Abstract
Radiation induced corrosion is one of the possible modes of materials degradation in the concept of long-term management of used nuclear fuel. Depending on the environmental conditions surrounding the used fuel container, a range of radiolysis products are expected to form that could impact the corrosion of the copper coating. For instance, γ-radiolysis of pure water produces molecular oxidants such as H2O2 and the radiolysis of humid air produces compounds such as NOx and HNO3. This review is confined to a discussion of the effect of γ-radiation on the corrosion of copper-coated containers. A simplified mixed-potential model is also presented to calculate the extent of copper corrosion by using the steady-state concentration of H2O2 generated during the first 300 years of emplacement, when the radiation field is significant.
1. Introduction
In common with all other programs worldwide, the Nuclear Waste Management Organization (NWMO) is developing a design for an underground deep geological repository (DGR) for the permanent disposal of used nuclear fuels. The repository concept is based on the accepted principle of isolation through the use of multiple engineered and natural barriers, with two of those engineered barriers being a copper used fuel container (UFC) surrounded by a compacted bentonite buffer material [1]. While this basic concept is similar to the Swedish and Finnish KBS-3 concept [2], there are a number of unique aspects of the NWMO design (Figure 1). These unique attributes arise from a number of factors, including the relatively small size of CANDU used fuel bundles compared with those for other reactor designs and the large volume of used fuel to be disposed of in Canada. For example, instead of the thick-walled copper-cast iron KBS-3 canister design, the NWMO design envisages the use of a thin copper coating as the corrosion barrier (the current reference thickness is 3 mm) applied to a carbon steel (C-steel) inner vessel, constructed from a standard pipe grade and which is designed to provide structural support against the various sources of external applied load. The use of a copper coating directly on steel eliminates the need for independent mechanical stability of the copper components; thus its thickness is defined by its corrosion allowance, which is expected to be limited under repository conditions. Furthermore, because the used fuel bundles are small (approximately 10 cm in diameter by 50 cm in length), there are a number of different viable arrangements for stacking them inside the container and, therefore, considerable flexibility in the size and shape of the UFC. The reference UFC design is 0.56 m in diameter, holds 48 used fuel bundles and is 2.5 m long (including the hemispherical ends designed to distribute the external load and to avoid tensile stresses in the region of the closure weld). The manageable size and mass (less than 3 tonnes when loaded with used fuel) make it feasible to emplace the containers with an integral layer of highly compacted bentonite (HCB) in a “buffer box” arrangement (Figure 1). The buffer box would be assembled in a shielded facility on the surface, with a single UFC per assembly, and then transported underground for emplacement in the disposal tunnels. This buffer box design allows greater quality control over the fabrication and installation of the HCB than is possible when the buffer is emplaced in situ in the DGR, and the small size of the buffer-box assembly allows for easier handling and more-efficient emplacement than is possible for other “supercontainer” designs with an integral canister and buffer material.
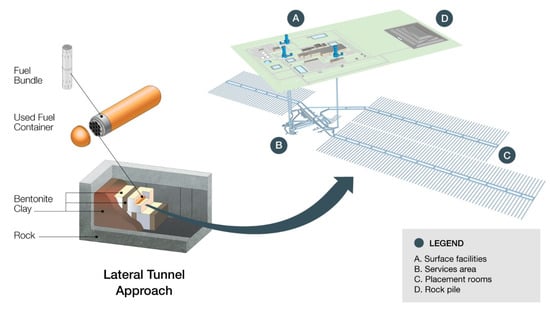
Figure 1.
Schematic of the design of the used fuel container, buffer box assembly, and deep geological repository for the disposal of used nuclear fuel in Canada.
The 3-mm-thick copper coating is expected to provide resistance to corrosion and to prevent a through-coating penetration for a period of at least one million years with a significant margin of safety [2]. The lifetime of the copper coating is estimated based on a series of corrosion allowances for each of the processes considered to occur under repository conditions. One of those processes is radiation-induced corrosion (RIC) due to the γ-irradiation emitted from the fission products trapped in the spent fuel matrix. The common understanding is that γ-radiation influences corrosion predominantly via radiolysis of the aqueous environment, producing redox active solution species, and not via direct interaction with the metal [3,4]. For the current discussion, we define RIC as including any effect of irradiation on the corrosion behaviour of the UFC, such as the production of oxidizing radiolysis products that may lead to an increase in the rate of corrosion, but also the apparent inhibition of corrosion induced by the formation of more-protective surface films.
The most important effect of radiation on the corrosion behaviour of the UFC is due to the production of oxidizing (and reducing) radiolysis products as a result of the absorption of ionizing radiation by the solvent (water). When water is exposed to ionizing radiation, it decomposes to form a range of chemically reactive species. The distribution of the water radiolysis products reaches homogeneity along the radiation track within 10−9 to 10−6 s. The chemical species formed at this time scale are referred to as primary radiolysis products and the yields are expressed in G-values. The G-value is defined as the number of molecules produced per unit of absorbed energy and has standard units of μmol/J. G-values depend strongly on the type of radiation (α, β, γ, etc.) and solvent properties. For γ−radiolysis of water, the primary radiolysis products and corresponding G-values (μmol/J) are shown in Table 1. The primary (or homogeneous) radiolysis yields are achieved in a very short time scale (<μs) when solute concentrations are less than 10−2 M. Since chemical reactions in condensed phases (liquid or solid) are slow, we can take the yields multiplied by the radiation dose rate (J/kg H2O/s = Gy/s) as the initial production rate of these species. Under continuous irradiation, these primary radiolysis products are formed continuously and react with each other, with additional H2O species (H2O, H+ and OH−), and with other solute species that may be present (such as O2 from the air in contact with the water, dissolved metal ions or anions). This results in the relatively rapid achievement of the pseudo-steady-state concentrations of the various radiolytic products. These steady-state concentrations are important in controlling corrosion in a steady-state radiation environment [4,5] and being able to predict them is, therefore, desirable.

Table 1.
The primary γ-radiolysis yields (G-values, μmol/J) in liquid water at 25 °C [6,7,8].
A question that arises is whether it is the dose rate or the total absorbed dose that is more important in determining the effects of irradiation on corrosion processes, such as the rate and extent of uniform corrosion or the ennoblement of the corrosion potential ECORR. It is the opinion of the authors that, of the two, the dose rate is the more important. Aqueous corrosion is a kinetic process, comprising a potentially large number of individual charge transfer (both interfacial and homogeneous) and chemical reactions, many in competition with each other. The rates of these individual processes depend on the concentration of the reacting species which, in the case of radiolytic species, depends on the dose rate. For long-lived radiolytic species and non-radiolytic reactants, the rate of mass transport may also be important. The overall rate of corrosion will be determined by the slowest of these individual reactions, the so-called rate-determining step. Furthermore, the value of the corrosion potential ECORR, a parameter of fundamental importance in corrosion science, is determined by the relative kinetics of the anodic (oxidation) and cathodic (reduction) reactions. Due to the fundamental role of these competing chemical and electrochemical kinetic processes, dose rate is more important that than the total dose in determining the corrosion behaviour. Due to the importance of the dose rate in determining the corrosion behaviour, care should be exercised when applying the results of corrosion experiments conducted at “high” dose rates (greater than, for example, a few tens of Gy/h) to the corrosion behaviour of used fuel containers exposed to “low” dose rates (less than a few Gy/h). For example, the dose rate is crucially important when discussing corrosion processes that depend on the value of ECORR, since the higher the dose rate the greater the ennoblement of ECORR and the more likely that processes such as passive film breakdown become.
The focus of this paper is the corrosion behaviour of the copper UFC due to the external γ-radiation field. The internal corrosion of the C-steel vessel due to entrained moisture inside the container, including the effects of irradiation, has been considered elsewhere [9]. Prior to reviewing the effects of irradiation on the corrosion of copper, the nature of the corrosive environment at the container surface is considered in some detail. The period of highest γ-radiation dose rate will coincide with thermal, redox, and moisture transients in the DGR near-field, which will impact the amount and type of radiolysis products that will be generated. These radiolysis products may induce corrosion of the UFC throughout the early transient period, as described in Section 2.2, and the relevant literature is described separately for unsaturated and saturated conditions. Finally, the implications for the long-term corrosion behaviour of the UFC are considered and an estimate is given for the corrosion allowance for RIC.
2. Nature of the Irradiation Environment
2.1. Magnitude and Duration of the Period of Irradiation
Due to the relatively thin wall of the copper-coated C-steel container, the radiation fields for the UFC are higher than those for the KBS-3 canister design, particularly in the axial direction due to the thinner dimensions of the hemispherical head. The combined copper and C-steel wall thickness is approximately 49 mm for the body of the container and 33 mm for the hemispherical head, for a nominal coating thickness of 3 mm. In comparison, the combined wall thickness of the copper corrosion barrier and cast-iron insert of the KBS-3 canister design is 80–100 mm [10]. However, used CANDU fuel is less radioactive than the used fuel from the pressurized or boiling reactors used in Sweden or Finland; thus, it requires less shielding, overall.
Figure 2 shows the time dependence of the γ-dose rate on the surface of the UFC at both the mid-point along the length (radial) and at the end of the hemispherical heads (axial) of the UFC, the latter location having the higher dose rate because of less shielding resulting from the reduced wall thickness. At the time of emplacement, the surface dose rate is of the order of 1 Gy/h, which compares with an average surface dose rate of 0.055 Gy/h for SKB’s KBS-3 canister [11]. The early decrease in dose rate is due to the decay of fission products and falls to insignificant levels after several hundred years. At longer times, the in-growth of γ-emitting actinides results in a low, but persistent, γ-dose rate, so that the dose continues to accumulate, even after 100,000 years (Figure 2b), although the dose rate changes only slightly.
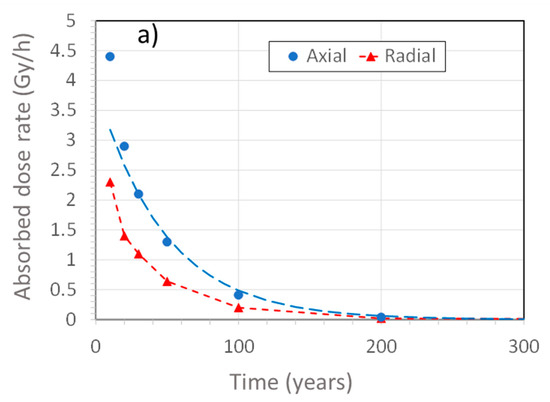
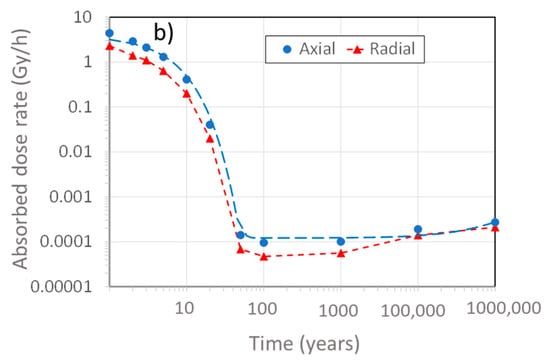
Figure 2.
Predicted absorbed dose rate on the surface of a copper-coated used fuel container as a function of the age of the used fuel. (a) Dose rates on the side (radial) and at the hemispherical head (axial), (b) long-term axial and radial dose rates. The points represent dose rates calculated at discrete times and the dashed curves represent an exponential fit up to 500 years, representing the period when the decay of fission products dominates the dose rate, and a linear fit for longer times, during which the γ-emission from actinides is dominant. The assumed age of the fuel at the time of emplacement is 30 years.
In addition to the irradiation of the interfacial region, the γ-field will extend into the HCB in the buffer box. Figure 3a shows the absorbed dose rate in the buffer as a function of distance away from the surface of the hemispherical head in the axial direction for various times. Over extended periods of time, the cumulative dose in the buffer amounts to several hundred kGy for some distance away from the container surface (Figure 3b).
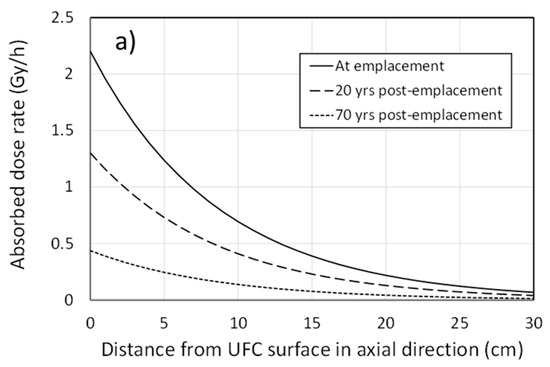
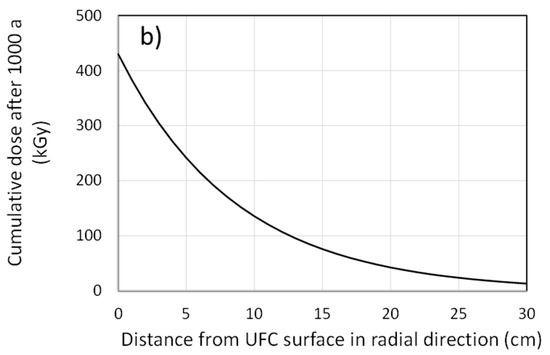
Figure 3.
Penetration of the radiation field into the compacted bentonite in the buffer-box assembly. (a) Distance dependence of the dose rate in the buffer in the axial direction at different times following emplacement, (b) distance dependence of the cumulative dose in the buffer in the radial direction after a period of 1000 years. Calculations are based on a half-layer value for compacted bentonite of 6 cm and an assumed age of the fuel at emplacement of 30 years.
2.2. Evolution of Near-Field Environment
In addition to the decrease in the external dose rate, other aspects of the near-field environment will also evolve over time [12]. The period of highest γ-dose rate is characterized by transients in the thermal, saturation, and redox conditions within the DGR, as the disturbances to the natural environment created by the excavation of the repository and the emplacement of heat- and radiation-emitting waste gradually diminish. In general, the near-field conditions evolve from an initial period of warm, oxidizing conditions to a long-term period of cool anoxic conditions similar to the pre-excavation environment within the host rock. The thermal transient will persist for a period of a few thousand years, with a peak temperature of 80–95 °C reached approximately 40–50 years after emplacement of the UFCs [13]. The duration of the saturation transient will depend on a number of factors, including the heat output of the UFC (which determines the dry-out characteristics) and the hydraulic conductivity of the host rock (which determines the period of time before full saturation of the buffer material). In addition to the formation of oxidizing radiolysis products, the near-field redox conditions are determined by the amount of residual atmospheric O2 trapped in the unsaturated pores of the buffer and backfill materials at the time of repository closure. Evidence for the rate of O2 consumption from full-scale in-situ experiments [14,15] suggests that this initially trapped O2 will be consumed within a period of a few weeks to a few months following repository closure.
In terms of the relative timescales, therefore, the various environmental transients will persist for the following periods (in order of increasing duration):
- Redox transient due to initially trapped O2: weeks to months following emplacement of the buffer boxes and the sealing of the disposal tunnels.
- Redox transient due to generation of radiolysis products (defined by the time for the external γ-dose rate to decrease to <0.1 Gy/h): approximately 150 years (Figure 2).
- Saturation transient: a few decades to tens of thousands of years, depending upon the local hydraulic conductivity and the properties of the host rock [12]
- Thermal transient (defined by the time for the UFC surface temperature to decrease to 30–50 °C): approximately 10,000 years [13].
With this general evolution of the near-field environment in mind, the nature of the environment in terms of radiolysis effects can be characterized as illustrated in Figure 4:
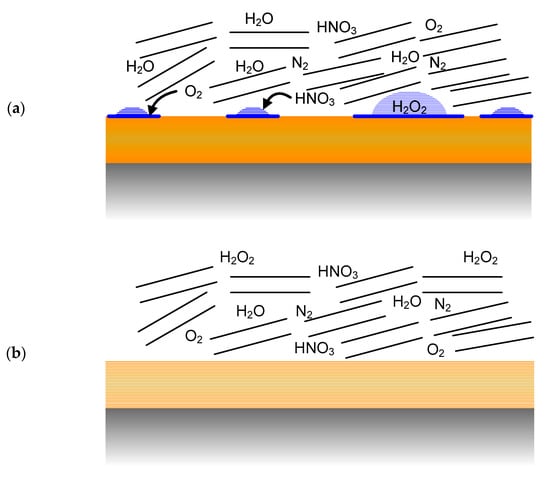
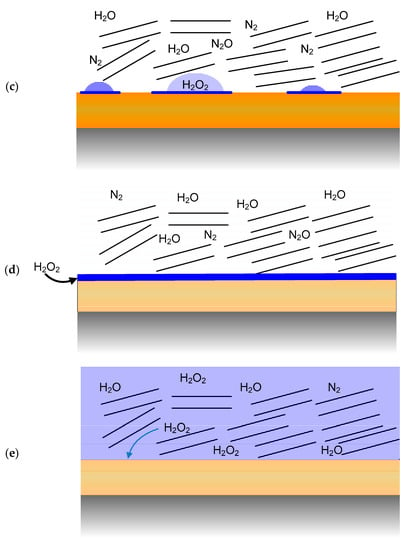
Figure 4.
Evolution of the environment at the container surface and the resulting major stable radiolysis products. Blue represents condensed H2O and the sets of parallel lines represent montmorillonite clay particles, (a) absorption of HNO3 formed by the radiolysis of humid air by liquid droplets on a non-uniformly wetted surface. (b) radiolysis of humid air during a period when the container surface is dry, (c) radiolysis of N2-H2O water following consumption of the initially trapped O2 and the radiolysis of liquid droplets following re-wetting of the container surface, (d) radiolysis of a thin surface water layer following complete wetting of the surface, with continued radiolysis of a humid N2-H2O atmosphere, (e) radiolysis of a bulk aqueous phase following complete saturation of the buffer box.
- Immediately following emplacement of the UFC in the buffer box, the container surface may be wetted (most likely by a discontinuous moisture film) as a result of the high relative humidity (RH) resulting from the moisture added to the dry bentonite to aid compaction of the shaped buffer blocks (Figure 4a).
- The UFC surface is expected to dry out rapidly as the initial moisture is driven away from the container surface by the thermal gradient. At this point (Figure 4b), the dry UFC surface would be in contact with a humid air atmosphere with residual trapped O2 and N2, as well as radiolysis products such as HNO3 and trace amounts of H2O2.
- After the peak in the container temperature (approximately 10 years post-emplacement), moisture will move back towards the UFC and re-wetting of the surface will occur, most likely non-uniformly at first (Figure 4c). By this time, however, the initially trapped O2 will have been consumed and the wetted container will be contacted by a humid N2-containing atmosphere. Species such as H2O2 and HNO3 may be present in water droplets, which may also contain species such as sodium chloride or other salt solutions (e.g., calcite and gypsum), as these compounds may initiate droplet formation by deliquescence.
- As saturation continues, the surface will become uniformly wetted as the near-field RH approaches 90–100% (Figure 4d). As noted above, the timing and duration of these two stages depends largely on the hydraulic properties of the host rock.
- Eventually, the near-field will completely saturate (Figure 4e) and the UFC will be exposed to the evolving bentonite pore-water chemistry, which will eventually equilibrate with the local ground water.
The nature of the UFC surface will also impact the evolution of the corrosive environment. The as-emplaced container will be covered or at least partly covered by an air-formed oxide and, possibly, organic and other contaminants from the fabrication procedure. Apart from machining of the cold spray deposit over the closure weld, and possibly decontamination from radiation, no other surface preparation will be applied to the container surface before it is placed in the buffer box. In the buffer box, the UFC will be in intimate contact with the compacted bentonite, although there may be a gap between the copper and the shaped buffer block at the top. The container surface will, therefore, be contacted by montmorillonite particles and by other components of the natural bentonite clay, including salts such as gypsum and calcite.
The consequences for the production of radiolysis products for each of these phases are considered in the next section.
2.3. Humid Air and Aqueous-Phase Radiolysis Models
Humid Air: A radiolysis kinetic model solves the rate equations of chemical species that are undergoing many different reactions in the presence of a continuous flux of γ-radiation [16,17]. The rate parameters that determine the overall radiolysis kinetics are the G-values and dose rate for primary radiolytic processes and the second-order rate constants of the elementary reactions. Hence, a robust radiolysis kinetic model requires identifying the necessary elementary reactions that contribute to determining the overall radiolysis kinetics, defining their rate constants, and estimating the steady-state concentrations. In this section, radiolysis models developed under two conditions of humid air and an aqueous phase are summarized. Using these models, the concentration of the radiolysis products (i.e., HNO3 and H2O2) that are important for copper corrosion have been calculated as a function of fuel age.
The radiation chemistry of moist and dry air systems has been studied extensively [18,19,20]. More recent interest in the radiation chemistry of the moist air system has been expressed in relation to the disposal of high-level nuclear waste in DGRs. It has been shown that humid air radiolysis produces HNO3 [18,21]. The absorption of HNO3 in condensed water droplets will lead to a decrease in local pH and an increase in the concentration of nitrate that could be an oxidant for copper and other materials [19,22]. To calculate the production rate of HNO3 in the gas phase by humid air radiolysis, a humid air radiolysis model (HARM) was developed by Morco et al. [19]. In this model, the absorption of radiation energy by the three main components of humid air (N2, O2, and H2O: as little as 10% RH) results in the formation of a range of primary products including water radiolysis products (H2, H2O2, •HO2, and •OH) and additional nitrogen species (HNO3, HNO2, and peroxynitric acid HO2NO2). Comparison of the radiolytically produced HNO3 and the sum of the N species in both 10% and 85% RH air indicates that the main process in the radiolysis of humid air is the production of HNO3. H2O2 is also produced through the •OH recombination reaction, however, its concentration is not significant as •OH is consumed by reaction with NO, NO2, HNO2, and HNO3 [23]. It is worth noting that the gas phase reactions occur at a slower rate compared with related reactions in the liquid phase and the system requires more time to reach steady state.
Morco et al. also showed that the overall production of HNO3 has a small dependence on relative humidity (RH) at a given dose rate (Figure 5) [19], whereas the overall production of HNO3 is proportional to dose rate at a given RH and temperature (Figure 6). To provide an estimate of the extreme upper bound of radiolytically produced HNO3 to be used in corrosion allowance calculations, the concentration of HNO3 was calculated as a function of fuel age (Figure 7). As can be seen, an accumulation of a millimolar concentration of HNO3 would take many years presuming that the HNO3 would not react with other materials in the DGR.
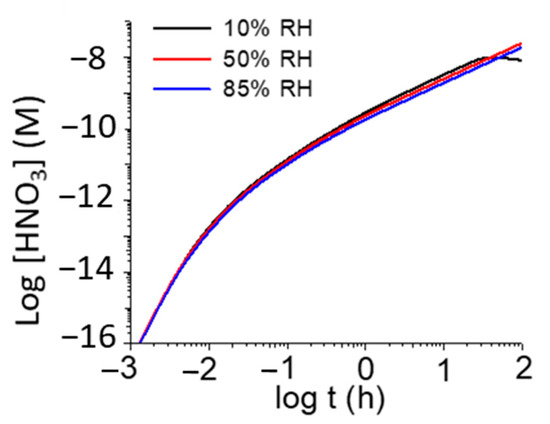
Figure 5.
Time evolution of [HNO3]g produced by γ-radiolysis for different RHs at 75 °C and a dose rate of 1 Gy/h. Reprinted with permission from Morco et al. [19]. Copyright 2017.
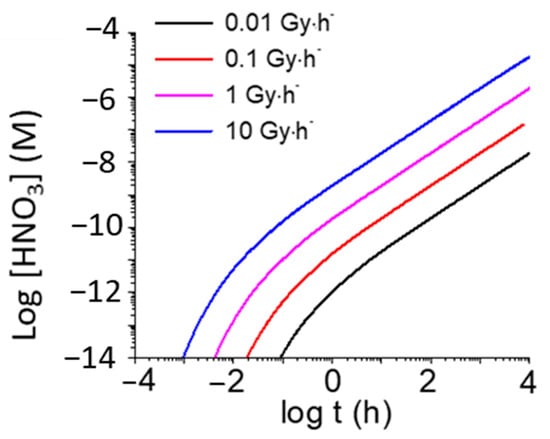
Figure 6.
Time evolution of [HNO3]g produced by γ-radiolysis of 85% air at 75 °C at different dose rates. Reprinted with permission from Morco et al. [19]. Copyright 2017.
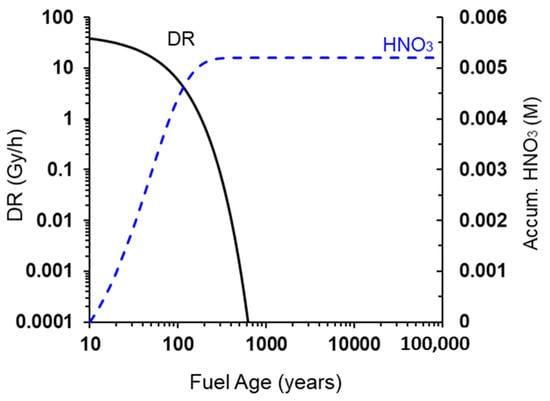
Figure 7.
Evolution of [HNO3] and dose rate as a function of fuel age. Reprinted with permission from Morco et al. [9]. Copyright 2017.
Aqueous Phase: The radiolysis of pure water (aqueous phase) in γ-radiation fields has also been studied extensively [6,17,24,25,26,27]. Specifically, Joseph et al. developed a model that was used to identify the key reactions that control the radiolysis product concentrations [24]. The pure water radiolysis kinetic model showed that H2O2 and H2 are the predominant water radiolysis molecular products. In the same work, the steady state concentrations of radiolysis products (i.e., •eaq− and •OH) that control the steady state concentration of H2O2 (Equation (1)) in both aerated and de-aerated conditions were obtained [24]. Later on Morco et al. presented a simplified expression for [H2O2]ss [23]:
where k6 and k18 are the rate constants (in units of mol−1·dm3·s−1) of reactions of •eaq− and •OH with H2O2, respectively, DR is the dose rate (Gy/s), ρ is the density of water (kg/dm3), and GH2O2 and GOH are the G-value for H2O2 production (0.07 μmol/J at 25 °C) and •OH production (0.28 μmol/J at 25 °C), respectively.
Under anoxic conditions, the steady-state [H2O2] for the simplified radiolysis model is given by (after substituting the expressions for [•eaq−]ss and [•OH]ss in Equation (1)):
where the steady-state [•eaq−] is determined by the balance between the primary radiolysis reaction (Ge− = 0.27 μmol/J at 25 °C) and the reaction of e− with H+ (rate constant k40) and the steady-state [•OH] is determined by the balance between the primary radiolysis reaction and the recombination reaction to produce H2O2 (rate constant k17).
Figure 8 shows the steady-state concentration of H2O2 under anoxic condition as a function of pH. The [H2O2] concentrations are based on the time-dependent dose rate for the UFC and are calculated for the highest dose rate on the container surface, namely in the axial orientation on the hemispherical heads.
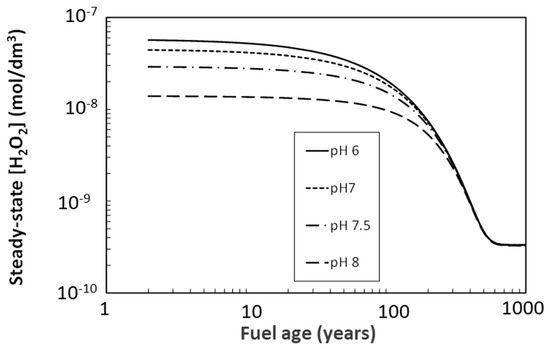
Figure 8.
Steady-state H2O2 concentration at the UFC surface as a function of fuel age based on a simplified radiolysis model for anoxic conditions.
Combined Effects: The impact of humid air radiolysis on water radiolysis kinetics was also investigated by coupling the transfer kinetics of nitrate from the gas phase to the solution phase [23]. The results show that the nitrate from HARM has no significant effect on the water radiolysis kinetics [23] at the dose rate (1 Gy/h), close to that expected in the Canadian DGR (~30 years old fuel). This is due to the slow production of HNO3 at low dose rates, and, as shown in Figure 6, the concentration of HNO3 is proportional to the dose rate.
2.4. Other Irradiation Considerations
There are a number of other unique aspects to the study of the effects of radiation on the corrosion behaviour of UFC materials that need to be considered. An important characteristic of the disposal system is that the ratio of the surface area of solid phases to the volume of aqueous or gaseous phases is very high. For example, the specific surface area of bentonite is 500 m2/g [28], so that the average water layer thickness in the saturated buffer box is only 0.5 nm. Therefore, the properties of interfaces and the nature of the solid phases will play an important role in the response of the entire system to irradiation.
Polished and degreased coupon surfaces are typically used in irradiation experiments, but the surface of the UFC will not be prepared similarly following the loading and sealing of the container in the encapsulation facility. Apart from a final machining of the cold-sprayed copper coating covering the final closure weld to facilitate ultrasonic inspection, no other surface cleaning/resurfacing will be performed. As a result, the container surface will at least be covered by an air-formed oxide, a feature that may be enhanced during the heat treatment of the cold-spray copper, which may occur at temperatures of 350 °C or higher.
Irradiation of semiconducting oxides of copper (CuO or Cu2O) will create electron–hole pairs due to the excitation of electrons from the valence band to the conduction band. This will have the effect of enhancing reduction reactions on p-type semiconductors (which normally have a deficiency of electrons) and promoting oxidation reactions for n-type semiconductors (which usually have a deficiency of holes) [29]. Independent of any effect of radiolysis, therefore, the corrosion potential (ECORR) of a metal surface covered by a p-type semiconducting oxide would shift to more positive potentials upon irradiation, and that of a metal/n-type oxide surface to more-negative potentials. Both cupric and cuprous oxides are p-type semiconductors [30,31,32,33,34], although n-type behaviour has also been reported [35,36]. In turn, a positive shift in ECORR upon irradiation (due to the presence of a p-type oxide) could increase the probability of localized film breakdown and pitting corrosion if aggressive anions, such as Cl−, are present in the environment. The degree of ennoblement of ECORR and the corresponding increase in susceptibility to film breakdown would depend on the dose rate and is expected to be relatively minor for the maximum dose rates at the surface of the UFC.
The rate of absorption of energy at an interface is different from that in the bulk due to differences in the electron-scattering properties of the two materials [29]. In general, if radiation passes from a material of low atomic number (Z) to a material of higher atomic number, the dose rate is enhanced in the low-Z material due to backscattering from the high-Z material. This phenomenon was illustrated by Airey et al. [29] by considering the effect of the orientation of irradiation on the dose rate at an interface between copper and graphite, with the graphite being a reasonable representation of bulk water due to the similarity in Z. If the radiation interacts with the graphite layer before the copper layer, then the dose rate in the graphite layer at the interface is approximately 50% higher due to the backscattering of electrons from the copper. This phenomenon is also important in radiation oncology, where the backscattering of radiation by titanium dental implants can increase the effective dose in the adjacent tissue by 15% [37]. The issue is of interest, here, because the majority of experimental studies involve the use of an external radiation source, with the orientation of irradiation being environment|oxide|metal rather than metal|oxide|environment, as in the actual repository. Based on the backscattering argument of Airey et al., therefore, most experimental studies would be conservative in that the effective dose rate in the environment at the copper surface would be higher than that in the repository (for a given source strength). However, backscattering only affects the yield of radiolytic species in the proximity of the interface. If the main radiolytic species of concern are stable molecular species produced at greater distances from the interface, then the overall impact of the backscattered electrons will be reduced.
The UFC is surrounded by a buffer material consisting of compacted bentonite clay. If energy transfer involving the aluminosilicate clay minerals affects the yield of radiolysis products, then there could be an impact on the corrosion behaviour of the container. The compacted bentonite buffer represents a system with a particularly high (clay) surface:area to (water) volume ratio. Clay minerals are layered structures comprising sheets of tetrahedral silicate and octahedral alumina, with varying degrees of substitution by silicon or aluminium for cations such as Fe2+, Fe3+, and Mg2+. Montmorillonite is an example of a 2:1 phyllosilicate, consisting of two silicate layers sandwiching a single alumina layer. The irradiation of compacted bentonite can produce radiolysis products by one of two mechanisms:
- (a)
- interfacial energy transfer at the clay/H2O interface involving either trapped excitons or ejected holes or electrons, and
- (b)
- energy absorption in the solution in larger pores, similar in nature to the radiolysis of bulk water.
Le Caër and co-workers have studied the effect of irradiation on the radiolytic production of H2 for natural and synthetic montmorillonites as a function of the availability of H2O [38,39,40]. With synthetic montmorillonite (and saponite), the yield of H2 exceeds that from the radiolysis of bulk water at all RH studied, with an enhancement by a factor of two to three at low RH, which decreases with increasing RH (Figure 9a). However, for natural bentonites, the yield of radiolytic H2 is less than that in bulk water at low RH but increases with RH, reaching the same yield as bulk water at 100% RH. This difference in behaviour was attributed, by Lainé et al., to the presence of Fe3+ in the natural clays that trapped electrons, resulting in reduction to Fe2+ rather than radiolytic H2 production [38].
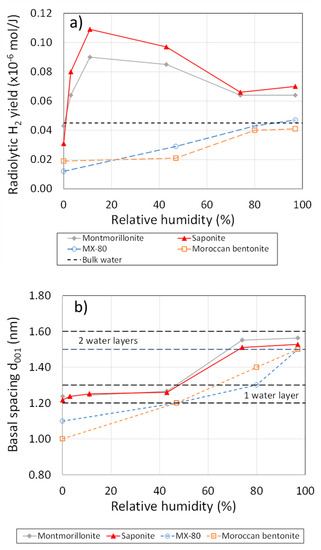
Figure 9.
(a): Radiolytic yield of H2 for unsaturated natural bentonite sand synthetic montmorillonites (based on the work of Fourdrin et al. 2013 and Lainé et al. 2017) [38,39], (a) radiolytic yield in unsaturated clays as a function of relative humidity. The yield in bulk solution is shown for comparison, (b) basal d-spacing and the corresponding number of adsorbed wate layers as a function of relative humidity.
It is also interesting to relate the production of radiolytic H2 to the amount of H2O in the system. Figure 9b shows the basal d-spacing of the different montmorillonite powder samples determined by X-ray diffraction as a function of RH. Basal d-spacings of 1.2–1.3 nm and 1.5–1.6 nm are characteristic of one and two water layers, respectively [38]. For RH values of up to 50%, the clay surface is covered by a single monolayer of H2O, with a second water layer forming at RH values exceeding 70%. Thus, the enhanced radiolytic yield of H2 (compared with that in bulk solution) exhibited by synthetic clays at RH ≤ 70% (Figure 9a) is the result of direct energy transfer to a surface H2O layer rather than due to radiolysis of a bulk aqueous phase. Even in saturated clays, much of the radiolysis will involve adsorbed H2O with bulk radiolysis reactions only occurring in large pore-water-filled pores between clay aggregates. Bulk radiolysis models, therefore, may need to be adjusted in order to properly account for radiolytic yields in compacted clay systems.
3. Effect of Irradiation on the Corrosion Behaviour of Copper
Studies of the effect of irradiation on the corrosion behaviour of copper have been conducted at different times over the past 40 years, including from the 1980′s, when copper was first being investigated as a candidate container material. However, because there was no apparent adverse effect of irradiation at low dose rates and because of the tendency towards thick-walled self-shielding container designs, these early studies were discontinued. More recently, there has been a rekindling of interest in such studies due to the proposed use of copper-coated UFC designs with higher surface dose rates. The Swedish Land and Environmental Court also raised some questions to SKB with regard to RIC on copper canisters in 2018. The questions pertained more to the effect of radiation on mechanical aspects such as stress corrosion cracking and hydrogen embrittlement, which are of less concern for the Canadian design of the UFC.
Here, relevant information from both historic and more-recent studies is reviewed. Since the period of highest radiation intensity will coincide with the early saturation transient in the evolution of the repository near-field, the behaviour of copper in irradiated humid air atmospheres is first reviewed. This analysis is supported by a review of relevant studies from the more-extensive work in bulk solutions.
3.1. γ-Radiation Effects in Humid Air
The initial environment around a sealed UFC is anticipated to be humid air due to the ambient humidity (based on the high saturation level of the buffer box of 89%) at the time of emplacement. As described in Section 2.3, at a sufficient dose rate, humid air radiolysis produces HNO3 that could absorb and then dissolve into water droplets and thereby affect the corrosion of copper.
Morco et al. calculated the production rate of HNO3(g) by humid air radiolysis as a function of dose rate for the range of dose rates relevant for the UFC [19]. To determine the production rate of HNO3(aq) in a water droplet, a volume ratio of air to that of the droplet of 100 was assumed and, by integrating the dose rate over the DGR lifetime, a bounding estimate of HNO3(aq) concentration was calculated. The results of this assessment were that ≤9.4 μm of Cu corrosion will occur over 106 years.
Experimental evidence obtained by Ibrahim et al. in humid air conditions confirmed that γ-radiation at low dose rates (~0.35 Gy/h) produced no measurable corrosion damage beyond that observed in the absence of radiation [41]. The results also showed the depth of corrosion penetration into the Cu was relatively minor compared to the lateral spread of corrosion patches across the surface. Their observations suggested that the distribution of condensed water was the dominant feature controlling the development of patches, a process possibly enhanced by the production of deliquescent HNO3 by the radiolysis of water vapour. It was also shown that, at higher relative humidity (RH), the spread of patches increases irrespective of the presence of a radiation field, indicating that the main factor driving the corrosion is the presence of aerated condensed water [42].
The effect of oxygen concentration on short-term Cu corrosion in a humid environment in the presence of high-dose-rate γ-radiation (3 kGy/h) was also studied to reflect the expected aerated and unsaturated early DGR environmental conditions [43]. Although during this period γ-fields will be highest, the expected dose rate on the copper coated container will be three orders of magnitude lower than the dose rate of 3 kGy/h used experimentally in this work. The effect of O2 concentration on Cu corrosion in a humid environment was investigated in the presence and absence of radiation for 96 h using different atmospheres, Ar, air (21% O2) and 35% O2 in Ar, with the humidity set at 85% RH and the temperature set to 75 °C in all cases. In the absence of radiation, the samples exhibited a greater number of patches with uniform lateral distribution in the higher oxygenated environments. When radiation was present, the corrosion progressed faster in all exposure environments and thicker oxide films were measured in the higher oxygenated environments. This is consistent with the model calculations showing the production of H2O2 as the main oxidant from radiolysis of water vapour is higher with O2 present in the humid atmosphere.
Turnbull et al. recently reported the results from examinations of irradiated Cu surfaces that were harvested from a shutdown nuclear research reactor [44]. These Cu samples had been exposed to about 40 years of gamma and neutron radiation (at ~0.015 Gy/h) under uncontrolled humid air conditions at a temperature of approximately 40 °C. For comparison, this dose rate is equivalent to that expected at the Cu surface of the UFC after around 200 years in a DGR. Surface analysis and metallographic examinations including etched cross section and micro-hardness measurements concluded that long-term gamma and neutron irradiation of Cu, of the dose rates and cumulative dose described above, did not affect the integrity of the Cu samples.
Björkbacka et al. also considered the effect of radiation on the corrosion of copper in humid atmospheres [20]. Experiments were conducted on polished copper cubes in humid air (60% and 100% RH) and humid Ar (100% RH) atmospheres, with and without radiation. Corrosion resulted in the non-uniform oxide formation on the surfaces of the samples, as observed by Ibrahim et al., with the extent of corrosion estimated based on the charge required to electrochemically remove the corrosion products at the end of the test. A number of interesting observations were made, including:
- irradiation in humid air (either 60% or 100% RH) increases the extent of corrosion by a factor of 7–9 compared with unirradiated conditions (dose rate 500 Gy/h, total dose of 48 kGy),
- irradiation in humid Ar (100% RH) increases the extent of corrosion by a factor of 8 compared with unirradiated conditions,
- since the enhancement factor in humid Ar is the same as in humid air, Björkbacka et al. [20] concluded that radiolytically produced HNO3 does not contribute to corrosion,
- irradiation under unsaturated conditions (either humid air tests or humid Ar) results in four-to-five times more corrosion than irradiation under saturated conditions,
- irradiation in “dry” air or “dry” Ar produced no corrosion, although the RH corresponding to “dry” conditions was not defined.
There had also been speculation that a period of prior irradiation could render the surface more electrochemically reactive and, in particular, that the surface might then support the anoxic corrosion of copper [20]. However, subsequent exposure of irradiated coupons to anoxic, unirradiated H2O did not result in additional corrosion and there was no evidence that the surface had become “activated” by exposure to γ-radiation.
In reference to the evolution of the near-field environment under unsaturated conditions illustrated schematically in Figure 4a–d, the results of these various experimental studies indicate that:
- At repository-relevant dose rates, there is little impact of irradiation on the corrosion rate of copper, even over extended timescales.
- At higher dose rates of the order of 100’s to 1000’s Gy/h, there is evidence for enhanced corrosion due to the formation of radiolysis products, with both HNO3 and H2O2 as possible radiolytic oxidants.
- There is some evidence that RIC does not occur in “dry” atmospheres (cf. Figure 4b), although the threshold RH above which there is a sufficiently thick water layer to support the electrochemically based radiation-induced corrosion reactions has not been defined.
- Modelling studies of the absorption of gaseous HNO3 by water droplets on the UFC surface (cf. Figure 4a) suggest a maximum depth of corrosion of <10 μm.
3.2. γ-Radiation Effects under Saturated Conditions
The probability of groundwaters diffusing through the bentonite clay buffer and reaching the outer copper surface, within the period of time when the radiation dose is still significant, is extremely low. If this did occur, the clay buffer could become saturated, and the ions adsorbed on the clay could then leach into the waters surrounding the UFC. In this event, the outer surface of the UFC would come into contact with highly saline water. To address UFC corrosion in saline water in the presence of γ-radiation, Morco et al. developed a groundwater radiolysis model (GWRM) for γ-radiolysis of water with different salinities [23]. The GWRM has been used to predict the concentrations of redox active chlorine species (e.g., HOCl, ClO2−, ClO3−) in addition to the standard water radiolysis products (e.g., H2O2, O2) that may impact copper corrosion. The GWRM calculations showed that, under continuous irradiation in the presence of Cl− ions, stronger oxidants such as H2O2 are slowly converted to progressively weaker oxidants with time: H2O2 → HOCl → HOCl2 → HOCl3. For copper corrosion, it is known that H2O2 and HOCl are effective oxidants, while the effectiveness of HOCl2 and HOCl3 in copper corrosion has yet to be investigated. The calculated near steady-state concentrations over a 1-h or 24-h irradiation period at 3 kGy/h range from 1 µM to 100 µM for H2O2, and from 10 µM to 3 µM for HOCl. GWRM calculation results showed the sum of [H2O2] and [O2] is much higher than the sum of the oxychloride species ([HOCl] + [ClO2−] + [ClO3−]), indicating that H2O2 and O2 are the main oxidants that should be considered for copper corrosion in the DGR. However, between these two oxidants, H2O2 is the stronger and kinetically more effective oxidant than O2 and will have more impact on corrosion. It is also known that O2 solubility decreases dramatically in high chloride concentration solutions, hence making O2 less effective than H2O2 [45]. The predictions from the GWRM were validated against experimental measurements of the concentrations of various species produced during the radiolysis of saline solutions, with good agreement found between the model and experiments. Therefore, while some of the rate constant used in the GWRM may have a limited basis, the uncertainty in these values does not appear to be significant.
The impact of a radiation dose rate of 2 kGy/h on copper corrosion in contact with water droplets (50 µL) with different initial pHs and cover gases in sealed quartz vials has also been studied [46]. The corrosion kinetics were investigated by measuring the dissolved copper concentration, the change in pH, and the morphology, elemental composition, and cross-section of oxides on a coupon surface, as a function of exposure duration. Figure 10 shows the optical images showing the evolution of surface morphology for copper coupons exposed to water droplets under aerated conditions with and without γ-radiation. As can be seen, in the presence of high field γ-radiation, corrosion was accelerated, and the features of corrosion products progressively changed with time. The impact of radiation is more pronounced by decreasing the pH in the water droplet, which increases the dissolution of Cu ions. During the course of exposure, as the concentration of Cu ions increases, the precipitation of Cu2O particles on the surface (as indicated by X-ray photoelectron spectroscopy) was noticeable. Once the entire surface is covered with copper oxide, no significant change in dissolved copper concentration and pH was observed. The results show that γ-radiation decreases pH and increases the duration of Stage 1. The decrease in pH increases the concentration of CuII(sol) and increases the overall rate of Cu2O particle growth in Stage 2 (stages are shown in Figure 10).
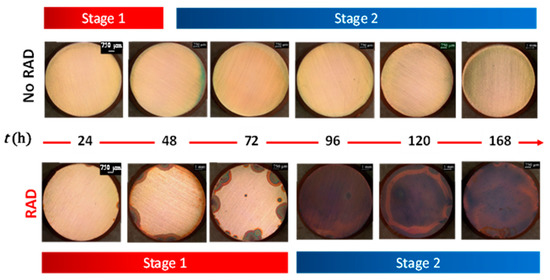
Figure 10.
Optical and SEM micrographs showing the evolution of surface morphology for copper coupons exposed to 2.54-cm deep solutions initially containing 5 mM H2SO4 (initial pH 2.0) [46]. Copyright© by WM Symposia. All Rights Reserved. Reprinted with permission.
In addition to these recent Canadian studies, there have been a number of Swedish studies of the RIC of oxygen-free copper in deaerated high-purity water [47,48,49,50]. Experiments were conducted at relatively high absorbed dose rates (80–770 Gy/h), but to total absorbed doses similar to those predicted for a KBS-3 container for periods up to 100 years in a DGR (i.e., on the order of 100 kGy). The initial study produced a number of issues of potential concern, which were subsequently resolved, including:
- the total amount of corrosion (as both precipitated oxide and dissolved in solution) far exceeded that predicted based on a coupled homogeneous radiolysis/surface kinetic model,
- the dissolved copper concentrations exceeded the solubility of either Cu2O or CuO,
- and there was evidence for localized attack, in the form of circular corrosion features (local depth of penetration of 0.8 μm).
Of these issues, the main concern was the inability to account for the extent of corrosion based on radiolytic yields predicted by bulk radiolysis models based on the assumption that •OH radicals were the predominant oxidant. The apparent discrepancy caused the investigators to conclude that an unknown process other than the bulk radiolytic production of oxidants was responsible for the radiation-induced corrosion [48,49,50]. Enhanced radiolytic production by electron–hole pair production by irradiation of a porous, large surface area, surface oxide was suggested as one possible explanation. Recently, however, the discrepancy between the extent of copper corrosion and the radiolytic yield of oxidants has been resolved by additional experimental studies and modelling, in which radiolytically produced O2 is shown to be the major oxidant [51]. The O2 is formed by the interfacial decomposition of H2O2
2H2O2 = O2 + 2H2O
Molecular O2 was shown to be the main oxidant based on a comparison of the extent of corrosion in sealed experiments (from which O2 could not escape) and N2-purged tests (in which O2 was lost), the former resulting in more corrosion.
In terms of the other two issues raised by the early studies, the high “dissolved” copper concentrations were found to be the result of the formation of colloids promoted by the use of pure H2O as the electrolyte in the experiments, which could be removed from solution by suitable filtration [50]. Lastly, although no explanation has been provided for the localized corrosion features, the extent of depth-wise penetration is minimal, even for accelerated conditions of a high dose rate. In general, localized features tend to spread laterally, not deeply, producing features similar to Ibrahim et al. [41].
Historically, a number of RIC studies were conducted in the 1980′s when copper was first being proposed as a candidate UFC material. Although these studies were conducted in support of the proposed use of a thick-walled copper/steel or copper/cast iron container design, the results are still relevant in the current context of a thinner-walled copper-coated design. In fact, these older data are particularly relevant. as many of the experiments were performed with repository-relevant dose rates.
A major focus of these early studies was whether γ-radiation would result in an increase in the rate of general corrosion and, given the general lack of oxidants for copper in the DGR, whether irradiation would be a significant source of additional oxidants. Figure 11a shows the general corrosion enhancement factor, i.e., the ratio of the irradiated corrosion rate to the rate measured under similar unirradiated conditions, as a function of dose rate. The detailed experimental conditions for each of the experiments, as identified by the data labels in Figure 11a, are summarized in Table 2. It is apparent that, for these UFC-relevant dose rates, there is no significant enhancement of the corrosion rate, under either aerated or deaerated conditions (Table 2). Except for the data point labelled “4” in the figure, which refers to a long-term exposure to a low dose rate in humid air, there is some indication of a trend to greater enhancement with increasing dose rate, but the evidence is not strong. Neither is there an indication that the enhancement factor is a function of the cumulative dose (Figure 11b).
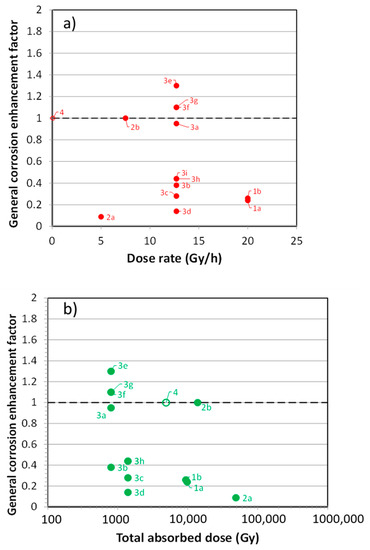
Figure 11.
(a) Enhancement factor for general corrosion of copper as a function of (a) dose rate and, (b) cumulative dose. The labels correspond to the studies listed in Table 2.

Table 2.
Summary of Experimental Studies on the Effect of Irradiation on the Enhancement of the Rate of General Corrosion of Copper Under Saturated Conditions.
A more-consistent trend from the data is that there is evidence for an inhibitive effect of radiation at these low dose rates: i.e., that the corrosion rate is lower in the presence of irradiation. Enhancement factors of <1 were reported in all three studies under saturated conditions, covering experiments in both bulk solution and compacted buffer material, in aerated and deaerated conditions, with temperatures from ambient to 150 °C, and for exposure times of up to 9800 h (Table 2). The investigators reported that the surface films on the irradiated coupons appeared visually to be more-compact and protective than those formed in the absence of radiation, but no further analyses were conducted [52,53,54]. Irradiation-induced passivation of the copper surface would explain this apparent inhibition in the presence of radiation, but most of these experiments were conducted in solutions with high [Cl−] (≥1 mol/L), in which copper would dissolve actively, with little tendency for passivation. It was partly because of this absence of any adverse effect of irradiation, that these early irradiation studies were curtailed without resolving the apparent inhibition mechanism.
Another interesting aspect of the work of Simpson is the study of the effect of irradiation orientation. As noted in Table 2, Simpson arranged the irradiation source so that, in some experiments, the copper coupons were irradiated “from behind” in the same manner as for the container (i.e., the γ-photons passed through the copper sample and then the environment), with other samples irradiated “indirectly” (i.e., the γ-photons passed through the environment and then the copper sample) [54]. As discussed above, the “indirect” irradiation orientation should result in a higher dose rate in the aqueous phase at the interface due to backscattering of electrons from the copper surface. In experiments where both irradiation orientations were used, there is indeed an enhanced effect for the “indirect” orientation, although in this case it is an enhancement of the degree of inhibition of the corrosion rate by irradiation.
The early irradiation studies also included in-situ electrochemical measurements of the corrosion potential (ECORR) and of the polarization behaviour of copper in the presence of irradiation. Figure 12 shows the time dependence of ECORR of oxygen-free copper in a deaerated synthetic saline ground water solution (Cl− concentration 0.97 mol/L) at a temperature of 150 °C as an Ir-192 γ-source (dose rate 27 Gy/h) was repeatedly inserted and retracted [52]. The initial decrease and subsequent increase in ECORR is not, in itself, an effect of irradiation, as similar behaviour was observed when copper was exposed to unirradiated solution. Once a steady-state ECORR value has been established after approximately 100 h, however, the insertion and retraction of the source have no effect on the potential at all. This behaviour is in contrast with that observed in a deaerated dilute ground water (J-13 water, 226 mg/L total dissolved solids) at a higher dose rate of 33 kGy/h where an instantaneous increase in ECORR of approximately 100 mV was observed [55]. The same behaviour could be replicated by the addition of a single drop of 30% H2O2 solution to an unirradiated solution (sufficient to produce a [H2O2] of 5 × 10−4 mol/L), which led to the conclusion that H2O2 is the key radiolysis product. Interestingly, both in the continuously irradiated experiment and for the chemical addition of H2O2, the ennobled ECORR value was not maintained but decreased over a period of a few hours to a value that was only 20–30 mV more positive than that immediately prior to irradiation or the addition of the droplet of H2O2 solution. This time dependent decrease in ECORR was attributed to the interfacial catalysis of the H2O2 decomposition reaction (to produce H2O and O2) by the copper surface, which is another mechanism by which the impact of irradiation could be reduced by the nature of the copper surface.
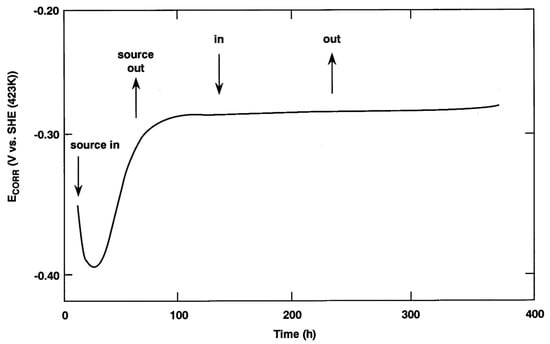
Figure 12.
Time dependence of the corrosion potential ECORR of a copper electrode in deaerated synthetic ground water solution (Cl− concentration 1 mol/dm3) at 150 °C with periodic insertion and retraction of a 192Ir γ-source, absorbed dose rate 27 Gy/h [52]. Reprinted with permission of Atomic Energy of Canada Limited. Copyright 1987.
The implication from this study that it is H2O2 that is the main radiolytic oxidant supporting corrosion is not inconsistent with the more-recent suggestion of Soroka et al. [51] that O2 is the species of concern. From an electron-balance point-of-view, the same amount of corrosion would result from the reaction of two H2O2 molecules as from the one O2 molecule that would result from the decomposition reaction (Equation (3)). Thus, in a sealed system from which molecular O2 cannot escape, the maximum amount of corrosion is indistinguishable for the two potential radiolytic oxidants.
3.3. Irradiation-Enhanced Localized Corrosion and EAC of Copper
Copper is expected to corrode uniformly under the environmental conditions expected within the buffer box, with little tendency for localized corrosion or environmentally assisted cracking (EAC) [2,56]. Although there are several mechanisms by which irradiation could theoretically induce these forms of corrosion, in practice, the dose rates at the UFC surface are too small to have any impact and no evidence for such processes has been observed under realistic irradiation conditions. Some of the possible mechanisms are discussed next.
3.3.1. Passivation of the Copper Surface and the Ennoblement of ECORR to a Value Exceeding the Film Breakdown Potential
Although there is anecdotal evidence for the formation of a more protective, possibly passive, film on copper at low dose rates that might then be susceptible to localized film breakdown, irradiation at repository-relevant dose rates does not result in the ennoblement of ECORR [52,53,54]. Only at dose rates of tens of kGy/h is a measurable effect of irradiation on ECORR observed [55], and then the observed shift in ECORR is transient and insufficient to increase the probability of pit initiation. These arguments are consistent with the absence of pitting on a large number of copper coupons irradiated under different conditions [53,54,57]. Björkbacka et al. did report evidence for the localized corrosion of copper following irradiation in deionized water at a dose rate of 360–760 Gy/h, but the maximum depth of penetration was on the order of 1 μm [47].
3.3.2. Localized Dissolution of Non-Uniformly Wetted Surfaces under Unsaturated Conditions
It has been suggested that the dissolution of gaseous HNO3 formed from the radiolysis of humid air in droplets of moisture on the UFC surface could induce localized corrosion [19]. Modelling of this process suggests a maximum penetration depth of approximately 10 μm. Alternatively, liquid-phase radiolysis of an Evans-type water droplet on the surface could produce a non-uniform distribution of oxidants in the centre compared with around the periphery of the droplet. However, long-term exposure of copper coupons to humid air environments with and without low-level irradiation (0.35 Gy/h) demonstrated little impact of irradiation [41]. Corrosion resulted in a patchy appearance of the surface under both irradiated and unirradiated conditions, with the patches corresponding to the location of the water droplets. Although statistical analysis was not possible on the samples, the patches were somewhat larger in the presence of radiation, possibly because of lateral spreading of the droplets due to the formation of deliquescent nitrate salts. Corrosion spreads laterally in the presence and absence of radiation, and there was no enhancement of the depth-wise penetration depth with radiation present.
3.3.3. Radiolytic Production of Stress Corrosion Cracking (SCC) Agents, Such as Ammonia or Nitrite
Copper is susceptible to SCC in environments containing ammonia and nitrite ions [58,59], both possible products of the radiolysis of humid atmospheres. Although nitrite ions are produced by the reduction of nitrate, the source of which is radiolytically produced HNO3, the low pH resulting from the absorption of nitric acid vapour would preclude film formation [60], which is a pre-requisite for cracking [58,59]. Radiolytic ammonia formation occurs in the absence of O2 [61], but then SCC would not be possible, as ammonia-induced cracking is a dissolution-based mechanism involving film formation and would not occur in the absence of an oxidant. It is not surprising, therefore, that no SCC has been reported following the long-term irradiation of stressed copper samples exposed to both humid air atmospheres [57] and bulk solution [62] for periods of one to four years with dose rates of up 5 kGy/h.
3.3.4. Radiolytically Enhanced Absorption of Hydrogen Leading to H-Induced Cracking Mechanisms
There is a report that the concentration of absorbed H increases with increasing cumulative dose [63]. Increased H concentrations, albeit under aggressive charging conditions, have been shown to affect the creep properties of copper [64] and have been associated with SCC in sulfide environments [65,66], neither of which should occur for the copper-coated UFC design because the copper layer is in intimate contact with the load-bearing steel substrate and because loading will be largely compressive in nature. Furthermore, the increase in absorbed H concentration due to irradiation was within the variability found for as-received copper of that grade [11]. Therefore, irradiation is not expected to induce H-related degradation mechanisms for a copper-coated UFC.
3.4. Effect of Irradiation on Material Properties
Exposure to neutron (and, to some extent, gamma) radiation is known to affect material properties, such as ductility and fracture toughness [67]. Energy transfer to the material by interaction with fast neutrons or by γ-photon interactions with electrons can induce damage in the material, typically measured in terms of the number of displacements (from their lattice sites) per atom (dpa). For copper, there is no measurable effect on mechanical and fracture properties for irradiation damage of <10−4 to 10−3 dpa [68].
In the Canadian reference container design, copper coatings have much lower mechanical requirements, as structural support is provided by the underlying steel. Nevertheless, a short summary of recent studies of the potential radiation damage to the UFC materials is presented below:
- Wu et al. estimated the neutron fluence to the inner surface of the C-steel liner of the UFC for the entire container service life of 106 years [9]. The estimated neutron fluence of 4.2 × 1014 n/cm2 would result in <10−6 dpa for the C-steel vessel, and significantly less for the copper coating as a result of shielding by the inner vessel. This accumulated damage is more than two orders of magnitude less than the minimum below which any observable effect on the mechanical or material properties of copper has been reported [68].
- As described in Section 3.1, a recent study by Turnbull et al. reported new results from examinations of irradiated Cu surfaces that were exposed to about 40 years of gamma and neutron radiation. Vickers hardness measurements were performed with a 100-g load of the cross section on both irradiated and non-irradiated copper surfaces. An analysis of the microhardness concluded that negligible irradiation hardening occurred to the irradiated Cu surface despite the constant flux of neutron and gamma irradiation during the operation of the research reactor [44].
- Similar analyses for the Swedish nuclear waste management program have also shown no effect of neutron and γ-radiation. The estimated damage to the copper shell of the KBS-3 canister design is <10−6 dpa after 105 years [69]. As with the study of Wu et al., such estimates ignore the potential beneficial effects of thermal annealing which, although relatively small at repository temperatures, will reduce the level of accumulated damage even further [9]. Unsurprisingly, Padovani et al. were unable to detect any effect of γ-irradiation to a total dose of 100 kGy (approximately equal to the accumulated dose for a KBS-3 canister for the first 300 years post-emplacement) on the microstructure of oxygen-free copper using high-resolution transmission electron microscopy, nanoindentation, positron annihilation spectroscopy, or electrical resistance measurements [70].
Thus, there is no evidence that long-term exposure to the low γ-dose rate or neutron flux will adversely affect the material and mechanical properties of the copper coating, even over periods of up to 106 years.
4. Expected Influence of Irradiation on the Performance of a Copper-Coated UFC
The thinner design of copper-coated UFCs, particularly in the hemispherical heads, provide significantly less radiation shielding than dual-vessel designs. Therefore, it is important to consider the maximum extent of radiation-induced corrosion to the copper coating of UFCs in a Canadian DGR environment. Due to the timescales of interest, it can be difficult to justify long-term predictions of the corrosion behaviour. One method for building confidence in such predictions is through the use of complementary modelling techniques and being able to demonstrate that the models produce similar predicted corrosion depths.
On the assumption that the fuel age is approximately 30-years-old at the time of emplacement [71], the external UFC surface γ-radiation dose rate is calculated to be around 1 Gy/h and to decrease substantially after 200 years to about 0.002 Gy/h [19]. The assessment of RIC is challenging due to uncertainty over the evolution of the near-field environment. As described in Section 2.2, in the Canadian crystalline geosphere, the dominant relevant environment to which the UFC will be exposed is likely to be a moderately humid atmosphere with a modest dose of γ-radiation (Figure 4c). Within the Canadian sedimentary geosphere, the most relevant environment is expected to be less humid, with an equivalently modest does of γ-radiation. Recent experimental results have confirmed that long-term exposure to low-dose-rate radiation in humid air conditions produces no measurable corrosion damage beyond that observed in the absence of radiation. In fact, the depth of corroded areas did not exceed a few micrometres (<10 µm), with and without radiation [41].
Turnbull et al. conducted a series of studies to investigate the effect of nitric acid on copper corrosion as the main oxidant produced under humid air radiolysis [22,60]. By using water droplets containing a range of concentrations of nitric acid exposed to aerated and de-aerated conditions, they demonstrated the slow ability of nitric acid to oxidize copper in the absence of oxygen. Separate work by Morco at al. provided a bounding estimate of <10 µm for corrosion damage on copper by nitric acid over the entire service life of the container [19].
We are also developing a mixed-potential model (MPM) for RIC, based on a similar model for copper corrosion in O2-containing Cl− environments [72]. For the preliminary version of the model, the cathodic reaction supporting the anodic dissolution of Cu is taken to be the reduction of radiolytically produced H2O2. The model is based on the assumption of saturated, anoxic conditions and does not currently take into account the possible decomposition of H2O2 to form O2 (and H2O); thus it can be considered to be conservative.
The anodic reaction involves the dissolution of Cu in Cl− solutions in the form of cuprous–chloro complex ions, such as CuCl2−:
with the transport of CuCl2− from the surface to the bulk environment being the rate-determining step (rds) [72] The cathodic reaction, the reduction of H2O2 to OH−,
is assumed to be under kinetic control, since the peroxide concentration is maintained at the UFC surface by the radiolysis process. The decomposition of hydrogen peroxide to oxygen and water is presumed not to occur. Rate parameters for the reduction of H2O2 are taken from the study of Vazquez et al. [73]. The interfacial [H2O2] is assumed to be equal to the steady-state concentration estimated from the simplified aqueous phase radiolysis model for deaerated conditions described by Equation (2). The pH of the pore-water in the buffer box will be buffered at a pH of 7–8, due to the presence of calcite in the bentonite, with a value of pH 7 used for the current calculations in order to be conservative, as this pH presumes the highest amount of peroxide will be produced within this pH range (Figure 8).
H2O2 + 2e− → 2OH−
Using the above assumptions, as well as the time-dependent interfacial [H2O2] for the UFC in the mixed-potential model, the time dependent ECORR and corrosion rate of the copper coating can be calculated, as shown in Figure 13. The corrosion rate is 0.2 μm/year initially and steadily decreases with increasing fuel age as the dose rate decreases. The value of ECORR also decreases with time as the concentration of radiolytically produced H2O2 decreases with decreasing dose rate. After the first 300 years, which corresponds to the period of highest activity, the depth of RIC is predicted to be 25 μm, which is similar in magnitude to the 9.4 μm predicted for corrosion by HNO3 absorbed in droplets under unsaturated conditions, and less than the value of 50 μm, suggested in a recent review by the NWMO to account for uncertainty in radiation-induced corrosion [2].
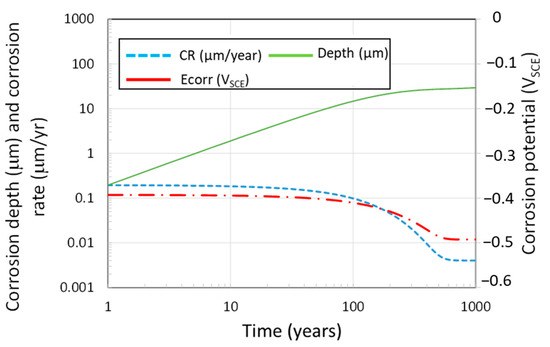
Figure 13.
Predicted evolution of the corrosion potential, corrosion rate, and the depth of radiation-induced corrosion of the copper UFC based on mixed-potential modelling and a simplified radiolysis model for the production of H2O2. The prediction was made for the highest dose rate on the copper surface corresponding to the axial orientation and for an assumed buffer pore-water pH 7.
It is also interesting to compare the MPM predictions with the experimentally measured RIC rates for copper. Björkbacka et al. measured the amount of dissolved copper resulting from the corrosion of copper samples in pure H2O at pH 5–6 at dose rates of 80 Gy/h and 770 Gy/h, from which corrosion rates of 0.19 μm/year and 1.2 μm/year can be estimated [48]. If we estimate the steady-state [H2O2] at pH 6 from Equation (2) and use it in the mixed-potential model, the predicted corrosion rates are 0.72 μm/year and 1.5 μm/year at dose rates of 80 Gy/h and 770 Gy/h, respectively, in reasonable agreement with the measured values. This agreement between experimental and predicted values is despite the difference in the anodic reactions in the experiment (copper dissolution as Cu+) and model (copper dissolution as CuCl2−), and may indicate that the corrosion rate was primarily under cathodic control in the experiment, i.e., determined by the rate of reduction of radiolytically produced oxidants.
Nevertheless, the reasonable agreement between model and prediction in the case of the MPM and between the predictions of the MPM and the model for corrosion by radiolytically produced HNO3 [19] builds confidence in our ability to predict the extent of RIC and suggests that the extent of corrosion for the UFC will be limited.
5. Conclusions
Copper-coated used fuel containers will be exposed to low-dose-rate γ-irradiation (1 Gy/h) following emplacement in a deep geological repository. The period of highest dose rate will correspond to the early thermal-saturation transient, during which the environment at the container surface will evolve from an initially warm, oxic, humid atmosphere to a long-term anoxic, saturated condition.
Based on the results of various experimental studies covering a range of environmental conditions and γ-radiation dose rates, it is concluded that the extent of radiation-induced corrosion of the copper coating will be minimal (of the order of a few tens of μm). For repository-relevant dose rates, there is no evidence for either an enhanced rate of general corrosion or for radiation-induced localized corrosion or environmentally assisted cracking. Although the rate of general corrosion can be enhanced at high dose rates (>>100 Gy/h), such effects are not considered to be relevant for repository conditions.
This conclusion of insignificant RIC is consistent with the results from two different modelling approaches. A conservative assessment of the extent of corrosion due to the absorption of radiolytically produced gaseous HNO3 by a water droplet formed under unsaturated conditions indicates a maximum depth of corrosion of <10 μm. Similarly, the results from a mixed-potential model for the RIC of copper under saturated conditions suggest a maximum corrosion depth of 20–30 μm.
Thus, although it is prudent to include a corrosion allowance in the lifetime prediction for a copper-coated UFC, RIC is not expected to be a major factor in determining the container lifetime.
Funding
Funding for this review was provided by the Nuclear Waste Management Organization, to whom the authors are grateful for permission to publish the work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Clara Wren and Ryan Morco for having valuable discussions and sharing their insight with regard to radiolysis modelling.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hall, D.S.; Keech, P.G. An Overview of the Canadian Corrosion Program for the Long-Term Management of Nuclear Waste. Corros. Eng. Sci. Technol. 2017, 52 (Suppl. 1), 2–5. [Google Scholar] [CrossRef]
- Hall, D.S.; Behazin, M.; Binns, W.J.; Keech, P.G. An Evaluation of Corrosion Processes Affecting Copper-Coated Nuclear Waste Containers in a Deep Geological Repository. Prog. Mater. Sci. 2021, 118, 100766. [Google Scholar] [CrossRef]
- Shoesmith, D.W.; King, F. The Effects of Gamma Radiation on the Corrosion of Candidate Materials for the Fabrication of Nuclear Waste Packages; AECL-11999; Atomic Energy of Canada Ltd.: Pinawa, MB, Canada, 1999. [Google Scholar]
- Daub, K.; Zhang, X.; Noël, J.J.; Wren, J.C. Effects of γ-Radiation versus H2O2 on Carbon Steel Corrosion. Electrochim. Acta 2010, 55, 2767–2776. [Google Scholar] [CrossRef]
- Knapp, Q.W.; Wren, J.C. Film Formation on Type-316L Stainless Steel as a Function of Potential: Probing the Role of Gamma-Radiation. Electrochim. Acta 2012, 80, 90–99. [Google Scholar] [CrossRef]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1990. [Google Scholar]
- Belloni, J.; Mostafavi, M.; Douki, T.; Spotheim-Maurizot, M. Radiation Chemistry from Basics to Applications in Material and Life Sciences; Editions EDP Sciences: Les Ulis, France, 2008. [Google Scholar]
- Elliot, A.J.; Chenier, M.P.; Ouellette, D.C. G-Values for γ-Irradiated Water as a Function of Temperature. Can. J. Chem. 1990, 68, 712–719. [Google Scholar] [CrossRef]
- Wu, M.; Behazin, M.; Nam, J.; Keech, P. Internal Corrosion of Used Fuel Container; Technical Report NWMO-TR-2019-02; NWMO: Toronto, ON, Canada, 2019. [Google Scholar]
- Johnsson, M.; Emilsson, G.; Emilsson, L. Mechanical Design Analysis for the Canister; Technical Report Posiva SKB Report 04; Posiva Oy: Eurajoki, Finland, 2018. [Google Scholar]
- SKB. Supplementary Information on Canister Integrity Issues; Technical Report SKB TR-19-15; SKB: Stockholm, Sweden, 2019; p. 135. [Google Scholar]
- King, F.; Hall, D.S.; Keech, P.G. Nature of the Near-Field Environment in a Deep Geological Repository and the Implications for the Corrosion Behaviour of the Container. Corros. Eng. Sci. Technol. 2017, 52 (Suppl. 1), 25–30. [Google Scholar] [CrossRef]
- Guo, R. Thermal Response of a Canadian Conceptual Deep Geological Repository in Crystalline Rock and a Method to Correct the Influence of the Near-Field Adiabatic Boundary Condition. Eng. Geol. 2017, 218, 50–62. [Google Scholar] [CrossRef]
- Johansson, L.; Stahlén, J.; Taborowski, T.; Pedersen, K.; Luukari, S.; Marmo, J. Microbial Release of Iron from Olkiluoto Rock Minerals; Posiva Working Report 2018–30; Olkiluoto: Helsinki, Finland, 2019; pp. 1–120. [Google Scholar]
- Giroud, N.; Tomonaga, Y.; Wersin, P.; Briggs, S.; King, F.; Vogt, T.; Diomidis, N. On the Fate of Oxygen in a Spent Fuel Emplacement Drift in Opalinus Clay. Appl. Geochem. 2018, 97, 270–278. [Google Scholar] [CrossRef]
- Ershov, B.G.; Gordeev, A.V. A Model for Radiolysis of Water and Aqueous Solutions of H2, H2O2 and O2. Radiat. Phys. Chem. 2008, 77, 928–935. [Google Scholar] [CrossRef]
- Boyd, A.W.; Carver, M.B.; Dixon, R.S. Computed and Experimental Product Concentrations in the Radiolysis of Water. Radiat. Phys. Chem. (1977) 1980, 15, 177–185. [Google Scholar] [CrossRef]
- Reed, D.T.; Van Konynenburg, R.A. Effect of Ionizing Radiation on Moist Air Systems. In Materials Research Society Symposium Proceedings; Materials Research Society: Warrendale, PA, USA, 1988; Volume 112, pp. 393–404. [Google Scholar]
- Morco, R.P.; Joseph, J.M.; Hall, D.S.; Medri, C.; Shoesmith, D.W.; Wren, J.C. Modelling of Radiolytic Production of HNO3 Relevant to Corrosion of a Used Fuel Container in Deep Geologic Repository Environments. Corros. Eng. Sci. Technol. 2017, 52 (Suppl. 1), 141–147. [Google Scholar] [CrossRef]
- Björkbacka, Å.; Johnson, C.M.; Leygraf, C.; Jonsson, M. Radiation Induced Corrosion of Copper in Humid Air and Argon Atmospheres. J. Electrochem. Soc. 2017, 164, C201–C206. [Google Scholar] [CrossRef]
- Johnson, G.R.D. The Radiation Chemistry of Nitrogen and Its Compounds. Inorganic and Theoretical Chemistry; Wiley: New York, NY, USA, 1967. [Google Scholar]
- Turnbull, J.; Szukalo, R.; Zagidulin, D.; Biesinger, M.; Shoesmith, D. The Kinetics of Copper Corrosion in Nitric Acid. Mater. Corros. 2020, 72, 348–360. [Google Scholar] [CrossRef]
- Morco, R.P. Gamma-Radiolysis Kinetics and Its Role in The Overall Dynamics of Materials Degradation. Ph.D. Thesis, University of Western Ontario, London, ON, Canada, 2020. [Google Scholar]
- Joseph, J.M.; Seon Choi, B.; Yakabuskie, P.; Wren, J.C. A Combined Experimental and Model Analysis on the Effect of PH and O2(Aq) on γ-Radiolytically Produced H2 and H2O2. Radiat. Phys. Chem. 2008, 77, 1009–1020. [Google Scholar] [CrossRef]
- Hochanadel, C.J. Effects of Cobalt γ-Radiation on Water and Aqueous Solutions. J. Phys. Chem. 1952, 56, 587–594. [Google Scholar] [CrossRef]
- Allen, A.O. The Radiation Chemistry of Water and Aqueous Solutions; Van Nostrand: Princeton, NJ, USA, 1961. [Google Scholar]
- Morawetz, H. Radiation Chemistry-Principles and Applications; Farhataziz, Rogers, M.A.J., Eds.; VCH: New York, NY, USA, 1987; p. 641. [Google Scholar]
- Dixon, D.A. Review of the T-H-M-C Properties of MX-80 Bentonite; NWMO TR-2019-07; Nuclear Waste Management Organisation: Toronto, ON, Canada, 2019. [Google Scholar]
- Airey, P.L. Effect of Irradiation on Electrode Processes. Part 1—The Hydrogen Electrode in Dilute Solutions. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1972, 68, 1299–1311. [Google Scholar] [CrossRef]
- Abrantes, L.M.; Castillo, L.M.; Norman, C.; Peter, L.M. A Photoelectrochemical Study of the Anodic Oxidation of Copper in Alkaline Solution. J. Electroanal. Chem. Interfacial Electrochem. 1984, 163, 209–221. [Google Scholar] [CrossRef]
- Collisi, U.; Strehblow, H.-H. A Photoelectrochemical Study of Passive Copper in Alkaline Solutions. J. Electroanal. Chem. Interfacial Electrochem. 1986, 210, 213–227. [Google Scholar] [CrossRef]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Kondo, J.N.; Domen, K.; Hara, M.; Shinohara, K.; Tanaka, A. Cu2O as a Photocatalyst for Overall Water Splitting under Visible Light Irradiation. Chem. Commun. 1998, 3, 357–358. [Google Scholar] [CrossRef]
- Yamashita, M.; Omura, K.; Hirayama, D. Passivating Behavior of Copper Anodes and Its Illumination Effects in Alkaline Solutions. Surf. Sci. 1980, 96, 443–460. [Google Scholar] [CrossRef]
- Isherwood, P.J.M.; Walls, J.M. Cupric Oxide-Based p-Type Transparent Conductors. Energy Procedia 2014, 60, 129–134. [Google Scholar] [CrossRef][Green Version]
- Aruchamy, A.; Fujishima, A. Photoanodic Behaviour of Cu2O Corrosion Layers on Copper Electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1989, 272, 125–136. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Liu, X.; Hou, J.; Sun, M.; Zeng, R. Study on the Mechanism of the Photoelectrochemical Effect on the Initial NaCl-Induced Atmospheric Corrosion Process of Pure Copper Exposed in Humidified Pure Air. J. Electrochem. Soc. 2018, 165, C608–C617. [Google Scholar] [CrossRef]
- Mian, T.A.; Van Putten, M.C., Jr.; Kramer, D.C.; Jacob, R.F.; Boyer, A.L. Backscatter Radiation at Bone-Titanium Interface from High-Energy x and Gamma Rays. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 1943–1947. [Google Scholar] [CrossRef]
- Lainé, M.; Balan, E.; Allard, T.; Paineau, E.; Jeunesse, P.; Mostafavi, M.; Robert, J.-L.; Le Caër, S. Reaction Mechanisms in Swelling Clays under Ionizing Radiation: Influence of the Water Amount and of the Nature of the Clay Mineral. RSC Adv. 2017, 7, 526–534. [Google Scholar] [CrossRef]
- Fourdrin, C.; Aarrachi, H.; Latrille, C.; Esnouf, S.; Bergaya, F.; Le Caër, S. Water Radiolysis in Exchanged-Montmorillonites: The H2 Production Mechanisms. Environ. Sci. Technol. 2013, 47, 9530–9537. [Google Scholar] [CrossRef]
- Le Caër, S. Water Radiolysis: Influence of Oxide Surfaces on H2 Production under Ionizing Radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef]
- Ibrahim, B.; Zagidulin, D.; Behazin, M.; Ramamurthy, S.; Wren, J.C.; Shoesmith, D.W. The Corrosion of Copper in Irradiated and Unirradiated Humid Air. Corros. Sci. 2018, 141, 53–62. [Google Scholar] [CrossRef]
- Ibrahim, B. The Corrosion Behaviour of Cu in Irradiated and Non-Irradiated Humid Air. Master’s Thesis, University of Western Ontario, London, ON, Canada, 2015. [Google Scholar]
- Ibrahim, B.; Zagidulin, D.; Smith, J.M.; Ramamurthy, S.; Wren, J.C.; Shoesmith, D.W. Radiolytic Corrosion of Cu Nuclear Waste Containers. In Proceedings of the 17th International Conference on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors, Ottawa, ON, Canada, 9–13 August 2015. [Google Scholar]
- Turnbull, J.; Behazin, M.; Smith, J.; Keech, P.G. The Impact of 40 Years of Radiation on the Integrity of Copper. J. Nucl. Mater. 2021. accepted for publication. [Google Scholar] [CrossRef]
- Sherwood, J.E.; Stagnitti, F.; Kokkinn, M.J.; Williams, W.D. A Standard Table for Predicting Equilibrium Dissolved Oxygen Concentrations in Salt Lakes Dominated by Sodium Chloride. Int. J. Salt Lake Res. 1992, 1, 1–6. [Google Scholar] [CrossRef]
- Wren, J.C.; Jean, A.; Naghizadeh, M.; Grandy, L.; Morco, R.; Joseph, J.M.; Behazin, M.; Keech, P.G. Radiation Induced Corrosion of Copper in Deep Geological Repositories—19493; WM Symposia, Inc.: Tempe, AZ, USA, 2019. [Google Scholar]
- Björkbacka, Å.; Hosseinpour, S.; Leygraf, C.; Jonsson, M. Radiation Induced Corrosion of Copper in Anoxic Aqueous Solution. Electrochem. Solid-State Lett. 2012, 15, C5–C7. [Google Scholar] [CrossRef][Green Version]
- Björkbacka, Å.; Hosseinpour, S.; Johnson, M.; Leygraf, C.; Jonsson, M. Radiation Induced Corrosion of Copper for Spent Nuclear Fuel Storage. Radiat. Phys. Chem. 2013, 92, 80–86. [Google Scholar] [CrossRef]
- Björkbacka, Å.; Yang, M.; Gasparrini, C.; Leygraf, C.; Jonsson, M. Kinetics and Mechanisms of Reactions between H2O2 and Copper and Copper Oxides. Dalton Trans. 2015, 44, 16045–16051. [Google Scholar] [CrossRef]
- Björkbacka, Å.; Johnson, C.M.; Leygraf, C.; Jonsson, M. Role of the Oxide Layer in Radiation-Induced Corrosion of Copper in Anoxic Water. J. Phys. Chem. C 2016, 120, 11450–11455. [Google Scholar] [CrossRef]
- Soroka, I.; Chae, N.; Jonsson, M. On the Mechanism of γ-Radiation-Induced Corrosion of Copper in Water. Corros. Sci. 2021, 182, 109279. [Google Scholar] [CrossRef]
- King, F.; Litke, C.D. The Corrosion of Copper in Synthetic Groundwater at 150 C. Part I. The Results of Short-Term Electrochemical Tests; Technical Report TR-428; Atomic Energy of Canada Ltd.: Pinawa, MB, Canada, 1987. [Google Scholar]
- Litke, C.D.; Ryan, S.R.; King, F. A Mechanistic Study of the Uniform Corrosion of Copper in Compacted Clay-Sand Soil; Atomic Energy of Canada Ltd.: Pinawa, MB, Canada, 1992; p. 141. [Google Scholar]
- Simpson, J.P. Experiments on Container Materials for Swiss High-Level Waste Disposal Projects Part; Nagra Technical Report, NTB 84-01; National Cooperative for the Disposal of Radioactive Waste: Wettingen, Switzerland, 1984; p. 100. [Google Scholar]
- Glass, R.S.; Van Konynenburg, R.A.; Overturf, G.E. Corrosion Processes of Austenitic Stainless Steels and Copper-Based Materials in Gamma-Irradiated Aqueous Environments. In Proceedings of the Corrosion 86, Houston, TX, USA, 17–21 March 1986. paper No. 258. [Google Scholar] [CrossRef][Green Version]
- King, F.; Lilja, C.; Pedersen, K.; Pitkänen, P.; Vähänen, M. An Update of the State-of-the-Art Report on the Corrosion of Copper under Expected Conditions in a Deep Geologic Repository; Technical Report SKB TR-10-67; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2010; p. 180. [Google Scholar]
- Yunker, W.H. Corrosion Behavior of Copper-Base Materials in a Gamma-Irradiated Environment; Report WHC-EP-0188; Westinghouse Hanford Company: Richland, WA, USA, 1990. [Google Scholar] [CrossRef][Green Version]
- King, F. Assessment of the Stress Corrosion Cracking of Copper Canisters; 2021-11 Working Report; Posiva Oy: Eurajoki, Finland, 2021. [Google Scholar]
- King, F.; Newman, R.C. Stress Corrosion Cracking of Copper Canisters; Technical Report SKB TR-10-04; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2010. [Google Scholar]
- Turnbull, J.; Szukalo, R.; Behazin, M.; Hall, D.; Zagidulin, D.; Ramamurthy, S.; Wren, J.C.; Shoesmith, D.W. The Effects of Cathodic Reagent Concentration and Small Solution Volumes on the Corrosion of Copper in Dilute Nitric Acid Solutions. Corrosion 2017, 74, 326–336. [Google Scholar] [CrossRef]
- Karasawa, H.; Ibe, E.; Uchida, S.; Etoh, Y.; Yasuda, T. Radiation Induced Decomposition of Nitrogen. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1991, 37, 193–197. [Google Scholar] [CrossRef]
- Ryan, S.R.; Clarke, C.F.; Ikeda, B.M.; King, F.; Litke, C.D. Investigation of the Long-Term Corrosion Behaviour of Selected Nuclear Fuel Waste Container Materials under Possible Disposal Vault Conditions; Technical Report TR-489, COG-94-55; Atomic Energy of Canada Ltd.: Pinawa, MB, Canada, 1994. [Google Scholar]
- Lousada, C.M.; Soroka, I.L.; Yagodzinskyy, Y.; Tarakina, N.V.; Todoshchenko, O.; Hänninen, H.; Korzhavyi, P.A.; Jonsson, M. Gamma Radiation Induces Hydrogen Absorption by Copper in Water. Sci. Rep. 2016, 6, 24234. [Google Scholar] [CrossRef]
- Yagodzinskyy, Y.; Malitckii, E.; Saukkonen, T.; Hänninen, H. Hydrogen-Enhanced Creep and Cracking of Oxygen-Free Phosphorus-Doped Copper. Scr. Mater. 2012, 67, 931–934. [Google Scholar] [CrossRef]
- Forsström, A.; Becker, R.; Hänninen, H.; Yagodzinskyy, Y.; Heikkilä, M. Sulphide-Induced Stress Corrosion Cracking and Hydrogen Absorption of Copper in Deoxygenated Water at 90 °C. Mater. Corros. 2021, 72, 317–332. [Google Scholar] [CrossRef]
- Hänninen, H.; Forsström, A.; Yagodzinskyy, Y. 11—Copper Behavior in Geological Nuclear Waste Disposal. In Nuclear Corrosion; Ritter, S., Ed.; Woodhead Publishing: Cambridge, UK, 2020; pp. 391–402. [Google Scholar] [CrossRef]
- English, C.A.; Hyde, J.M. 6.08—Radiation Embrittlement of Reactor Pressure Vessel Steels. In Comprehensive Structural Integrity; Milne, I., Ritchie, R.O., Karihaloo, B., Eds.; Pergamon: Oxford, UK, 2003; pp. 351–398. [Google Scholar] [CrossRef]
- Li, M.; Zinkle, S.J. Physical and Mechanical Properties of Copper and Copper Alloys. Compr. Nucl. Mater. 2012, 4, 667–690. [Google Scholar]
- Yang, Q.; Toijer, E.; Olsson, P. Analysis of Radiation Damage in the KBS-3 Canister Materials; Technical Report SKB TR-19-14; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2019. [Google Scholar]
- Padovani, C.; Pletser, D.; Jurkschat, K.; Armstrong, D.; Dugdale, S.; Brunt, D.; Faulkner, R.; Was, G.; Johansson, A.J. Assessment of Microstructural Changes in Copper Due to Gamma Radiation Damage; Technical Report SKB TR-19-12; Swedish Nuclear Fuel and Waste Management Co.: Solna, Sweden, 2019. [Google Scholar]
- Keech, P.G.; Behazin, M.; Binns, W.J.; Briggs, S. An Update on the Copper Corrosion Program for the Long-term Management of Used Nuclear Fuel in Canada. Mater. Corros. 2020, 72, 25–31. [Google Scholar] [CrossRef]
- King, F.; Litke, C.D.; Quinn, M.J.; LeNeveu, D.M. The Measurement and Prediction of the Corrosion Potential of Copper in Chloride Solutions as a Function of Oxygen Concentration and Mass-Transfer Coefficient. Corros. Sci. 1995, 37, 833–851. [Google Scholar] [CrossRef]
- Vazquez, M.V.; de Sanchez, S.R.; Calvo, E.J.; Schiffrin, D.J. The Electrochemical Reduction of Hydrogen Peroxide on Polycrystalline Copper in Borax Buffer. J. Electroanal. Chem. 1994, 374, 179–187. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).