Development of Multifunctional CoAl Based Layered Double Hydroxide Protective Film on Aluminum Alloy
Abstract
:1. Introduction
2. Materials and Synthesis
2.1. Pretreatment
2.2. Synthesis of CoAl-LDHs
3. Characterization
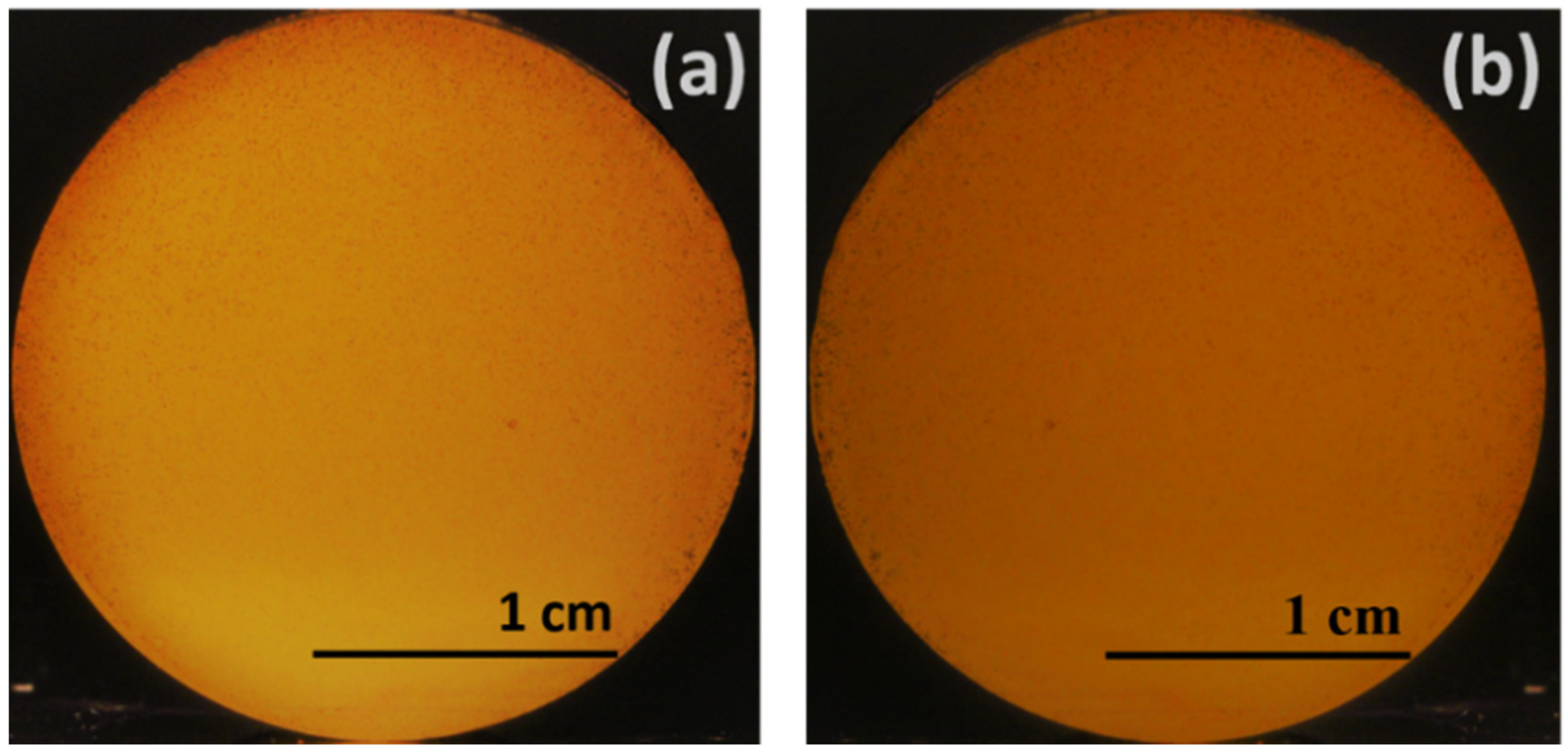
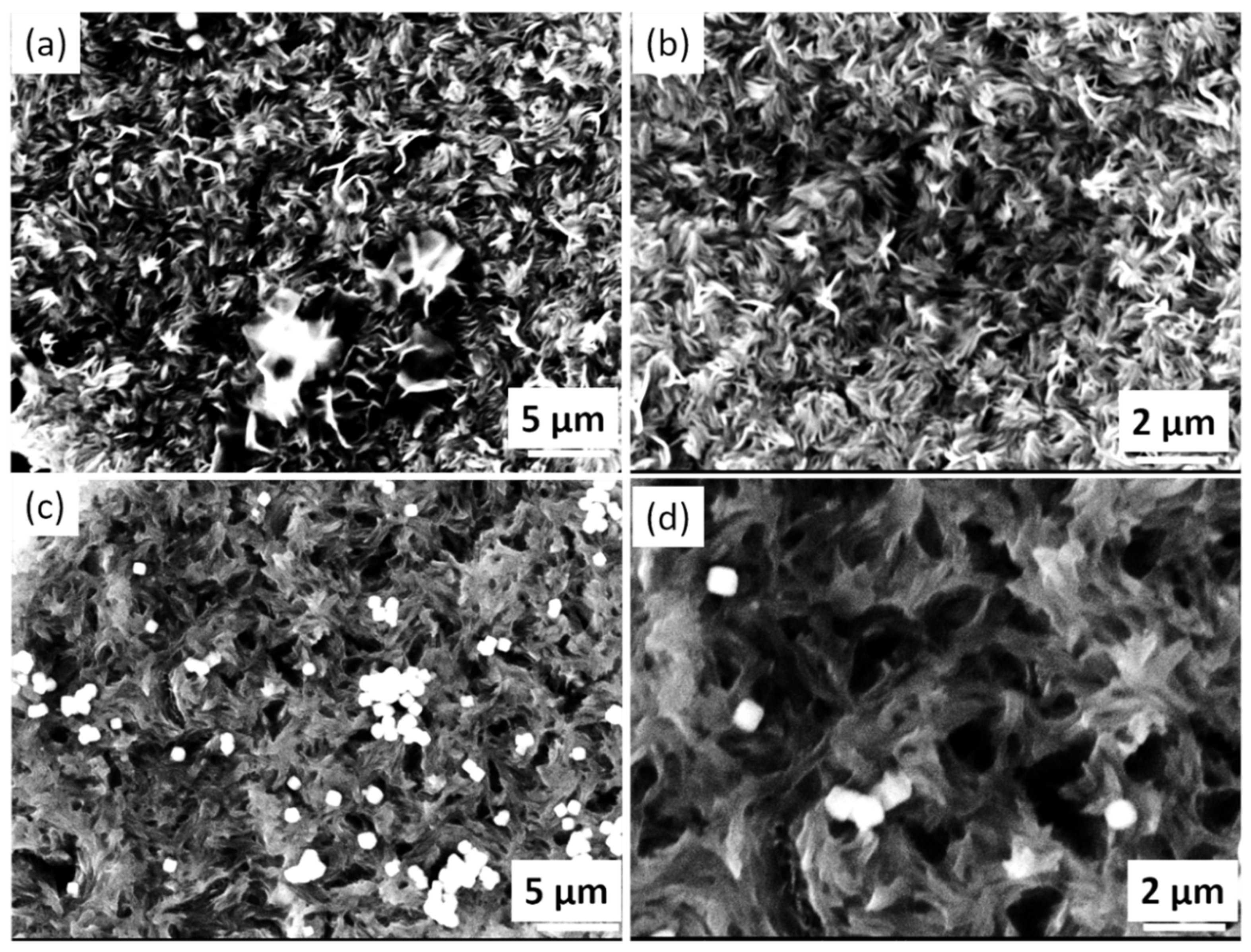
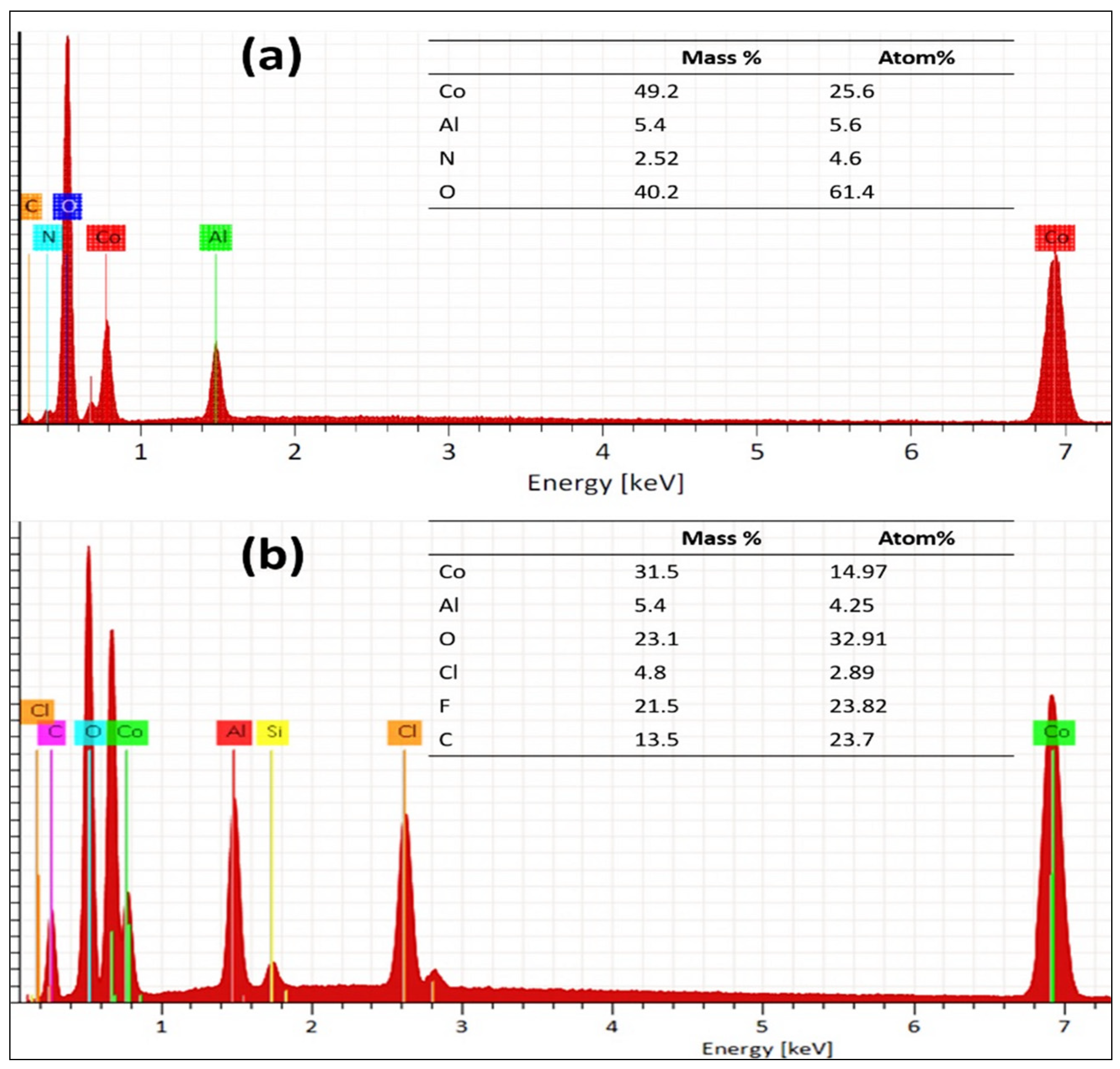
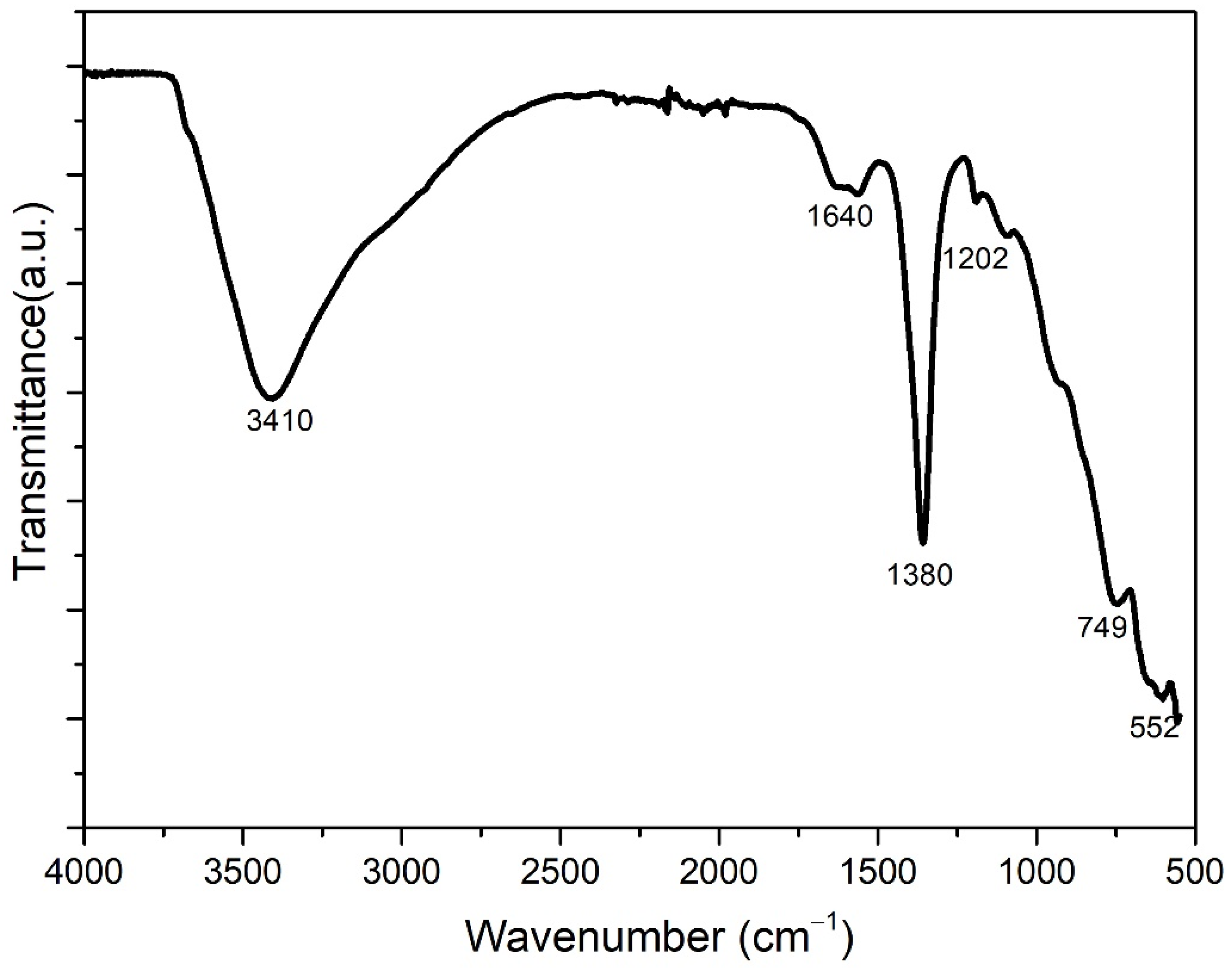
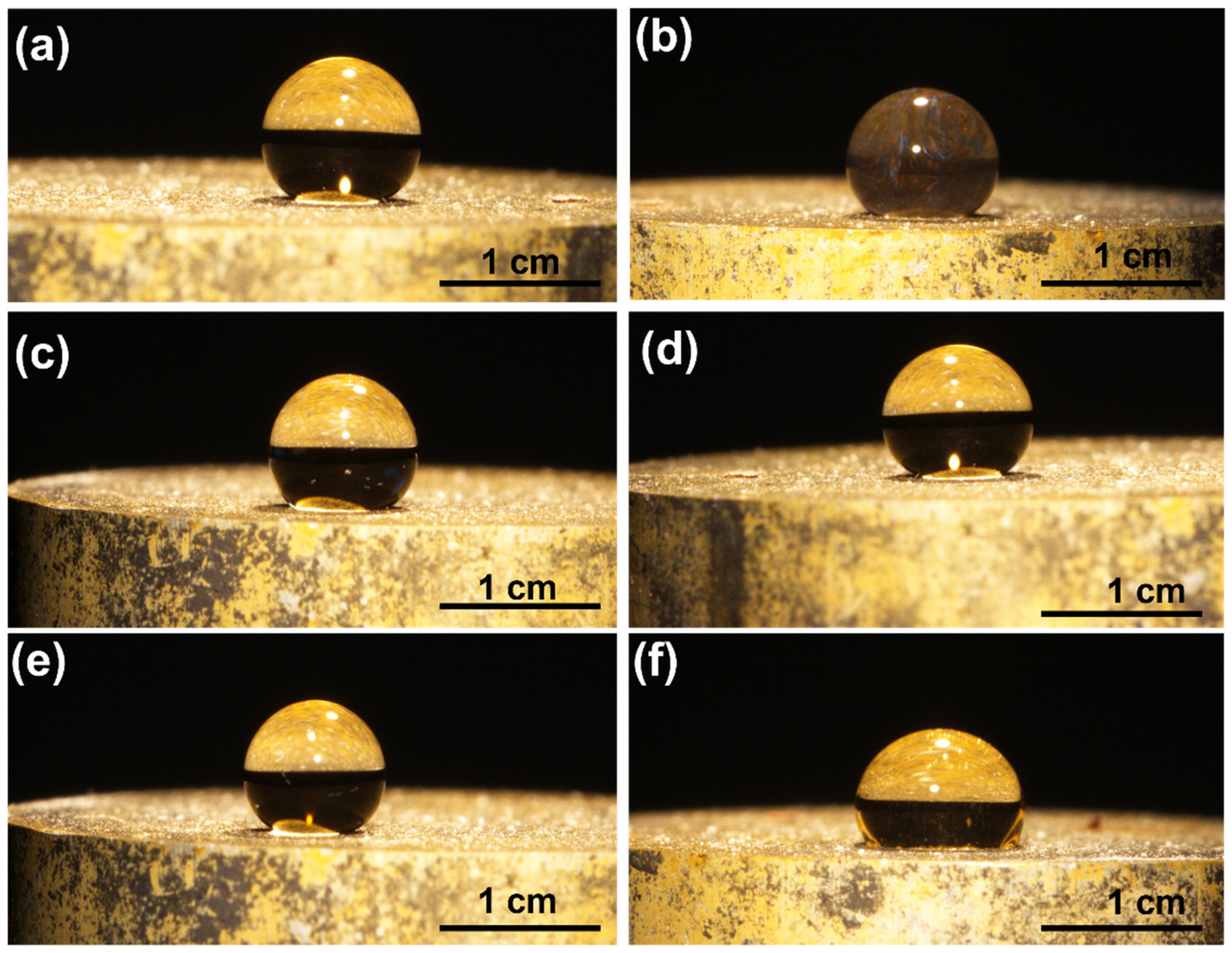
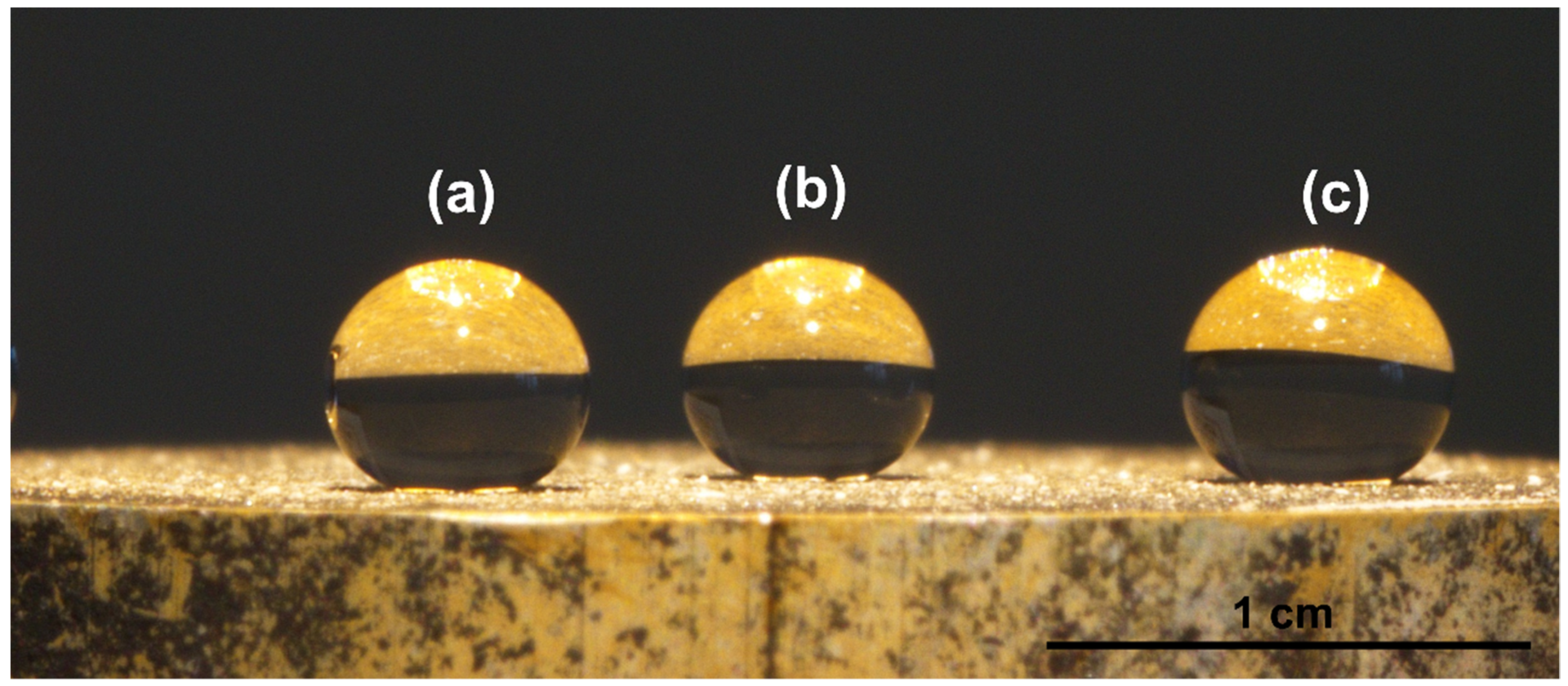
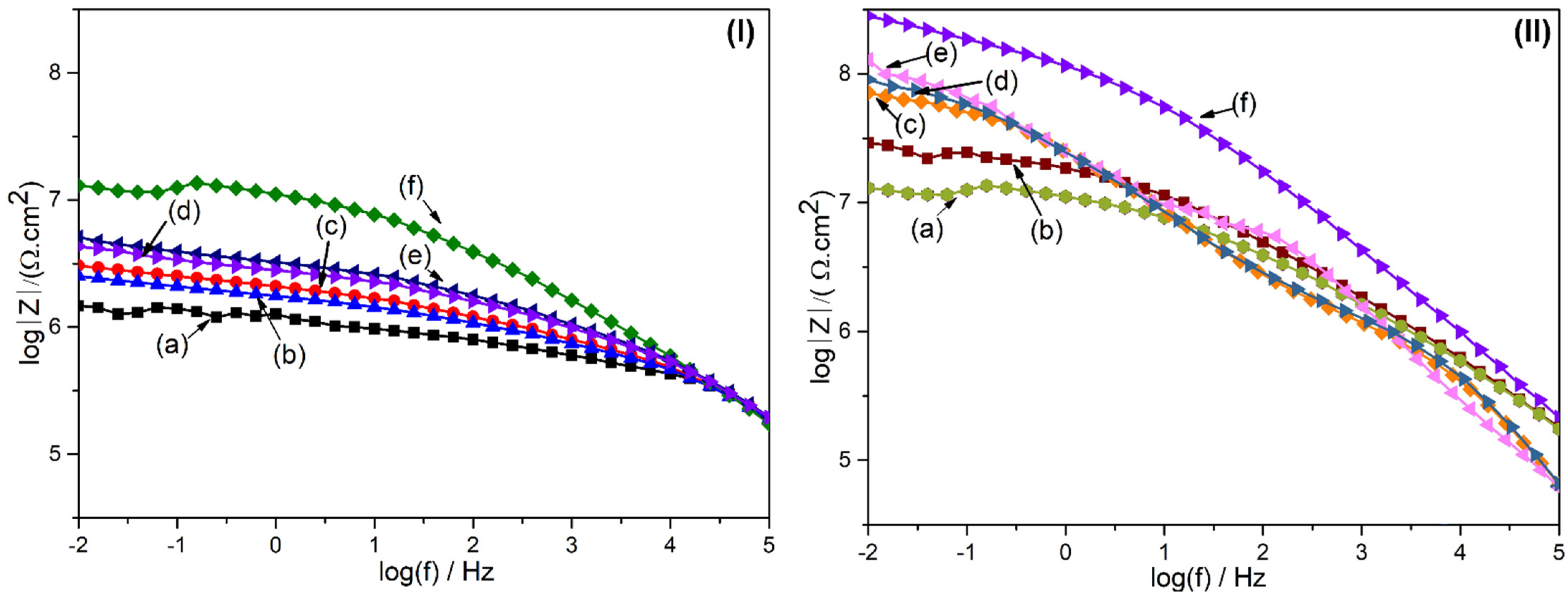
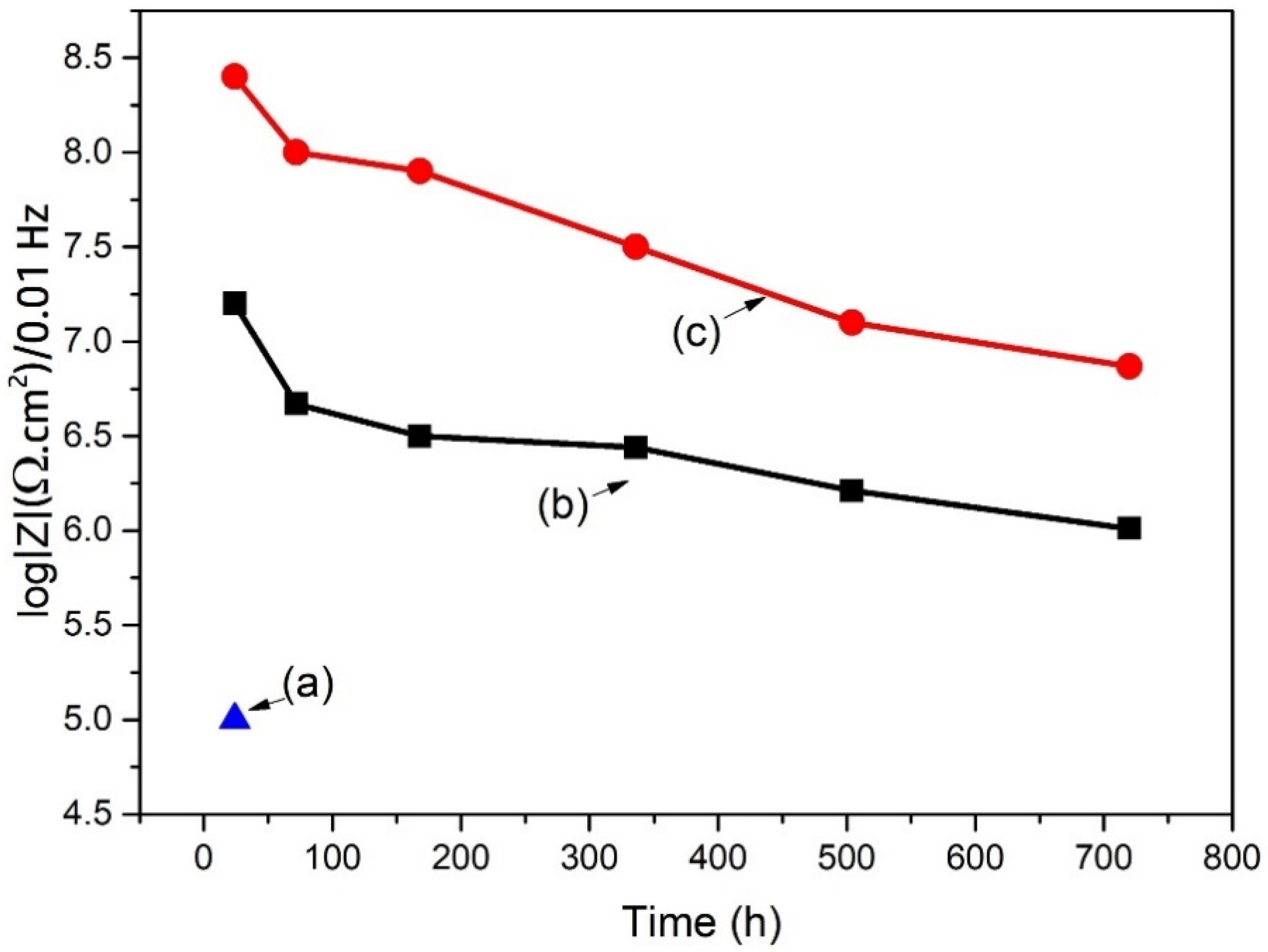
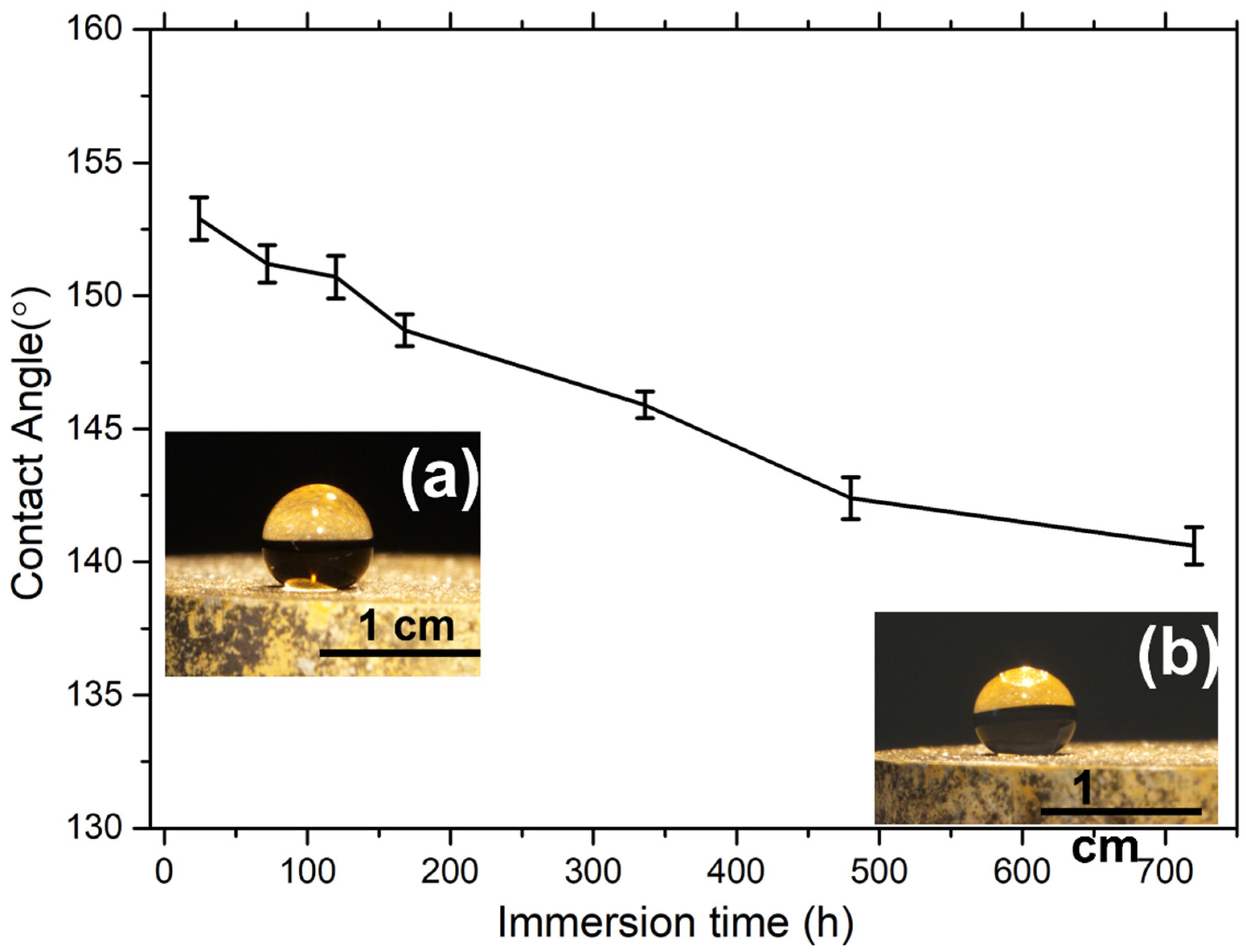
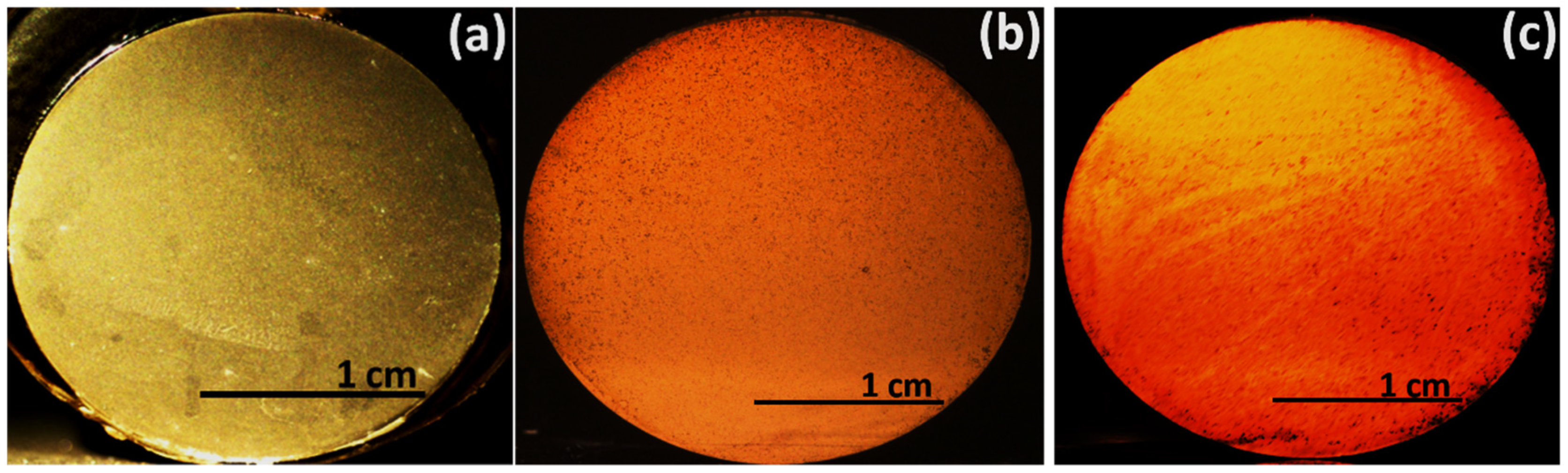
4. Experimental Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iqbal, M.A.; Sun, L.; Barrett, A.T.; Fedel, M. Layered double hydroxide protective films developed on aluminum and aluminum alloys: Synthetic methods and anti-corrosion mechanisms. Coatings 2020, 10, 428. [Google Scholar] [CrossRef]
- Wu, M.J.; Wu, J.Z.; Zhang, J.; Chen, H.; Zhou, J.Z.; Qian, G.R.; Xu, Z.P. (Gordon); Du, Z.; Rao, Q.L. A review on fabricating heterostructures from layered double hydroxides for enhanced photocatalytic activities. Catal. Sci. Technol. 2018, 8, 1207–1228. [Google Scholar] [CrossRef]
- Zheludkevich, M.; Poznyak, S.; Rodrigues, L.; Raps, D.; Hack, T.; Dick, L.F.P.; Nunes, T.; Ferreira, M. Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, F.; Evans, D.G.; Duan, X. Layered double hydroxide films: Synthesis, properties and applications. Chem. Commun. 2010, 46, 5197–5210. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Asghar, H.; Fedel, M. Sorption of As(V) from aqueous solution using in situ growth MgAl–NO3 layered double hydroxide thin film developed on AA6082. SN Appl. Sci. 2019, 1, 666. [Google Scholar] [CrossRef] [Green Version]
- Shirin, V.A.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.; Pramod, K. Advanced drug delivery applications of layered double hydroxide. J. Control. Release 2021, 330, 398–426. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wei, M. Layered double hydroxide-based catalysts: Recent advances in preparation, structure, and applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Xuan, X.; Qian, M.; Han, L.; Wan, L.; Li, Y.; Lu, T.; Pan, L.; Niu, Y.; Gong, S. In-situ growth of hollow NiCo layered double hydroxide on carbon substrate for flexible supercapacitor. Electrochim. Acta 2019, 321, 134710. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Fedel, M. Ordering and disordering of in situ grown MgAl-layered double hydroxide and its effect on the structural and corrosion resistance properties. Int. J. Miner. Metall. Mater. 2019, 26, 1570–1577. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Asghar, H.; Fedel, M. Double doped cerium-based superhydrophobic layered double hydroxide protective films grown on anodic aluminium surface. J. Alloys Compd. 2020, 844, 156112. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Li, X.; Lin, J.; Wang, C.; Dong, S.; Lin, C. Enhanced corrosion resistance of superhydrophobic layered double hydroxide films with long-term stability on Al substrate. ACS Appl. Mater. Interfaces 2018, 10, 15150–15162. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Secchi, M.; Iqbal, M.A.; Montagna, M.; Zanella, C.; Fedel, M. MgAl-LDH/graphene protective film: Insight into LDH-graphene interaction. Surf. Coat. Technol. 2020, 401, 126253. [Google Scholar] [CrossRef]
- Guo, X.; Xu, S.; Zhao, L.; Lu, W.; Zhang, F.; Evans, D.G.; Duan, X. One-step hydrothermal crystallization of a layered double hydroxide/alumina bilayer film on aluminum and its corrosion resistance properties. Langmuir 2009, 25, 9894–9897. [Google Scholar] [CrossRef] [PubMed]
- Tedim, J.; Kuznetsova, A.I.; Salak, A.N.; Montemor, F.; Snihirova, D.; Pilz, M.; Zheludkevich, M.L.; Ferreira, M.G.S. Zn–Al layered double hydroxides as chloride nanotraps in active protective coatings. Corros. Sci. 2012, 55, 1–4. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, Z.; Jiang, B.; Sun, Y.; Chen, Z.; Gao, X.; Yang, N. Ni-Al layered double hydroxides (LDHs) coated superhydrophobic mesh with flower-like hierarchical structure for oil/water separation. Appl. Surf. Sci. 2019, 490, 145–156. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, H.; You, Z.; Xu, F.; Jiang, G.; Lv, S.; Zhang, R.; Yang, H. Preparation and anti-icing properties of a superhydrophobic silicone coating on asphalt mixture. Constr. Build. Mater. 2018, 189, 227–235. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhang, Y.; Yu, M.; Liu, J. Fabrication of superhydrophobic layered double hydroxides films with different metal cations on anodized aluminum 2198 alloy. Mater. Lett. 2015, 142, 137–140. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Asghar, H.; Fedel, M. Ce-Doped-MgAl Superhydrophobic Layered Double Hydroxide for Enhanced Corrosion Resistance Properties. Solids 2021, 2, 76–86. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, L.; Chen, H.; Xu, S.; Evans, D.G.; Duan, X. Corrosion resistance of superhydrophobic layered double hydroxide films on aluminum. Angew. Chem. 2008, 120, 2500–2503. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z. Facile synthesis of superhydrophobic three-metal-component layered double hydroxide films on aluminum foils for highly improved corrosion inhibition. New J. Chem. 2019, 43, 2289–2298. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, F.; Fu, S.; Duan, X. In situ microstructure control of oriented layered double hydroxide monolayer films with curved hexagonal crystals as superhydrophobic materials. Adv. Mater. 2006, 18, 3089–3093. [Google Scholar] [CrossRef]
- Xu, F.; Wang, T.; Chen, H.; Bohling, J.; Maurice, A.M.; Wu, L.; Zhou, S. Preparation of photocatalytic TiO2-based self-cleaning coatings for painted surface without interlayer. Prog. Org. Coat. 2017, 113, 15–24. [Google Scholar] [CrossRef]
- Song, K.; Kim, I.; Bang, S.; Jung, J.-Y.; Nam, Y. Corrosion resistance of water repellent aluminum surfaces with various wetting morphologies. Appl. Surf. Sci. 2018, 467–468, 1046–1052. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Li, Y.; Yu, M.; Li, S.; Xue, B. A facile approach to superhydrophobic LiAl-layered double hydroxide film on Al–Li alloy substrate. J. Coat. Technol. Res. 2015, 12, 595–601. [Google Scholar] [CrossRef]
- Cho, D.-K.; Park, I.-K. Evolution of structural and chemical properties of CoAl-based layered double hydroxides grown on silicon substrate. Ceram. Int. 2018, 44, 8556–8561. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Lu, C.; Lin, J.-M. Improved chemiluminescence in fenton-like reaction via dodecylbenzene-sulfonate-intercalated layered double hydroxides. J. Phys. Chem. C 2012, 116, 14711–14716. [Google Scholar] [CrossRef]
- Baliarsingh, N.; Parida, K.M.; Pradhan, G.C. Effects of Co, Ni, Cu, and Zn on photophysical and photocatalytic properties of carbonate intercalated MII/Cr LDHs for enhanced photodegradation of methyl orange. Ind. Eng. Chem. Res. 2014, 53, 3834–3841. [Google Scholar] [CrossRef]
- Bai, J.; Liu, Y.; Yin, X.; Duan, H.; Ma, J. Efficient removal of nitrobenzene by Fenton-like process with Co-Fe layered double hydroxide. Appl. Surf. Sci. 2017, 416, 45–50. [Google Scholar] [CrossRef]
- Gong, C.; Chen, F.; Yang, Q.; Luo, K.; Yao, F.; Wang, S.; Wang, X.; Wu, J.; Li, X.; Wang, D.; et al. Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B. Chem. Eng. J. 2017, 321, 222–232. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Martens, W.N.; Frost, R.L. Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J. Phys. Chem. C 2009, 114, 111–119. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wang, Y.; Zhou, X.; Yin, M.; Pu, J.; Yuan, N.; Ding, J. Superhydrophobic and Self-Healing Mg-Al Layered Double Hydroxide/Silane Composite Coatings on the Mg Alloy Surface with a Long-Term Anti-corrosion Lifetime. Langmuir 2021, 37, 8129–8138. [Google Scholar] [CrossRef]
- Aisawa, S.; Hirahara, H.; Uchiyama, H.; Takahashi, S.; Narita, E. Synthesis and thermal decomposition of Mn–Al layered Double hydroxides. J. Solid State Chem. 2002, 167, 152–159. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Kloprogge, J.; Frost, R.L. Fourier transform infrared and raman spectroscopic study of the local structure of Mg-, Ni-, and Co-hydrotalcites. J. Solid State Chem. 1999, 146, 506–515. [Google Scholar] [CrossRef]
- Ni, Z.; Xia, S.; Fang, C.; Wang, L.; Hu, J. Synthesis, characterization and thermal property of Cu/Co/Mg/Al hydrotalcite like compounds. Rare Met. Mater. Eng. S 2008, 2, 634–637. [Google Scholar]
- Wang, Z.; Shen, X.; Qian, T.; Xu, K.; Sun, Q.; Jin, C. Fabrication of superhydrophobic Mg/Al layered double hydroxide (LDH) coatings on medium density fiberboards (MDFs) with flame retardancy. Materials 2018, 11, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, T.; Xu, S.; Peng, Q.; Zhao, L.; Zhao, X.; Lei, X.; Zhang, F. Self-healing of layered double hydroxide film by dissolution/recrystallization for corrosion protection of aluminum. J. Electrochem. Soc. 2013, 160, C480–C486. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; He, H.; Sun, W.; Zong, Q.; Liu, G. Enhanced corrosion resistance of MgAl hydrotalcite conversion coating on aluminum by chemical conversion treatment. Surf. Coat. Technol. 2013, 235, 484–488. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Fedel, M. Protective Cerium-Based Layered Double Hydroxides Thin Films Developed on Anodized AA6082. Adv. Mater. Sci. Eng. 2020, 2020, 5785393. [Google Scholar] [CrossRef]
- Lin, K.; Luo, X.; Pan, X.; Zhang, C.; Liu, Y. Enhanced corrosion resistance of LiAl-layered double hydroxide (LDH) coating modified with a Schiff base salt on aluminum alloy by one step in-situ synthesis at low temperature. Appl. Surf. Sci. 2019, 463, 1085–1096. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Sun, L.; Asghar, H.; Fedel, M. Chlorides entrapment capability of various in-situ grown NiAl-LDHs: Structural and corrosion resistance properties. Coatings 2020, 10, 384. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, M.A.; Asghar, H.; Fedel, M. Development of Multifunctional CoAl Based Layered Double Hydroxide Protective Film on Aluminum Alloy. Corros. Mater. Degrad. 2021, 2, 708-720. https://doi.org/10.3390/cmd2040038
Iqbal MA, Asghar H, Fedel M. Development of Multifunctional CoAl Based Layered Double Hydroxide Protective Film on Aluminum Alloy. Corrosion and Materials Degradation. 2021; 2(4):708-720. https://doi.org/10.3390/cmd2040038
Chicago/Turabian StyleIqbal, Muhammad Ahsan, Humaira Asghar, and Michele Fedel. 2021. "Development of Multifunctional CoAl Based Layered Double Hydroxide Protective Film on Aluminum Alloy" Corrosion and Materials Degradation 2, no. 4: 708-720. https://doi.org/10.3390/cmd2040038
APA StyleIqbal, M. A., Asghar, H., & Fedel, M. (2021). Development of Multifunctional CoAl Based Layered Double Hydroxide Protective Film on Aluminum Alloy. Corrosion and Materials Degradation, 2(4), 708-720. https://doi.org/10.3390/cmd2040038





