Studies on the Chemical Etching and Corrosion Resistance of Ultrathin Laminated Alumina/Titania Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Deposition of Thin Films
2.2. Etching and Characterization of Thin Films
2.3. Testing of the Coatings
3. Results
3.1. Structure, Composition, and Morphology of the Coatings
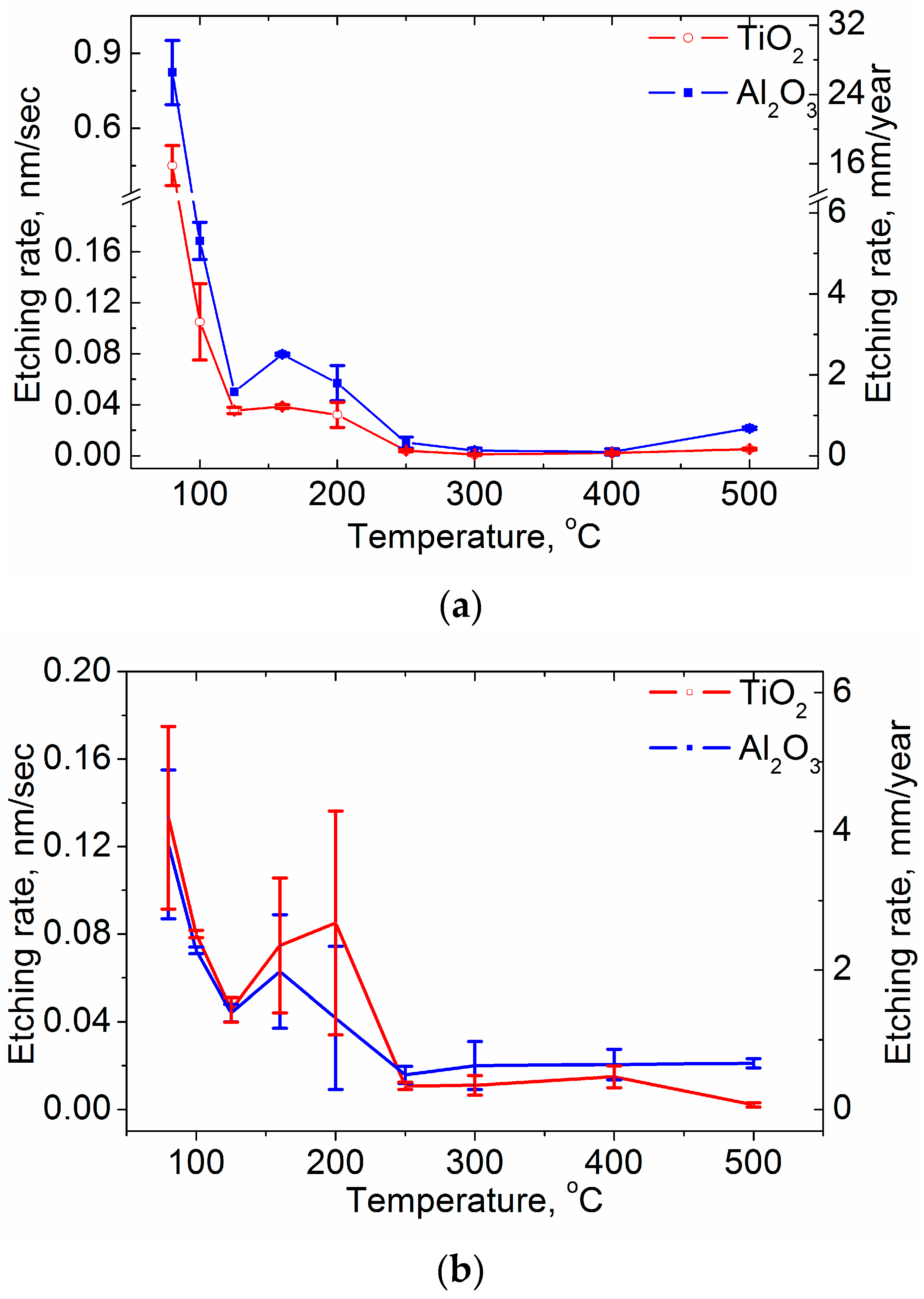
3.2. Chemical Etching Results of the Coatings
3.3. Microscopy Study of the Etched Coatings
3.4. Electrochemical Studies
3.4.1. Results of the Voltammetry Measurements
3.4.2. Results of the EIS Analyses
3.4.3. Immersion Tests
3.5. Mechanical Properties of the Laminated Coatings
4. Discussion
4.1. Role of the Metal Surface Pretreatment
4.2. Porosity of the Coatings
4.3. Potential Applicability of ALD Coatings in Industry
5. Conclusions
- All of the coatings tested provided a certain level of protection to both substrate types, but there is no ideal TG value that would guarantee the best performance for all the properties tested.
- Coatings deposited at TG ≤ 100 °C had the lowest protective properties due to the high content of residual impurities resulting from incomplete surface reactions in the ALD processes.
- The total growth rate of the coatings was higher and the chlorine residue content lower for the coatings deposited on SS substrates at TG = 100–125 °C compared to coatings deposited on Si substrates in the same run; the effect somewhat improves the performance of low-temperature ALD coatings in protecting SS substrates.
- The best corrosion resistance appeared with a fully amorphous laminated coating deposited at 125 °C. Still, due to its poor abrasion resistance, it may need to be coated with a paint/varnish layer.
- Coatings deposited at TG ≥ 250 °C showed the best resistance to chemical etching and the physical treatments due to the crystalline titania (anatase) sublayers. However, raising the TG to 500 °C will cause some decomposition of the aluminum precursor TMA.
- The inhomogeneity of the interfaces in the laminates grown at 160 °C and initial crystallization of titania in the laminates grown at TG = 200 °C both increase the defectiveness of the coatings and lead to slowing the increase in the etching resistance efficiency and, at the same time, lead to a decrease in the corrosion protection of the coatings prepared at these temperatures. Thus, we do not recommend the use of this temperature range for the preparation of laminate coatings.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AISI | American Iron and Steel Institute |
| SS | Stainless steel |
| ALD | Atomic layer deposition |
| TMA | Trimethylaluminum |
| XRF | X-ray fluorescence |
| EPMA | Electron probe microanalysis |
| XRD | X-ray diffraction |
| GIXRD | Grazing incidence X-ray diffraction |
| PB | Parallel beam (geometry) |
| HR-SEM | High-resolution scanning electron microscope |
| FIB | Focused ion beam |
| SEM | Scanning electron microscope |
| STEM | Scanning transmission electron microscope |
| HR-(S)TEM | High-resolution (scanning) transmission electron microscope |
| SCE | Saturated calomel electrode |
| LSV | Linear sweep voltammetry |
| OCP | Open-circuit potential |
| EIS | Electrochemical impedance spectrometry |
| RMS | Root mean square |
| AC | Alternating current |
| CPE | Constant phase element |
| ASTM | American Society for Testing and Materials |
| GenAI | Generative artificial intelligence |
| BF | Bright field |
| HAADF | High-angle annular dark field |
| RBS | Rutherford backscattered spectroscopy |
| TOF-ERDA | Time-of-flight elastic recoil detection analysis |
| EC | (Electrical) equivalent circuit |
Appendix A
References
- McGuire, M. Stainless Steels for Design Engineers; ASM International: Novelty, OH, USA, 2008; pp. 161–171. [Google Scholar]
- Norton, J.; Baxter, D.; Santorelli, R.; Bregani, F. The corrosion of AISI 310 stainless steel exposed to sulphidizing/oxidizing/carburizing atmospheres at 600 °C. Corros. Sci. 1993, 35, 1085–1090. [Google Scholar] [CrossRef]
- Roberge, P.R. Handbook of Corrosion Engineering; McGraw-Hill: New York, NY, USA, 2000; ISBN 0-07-076516-2. [Google Scholar]
- Padhi, N.; Kamal, S.; Chandra, R.; Kamachi Mudali, U.; Raj, B. Corrosion performance of TiO2 coated type 304L stainless steel in nitric acid medium. Surf. Coat. Techol. 2010, 204, 2782–2788. [Google Scholar] [CrossRef]
- Sridharan, M.; Sillassen, M.; Bøttiger, J.; Chevallier, J.; Birkedal, H. Pulsed DC magnetron sputtered Al2O3 films and their hardness. Surf. Coatings Technol. 2007, 202, 920–924. [Google Scholar] [CrossRef]
- Bera, S.; Rout, T.K.; Udayabhanu, G.; Narayan, R. Comparative Study of Corrosion Protection of Sol–Gel Coatings with Different Organic Functionality on Al-2024 substrate. Progr. Org. Coat. 2015, 88, 293–303. [Google Scholar] [CrossRef]
- Lazar, A.-M.; Yespica, W.P.; Marcelin, S.; Pébère, N.; Samélor, D.; Tendero, C.; Vahlas, C. Corrosion protection of 304L stainless steel by chemical vapor deposited alumina coatings. Corros. Sci. 2014, 81, 125–131. [Google Scholar] [CrossRef]
- Sugama, T. CVD-titanium carbonitride coatings as corrosion-preventing barriers for steel in acid-brine steam at 200 °C. Mater. Lett. 1999, 38, 227–234. [Google Scholar] [CrossRef]
- Lauwerens, W.; De Boeck, A.; Thijs, M.; Claessens, S.; Van Stappen, M.; Steenackers, P. PVD Al–Ti and Al–Mn coatings for high temperature corrosion protection of sheet steel. Surf. Coat. Technol. 2001, 146, 27–32. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Review Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Films 2002, 409, 138–146. [Google Scholar] [CrossRef]
- Matero, R.; Ritala, M.; Leskelä, M.; Salo, T.; Aromaa, J.; Forsen, O. Atomic layer deposited thin films for corrosion protection. J. Phys. IV 1999, 9, 493–499. [Google Scholar] [CrossRef]
- Shan, C.X.; Hou, X.; Choy, K.-L. Corrosion Resistance of TiO2 Films Grown on Stainless Steel by Atomic Layer Deposition. Surf. Coatings Technol. 2008, 202, 2399–2402. [Google Scholar] [CrossRef]
- Abdulagatov, A.I.; Yan, Y.; Cooper, J.R.; Zhang, Y.; Gibbs, Z.M.; Cavanagh, A.S.; Yang, R.G.; Lee, Y.C.; George, S.M. Al2O3 and TiO2 Atomic layer deposition on copper for water corrosion resistance. Appl. Mater. Interfaces 2011, 3, 4593–4601. [Google Scholar] [CrossRef]
- Correa, G.C.; Bao, B.; Strandwitz, N.C. Chemical Stability of Titania and Alumina Thin Films Formed by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2015, 7, 14816–14821. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A.; Guzman, L.; Fedrizzi, L. Chemical and electrochemical characterization of TiO2/Al2O3 atomic layer depositions on AZ-31 magnesium alloy. J. Coat. Technol. Res. 2012, 9, 347–355. [Google Scholar] [CrossRef]
- Merisalu, M.; Aarik, L.; Piirsoo, H.-M.; Kozlova, J.; Tarre, A.; Zabels, R.; Wessing, J.; Brieva, A.; Sammelselg, V. Nanostructured Coating for Aluminum Alloys Used in Aerospace Applications. J. Electrochem. Soc. 2022, 169, 071503. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A.; Guzman, L.; Fedrizzi, L. Corrosion protection of AISI 316 stainless steel by ALD alumina/titania nanometric coatings. J. Coatings Technol. Res. 2011, 8, 655–659. [Google Scholar] [CrossRef]
- Marin, E.; Guzman, L.; Lanzutti, A.; Ensinger, W.; Fedrizzi, L. Multilayer Al2O3/TiO2 Atomic Layer Deposition coatings for the corrosion protection of stainless steel. Thin Solid Film. 2012, 522, 283–288. [Google Scholar] [CrossRef]
- Härkönen, E.; Diaz, B.; Swiatowska, J.; Maurice, V.; Seyeux, A.; Vehkamäki, M.; Sajavaara, T.; Fenker, M.; Marcus, P.; Ritala, M. Corrosion protection of steel with oxide nanolaminates grown by Atomic Layer Deposition. J. Electrochem. Soc. 2011, 158, C369–C378. [Google Scholar] [CrossRef]
- ISO9227:2022; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. The International Organization for Standardization (ISO): Geneva, Switzerland, 2022. Available online: https://www.iso.org/standard/81744.html (accessed on 27 July 2025).
- Kong, J.-Z.; Xu, P.; Cao, Y.-Q.; Li, A.-D.; Wang, Q.-Z.; Zhou, F. Improved corrosion protection of CrN hard coating on steel sealed with TiOxNy-TiN composite layers. Surf. Coat. Technol. 2020, 381, 125108. [Google Scholar] [CrossRef]
- Lim, J.W.; Yun, S.J.; Kim, H.-T. Characteristics of AlxTi1−xOy films grown by Plasma-Enhanced Atomic Layer. J. Electrochem. Soc. 2007, 154, G239–G243. [Google Scholar] [CrossRef]
- Kukli, K.; Ritala, M.; Leskelä, M.; Sundqvist, J.; Oberbeck, L.; Heitmann, J.; Schröder, U.; Aarik, J.; Aidla, A. Influence of TiO2 incorporation in HfO2 and Al2O3 based capacitor dielectrics. Thin Solid Film. 2007, 515, 6447–6451. [Google Scholar] [CrossRef]
- Jõgi, I.; Kukli, K.; Kemell, M.; Ritala, M.; Leskelä, M. Electrical characterization of AlxTiyOzmixtures and Al2O3–TiO2–Al2O3 nanolaminates. J. Appl. Phys. 2007, 102, 114114. [Google Scholar] [CrossRef]
- Abaffy, N.B.; McCulloch, D.G.; Partridge, J.G.; Evans, P.J.; Triani, G. Engineering titanium and aluminum oxide composites using atomic layer deposition. J. Appl. Phys. 2011, 110, 123514. [Google Scholar] [CrossRef]
- Härkönen, E.; Diaz, B.; Swiatowska, J.; Maurice, V.; Seyeux, A.; Fenker, M.; Toth, L.; Radnoczi, G.; Marcus, P.; Ritala, M. AlxTayOz Mixture coatings prepared using Atomic Layer Deposition for corrosion protection of steel. Chem. Vap. Depos. 2013, 19, 194–203. [Google Scholar] [CrossRef]
- Daubert, J.S.; Hill, G.T.; Gotsch, H.N.; Gremaud, A.P.; Ovental, J.S.; Philip, S.; Williams, P.S.; Oldham, C.N.; Gregory, N.; Parsons, G.N. Corrosion Protection of Copper Using Al2O3, TiO2, ZnO, HfO2, and ZrO2 Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2017, 9, 4192–4201. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, C.; Dai, Y.; Lan, Z.; Liu, C. Electrochemical and structural properties of ultrathin atomic layer deposition coatings for corrosion resistance on aluminum alloy. Ceram. Int. 2025, 51, 12123–12137. [Google Scholar] [CrossRef]
- Santinacci, L. Atomic layer deposition: An efficient tool for corrosion protection. Curr. Opin. Colloid Interface Sci. 2023, 63, 101674. [Google Scholar] [CrossRef]
- Sammelselg, V.; Netšipailo, I.; Aidla, A.; Tarre, A.; Aarik, L.; Asari, J.; Ritslaid, P.; Aarik, J. Chemical resistance of thin film materials based on metal oxides grown by atomic layer deposition. Thin Solid Films 2013, 542, 219–224. [Google Scholar] [CrossRef]
- Aarik, L.; Kozlova, J.; Mändar, H.; Aarik, J.; Sammelselg, V. Chemical resistance of TiO2 and Al2O3 single-layer and multilayer coatings atomic layer deposited from hydrogen-free precursors on silicon and stainless steel. Mater. Chem. Phys. 2019, 228, 285–292. [Google Scholar] [CrossRef]
- Arroval, T.; Aarik, L.; Rammula, R.; Kruusla, V.; Aarik, J. Effect of substrate-enhanced and inhibited growth on atomic layer deposition and properties of aluminum-titanium oxide films. Thin Solid Films 2016, 600, 119–125. [Google Scholar] [CrossRef]
- Pärna, R.; Nõmmiste, E.; Kikas, A.; Jussila, O.; Hirsimäki, M.; Valden, M.; Kisand, V. Electron spectroscopic study of passive oxide layer formation on Fe-19Cr-18Ni-1Al-TiC austenitic stainless steel. J. Electron Spectrosc. Relat. Phenom. 2010, 182, 108–114. [Google Scholar] [CrossRef]
- Sammelselg, V.; Rauhala, E.; Arstila, K.; Zakharov, A.; Aarik, J.; Kikas, A.; Karlis, J.; Tarre, A.; Seppälä, A.; Asari, J.; et al. Study of thin oxide films by electron, ion, and synchrotron radiation beams. Microchim. Acta 2002, 139, 165–169. [Google Scholar] [CrossRef]
- Brug, G.J.; Van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Zoltowski, P. On the electrical capacitance of interfaces exhibiting constant phase element behavior. J. Electroanal. Chem. 1998, 443, 149–154. [Google Scholar] [CrossRef]
- ASTM G48-11(2020)e1; Standard Test Methods for Pitting and Crevice Corrosion Resistance of Stainless Steels and Related Alloys by Use of Ferric Chloride Solution. ASTM International: West Conshohocken, PA, USA, 2020.
- Mamedov, S. Characterization of TiO2 Nanopowders by Raman Spectroscopy. Spectrosc. Suppl. 2020, 35, 41–49. [Google Scholar]
- Aarik, J.; Aidla, A.; Kiisler, A.; Uustare, T.; Sammelselg, V. Effect of crystal structure on optical properties of TiO2 films grown by atomic layer deposition. Thin Solid Films 1997, 305, 270–273. [Google Scholar] [CrossRef]
- Sammelselg, V.; Tarre, A.; Lu, J.; Aarik, J.; Niilisk, A.; Uustare, T.; Netšipailo, I.; Rammula, R.; Pärna, R.; Rosental, A. Structural characterization of TiO2–Cr2O3 nanolaminates grown by atomic layer deposition. Surf. Coatings Technol. 2010, 204, 2015–2018. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Mändar, H.; Sammelselg, V. Anomalous effect of temperature on atomic layer deposition of titanium dioxide. J. Cryst. Growth 2000, 220, 531–537. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Coy, E.; Viter, R.; Nowaczyk, G.; Jancelewicz, M.; Baleviciute, I.; Załęski, K.; Jurga, S. Study on Structural, Mechanical, and Optical Properties of Al2O3−TiO2 Nanolaminates Prepared by Atomic Layer Deposition. J. Phys. Chem. C 2015, 119, 20591–20599. [Google Scholar] [CrossRef]
- Mondal, J.; Marques, A.; Aarik, L.; Kozlova, J.; Simões, A.; Sammelselg, V. Development of a Thin Ceramic-Graphene Nanolaminate Coating for Corrosion Protection of Stainless Steel. Corros. Sci. 2016, 105, 161–169. [Google Scholar] [CrossRef]
- ASTM G46–94; Standard Guide for Examination and Evaluation of Pitting Corrosion. American Society for Testing of Materials: West Conshohocken, PA, USA, 1999.
- Ylivaara, O.M.E.; Langner, A.; Liu, X.; Schneider, D.; Julin, J.; Arstila, K.; Sintonen, S.; Ali, S.; Lipsanen, H.; Sajavaara, T.; et al. Mechanical and optical properties of as-grown and thermally annealed titanium dioxide from titanium tetrachloride and water by atomic layer deposition. Thin Solid Films 2021, 732, 138758. [Google Scholar] [CrossRef]
- Tato, W.; Landolt, D. Electrochemical determination of the porosity of single and duplex PVD coatings of titanium and titanium nitride on brass. J. Electrochem. Soc. 1998, 145, 4173–4181. [Google Scholar] [CrossRef]
- Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Normand, B.; Härkönen, E.; Ritala, M.; Marcus, P. Electrochemical and time-of-flight secondary ion mass spectrometry analysis of ultra-thin metal oxide (Al2O3 and Ta2O5) coatings deposited by atomic layer deposition on stainless steel. Electrochim. Acta 2011, 56, 10516–10523. [Google Scholar] [CrossRef]
- Chou, N.H. Atomic Layer Deposition: Significance and Application in Modern Microelectronics. Available online: https://www.materion.com/en/insights/blog/atomic-layer-deposition-significance-application-in-modern-microelectronics (accessed on 13 July 2025).
- Sekkat, A.; Weber, M.; López-Sánchez, J.; Rabat, H.; Hong, D.; Rubio-Zuazo, J.; Bellet, D.; Chichignoud, G.; Kaminski-Cachopo, A.; Muñoz-Rojas, D. Selective spatial atomic layer deposition of Cu, Cu2O and CuO thin films in the open air: Reality or fiction? Mater. Today Chem. 2023, 29, 101431. [Google Scholar] [CrossRef]
- Sammelselg, V.; Aarik, L.; Merisalu, M. Method of Preparing Corrosion Resistant Coatings. WO2014102758A1, 3 July 2014. Available online: http://www.google.com/patents/WO2014102758A1?cl=en (accessed on 27 July 2025).
- Sammelselg, V.; Kostamo, J.; Bayerl, W.; Aarik, J.; Aarik, L.; Lindfors, S.; Adam, P.; Poutianen, J. Protecting an Interior of a Gas Container with an ALD Coating. WO20151324443A1, 11 September 2015. Available online: http://www.google.com/patents/WO2015132443A1?cl=en (accessed on 27 July 2025).

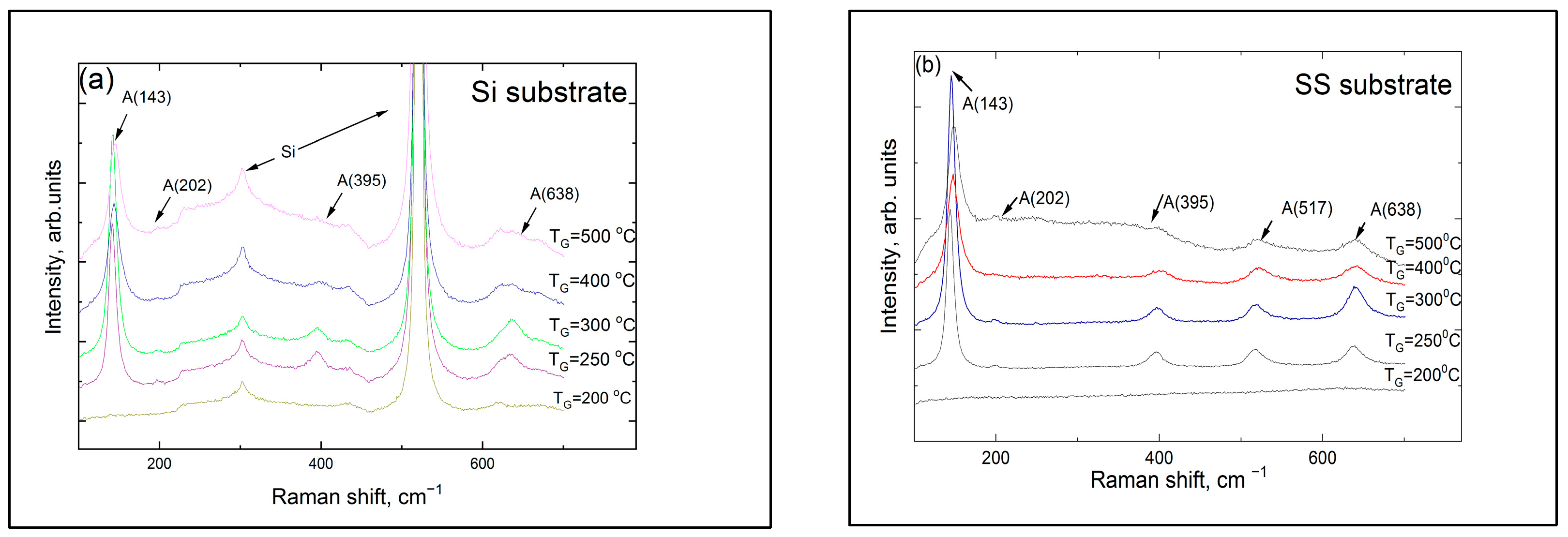
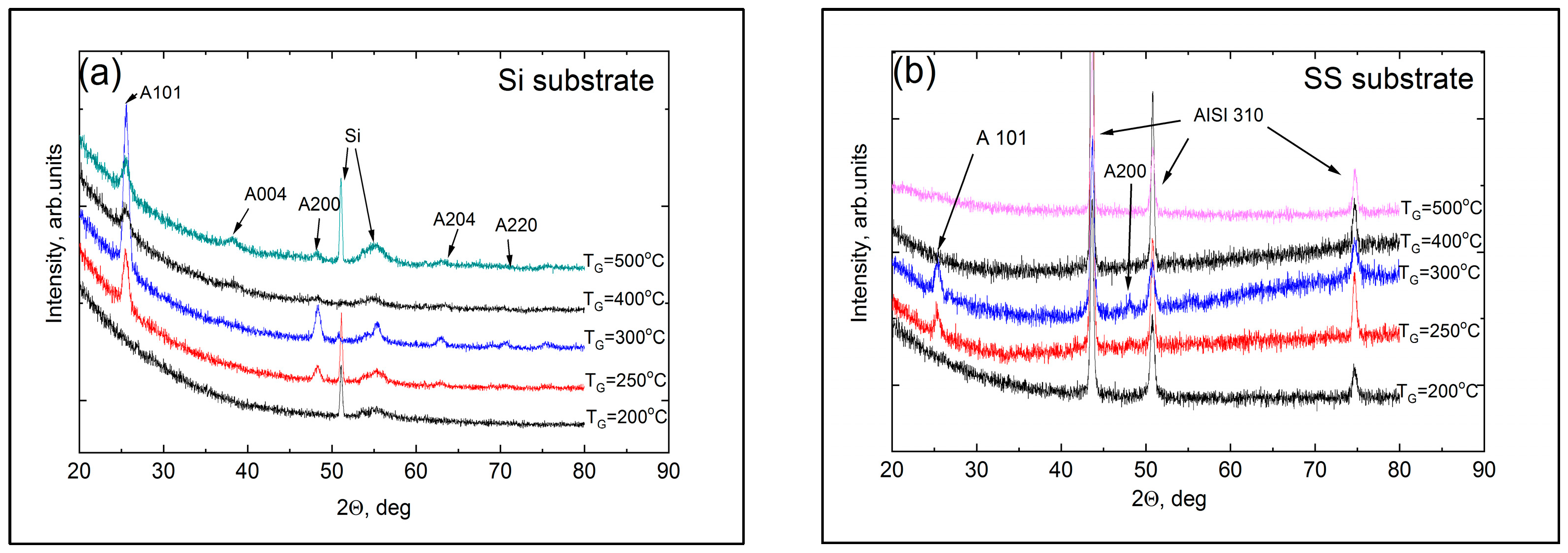

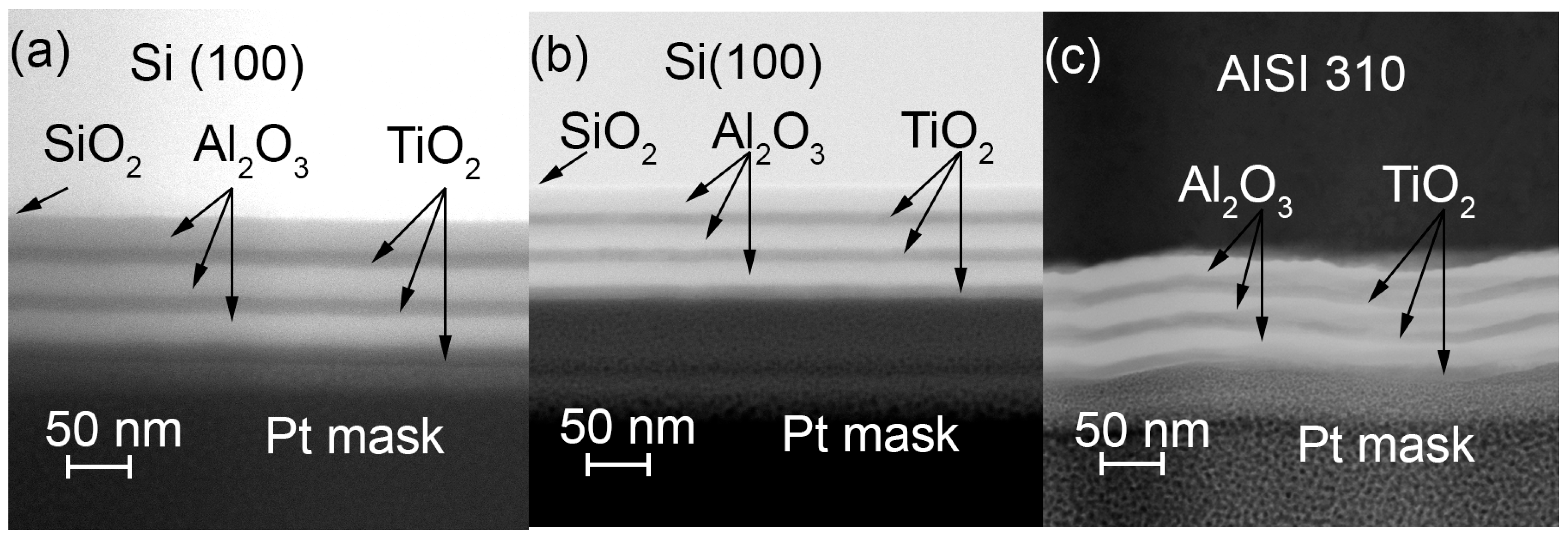

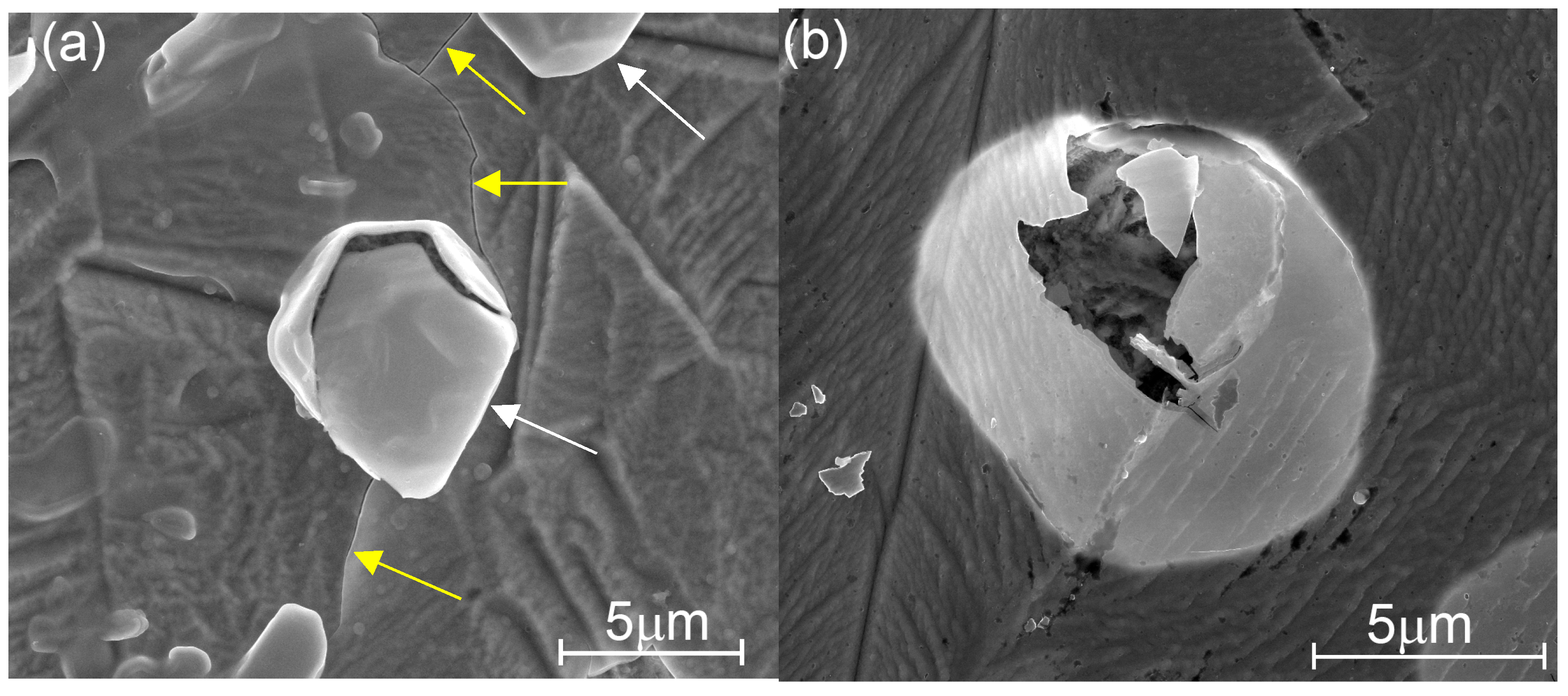

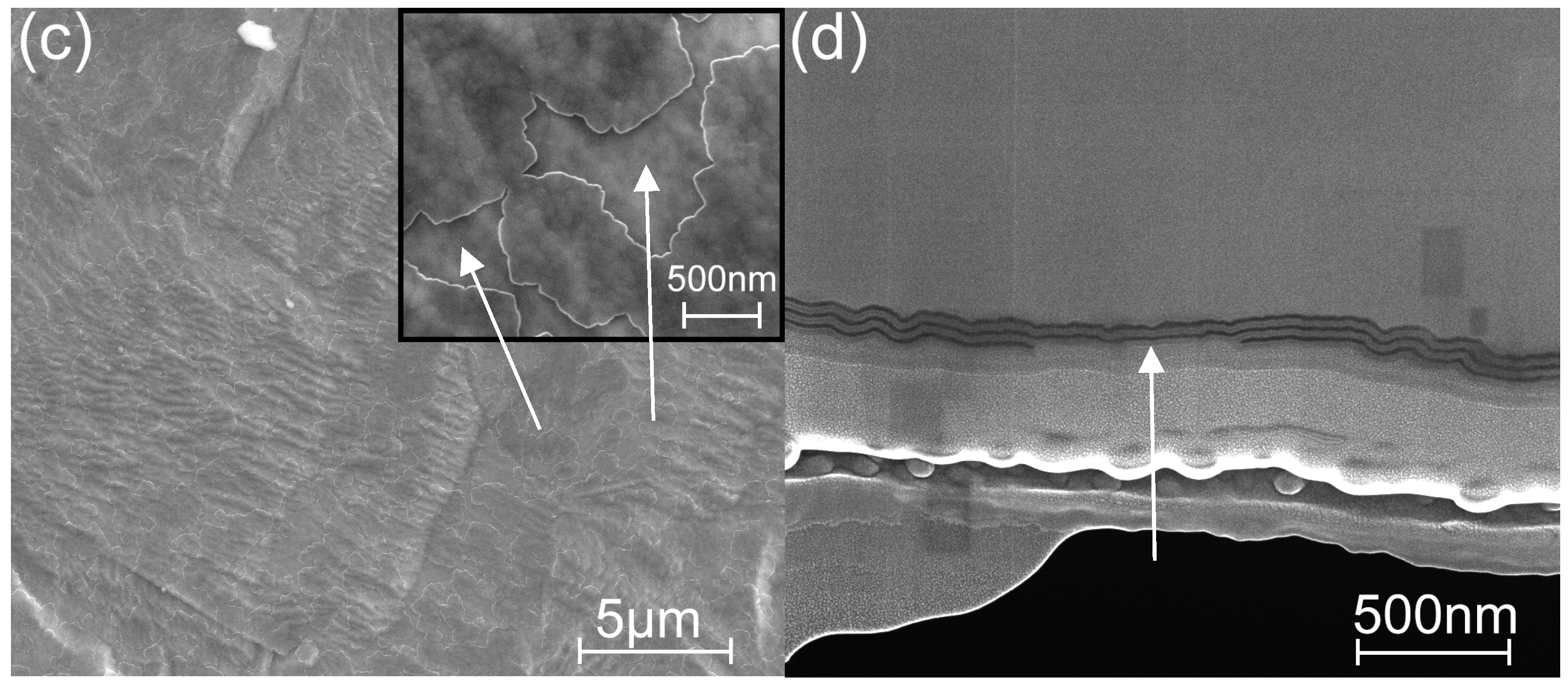

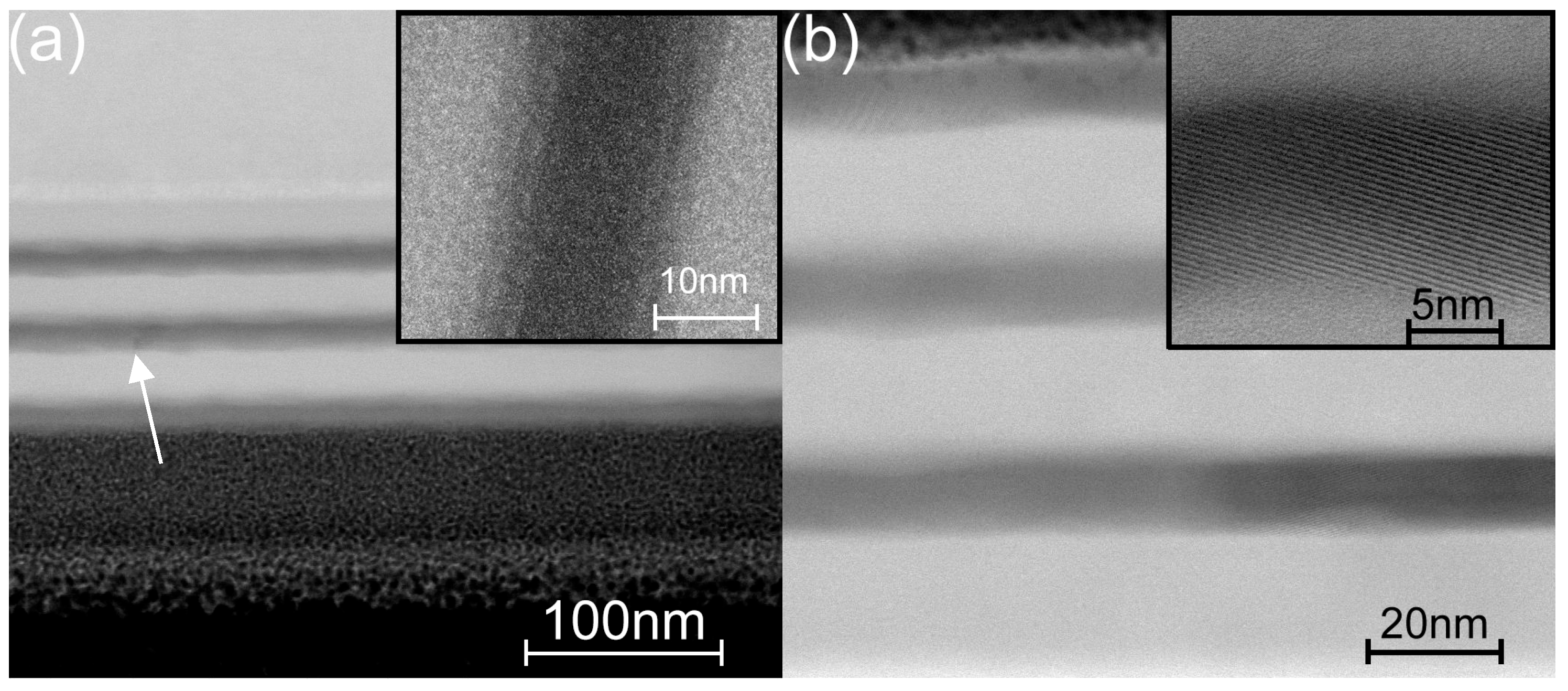
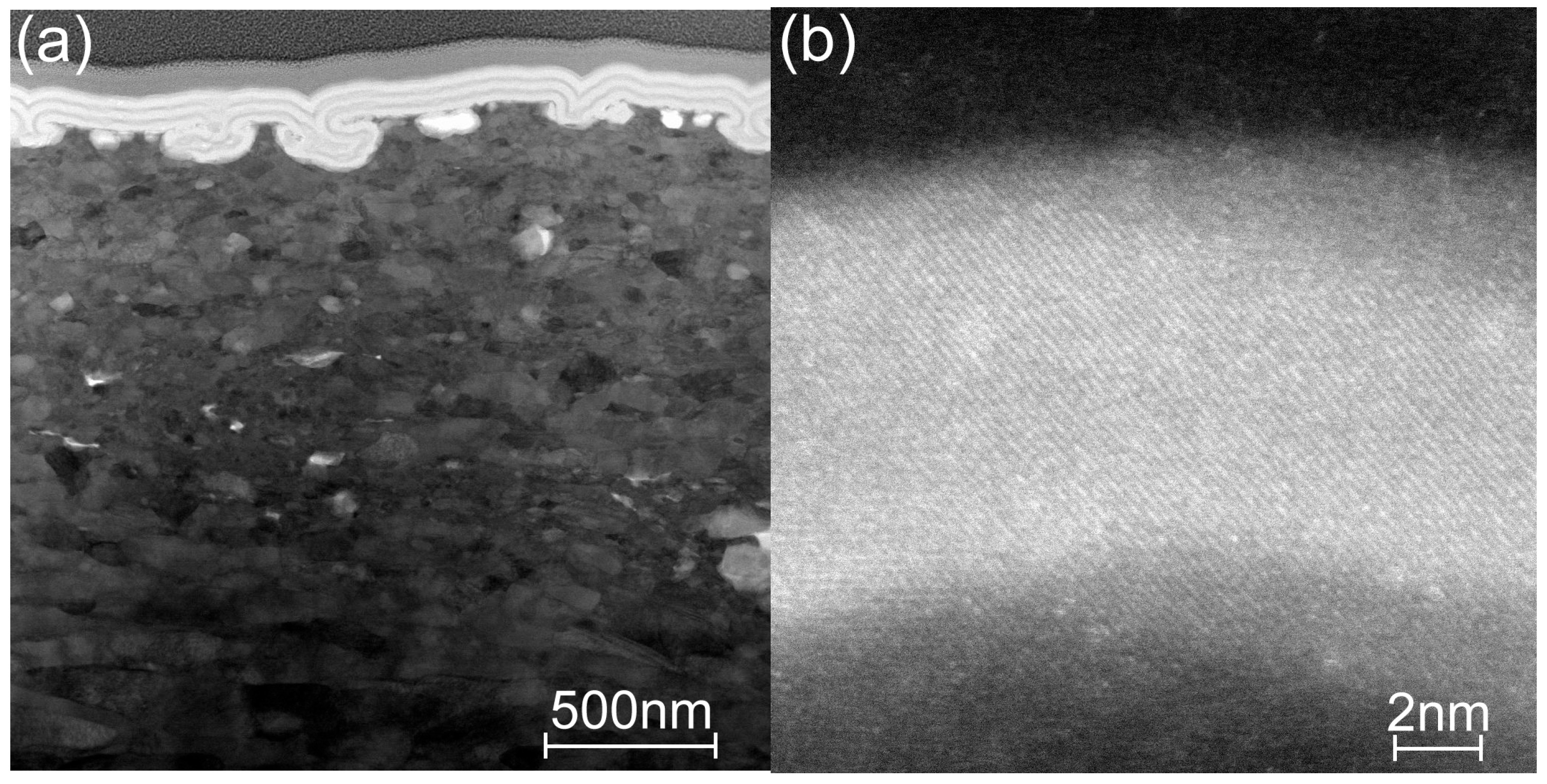


| TG, °C | Crystal Structure of Titania Sublayers | Coatings on Si Substrates | Coatings on AISI310 Substrates | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial Thickness 1, d0, nm | Etching Rate 2, ke, nm/s | Etching Time, min | Initial Thickness, nm | Etching Rate 2, ke, nm/s | Etching Time, min | ||||
| TiO2 | Al2O3 | TiO2 | Al2O3 | ||||||
| 80 | Amorphous | 106.0 | 0.37 | 0.70 | 1 | 108.2 | 0.09 | 0.09 | 5 |
| Amorphous | 110.0 | 0.78 | 0.82 | 1 | 157.1 | 0.18 | 0.16 | 4 | |
| 100 | Amorphous | 107.0 | 0.10 | 0.15 | 3 | 130.4 | 0.08 | 0.07 | 5 |
| Amorphous | 111.3 | 0.17 | 0.16 | 3 | 138.1 | 0.08 | 0.07 | 5 | |
| 125 | Amorphous | 133.0 | 0.03 | 0.05 | 10 | 111.9 | 0.05 | 0.04 | 10 |
| Amorphous | 138.0 | 0.05 | 0.04 | 7.5 | 121.3 | 0.04 | 0.05 | 7.5 | |
| 160 | Amorphous? | 92.4 | 0.06 | 0.15 | 7.5 | 117.9 | 0.03 | 0.01 | 5 |
| Amorphous? | 118.0 | 0.06 | 0.06 | 7.5 | 115.9 | 0.04 | 0.04 | 5 | |
| 200 | Amorphous with nanocrystallites | 143.9 | 0.08 | 0.24 | 7.5 | 133.0 | 0.11 | 0.09 | 3 |
| Amorphous with nanocrystallites | 148.8 | 0.07 | 0.27 | 7.5 | 207.4 | 0.14 | 0.07 | 3 | |
| 250 | Crystalline (anatase) | 115.7 | 0.00 | 0.01 | 10 | 109.5 | 0.01 | 0.01 | 10 |
| Crystalline (anatase) | 102.2 | 0.02 | 0.04 | 30 | 110.4 | 0.01 | 0.02 | 10 | |
| 300 | Crystalline (anatase) | 93.4 | 0.00 | 0.00 | 10 | 126.3 | 0.00 | 0.00 | 10 |
| Crystalline (anatase) | 94.6 | 0.01 | 0.02 | 60 | 96.4 | 0.02 | 0.03 | 10 | |
| 400 | Crystalline (anatase) | 94.0 | 0.00 | 0.00 | 10 | 86.1 | 0.01 | 0.02 | 10 |
| Crystalline (anatase) | 82.9 | 0.01 | 0.03 | 30 | 93.7 | 0.02 | 0.02 | 7.5 | |
| Crystalline (anatase) | 82.9 | 0.01 | 0.02 | 60 | - | - | - | - | |
| 500 | Crystalline (anatase) | 88.6 | 0.01 | 0.02 | 10 | 171.8 | 0.00 | 0.02 | 7.5 |
| Crystalline (anatase) | 104.1 | 0.00 | 0.04 | 30 | 92.0 | 0.00 | 0.02 | 5 | |
| TG/substrate | Coating Mass thickness, µg/cm2 | Element Content, mass% | |||||
|---|---|---|---|---|---|---|---|
| C | O | Al | Ti | Cl | Ratio for Cl 1 | ||
| 100 °C/Si 2 | 31.60 | 1.2 ± 0.9 | 41.0 ± 1.1 | 24.6 ± 0.1 | 30.4 ± 1.0 | 2.8 ± 0.2 | 1.2 |
| 100 °C/SS | 45.81 | 2.5 ± 0.1 | 42.1 ± 0.3 | 21.9 ± 0.01 | 31.1 ± 0.1 | 2.4 ± 0.1 | |
| 125 °C/Si | 23.18 | 3.1 ± 0.2 | 36.9 ± 0.2 | 25.5 ± 0.02 | 32.5 ± 0.1 | 2.0 ± 0.2 | 1.4 |
| 125 °C/SS | 35.21 | 5.3 ± 0.1 | 36.7 ± 0.3 | 27.1 ± 0.01 | 29.5 ± 0.1 | 1.4 ± 0.2 | |
| Samples | Parameters 1 | |||
|---|---|---|---|---|
| jcor, nA | j−0.1 V, nA | jmax; 2 V, mA | Epit, V | |
| Bare SS | 49.0 | 244 | 105 | 1.1 |
| Coated SS: 125 °C | 0.04 | 0.5 | 7.2 | 1.3 |
| Coated SS: 250 °C | 0.82 | 1.9 | 0.06 | >2.0 |
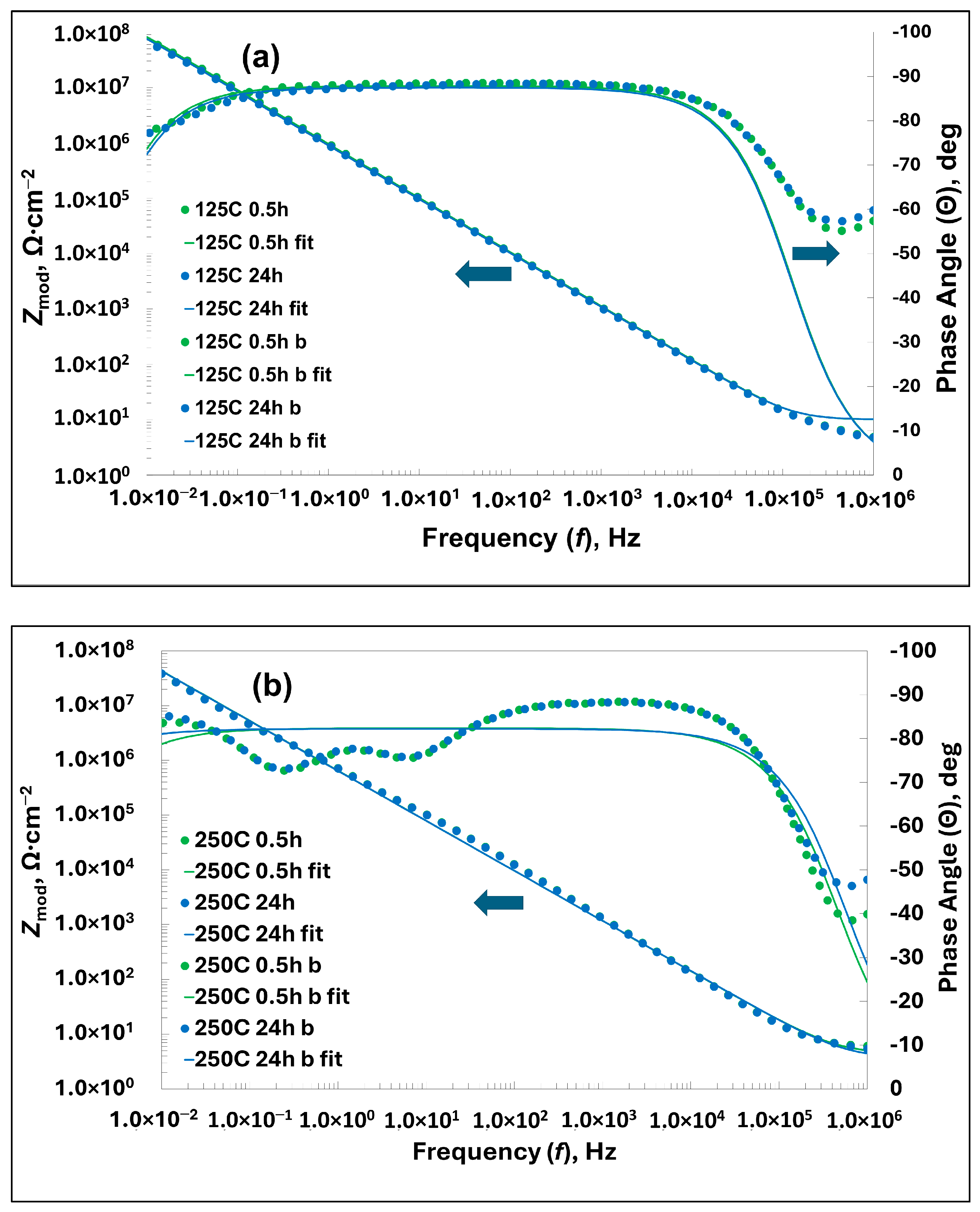
| Samples (TG, Immersion Time) | Experimental Data 1 | Parameters 2,3 | |||||
|---|---|---|---|---|---|---|---|
| Zmod(10 mHz), Ω cm−2 | Θmax, deg | Rs, Ω cm−2 | Rlam, Ω cm−2 | n | Qlam, F cm−2 sn−1 | χ2 | |
| 125 °C, 0.5 h | 7.4 × 107 | −88.5 | 3.5 | 3.7 × 108 | 0.97 | 1.8 × 10−7 | 0.017 |
| 125 °C, 24 h | 6.7× 107 | −88.3 | 3.2 | 3.2 × 108 | 0.96 | 1.9× 10−7 | 0.018 |
| 250 °C, 0.5 h | 3.9× 107 | −88.4 | 4.3 | 4.7 × 108 | 0.92 | 2.9× 10−7 | 0.035 |
| 250 °C, 24 h | 3.8× 107 | −88.3 | 3.4 | 2.1 × 108 | 0.91 | 2.9× 10−7 | 0.034 |
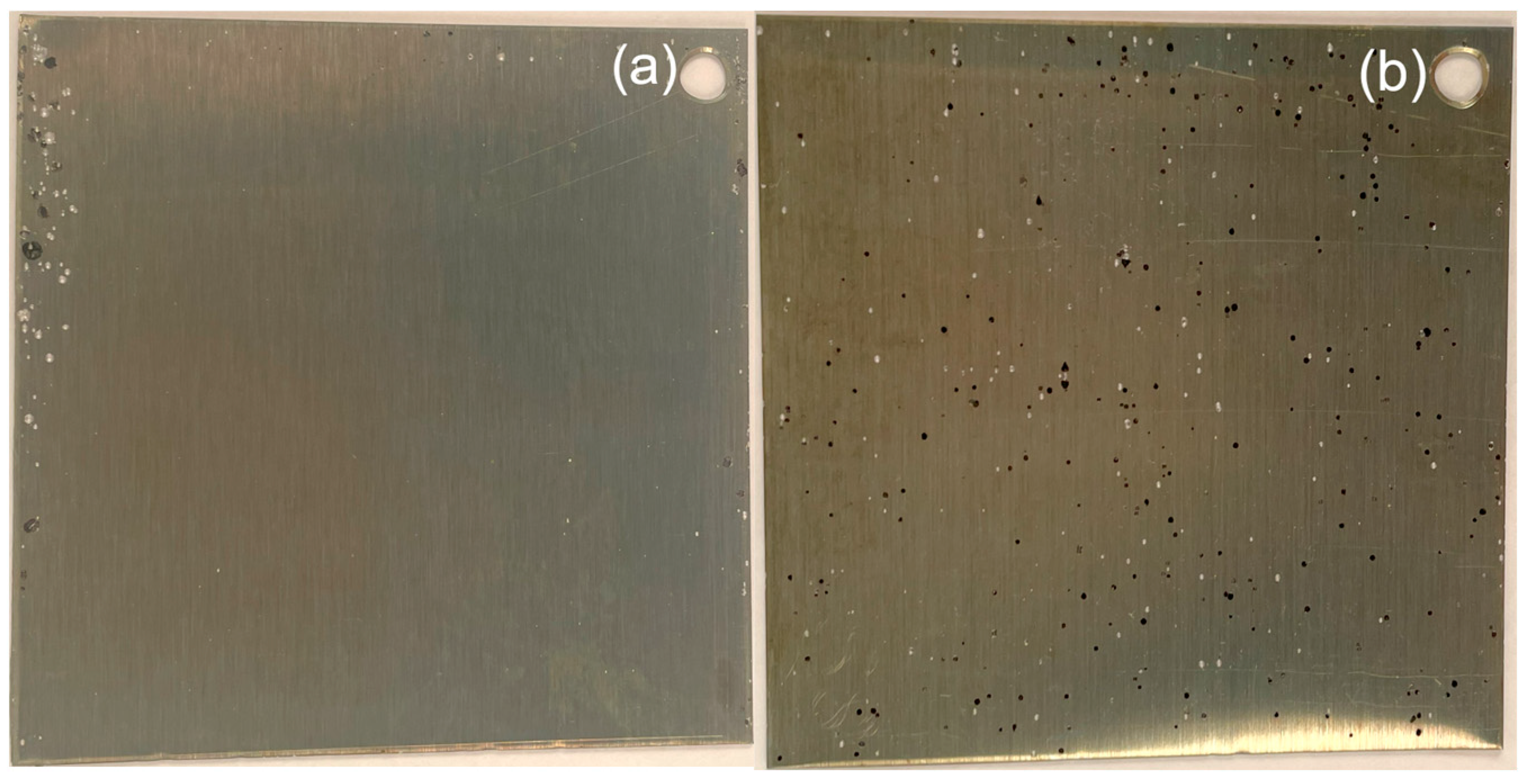
| Sample TG, °C | Number of Pits During Immersion | Final Pit Density, m−2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||||||||
| Bright Pits | Dark Pits | All Pits | Bright Pits | Dark Pits | All Pits | Bright Pits | Dark Pits | All Pits | ||
| 125 | - | 1 | 1 | 2 | - | 2 | 3 | - | 3 | 1.9 × 103 |
| 200 | 3 | 16 | 19 | 31 | 47 | 78 | 70 | 48 | 118 | 7.4 × 104 |
| 250 | 1 | 22 | 23 | 22 | 71 | 93 | 78 | 153 | 231 | 1.4 × 105 |
| 300 | 10 | 21 | 31 | 38 | 40 | 78 | 97 | 115 | 212 | 1.3 × 105 |
| Sample/Parameter | jp0, A·cm−2 | jp, A·cm−2 | P, % |
|---|---|---|---|
| SS/Laminate (125 °C) | 4.92 × 10−8 | 4.76 × 10−11 | 0.097 |
| SS/Laminate (250 °C) | 9.44 × 10−10 | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netšipailo, I.; Aarik, L.; Kozlova, J.; Tarre, A.; Merisalu, M.; Aab, K.; Mändar, H.; Ritslaid, P.; Sammelselg, V. Studies on the Chemical Etching and Corrosion Resistance of Ultrathin Laminated Alumina/Titania Coatings. Corros. Mater. Degrad. 2025, 6, 36. https://doi.org/10.3390/cmd6030036
Netšipailo I, Aarik L, Kozlova J, Tarre A, Merisalu M, Aab K, Mändar H, Ritslaid P, Sammelselg V. Studies on the Chemical Etching and Corrosion Resistance of Ultrathin Laminated Alumina/Titania Coatings. Corrosion and Materials Degradation. 2025; 6(3):36. https://doi.org/10.3390/cmd6030036
Chicago/Turabian StyleNetšipailo, Ivan, Lauri Aarik, Jekaterina Kozlova, Aivar Tarre, Maido Merisalu, Kaisa Aab, Hugo Mändar, Peeter Ritslaid, and Väino Sammelselg. 2025. "Studies on the Chemical Etching and Corrosion Resistance of Ultrathin Laminated Alumina/Titania Coatings" Corrosion and Materials Degradation 6, no. 3: 36. https://doi.org/10.3390/cmd6030036
APA StyleNetšipailo, I., Aarik, L., Kozlova, J., Tarre, A., Merisalu, M., Aab, K., Mändar, H., Ritslaid, P., & Sammelselg, V. (2025). Studies on the Chemical Etching and Corrosion Resistance of Ultrathin Laminated Alumina/Titania Coatings. Corrosion and Materials Degradation, 6(3), 36. https://doi.org/10.3390/cmd6030036








