A Review of the Effect of Irradiation on the Corrosion of Copper-Coated Used Fuel Containers
Abstract
1. Introduction
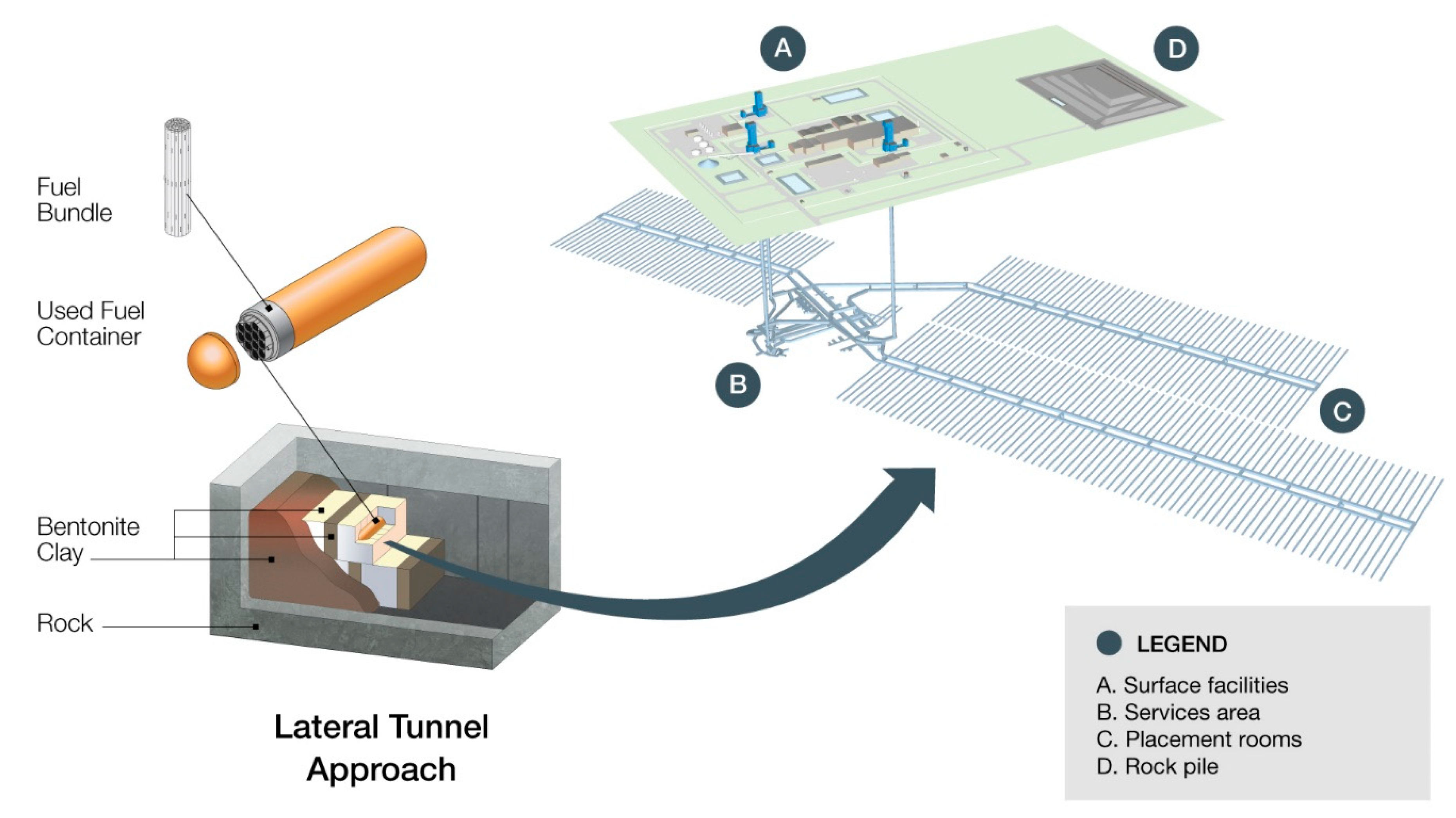
2. Nature of the Irradiation Environment
2.1. Magnitude and Duration of the Period of Irradiation
2.2. Evolution of Near-Field Environment
- Redox transient due to initially trapped O2: weeks to months following emplacement of the buffer boxes and the sealing of the disposal tunnels.
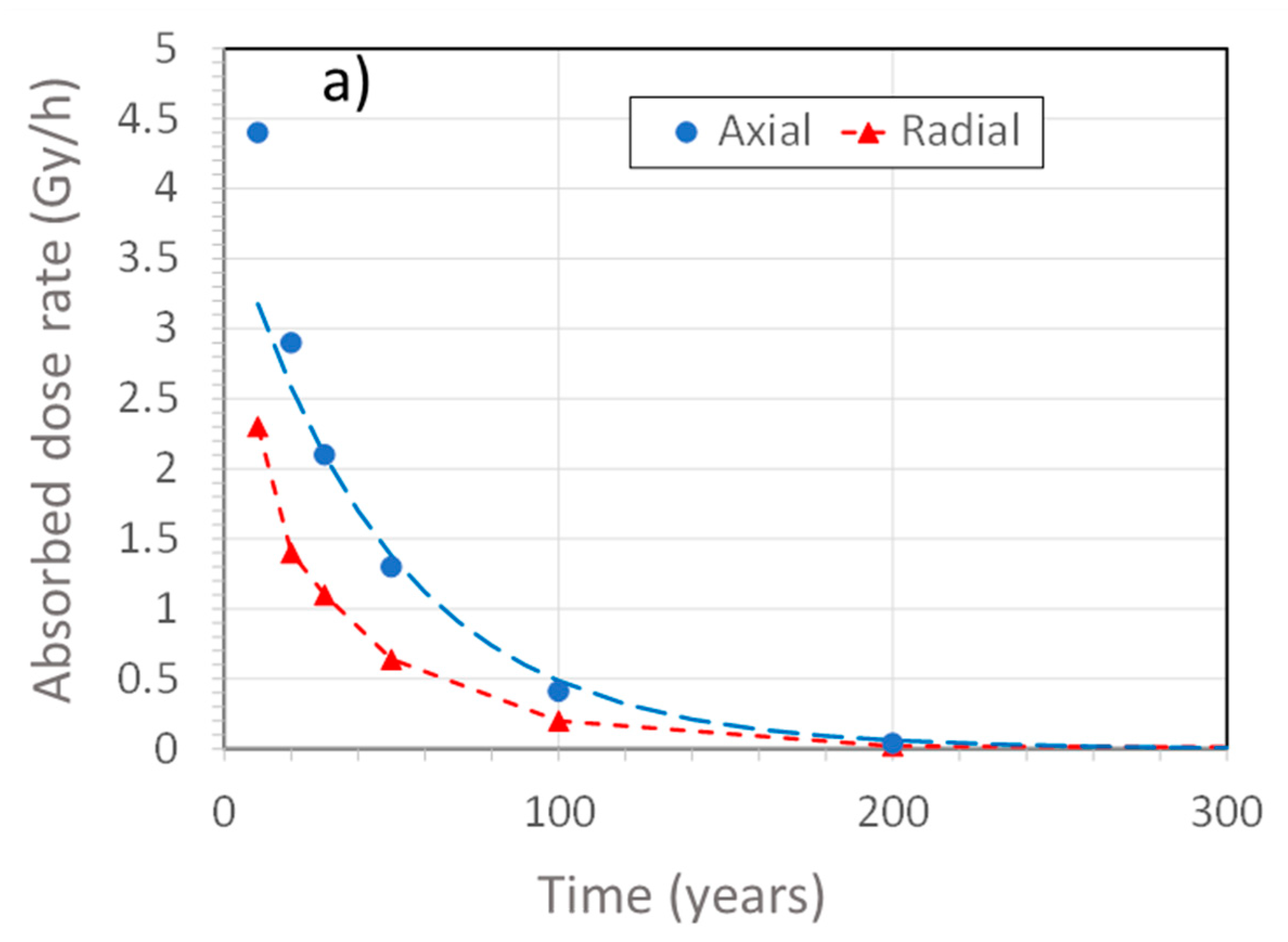
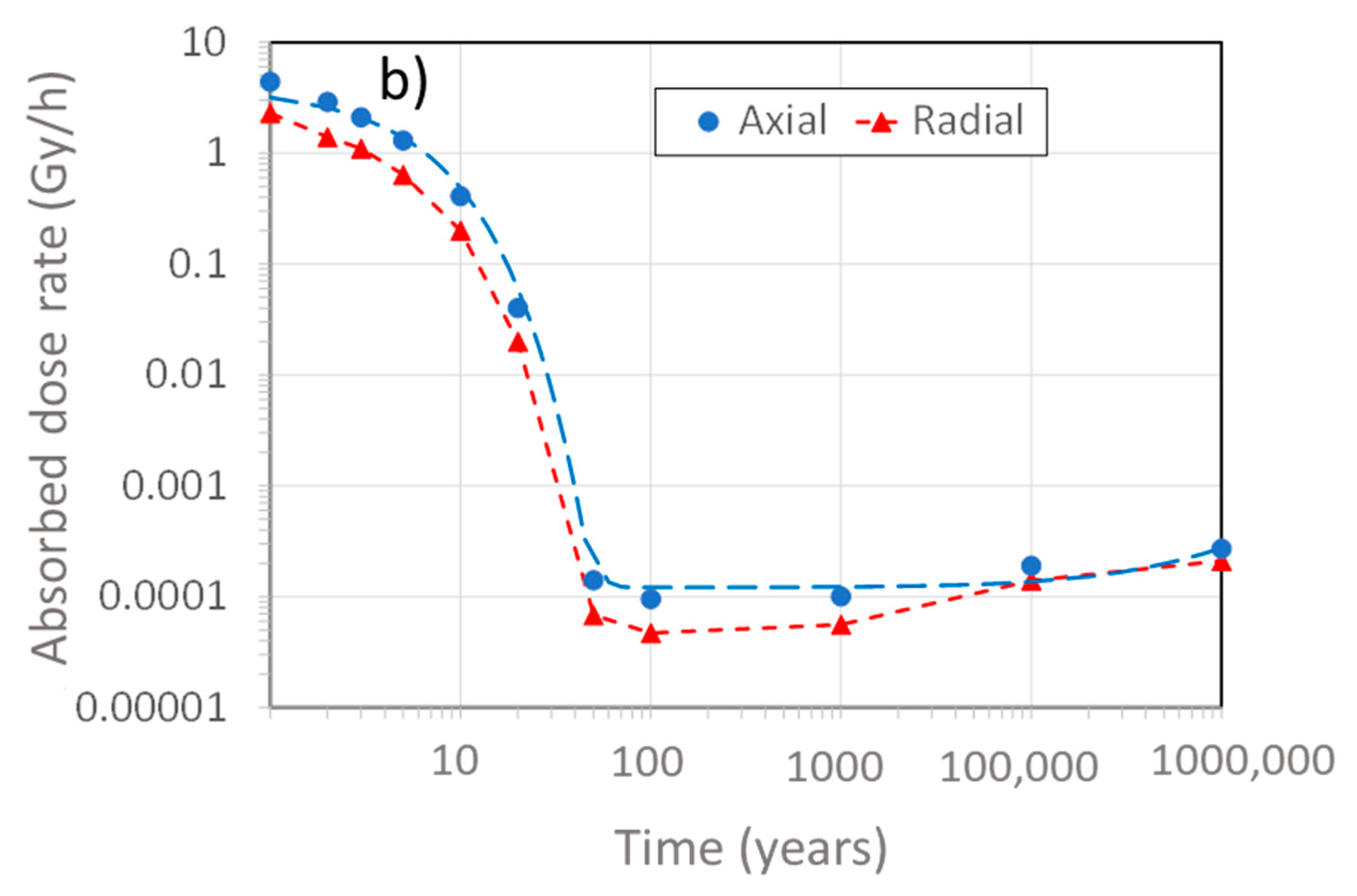
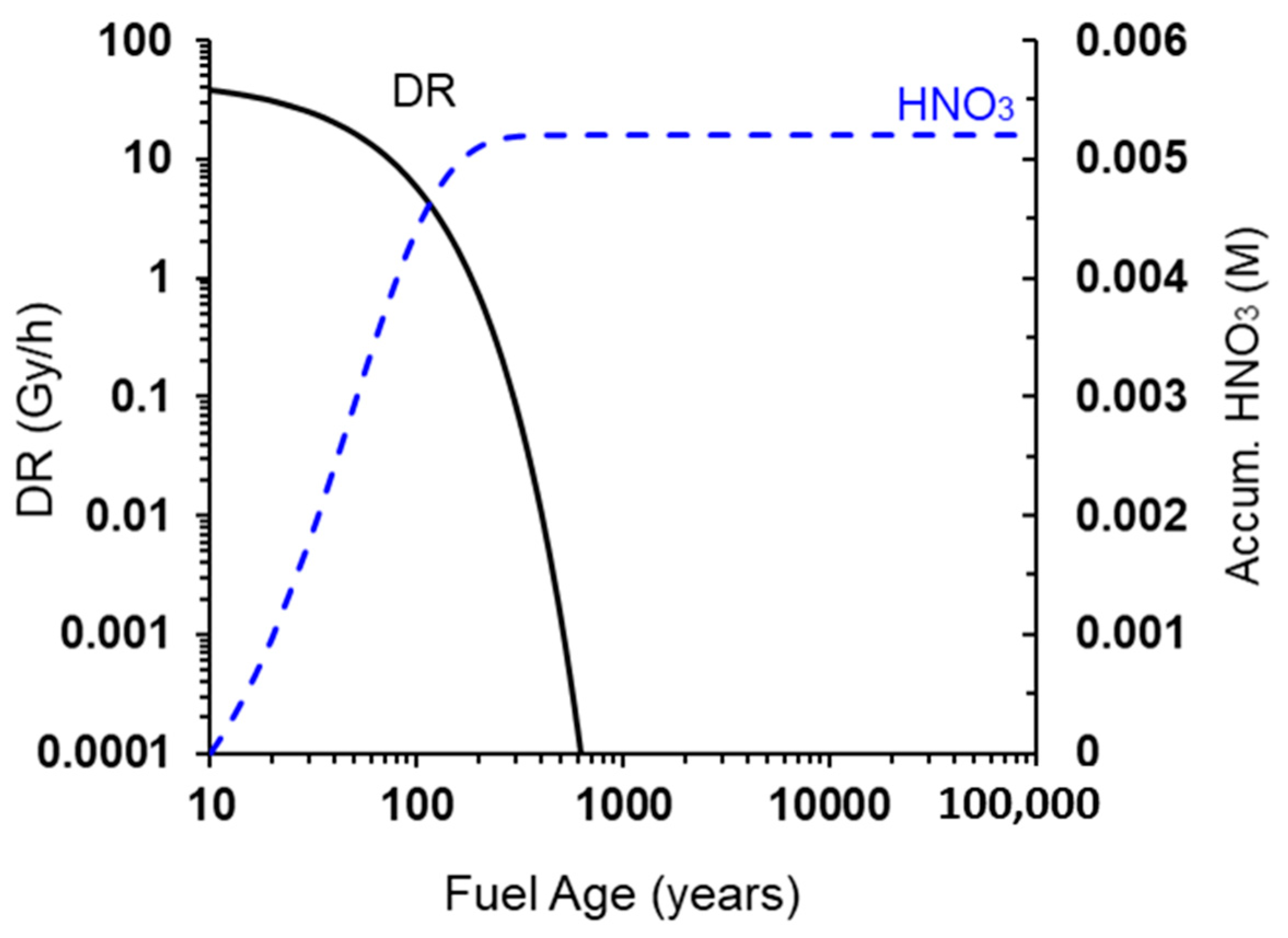
- Redox transient due to generation of radiolysis products (defined by the time for the external γ-dose rate to decrease to <0.1 Gy/h): approximately 150 years (Figure 2).
- Saturation transient: a few decades to tens of thousands of years, depending upon the local hydraulic conductivity and the properties of the host rock [12]
- Thermal transient (defined by the time for the UFC surface temperature to decrease to 30–50 °C): approximately 10,000 years [13].
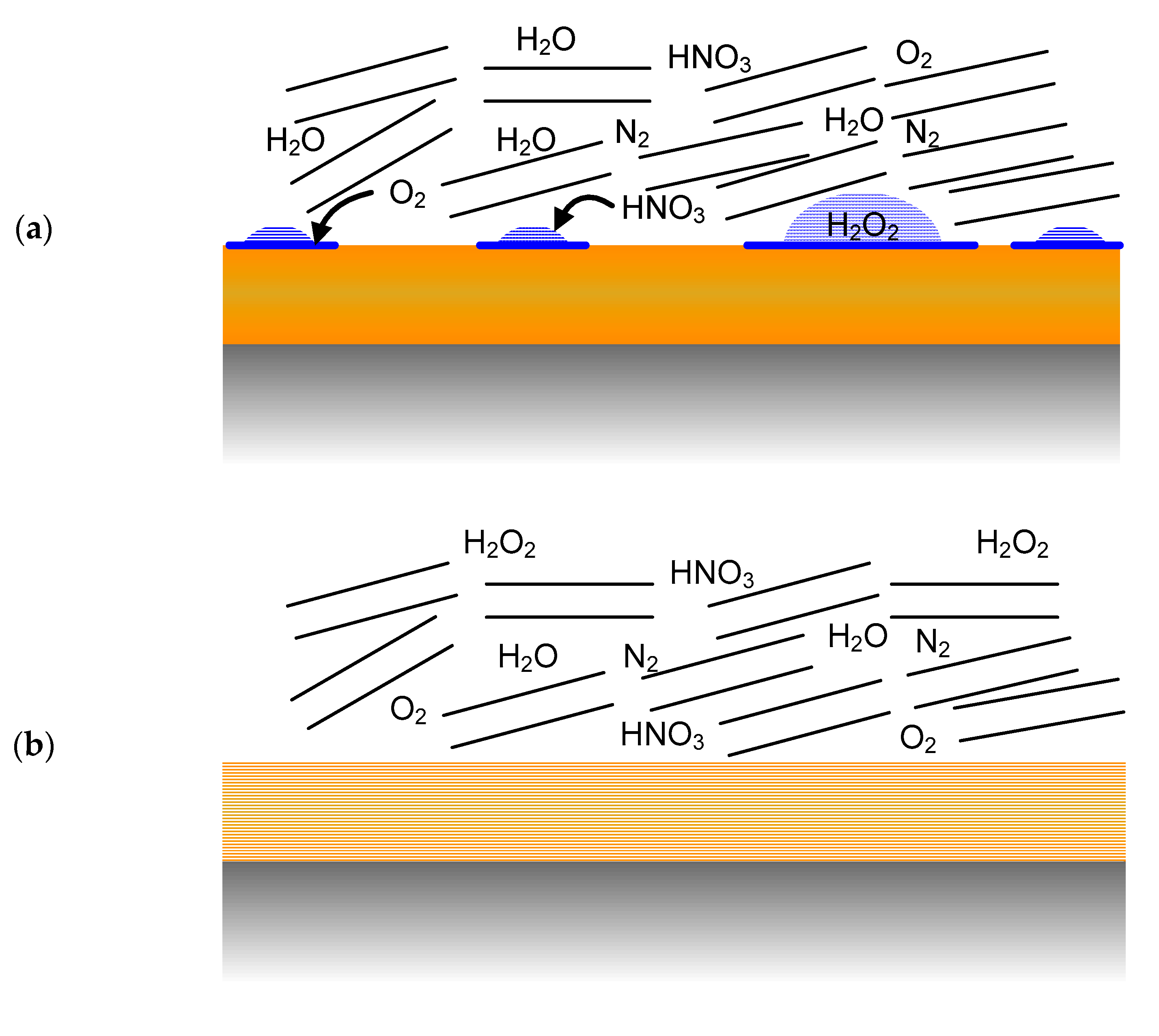
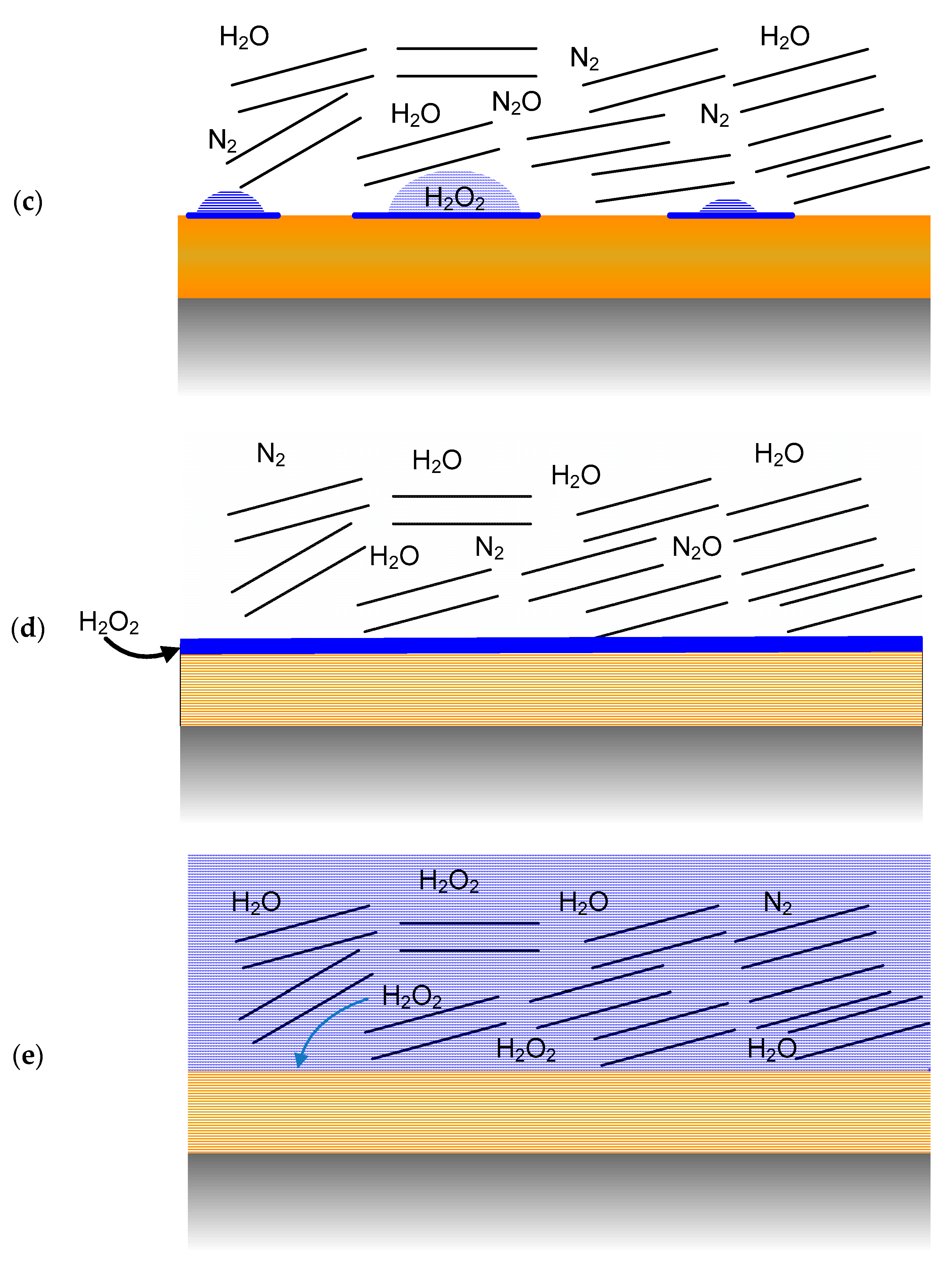
- Immediately following emplacement of the UFC in the buffer box, the container surface may be wetted (most likely by a discontinuous moisture film) as a result of the high relative humidity (RH) resulting from the moisture added to the dry bentonite to aid compaction of the shaped buffer blocks (Figure 4a).
- The UFC surface is expected to dry out rapidly as the initial moisture is driven away from the container surface by the thermal gradient. At this point (Figure 4b), the dry UFC surface would be in contact with a humid air atmosphere with residual trapped O2 and N2, as well as radiolysis products such as HNO3 and trace amounts of H2O2.
- After the peak in the container temperature (approximately 10 years post-emplacement), moisture will move back towards the UFC and re-wetting of the surface will occur, most likely non-uniformly at first (Figure 4c). By this time, however, the initially trapped O2 will have been consumed and the wetted container will be contacted by a humid N2-containing atmosphere. Species such as H2O2 and HNO3 may be present in water droplets, which may also contain species such as sodium chloride or other salt solutions (e.g., calcite and gypsum), as these compounds may initiate droplet formation by deliquescence.
- As saturation continues, the surface will become uniformly wetted as the near-field RH approaches 90–100% (Figure 4d). As noted above, the timing and duration of these two stages depends largely on the hydraulic properties of the host rock.
- Eventually, the near-field will completely saturate (Figure 4e) and the UFC will be exposed to the evolving bentonite pore-water chemistry, which will eventually equilibrate with the local ground water.
2.3. Humid Air and Aqueous-Phase Radiolysis Models
2.4. Other Irradiation Considerations
- (a)
- interfacial energy transfer at the clay/H2O interface involving either trapped excitons or ejected holes or electrons, and
- (b)
- energy absorption in the solution in larger pores, similar in nature to the radiolysis of bulk water.
3. Effect of Irradiation on the Corrosion Behaviour of Copper
3.1. γ-Radiation Effects in Humid Air
- irradiation in humid air (either 60% or 100% RH) increases the extent of corrosion by a factor of 7–9 compared with unirradiated conditions (dose rate 500 Gy/h, total dose of 48 kGy),
- irradiation in humid Ar (100% RH) increases the extent of corrosion by a factor of 8 compared with unirradiated conditions,
- since the enhancement factor in humid Ar is the same as in humid air, Björkbacka et al. [20] concluded that radiolytically produced HNO3 does not contribute to corrosion,
- irradiation under unsaturated conditions (either humid air tests or humid Ar) results in four-to-five times more corrosion than irradiation under saturated conditions,
- irradiation in “dry” air or “dry” Ar produced no corrosion, although the RH corresponding to “dry” conditions was not defined.
- At repository-relevant dose rates, there is little impact of irradiation on the corrosion rate of copper, even over extended timescales.
- At higher dose rates of the order of 100’s to 1000’s Gy/h, there is evidence for enhanced corrosion due to the formation of radiolysis products, with both HNO3 and H2O2 as possible radiolytic oxidants.
- There is some evidence that RIC does not occur in “dry” atmospheres (cf. Figure 4b), although the threshold RH above which there is a sufficiently thick water layer to support the electrochemically based radiation-induced corrosion reactions has not been defined.
- Modelling studies of the absorption of gaseous HNO3 by water droplets on the UFC surface (cf. Figure 4a) suggest a maximum depth of corrosion of <10 μm.
3.2. γ-Radiation Effects under Saturated Conditions
- the total amount of corrosion (as both precipitated oxide and dissolved in solution) far exceeded that predicted based on a coupled homogeneous radiolysis/surface kinetic model,
- the dissolved copper concentrations exceeded the solubility of either Cu2O or CuO,
- and there was evidence for localized attack, in the form of circular corrosion features (local depth of penetration of 0.8 μm).
3.3. Irradiation-Enhanced Localized Corrosion and EAC of Copper
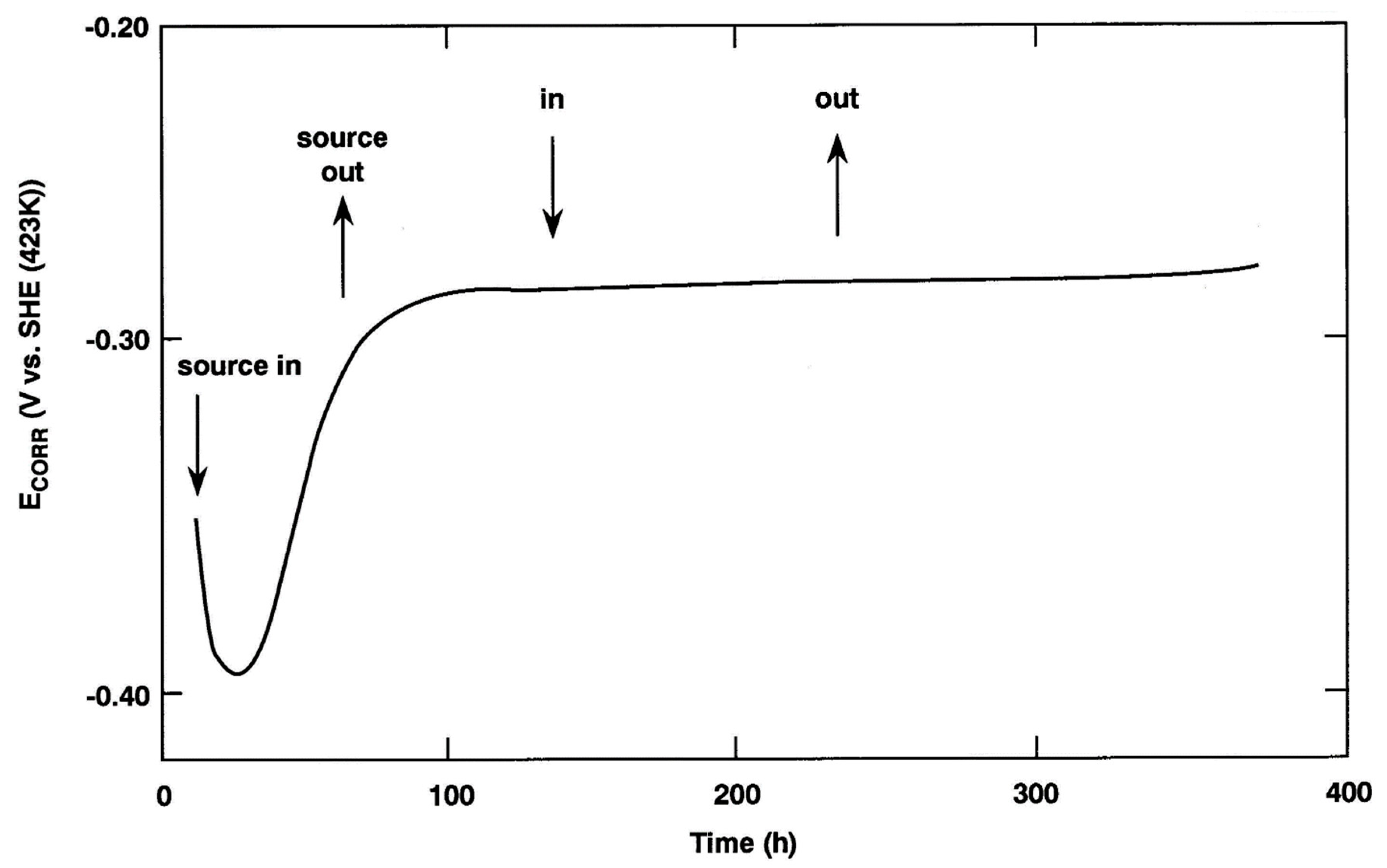
3.3.1. Passivation of the Copper Surface and the Ennoblement of ECORR to a Value Exceeding the Film Breakdown Potential
3.3.2. Localized Dissolution of Non-Uniformly Wetted Surfaces under Unsaturated Conditions
3.3.3. Radiolytic Production of Stress Corrosion Cracking (SCC) Agents, Such as Ammonia or Nitrite
3.3.4. Radiolytically Enhanced Absorption of Hydrogen Leading to H-Induced Cracking Mechanisms
3.4. Effect of Irradiation on Material Properties
- Wu et al. estimated the neutron fluence to the inner surface of the C-steel liner of the UFC for the entire container service life of 106 years [9]. The estimated neutron fluence of 4.2 × 1014 n/cm2 would result in <10−6 dpa for the C-steel vessel, and significantly less for the copper coating as a result of shielding by the inner vessel. This accumulated damage is more than two orders of magnitude less than the minimum below which any observable effect on the mechanical or material properties of copper has been reported [68].
- As described in Section 3.1, a recent study by Turnbull et al. reported new results from examinations of irradiated Cu surfaces that were exposed to about 40 years of gamma and neutron radiation. Vickers hardness measurements were performed with a 100-g load of the cross section on both irradiated and non-irradiated copper surfaces. An analysis of the microhardness concluded that negligible irradiation hardening occurred to the irradiated Cu surface despite the constant flux of neutron and gamma irradiation during the operation of the research reactor [44].
- Similar analyses for the Swedish nuclear waste management program have also shown no effect of neutron and γ-radiation. The estimated damage to the copper shell of the KBS-3 canister design is <10−6 dpa after 105 years [69]. As with the study of Wu et al., such estimates ignore the potential beneficial effects of thermal annealing which, although relatively small at repository temperatures, will reduce the level of accumulated damage even further [9]. Unsurprisingly, Padovani et al. were unable to detect any effect of γ-irradiation to a total dose of 100 kGy (approximately equal to the accumulated dose for a KBS-3 canister for the first 300 years post-emplacement) on the microstructure of oxygen-free copper using high-resolution transmission electron microscopy, nanoindentation, positron annihilation spectroscopy, or electrical resistance measurements [70].
4. Expected Influence of Irradiation on the Performance of a Copper-Coated UFC
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, D.S.; Keech, P.G. An Overview of the Canadian Corrosion Program for the Long-Term Management of Nuclear Waste. Corros. Eng. Sci. Technol. 2017, 52 (Suppl. 1), 2–5. [Google Scholar] [CrossRef]
- Hall, D.S.; Behazin, M.; Binns, W.J.; Keech, P.G. An Evaluation of Corrosion Processes Affecting Copper-Coated Nuclear Waste Containers in a Deep Geological Repository. Prog. Mater. Sci. 2021, 118, 100766. [Google Scholar] [CrossRef]
- Shoesmith, D.W.; King, F. The Effects of Gamma Radiation on the Corrosion of Candidate Materials for the Fabrication of Nuclear Waste Packages; AECL-11999; Atomic Energy of Canada Ltd.: Pinawa, MB, Canada, 1999. [Google Scholar]
- Daub, K.; Zhang, X.; Noël, J.J.; Wren, J.C. Effects of γ-Radiation versus H2O2 on Carbon Steel Corrosion. Electrochim. Acta 2010, 55, 2767–2776. [Google Scholar] [CrossRef]
- Knapp, Q.W.; Wren, J.C. Film Formation on Type-316L Stainless Steel as a Function of Potential: Probing the Role of Gamma-Radiation. Electrochim. Acta 2012, 80, 90–99. [Google Scholar] [CrossRef]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1990. [Google Scholar]
- Belloni, J.; Mostafavi, M.; Douki, T.; Spotheim-Maurizot, M. Radiation Chemistry from Basics to Applications in Material and Life Sciences; Editions EDP Sciences: Les Ulis, France, 2008. [Google Scholar]
- Elliot, A.J.; Chenier, M.P.; Ouellette, D.C. G-Values for γ-Irradiated Water as a Function of Temperature. Can. J. Chem. 1990, 68, 712–719. [Google Scholar] [CrossRef]
- Wu, M.; Behazin, M.; Nam, J.; Keech, P. Internal Corrosion of Used Fuel Container; Technical Report NWMO-TR-2019-02; NWMO: Toronto, ON, Canada, 2019. [Google Scholar]
- Johnsson, M.; Emilsson, G.; Emilsson, L. Mechanical Design Analysis for the Canister; Technical Report Posiva SKB Report 04; Posiva Oy: Eurajoki, Finland, 2018. [Google Scholar]
- SKB. Supplementary Information on Canister Integrity Issues; Technical Report SKB TR-19-15; SKB: Stockholm, Sweden, 2019; p. 135. [Google Scholar]
- King, F.; Hall, D.S.; Keech, P.G. Nature of the Near-Field Environment in a Deep Geological Repository and the Implications for the Corrosion Behaviour of the Container. Corros. Eng. Sci. Technol. 2017, 52 (Suppl. 1), 25–30. [Google Scholar] [CrossRef]
- Guo, R. Thermal Response of a Canadian Conceptual Deep Geological Repository in Crystalline Rock and a Method to Correct the Influence of the Near-Field Adiabatic Boundary Condition. Eng. Geol. 2017, 218, 50–62. [Google Scholar] [CrossRef]
- Johansson, L.; Stahlén, J.; Taborowski, T.; Pedersen, K.; Luukari, S.; Marmo, J. Microbial Release of Iron from Olkiluoto Rock Minerals; Posiva Working Report 2018–30; Olkiluoto: Helsinki, Finland, 2019; pp. 1–120. [Google Scholar]
- Giroud, N.; Tomonaga, Y.; Wersin, P.; Briggs, S.; King, F.; Vogt, T.; Diomidis, N. On the Fate of Oxygen in a Spent Fuel Emplacement Drift in Opalinus Clay. Appl. Geochem. 2018, 97, 270–278. [Google Scholar] [CrossRef]
- Ershov, B.G.; Gordeev, A.V. A Model for Radiolysis of Water and Aqueous Solutions of H2, H2O2 and O2. Radiat. Phys. Chem. 2008, 77, 928–935. [Google Scholar] [CrossRef]
- Boyd, A.W.; Carver, M.B.; Dixon, R.S. Computed and Experimental Product Concentrations in the Radiolysis of Water. Radiat. Phys. Chem. (1977) 1980, 15, 177–185. [Google Scholar] [CrossRef]
- Reed, D.T.; Van Konynenburg, R.A. Effect of Ionizing Radiation on Moist Air Systems. In Materials Research Society Symposium Proceedings; Materials Research Society: Warrendale, PA, USA, 1988; Volume 112, pp. 393–404. [Google Scholar]
- Morco, R.P.; Joseph, J.M.; Hall, D.S.; Medri, C.; Shoesmith, D.W.; Wren, J.C. Modelling of Radiolytic Production of HNO3 Relevant to Corrosion of a Used Fuel Container in Deep Geologic Repository Environments. Corros. Eng. Sci. Technol. 2017, 52 (Suppl. 1), 141–147. [Google Scholar] [CrossRef]
- Björkbacka, Å.; Johnson, C.M.; Leygraf, C.; Jonsson, M. Radiation Induced Corrosion of Copper in Humid Air and Argon Atmospheres. J. Electrochem. Soc. 2017, 164, C201–C206. [Google Scholar] [CrossRef]
- Johnson, G.R.D. The Radiation Chemistry of Nitrogen and Its Compounds. Inorganic and Theoretical Chemistry; Wiley: New York, NY, USA, 1967. [Google Scholar]
- Turnbull, J.; Szukalo, R.; Zagidulin, D.; Biesinger, M.; Shoesmith, D. The Kinetics of Copper Corrosion in Nitric Acid. Mater. Corros. 2020, 72, 348–360. [Google Scholar] [CrossRef]
- Morco, R.P. Gamma-Radiolysis Kinetics and Its Role in The Overall Dynamics of Materials Degradation. Ph.D. Thesis, University of Western Ontario, London, ON, Canada, 2020. [Google Scholar]
- Joseph, J.M.; Seon Choi, B.; Yakabuskie, P.; Wren, J.C. A Combined Experimental and Model Analysis on the Effect of PH and O2(Aq) on γ-Radiolytically Produced H2 and H2O2. Radiat. Phys. Chem. 2008, 77, 1009–1020. [Google Scholar] [CrossRef]
- Hochanadel, C.J. Effects of Cobalt γ-Radiation on Water and Aqueous Solutions. J. Phys. Chem. 1952, 56, 587–594. [Google Scholar] [CrossRef]
- Allen, A.O. The Radiation Chemistry of Water and Aqueous Solutions; Van Nostrand: Princeton, NJ, USA, 1961. [Google Scholar]
- Morawetz, H. Radiation Chemistry-Principles and Applications; Farhataziz, Rogers, M.A.J., Eds.; VCH: New York, NY, USA, 1987; p. 641. [Google Scholar]
- Dixon, D.A. Review of the T-H-M-C Properties of MX-80 Bentonite; NWMO TR-2019-07; Nuclear Waste Management Organisation: Toronto, ON, Canada, 2019. [Google Scholar]
- Airey, P.L. Effect of Irradiation on Electrode Processes. Part 1—The Hydrogen Electrode in Dilute Solutions. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1972, 68, 1299–1311. [Google Scholar] [CrossRef]
- Abrantes, L.M.; Castillo, L.M.; Norman, C.; Peter, L.M. A Photoelectrochemical Study of the Anodic Oxidation of Copper in Alkaline Solution. J. Electroanal. Chem. Interfacial Electrochem. 1984, 163, 209–221. [Google Scholar] [CrossRef]
- Collisi, U.; Strehblow, H.-H. A Photoelectrochemical Study of Passive Copper in Alkaline Solutions. J. Electroanal. Chem. Interfacial Electrochem. 1986, 210, 213–227. [Google Scholar] [CrossRef]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Kondo, J.N.; Domen, K.; Hara, M.; Shinohara, K.; Tanaka, A. Cu2O as a Photocatalyst for Overall Water Splitting under Visible Light Irradiation. Chem. Commun. 1998, 3, 357–358. [Google Scholar] [CrossRef]
- Yamashita, M.; Omura, K.; Hirayama, D. Passivating Behavior of Copper Anodes and Its Illumination Effects in Alkaline Solutions. Surf. Sci. 1980, 96, 443–460. [Google Scholar] [CrossRef]
- Isherwood, P.J.M.; Walls, J.M. Cupric Oxide-Based p-Type Transparent Conductors. Energy Procedia 2014, 60, 129–134. [Google Scholar] [CrossRef][Green Version]
- Aruchamy, A.; Fujishima, A. Photoanodic Behaviour of Cu2O Corrosion Layers on Copper Electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1989, 272, 125–136. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Liu, X.; Hou, J.; Sun, M.; Zeng, R. Study on the Mechanism of the Photoelectrochemical Effect on the Initial NaCl-Induced Atmospheric Corrosion Process of Pure Copper Exposed in Humidified Pure Air. J. Electrochem. Soc. 2018, 165, C608–C617. [Google Scholar] [CrossRef]
- Mian, T.A.; Van Putten, M.C., Jr.; Kramer, D.C.; Jacob, R.F.; Boyer, A.L. Backscatter Radiation at Bone-Titanium Interface from High-Energy x and Gamma Rays. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 1943–1947. [Google Scholar] [CrossRef]
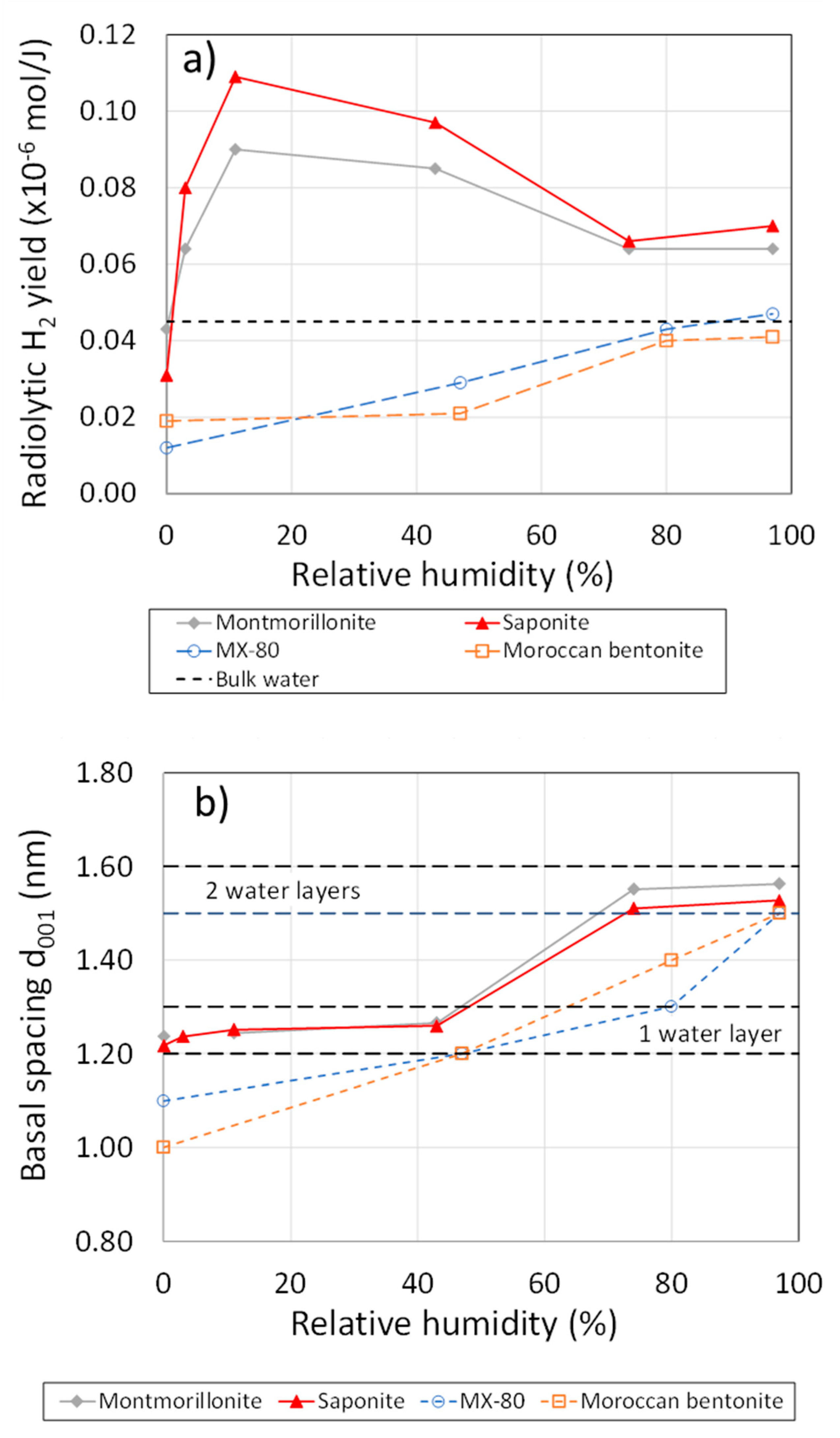
- Lainé, M.; Balan, E.; Allard, T.; Paineau, E.; Jeunesse, P.; Mostafavi, M.; Robert, J.-L.; Le Caër, S. Reaction Mechanisms in Swelling Clays under Ionizing Radiation: Influence of the Water Amount and of the Nature of the Clay Mineral. RSC Adv. 2017, 7, 526–534. [Google Scholar] [CrossRef]
- Fourdrin, C.; Aarrachi, H.; Latrille, C.; Esnouf, S.; Bergaya, F.; Le Caër, S. Water Radiolysis in Exchanged-Montmorillonites: The H2 Production Mechanisms. Environ. Sci. Technol. 2013, 47, 9530–9537. [Google Scholar] [CrossRef]
- Le Caër, S. Water Radiolysis: Influence of Oxide Surfaces on H2 Production under Ionizing Radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef]
- Ibrahim, B.; Zagidulin, D.; Behazin, M.; Ramamurthy, S.; Wren, J.C.; Shoesmith, D.W. The Corrosion of Copper in Irradiated and Unirradiated Humid Air. Corros. Sci. 2018, 141, 53–62. [Google Scholar] [CrossRef]
- Ibrahim, B. The Corrosion Behaviour of Cu in Irradiated and Non-Irradiated Humid Air. Master’s Thesis, University of Western Ontario, London, ON, Canada, 2015. [Google Scholar]
- Ibrahim, B.; Zagidulin, D.; Smith, J.M.; Ramamurthy, S.; Wren, J.C.; Shoesmith, D.W. Radiolytic Corrosion of Cu Nuclear Waste Containers. In Proceedings of the 17th International Conference on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors, Ottawa, ON, Canada, 9–13 August 2015. [Google Scholar]
- Turnbull, J.; Behazin, M.; Smith, J.; Keech, P.G. The Impact of 40 Years of Radiation on the Integrity of Copper. J. Nucl. Mater. 2021. accepted for publication. [Google Scholar] [CrossRef]
- Sherwood, J.E.; Stagnitti, F.; Kokkinn, M.J.; Williams, W.D. A Standard Table for Predicting Equilibrium Dissolved Oxygen Concentrations in Salt Lakes Dominated by Sodium Chloride. Int. J. Salt Lake Res. 1992, 1, 1–6. [Google Scholar] [CrossRef]
- Wren, J.C.; Jean, A.; Naghizadeh, M.; Grandy, L.; Morco, R.; Joseph, J.M.; Behazin, M.; Keech, P.G. Radiation Induced Corrosion of Copper in Deep Geological Repositories—19493; WM Symposia, Inc.: Tempe, AZ, USA, 2019. [Google Scholar]
- Björkbacka, Å.; Hosseinpour, S.; Leygraf, C.; Jonsson, M. Radiation Induced Corrosion of Copper in Anoxic Aqueous Solution. Electrochem. Solid-State Lett. 2012, 15, C5–C7. [Google Scholar] [CrossRef][Green Version]
- Björkbacka, Å.; Hosseinpour, S.; Johnson, M.; Leygraf, C.; Jonsson, M. Radiation Induced Corrosion of Copper for Spent Nuclear Fuel Storage. Radiat. Phys. Chem. 2013, 92, 80–86. [Google Scholar] [CrossRef]
- Björkbacka, Å.; Yang, M.; Gasparrini, C.; Leygraf, C.; Jonsson, M. Kinetics and Mechanisms of Reactions between H2O2 and Copper and Copper Oxides. Dalton Trans. 2015, 44, 16045–16051. [Google Scholar] [CrossRef]
- Björkbacka, Å.; Johnson, C.M.; Leygraf, C.; Jonsson, M. Role of the Oxide Layer in Radiation-Induced Corrosion of Copper in Anoxic Water. J. Phys. Chem. C 2016, 120, 11450–11455. [Google Scholar] [CrossRef]
- Soroka, I.; Chae, N.; Jonsson, M. On the Mechanism of γ-Radiation-Induced Corrosion of Copper in Water. Corros. Sci. 2021, 182, 109279. [Google Scholar] [CrossRef]
- King, F.; Litke, C.D. The Corrosion of Copper in Synthetic Groundwater at 150 C. Part I. The Results of Short-Term Electrochemical Tests; Technical Report TR-428; Atomic Energy of Canada Ltd.: Pinawa, MB, Canada, 1987. [Google Scholar]
- Litke, C.D.; Ryan, S.R.; King, F. A Mechanistic Study of the Uniform Corrosion of Copper in Compacted Clay-Sand Soil; Atomic Energy of Canada Ltd.: Pinawa, MB, Canada, 1992; p. 141. [Google Scholar]
- Simpson, J.P. Experiments on Container Materials for Swiss High-Level Waste Disposal Projects Part; Nagra Technical Report, NTB 84-01; National Cooperative for the Disposal of Radioactive Waste: Wettingen, Switzerland, 1984; p. 100. [Google Scholar]
- Glass, R.S.; Van Konynenburg, R.A.; Overturf, G.E. Corrosion Processes of Austenitic Stainless Steels and Copper-Based Materials in Gamma-Irradiated Aqueous Environments. In Proceedings of the Corrosion 86, Houston, TX, USA, 17–21 March 1986. paper No. 258. [Google Scholar] [CrossRef][Green Version]
- King, F.; Lilja, C.; Pedersen, K.; Pitkänen, P.; Vähänen, M. An Update of the State-of-the-Art Report on the Corrosion of Copper under Expected Conditions in a Deep Geologic Repository; Technical Report SKB TR-10-67; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2010; p. 180. [Google Scholar]
- Yunker, W.H. Corrosion Behavior of Copper-Base Materials in a Gamma-Irradiated Environment; Report WHC-EP-0188; Westinghouse Hanford Company: Richland, WA, USA, 1990. [Google Scholar] [CrossRef][Green Version]
- King, F. Assessment of the Stress Corrosion Cracking of Copper Canisters; 2021-11 Working Report; Posiva Oy: Eurajoki, Finland, 2021. [Google Scholar]
- King, F.; Newman, R.C. Stress Corrosion Cracking of Copper Canisters; Technical Report SKB TR-10-04; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2010. [Google Scholar]
- Turnbull, J.; Szukalo, R.; Behazin, M.; Hall, D.; Zagidulin, D.; Ramamurthy, S.; Wren, J.C.; Shoesmith, D.W. The Effects of Cathodic Reagent Concentration and Small Solution Volumes on the Corrosion of Copper in Dilute Nitric Acid Solutions. Corrosion 2017, 74, 326–336. [Google Scholar] [CrossRef]
- Karasawa, H.; Ibe, E.; Uchida, S.; Etoh, Y.; Yasuda, T. Radiation Induced Decomposition of Nitrogen. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1991, 37, 193–197. [Google Scholar] [CrossRef]
- Ryan, S.R.; Clarke, C.F.; Ikeda, B.M.; King, F.; Litke, C.D. Investigation of the Long-Term Corrosion Behaviour of Selected Nuclear Fuel Waste Container Materials under Possible Disposal Vault Conditions; Technical Report TR-489, COG-94-55; Atomic Energy of Canada Ltd.: Pinawa, MB, Canada, 1994. [Google Scholar]
- Lousada, C.M.; Soroka, I.L.; Yagodzinskyy, Y.; Tarakina, N.V.; Todoshchenko, O.; Hänninen, H.; Korzhavyi, P.A.; Jonsson, M. Gamma Radiation Induces Hydrogen Absorption by Copper in Water. Sci. Rep. 2016, 6, 24234. [Google Scholar] [CrossRef]
- Yagodzinskyy, Y.; Malitckii, E.; Saukkonen, T.; Hänninen, H. Hydrogen-Enhanced Creep and Cracking of Oxygen-Free Phosphorus-Doped Copper. Scr. Mater. 2012, 67, 931–934. [Google Scholar] [CrossRef]
- Forsström, A.; Becker, R.; Hänninen, H.; Yagodzinskyy, Y.; Heikkilä, M. Sulphide-Induced Stress Corrosion Cracking and Hydrogen Absorption of Copper in Deoxygenated Water at 90 °C. Mater. Corros. 2021, 72, 317–332. [Google Scholar] [CrossRef]
- Hänninen, H.; Forsström, A.; Yagodzinskyy, Y. 11—Copper Behavior in Geological Nuclear Waste Disposal. In Nuclear Corrosion; Ritter, S., Ed.; Woodhead Publishing: Cambridge, UK, 2020; pp. 391–402. [Google Scholar] [CrossRef]
- English, C.A.; Hyde, J.M. 6.08—Radiation Embrittlement of Reactor Pressure Vessel Steels. In Comprehensive Structural Integrity; Milne, I., Ritchie, R.O., Karihaloo, B., Eds.; Pergamon: Oxford, UK, 2003; pp. 351–398. [Google Scholar] [CrossRef]
- Li, M.; Zinkle, S.J. Physical and Mechanical Properties of Copper and Copper Alloys. Compr. Nucl. Mater. 2012, 4, 667–690. [Google Scholar]
- Yang, Q.; Toijer, E.; Olsson, P. Analysis of Radiation Damage in the KBS-3 Canister Materials; Technical Report SKB TR-19-14; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2019. [Google Scholar]
- Padovani, C.; Pletser, D.; Jurkschat, K.; Armstrong, D.; Dugdale, S.; Brunt, D.; Faulkner, R.; Was, G.; Johansson, A.J. Assessment of Microstructural Changes in Copper Due to Gamma Radiation Damage; Technical Report SKB TR-19-12; Swedish Nuclear Fuel and Waste Management Co.: Solna, Sweden, 2019. [Google Scholar]
- Keech, P.G.; Behazin, M.; Binns, W.J.; Briggs, S. An Update on the Copper Corrosion Program for the Long-term Management of Used Nuclear Fuel in Canada. Mater. Corros. 2020, 72, 25–31. [Google Scholar] [CrossRef]
- King, F.; Litke, C.D.; Quinn, M.J.; LeNeveu, D.M. The Measurement and Prediction of the Corrosion Potential of Copper in Chloride Solutions as a Function of Oxygen Concentration and Mass-Transfer Coefficient. Corros. Sci. 1995, 37, 833–851. [Google Scholar] [CrossRef]
- Vazquez, M.V.; de Sanchez, S.R.; Calvo, E.J.; Schiffrin, D.J. The Electrochemical Reduction of Hydrogen Peroxide on Polycrystalline Copper in Borax Buffer. J. Electroanal. Chem. 1994, 374, 179–187. [Google Scholar] [CrossRef]








| Species | H2O | •eaq− | H+ | •OH | •H | •HO2 | H2 | H2O2 |
| G-value | −0.43 | 0.27 | 0.27 | 0.28 | 0.06 | 0 | 0.05 | 0.07 |
| Data Label | Reference | Dose Rate (Gy/h) | Enhancement Factor | Grade of Copper * | Environment | Temperature (°C) | Duration (h) | Comments |
|---|---|---|---|---|---|---|---|---|
| 1a | King and Litke (1987) [52] | 20 | 0.24 | CDA101 | Ar-deaerated SCSSS ** | 150 | 490 | |
| 1b | 20 | 0.26 | CDA101 | Aerated SCSSS | 150 | 470 | ||
| 2a | Litke et al. (1992) [53] | 5 | 0.089 | CDA101 | Aerated SCSSS, compacted 50:50 sand:bentonite buffer | 100 | 9768 | |
| 2b | 7.5 | 1 | CDA101 | Aerated SCSSS, compacted 50:50 sand:bentonite buffer | RT *** | 1848 | ||
| 3a | Simpson (1984) [54] | 12.7 | 0.95 | PDO | Deaerated 1 mol/L NaCl | RT | 63 | Irradiated from behind |
| 3b | 12.7 | 0.38 | PDO | Deaerated 1 mol/L NaCl | RT | 63 | Irradiated “indirectly” | |
| 3c | 12.7 | 0.28 | PDO | Deaerated 0.1 mol/L NaCl | RT | 110 | Irradiated from behind | |
| 3d | 12.7 | 0.14 | PDO | Deaerated 0.1 mol/L NaCl | RT | 110 | Irradiated “indirectly” | |
| 3e | 12.7 | 1.3 | PDO | Deaerated 0.1 mol/L NaNO3 | RT | 63 | Irradiated from behind | |
| 3f | 12.7 | 1.1 | PDO | Deaerated 0.05 mol/L Na2SO4 + Fe2SiO4 | RT | 63 | Irradiated from behind | |
| 3g | 12.7 | 1.1 | PDO | Deaerated 0.05 mol/L Na2SO4 + Fe2SiO4 | RT | 63 | Irradiated “indirectly” | |
| 3h | 12.7 | 0.44 | PDO | Deaerated Säckingen ground water | RT | 110 | Irradiated from behind | |
| 3i | 12.7 | 0.44 | PDO | Deaerated Säckingen ground water | RT | 110 | Irradiated “indirectly” | |
| 4 | Turnbull et al. (2021) [44] | 0.015 | 1 | unknown | Humid air | RT | 325,440 | From archive research reactor component |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, F.; Behazin, M. A Review of the Effect of Irradiation on the Corrosion of Copper-Coated Used Fuel Containers. Corros. Mater. Degrad. 2021, 2, 678-707. https://doi.org/10.3390/cmd2040037
King F, Behazin M. A Review of the Effect of Irradiation on the Corrosion of Copper-Coated Used Fuel Containers. Corrosion and Materials Degradation. 2021; 2(4):678-707. https://doi.org/10.3390/cmd2040037
Chicago/Turabian StyleKing, Fraser, and Mehran Behazin. 2021. "A Review of the Effect of Irradiation on the Corrosion of Copper-Coated Used Fuel Containers" Corrosion and Materials Degradation 2, no. 4: 678-707. https://doi.org/10.3390/cmd2040037
APA StyleKing, F., & Behazin, M. (2021). A Review of the Effect of Irradiation on the Corrosion of Copper-Coated Used Fuel Containers. Corrosion and Materials Degradation, 2(4), 678-707. https://doi.org/10.3390/cmd2040037






