Abstract
The current study examined the possible predictors of dropout during a five-week light treatment (LT) with a gradual advance protocol in 93 patients with unipolar non-seasonal depression and evening chronotypes by comparing their clinical characteristics and performing a logistic regression analysis. Nineteen out of ninety-three (20%) subjects (80% female, 46.5 ± 11.7 years old) dropped out during the 5-week light treatment. Treatment non-adherence (i.e., receiving LT for less than 80% of the prescribed duration) over the first treatment week predicted a five-fold increase in risk of dropout during light therapy (OR: 5.85, CI: 1.41–24.21) after controlling for potential confounders, including age, gender, treatment group, rise time at the baseline, patient expectation, and treatment-emergent adverse events. There is a need to incorporate strategies to enhance treatment adherence and retention in both research and clinical settings. Chinese clinical trial registry (ChiCTR-IOR-15006937).
1. Introduction
Depression is a common mental health problem and a leading cause of mental health-related burdens across the globe [1]. It is associated with a range of adverse outcomes, including reduced daytime functioning, a wide range of secondary disorders, and increased early mortality due to physical disorders and suicide [2]. Emerging evidence has shown that bright light therapy (BLT) could be an efficacious treatment for non-seasonal depression, either as a monotherapy or an adjunct therapy to antidepressants [3,4,5]. Several randomized controlled trials have demonstrated the efficacy of adjunctive bright light therapy in reducing depressive symptoms of non-seasonal depression in major depressive disorders (MDDs) [6,7,8]. A meta-analysis which included 23 randomized controlled trials (RCTs) demonstrated a beneficial effect of light therapy in patients with non-seasonal depression with a standardized mean difference (SMD) in depression score of −0.40 (95% CI −0.60 to −0.21) [3].
Previous research showed that light therapy was generally well-tolerated and that adverse effects were mild and transient [4]. However, wide variation in dropout rates in trials of adjunctive light therapy in non-seasonal depression were observed, varying from 13% in a study with patients of unipolar depression [7] to 44% [9] in studies involving bipolar depression patients. The mean attrition rate was 17% in a systematic review involving light therapy studies on patients with intrinsic circadian rhythm sleep disorders and neuropsychiatric disorders [10]. Not only do dropouts significantly hamper treatment effects [11], they may also influence study power and generalizability and cause potential bias, as the remaining participants might differ from the overall sample recruited at the baseline [12].
Furthermore, unipolar depression patients with concomitant eveningness (i.e., a circadian preference towards a later timing for rest and activity; individuals with such chronotypes also being referred to as ‘night owls’) may have a higher propensity to drop out in interventions (e.g., adjunctive light therapy), as they tend to have lower self-control [13], more lifestyle irregularity [14], higher impulsivity [15], and greater alcohol and substance use [16], which could all contribute to poorer treatment adherence and worse outcomes. Eveningness is common among patients with MDD, with a prevalence rate of around 20% [17]. Depressed patients with eveningness have been found to have poorer outcomes of depression, including higher non-remission rates and suicidality [17,18,19]. An improved understanding of the factors associated with these patients’ tendencies to drop out might shed light on how to improve subject retention and enhance therapeutic effects in this vulnerable group.
In the treatment of depression, demographics and types of treatment or medications have been implicated as predictors of dropout [20,21,22]. On the other hand, dropout in light therapy trials could also be related to a variety of factors, including patient expectations, treatment efficacy, and adverse events, but such reasons have been underreported in light treatment studies [10]. Light therapy was reported to have a quick onset of therapeutic effect, as early as 1 week [23]. However, in an antidepressant trial involving 1008 adults with MDD, a higher burden of side effects as early as 4 days post-treatment was associated with poorer treatment outcomes [24]. In a randomized controlled trial (RCT) of depression treatment involving 1646 Japanese adults with non-psychotic MDD, those who dropped out of treatment were found to be less adherent to treatment even at the first week of treatment [25]. Thus, the limited evidence showed that non-adherence to treatment in the early weeks may impact dropout rates and the treatment outcomes. However, there is a dearth of data focusing on treatment adherence and change in clinical outcomes in the initial weeks which might be informative for predicting dropout in the course of light treatment. The current study aimed to investigate baseline demographic data, treatment expectations, sleep patterns, and clinical differences as potential predictors of dropout in a 5-week trial of light treatment with a gradual advance protocol and to explore the possible role of side effects, treatment adherence, and change in clinical outcomes in the first week as early predictors of dropout. Adherence in this study was defined as receiving 80% or more of the prescribed duration of light therapy in a week; this amount was arbitrarily chosen, as a previous study on seasonal affective disorder having found that adherence to the prescribed duration of exposure averaged 83% among the completers of four weeks of light treatment [26]. Dropout was defined as premature termination of treatment prior to the 5-week endpoint.
2. Results
2.1. Baseline Characteristics
A total of 93 participants were included in the analysis. The mean age was 46.5 ± 11.7 (years ± SD) and 80% were female. Nineteen out of ninety-three (20%) of the participants dropped out during the five weeks of light treatment (Figure 1, subject flow diagram). There were no differences in age, gender, education level, living status, monthly income, and season of enrollment to the study. The expectation towards the effectiveness of treatment in both groups was also similar. Sleep diary data showed later rising times in the Dropout group than in the Completer group (11:12 h ± 02:27 vs. 10:13 h ± 01:54 (time ± SD, p = 0.066)), but there were no differences in sleep duration or sleep midpoint. The duration of depressive illness appeared to be longer in the Dropout group but was not statistically significant (17.0 ± 13.9 vs. 12.9 ± 10.1 (years ± SD, p = 0.19)). The baseline scores for 17-HDS, atypical symptom score for SIGH, HAMA, HADS, ISI, SF-36, CFS, YMRS, and MEQ were not significantly different between the two groups (Table 1).
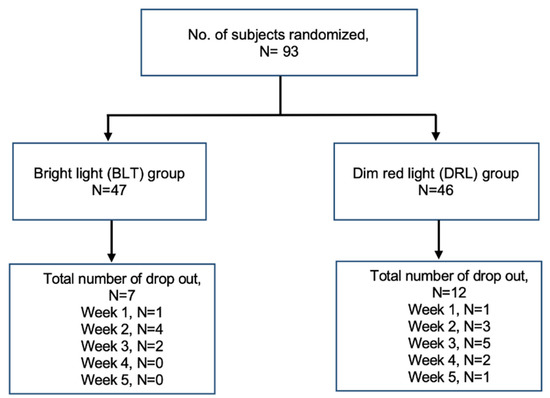
Figure 1.
Subject flow diagram.

Table 1.
Baseline demographics and clinical characteristics of the Dropout group versus the Completer group (means ± standard deviations).
2.2. Treatment Characteristics
Seven out of forty-seven subjects (15%) and 12 out of 46 subjects (26%) dropped out in the bright light therapy group and dim red light group, respectively (p = 0.41). Over 70% of subjects were put on psychotropic medications. There was no difference in the prescription of psychotropic medications between the two groups (Table 2).

Table 2.
Light treatment allocation and the prescription of psychotropic medications for the Dropout and Completer groups.
2.3. Early Treatment Adherence and Clinical Outcome Changes
As two subjects (one from the bright light therapy group and one from the dim red light group) dropped out before the first-week assessment, they were excluded from the analysis of early treatment adherence and change in clinical outcomes. Both prescribed and recorded light therapy start times appeared to be later in the Dropout group but were not significantly different. However, the total weekly duration of light therapy in the first week was significantly higher in the Completer than in the Dropout group (174 ± 54 vs. 110 ± 82 (minutes ± SD, p = 0.007)). The percentage of participants who received LT treatment for more than 80% of the prescribed duration was also higher for the completers than for the dropouts, 62% vs. 23% (p = 0.004), respectively. There was no difference in the incidence of treatment-emergent adverse events at week 1 between the dropouts and the completers. Regarding clinical outcomes, both groups showed a trend towards improvement with a reduction in mean scores for 17-HDS, HAMA, HADS, BSSI, and CFS, without significant group differences. YMRS score was slightly higher in the Dropout group as compared to the Completer group, but the magnitude of change (−0.2 ± 1.8 vs. 0.8 ± 2.1) was unlikely to be clinically significant. The overall rate for missing data was 5% (Table 3).

Table 3.
Treatment adherence and early changes in clinical outcomes from baseline to week 1.
2.4. Predictors for Dropout
In the logistic regression, Model 1 included the variables with p < 0.1 in the univariate analysis (i.e., the change of YMRS score in week 1 and rise time at the baseline). The categorical variable of “getting less than 80% duration of light therapy in the first week” was chosen over the continuous variable of “the weekly total duration of light therapy performed” to allow easier identification of at-risk individuals in the clinical setting. Those who received less than 80% of the total duration of light therapy prescribed in the first week had a 4.6-fold higher risk of dropout during weeks 2 to 5 of the light therapy treatment program (OR: 4.69, CI: 1.32–16.66, p = 0.017). Model 2 additionally adjusted for age, gender, light treatment, patient expectation, and TEAE at the first week of treatment and showed similar results (OR: 5.85, CI: 1.41–24.21, p = 0.015) (Table 4).

Table 4.
Logistic regression analysis for the predictors of dropout.
3. Discussion
This study aimed to examine the effect of early treatment adherence and changes in clinical outcomes on the risk of dropout during a light treatment intervention. The results of our study showed that the overall dropout rate during the intervention phase was 20% (19 out of 93). Those subjects who received less than 80% of the total prescribed duration of light therapy in the first week had a five-fold increased risk of dropout during the course of light treatment and the risk was independent of other confounding variables, including age, gender, type of light treatment, rise time at baseline, patient expectation, and treatment-emergent adverse events.
3.1. Dropout Rate
The overall dropout rate of 20% in our study was comparable to the overall rate of dropout in mental health treatment—around 17–19% [27]. The mean attrition rate during the light treatment phase was 17% across studies of intrinsic circadian rhythm sleep disorders and neuropsychiatric illness [10]. The 20% rate for our study is slightly higher than those of other randomized controlled trials that focused on adult patients with non-seasonal MDD in the out-patient setting, which reported 9 and 13%, respectively [6,7]. This could be attributable to an exclusive evening-type sample characterized by more sedentary activity and less moderate-to-vigorous physical activity [28], unhealthy eating habits [29], lower self-control [13], and an overall more irregular lifestyle [14], hence greater difficulty in engaging in treatment. This suggests that for this particular group of patients, better strategies should be developed to reduce dropout and enhance treatment adherence. One might expect the attrition rate to be higher in the control group than in the intervention group. The dropout rate in this trial was numerically higher in the DRL group than the BLT group (26% vs. 15%) but did not reach statistical significance. This is consistent with the findings of 14% and 8% dropout in the intervention group versus the control group for light therapy studies in a systematic review [10]. Similar to another study focusing on adherence in light therapy, patient expectations were found not to be related to dropout [26]. The expectation score also did not differ between the BLT group and the DRL group in this trial [8]. There was a significant difference in the change of YMRS from baseline to week 1 between the bright light therapy (BLT) and dim red light (DRL) groups (−0.2 ± 1.8 vs. 0.8 ± 2.1, p = 0.046). None of the subjects had reached the cut-off of 12, which was the general cut-off for hypomania [30]. This suggested that bright light therapy is a safe antidepressant treatment with respect to hypomanic swings in depressed patients with eveningness.
3.2. Monitoring of Adherence
Our study highlighted the importance of adherence to light treatment in the initial week of intervention. However, monitoring adherence to light therapy remains a clinical challenge. Some studies have used self-reported treatment logs to capture adherence information [7,8,31]. Nonetheless, a pilot study focused on the adherence of bright light treatment in patients with seasonal affective disorder found no correlation between patient self-reports and the objectively measured durations of light box use objectively measured by a built-in meter [26]. This implies that a more objective method might be needed in future light therapy studies to monitor adherence. In this study, we defined compliance based on the duration of light therapy undergone [8]. A light data logger (HOBO U9 light on/off logger) was used in one study on bipolar depression to confirm appropriate, missed, or identified ill-timed light therapy sessions [31]. The light sensor on the actiwatch has been used to capture continuous light data during and around interventions, but there could be a potential problem in measuring light exposure at the wrist, as it is at a distance from the cornea and might be covered by clothes in winter months [32]. Figueiro et al. found that a light sensor placed as a pendant around the neck or pinned on the torso yielded similar photopic illuminance data as compared to a light sensor near the cornea [32]. Such measurements could be potentially useful as objective measurements of light exposure and serve as proxies for bright light treatment adherence.
3.3. Strategies to Enhance Adherence
In patients receiving antidepressant treatment, the systematic review by Pampallona et al. found a variety of interventions (for example, patient education and medication clinics) that have been used to enhance adherence to medications. Although the heterogeneity of the studies precluded a solid conclusion, the evidence consistently indicated that adherence to treatment can be increased through interventions [33]. Different approaches to adherence management for light therapy have been used, including supervision, education, reminders, environmental modification, etc. [10], but there is a paucity of data that would allow the systematic evaluation of their efficacy in improving adherence. In terms of optimizing treatment environments and support, BLT has been used an adjunct treatment with antidepressants in hospitalized patients with depression and was shown to hasten the effect of antidepressants [34,35]. The in-patient setting could be a supportive environment in which to initiate light therapy and consolidate its implementation. A recent study that tested the use of BLT for depressive symptoms in an acute psychiatric in-patient unit found a dropout rate of 9% and an adherence rate to daily BLT of 94%, even though participants were allowed to skip any session [36]. This study suggests that it is feasible to start BLT in acute psychiatric units to enhance early treatment adherence, although it is unclear whether the benefits could be extended after a patient is discharged from the hospital, as BLT might require several weeks for the treatment of depressive symptoms [7,31]. Further study is needed to provide confirmatory evidence that initiating BLT in acute in-patient units has greater benefits than in the out-patient setting.
Cognitive behavioral therapy (CBT) has also been used to enhance medication adherence in patients with major depressive disorder [37]. There is currently no trial evaluating whether CBT could enhance adherence to BLT and retention in treatment in patients with depression. There have been several trials that investigated the efficacy of a combination of CBT with BLT for young patients with delayed sleep phase. Gradisar et al. evaluated the use of combined CBT and concurrent BLT for adolescents with delayed sleep phase disorder (DSPD) and found that the combination treatment resulted in a greater improvement in sleep parameters, with more sustained effects in the group with CBT. There was a numerically lower rate of dropout of 11% (3 out of 26) in the CBT group and of 26% (6 out of 23) in the waitlist group, though characteristics of dropout were not described [38]. On the other hand, Danielsson et al. found an overall rate of protocol failure because of “never use the light box” in 16% (19 out 57) patients with delayed sleep phase disorder in a study of 4-week CBT following either 2 weeks of BLT or the control condition; however, the impact of CBT on BLT could not be evaluated, as BLT was completed before CBT [39]. Interestingly, in a larger trial involving 102 adolescents who complained of delayed sleep, the combination of light flash therapy plus CBT resulted in a seven-fold greater increase in bedtime compliance than that observed in the group with sham plus CBT [40]. This suggests that, reciprocally, BLT might also improve adherence to prescribed sleep schedules in CBT.
As the use of light treatment represents a behavioral change and often involves lifestyle modification (e.g., an earlier rise time, less use of electronic devices at night, etc.), motivational interviewing (MI) could be another strategy to enhance adherence to treatment. MI is a person-centered, goal-oriented method of communication for eliciting and strengthening intrinsic motivation for positive change [41]. MI has been evaluated for reducing depressive symptoms and was found to improve the trajectory of depression and increase receipt of antidepressants in the primary care setting [42]. It has also been used in patients with depression and comorbid substance use disorder to reduce substance use [43,44]. MI has been employed as a component in cognitive behavioral therapy for insomnia in patients with bipolar disorder (CBTI-BD) [45]. The overall treatment package yielded significant results in terms of reducing the number of days in a mood episode. The dropout rate after randomization, however, did not differ between the CBT-BD group and the control group (20% vs. 21%, respectively), albeit the component of MI was not separately evaluated in this trial. A combination of BLT with or without MI/CBT has not been tested in patients with depression; future trials may consider integrating MI techniques or using CBT as an adjunct to BLT to further improve adherence and outcomes.
3.4. Limitations
This was a prospective study that involved a well-defined patient group with unipolar depression and evening preference with weekly follow-up on the clinical outcomes during the intervention phase which allowed us to capture information about early changes in symptoms and adherence with respect to dropout. However, the measurement of adherence was subjective and actual treatment durations may have been overestimated. Future trials using objective measurements (e.g., measurements obtained by light-logging devices) may provide more accurate records. Secondly, the subjects included in this study were patients with unipolar nonseasonal depression and exclusively evening chronotypes, which might limit the generalizability of the results to other patients.
4. Materials and Methods
4.1. Participants
The current study analyzed the data collected in a randomized controlled trial (RCT) of adjunctive bright light therapy in patients with non-seasonal depression and concomitant eveningness. The detailed study procedure has been reported elsewhere [8,46]. In sum, 93 adult patients with a diagnosis of non-seasonal unipolar depression were recruited. They were assessed using the Mini-International Neuropsychiatric Interview (MINI) [47], the seasonal pattern specifier of DSM-V [48], and the 17-Item Hamilton Depression Score (17-HDS) component of the Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement (SIGH-ADS) [49]. The inclusion criteria for this study included: (i) Chinese subjects aged 18–65 years old; (ii) being capable of giving informed consent; (iii) meeting the diagnostic criteria of MDD according to MINI; (iv) scoring at least 14 on the 17-HDS; and (vi) scoring 41 or less in the Morningness–Eveningness Questionnaire (i.e., having an evening chronotype) [50]. Subjects with the conditions listed below were excluded from the study: (i) MDD fulfilling the seasonal pattern specifier of the DSM-V; (ii) a current diagnosis of substance abuse or dependence; a current or past history of manic or hypomanic episodes, schizophrenia, personality disorder, mental retardation, or organic mental disorder; (iii) significant suicidal risk in the opinion of the investigator, or a moderate or higher level of suicidality as assessed by the Suicidality Module of MINI, or having made a suicide attempt in the past 3 months; (iv) a history of light-induced migraine/epilepsy; (v) current use of photosensitizing medications; (vi) presence of eye disease, e.g., retinal blindness, severe cataracts, glaucoma; (vii) current therapy with drugs that could potentially interfere with circadian rhythms, i.e., lithium, exogenous melatonin, melatonergic antidepressants within past 3 months; (viii) shift worker; (ix) trans-meridian flight in the past 3 months or during the study; (x) significant medical condition/hearing impairment/speech deficit which might lead to incapability of completing the clinical interview. The trial was registered with the Chinese clinical trial registry (ChiCTR-IOR-15006937).
4.2. Intervention
Eligible participants were randomized to five weeks of either the active intervention group, which received 10,000 lux bright white light therapy (NatureBright Company, Irvine, CA, USA) (BLT group), or the placebo group, which received 50 lux dim red light (DRL group). Both groups received light therapy at home for 30 min a day; the initial timing of the light therapy was set at the habitual wake time, which was determined by the 1-week sleep diary prior to the baseline session. A prescriber reviewed the treatment log and sleep diary at each treatment week to determine the timing of the light therapy according to a gradual advance protocol: if the participant received adequate appropriately timed light therapy over the past week (defined as more than 50% of the total weekly duration of light therapy received, with timing overlapped with or earlier than the prescribed time of light therapy), the timing of the light therapy would be advanced for 30 min. On the other hand, if the participant was not able to achieve adequate appropriately timed treatment, the timing of the light therapy would be kept the same in the following week [8]. Each participant was prescribed a start time for light therapy by a psychiatrist, and they also recorded the actual light therapy start and end time in a treatment log. They were followed up weekly during the five-week light therapy. All participants continued their usual psychiatric treatment.
4.3. Outcomes
At the baseline, demographic, sleep, and clinical characteristics of the participants were collected. They were asked to rate their expectation towards the effectiveness of light therapy in improving their mood symptoms on a Likert Scale from 1 (not at all) to 100 (very much) prior to the start of treatment. In each of the follow-ups, participants were assessed for their clinical outcomes and side effects and returned their completed sleep dairies. The incidence of a treatment-emergent adverse event (TEAE) was defined as a change from baseline to at least moderate severity at follow-up. A range of clinical outcomes were measured weekly during the intervention, including the 17-HDS [49], Hamilton Anxiety Scale (HAM-A) [51], Hospital Anxiety and Depression Scale (HADS) [52], Insomnia Severity Index (ISI) [53], Beck’s Scale for Suicide Ideation (BSSI) [54], Chalder Fatigue Scale (CFS) [55], and quality of life assessed by the Short-Form 36-Item Health Survey (SF36) [56]. The self-reported questionnaires were translated and validated in Chinese [57,58,59,60,61]. The internal consistency (Cronbach’s alpha) of the results for the 17-HDS, HAMA, HADS, ISI, BSSI, CFS, and SF36 were 0.81, 0.92, 0.79, 0.87, 0.67, 0.91, and 0.91, respectively. The primary outcomes of the RCT were the cumulative rate of achieving remission of depression (defined as a 17-HDS score of 7 or less) and the time to achieve remission during the 5 weeks of treatment and 5-month follow-up after the light treatment [8].
4.4. Statistical Analysis
The data in the current study were analyzed on an intention-to-treat basis. Chi-square tests and t-tests were employed to compare the baseline demographic and clinical characteristics between the dropouts and the completers. Logistic regression analysis was performed, with dropout as the dependent variable. As this study aimed to investigate whether early symptom improvement could affect dropout, subjects who dropped out prior to the assessment at the end of the first week were excluded from the logistic regression analysis (N = 2). The independent variables selected for the present analysis included variables with p < 0.1 in the univariate analysis. Potential confounders (age, gender, treatment allocation, patient expectation, treatment-emergent adverse events) were included as independent variables in the adjusted model. The adjusted odds ratios (ORs) and 95% confidence intervals (CIs) are presented for the independent variables. Two-sided tests were used and a p-value of less than 0.05 was considered statistically significant. The data were analyzed using the Statistical Package System for Windows v25.0 (SPSS, IBM Corp., Chicago, IL, USA).
5. Conclusions
This study found that adherence to light therapy of 80% or less of the duration prescribed in the first week of treatment predicted a five-fold increase in the risk of dropout during the light treatment phase irrespective of patient expectations, adverse events, and treatment group. Future trials should enhance light therapy adherence monitoring by introducing objective measurements and should incorporate strategies to enhance adherence and treatment retention.
Author Contributions
Conceptualization, J.W.Y.C., S.X.L., J.Z. and Y.K.W.; methodology, J.W.Y.C., S.X.L., N.Y.C., J.Z. and Y.K.W.; investigation: J.W.Y.C., S.W.H.C., J.Z. and Y.K.W.; formal analysis, J.W.Y.C.; writing—original draft preparation, J.W.Y.C.; writing—review and editing, J.W.Y.C., S.X.L., S.W.H.C., N.Y.C., J.Z. and Y.K.W.; funding acquisition, Y.K.W.; supervision, Y.K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Health and Medical Research Fund, the Food and Health Bureau, the Government of Hong Kong Special Administrative Region (grant number: 12131131). The funder (the Food and Health Bureau, Hong Kong) of the study had no role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or in the decision to submit the article for publication.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Joint Chinese University of Hong Kong New Territories East Cluster Clinical Research Ethics Committee (CREC ref. No.: 2014.505; date of approval: 10 November 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Reasonable requests to access the related data will be considered on a case-by-case basis and should be made to the corresponding author. Ethical approval for data-sharing agreements is required to share data in order to protect participant confidentiality.
Conflicts of Interest
This was not an industry-supported study. JWYC reported grants from the General Research Fund of the University Grants Committee, Hong Kong SAR, and personal fees for joining an expert panel meeting of Eisai Co., Ltd. YKW reported grants from the General Research Fund of the University Grants Committee, Health and Medical Research Fund, Hong Kong SAR, and received personal fees for delivering a lecture from Eisai Co., Ltd. and sponsorship from Lundbeck HK Limited. All the other authors have indicated no financial conflict of interest.
References
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020, 126, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Bromet, E.J. The Epidemiology of Depression Across Cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef]
- Tao, L.; Jiang, R.; Zhang, K.; Qian, Z.; Chen, P.; Lv, Y.; Yao, Y. Light therapy in non-seasonal depression: An update meta-analysis. Psychiatry Res. 2020, 291, 113247. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Eisen, R.; Bhatt, M.; Bhatnagar, N.; de Souza, R.; Thabane, L.; Samaan, Z. Light therapy for non-seasonal depression: Systematic review and meta-analysis. BJPsych Open 2016, 2, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.W.; Teng, M.; Jung, Y.-E.; Evans, V.C.; Gottlieb, J.F.; Chakrabarty, T.; Michalak, E.E.; Murphy, J.K.; Yatham, L.N.; Sit, D.K. Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can. J. Psychiatry 2020, 65, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Martiny, K. Adjunctive bright light in non-seasonal major depression. Acta Psychiatr. Scand. 2004, 110, 7–28. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.W.; Levitt, A.J.; Levitan, R.D.; Michalak, E.E.; Cheung, A.H.; Morehouse, R.; Tam, E.M. Efficacy of Bright Light Treatment, Fluoxetine, and the Combination in Patients with Nonseasonal Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2016, 73, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.; Lam, S.; Li, S.X.; Chau, S.W.; Chan, S.; Chan, N.; Zhang, J.; Wing, Y. Adjunctive bright light treatment with gradual advance in unipolar major depressive disorder with evening chronotype—A randomized controlled trial. Psychol. Med. 2020, 52, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Dauphinais, D.R.; Rosenthal, J.Z.; Terman, M.; DiFebo, H.M.; Tuggle, C.; Rosenthal, N.E. Controlled trial of safety and efficacy of bright light therapy vs. negative air ions in patients with bipolar depression. Psychiatry Res. 2012, 196, 57–61. [Google Scholar] [CrossRef]
- Faulkner, S.M.; Dijk, D.-J.; Drake, R.J.; Bee, P.E. Adherence and acceptability of light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuropsychiatric illness: A systematic review. Sleep Health 2020, 6, 690–701. [Google Scholar] [CrossRef]
- Masand, P.S. Tolerability and adherence issues in antidepressant therapy. Clin. Ther. 2003, 25, 2289–2304. [Google Scholar] [CrossRef]
- Fewtrell, M.S.; Kennedy, K.; Singhal, A.; Martin, R.M.; Ness, A.; Hadders-Algra, M.; Lucas, A. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch. Dis. Child 2008, 93, 458–461. [Google Scholar] [CrossRef]
- Digdon, N.L.; Howell, A.J. College Students Who Have an Eveningness Preference Report Lower Self-Control and Greater Procrastination. Chrono. Int. 2008, 25, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Monk, T.H.; Buysse, D.J.; Potts, J.M.; DeGrazia, J.M.; Kupfer, D.J. Morningness-Eveningness and Lifestyle Regularity. Chrono. Int. 2004, 21, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Selvi, Y.; Aydin, A.; Atli, A.; Boysan, M.; Selvi, F.; Besiroglu, L. Chronotype Differences in Suicidal Behavior and Impulsivity Among Suicide Attempters. Chrono. Int. 2011, 28, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Hasler, B.P.; Franzen, P.L.; de Zambotti, M.; Prouty, D.; Brown, S.A.; Tapert, S.F.; Pfefferbaum, A.; Pohl, K.M.; Sullivan, E.V.; De Bellis, M.D.; et al. Eveningness and Later Sleep Timing Are Associated with Greater Risk for Alcohol and Marijuana Use in Adolescence: Initial Findings from the National Consortium on Alcohol and Neurodevelopment in Adolescence Study. Alcohol. Clin. Exp. Res. 2017, 41, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Lam, S.P.; Li, S.X.; Yu, M.W.M.; Chan, N.Y.; Zhang, J.; Wing, Y.-K. Eveningness and Insomnia: Independent Risk Factors of Nonremission in Major Depressive Disorder. Sleep 2014, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Rumble, M.E.; Dickson, D.; McCall, W.; Krystal, A.D.; Case, D.; Rosenquist, P.B.; Benca, R.M. The relationship of person-specific eveningness chronotype, greater seasonality, and less rhythmicity to suicidal behavior: A literature review. J. Affect. Disord. 2018, 227, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Selvi, Y.; Aydin, A.; Boysan, M.; Atli, A.; Agargun, M.Y.; Besiroglu, L.A. Associations between chronotype, sleep quality, suicidality, and depressive symptoms in patients with major depression and healthy controls. Chrono. Int. 2010, 27, 1813–1828. [Google Scholar] [CrossRef] [PubMed]
- Simons, A.D.; Levine, J.L.; Lustman, P.J.; Murphy, G.E. Patient attrition in a comparative outcome study of depression: A follow-up report. J. Affect. Disord. 1984, 6, 163–173. [Google Scholar] [CrossRef]
- Pinto-Meza, A.; Fernández, A.; Bruffaerts, R.; Alonso, J.; Kovess, V.; De Graaf, R.; De Girolamo, G.; Matschinger, H.; Haro, J.M. Dropping out of mental health treatment among patients with depression and anxiety by type of provider: Results of the European Study of the Epidemiology of Mental Disorders. Soc. Psychiatry 2011, 46, 273–280. [Google Scholar]
- Pradier, M.F.; Jr, T.H.M.; Hughes, M.; Perlis, R.H.; Doshi-Velez, F. Predicting treatment dropout after antidepressant initiation. Transl. Psychiatry 2020, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Kripke, D.F. Light treatment for nonseasonal depression: Speed, efficacy, and combined treatment. J. Affect. Disord. 1998, 49, 109–117. [Google Scholar] [CrossRef]
- Braund, T.A.; Tillman, G.; Palmer, D.M.; Gordon, E.; Rush, A.J.; Harris, A.W. Antidepressant side effects and their impact on treatment outcome in people with major depressive disorder: An iSPOT-D report. Transl Psychiatry 2021, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.A.; Kato, T.; Akechi, T.; Shimodera, S.; Okada, N.; Yanai, I.; Kinou, K. Dropouts in an Antidepressant Trial: How Do They Fare Afterwards? Psychother Psychosom 2018, 87, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Michalak, E.E.; Murray, G.; Wilkinson, C.; Dowrick, C.; Lam, R.W. A pilot study of adherence with light treatment for seasonal affective disorder. Psychiatry Res. 2007, 149, 315–320. [Google Scholar] [CrossRef]
- Edlund, M.J.; Wang, P.S.; Berglund, P.A.; Katz, S.J.; Lin, E.; Kessler, R.C. Dropping Out of Mental Health Treatment: Patterns and Predictors Among Epidemiological Survey Respondents in the United States and Ontario. Am. J. Psychiatry 2002, 159, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi Frisk, M.; Hedner, J.; Grote, L.; Ekblom, Ö.; Arvidsson, D.; Bergström, G.; Zou, D. Eveningness is associated with sedentary behavior and increased 10-year risk of cardiovascular disease: The SCAPIS pilot cohort. Sci. Rep. 2022, 12, 8203. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.P.; Guimarães, K.C.; Soares, A.G.N.S.; Marqueze, E.C.; Moreno, C.R.C.; Mota, M.C.; Crispim, C.A. Role of chronotype in dietary intake, meal timing, and obesity: A systematic review. Nutr. Rev. 2022. [Google Scholar] [CrossRef] [PubMed]
- Suppes, T.; Mintz, J.; McElroy, S.L.; Altshuler, L.L.; Kupka, R.W.; Frye, M.A.; Post, R.M. Mixed hypomania in 908 patients with bipolar disorder evaluated prospectively in the Stanley Foundation Bipolar Treatment Network: A sex-specific phenomenon. Arch. Gen. Psychiatry 2005, 62, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Sit, D.K.; McGowan, J.; Wiltrout, C.; Diler, R.S.; Dills, J.; Luther, J.; Yang, A.; Ciolino, J.D.; Seltman, H.; Wisniewski, S.R.; et al. Adjunctive Bright Light Therapy for Bipolar Depression: A Randomized Double-Blind Placebo-Controlled Trial. Am. J. Psychiatry 2018, 175, 131–139. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Hamner, R.; Bierman, A.; Rea, M.S. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light. Res. Technol. 2013, 45, 421–434. [Google Scholar] [CrossRef]
- Pampallona, S.; Bollini, P.; Tibaldi, G.; Kupelnick, B.; Munizza, C. Patient adherence in the treatment of depression. Br. J. Psychiatry 2002, 180, 104–109. [Google Scholar] [CrossRef]
- Ozdemir, P.G.; Boysan, M.; Smolensky, M.H.; Selvi, Y.; Aydin, A.; Yilmaz, E. Comparison of Venlafaxine Alone Versus Venlafaxine Plus Bright Light Therapy Combination for Severe Major Depressive Disorder. J. Clin. Psychiatry 2015, 76, e645–e654. [Google Scholar] [CrossRef]
- Benedetti, F.; Colombo, C.; Pontiggia, A.; Bernasconi, A.; Florita, M.; Smeraldi, E. Morning light treatment hastens the antidepressant effect of citalopram: A placebo-controlled trial. J. Clin. Psychiatry 2003, 64, 648–653. [Google Scholar] [CrossRef]
- Trinh, A.; Jain, P.; Sabahath, S.; Wang, D.; Megna, J.L.; Leontieva, L. Feasibility of Adjunctive Bright Light Therapy for Depressive Symptoms on an Acute Psychiatric Floor. Cureus 2021, 13, e13945. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.; Bu, P.; Yang, Y.; Dong, L.; Sun, T.; Shi, Y. Group cognitive behavioural therapy can reduce stigma and improve treatment compliance in major depressive disorder patients. Early Interv. Psychiatry 2020, 14, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Gradisar, M.; Dohnt, H.; Gardner, G.; Paine, S.; Starkey, K.; Menne, A.; Slater, A.; Wright, H.; Hudson, J.; Weaver, E.; et al. A Randomized Controlled Trial of Cognitive-Behavior Therapy Plus Bright Light Therapy for Adolescent Delayed Sleep Phase Disorder. Sleep 2011, 34, 1671–1680. [Google Scholar]
- Danielsson, K.; Jansson-Fröjmark, M.; Broman, J.-E.; Markström, A. Cognitive Behavioral Therapy as an Adjunct Treatment to Light Therapy for Delayed Sleep Phase Disorder in Young Adults: A Randomized Controlled Feasibility Study. Behav. Sleep Med. 2016, 14, 212–232. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, K.A.; Mashash, M.; Williams, R.; Batchelder, H.; Starr-Glass, L.; Zeitzer, J.M. Effect of Light Flashes vs Sham Therapy During Sleep With Adjunct Cognitive Behavioral Therapy on Sleep Quality among Adolescents: A Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e1911944. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Rose, G.S. Toward a theory of motivational interviewing. Am. Psychol. 2009, 64, 527–537. [Google Scholar] [CrossRef]
- Keeley, R.D.; Brody, D.S.; Engel, M.; Burke, B.L.; Nordstrom, K.; Moralez, E.; Dickinson, L.M.; Emsermann, C. Motivational interviewing improves depression outcome in primary care: A cluster randomized trial. J. Consult. Clin. Psychol. 2016, 84, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Satre, D.D.; Leibowitz, A.; Sterling, S.A.; Lu, Y.; Travis, A.; Weisner, C. A randomized clinical trial of Motivational Interviewing to reduce alcohol and drug use among patients with depression. J. Consult. Clin. Psychol. 2016, 84, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Satre, D.D.; Delucchi, K.; Lichtmacher, J.; Sterling, S.A.; Weisner, C. Motivational interviewing to reduce hazardous drinking and drug use among depression patients. J. Subst. Abus. Treat. 2013, 44, 323–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harvey, A.G.; Soehner, A.M.; Kaplan, K.A.; Hein, K.; Lee, J.; Kanady, J.; Li, D.; Rabe-Hesketh, S.; Ketter, T.A.; Neylan, T.C.; et al. Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: A pilot randomized controlled trial. J. Consult. Clin. Psychol. 2015, 83, 564–577. [Google Scholar] [CrossRef]
- Chan, J.W.; Chan, N.Y.; Li, S.X.; Lam, S.P.; Chau SW, H.; Liu, Y.; Wing, Y.K. Change in circadian preference predicts sustained treatment outcomes in patients with unipolar depression and evening preference. J. Clin. Sleep Med. 2021, 18, 523–531. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Balker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. S20), 22–33. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Williams, J.B.; Terman, M. Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement (SIGH-ADS); New York State Psychiatric Insititute: New York, NY, USA, 2003. [Google Scholar]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar]
- Maier, W.; Buller, R.; Philipp, M.; Heuser, I. The Hamilton Anxiety Scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 1988, 14, 61–68. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallieres, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ranieri, W.F. Scale for Suicide Ideation: Psychometric properties of a self-report version. J. Clin. Psychol. 1988, 44, 499–505. [Google Scholar] [CrossRef]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef]
- Hays, R.D.; Sherbourne, C.D.; Mazel, R.M. The rand 36-item health survey 1.0. Health Econ. 1993, 2, 217–227. [Google Scholar] [CrossRef]
- Leung, C.M.; Wing, Y.K.; Kwong, P.K.; Shum, A.L. Validation of the Chinese-Cantonese version of the Hospital Anxiety and Depression Scale and comparison with the Hamilton Rating Scale of Depression. Acta Psychiatr. Scand. 1999, 100, 456–461. [Google Scholar] [CrossRef]
- Yu, D.S. Insomnia Severity Index: Psychometric properties with Chinese community-dwelling older people. J. Adv. Nurs. 2010, 66, 2350–2359. [Google Scholar] [CrossRef]
- Li, X.Y.; Phillips, M.R.; Tong, Y.S.; Li, K.J.; Zhang, Y.L.; Zhang, Y.P.; Yajua, N. Reliability and Validity of the Chinese version of Beck Suicide Ideation Scale (BSI-CV) in adult community residents. Chin. Ment. Health J. 2010, 24, 250–255. [Google Scholar]
- Wong, W.S.; Fielding, R. Construct validity of the Chinese version of the Chalder Fatigue Scale in a Chinese community sample. Psychosomatics 2010, 68, 89–93. [Google Scholar] [CrossRef]
- Lam, C.L.; Gandek, B.; Ren, X.S.; Chan, M. Tests of Scaling Assumptions and Construct Validity of the Chinese (HK) Version of the SF-36 Health Survey. J. Clin. Epidemiol. 1998, 51, 1139–1147. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).