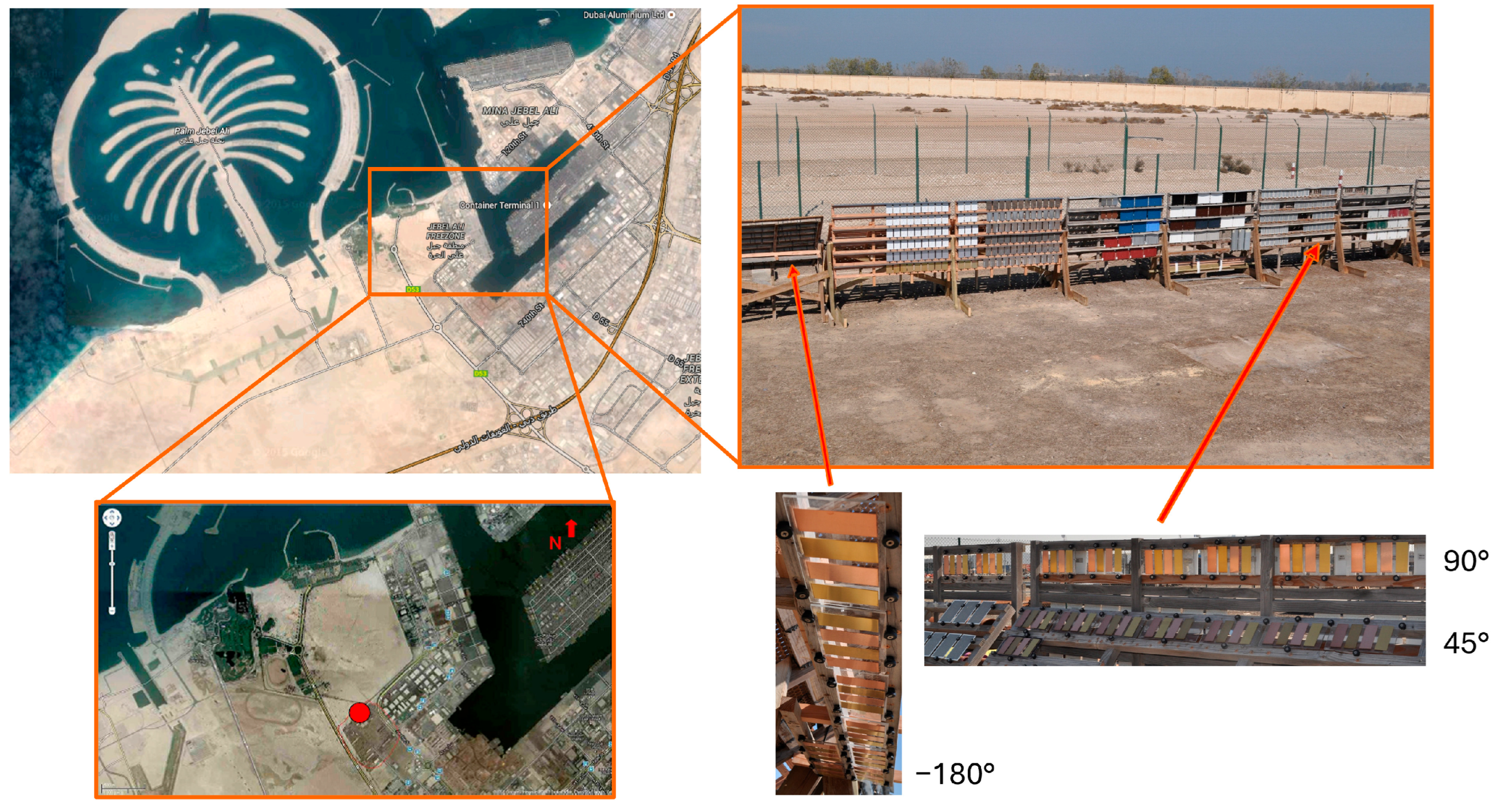
3.1. Copper Alloy Patinas Under Marine–Desert (Dubai) and Marine (Brest) Exposure: Visual Appearance and Characteristics
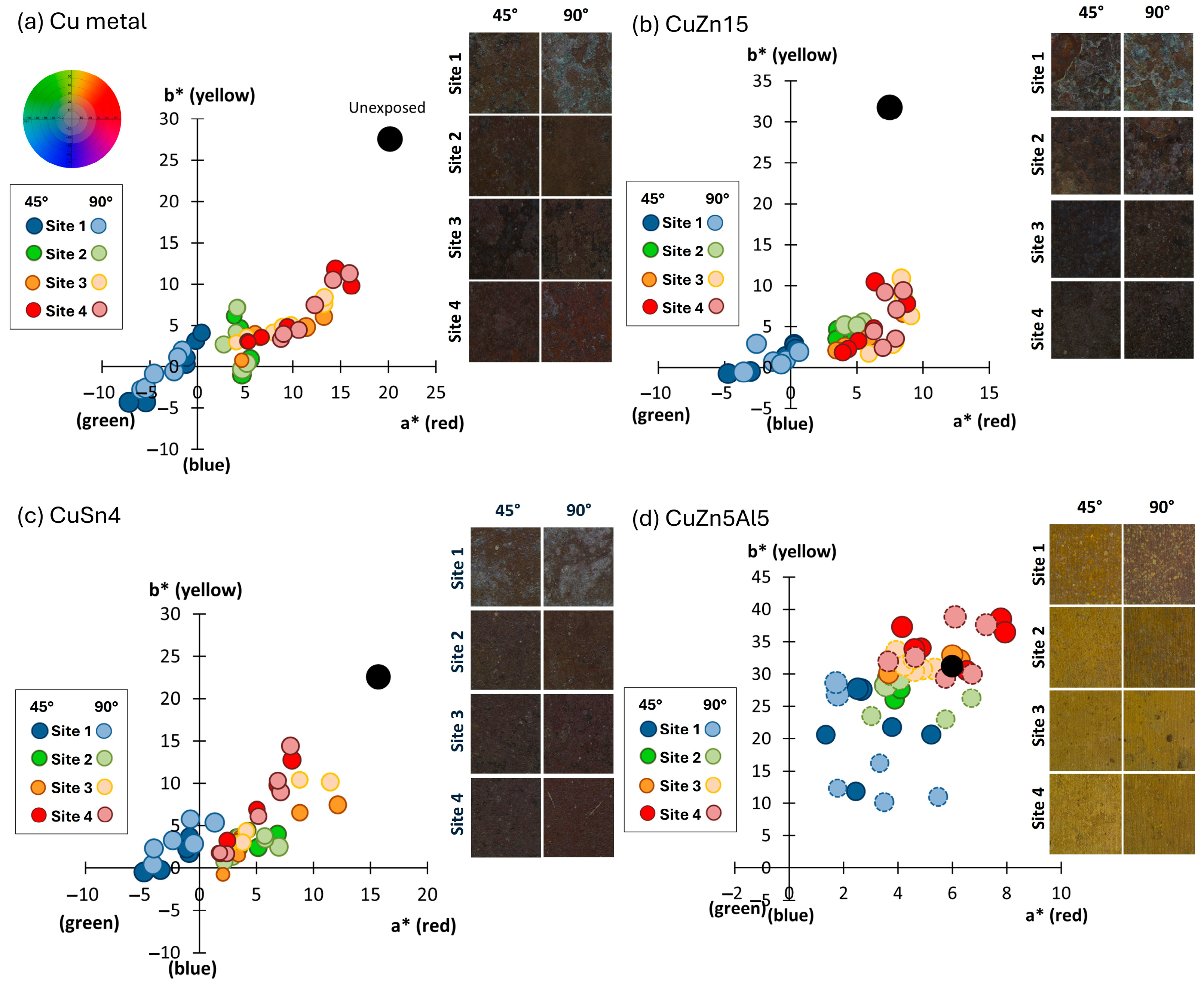
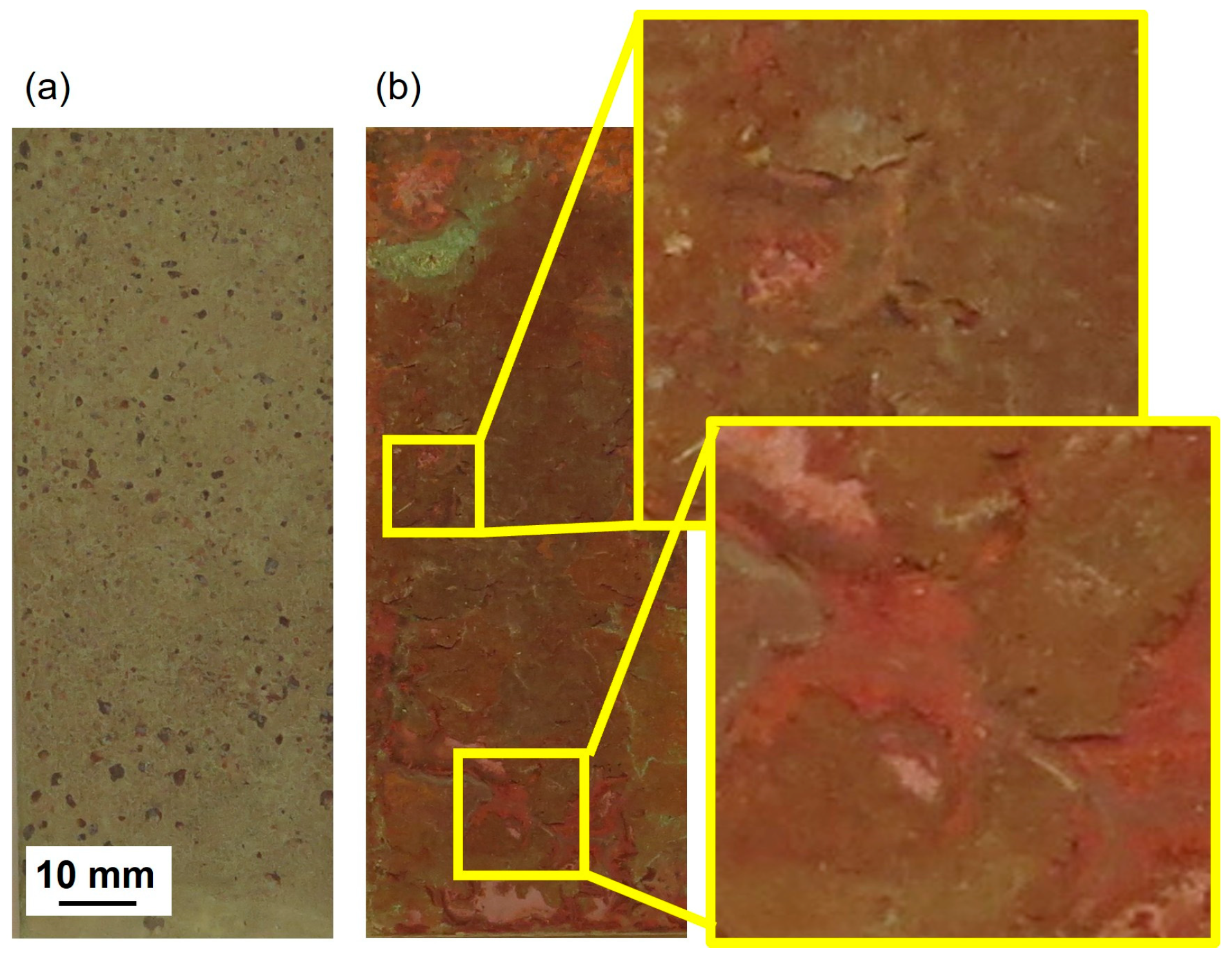
The visual appearance and corresponding colorimetric characteristics of Cu metal and the three Cu-based alloys (CuZn15, CuSn4, and CuZn5Al5) exposed for 1, 2, 3, and 4 years in Dubai are presented in
Figure 2 for surfaces inclined 45, 90, and −180° from the horizontal, facing south.
All materials and inclinations revealed a colorimetric appearance that ended up in the yellow-reddish quadrant (a*,b*) with considerably reduced lightness (L*). The reduced lightness was predominantly governed by the presence of sand firmly attached onto all exposed surfaces. The golden alloy, CuZn5Al5 (
Figure 2d), showed a similar appearance as Cu metal (
Figure 2a), CuZn15 (
Figure 2b), and CuSn4 (
Figure 2c), even though being slightly more yellowish for the surfaces exposed 45° from the horizontal. The yellowish appearance could be connected to the extensive presence of sand. All coupons exposed up-side down (−180°) revealed, independent on material, the largest change in lightness, compared to coupons exposed 45° and 90°. The lack of, or minor contribution of the blue and greenish components, illustrates the low levels of chloride deposition (annual average of 30–90 mg/m
2, day) and a relatively long distance of the test site from the seashore (2 km). The total color difference (ΔE) between the unexposed and the exposed surfaces was clearly visible,
Figure 2e with ΔE values >> 3 defined as visual differences. Largest changes in appearance were observed for surfaces inclined 45° for CuZn15, closely followed by Cu metal ≈ CuSn4 >> CuZn5Al5. All surfaces inclined 90° and −180° showed small differences between the inclinations but larger changes in appearance compared to corresponding surfaces inclined 45°. Unexpectedly, the CuZn5Al5 inclined 90° and −180° showed a similar change in visual appearance as the Cu metal, CuZn15, and CuSn4 inclined 45°. This may be related to the presence of embedded sand and dust particles within the patina, as described below.
Aesthetic changes (and stereomicrograph images after 4 years), similar to those observed for Cu metal and the alloys in Dubai, have previously been reported by the authors for the same alloys when freely exposed at inclinations of 45° and 90° at sites located approximately 1.5 km (site 3) and 40 km (site 4) from the marine seashore of Brest, France [
14]. The test sites located closer to the seashore (<5 m: site 1, and 20–30 m: site 2) of 10–100 times higher deposition rates of chlorides resulted in patinas of a more blue-greenish appearances for Cu metal, CuZn15, and CuSn4, whereas the color appearance of the CuZn5Al5 alloy remained in the red-yellow quadrant,
Figure 3 [
14,
18]. The results illustrate the importance of the extent of chloride deposition and repeated dry and wet periods resulting in a patina of chloride-rich corrosion products on Cu metal, CuZn15, and CuSn4, giving them a blue-greenish appearance [
1].
3.2. Field Exposure in the Marine–Desert Atmosphere of Dubai Compared to Marine Sites of Varying Chloride Deposition in Brest–Patina Formation and Composition
According to previous multianalytical characterizations of the patina formed at site 1, with the highest chloride deposition rate of the investigated marine sites [
14,
18], Cu metal developed a two-layered type patina with cuprite (Cu
2O) as the inner layer and atacamite (Cu
2(OH)
3Cl) as the outer layer, sometimes containing nantokite (CuCl). Alloy-specific products such as SnO
2 (Cu4Sn), Zn hydroxycarbonates (Cu15Zn), and Zn/Al oxides/hydroxides (CuZn5Al5) were also detected, with Zn- and Zn/Al-rich phases providing protective barrier effects. As a result, Cu15Zn and CuZn5Al5 showed greater resistance to chloride-induced corrosion than Cu metal and Cu4Sn. Over time, the outer layer compacted and adhered more strongly, reducing the corrosion rates. Spallation or flaking of corrosion products, mainly linked to CuCl-Cu
2(OH)
3Cl formation, was most severe for Cu metal and Cu4Sn, minor for Cu15Zn, and absent for CuZn5Al5. The patina thickness strongly influenced the visual appearance, with CuZn5Al5 retaining a lustrous golden finish after four years, while the other materials developed brown–red-bluish tones. This is attributed to the presence of Cu
2O, ZnO, Al
2O
3, and SnO
2 in the inner layer of the patina of the CuZn5Al5, along with the incorporation of SnO
2, Zn
5(CO
3)
2(OH)
6, Zn
6Al
2(OH)
16CO
3·4H
2O, and Zn
5(OH)
8Cl
2·H
2O in the outer patina layer, predominantly composed of Cu
2(OH)
3Cl, able to mitigate chloride-induced corrosion compared to pure Cu metal [
15]. Similar patina compositions were observed at sites 2, 3, and 4, with the difference that the extent of chloride-containing corrosion products was reduced, though still present, with increasing distances from the seashore (i.e., reduced amounts of chloride deposition).
Analysis of corrosion products of the patinas formed on Cu metal, CuZn15, CuSn4, and CuZn5Al5 exposed at 45° from the horizontal in Dubai by means of SEM/EDS and XRD after 9 months and 1 year of exposure.
Figure 4 revealed the presence of almost the same corrosion products as observed in the patinas formed at the marine site in Brest [
15], as summarized above, though with considerably lower extent of chloride-rich corrosion products such as Cu
2(OH)
3Cl and CuCl.
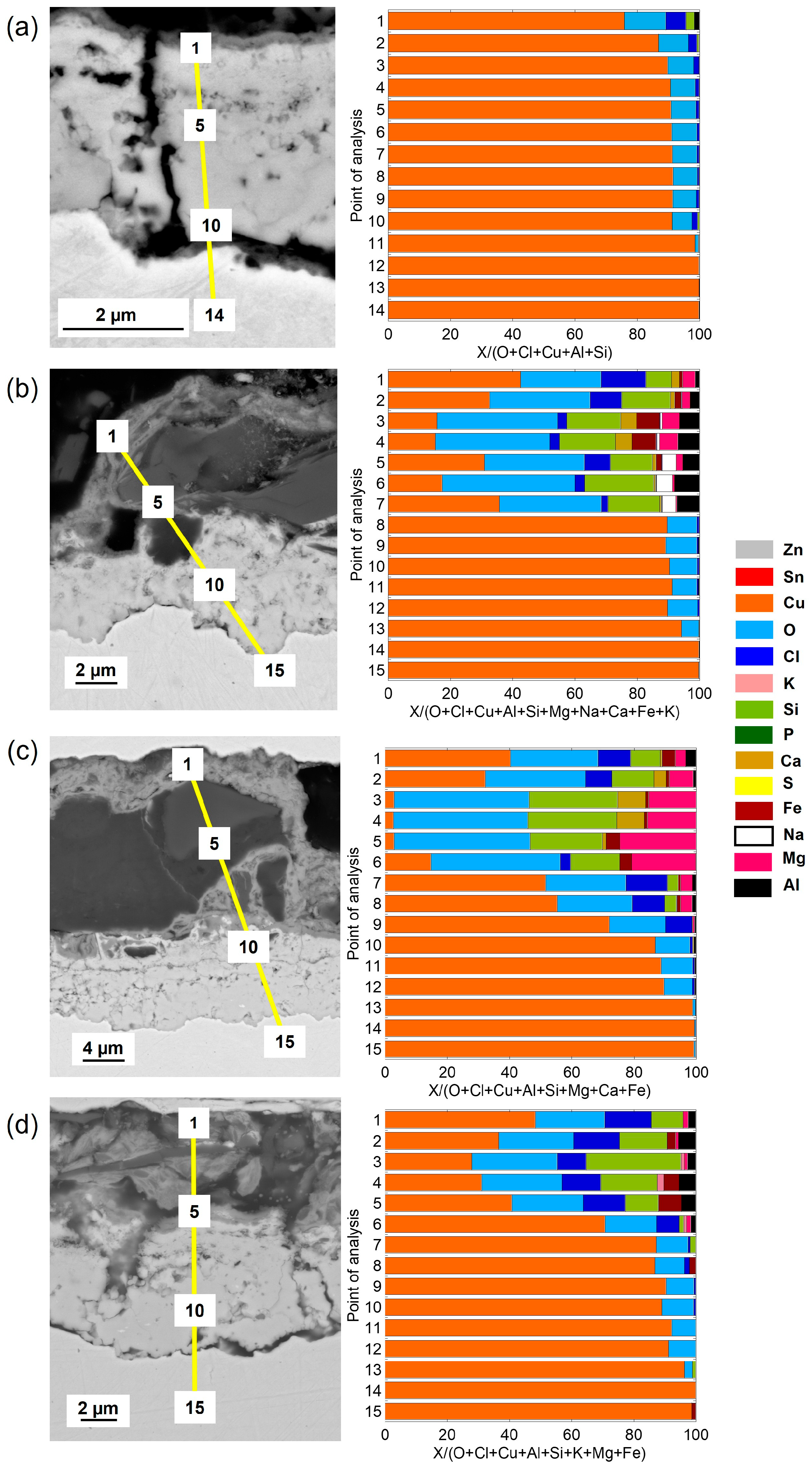
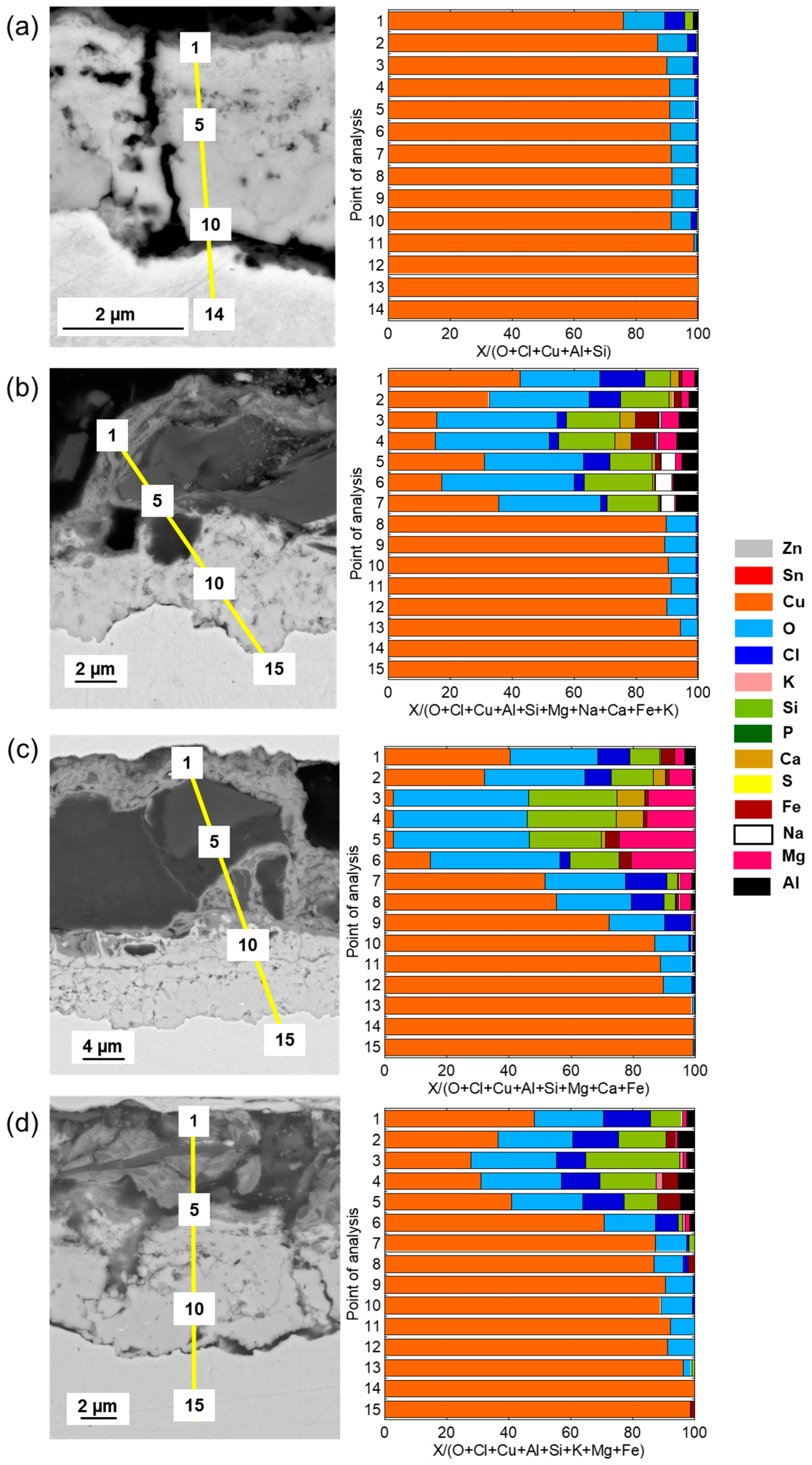
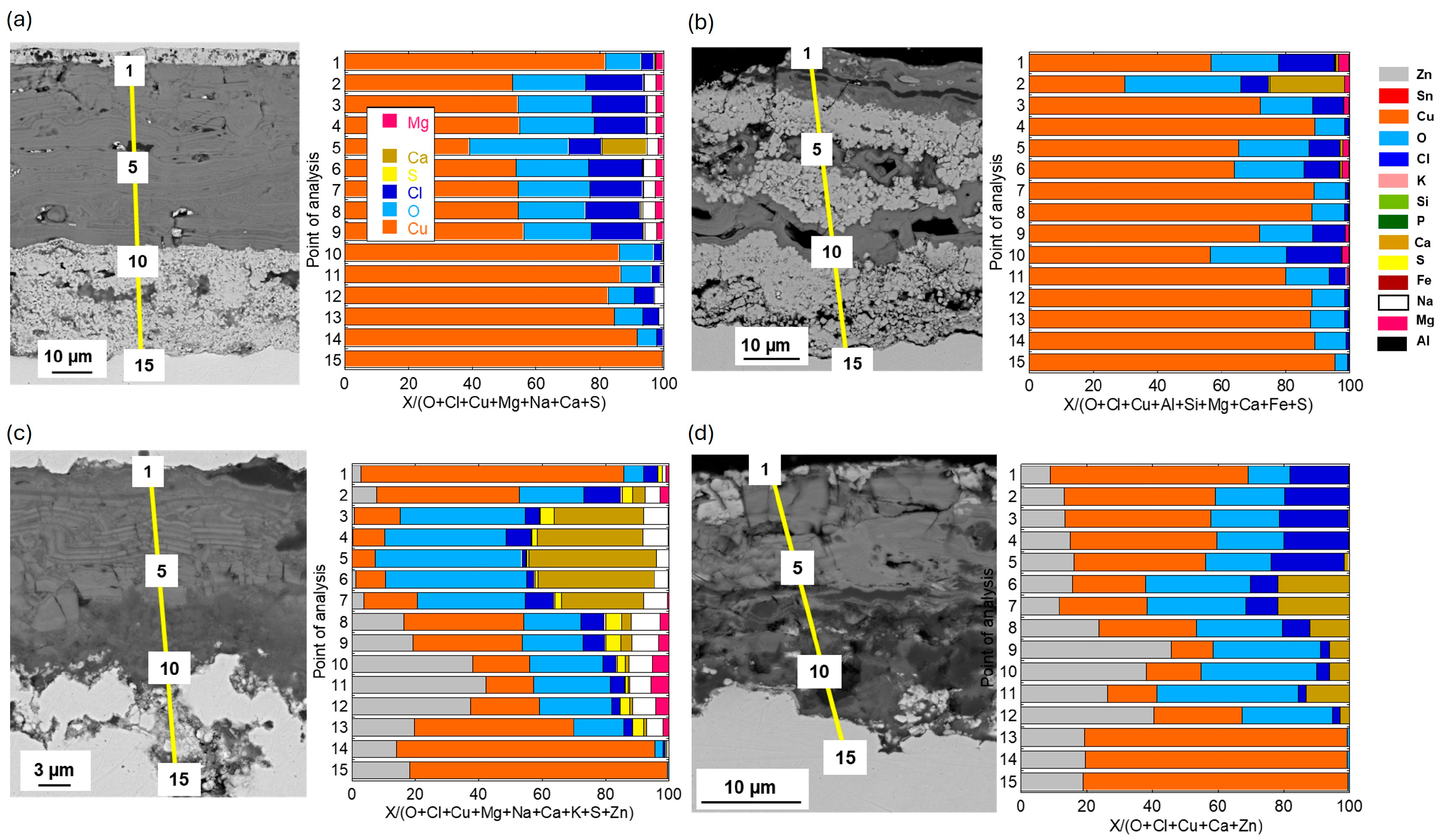
Cross-sectional SEM images and compositional analysis of the patina on Cu metal after 1 year of exposure,
Figure 5a, show a relatively thin (≈2–3 µm) two-layer patina with an inner layer of Cu–oxides with small amounts of Cl covered by a thin (<0.5 µm) outer layer containing chlorine (Cl) in addition to aluminum (Al) and silicon (Si), the latter most probably from deposited sand residues. With increasing exposure time, from 2 up to 4 years, the outer patina layer had evolved into varying thicknesses (12–30 µm) with elevated levels of Cl and sand/dust/construction-derived particles (Al, Mg, Fe, Ca, K, Si), embedded in the layer, contributing to its complex chemistry, heterogeneity, and varying thickness (
Figure 5b–d).
The patina of the CuZn15 alloy was slightly thicker (≈3–4 µm) than observed for Cu metal after 1 year of exposure with an inner part of the patina composed of varying proportions of Zn- and Cu-rich constituents in striated layers and small amounts of oxygen (O) and Cl,
Figure 6a. After 2–4 years, the patina thickness largely varied over the surface from typically 15 to 30 µm. The striped areas of Cu- and Zn-rich patina constituents formed an inner layer porous layer being covered by an outer highly heterogeneous layer also containing Zn- and Cu-rich corrosion products with embedded sand/dust particles rich in Ca, S, Na, Mg, Al, and Fe,
Figure 6b–d.
Similar to previous findings at the marine sites in Brest, the bronze alloy, CuSn4, revealed after 1 year of exposure a relatively thick, ≈10–12 µm, and porous striated patina containing both Cu- and Sn-rich oxidized areas with small amounts of Cl integrated within the layers, as seen in
Figure 7.
No Sn and slightly more Cl, combined with sand and construction particles, were present in the outermost top layer. Longer time periods, 2–4 years, as seen in
Figure 7b–d, resulted in thicker but similarly striated oxidized Cu and Sn layers (up to ≈15–20 µm in thickness), with Cl integrated within the layer and a few µm thick top layers with embedded sand and dust particles. The presence of Cl within the layer is believed to be connected to the initial formation of CuCl, the corrosion product that readily converts to Cu
2(OH)
3Cl in the presence of moisture and oxygen, causing a volume expansion within the patina, which causes physical stress in the porous layer, which in turn leads to flaking/spallation of corrosion products [
1,
14,
15]. From the XRD patterns,
Figure 4, the presence of both CuCl and Cu
2(OH)
3Cl could be confirmed in the CuSn4 patina. Flaking/spallation has previously been reported to take place for both Cu metal and Cu4Sn exposed at near seashore marine conditions [
14,
15]. However, in the desert climate of Dubai, no visual flaking was observed on the CuSn4 samples exposed at 45°, which could be a result of the low deposition rates of chlorides. However, as discussed below, flaking was clearly taking place for the CuSn4 samples inclined upside-down (−180°). No evidence of either CuCl or flaking was observed for the Cu metal exposed in Dubai.
In agreement with previous observations for the CuZn5Al5 alloy at both urban and marine conditions, the patina formation was slower compared with Cu metal, CuZn15, and CuSn4 [
8,
18]. Cross-sectional investigations showed a two-layer patina with an inner layer with a thickness of ≈1.5 µm and an outer layer increasing in thickness with time to a total patina thickness of ≈5 µm after 4 years, respectively, as seen in
Figure 8. The patina morphology with striated layers of Cu- and Zn-rich corrosion products observed for CuZn15 was also observed for the CuZn5Al5 alloy after 1 year of exposure including small amounts of Sn and Cl, as seen in
Figure 8a. Sand and construction particles as well as Cl were mainly observed in the top layer. Different to CuZn15, the patina growth (corrosion rate) was considerably lower judged from the relatively thin patina after 4 years (≈5 µm compared to 15–30 µm), as seen in
Figure 8c,d. This is connected to the presence of Al
2O
3, ZnO, and SnO
2 at the bulk/patina interface as described elsewhere [
18,
19] and the formation and intercalation of different Zn-, Al-, and Sn-rich corrosion products in addition to Cu-constituents acting as efficient barriers, thereby suppressing chloride-induced corrosion.
3.3. Effect of Surface Inclination on Patina Flaking/Spallation–Visual Observations
The literature findings show surface inclination to strongly influence patina formation on Cu metal and its alloys [
20]. Upward-facing surfaces (e.g., 45°) in marine atmospheres generally experience longer wetting times and higher deposition of chlorides, aerosols, and dust, leading to thicker, more porous, chloride-rich outer patinas, whereas downward-facing surfaces develop thinner, denser, and more adherent films [
21,
22]. As already discussed above, spallation has been shown to be primarily driven by the formation of CuCl at the patina/metal interface, which transforms to Cu
2(OH)
3Clwith volume expansion, generating internal stresses [
1,
15]. These processes can explain the observation of spallation/flaking of corrosion products on Cu and Cu–Sn alloys at marine exposure conditions (Brest, France, near the seashore–site 2), in contrast to the more adherent patinas on Zn- and Al-containing alloys [
14,
15]. While multiple studies confirm the role of chloride deposition, patina heterogeneity, and environmental microclimates on spallation, field data directly comparing spallation as a function of inclination (e.g., 45° vs. upside-down) remain scarce.
The effect of chloride-driven spallation and surface inclination was therefore investigated during 3 years in the marine environment of Brest at three sites of largely different annual mean chloride deposition rates (sites 1, 2, and 4), as seen in
Figure 9.
Like findings for surfaces inclined 45° [
14], the extent of green-bluish patina formation was considerable for Cu metal and the alloys (CuZn15, CuSn4, and CuZn5Al5) also exposed up-side down (−180°) at site 1 located < 5 m from the seashore (not in the splash zone), with substantial flaking taking place on Cu metal, and even more pronounced on CuSn4,
Figure 9a. No flaking was observed for the Zn-containing Cu alloys (CuZn15, CuZn5Al5). The underlying mechanisms for the lack of flaking/spallation have been reported to be related to the integration of corrosion products of Zn (Zn
5(CO
3)
2(OH)
6 and Zn
5(OH)
8Cl
2·H
2O) in the Cu-rich patina of CuZn15, and the additional presence of Cu
2O, ZnO, Al
2O
3, and SnO
2 in the inner layer of the patina of the CuZn5Al5 alloy and Zn- and Sn-rich corrosion products (SnO
2, Zn
5(CO
3)
2(OH)
6, Zn
6Al
2(OH)
16CO
3·4H
2O, and Zn
5(OH)
8Cl
2·H
2O in the outer patina) predominantly composed of Cu
2(OH)
3Cl, able to mitigate chloride-induced corrosion compared to pure Cu metal [
15]. At site 2, 20–30 m from the seashore with 6–7 times lower deposition rates of chlorides than site 1, the effect of chloride-driven spallation was minor and not visually observed for any surface inclination (45°, −180°), as seen in
Figure 9b. Similar findings were seen at site 4 with even considerably lower (40–70 times) mean annual chloride deposition rates than site 1, as seen in
Figure 9c. The extent of flaking was in general more severe for surfaces inclined 90° compared to 45°, also consistent with differences in corrosion rates [
14].
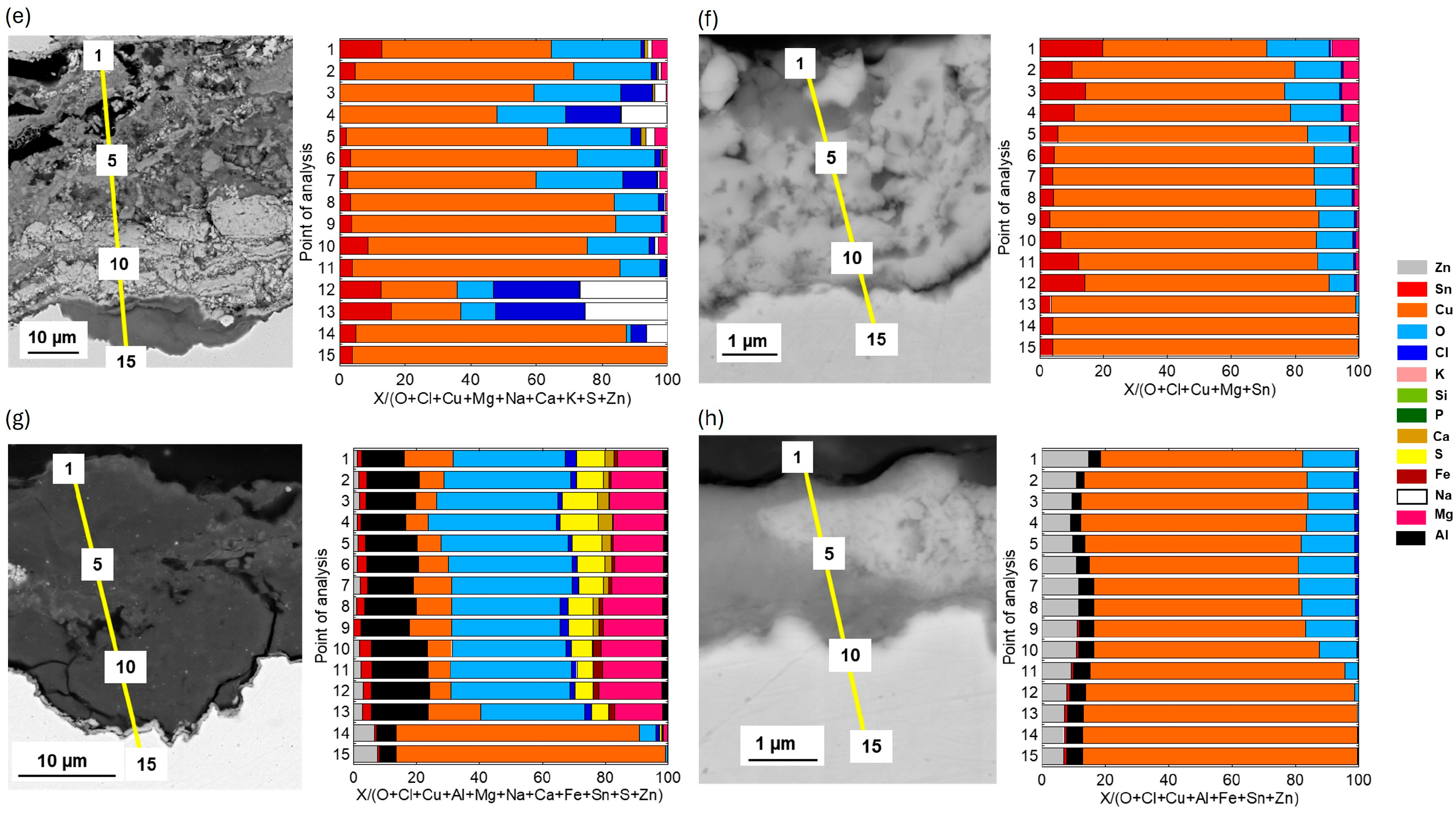
SEM/EDS cross-sectional analysis of the upside-down coupons (−180°) of Cu metal, CuZn15, CuSn4, and CuZn5Al5 is presented in
Figure 10 after 2 and 3 years of exposure. Cu metal revealed a very thick patina with some sections being a two-layer type composed of a 25–30 µm thick inner layer mainly composed of Cu-oxides (Cu
2O) and small amounts of Cl, and a thick (≈40 µm) compact layer of chloride-rich Cu corrosion products (Cu
2(OH)
3Cl),
Figure 10a,b. Other sections of the patina showed highly porous, thick (40–50 µm) layered patina structures rich in Cu-oxides alternating with Cu-, O, and Cl-rich corrosion products as well as sand and dust particles (Si, Al, Ca, Fe, Mg). The latter sections could most probably be easily flaked off.
The patina of the CuZn15 coupons was considerably generally thinner than the Cu metal patina consisting of sections with an inner, approximately 15 µm thick layer rich in mainly Cu and Zn-rich corrosion products and small amounts of Cl, and an outer layer (≈10 µm) consisting of Cu and Zn as well as larger amounts of Cl. Other sections showed a similar composition but was considerably thinner, ≈2 µm,
Figure 10c,d. The CuSn4 patina was highly heterogeneous and largely varied in thickness from 5–6 µm to 50–60 µm, most probably because of flaked off corrosion products. The patina showed layered structures rich in Cu, Sn, O, and of varying amounts of Cl as well as embedded sand/dust-particles,
Figure 10e,f. The CuZn5Al5 coupons showed a patina of Zn-, Cu-, Al-, and Sn-rich corrosion products being considerably thinner (2–3 µm) than the patinas on Cu metal, CuZn15, and CuSn4. Only very small amounts of Cl were observed in the patina, like the observations at 45°, showing the Zn- and Al-rich corrosion products able to hinder chloride-induced corrosion, explaining the thinner patina layers. Some sections of the patina revealed also for this alloy embedded sand/dust/construction particles, as seen in
Figure 10g,h.
Exposure in Dubai for 4 years, a site characterized by considerably lower deposition rates of chlorides (30–45 times lower annual mean deposition rates) than sites 1 and 2 in Brest, did not result in any observed flaking/spallation of the Cu metal surfaces inclined either 45° or upside-down (−180°). As described above, this was attributed to no or minor amounts of CuCl within the patina, resulting in volume expansion during the formation of Cu
2(OH)
3Cl, whereas small amounts of both phases were observed for CuSn4,
Figure 4. Nevertheless, no flaking was visually observed for surfaces inclined 45° from the horizontal, but clearly taking place on surfaces exposed up-side down (−180°), as seen in
Figure 11.
The low deposition rates of chlorides measured at the Dubai site could not explain this flaking behavior as no flaking was observed for CuSn4 at the marine test sites of similar chloride deposition rates, i.e., sites 3 and 4. However, chlorine was evidently observed within sections of the upside-down patina of CuSn4 in Dubai, see
Figure 10e, which possibly could be connected to CuCl and Cu
2(OH)
3Cl formation. The patinas were further locally considerably thicker, and the morphology different,
Figure 10f, compared with the patina of coupons with a surface inclination of 45°,
Figure 7. This could be an effect of condensation-forming droplets, local concentration of pollutants including Cl on the upside-down facing surfaces of the coupons due to large variations in daily temperatures which locally lead to longer wetting times with induced stresses, poor adhesion, and possible flaking of corrosion products. In combination with the incorporation of sand and construction particles into the patina, the patina may have become even more brittle. However, further analysis and investigations are required to properly understand these flaking processes. Like the findings at the marine sites, no flaking/spallation of corrosion products was observed for the Zn-containing alloys (CuZn15 and CuZn5Al5) for any surface inclination though being highly corroded, forming heterogeneous patinas with embedded sand and dust/construction particles. Changes in visual appearance of Cu metal, CuZn15, CuSn2, and CuZn5Al5 surfaces inclined 45° and upside-down (−180°) after 1, 2, 3, and 4 years of exposure are presented in
Figures S1–S4 (Supporting Information).