The Effect of Light Therapy on Electroencephalographic Sleep in Sleep and Circadian Rhythm Disorders: A Scoping Review
Abstract
:1. Introduction
1.1. Rationale
1.2. Objective
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
- Population: Participants with a sleep or circadian rhythm disorder.
- Intervention: The intervention light therapy had to include either:
- Intensity of light greater than or equal to control light condition;
- Clock time (outside of regular light hours).
- 3.
- Comparison: The control light condition had to include either:
- Intensity of light less than or equal to intervention light condition;
- Clock time (regular light hours).
- 4.
- Outcome: Sleep macro- or micro-architecture assessed with EEG or polysomnography recordings. Both nighttime and daytime sleep were included. Time in bed (TIB), TST, wake after sleep onset (WASO), sleep efficiency (SE), SOL and the duration of non-rapid eye movement (NREM) and rapid eye movement (REM) sleep were included as macro-architecture measures of sleep. Sleep micro-architecture was measured using EEG power spectral analysis, including any of the frequency bands (delta, theta, alpha, sigma and beta).
- 5.
- Study type: Laboratory or clinic-based studies where randomization had been used to assign participants to conditions (parallel trials) or the order in which they were exposed to conditions (cross-over trials).
2.3. Information Sources and Search
2.4. Selection of Sources of Evidence
2.5. Data Charting Process and Data Items
2.6. The Critical Appraisal of Individual Sources of Evidence
2.7. Synthesis of Results
3. Results
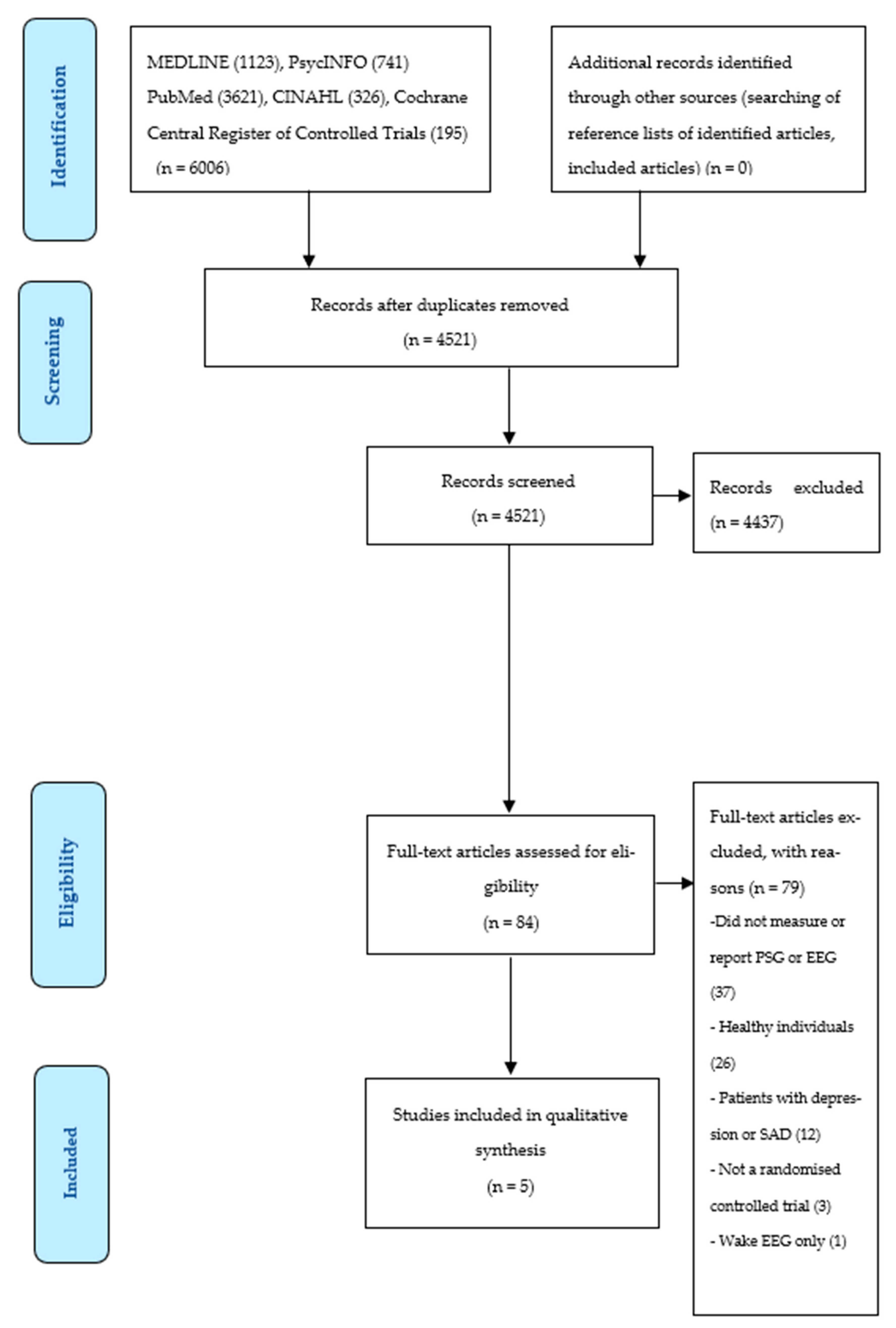
3.1. Selection of Sources of Evidence
3.2. Characteristics of Sources of Evidence
3.3. Results of Sources of Evidence
3.4. Synthesis of Results
3.4.1. Insomnia Studies
3.4.2. DWSPD Study
3.4.3. Sleep EEG Micro-Architecture
4. Discussion
4.1. Summary of Evidence
4.1.1. Patients with Insomnia
4.1.2. Patients with DSWPD
4.1.3. Lack of Sleep EEG Micro-Architecture Outcomes
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CINAHL | Cumulative Index to Nursing and Allied Health Literature |
| DSWPD | Delayed sleep–wake phase disorder |
| EEG | Electroencephalography |
| NREM | Non-rapid eye movement |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews |
| REM | Rapid eye movement |
| SCN | Suprachiasmatic nucleus |
| SE | Sleep efficiency |
| SOL | Sleep onset latency |
| TIB | Time in bed |
| TST | Total sleep time |
| WASO | Wake after sleep onset |
References
- Aschoff, J.; Pöppel, E.; Wever, R. Circadian rhythms in men under the influence of light-dark cycles of various periods. Pflug. Arch. 1969, 306, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Wever, R.A. Phase shifts of human circadian rhythms due to shifts of artificial zeitgebers. Chronobiologia 1980, 7, 303–327. [Google Scholar] [PubMed]
- Czeisler, C.A.; Kronauer, R.E.; Allan, J.S.; Duffy, J.F.; Jewett, M.E.; Brown, E.N.; Ronda, J.M. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science 1989, 244, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.F.; Wright, K.P., Jr. Entrainment of the human circadian system by light. J. Biol. Rhythm. 2005, 20, 326–338. [Google Scholar] [CrossRef]
- Yetish, G.; Kaplan, H.; Gurven, M.; Wood, B.; Pontzer, H.; Manger, P.R.; Wilson, C.; McGregor, R.; Siegel, J.M. Natural sleep and its seasonal variations in three pre-industrial societies. Curr. Biol. 2015, 25, 2862–2868. [Google Scholar] [CrossRef] [Green Version]
- Pilz, L.K.; Levandovski, R.; Oliveira, M.A.; Hidalgo, M.P.; Roenneberg, T. Sleep and light exposure across different levels of urbanisation in Brazilian communities. Sci. Rep. 2018, 8, 11389. [Google Scholar] [CrossRef] [Green Version]
- Stevens, R.G.; Zhu, Y. Electric light, particularly at night, disrupts human circadian rhythmicity: Is that a problem? Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140120. [Google Scholar] [CrossRef] [Green Version]
- Wright, K.P., Jr.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013, 23, 1554–1558. [Google Scholar] [CrossRef] [Green Version]
- aan het Rot, M.; Moskowitz, D.S.; Young, S.N. Exposure to bright light is associated with positive social interaction and good mood over short time periods: A naturalistic study in mildly seasonal people. J. Psychiatr. Res. 2008, 42, 311–319. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [Green Version]
- Hubert, M.; Dumont, M.; Paquet, J. Seasonal and diurnal patterns of human illumination under natural conditions. Chronobiol. Int. 1998, 15, 59–70. [Google Scholar] [CrossRef]
- Schweizer, C.; Edwards, R.D.; Bayer-Oglesby, L.; Gauderman, W.J.; Ilacqua, V.; Jantunen, M.J.; Lai, H.K.; Nieuwenhuijsen, M.; Künzli, N. Indoor time-microenvironment-activity patterns in seven regions of Europe. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Cinzano, P.; Falchi, F.; Elvidge, C.D. The first world atlas of the artificial night sky brightness. Mon. Not. R. Astron. Soc. 2001, 328, 689–707. [Google Scholar] [CrossRef] [Green Version]
- Kubota, T.; Uchiyama, M.; Suzuki, H.; Shibui, K.; Kim, K.; Tan, X.; Tagaya, H.; Okawa, M.; Inoue, S. Effects of nocturnal bright light on saliva melatonin, core body temperature and sleep propensity rhythms in human subjects. Neurosci. Res. 2002, 42, 115–122. [Google Scholar] [CrossRef]
- Thapan, K.; Arendt, J.; Skene, D.J. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J. Physiol. 2001, 535, 261–267. [Google Scholar] [CrossRef]
- Zeitzer, J.M.; Dijk, D.J.; Kronauer, R.E.; Brown, E.N.; Czeisler, C.A. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J. Physiol. 2000, 526, 695–702. [Google Scholar] [CrossRef]
- Chang, A.M.; Santhi, N.; St Hilaire, M.; Gronfier, C.; Bradstreet, D.S.; Duffy, J.F.; Lockley, S.W.; Kronauer, R.E.; Czeisler, C.A. Human responses to bright light of different durations. J. Physiol. 2012, 590, 3103–3112. [Google Scholar] [CrossRef] [Green Version]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [Green Version]
- Brainard, G.C.; Hanifin, J.P.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J. Neurosci. 2001, 21, 6405–6412. [Google Scholar] [CrossRef] [Green Version]
- Bailes, H.J.; Lucas, R.J. Human melanopsin forms a pigment maximally sensitive to blue light (λmax ≈ 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc. Biol. Sci. 2013, 280, 20122987. [Google Scholar] [CrossRef] [Green Version]
- Prayag, A.S.; Najjar, R.P.; Gronfier, C. Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J. Pineal Res. 2019, 66, e12562. [Google Scholar] [CrossRef]
- Phillips, A.J.K.; Vidafar, P.; Burns, A.C.; McGlashan, E.M.; Anderson, C.; Rajaratnam, S.M.W.; Lockley, S.W.; Cain, S.W. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc. Natl. Acad. Sci. USA 2019, 116, 12019–12024. [Google Scholar] [CrossRef] [Green Version]
- van Maanen, A.; Meijer, A.M.; van der Heijden, K.B.; Oort, F.J. The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 29, 52–62. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Okamoto, N.; Tomioka, K.; Nezu, S.; Ikada, Y.; Kurumatani, N. Effect of exposure to evening light on sleep initiation in the elderly: A longitudinal analysis for repeated measurements in home settings. Chronobiol. Int. 2014, 31, 461–467. [Google Scholar] [CrossRef]
- Goulet, G.; Mongrain, V.; Desrosiers, C.; Paquet, J.; Dumont, M. Daily light exposure in morning-type and evening-type individuals. J. Biol. Rhythm. 2007, 22, 151–158. [Google Scholar] [CrossRef]
- Cain, S.W.; McGlashan, E.M.; Vidafar, P.; Mustafovska, J.; Curran, S.P.N.; Wang, X.; Mohamed, A.; Kalavally, V.; Phillips, A.J.K. Evening home lighting adversely impacts the circadian system and sleep. Sci. Rep. 2020, 10, 19110. [Google Scholar] [CrossRef]
- Leger, D.; Bayon, V.; Elbaz, M.; Philip, P.; Choudat, D. Underexposure to light at work and its association to insomnia and sleepiness: A cross-sectional study of 13,296 workers of one transportation company. J. Psychosom. Res. 2011, 70, 29–36. [Google Scholar] [CrossRef]
- Hölker, F.; Moss, T.; Griefahn, B.; Kloas, W.; Voigt, C.C.; Henckel, D.; Hänel, A.; Kappeler, P.M.; Völker, S.; Schwope, A. The dark side of light: A transdisciplinary research agenda for light pollution policy. Ecol. Soc. 2010, 15, 13. [Google Scholar] [CrossRef]
- Cinzano, P. The growth of light pollution in north-eastern Italy from 1960 to 1995. Mem. Soc. Astron. Ital. 2000, 71, 159–165. [Google Scholar]
- Bunnell, D.E.; Treiber, S.P.; Phillips, N.H.; Berger, R.J. Effects of evening bright light exposure on melatonin, body temperature and sleep. J. Sleep Res. 1992, 1, 17–23. [Google Scholar] [CrossRef]
- Kubota, T.; Uchiyama, M.; Hirokawa, G.; Ozaki, S.; Hayasi, M.; Okawa, M. Effects of evening light on body temperature. Psychiatry Clin. Neurosci. 1998, 52, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Ruger, M.; Gordijn, M.C.; Beersma, D.G.; de Vries, B.; Daan, S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: Comparison of daytime and nighttime exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1413–R1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komada, Y.; Tanaka, H.; Yamamoto, Y.; Shirakawa, S.; Yamazaki, K. Effects of bright light pre-exposure on sleep onset process. Psychiatry Clin. Neurosci. 2000, 54, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, S.; Paquet, J.; Selmaoui, B.; Rufiange, M.; Dumont, M. Vigilance levels during and after bright light exposure in the first half of the night. Chronobiol. Int. 2003, 20, 1019–1038. [Google Scholar] [CrossRef]
- Tzischinsky, O.; Lavie, P. The effects of evening bright light on next-day sleep propensity. J. Biol. Rhythm. 1997, 12, 259–265. [Google Scholar] [CrossRef]
- Gradisar, M.; Wolfson, A.R.; Harvey, A.G.; Hale, L.; Rosenberg, R.; Czeisler, C.A. The sleep and technology use of Americans: Findings from the National Sleep Foundation’s 2011 Sleep in America poll. J. Clin. Sleep Med. 2013, 9, 1291–1299. [Google Scholar] [CrossRef]
- Fossum, I.N.; Nordnes, L.T.; Storemark, S.S.; Bjorvatn, B.; Pallesen, S. The association between use of electronic media in bed before going to sleep and insomnia symptoms, daytime sleepiness, morningness, and chronotype. Behav. Sleep Med. 2014, 12, 343–357. [Google Scholar] [CrossRef]
- Brunborg, G.S.; Mentzoni, R.A.; Molde, H.; Myrseth, H.; Skouverøe, K.J.; Bjorvatn, B.; Pallesen, S. The relationship between media use in the bedroom, sleep habits and symptoms of insomnia. J. Sleep Res. 2011, 20, 569–575. [Google Scholar] [CrossRef]
- Lastella, M.; Rigney, G.; Browne, M.; Sargent, C. Electronic device use in bed reduces sleep duration and quality in adults. Sleep Biol. Rhythm. 2020, 18, 121–129. [Google Scholar] [CrossRef]
- Higuchi, S.; Motohashi, Y.; Liu, Y.; Maeda, A. Effects of playing a computer game using a bright display on presleep physiological variables, sleep latency, slow wave sleep and REM sleep. J. Sleep Res. 2005, 14, 267–273. [Google Scholar] [CrossRef]
- Exelmans, L.; Van den Bulck, J. Bedtime mobile phone use and sleep in adults. Soc. Sci. Med. 2016, 148, 93–101. [Google Scholar] [CrossRef]
- Münch, M.; Kobialka, S.; Steiner, R.; Oelhafen, P.; Wirz-Justice, A.; Cajochen, C. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1421–R1428. [Google Scholar] [CrossRef] [Green Version]
- McEnany, G.W.; Lee, K.A. Effects of light therapy on sleep, mood, and temperature in women with nonseasonal major depression. Issues Ment. Health Nurs. 2005, 26, 781–794. [Google Scholar] [CrossRef]
- Rahman, S.A.; Shapiro, C.M.; Wang, F.; Ainlay, H.; Kazmi, S.; Brown, T.J.; Casper, R.F. Effects of filtering visual short wavelengths during nocturnal shiftwork on sleep and performance. Chronobiol. Int. 2013, 30, 951–962. [Google Scholar] [CrossRef] [Green Version]
- Santhi, N.; Thorne, H.C.; van der Veen, D.R.; Johnsen, S.; Mills, S.L.; Hommes, V.; Schlangen, L.J.; Archer, S.N.; Dijk, D.J. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J. Pineal Res. 2012, 53, 47–59. [Google Scholar] [CrossRef]
- Edinger, J.D.; Fins, A.I.; Glenn, D.M.; Sullivan, R.J., Jr.; Bastian, L.A.; Marsh, G.R.; Dailey, D.; Hope, T.V.; Young, M.; Shaw, E.; et al. Insomnia and the eye of the beholder: Are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? J. Consult. Clin. Psychol. 2000, 68, 586–593. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F. III.; Monk, T.H.; Hoch, C.C.; Yeager, A.L.; Kupfer, D.J. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep 1991, 14, 331–338. [Google Scholar]
- Vitiello, M.V.; Larsen, L.H.; Moe, K.E. Age-related sleep change: Gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women. J. Psychosom. Res. 2004, 56, 503–510. [Google Scholar] [CrossRef]
- Rechtschaffen, A.; Kales, A. A Manual of Standardized TerminologyTechniques and Scoring Systems for Sleep Stages of Human Subjects; Public Health Services, U.S. Government Printing Office: Washington, DC, USA, 1968.
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S.F. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification, 1st ed.; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Silber, M.H.; Ancoli-Israel, S.; Bonnet, M.H.; Chokroverty, S.; Grigg-Damberger, M.M.; Hirshkowitz, M.; Kapen, S.; Keenan, S.A.; Kryger, M.H.; Penzel, T.; et al. The visual scoring of sleep in adults. J. Clin. Sleep Med. 2007, 3, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Biswal, S.; Kulas, J.; Sun, H.; Goparaju, B.; Westover, M.B.; Bianchi, M.T.; Sun, J. SLEEPNET: Automated sleep staging system via deep learning. arXiv 2017, arXiv:1707.08262. [Google Scholar]
- Mendonça, F.; Mostafa, S.S.; Morgado-Dias, F.; Ravelo-Garcia, A.G.; Penzel, T. A review of approaches for sleep quality analysis. IEEE Access 2019, 7, 24527–24546. [Google Scholar] [CrossRef]
- O’Donnell, D.; Silva, E.J.; Münch, M.; Ronda, J.M.; Wang, W.; Duffy, J.F. Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. J. Sleep Res. 2009, 18, 254–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, N.E.; Joseph-Vanderpool, J.R.; Levendosky, A.A.; Johnston, S.H.; Allen, R.; Kelly, K.A.; Souetre, E.; Schultz, P.M.; Starz, K.E. Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep 1990, 13, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Friedman, L.; Zeitzer, J.M.; Kushida, C.; Zhdanova, I.; Noda, A.; Lee, T.; Schneider, B.; Guilleminault, C.; Sheikh, J.; Yesavage, J.A. Scheduled bright light for treatment of insomnia in older adults. J. Am. Geriatr. Soc. 2009, 57, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Suhner, A.G.; Murphy, P.J.; Campbell, S.S. Failure of timed bright light exposure to alleviate age-related sleep maintenance insomnia. J. Am. Geriatr. Soc. 2002, 50, 617–623. [Google Scholar] [CrossRef]
- Murphy, P.J.; Campbell, S.S. Enhanced performance in elderly subjects following bright light treatment of sleep maintenance insomnia. J. Sleep Res. 1996, 5, 165–172. [Google Scholar] [CrossRef]
- Youngstedt, S.D.; Kripke, D.F.; Elliott, J.A.; Rex, K.M. Circadian phase-shifting effects of a laboratory environment: A clinical trial with bright and dim light. J. Circadian Rhythm. 2005, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Lack, L.C.; Mercer, J.D.; Wright, H. Circadian rhythms of early morning awakening insomniacs. J. Sleep Res. 1996, 5, 211–219. [Google Scholar] [CrossRef]
- Campbell, S.S.; Dawson, D.; Anderson, M.W. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J. Am. Geriatr. Soc. 1993, 41, 829–836. [Google Scholar] [CrossRef]
- Lack, L.; Wright, H. The effect of evening bright light in delaying the circadian rhythms and lengthening the sleep of early morning awakening insomniacs. Sleep 1993, 16, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Semo, M.; Lupi, D.; Peirson, S.N.; Butler, J.N.; Foster, R.G. Light-induced c-fos in melanopsin retinal ganglion cells of young and aged rodless/coneless (rd/rd cl) mice. Eur. J. Neurosci. 2003, 18, 3007–3017. [Google Scholar] [CrossRef]
- Kessel, L.; Lundeman, J.H.; Herbst, K.; Andersen, T.V.; Larsen, M. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J. Cataract. Refract. Surg. 2010, 36, 308–312. [Google Scholar] [CrossRef]
- Swaab, D.F.; Fliers, E.; Partiman, T.S. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985, 342, 37–44. [Google Scholar] [CrossRef]
- Sample, P.A.; Esterson, F.D.; Weinreb, R.N.; Boynton, R.M. The aging lens: In Vivo assessment of light absorption in 84 human eyes. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1306–1311. [Google Scholar]
- Bitsios, P.; Prettyman, R.; Szabadi, E. Changes in autonomic function with age: A study of pupillary kinetics in healthy young and old people. Age Ageing 1996, 25, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Najjar, R.P.; Chiquet, C.; Teikari, P.; Cornut, P.L.; Claustrat, B.; Denis, P.; Cooper, H.M.; Gronfier, C. Aging of non-visual spectral sensitivity to light in humans: Compensatory mechanisms? PLoS ONE 2014, 9, e85837. [Google Scholar] [CrossRef] [Green Version]
- Brainard, G.C.; Rollag, M.D.; Hanifin, J.P. Photic regulation of melatonin in humans: Ocular and neural signal transduction. J. Biol. Rhythm. 1997, 12, 537–546. [Google Scholar] [CrossRef]
- Liu, R.Y.; Zhou, J.N.; Hoogendijk, W.J.; van Heerikhuize, J.; Kamphorst, W.; Unmehopa, U.A.; Hofman, M.A.; Swaab, D.F. Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. J. Neuropathol. Exp. Neurol. 2000, 59, 314–322. [Google Scholar] [CrossRef]
- Gooley, J.J.; Chamberlain, K.; Smith, K.A.; Khalsa, S.B.; Rajaratnam, S.M.; Van Reen, E.; Zeitzer, J.M.; Czeisler, C.A.; Lockley, S.W. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 2011, 96, E463–E472. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, S.; Ishibashi, K.; Aritake, S.; Enomoto, M.; Hida, A.; Tamura, M.; Kozaki, T.; Motohashi, Y.; Mishima, K. Inter-individual difference in pupil size correlates to suppression of melatonin by exposure to light. Neurosci. Lett. 2008, 440, 23–26. [Google Scholar] [CrossRef]
- Chang, A.M.; Scheer, F.A.; Czeisler, C.A. The human circadian system adapts to prior photic history. J. Physiol. 2011, 589, 1095–1102. [Google Scholar] [CrossRef]
- Hébert, M.; Martin, S.K.; Lee, C.; Eastman, C.I. The efffects of prior light history on the suppression of melatonin by light in humans. J. Pineal Res. 2002, 33, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Münch, M.; Nowozin, C.; Regente, J.; Bes, F.; De Zeeuw, J.; Hädel, S.; Wahnschaffe, A.; Kunz, D. Blue-enriched morning light as a countermeasure to light at the wrong time: Effects on cognition, sleepiness, sleep, and circadian phase. Neuropsychobiology 2016, 74, 207–218. [Google Scholar] [CrossRef]
- Smith, K.A.; Schoen, M.W.; Czeisler, C.A. Adaptation of human pineal melatonin suppression by recent photic history. J. Clin. Endocrinol. Metab. 2004, 89, 3610–3614. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Chen, R.; Kim, H.; Etchegaray, J.P.; Weaver, D.R.; Lee, C. The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc. Natl. Acad. Sci. USA 2011, 108, 16451–16456. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, R.; Tsuchiya, Y.; Tokuda, I.; Matsuo, T.; Sato, M.; Node, K.; Nishida, E.; Akashi, M. The mammalian circadian clock protein period counteracts cryptochrome in phosphorylation dynamics of circadian locomotor output cycles kaput (CLOCK). J. Biol. Chem. 2014, 289, 32064–32072. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.; Jaskolski, M.; Davis, S.J. Circadian oscillator proteins across the kingdoms of life: Structural aspects. BMC Biol. 2019, 17, 13. [Google Scholar] [CrossRef] [Green Version]
- Pittendrigh, C.S.; Daan, S. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J. Comp. Physiol. 1976, 106, 253–266. [Google Scholar] [CrossRef]
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’Hagan, J.B.; et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tekieh, T.; Lockley, S.W.; Robinson, P.A.; McCloskey, S.; Zobaer, M.S.; Postnova, S. Modeling melanopsin-mediated effects of light on circadian phase, melatonin suppression.n, and subjective sleepiness. J. Pineal Res. 2020, 69, e12681. [Google Scholar] [CrossRef] [PubMed]
- Spitschan, M.; Stefani, O.; Blattner, P.; Gronfier, C.; Lockley, S.W.; Lucas, R.J. How to report light exposure in human chronobiology and sleep research experiments. Clocks Sleep 2019, 1, 280–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sack, R.L.; Auckley, D.; Auger, R.R.; Carskadon, M.A.; Wright, K.P., Jr.; Vitiello, M.V.; Zhdanova, I.V. Circadian rhythm sleep disorders: Part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. Sleep 2007, 30, 1484–1501. [Google Scholar] [CrossRef] [Green Version]
- Cajochen, C.; Dijk, D.J.; Borbely, A.A. Dynamics of EEG slow-wave activity and core body temperature in human sleep after exposure to bright light. Sleep 1992, 15, 337–343. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Steiner, R.; Oelhafen, P.; Lang, D.; Gotz, T.; Krebs, J.; Cajochen, C. Acute exposure to evening blue-enriched light impacts on human sleep. J. Sleep Res. 2013, 22, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Cajochen, C.; Krauchi, K.; Danilenko, K.V.; Wirz-Justice, A. Evening administration of melatonin and bright light: Interactions on the EEG during sleep and wakefulness. J. Sleep Res. 1998, 7, 145–157. [Google Scholar] [CrossRef]
- Rahman, S.A.; St Hilaire, M.A.; Lockley, S.W. The effects of spectral tuning of evening ambient light on melatonin suppression, alertness and sleep. Physiol. Behav. 2017, 177, 221–229. [Google Scholar] [CrossRef]
- Rångtell, F.H.; Ekstrand, E.; Rapp, L.; Lagermalm, A.; Liethof, L.; Búcaro, M.O.; Lingfors, D.; Broman, J.E.; Schiöth, H.B.; Benedict, C. Two hours of evening reading on a self-luminous tablet vs. reading a physical book does not alter sleep after daytime bright light exposure. Sleep Med. 2016, 23, 111–118. [Google Scholar] [CrossRef]
- Schwarz, J.F.; Åkerstedt, T.; Lindberg, E.; Gruber, G.; Fischer, H.; Theorell-Haglöw, J. Age affects sleep microstructure more than sleep macrostructure. J. Sleep Res. 2017, 26, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Even, C.; Schröder, C.M.; Friedman, S.; Rouillon, F. Efficacy of light therapy in nonseasonal depression: A systematic review. J. Affect. Disord. 2008, 108, 11–23. [Google Scholar] [CrossRef]
- Faulkner, S.M.; Bee, P.E.; Meyer, N.; Dijk, D.J.; Drake, R.J. Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 46, 108–123. [Google Scholar] [CrossRef]
- Penders, T.M.; Stanciu, C.N.; Schoemann, A.M.; Ninan, P.T.; Bloch, R.; Saeed, S.A. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: A systematic review and meta-analysis. Prim. Care Companion CNS Disord. 2016, 18, 26717. [Google Scholar] [CrossRef]
- Forbes, D.; Blake, C.M.; Thiessen, E.J.; Peacock, S.; Hawranik, P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst. Rev. 2014, 2, Cd003946. [Google Scholar] [CrossRef] [Green Version]
- Hjetland, G.J.; Pallesen, S.; Thun, E.; Kolberg, E.; Nordhus, I.H.; Flo, E. Light interventions and sleep, circadian, behavioral, and psychological disturbances in dementia: A systematic review of methods and outcomes. Sleep Med. Rev. 2020, 52, 101310. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Mannion, H.; Sezgin, D.; O’Donovan, M.R.; Liew, A.; Molloy, D.W. Non-pharmacological treatments for sleep disturbance in mild cognitive impairment and dementia: A systematic review and meta-analysis. Maturitas 2019, 127, 82–94. [Google Scholar] [CrossRef]
| Patients with Delayed Sleep Phase Syndrome and Insomnia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Authors, Date, Country | Design | Primary Outcome | Sample Size Sex | Age (Mean ± SD, Range) | Population | Intervention Light | Control Light | Sleep Macro-Architecture Variable Reported |
| Rosenthal et al. (1990), USA | Cross-over RCT Duration: 2 weeks | MSLT, core body temperature | I: 15, C: 17, sex not specified | NR | Delayed sleep phase syndrome | Illuminance: 2500 lx Clock time: 06:00–09:00 h for 2 h CCT: NR Wavelength: NR | Illuminance: 300 lx Clock time: 06:00–09:00 h for 2 h CCT: NR Wavelength: NR | SOL |
| Murphy and Campbell (1996), USA | Parallel pseudo-RCT Duration: Twice per week for 3 months | Core body temperature and performance tasks | 8 F, 8 M (13 completers) | 73.1 y, (60–82 y) | Insomnia | Illuminance: >4000 lx Clock time: 21:00–23:00 CCT: NR Wavelength: NR | Illuminance: >4000 lx Clock time: 15:00–17:00 CCT: NR Wavelength: NR | SE (%) -Averaged 2 nights |
| Suhner et al. (2002), USA | Parallel RCT Duration: Twice per week for 3 months | Sleep EEG and core body temperature | 7 F, 8 M (I: 9 completers, C: 5 completers) | 71.5 y, (63–84 y) | Insomnia | Illuminance: >4000 lx Clock time: 21:00–23:00 CCT: NR Wavelength: NR | Illuminance: >4000 lx Clock time: 15:00–17:00 CCT: NR Wavelength: NR | TIB (min) TST (min) WASO (min) SE (%)SOL (min) S1 (% TST) S2 (% TST) S3 (% TST) S4 (% TST) REM (% TST) -Averaged 2 nights |
| Youngstedt et al. (2005), USA | Parallel RCT Duration: 4 days for each condition | Mood, sleep EEG and melatonin | 49 F, 23 M (older adults) 15 F, 15 M (young adults) | (60–79 y) (Older adults) (20–40 y) (Young adults) | Insomnia and/or depression Healthy | Illuminance: 3000 lx Clock time: Intervention 1: 1–3 h after awakening and 2 h before bedtime Intervention 2: 6–10 h after awakening CCT: NR Wavelength: NR | Illuminance: 1 lx Clock time: 6–10 h after awakening CCT: NR Wavelength: NR | TST † WASO † SE † -Averaged 4 nights |
| Friedman et al. (2009), USA | Parallel RCT, single-blinded Duration: 12 weeks | Sleep EEG and melatonin | 36 F, 25 M (49 completers) | 63.6 ± 7.1 y, (54–78 y) | Insomnia | Illuminance: ~4000 lx Clock time: Intervention 1: 15 min after awakening for 45 min Intervention 2: 1 h before bedtime for 45 min CCT: NRWavelength: NR | Illuminance: ~65 lx Clock time: Control 1: 15 min after awakening for 45 min Control 2: 1 h before bedtime for 45 min CCT: NR Wavelength: NR | TIB (min) TST (min) WASO (min) SE (%) S1 (%) S2 (%) S3 (%) S4 (%) REM (%) -Averaged 2 nights |
| Patients with Delayed Sleep Phase Syndrome and Insomnia | |||||||
|---|---|---|---|---|---|---|---|
| Author/s, Date | Macro-Architecture Measures | ||||||
| TIB | TST | WASO | SE | SOL | NREM | REM | |
| Rosenthal et al. (1990) o | ↑ | ||||||
| Murphy and Campbell (1996) • | ↑ | ||||||
| Suhner et al. (2002) • | ns | ns | ns | ns | ns | ns | |
| Youngstedt et al. (2005) • | ns | ns | ns | ||||
| Friedman et al. (2009) • | ns | ns | ns | ns | ns | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pun, T.B.; Phillips, C.L.; Marshall, N.S.; Comas, M.; Hoyos, C.M.; D’Rozario, A.L.; Bartlett, D.J.; Davis, W.; Hu, W.; Naismith, S.L.; et al. The Effect of Light Therapy on Electroencephalographic Sleep in Sleep and Circadian Rhythm Disorders: A Scoping Review. Clocks & Sleep 2022, 4, 358-373. https://doi.org/10.3390/clockssleep4030030
Pun TB, Phillips CL, Marshall NS, Comas M, Hoyos CM, D’Rozario AL, Bartlett DJ, Davis W, Hu W, Naismith SL, et al. The Effect of Light Therapy on Electroencephalographic Sleep in Sleep and Circadian Rhythm Disorders: A Scoping Review. Clocks & Sleep. 2022; 4(3):358-373. https://doi.org/10.3390/clockssleep4030030
Chicago/Turabian StylePun, Teha B., Craig L. Phillips, Nathaniel S. Marshall, Maria Comas, Camilla M. Hoyos, Angela L. D’Rozario, Delwyn J. Bartlett, Wendy Davis, Wenye Hu, Sharon L. Naismith, and et al. 2022. "The Effect of Light Therapy on Electroencephalographic Sleep in Sleep and Circadian Rhythm Disorders: A Scoping Review" Clocks & Sleep 4, no. 3: 358-373. https://doi.org/10.3390/clockssleep4030030
APA StylePun, T. B., Phillips, C. L., Marshall, N. S., Comas, M., Hoyos, C. M., D’Rozario, A. L., Bartlett, D. J., Davis, W., Hu, W., Naismith, S. L., Cain, S., Postnova, S., Grunstein, R. R., & Gordon, C. J. (2022). The Effect of Light Therapy on Electroencephalographic Sleep in Sleep and Circadian Rhythm Disorders: A Scoping Review. Clocks & Sleep, 4(3), 358-373. https://doi.org/10.3390/clockssleep4030030








