Oxidation of HfB2-HfO2-SiC Ceramics Modified with Ti2AlC Under Subsonic Dissociated Airflow
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Test Facility
2.3. Material Investigation
3. Results and Discussion
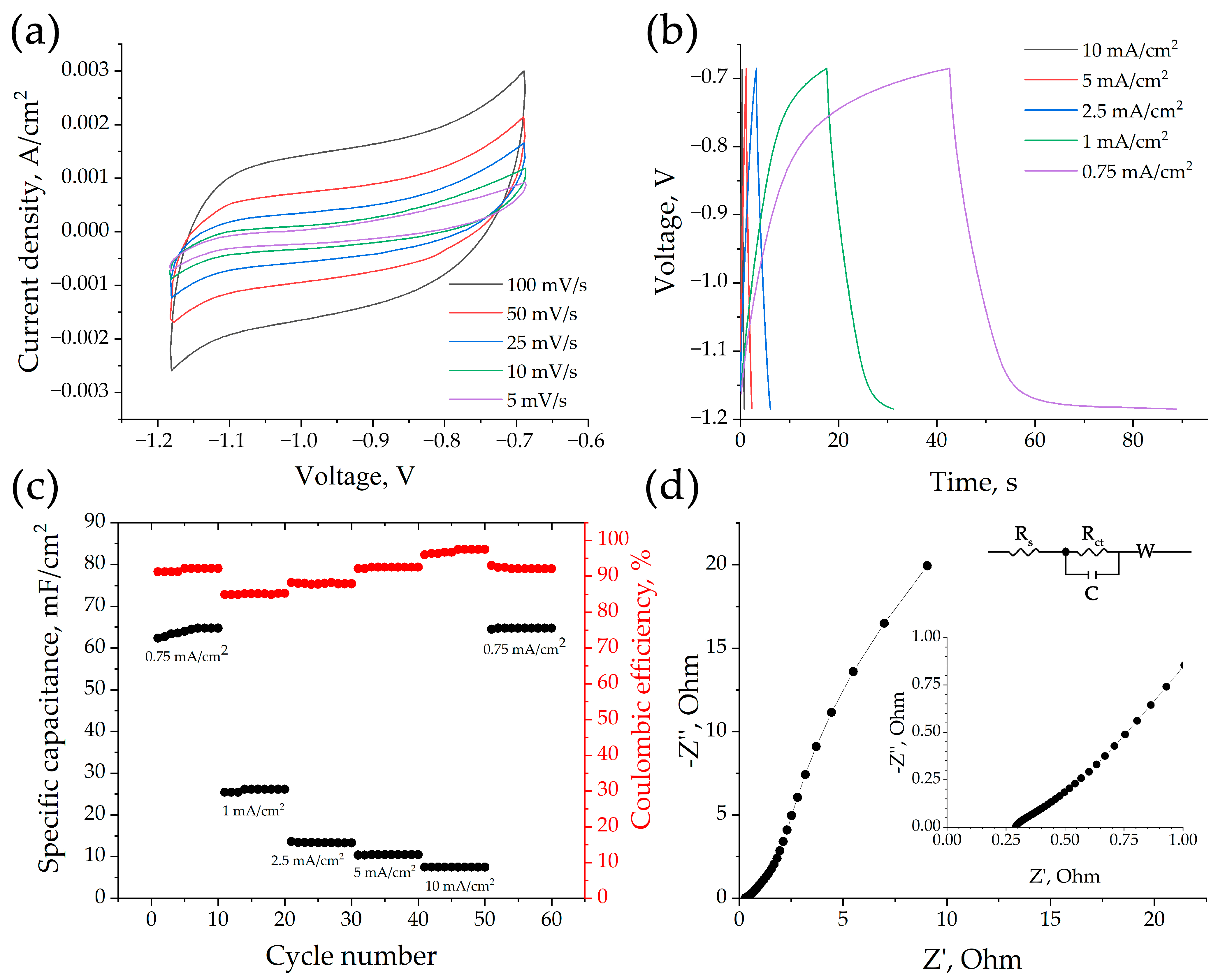
3.1. Electrochemical Properties of HfB2-HfO2-SiC-Based Sample
3.2. Exposure of the HfB2-HfO2-SiC-Based Sample Surface to Dissociated Airflows
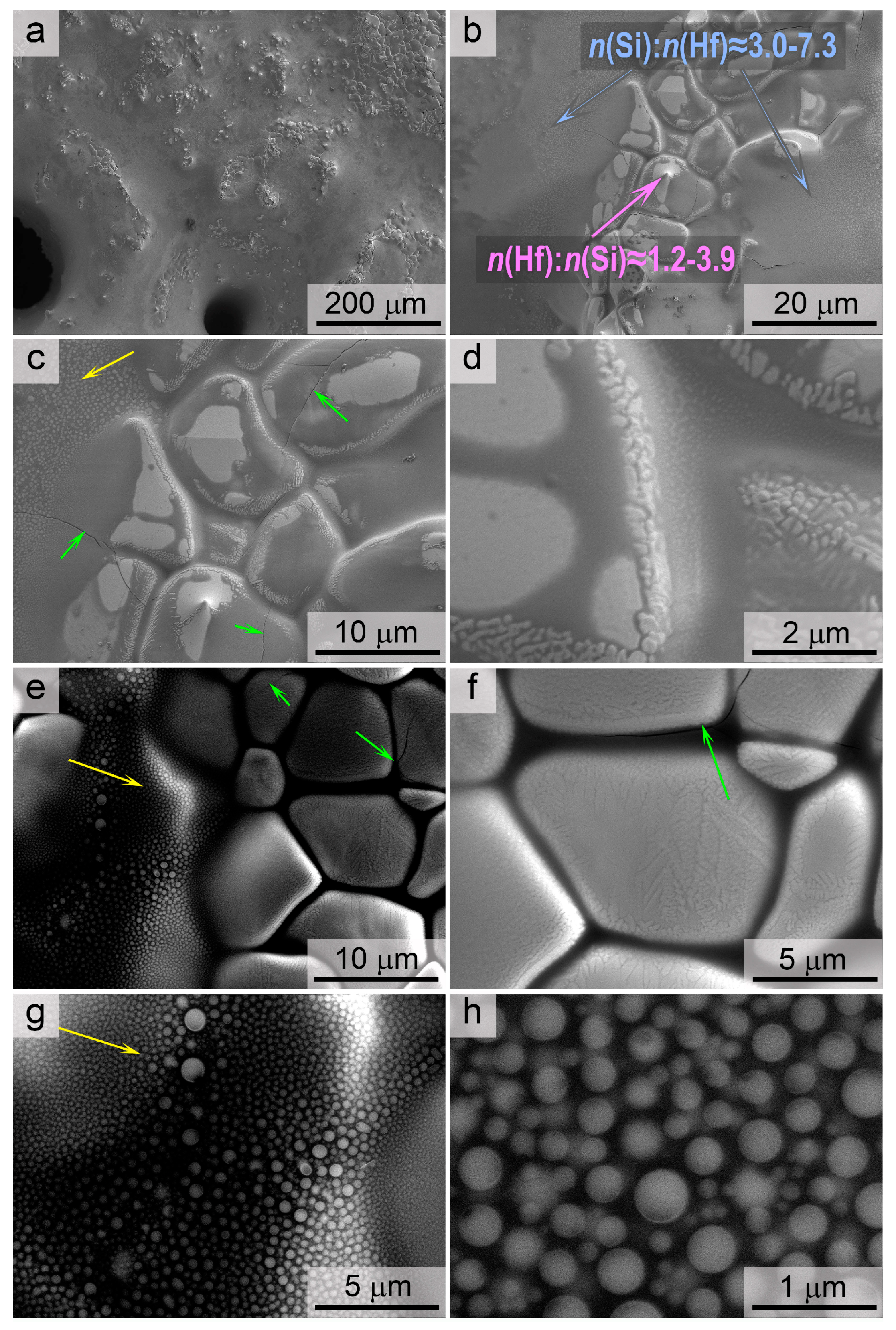
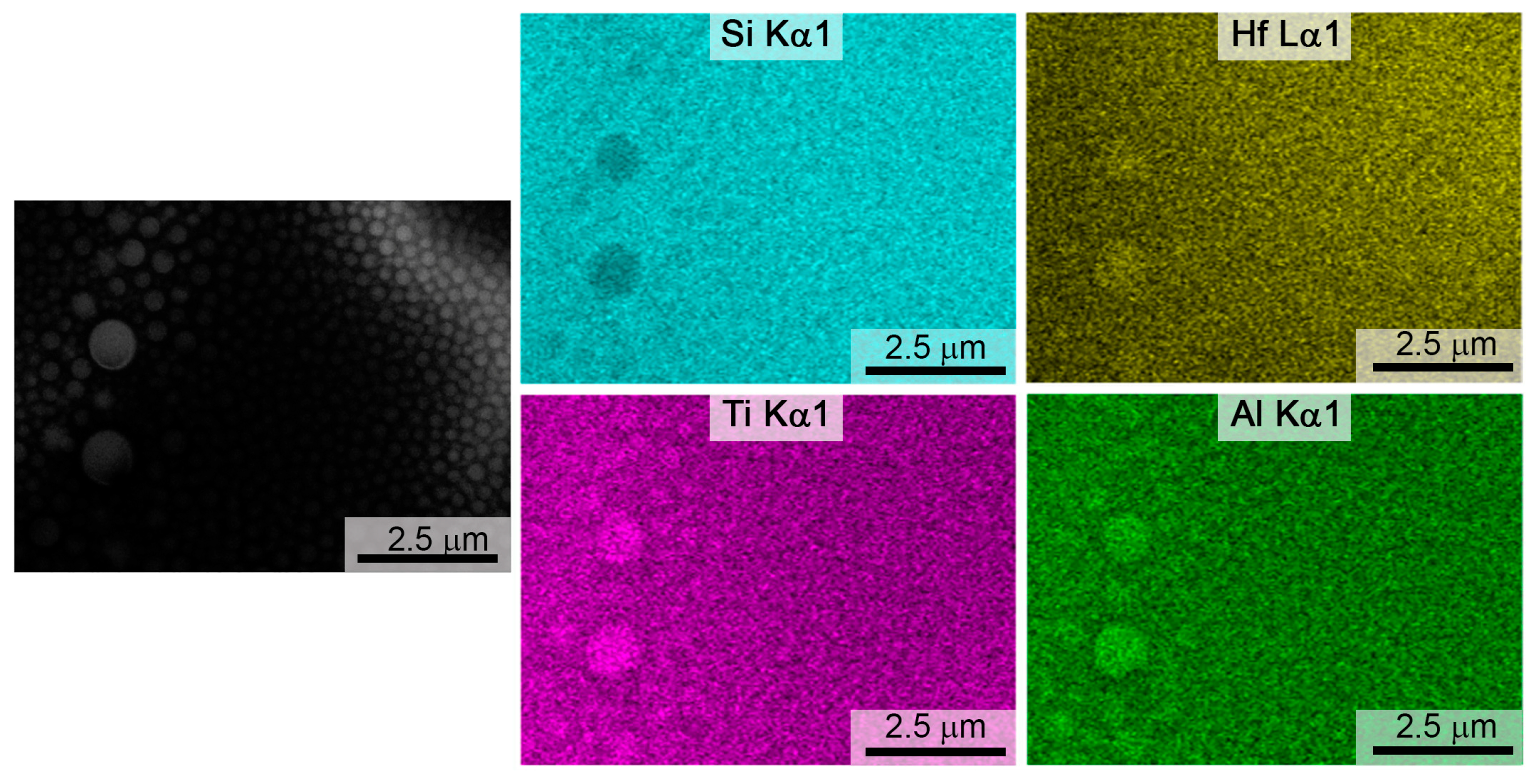
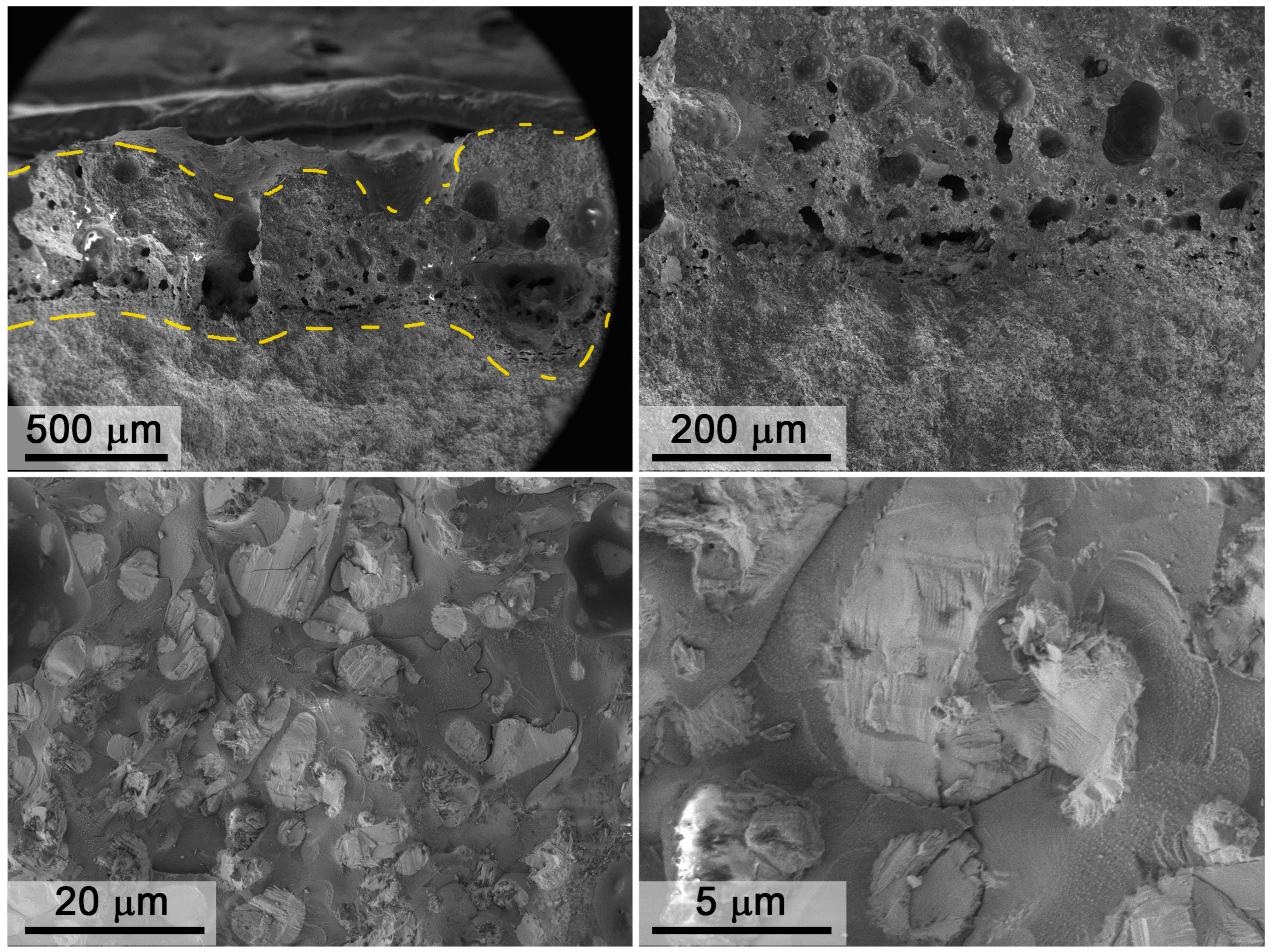
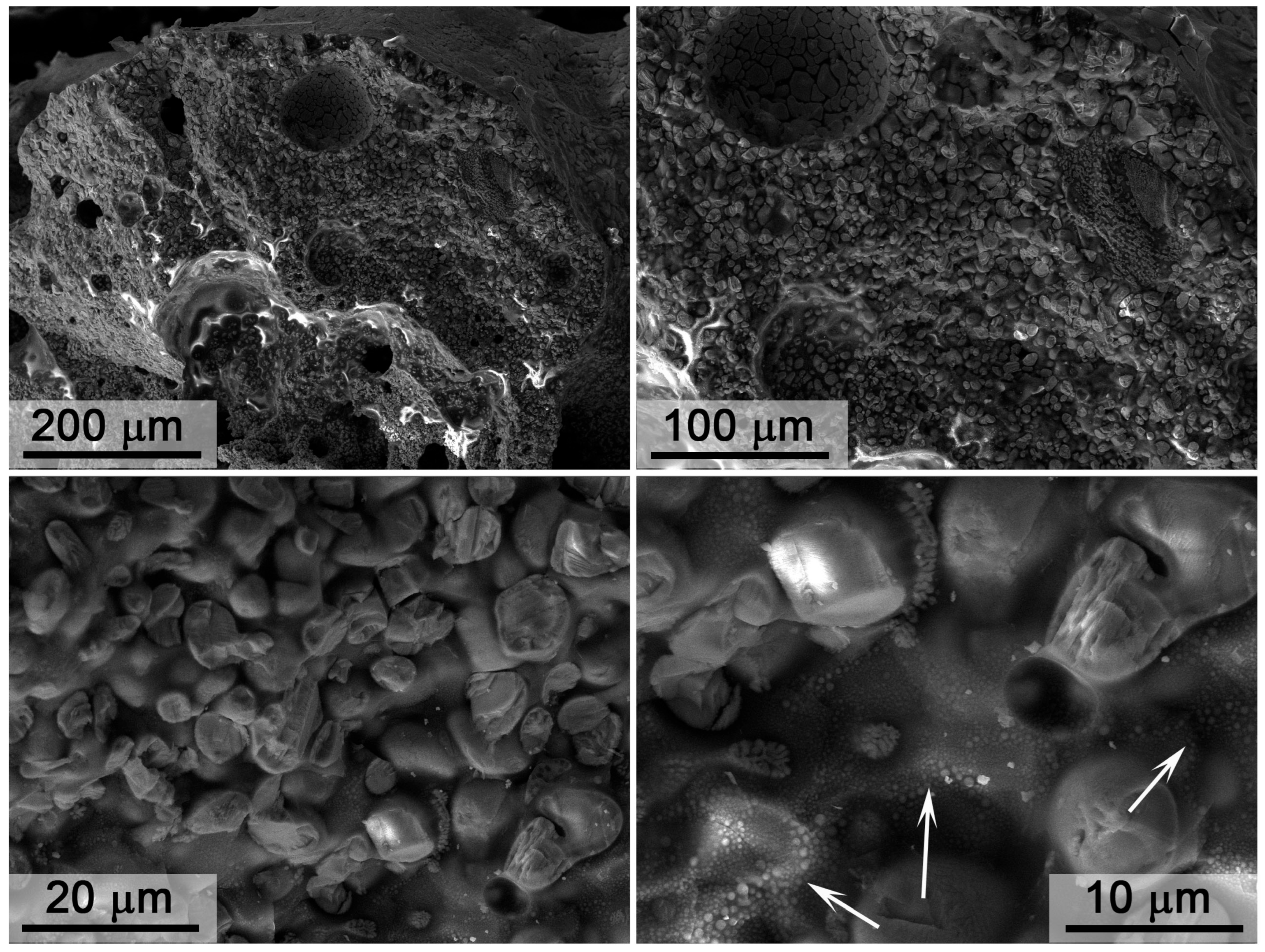
3.3. Investigation of Surface Degradation of HfB2-HfO2-SiC-Based Material After Exposure to Subsonic Flow of Dissociated Air
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonenko, E.P.; Sevast’yanov, D.V.; Simonenko, N.P.; Sevast’yanov, V.G.; Kuznetsov, N.T. Promising Ultra-High-Temperature Ceramic Materials for Aerospace Applications. Russ. J. Inorg. Chem. 2013, 58, 1669–1693. [Google Scholar] [CrossRef]
- Silvestroni, L.; Savino, R.; Cecere, A.; Mungiguerra, S. Failure Tolerant Functionally Graded Fiber-Reinforced UHTCs Exposed to Hypersonic Flows. Compos. Part A Appl. Sci. Manuf. 2024, 185, 108293. [Google Scholar] [CrossRef]
- Cecere, A.; Savino, R.; Allouis, C.; Monteverde, F. Heat Transfer in Ultra-High Temperature Advanced Ceramics under High Enthalpy Arc-Jet Conditions. Int. J. Heat Mass Transf. 2015, 91, 747–755. [Google Scholar] [CrossRef]
- Parthasarathy, T.A.; Petry, M.D.; Cinibulk, M.K.; Mathur, T.; Gruber, M.R. Thermal and Oxidation Response of UHTC Leading Edge Samples Exposed to Simulated Hypersonic Flight Conditions. J. Am. Ceram. Soc. 2013, 96, 907–915. [Google Scholar] [CrossRef]
- Squire, T.H.; Marschall, J. Material Property Requirements for Analysis and Design of UHTC Components in Hypersonic Applications. J. Eur. Ceram. Soc. 2010, 30, 2239–2251. [Google Scholar] [CrossRef]
- Gupta, K.; Murtaza, Q.; Yuvraj, N. Development of ZrB2-SiC Plasma-Sprayed Ceramic Coating for Thermo-Chemical Protection in Hypersonic Vehicles. J. Mater. Eng. Perform. 2024, 33, 1401–1410. [Google Scholar] [CrossRef]
- Li, P.; Ge, Y.; Jin, X.; Lu, P.; Hou, C.; Wang, H.; Fan, X. Cyclic Thermal Shock Behaviors of ZrB2-SiC Laminated Ceramics Sintered with Different-Sized Particles for Each Sublayer. J. Aust. Ceram. Soc. 2023, 59, 645–655. [Google Scholar] [CrossRef]
- Paksoy, A.; Yıldırım, İ.D.; Arabi, S.; Güngör, A.; Erdem, E.; Balcı-Çağıran, Ö. Enhanced Performance and Cycling Behavior in Symmetric Supercapacitors Developed by Pure HfB2 and HfB2-SiC Composites. J. Alloys Compd. 2024, 983, 173749. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Wu, Z. Uncovering the Effects of Chemical Disorder on the Irradiation Resistance of High-Entropy Carbide Ceramics. Acta Mater. 2024, 277, 120187. [Google Scholar] [CrossRef]
- Barbarossa, S.; Orrù, R.; Cao, G.; Balbo, A.; Zanotto, F.; Sani, E. Optical Properties of Bulk High-Entropy Diborides for Solar Energy Applications. J. Alloys Compd. 2023, 935, 167965. [Google Scholar] [CrossRef]
- Chen, Y. Ultra-High-Temperature Ceramic Materials Modified By Graphene: An Overview. Ceram. Silik. 2023, 67, 260–296. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sesso, M.L.; Franks, G.V. Effect of Internal Lattice Structure on the Flexural Strength of 3D Printed Hierarchical Porous Ultra-High Temperature Ceramic (ZrB2). J. Eur. Ceram. Soc. 2023, 43, 1762–1776. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, W.; Zhang, M.; Zeng, C.; Huang, Q. Enhanced Mechanical and Ablation Properties of Lamellar Porous ZrB2-SiC Ceramics by Highly Textured Pyrolytic Carbon. Corros. Sci. 2025, 250, 112882. [Google Scholar] [CrossRef]
- Zhao, K.; Ye, F.; Cheng, L.; Yang, J.; Chen, X. An Overview of Ultra-High Temperature Ceramic for Thermal Insulation: Structure and Composition Design with Thermal Conductivity Regulation. J. Eur. Ceram. Soc. 2023, 43, 7241–7262. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Kotov, M.A.; Yakimov, M.Y.; Lukomskii, I.V.; Galkin, S.S.; Shemyakin, A.N.; Solovyov, N.G.; Lysenkov, A.S.; et al. Oxidation of Ceramic Materials Based on HfB2-SiC under the Influence of Supersonic CO2 Jets and Additional Laser Heating. Int. J. Mol. Sci. 2023, 24, 13634. [Google Scholar] [CrossRef]
- Jin, H.; Meng, S.; Zhang, X.; Zeng, Q.; Niu, J. Effects of Oxidation Temperature, Time, and Ambient Pressure on the Oxidation of ZrB2–SiC–graphite Composites in Atomic Oxygen. J. Eur. Ceram. Soc. 2016, 36, 1855–1861. [Google Scholar] [CrossRef]
- Sciti, D.; Vinci, A.; Zoli, L.; Galizia, P.; Mor, M.; Fahrenholtz, W.; Mungiguerra, S.; Savino, R.; Caporale, A.M.; Airoldi, A. Elevated Temperature Performance: Arc-Jet Testing of Carbon Fiber Reinforced ZrB2 Bars up to 2200 °C for Strength Retention Assessment. J. Adv. Ceram. 2025, 14, 9221022. [Google Scholar] [CrossRef]
- Nisar, A.; Zhang, C.; Boesl, B.; Agarwal, A. Synthesis of Hf6Ta2O17 Superstructure via Spark Plasma Sintering for Improved Oxidation Resistance of Multi-Component Ultra-High Temperature Ceramics. Ceram. Int. 2023, 49, 783–791. [Google Scholar] [CrossRef]
- Meng, S.; Zeng, Q.; Jin, H.; Wang, L.; Xu, C. Evaluation of Atomic Oxygen Catalytic Coefficient of ZrB2–SiC by Laser-Induced Fluorescence up to 1473 K. Meas. Sci. Technol. 2018, 29, 075207. [Google Scholar] [CrossRef]
- Paksoy, A.; Buldu-Akturk, M.; Arabi, S.; Erdem, E.; Balcı-Çağıran, Ö. Synthesis and Capacitive Performance of ZrB2 and Its Composites as Supercapacitor Electrodes. Solid State Sci. 2023, 142, 107256. [Google Scholar] [CrossRef]
- Rezaie, A.; Fahrenholtz, W.G.; Hilmas, G.E. Effect of Hot Pressing Time and Temperature on the Microstructure and Mechanical Properties of ZrB2–SiC. J. Mater. Sci. 2007, 42, 2735–2744. [Google Scholar] [CrossRef]
- Zhu, S.; Fahrenholtz, W.G.; Hilmas, G.E. Influence of Silicon Carbide Particle Size on the Microstructure and Mechanical Properties of Zirconium Diboride–silicon Carbide Ceramics. J. Eur. Ceram. Soc. 2007, 27, 2077–2083. [Google Scholar] [CrossRef]
- Guo, S.-Q. Densification of ZrB2-Based Composites and Their Mechanical and Physical Properties: A Review. J. Eur. Ceram. Soc. 2009, 29, 995–1011. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, Y.; Yang, B.; Wang, T.; Hu, Y.; Sun, D.; Li, R.; Yin, S.; Li, J.; Feng, Z.; et al. Reactive Sintering of Tungsten-Doped High Strength ZrB2–SiC Porous Ceramics Using Metastable Precursors. Mater. Res. Bull. 2014, 51, 19–23. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Papynov, E.K.; Shichalin, O.O.; Belov, A.A.; Nagornov, I.A.; Simonenko, T.L.; Gorobtsov, P.Y.; Teplonogova, M.A.; Mokrushin, A.S.; Simonenko, N.P.; et al. Reactive Spark Plasma Sintering and Oxidation of ZrB2-SiC and ZrB2-HfB2-SiC Ceramic Materials. Ceramics 2024, 7, 1566–1583. [Google Scholar] [CrossRef]
- Nguyen, V.-Q.; Kim, J.-S.; Seo, H.; Lee, S.-H. Effects of High-Energy Ball Milling and Reactive Spark Plasma Sintering on the Densification, Microstructure, and Mechanical Properties of Ultra-Fine and X-Ray Pure ZrB2-SiC Composites. J. Eur. Ceram. Soc. 2024, 44, 116714. [Google Scholar] [CrossRef]
- Fazili, A.; Irankhah, R.; Shirani, M.; Nikzad, L.; Razavi, M.; Asadian, K. Comparison of ZrB2-SiC Composites Fabricated Through Reactive and Non-Reactive Methods. J. Mater. Eng. Perform. 2023, 32, 10728–10739. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Lysenkov, A.S.; Sevastyanov, V.G.; Kuznetsov, N.T. Reactive Hot Pressing of HfB2–SiC–Ta4HfC5 Ultra-High Temperature Ceramics. Russ. J. Inorg. Chem. 2020, 65, 446–457. [Google Scholar] [CrossRef]
- Shahedi Asl, M.; Nayebi, B.; Shokouhimehr, M. TEM Characterization of Spark Plasma Sintered ZrB2–SiC–graphene Nanocomposite. Ceram. Int. 2018, 44, 15269–15273. [Google Scholar] [CrossRef]
- Wang, H.; Lee, S.-H.; Feng, L. HfB2–SiC Composite Prepared by Reactive Spark Plasma Sintering. Ceram. Int. 2014, 40, 11009–11013. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Chaplygin, A.V.; Lukomskii, I.V.; Galkin, S.S.; Lysenkov, A.S.; Nagornov, I.A.; Mokrushin, A.S.; Kolesnikov, A.F.; Kuznetsov, N.T. Surface Degradation of Ultrahigh-Temperature Ceramics Based on HfB2–30vol%SiC in Subsonic Nitrogen Plasma Flow. Int. J. Refract. Met. Hard Mater. 2025, 130, 107139. [Google Scholar] [CrossRef]
- Mehdipour, M.; Balak, Z.; Azizieh, M.; Shahedi Asl, M. Optimization of SPS Parameters and Graphene Content with the Aim of Improving Densification and Flexural Strength of ZrB2-SiC Composites. JOM 2025, 77, 2001–2012. [Google Scholar] [CrossRef]
- Zoli, L.; Servadei, F.; Failla, S.; Mor, M.; Vinci, A.; Galizia, P.; Sciti, D. ZrB2–SiC Ceramics Toughened with Oriented Paper-Derived Graphite for a Sustainable Approach. J. Adv. Ceram. 2024, 13, 207–219. [Google Scholar] [CrossRef]
- Tripathi, S.; Bhadauria, A.; Tiwari, A.; Tiwari, A.K. Effect of Carbonaceous Reinforcements on Mechanical Properties of ZrB2-SiC Composites via Nanoindentation Study. Diam. Relat. Mater. 2023, 140, 110537. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; An, Y.; Hu, N. High Thermal-Conductivity RGO/ZrB2-SiC Ceramics Consolidated from ZrB2-SiC Particles Decorated GO Hybrid Foam with Enhanced Thermal Shock Resistance. J. Eur. Ceram. Soc. 2020, 40, 2760–2767. [Google Scholar] [CrossRef]
- He, L.; Wu, J.; Meng, Q.; Sun, Y.; Pan, J.; Tian, Y.; Wu, F. Study on Low Content Short Carbon Fibers to Enhance Mechanical Properties of ZrB2-SiC Composites with Improved Damage Tolerance. Mater. Today Commun. 2025, 42, 111391. [Google Scholar] [CrossRef]
- Gardini, D.; Backman, L.; Kaczmarek, P.; Capiani, C.; Sangiorgi, A.; Melandri, C.; Silvestroni, L. Viable Route to the Manufacture of Short Carbon Fiber-Rich UHTC Complex Shapes with Enhanced Toughness. Compos. Part B Eng. 2024, 277, 111373. [Google Scholar] [CrossRef]
- Murthy, S.S.N.; Patel, M.; Panigrahi, B.B. Processing of Continuous Carbon Fibre Reinforced ZrB2-SiC Composite Through In-Situ Matrix Development. Trans. Indian Inst. Met. 2024, 77, 2461–2469. [Google Scholar] [CrossRef]
- Gupta, N.; Mukhopadhyay, A.; Pavani, K.; Basu, B. Spark Plasma Sintering of Novel ZrB2–SiC–TiSi2 Composites with Better Mechanical Properties. Mater. Sci. Eng. A 2012, 534, 111–118. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Shahedi Asl, M.; Zakeri, M.; Farvizi, M. On the Reactive Spark Plasma Sinterability of ZrB2–SiC–TiN Composite. J. Alloys Compd. 2022, 909, 164611. [Google Scholar] [CrossRef]
- Xia, C.; Delbari, S.A.; Ahmadi, Z.; Shahedi Asl, M.; Ghassemi Kakroudi, M.; Van Le, Q.; Sabahi Namini, A.; Mohammadi, M.; Shokouhimehr, M. Electron Microscopy Study of ZrB2–SiC–AlN Composites: Hot-Pressing vs. Pressureless Sintering. Ceram. Int. 2020, 46, 29334–29338. [Google Scholar] [CrossRef]
- Nasiri, Z.; Mashhadi, M. Microstructure and Mechanical Behavior of Ternary Phase ZrB2-SiC-AlN Nanocomposite. Int. J. Refract. Met. Hard Mater. 2019, 78, 186–192. [Google Scholar] [CrossRef]
- Sengupta, P.; Manna, I. Role of TiC and WC Addition on the Mechanism and Kinetics of Isothermal Oxidation and High-Temperature Stability of ZrB2–SiC Composites. High Temp. Corros. Mater. 2024, 101, 57–83. [Google Scholar] [CrossRef]
- Duan, X.; Wei, C.; Geng, X.; Meng, F.; Zhou, L.; Wang, P.; Dong, Z.; Dong, S. Effects of Adding Si3N4 Whiskers to ZrB2–SiC Ceramics on Microstructure, Mechanical Properties and Oxidation Resistance. Ceram. Int. 2024, 50, 43153–43164. [Google Scholar] [CrossRef]
- Sarhangian, M.; Mashhadi, M. The Impact of Si3N4 Incorporation on the Mechanical Characteristics of ZrB2–SiC Nanocomposite Sintered via Pressureless Method. Heliyon 2024, 10, e33269. [Google Scholar] [CrossRef]
- Wei, C.; Liu, L.; Sun, M.; Wang, P.; Meng, F.; Chen, D.; Han, Q.; Yu, Z. Ablation Performance and Mechanism of Fibrous Monolithic ZrB2-SiC Ceramics Prepared by Wet-Spinning Co-Extrusion Method under Plasma Flame. Ceram. Int. 2024, 50, 11548–11556. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, D.; Fu, Q. Ablation Resistant Behavior of Silicide Modified HfB2-SiC Coating on Graphite by Spark Plasma Sintering: Role of MeSi2 Addition. J. Eur. Ceram. Soc. 2024, 44, 6286–6297. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Nagornov, I.A.; Lysenkov, A.S.; Papynov, E.K.; Shichalin, O.O.; Belov, A.A.; Kolodeznikov, E.S.; Mokrushin, A.S.; Simonenko, N.P.; Kuznetsov, N.T. Effect of Ti2AlC on Sintering of Ultrahigh-Temperature Ceramics Based on HfB2–HfO2–SiC System. Russ. J. Inorg. Chem. 2024, 69, 2151–2163. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Nagornov, I.A.; Mokrushin, A.S.; Sapronova, V.M.; Gorobtsov, P.Y.; Simonenko, N.P.; Kuznetsov, N.T. Synthesis of Ti2AlC in KBr Melt: Effect of Temperature and Component Ratio. Russ. J. Inorg. Chem. 2024, 69, 1744–1753. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Nagornov, I.A.; Mokrushin, A.S.; Kashevsky, S.V.; Gorban, Y.M.; Simonenko, T.L.; Simonenko, N.P.; Kuznetsov, N.T. Low Temperature Chemoresistive Oxygen Sensors Based on Titanium-Containing Ti2CTx and Ti3C2Tx MXenes. Materials 2023, 16, 4506. [Google Scholar] [CrossRef]
- Chaplygin, A.V.; Vasil’evskii, S.A.; Galkin, S.S.; Kolesnikov, A.F. Thermal State of Uncooled Quartz Discharge Channel of Powerful High-Frequency Induction Plasmatron. Phys. Kinet. Gas Dyn. 2022, 23, 38–56. [Google Scholar] [CrossRef]
- Gordeev, A. Overview of Characteristics and Experiments in IPM Plasmatrons. VKI RTO AVT/VKI Spec. Course Meas. Tech. High Enthalpy Plasma Flows1999, RTO EN-8, Belgium. Available online: https://apps.dtic.mil/sti/citations/ADP010736 (accessed on 1 June 2025).
- ASTM E457-08(2015); Standard Test Method for Measuring Heat-Transfer Rate Using a Thermal Capacitance. ASTM International: West Conshohocken, PA, USA. Available online: http://www.astm.org (accessed on 1 June 2025).
- Li, X.; Wei, Z.; Zu, Y.; Chen, G.; Fu, X.; Zhou, W. Phase Composition and Electrochemical Properties of (Hf0.2Zr0.2Ta0.2Mo0.2Ti0.2)B2 High-Entropy Ceramics. J. Ceram. 2023, 44, 688–694. [Google Scholar] [CrossRef]
- Khayyam Nekouei, R.S.; Mofarah, S.; Maroufi, S.; Tudela, I.; Sahajwalla, V. Determination of the Optimum Potential Window for Super- and Pseudocapacitance Electrodes via in-Depth Electrochemical Impedance Spectroscopy Analysis. J. Energy Storage 2022, 56, 106137. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Gordeev, A.N.; Kolesnikov, A.F.; Lysenkov, A.S.; Nagornov, I.A.; Sevastyanov, V.G.; Kuznetsov, N.T. The Effects of Subsonic and Supersonic Dissociated Air Flow on the Surface of Ultra-High-Temperature HfB2-30 Vol% SiC Ceramics Obtained Using the Sol-Gel Method. J. Eur. Ceram. Soc. 2020, 40, 1093–1102. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Gordeev, A.N.; Kolesnikov, A.F.; Sevastyanov, V.G.; Kuznetsov, N.T. Impact of a Subsonic Dissociated Air Flow on the Surface of HfB2–30 Vol% SiC UHTC Produced by the Sol–Gel Method. Russ. J. Inorg. Chem. 2018, 63, 1345–1355. [Google Scholar] [CrossRef]
- Sevastyanov, V.G.; Simonenko, E.P.; Gordeev, A.N.; Simonenko, N.P.; Kolesnikov, A.F.; Papynov, E.K.; Shichalin, O.O.; Avramenko, V.A.; Kuznetsov, N.T. HfB2-SiC (10–20 Vol%) Ceramic Materials: Manufacture and Behavior under Long-Term Exposure to Dissociated Air Streams. Russ. J. Inorg. Chem. 2014, 59, 1361–1382. [Google Scholar] [CrossRef]
- Marschall, J.; Pejakovic, D.; Fahrenholtz, W.G.; Hilmas, G.E.; Panerai, F.; Chazot, O. Temperature Jump Phenomenon during Plasmatron Testing of ZrB2-SiC Ultrahigh-Temperature Ceramics. J. Thermophys. Heat Transf. 2012, 26, 559–572. [Google Scholar] [CrossRef]
- Ruh, R.; Corfield, P.W.R. Crystal Structure of Monoclinic Hafnia and Comparison with Monoclinic Zirconia. J. Am. Ceram. Soc. 1970, 53, 126–129. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Fluorite Structure. In Crystal Structures, 2nd ed.; Interscience Publishers: New York, NY, USA, 1963; pp. 239–444. [Google Scholar]
- Holleck, H. Legierungsverhalten von HfB2 Mit Uran- Und Übergangsmetalldiboriden. J. Nucl. Mater. 1967, 21, 14–20. [Google Scholar] [CrossRef]
- Shulishova, O.I.; Shcherbak, I.A. Superconductivity of the Borides of Transition and Rare-Earth Metals. Inorg. Mater. 1967, 3, 1304–1306. [Google Scholar]
- Aigner, K.; Lengauer, W.; Rafaja, D.; Ettmayer, P. Lattice Parameters and Thermal Expansion of Ti(CxN1−x), Zr(CxN1−x), Hf(CxN1−x) and TiN1−x from 298 to 1473 K as Investigated by High-Temperature X-Ray Diffraction. J. Alloys Compd. 1994, 215, 121–126. [Google Scholar] [CrossRef]
- Declémy, A.; Oliviero, E.; Beaufort, M.F.; Barbot, J.F.; David, M.L.; Blanchard, C.; Tessier, Y.; Ntsoenzok, E. An IR-Reflectivity and X-Ray Diffraction Study of High Energy He-Ion Implantation-Induced Damage in 4H–SiC. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2002, 186, 318–323. [Google Scholar] [CrossRef]
- Yoshida, S.; Hijikata, Y.; Yaguchi, H. Nondestructive and Contactless Characterization Method for Spatial Mapping of the Thickness and Electrical Properties in Homo-Epitaxially Grown SiC Epilayers Using Infrared Reflectance Spectroscopy. In Physics and Technology of Silicon Carbide Devices; InTech: Daejeon, Korea, 2012. [Google Scholar]
- Hofmeister, A.M.; Pitman, K.M.; Goncharov, A.F.; Speck, A.K. Optical Constamts of Silicon Carbide for Astrophysical Applications. II. Extending Optical Functions from Infrared to Ultraviolet Using Single-Crystal Absorptionspectra. Astrophys. J. 2009, 696, 1502–1516. [Google Scholar] [CrossRef]
- Pavlikov, A.V.; Latukhina, N.V.; Chepurnov, V.I.; Timoshenko, V.Y. Structural and Optical Properties of Silicon-Carbide Nanowires Produced by the High-Temperature Carbonization of Silicon Nanostructures. Semiconductors 2017, 51, 402–406. [Google Scholar] [CrossRef]
- Shcherbakov, I.P.; Narykova, M.V.; Chmel, A.E. Evolution of Structural Defects of SiO2/TiO2 Glasses with a Change in TiO2 Concentration from Zero to Phase Separation. Phys. Solid State 2023, 65, 588. [Google Scholar] [CrossRef]
- Kim, H.J.; Roh, D.K.; Chang, J.H.; Kim, D.-S. SiO2 Coated Platy TiO2 Designed for Noble UV/IR-Shielding Materials. Ceram. Int. 2019, 45, 16880–16885. [Google Scholar] [CrossRef]
- Bechelany, M.; Brioude, A.; Cornu, D.; Ferro, G.; Miele, P. A Raman Spectroscopy Study of Individual SiC Nanowires. Adv. Funct. Mater. 2007, 17, 939–943. [Google Scholar] [CrossRef]
- Quintard, P.E.; Barbéris, P.; Mirgorodsky, A.P.; Merle-Méjean, T. Comparative Lattice-Dynamical Study of the Raman Spectra of Monoclinic and Tetragonal Phases of Zirconia and Hafnia. J. Am. Ceram. Soc. 2002, 85, 1745–1749. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kürti, J.; Koltai, J.; Kavan, L. Raman Spectra of Titanium Dioxide (Anatase, Rutile) with Identified Oxygen Isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 2012, 14, 14567. [Google Scholar] [CrossRef]
- Öhman, S.; Qiu, R.; Edvinsson, T.; Bäcke, O.; Törndahl, T.; Boman, M. Selective Kinetic Growth and Role of Local Coordination in Forming Al2TiO5 Based Coatings at Lower Temperatures. Mater. Adv. 2021, 2, 5737–5751. [Google Scholar] [CrossRef]

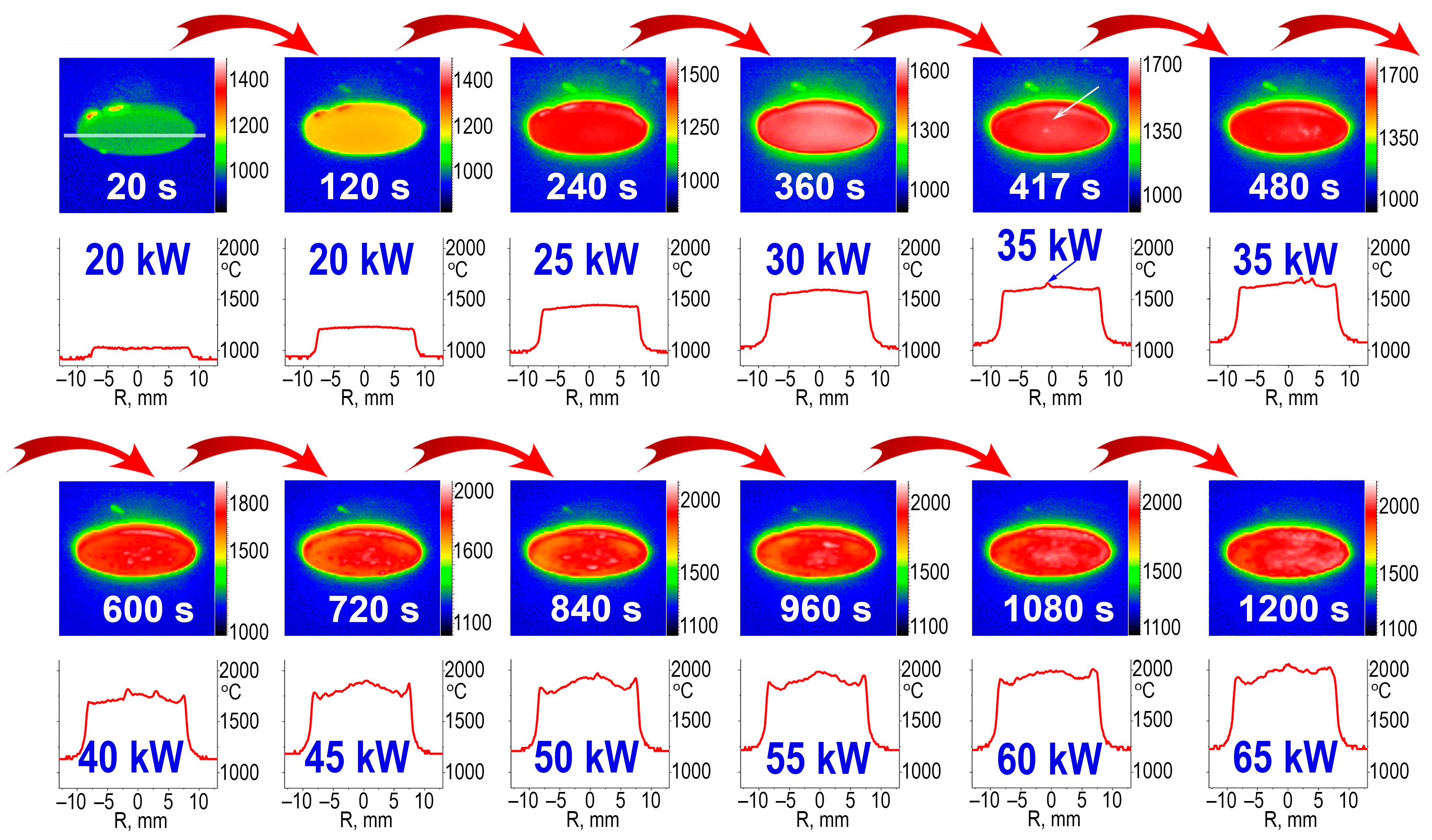



| N, kW | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 55 | 60 | 65 |
| q, W/cm2 | 92 | 125 | 158 | 186 | 201 | 222 | 247 | 266 | 274 | 297 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonenko, E.P.; Chaplygin, A.V.; Simonenko, N.P.; Lukomskii, I.V.; Galkin, S.S.; Lysenkov, A.S.; Nagornov, I.A.; Mokrushin, A.S.; Simonenko, T.L.; Kolesnikov, A.F.; et al. Oxidation of HfB2-HfO2-SiC Ceramics Modified with Ti2AlC Under Subsonic Dissociated Airflow. Corros. Mater. Degrad. 2025, 6, 35. https://doi.org/10.3390/cmd6030035
Simonenko EP, Chaplygin AV, Simonenko NP, Lukomskii IV, Galkin SS, Lysenkov AS, Nagornov IA, Mokrushin AS, Simonenko TL, Kolesnikov AF, et al. Oxidation of HfB2-HfO2-SiC Ceramics Modified with Ti2AlC Under Subsonic Dissociated Airflow. Corrosion and Materials Degradation. 2025; 6(3):35. https://doi.org/10.3390/cmd6030035
Chicago/Turabian StyleSimonenko, Elizaveta P., Aleksey V. Chaplygin, Nikolay P. Simonenko, Ilya V. Lukomskii, Semen S. Galkin, Anton S. Lysenkov, Ilya A. Nagornov, Artem S. Mokrushin, Tatiana L. Simonenko, Anatoly F. Kolesnikov, and et al. 2025. "Oxidation of HfB2-HfO2-SiC Ceramics Modified with Ti2AlC Under Subsonic Dissociated Airflow" Corrosion and Materials Degradation 6, no. 3: 35. https://doi.org/10.3390/cmd6030035
APA StyleSimonenko, E. P., Chaplygin, A. V., Simonenko, N. P., Lukomskii, I. V., Galkin, S. S., Lysenkov, A. S., Nagornov, I. A., Mokrushin, A. S., Simonenko, T. L., Kolesnikov, A. F., & Kuznetsov, N. T. (2025). Oxidation of HfB2-HfO2-SiC Ceramics Modified with Ti2AlC Under Subsonic Dissociated Airflow. Corrosion and Materials Degradation, 6(3), 35. https://doi.org/10.3390/cmd6030035







