Role of Hydrogen in Metal Oxidation—Implication to Irradiation Enhanced Corrosion of Ni-Based Alloys and Stainless Steels in High Temperature Water
Abstract
:1. Introduction
2. Section 1—High Temperature Oxidation of Ni Based Alloy in Various Environments with Hydrogen
2.1. Experimental
2.2. Results and Discussion
2.2.1. Oxidation Behavior of Metallic Ni with Hydrogen Permeation in Air
2.2.2. Oxidation Behavior of Alloy 600 with Hydrogen Permeation in High Temperature Water
2.3. Role of Hydrogen in Oxidation Process
3. Section 2—The Effects of Neutron Flux/Fluence on Oxidation Kinetics and SCC of 316L and 316LN Stainless Steels—Possible Role of Transmuted H from N
3.1. Experimental
3.1.1. Materials and Test Specimen Geometry
3.1.2. Irradiation History and Operating Conditions
3.2. Results and Discussion
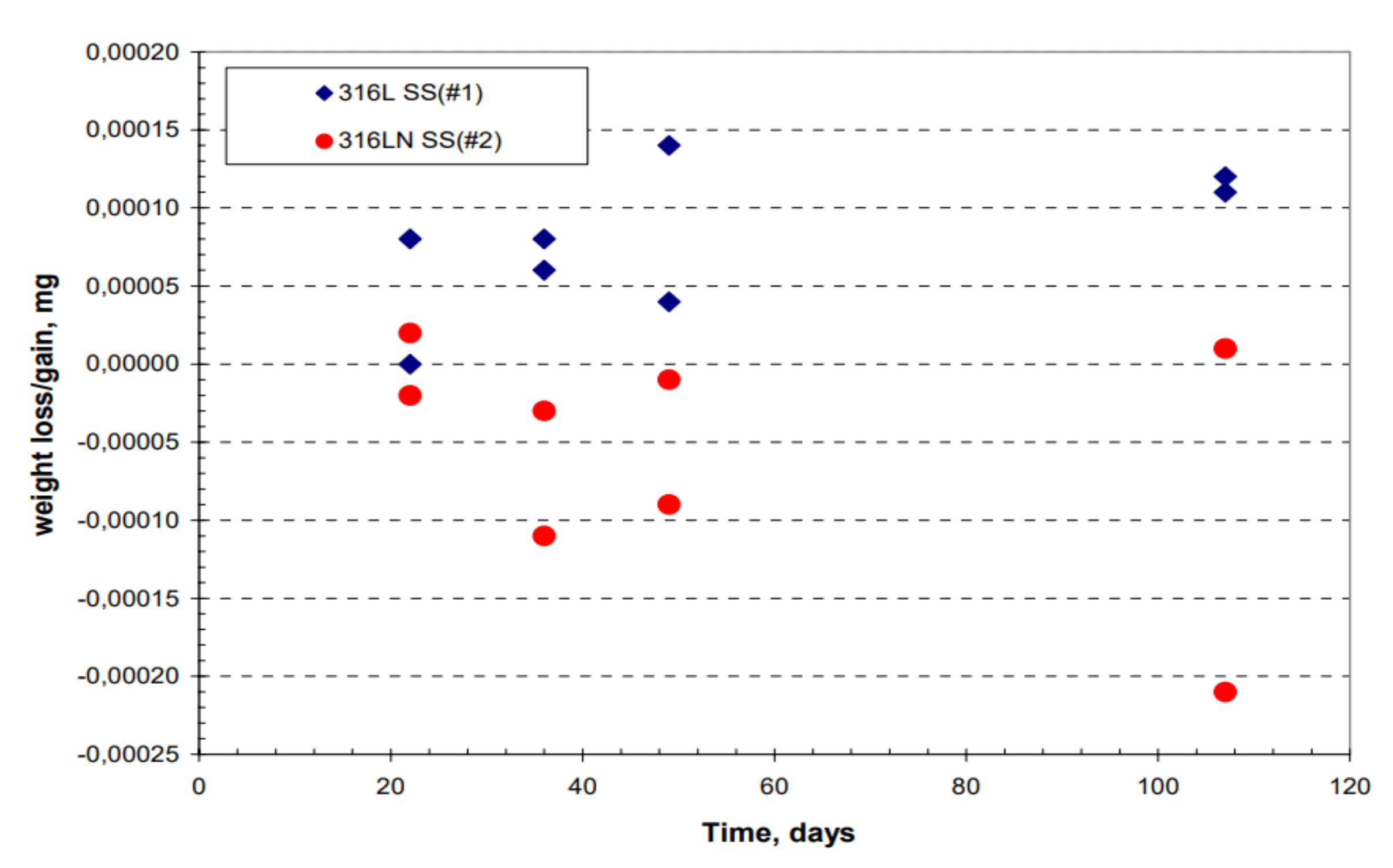
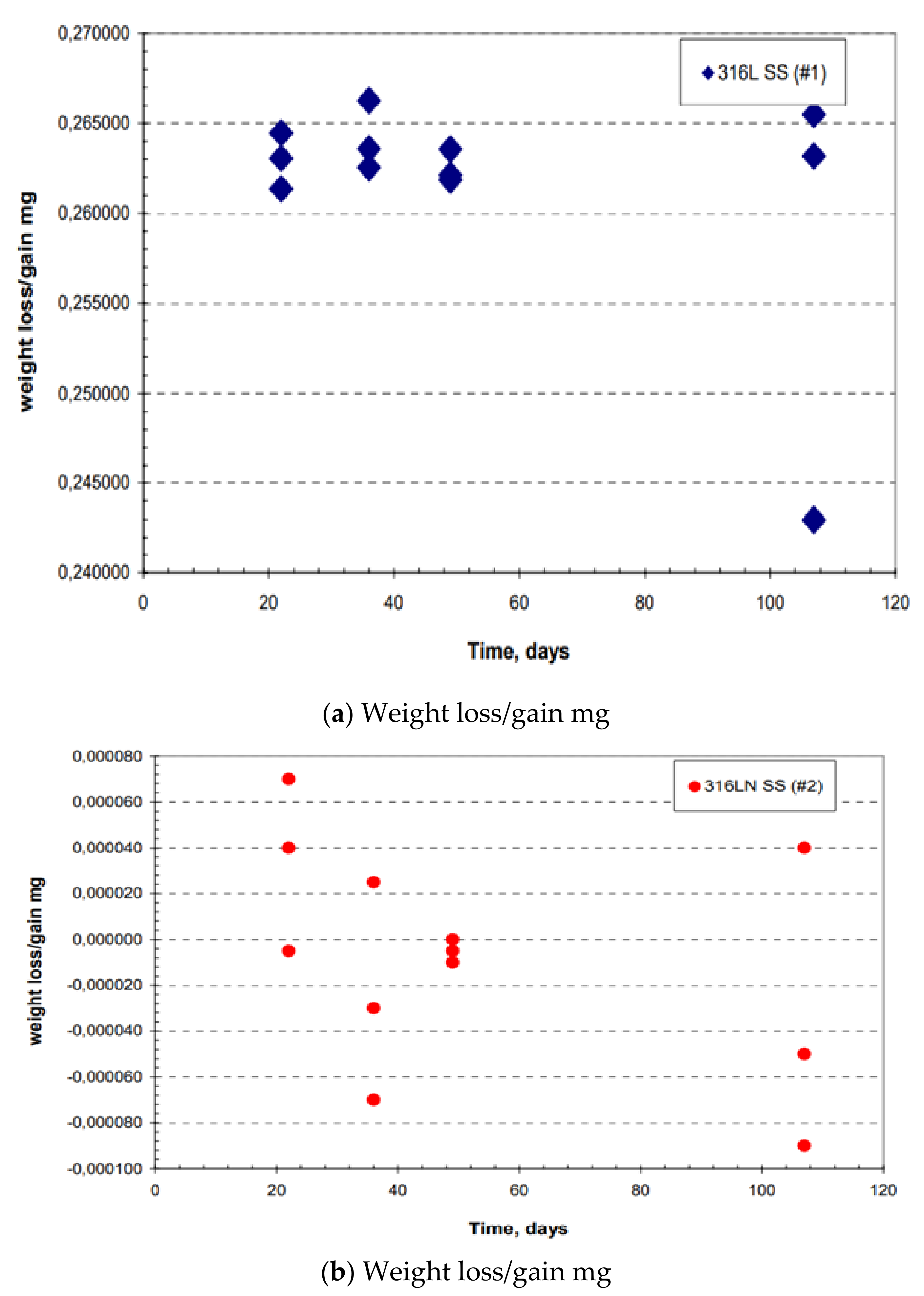
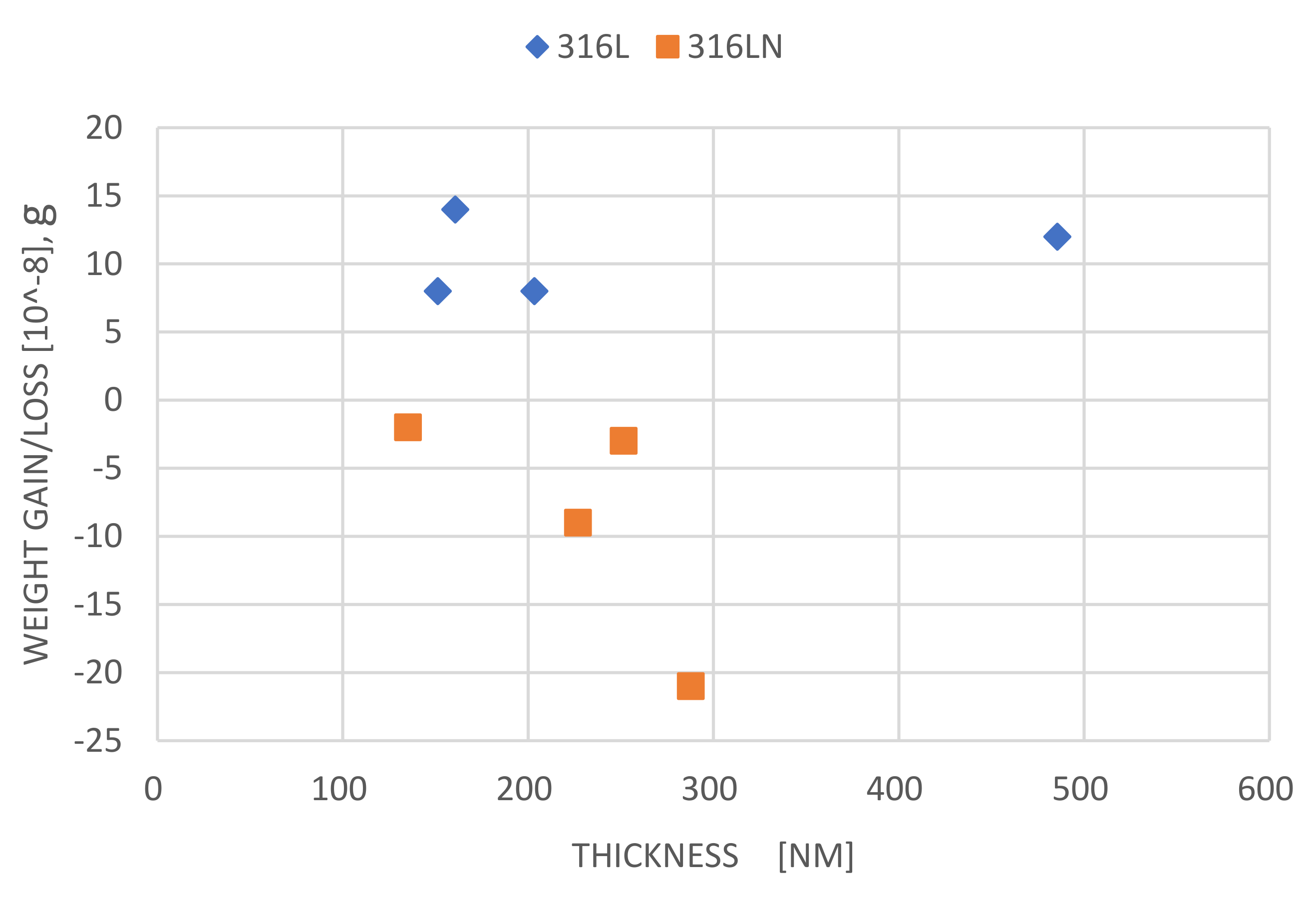
3.2.1. Weight Loss Data of Plate and Creviced Samples
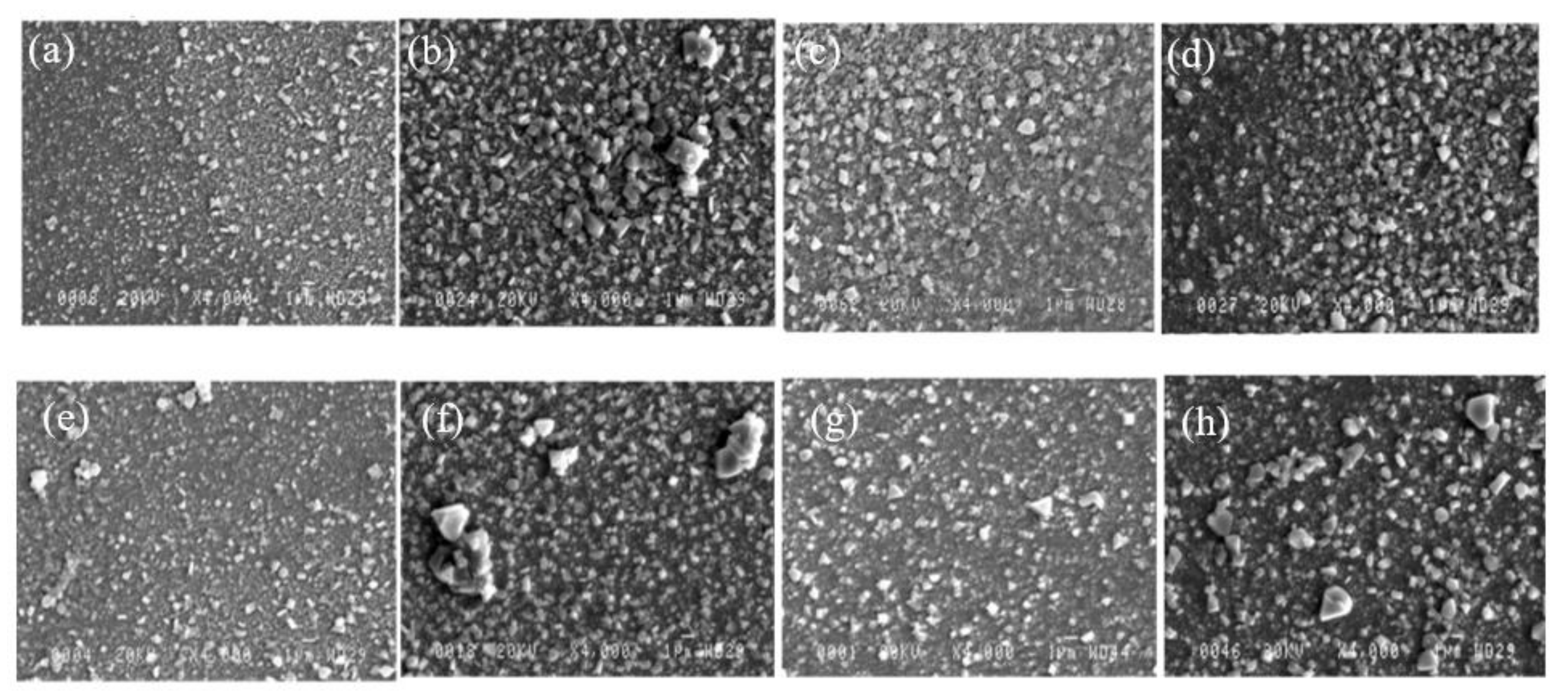
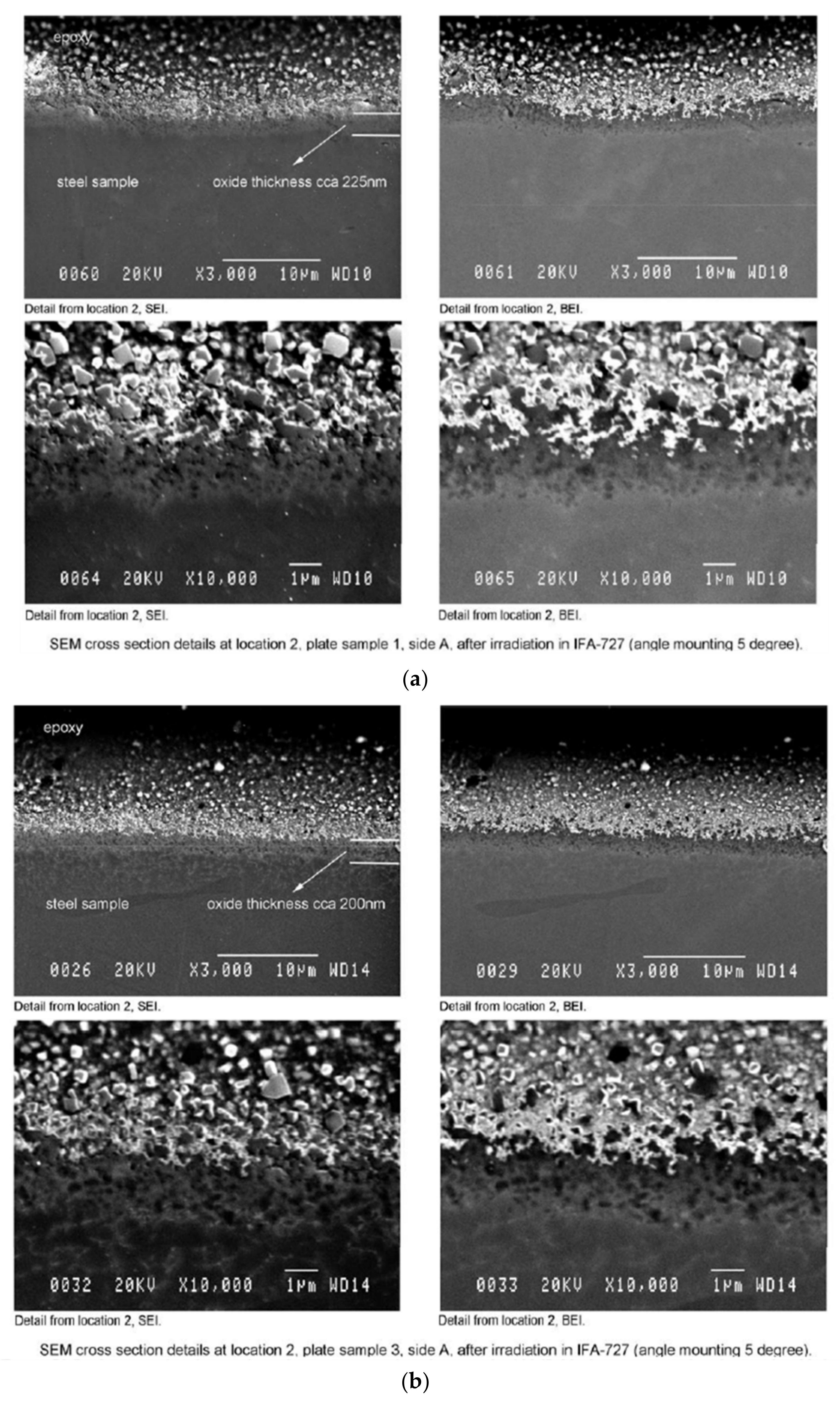
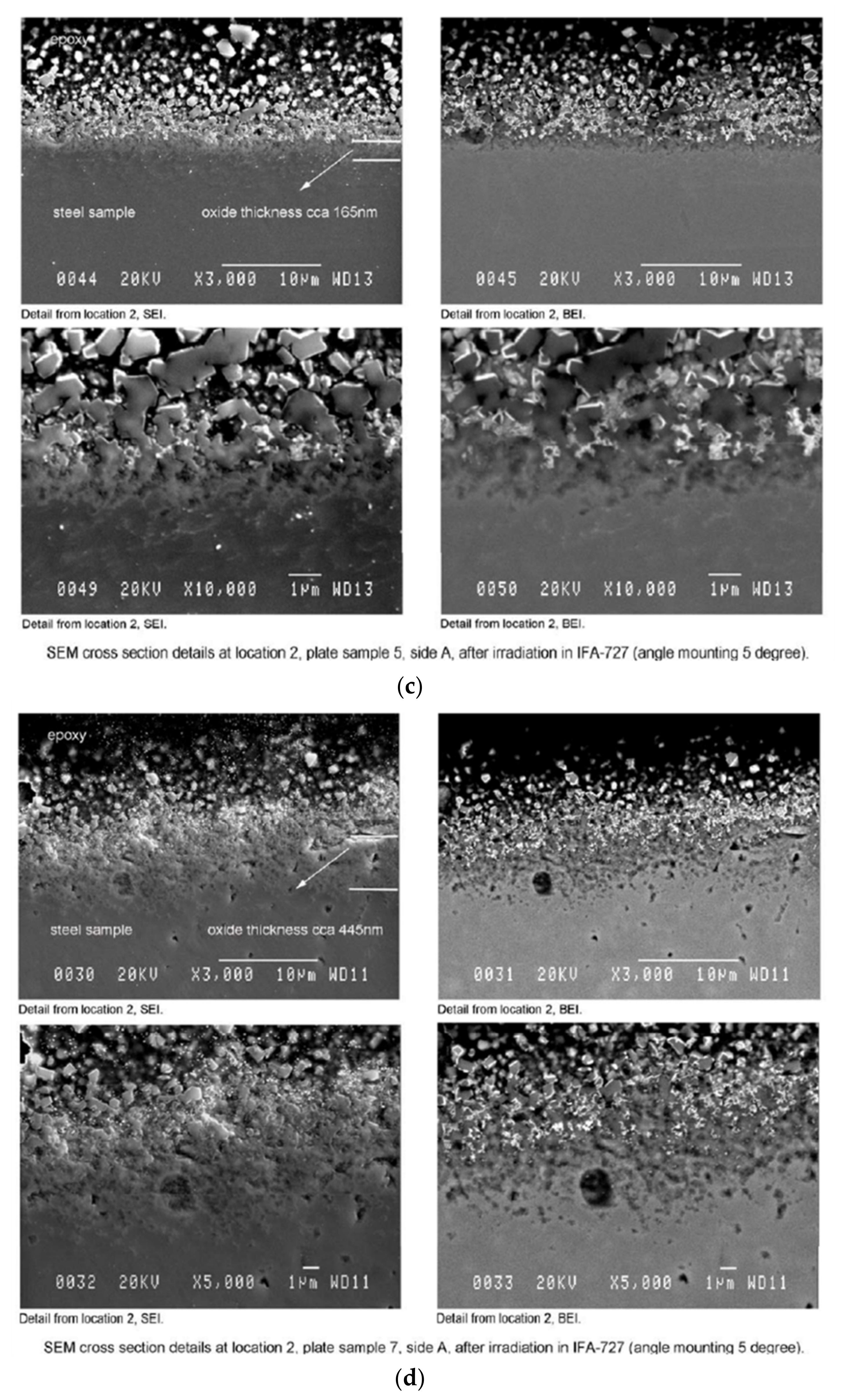
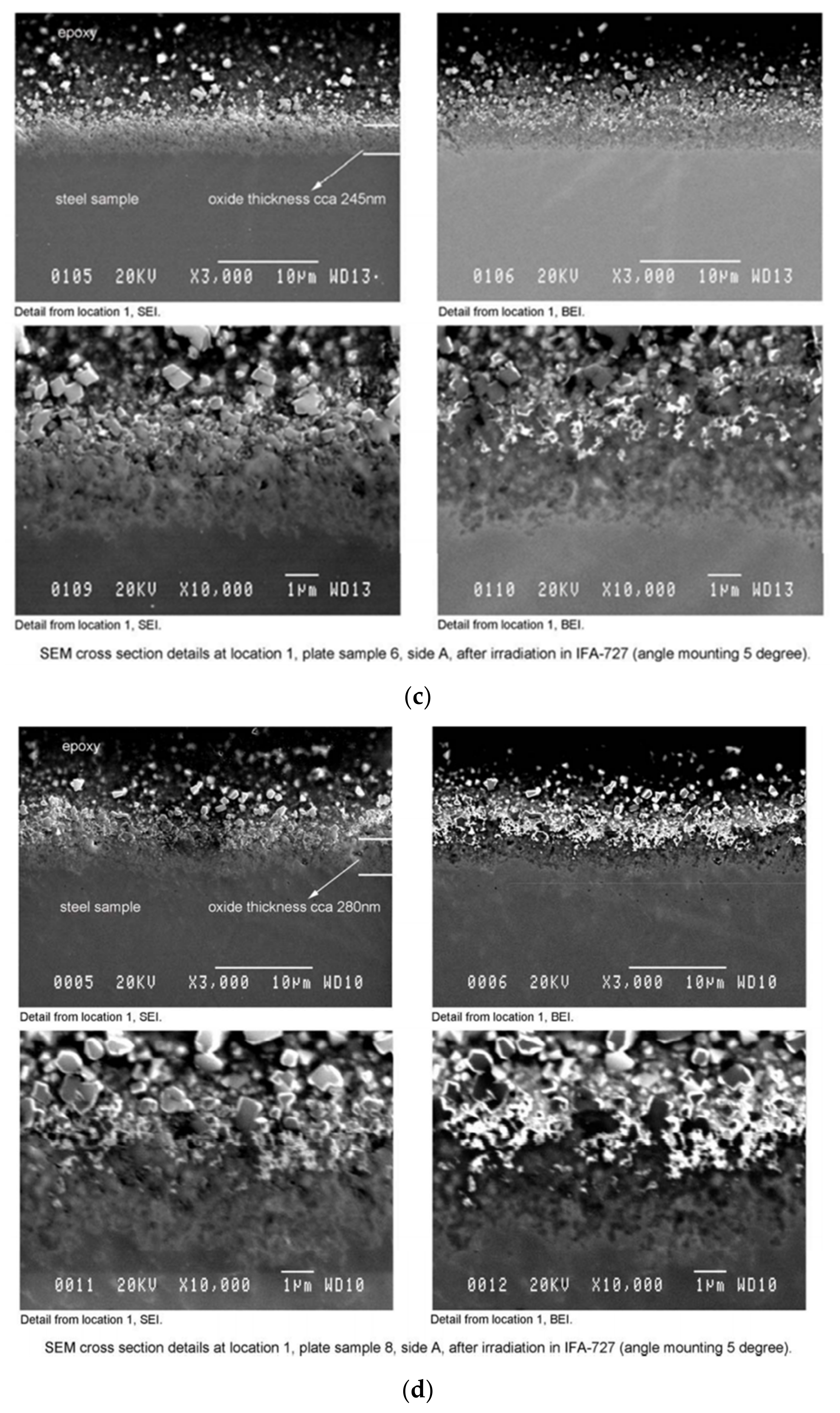
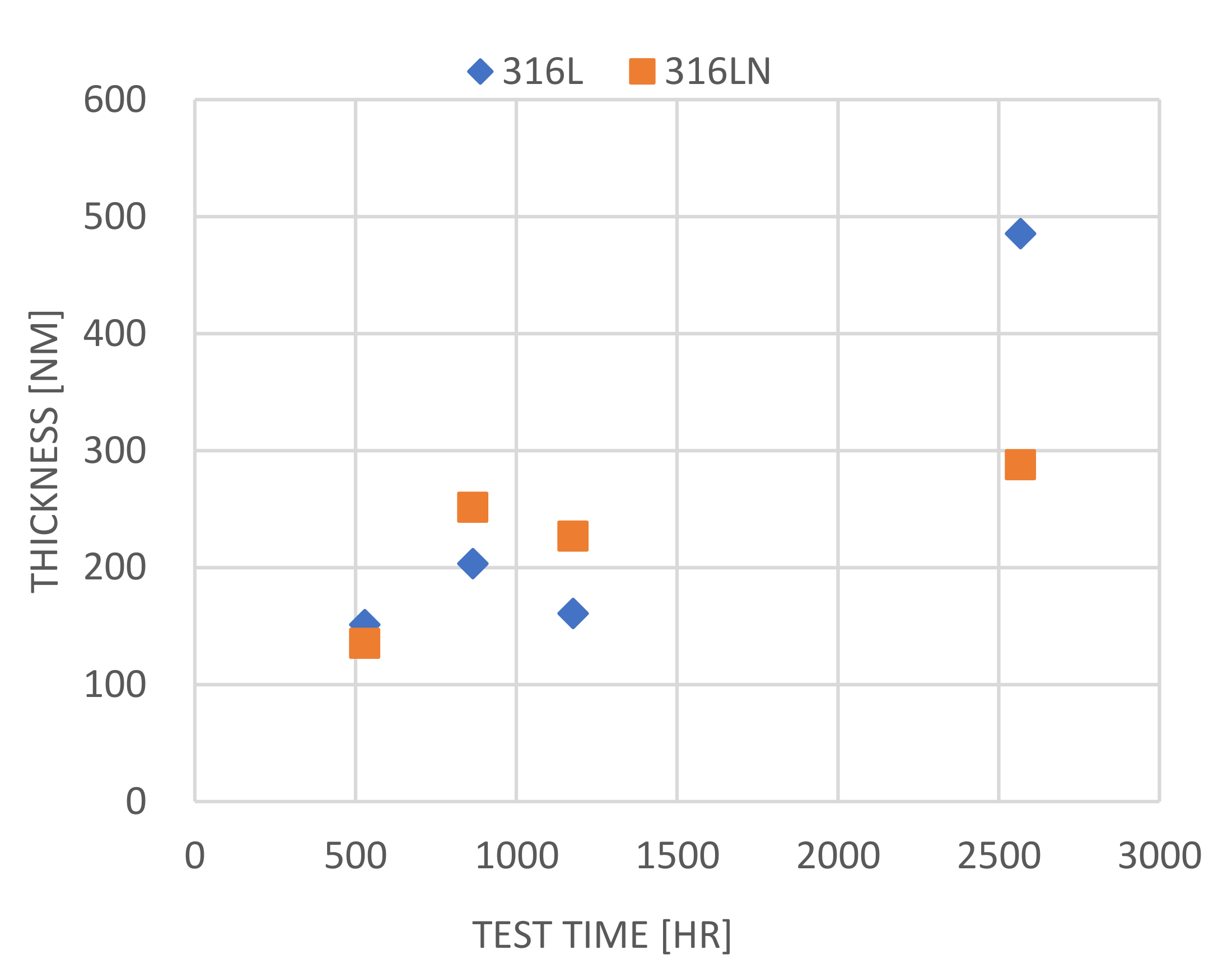
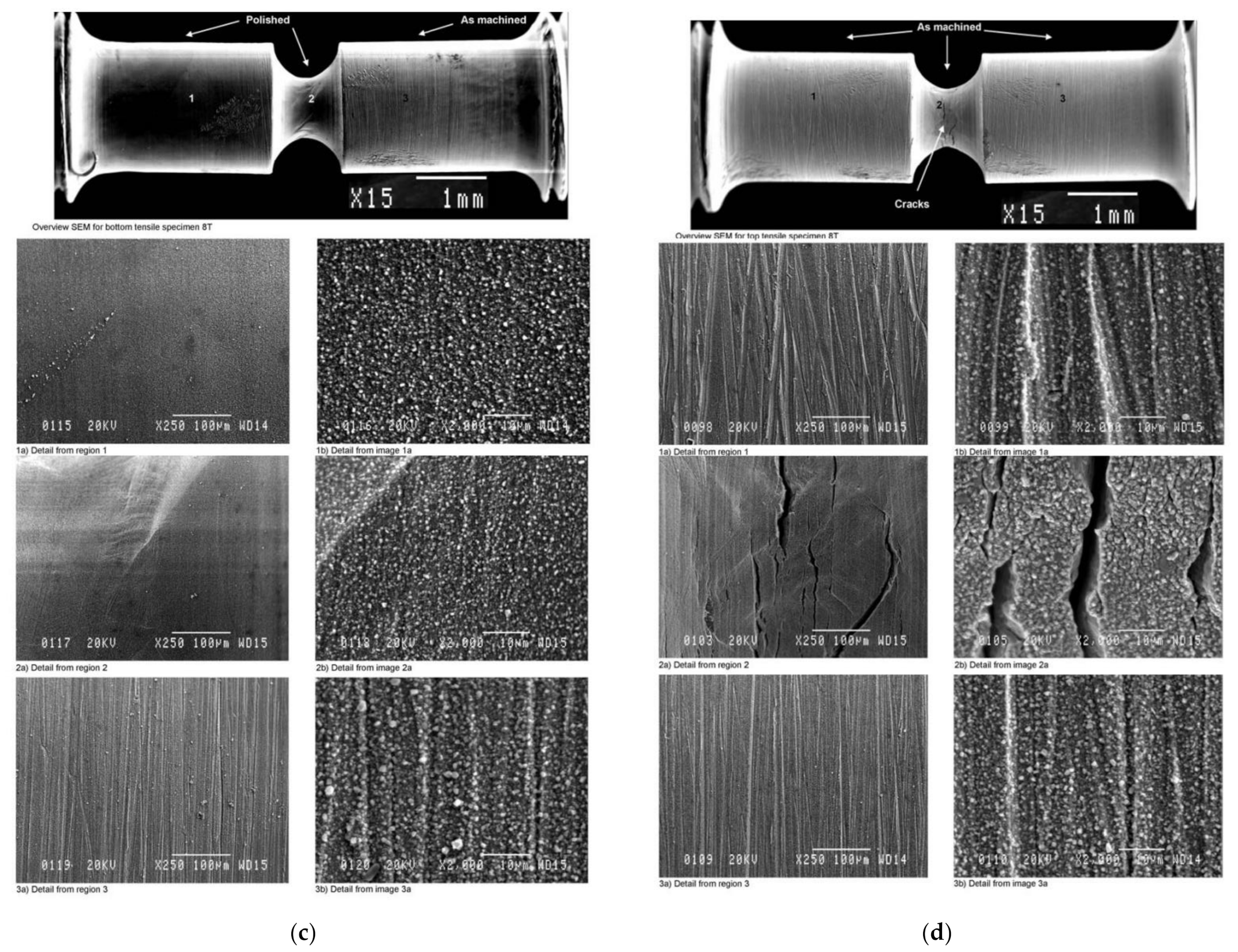
3.2.2. SEM Observation–Surface and Cross-Section
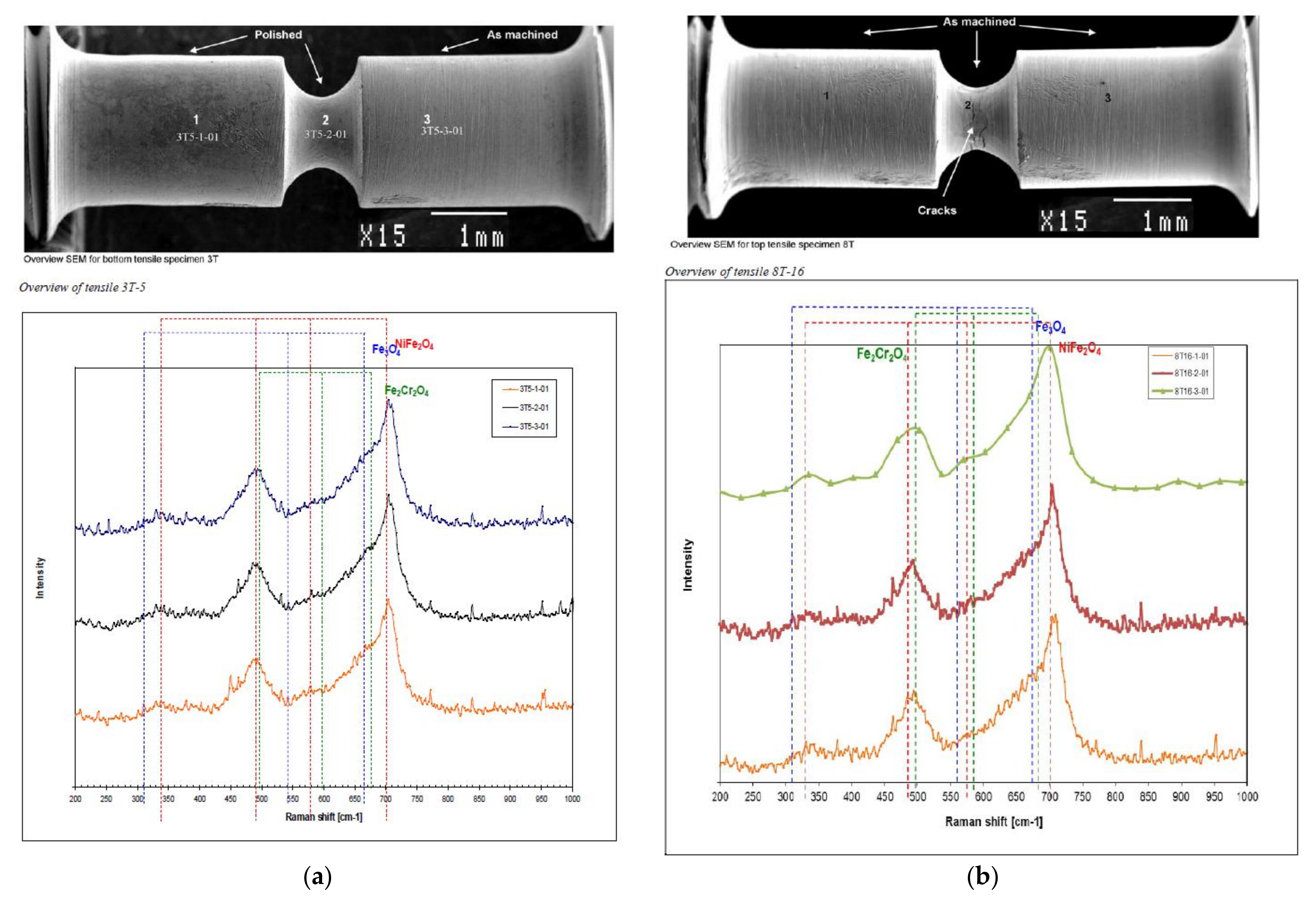
3.2.3. Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Takiguchi, H.; Ullberg, M.; Uchida, S. Optimization of dissolved hydrogen concentration for control of primary coolant radiolysis in pressurized water reactors. J. Nucl. Sci. Technol. 2004, 41, 601–609. [Google Scholar] [CrossRef]
- Economy, G.; Jacko, R.J.; Begley, J.A.; Pement, F.W. Influence of Hydrogen Partial Pressure on the IGSCC Behavior of Alloy 600 Tubing in 360/Sup 0/C Water or 400/Sup 0/C Steam; National Association of Corrosion Engineers: Houston, TX, USA, 1987. [Google Scholar]
- Domian, H.A.; Emanuelson, R.H.; Sarver, L.W.; Theus, G.J.; Katz, L. Effect of microstructure on stress corrosion cracking of Alloy 600 in high purity water. Corrosion 1977, 33, 26–38. [Google Scholar] [CrossRef]
- Jambon, F.; Marchetti, L.; Jomard, F.; Chêne, J. Mechanism of hydrogen absorption during the exposure of alloy 600-like single-crystals to PWR primary simulated media. J. Nucl. Mater. 2011, 414, 386–392. [Google Scholar] [CrossRef]
- Xiong, Y.; Watanabe, Y.; Shibayama, Y. Effects of dissolved hydrogen on low-cycle fatigue behaviors and hydrogen uptake of 316LN austenitic stainless steel in simulated pressurized water reactor primary water. Int. J. Fatigue 2020, 134, 105457. [Google Scholar] [CrossRef]
- Hou, J.; Peng, Q.J.; Sakaguchi, K.; Takeda, Y.; Kuniya, J.; Shoji, T. Effect of hydrogen in Inconel Alloy 600 on corrosion in high temperature oxygenated water. Corros. Sci. 2010, 52, 1098–1101. [Google Scholar] [CrossRef]
- Wang, Z.; Taleda, Y. Mechanistic Understanding of the Role of Hydrogen in Modification of Oxide Film of Alloy 600 in High Temperature High Pressure Water Environment. Corros. Sci. 2020, 170, 108656. [Google Scholar] [CrossRef]
- Wang, Z.; Takeda, Y. Roles of permeated hydrogen in the oxidation process of Ni-based alloy in high temperature water environment. Corros. Sci. 2021, 179, 109139. [Google Scholar] [CrossRef]
- Ziemniak, S.E.; Hanson, M. Corrosion behavior of NiCrFe Alloy 600 in high temperature, hydrogenated water. Corros. Sci. 2006, 48, 498–521. [Google Scholar] [CrossRef] [Green Version]
- Kuang, W.; Wu, X.; Han, E.H.; Rao, J. The mechanism of oxide film formation on Alloy 690 in oxygenated high temperature water. Corros. Sci. 2011, 53, 3853–3860. [Google Scholar] [CrossRef]
- Fournier, L.; Calonne, O.; Combrade, P.; Scott, P.; Chou, P.; Pathania, R. Grain boundary oxidation and embrittlement prior to crack initiation in Alloy 600 in PWR primary water. In Proceedings of the 15th International Conference on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors; Springer: Cham, Switzerland, 2011; pp. 1491–1501. [Google Scholar]
- Kuang, W.; Song, M.; Was, G.S. Insights into the stress corrosion cracking of solution annealed alloy 690 in simulated pressurized water reactor primary water under dynamic straining. Acta Mater. 2018, 151, 321–333. [Google Scholar] [CrossRef]
- Tveten, B.; Hultquist, G.; Norby, T. Hydrogen in chromium: Influence on the high-temperature oxidation kinetics in O2, oxide-growth mechanisms, and scale adherence. Oxid. Met. 1999, 51, 221–233. [Google Scholar] [CrossRef]
- Fukai, Y.; Akuma, N. Formation of superabundant vacancies in Pd hydride under high hydrogen pressures. Phys. Rev. Lett. 1994, 73, 1640–1643. [Google Scholar] [CrossRef]
- Fukai, Y. Superabundant Vacancies Formed in Metal Hydrogen Alloys. Phys. Scr. 2003, 103, 11–14. [Google Scholar] [CrossRef]
- Fukai, Y.F.Y.; Ōkuma, N.Ō.N. Evidence of copious vacancy formation in Ni and Pd under a high hydrogen pressure. Jpn. J. Appl. Phys. 1993, 32, L1256–L1259. [Google Scholar] [CrossRef]
- Fukai, Y.; Ishii, Y.; Goto, Y.; Watanabe, K. Formation of superabundant vacancies in Pd-H alloys. J. Alloys Compd. 2000, 313, 121–132. [Google Scholar] [CrossRef]
- Li, M.; Xie, D.G.; Ma, E.; Li, J.; Zhang, X.X.; Shan, Z.W. Effect of hydrogen on the integrity of aluminium-oxide interface at elevated temperatures. Nat. Commun. 2017, 8, 14564. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Takeda, Y. Hydrogen Assisted Oxidation Behavior of Alloy 600 in High Temperature Air Environment by in-situ H Charging Method. In Proceedings of the 19th International Conference on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors, Boston, MA, USA, 18–22 August 2019; American Nuclear Society: La Grange Park, IL, USA, 2019; pp. 1304–1310. [Google Scholar]
- Wang, Z.; Takeda, Y. Elemental Distribution in Oxide Film Formed by Hydrogen Penetration Using Dual-Exposed Specimen; IICAPP: Dublin, Ireland, 2019. [Google Scholar]
- Yang, Z.; Walker, M.S.; Singh, P.; Stevenson, J.W.; Norby, T. Oxidation Behavior of Ferritic Stainless Steels under SOFC Interconnect Exposure Conditions. J. Electrochem. Soc. 2004, 151, B669–B678. [Google Scholar] [CrossRef]
- Yang, Z.; Walker, M.S.; Singh, P.; Stevenson, J.W. Anomalous Corrosion Behavior of Stainless Steels under SOFC Interconnect Exposure Conditions, Electrochem. Solid-State Lett. 2003, 6, B35–B37. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, G.; Singh, P.; Stevenson, J.W. Effects of water vapor on oxidation behavior of ferritic stainless steels under solid oxide fuel cell interconnect exposure conditions. Solid State Ion. 2005, 176, 1495–1503. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, G.G.; Walker, M.S.; Wang, C.M.; Stevenson, J.W.; Singh, P. High temperature oxidation/corrosion behavior of metals and alloys under a hydrogen gradient. Int. J. Hydrogen Energy 2007, 32, 3770–3777. [Google Scholar] [CrossRef]
- Pankove, J.I.; Johnson, N.M. Hydrogen in Semiconductors; Academic Press: Boston, MA, USA, 1991. [Google Scholar]
- Norby, T. The influence of hydrogen defects on transport properties of oxides. Am. Ceram. Soc. Inc. Nonstoichiom. Compd. Adv. Ceram. 1987, 23, 107–123. [Google Scholar]
- Norby, T. Protonic defects in oxide and their possible role in high temperature oxidation. J. Phys. IV 1993, 3, 99–106. [Google Scholar] [CrossRef]
- Wallinder, D.; Hultquist, G.; Tveten, B.; Hörnlund, E. Hydrogen in chromium: Influence on corrosion potential and anodic dissolution in neutral NaCl solution. Corros. Sci. 2001, 43, 1267–1281. [Google Scholar] [CrossRef]
- Wallinder, D.; Hörnlund, E.; Hultquist, G. Influence of Hydrogen in Iron and in Two Stainless Steels on Aqueous and Gaseous Corrosion. J. Electrochem. Soc. 2002, 149, B393. [Google Scholar] [CrossRef]
- Hultquist, G.; Tveten, B.; Norby, T. Hydrogen in chromium: Influence on the high-temperature oxidation kinetics in H2O, oxide-growth mechanisms, and scale adherence. Oxid. Met. 1999, 54, 1–10. [Google Scholar] [CrossRef]
- Das, N.K.; Suzuki, K.; Ogawa, K.; Shoji, T. Early stage SCC initiation analysis of fcc Fe-Cr-Ni ternary alloy at 288 °C: A quantum chemical molecular dynamics approach. Corros. Sci. 2009, 51, 908–913. [Google Scholar] [CrossRef]
- Das, N.K.; Suzuki, K.; Takeda, Y.; Ogawa, K.; Shoji, T. Quantum chemical molecular dynamics study of stress corrosion cracking behavior for fcc Fe and Fe-Cr surfaces. Corros. Sci. 2008, 50, 1701–1706. [Google Scholar] [CrossRef]
- Das, N.K.; Shoji, T. An atomic study of hydrogen effect on the early stage oxidation of transition metal surfaces. Int. J. Hydrogen Energy 2013, 38, 1644–1656. [Google Scholar] [CrossRef]
- Das, N.K.; Shoji, T. Early stage oxidation of Ni-Cr binary alloy 111, 110 and 100 surfaces: A combined density functional and quantum chemical molecular dynamics study. Corros. Sci. 2013, 73, 18–31. [Google Scholar] [CrossRef]
- Xu, J.; Shoji, T. The corrosion behavior of Alloy 182 in a cyclic hydrogenated and oxygenated water chemistry in high temperature aqueous environment. Corros. Sci. 2016, 104, 248–259. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Han, E.H.; Ke, W. Analysis of surface oxide film formed on eletropolished alloy 690tt in high temperature and high pressure water with sequentially dissolved hydrogen and oxygen. Jinshu Xuebao/Acta Metall. Sin. 2015, 51, 85–92. [Google Scholar] [CrossRef]
- Mendonça, R.; Bosch, R.W.; van Renterghem, W.; Vankeerberghen, M.; Figueiredo, C.d. Effect of temperature and dissolved hydrogen on oxide films formed on Ni and Alloy 182 in simulated PWR water. J. Nucl. Mater. 2016, 477, 280–291. [Google Scholar] [CrossRef]
- Attanasio, S.A.; Morton, D.S.; Ando, M.A.; Panayotou, N.F.; Thompson, C.D. Measurement of the Nickel/Nickel Oxide Phase Transition in High Temperature Hydrogenated Water Using the Contact Electric Resistance CER Technique; Lockheed Martin Corporation: Schenectady, NY, USA, 2001. [Google Scholar]
- Terachi, T.; Totsuka, N.; Yamada, T.; Nakagawa, T.; Deguchi, H.; Horiuchi, M.; Oshitani, M. Influence of dissolved hydrogen on structure of oxide film on alloy 600 formed in primary water of pressurized water reactors. J. Nucl. Sci. Technol. 2003, 40, 509–516. [Google Scholar] [CrossRef]
- Andresen, P.L.; Hickling, J.; Ahluwalia, A.; Wilson, J. Effects of hydrogen on stress corrosion crack growth rate of nickel alloys in high-temperature water. Corrosion 2008, 64, 707–720. [Google Scholar] [CrossRef]
- Totsuka, N.; Kamaya, M.; Nakajima, N.; Mitsuda, H. SS, A New Evaluation Method for Short Crack Growth and Influence of Dissolved Hydrogen on PWSCC of Alloy 600 the Principles of Crack Growth Rate Evaluation Method. In Proceedings of the 10th International Conference on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors, Lake Tahoe, NE, USA, 5–9 August 2001. [Google Scholar]
- Guerre, C.; Laghoutaris, P.; Chêne, J.; Marchetti, L.; Molins, R.; Duhamel, C.; Sennour, M. Stress Corrosion Cracking of Alloy 600 in PWR Primary Water: Influence of Chromium, Hydrogen and oxygen Diffusion. In Proceedings of the 15th International Conference on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Morton, D.; Fish, J.; Schurman, M.K.; Attanasio, S.A. Influence of Dissolved Hydrogen on Nickel Alloy SCC in High Temperature Water. In CORROSION 99; OnePetro: Richardson, TX, USA, 1999. [Google Scholar]
- Andresen, P.L.; Morra, M.M. IGSCC of non-sensitized stainless steels in high temperature water. J. Nucl. Mater. 2008, 383, 97–111. [Google Scholar] [CrossRef]
- Nakagawa, T.; Totsuka, N.; Terachi, T.; Nakajima, N. Influence of Dissolved Hydrogen on Oxide Film and PWSCC of Alloy 600 in PWR Primary Water. J. Nucl. Sci. Technol. 2003, 40, 39–43. [Google Scholar] [CrossRef]
- Andresen, P.L.; Chou, P. Effects of Hydrogen on SCC Growth Rate of NI Alloys in BWR Water. In Proceedings of the 15th International Conference on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Mitsubishi Research Institute Inc. (Ed.) NISA Project on Enhancement of Ageing Management and Maintenance of Nuclear Power Stations 2006.4–2011.3. In Proceedings of the Inetrnational Symposium on the Ageing Management & Maintenance of Nuclear Power Plants, ISaG2020, Tokyo, Japan, 27–28 May 2010. [Google Scholar]
- Kim, J.-C. Calculation of relative axial gamma flux profile for IFA-727, CP-Note 10-01. 5 January 2010. [Google Scholar]
- Kim, J.-C. Fluence determination for IFA-727.1 using flux monitor wire results, CP-Note 09-40. 30 November 2009. [Google Scholar]
- Takeda, Y.; Sato, T.; Yamauchi, D.; Shoji, T.; Ohji, A. Non-lnear dynamics of the morphology at the oxide/metal interface of austenitic steels in simulated light water reactor environmenets and its implications for SCC initiation. In Proceedings of the 15th International Conference on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors; Springer: Cham, Switzerland, 2011; pp. 409–419. [Google Scholar]
- Harasawa, S. Radioisotope; Corona Publishing Co., Ltd.: Tokyo, Japan, 1979. (In Japanese) [Google Scholar]
- Das, N.K.; Suzuki, K.; Taakeda, Y.; Ogawa, K.; Shoji, T. Stress Corrosion Cracking Analysis of Fe 111 and Fe-Cr 111 Surfaces at Different Temperatures by Quantum Chemical Molecular Dynamics. In Proceedings of the 16th Pacific Basin Nuclear Conference, Aomori, Japan, 1 July 2008. [Google Scholar]
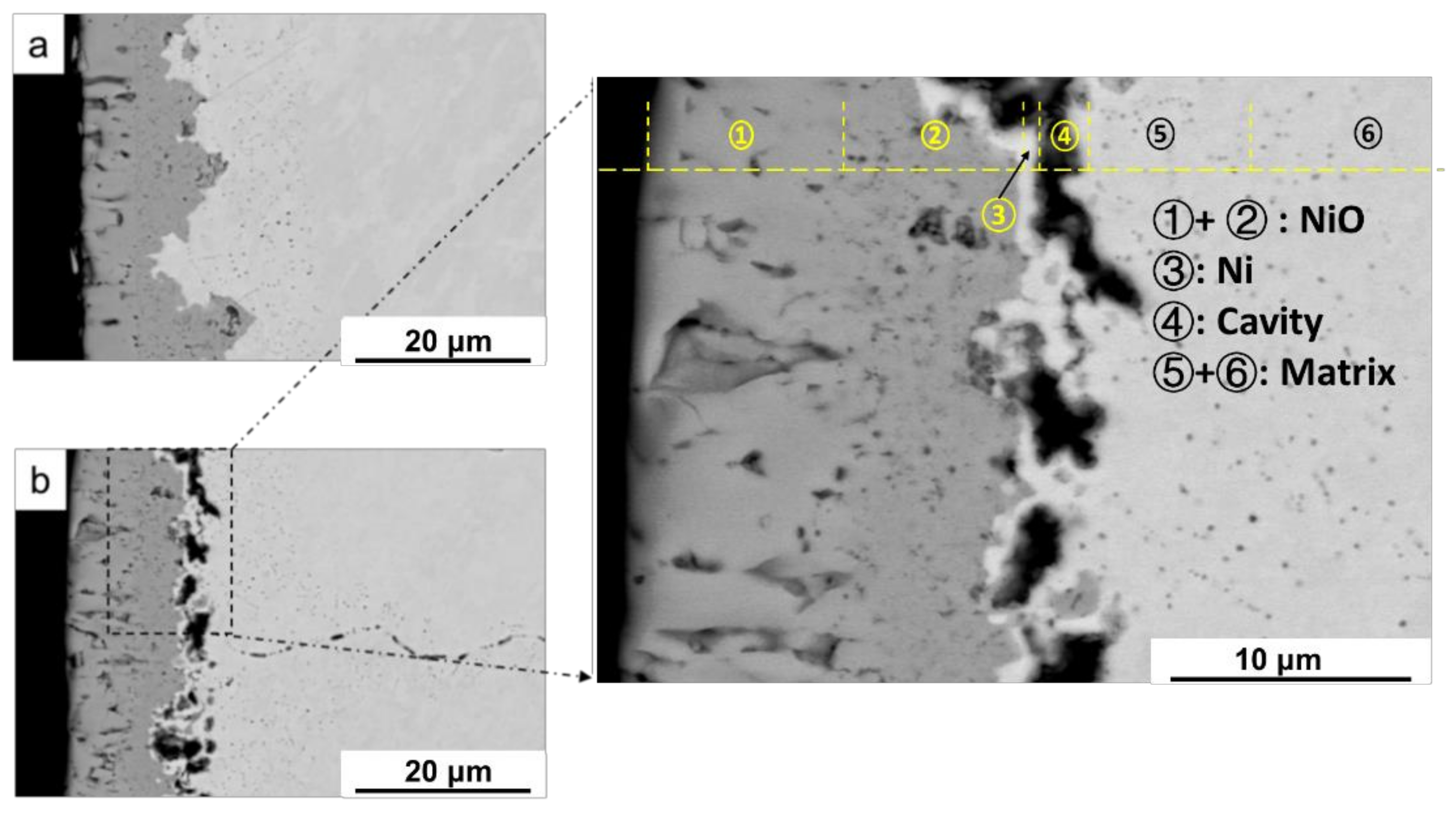
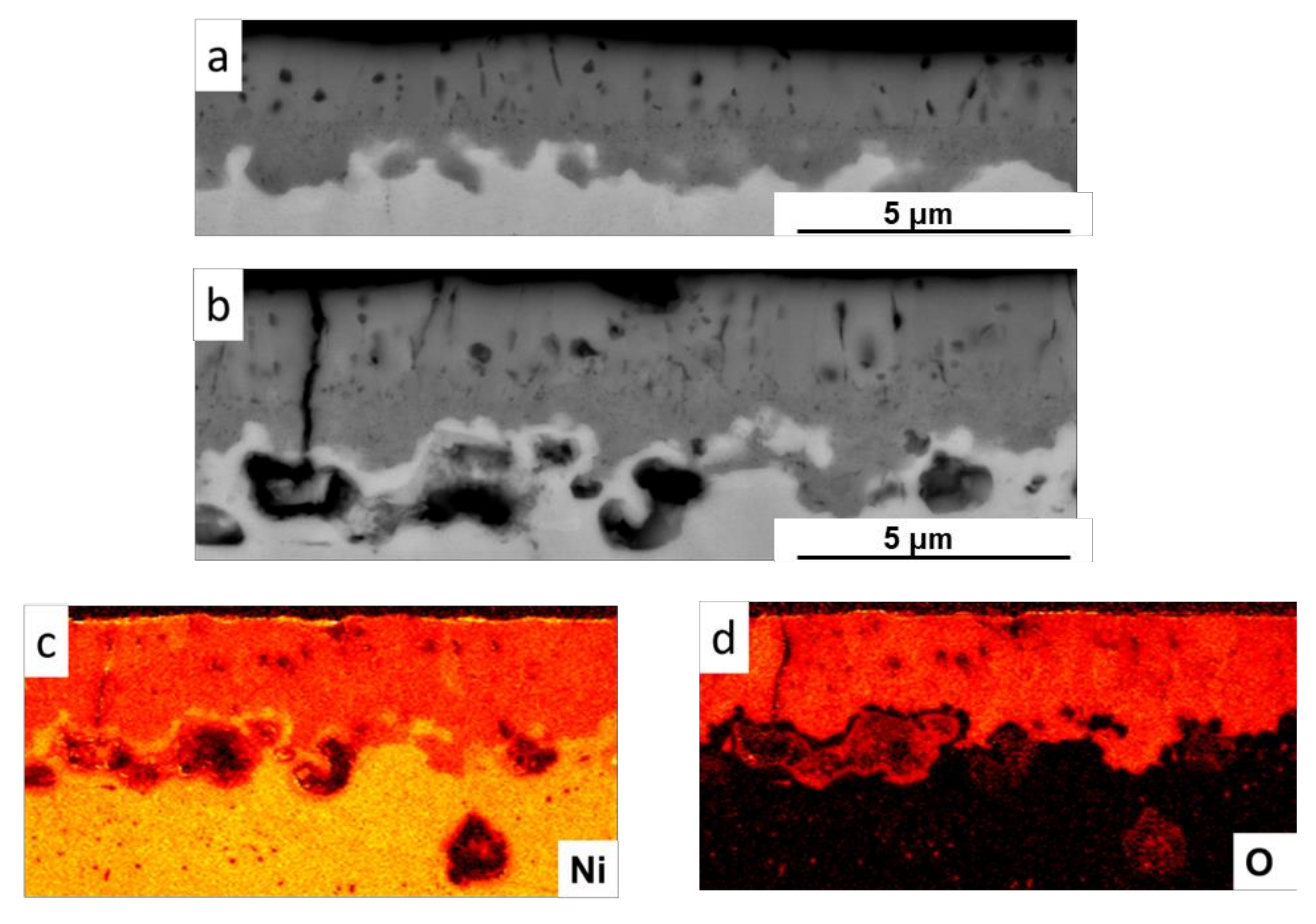
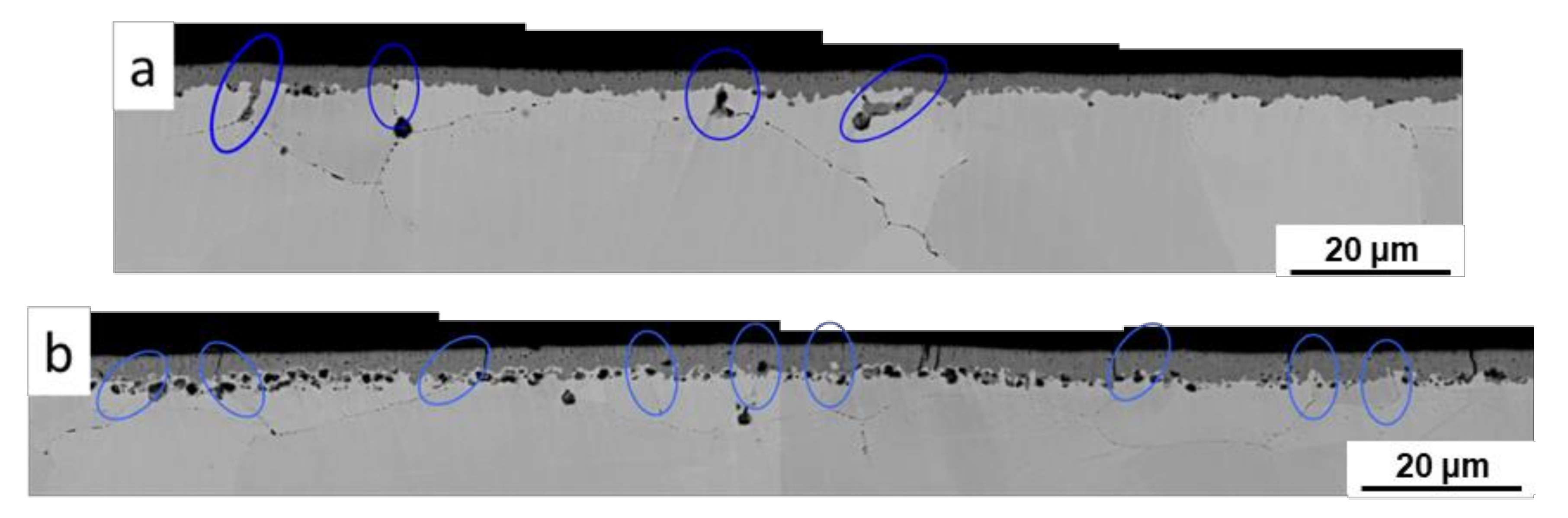
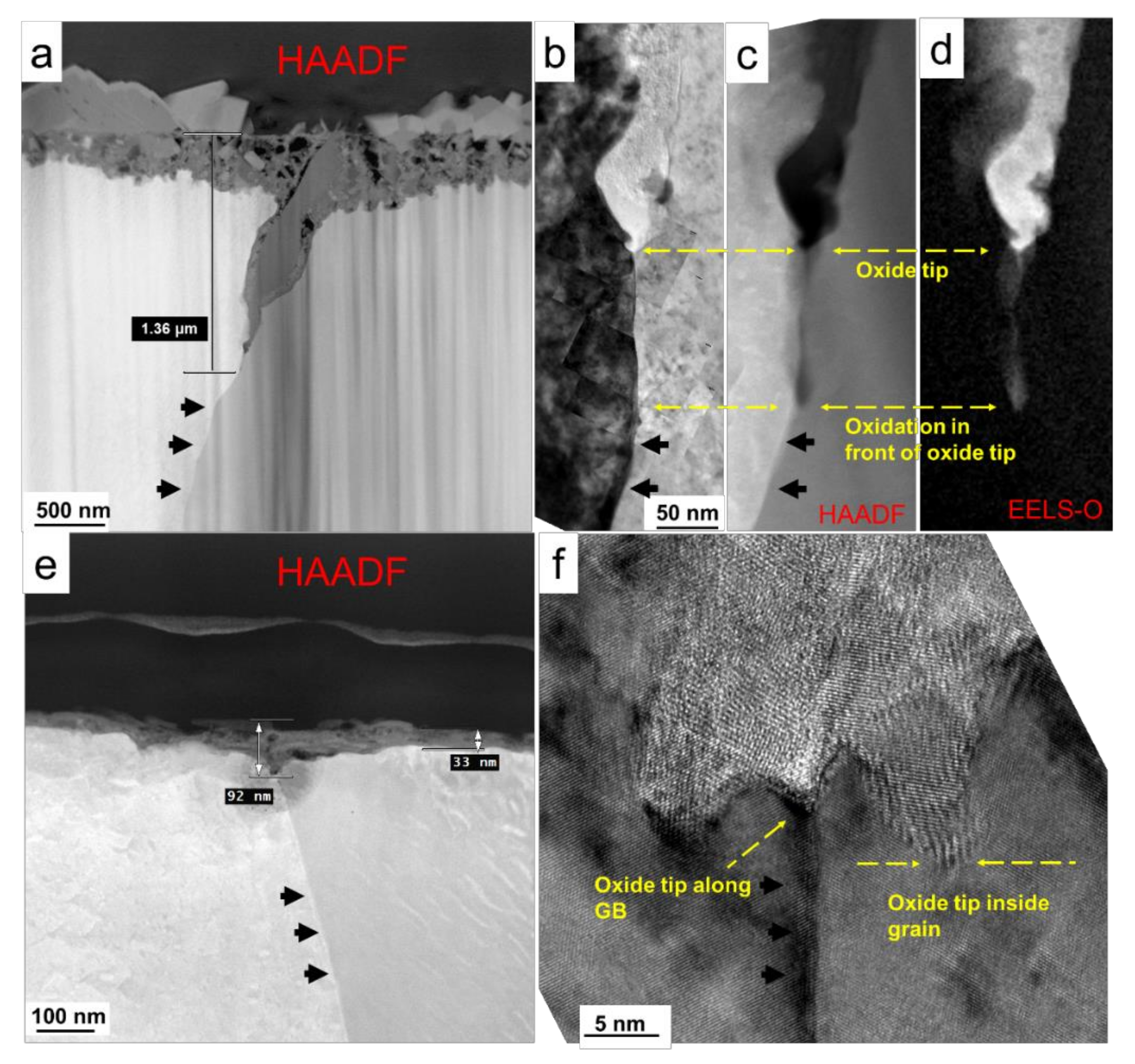
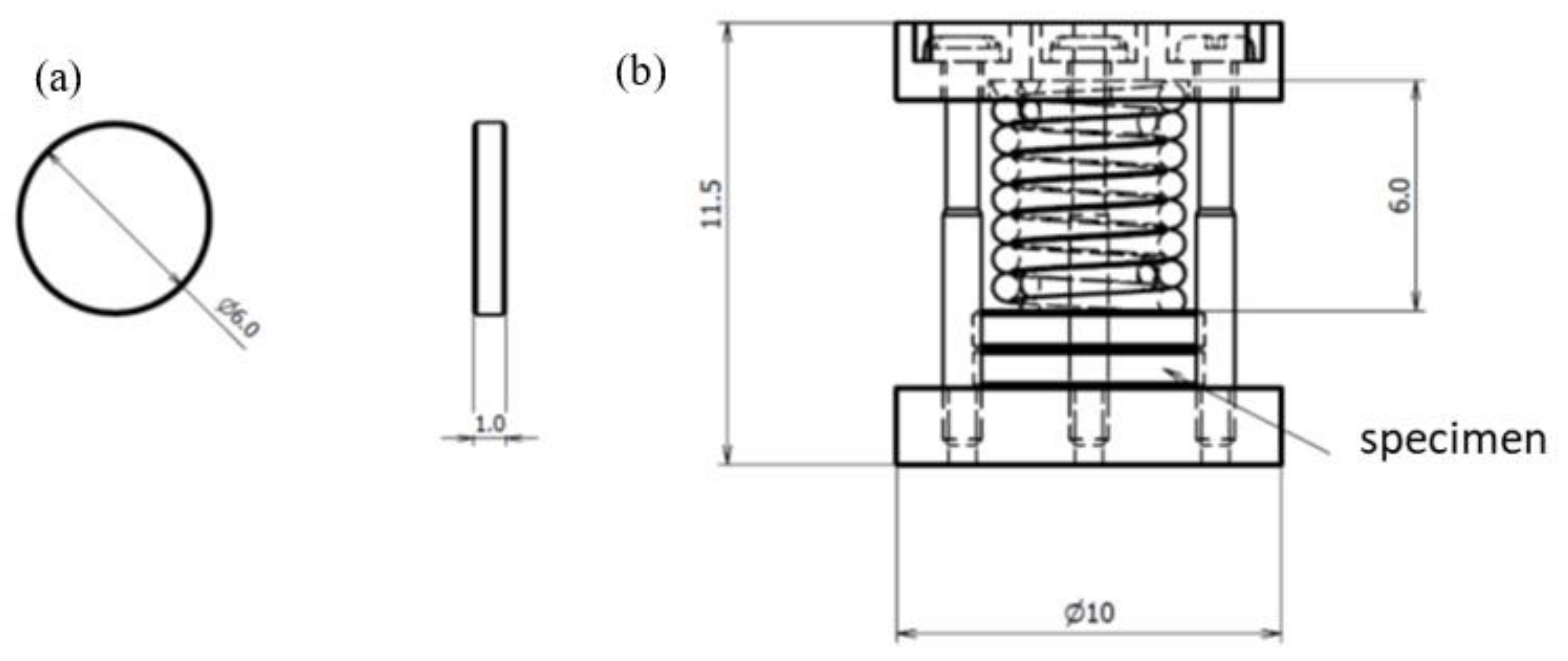



| Alloy | C | Si | Mn | P | S | Ni | Cr | Mo | Fe | N |
|---|---|---|---|---|---|---|---|---|---|---|
| 316 #1 | 0.018 | 0.47 | 0.82 | 0.028 | 0.001 | 12.06 | 17.68 | 2.17 | Bal. | 0.039 |
| 316 #2 (Nucl. grade) | 0.020 | 0.50 | 0.81 | 0.025 | 0.002 | 12.89 | 16.59 | 2.39 | Bal. | 0.094 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shoji, T. Role of Hydrogen in Metal Oxidation—Implication to Irradiation Enhanced Corrosion of Ni-Based Alloys and Stainless Steels in High Temperature Water. Corros. Mater. Degrad. 2022, 3, 281-302. https://doi.org/10.3390/cmd3020017
Wang Z, Shoji T. Role of Hydrogen in Metal Oxidation—Implication to Irradiation Enhanced Corrosion of Ni-Based Alloys and Stainless Steels in High Temperature Water. Corrosion and Materials Degradation. 2022; 3(2):281-302. https://doi.org/10.3390/cmd3020017
Chicago/Turabian StyleWang, Zihao, and Tetsuo Shoji. 2022. "Role of Hydrogen in Metal Oxidation—Implication to Irradiation Enhanced Corrosion of Ni-Based Alloys and Stainless Steels in High Temperature Water" Corrosion and Materials Degradation 3, no. 2: 281-302. https://doi.org/10.3390/cmd3020017
APA StyleWang, Z., & Shoji, T. (2022). Role of Hydrogen in Metal Oxidation—Implication to Irradiation Enhanced Corrosion of Ni-Based Alloys and Stainless Steels in High Temperature Water. Corrosion and Materials Degradation, 3(2), 281-302. https://doi.org/10.3390/cmd3020017






